Abstract
Background:
Microbiota-based treatments reduce the incidence of recurrent Clostridioides difficile infections (rCDIs), but prospectively collected safety data needed to broaden patient access and protect public health have been limited.
Objectives:
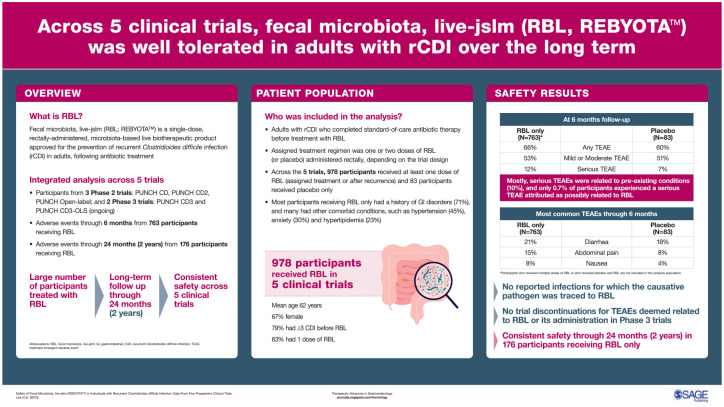
We provide cumulative safety data from five prospective clinical trials evaluating fecal microbiota, live-jslm (RBL) – the first microbiota-based live biotherapeutic product approved by the US Food and Drug Administration – for preventing rCDI in adults.
Design:
Integrated safety analysis includes three phase II trials (PUNCH CD, PUNCH CD2, PUNCH Open-Label) and two phase III trials (PUNCH CD3, PUNCH CD3-OLS) of RBL.
Methods:
Trial participants were at least 18 years of age with documented rCDI who completed standard-of-care antibiotic therapy before treatment with RBL. Assigned study treatment regimen was one or two doses of RBL (or placebo) administered rectally, depending on the trial design. In four of the five trials, participants with CDI recurrence within 8 weeks after RBL or placebo administration were eligible for treatment with open-label RBL. Treatment-emergent adverse events (TEAEs) were recorded for at least 6 months following last study treatment; in PUNCH CD2 and PUNCH Open-Label trials, TEAEs and serious TEAEs were collected through 12 and 24 months, respectively.
Results:
Among the five trials, 978 participants received at least one dose of RBL (assigned treatment or after recurrence) and 83 participants received placebo only. TEAEs were reported in 60.2% of Placebo Only participants and 66.4% of RBL Only participants. Only abdominal pain, nausea, and flatulence were significantly higher in the RBL Only group compared with the Placebo Only group. Most TEAEs were mild or moderate in severity and were most frequently related to preexisting conditions. There were no reported infections for which the causative pathogen was traced to RBL. Potentially life-threatening TEAEs were infrequent (3.0% of participants).
Conclusion:
Across five clinical trials, RBL was well tolerated in adults with rCDI. In aggregate, these data consistently demonstrated the safety of RBL.
Keywords: CDI, Clostridioides difficile, dysbiosis, fecal microbiota transplantation, live biotherapeutic product, RBX2660, microbiome-based therapeutic, microbiome restoration, rCDI, safety
Graphical Abstract
Introduction
Clostridioides difficile infection (CDI) is the most common cause of infectious diarrhea in hospitalized patients and has been designated an urgent public health threat by the US Centers for Disease Control and Prevention (CDC).1–3 CDI can be debilitating and potentially fatal, with a 30-day mortality rate of 9% in patients 65 years of age and older with hospital-associated CDI. 4
The healthy human colon contains a diverse composition of microbiota, up to 90% of which belong to the Bacteroidetes and Firmicutes phyla. 5 These bacteria work symbiotically to maintain overall health through functions such as colonization resistance, a phenomenon of pathogen exclusion via complex niche occupation, biofilm formation, and microbial metabolic activity. 6
C. difficile is an anaerobic, spore-forming, toxin-producing bacterium that can be acquired from the environment via the fecal-oral route. 7 A diverse and healthy gut microbiota is able to draw on multiple colonization resistance mechanisms to prevent C. difficile spore germination and outgrowth of vegetative cells, including competing for key nutrients, producing inhibitory bile acids, short-chain fatty acids, and bacteriocins, and lowering the luminal pH. 8 Multiple risk factors for CDI have been identified and include advanced age, hospitalization, underlying comorbidities, and antibiotic use.5,9Via their elimination pathways through the intestinal tract, broad-spectrum antibiotics destroy gut microbes – creating a condition termed dysbiosis – that results in reduced colonization resistance, enabling the once dormant spores to germinate, proliferate into toxin-producing vegetative cells, and cause diarrheal disease. 10
Major medical societies globally recommend antimicrobial treatment for CDI with either fidaxomicin or vancomycin, which help eliminate the vegetative, toxin-producing phase of C. difficile.11,12 However, these antibiotics either do not affect (vancomycin) or do not fully eliminate (fidaxomicin) C. difficile spores, which can persist and germinate in a setting of persistent dysbiosis and thereby increase the risk for recurrent CDI (rCDI). 13 Of patients who develop an initial CDI, up to 35% may recur within the first 8 weeks following completion of antimicrobial therapy for that episode.7,14–17 rCDI is challenging to treat because the risks of recurrence, morbidity, and mortality increase with each subsequent infection, placing a significant burden on both the patients themselves and on the healthcare system. 18
A promising therapeutic option to break the cycle of rCDI is the instillation of healthy donor stool through fecal microbiota transplantation (FMT).19,20 The goal of FMT is to restore the diversity of the gut microbiome, counteract dysbiosis, and allow the microbiota to regain colonization resistance 20 following standard-of-care antimicrobial therapy. Since 2021, the American College of Gastroenterology, the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, and the European Society of Clinical Microbiology and Infectious Diseases clinical practice guidelines recommend FMT for patients with two or more recurrences of CDI who have failed appropriate antibiotic treatments.11,12,21 FMT is generally considered safe in the short term when rigorous donor screening procedures are employed. However, data and long-term follow-up from prospective, placebo-controlled trials of FMT are limited. 22 Furthermore, a lack of standardized manufacturing processes, route of administration, dose, and potency are viewed as challenges with FMT. In an effort to help address these issues and to meet requirements for eventual approval of a regulated drug product, the field of live biotherapeutic products emerged. 23
Fecal microbiota, live-jslm (REBYOTA™; RBL, previously known as RBX2660; Ferring Pharmaceuticals Inc., Parsippany, NJ, USA) is the first FDA-approved, single-dose, microbiota-based live biotherapeutic product for the prevention of rCDI in adults following antibiotic treatment for rCDI. 24 Within 24–72 h of completing standard-of-care antibiotic therapy, RBL is administered rectally as a 150-mL microbiota suspension containing a broad consortium of spore-forming and non-spore-forming bacteria, including Bacteroidetes and Firmicutes. RBL is manufactured according to good manufacturing practices and undergoes standardized screening procedures and pathogen testing in alignment with FDA requirements to help ensure patient safety. 25 The efficacy and safety of RBL has been assessed in five prospective clinical trials comprising more than 1000 total participants25–29; four of the five trials are complete and one is ongoing. We report the current integrated RBL safety results from the largest safety database of any microbiota-based live biotherapeutic product.
Methods
RBL
RBL is manufactured from human fecal matter sourced from qualified donors. Stool donations are collected at the manufacturing site, stored under controlled conditions, and can be traced back to a specific donor, date and health status at the time of donation. Donors are screened and blood and fecal matter are tested for a panel of transmissible pathogens. Screening and testing protocols have evolved over time in line with FDA recommendations; these protocols also closely align with international guidelines.30,31 Current pathogen testing includes but is not limited to HIV, hepatitis A/B/C, syphilis, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; agent for COVID-19), enteropathogenic Escherichia coli (E. coli), Shiga toxin-producing E. coli, norovirus, rotavirus, adenovirus, vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, other antibiotic-resistant bacterial strains, Vibrio, Listeria, intestinal parasites, and other enteric pathogens. Donors do not have dietary restrictions with respect to potential food allergens, and RBL may contain food allergens. The potential for RBL to cause adverse reactions due to food allergens is unknown. The fecal microbiota suspension is the filtrate generated by processing the fecal matter in a pre-defined ratio with a solution of polyethylene glycol (PEG) 3350 and saline. Each 150 mL dose of RBL contains between 1 × 108 and 5 × 1010 colony forming units (CFU) per mL of fecal microbes, including >1 × 105 CFU/mL of Bacteroides, and contains not greater than 5.97 g of PEG3350 in saline.
Trial design
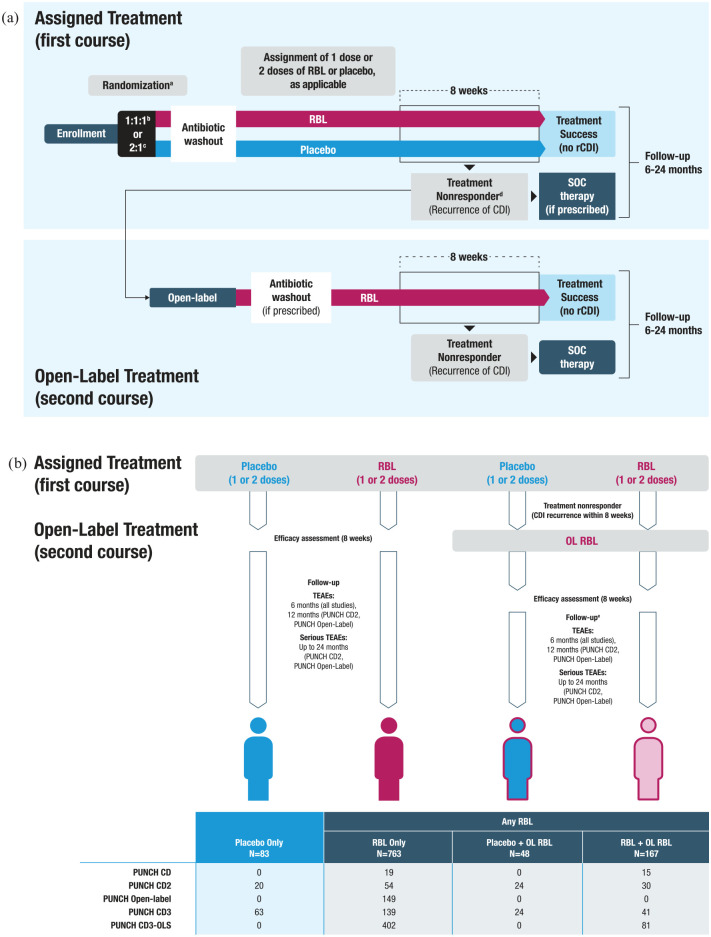
Safety data from five prospective trials were combined (three phase II and two phase III; Figure 1(a) and Supplemental Table 1).25,26,29 These trials had similar protocols and evaluated the same product that was generated using standardized manufacturing practices and iteratively evolving pathogen screening to help ensure product safety. All trials are complete except for one ongoing trial (PUNCH CD3-OLS); data from participants enrolled into that trial between 30 July 2019 and 25 March 2022 were incorporated into this analysis. All trials enrolled adults at least 18 years of age with rCDI who received antibiotics for their enrolling CDI episode before study treatment. Dosing regimens differed between the trials in that either a single dose or two doses of RBL and/or placebo administered 7 ± 2 days apart may have been administered. The two-dose regimen was considered to be one treatment course. Most participants that received two doses of RBL received product from different donors. Four trials (PUNCH CD, PUNCH CD2, PUNCH CD3, and PUNCH CD3-OLS) allowed an open-label (OL) treatment (one or two doses, depending on the trial) with RBL if a CDI recurrence was confirmed within 8 weeks of initial treatment course. Participants were followed regardless of whether they received OL treatment. All trials included at least 6 months of follow-up after the last dose of RBL or placebo (e.g. the follow-up schedule restarted to allow for 6 months of follow-up after receipt of OL treatment course); PUNCH CD2 and PUNCH Open-Label trials included 2 years of follow-up safety data after the last treatment course.
Figure 1.
Overview of trial designs and treatment group assignment.
aNo randomization in PUNCH CD, PUNCH Open-Label, and PUNCH CD3-OLS.
bPUNCH CD2 participants were stratified at baseline by the type of antibiotic used: vancomycin, fidaxomicin, or metronidazole.
cPUNCH CD3 participants were stratified at baseline by the type of antibiotic used: vancomycin, vancomycin in combination, fidaxomicin, other.
dTreatment nonresponders could have received SOC or OL treatment.
eIn the case a participant received a second course of therapy, the duration of safety follow-up was reset to allow 6 months of follow-up after OL treatment.
CDI, Clostridioides difficile infection; OL, open-label; RBL, fecal microbiota, live-jslm; rCDI, recurrent Clostridioides difficile infection; SOC, standard-of-care; TEAE, treatment-emergent adverse event.
In-office visits occurred during the first 8 weeks after treatment. Telephone visits occurred during the first 8 weeks and at months 3 or 4, and 6 after treatment (minor scheduling differences occurred depending on the trial); and also at months 12 and 24 in PUNCH CD2 and PUNCH Open-Label trials.
All trials included stopping rules indicating study pause or termination if a pathogenic intestinal infection was detected for which RBL or the administration procedure was a considered probable cause, or if any series of events of major significance such as death or other serious outcome for which a causal connection to RBL or its administration was plausible and represented an excess of the important adverse event(s) (AEs).
Safety analysis
The safety population was defined as any participant who received study treatment (RBL or placebo). Because participants may have received a combination of placebo and RBL, participants were assigned to only one of the four treatment groups for the safety analysis (i.e. treatment group assignment was mutually exclusive) (Figure 1(b)). Participants who received one treatment course are included in the Placebo Only or RBL Only groups. If a participant received a second course of OL RBL treatment following a confirmed recurrence of CDI, they are included in the ‘+ OL RBL’ groups and separated depending on their first course of treatment. All participants who received any RBL (assigned or OL treatment) are denoted as the ‘Any RBL’ group.
Safety analyses are presented from baseline through 6 months after last treatment. In the event a participant received OL RBL, the duration of safety follow-up was reset to allow 6 months of follow-up after OL treatment. AEs were defined as any untoward medical occurrence in a clinical investigation participant associated with the use of investigational product (IP), which does not necessarily have a causal relationship with the treatment. An AE can therefore be any unfavorable and/or unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of IP. Treatment-emergent adverse events (TEAEs) were defined as any AE occurring on or after the day of first treatment and were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0. TEAEs are presented by MedDRA System Organ Classes (type) and Preferred Terms (PTs; exact description of medical condition). TEAEs leading to trial discontinuation were recorded in PUNCH CD3 and PUNCH CD3-OLS. Serious adverse events (SAEs) were defined as an AE or adverse reaction meeting any of the following outcomes: event resulted in death; event was life-threatening; event required hospitalization >24 h or prolongation of an existing hospitalization; persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions; congenital anomaly/birth defect; important medical event. SAEs with an onset on or after the day of first treatment are referred to as serious TEAEs. For long-term safety data from PUNCH CD2 and PUNCH Open-Label trials, TEAEs (AEs and SAEs) were collected at 6 and 12 months of follow-up; events between 12 and 24 months were collected during the trial exit interview and captured serious TEAEs after the last dose of RBL or placebo.
Solicited AEs were collected from participants via a daily diary from the date of enrollment through the seventh day after receiving the assigned study treatment (PUNCH CD, PUNCH CD3, and PUNCH CD3-OLS) or through the seventh day after receiving the second dose of assigned study treatment (PUNCH CD2 and PUNCH Open-Label). For participants who had a confirmed CDI recurrence within 8 weeks of the initial treatment course and opted to receive OL RBL, solicited AEs were collected in a new posttreatment diary from the day of the first OL RBL treatment to the seventh day after receiving the last OL RBL treatment. The solicited events were flatulence, abdominal distension or bloating, increased diarrhea, abdominal pain or cramping, constipation, fever, chills/severe shivering, rectal irritation or pain, rectal bleeding, nausea, and vomiting. The investigator assessed the frequency and severity of these events to determine if an AE report was warranted.
Statistical analysis
Risk difference (RD) analysis for TEAEs in the double-blind, placebo-controlled trials PUNCH CD2 and PUNCH CD3 was based on medical conditions (by PT) with ⩾ 5% incidence within a treatment group and PTs that were anticipated AEs or major complications of CDI. The RD was calculated as the difference in the percentage between the two treatment columns, RBL and placebo. The 95% confidence intervals (CIs; two-sided) were calculated using a normal distribution approximation.
Results
Participant demographics and disposition
Demographics and disposition of the 1061 participants in the safety population are detailed in Table 1. Across treatment groups, most participants were white females who had experienced at least three episodes (i.e. at least two recurrences) of CDI before enrollment and had received vancomycin for the enrolling episode. A total of 48 participants treated with placebo and 167 treated with RBL experienced CDI recurrence and opted to receive OL RBL treatment. Across all trials, 978 participants received at least one dose of RBL.
Table 1.
Participant demographics, baseline disease, and disposition.
| Number of participants, n (%) | |||||
|---|---|---|---|---|---|
| Placebo Only | RBL | ||||
| RBL Only | Placebo + OL RBL | RBL + OL RBL | Any RBL | ||
| N = 83 | N = 763 | N = 48 | N = 167 | N = 978 | |
| Age, mean (SD) | 58.1 (16.5) | 61.4 (17.6) | 58.0 (18.2) | 62.8 (18.3) | 61.5 (17.8) |
| <65 years, n (%) | 52 (62.7) | 395 (51.8) | 27 (56.3) | 79 (47.3) | 501 (51.2) |
| ⩾65 years, n (%) | 31 (37.3) | 368 (48.2) | 21 (43.8) | 88 (52.7) | 477 (48.8) |
| Female, n (%) | 60 (72.3) | 516 (67.6) | 30 (62.5) | 111 (66.5) | 657 (67.2) |
| Race group, n (%) | |||||
| White | 75 (90.4) | 713 (93.4) | 46 (95.8) | 158 (94.6) | 917 (93.8) |
| Other than White | 8 (9.6) | 49 (6.4) | 2 (4.2) | 9 (5.4) | 60 (6.1) |
| Not reported | 0 | 1 (0.1) | 0 | 0 | 1 (0.1) |
| No. previous CDI episodes before trial entry, n (%) | |||||
| 1 a –3 | 60 (72.3) | 450 (59.0) | 23 (47.9) | 98 (58.7) | 571 (58.4) |
| ⩾3 | 57 (68.7) | 595 (78.0) | 40 (83.3) | 133 (79.6) | 768 (78.5) |
| Antibiotic for qualifying CDI episode, n (%) | |||||
| Vancomycin alone | 73 (88.0) | 623 (81.7) | 45 (93.8) | 151 (90.4) | 819 (83.7) |
| Vancomycin in combination | 2 (2.4) | 4 (0.5) | 0 | 1 (0.6) | 5 (0.5) |
| Fidaxomicin | 5 (6.0) | 65 (8.5) | 3 (6.3) | 6 (3.6) | 74 (7.6) |
| Other | 3 (3.6) | 45 (5.9) | 0 | 8 (4.8) | 53 (5.4) |
| Unknown | 0 | 20 (2.6) | 0 | 1 (0.6) | 21 (2.1) |
| Participant disposition | |||||
| Received treatment | 83 (100.0) | 763 (100.0) | 48 (100.0) | 167 (100.0) | 978 (100.0) |
| Completed 8-week follow-up b | 78 (94.0) | 672 (88.1) | 42 (87.5) | 146 (87.4) | 860 (87.9) |
| Completed 6-month follow-up b | 75 (90.4) | 583 (76.4) | 42 (87.5) | 126 (75.4) | 751 (76.8) |
| Completed 24-month follow-up b | 16 (19.3) | 145 (19.0) | 19 (39.6) | 18 (10.8) | 182 (18.6) |
CDI history for PUNCH CD3-OLS was incomplete at the time of this analysis.
After last treatment.
CDI, Clostridioides difficile infection; OL, open-label; RBL, fecal microbiota, live-jslm.
Percentage is calculated using the number of participants in the column heading as the denominator.
A greater percentage of participants in the RBL Only group were 65 years or older than in the Placebo Only group [48.2% (368 of 763) versus 37.3% (31 of 83)] (Table 1). A greater percentage of participants in the RBL Only [78.0% (595 of 763)], Placebo + OL RBL [83.3% (40 of 48)], and RBL + OL RBL [79.6% (133 of 167)] groups had at least three CDI episodes before trial entry compared with the Placebo Only group (68.7% [57 of 83]). Regarding medical history, a greater percentage of participants treated with RBL compared with Placebo Only had a history of gastrointestinal disorders, with gastroesophageal reflux disease, hemorrhoids, and a history of diverticular disease being the top three medical conditions [Any RBL: 70.4% (689 of 978) versus Placebo Only: 48.2% (40 of 83)] (Supplemental Table 2).
Across treatment groups, completion of the 8-week follow-up period ranged from 87.0% to 94.0% of participants, with 860 participants (87.9%) completing in the Any RBL group and 78 participants (94.0%) completing in the Placebo Only group (Table 1). The primary reason for discontinuation between treatment and 8 weeks of follow-up was withdrawal by participant in both the Placebo Only [100% (5 of 5)] and Any RBL [45.9% (34 of 74)] groups (Supplemental Table 3). Only 28 participants (2.6%) discontinued between the 8-week and 6-month follow-up, with the primary reason being ‘lost to follow-up’. A total of 751 participants (76.8%) who received any RBL (assigned or OL treatment) completed 6 months of follow-up after last treatment (Table 1). The difference between discontinuation and trial completion is attributable to the ongoing nature of PUNCH CD3-OLS (8-week follow-up data were not available for 44 PUNCH CD3-OLS participants at the time of this analysis).
In PUNCH CD2 and PUNCH Open-Label trials, 84.2% (16 of 19) of participants in the Placebo Only group and 82% (182 of 222) of participants in the Any RBL group who began long-term follow-up completed 24 months of follow-up (Table 1 and Supplemental Table 4).
Treatment exposure
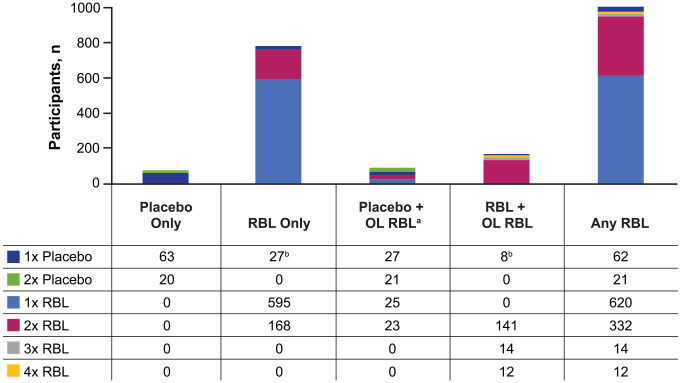
In PUNCH CD, PUNCH CD3, and PUNCH CD3-OLS, a complete treatment course consisted of one dose; in PUNCH CD2 and PUNCH Open-Label, a complete treatment course consisted of two doses administered approximately 1 week apart. With the exception of PUNCH-Open-Label, these trials offered an OL course of RBL to participants who experienced a CDI recurrence within 8 weeks of completing treatment. PUNCH CD2 offered up to two OL doses, and therefore, the number of protocol-defined exposures to RBL ranged from one to four doses.
Among 978 participants who received at least one dose of RBL (assigned treatment or after recurrence), 620 (63.4%) received one dose, 332 (33.9%) received two doses, 14 (1.4%) received three doses, and 12 (1.2%) received four doses (Figure 2). Eighty-three participants received placebo only.
Figure 2.
Exposure to study treatment by treatment groups.
aParticipants who received blinded placebo + OL RBL (n = 48) are counted for both treatments.
bParticipants in PUNCH CD2, group C, were assigned to receive a placebo dose after their blinded dose of RBL. This did not impact the treatment group assignment for the integrated analysis.
OL, open-label; RBL, fecal microbiota, live-jslm.
Safety
Across all treatment groups, most participants experienced TEAEs that were mild or moderate in severity and were most frequently related to preexisting conditions (Figure 3 and Supplemental Table 5). Through 6 months, TEAEs were reported in 60.2% (50 of 83) of Placebo Only participants and 66.4% (507 of 763) of RBL Only participants; most participants experienced mild [Placebo Only, 15.7% (13 of 83); RBL Only, 23.7% (181 of 763)] or moderate [Placebo Only, 34.9% (29 of 83); RBL Only, 28.8% (220 of 763)] TEAEs by maximum severity. Following OL RBL treatment, TEAEs were reported in 70.8% (34 of 48) of participants who received placebo as a first treatment course (Placebo + OL RBL) and 58.7% (98 of 167) of participants who received RBL as a first treatment course (RBL + OL RBL). Most participants who received OL treatment experienced TEAEs that were at most mild [Placebo + OL RBL, 25.0% (12 of 48); RBL + OL RBL, 16.2% (27 of 167)] or moderate [Placebo + OL RBL, 35.4% (17 of 48); RBL + OL RBL, 26.3% (44 of 167)] in severity, and similar to the rates reported in participants who received only one course of treatment.
Figure 3.
TEAEs by treatment groups and courses through 6 months. (a) Percentage of participants with TEAEs by maximum severity. Participants with multiple events were counted according to the event with the maximum severity. The severity grade of events with a missing severity grade were categorized as the maximum severity. (b) Percentage of participants with gastrointestinal disorder system organ class TEAEs by maximum severity.
aSafety follow-up was reset to allow for 6 months of follow-up after receiving OL treatment.
bIncludes any TEAE experienced by a participant in the three groups (RBL Only, Placebo + OL RBL, and RBL + OL RBL) that were exposed to RBL, which includes events after blinded placebo in the Placebo + OL RBL group.
Percentage is calculated using the number of participants in the column heading as the denominator.
CDI, Clostridioides difficile infection; OL, open-label; RBL, fecal microbiota, live-jslm; TEAE, treatment-emergent adverse event.
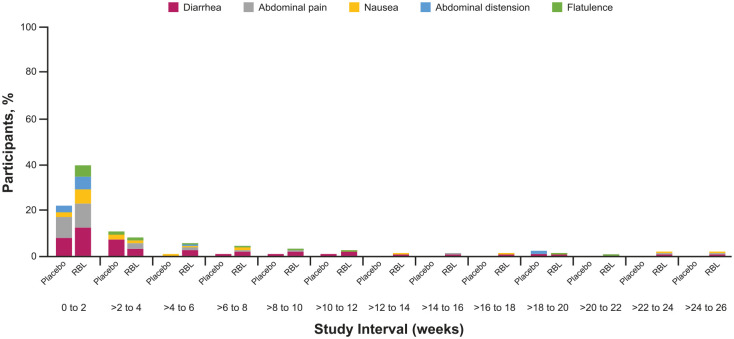
The most common TEAEs across all treatment groups and courses were in the gastrointestinal disorders system organ class and included diarrhea, abdominal pain, and nausea (Placebo Only: 18.1, 8.4, 3.6%, respectively; RBL Only: 21.2, 15.1, 8.4%, respectively; Table 2). These TEAEs occurred in close proximity to treatment in both the Placebo Only and RBL Only groups (Figure 4). No unexpected TEAEs were reported. The RDs for TEAEs were calculated for the double-blind, placebo-controlled trials, PUNCH CD2 and PUNCH CD3. Only abdominal pain [RD, 11.3; 95% CI, 3.1–19.5 (p = 0.0209)], nausea [RD, 7.3; 95% CI, 1.3–13.2 (p = 0.0614)], and flatulence [RD, 6; 95% CI, 1.7–10.4 (p = 0.0445)] were higher in the RBL Only group compared with the Placebo Only group (Supplemental Figure 1). In a separate analysis of time to onset and duration of gastrointestinal TEAEs in PUNCH CD2 and PUNCH CD3 participants, the median time to onset was 8.5 days [interquartile range (IQR): 3 days, 20 days] and 8 days [IQR: 3 days, 25 days] and the median duration was 3 days (IQR: 1 day, 15 days) and 1 day (IQR: 1 day, 5 days) for Placebo Only and RBL Only participants, respectively.
Table 2.
Treatment-emergent adverse events in ⩾5% of participants in any treatment group.
| MedDRA system organ class and preferred term | Number of participants, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| RBL | |||||||
| First (only) treatment course | First treatment course | Second treatment course | All courses | ||||
| Placebo Only | RBL Only | Placebo + OL RBL | RBL + OL RBL | Placebo + OL RBL | RBL + OL RBL | Any RBL | |
| N = 83 | N = 763 | N = 48 | N = 167 | N = 48 | N = 167 | N = 978 | |
| Gastrointestinal disorders | |||||||
| Diarrhea | 15 (18.1) | 162 (21.2) | 5 (10.4) | 26 (15.6) | 13 (27.1) | 34 (20.4) | 226 (23.1) |
| Abdominal pain | 7 (8.4) | 115 (15.1) | 9 (18.8) | 22 (13.2) | 7 (14.6) | 16 (9.6) | 160 (16.4) |
| Nausea | 3 (3.6) | 64 (8.4) | 2 (4.2) | 10 (6.0) | 7 (14.6) | 11 (6.6) | 91 (9.3) |
| Flatulence | 1 (1.2) | 54 (7.1) | 2 (4.2) | 10 (6.0) | 3 (6.3) | 7 (4.2) | 72 (7.4) |
| Constipation | 5 (6.0) | 40 (5.2) | 2 (4.2) | 10 (6.0) | 2 (4.2) | 13 (7.8) | 63 (6.4) |
| Abdominal distension | 3 (3.6) | 51 (6.7) | 3 (6.3) | 9 (5.4) | 3 (6.3) | 6 (3.6) | 69 (7.1) |
| General disorders and administration site conditions | |||||||
| Chills | 4 (4.8) | 25 (3.3) | 3 (6.3) | 10 (6.0) | 2 (4.2) | 6 (3.6) | 44 (4.5) |
| Pyrexia | 4 (4.8) | 22 (2.9) | 2 (4.2) | 6 (3.6) | 2 (4.2) | 9 (5.4) | 39 (4.0) |
| Infections and infestations | |||||||
| Urinary tract infection | 4 (4.8) | 43 (5.6) | 0 | 3 (1.8) | 1 (2.1) | 17 (10.2) | 64 (6.5) |
| Upper respiratory tract infection | 5 (6.0) | 19 (2.5) | 0 | 0 | 1 (2.1) | 3 (1.8) | 23 (2.4) |
| Viral upper respiratory tract infection | 2 (2.4) | 15 (2.0) | 0 | 0 | 3 (6.3) | 2 (1.2) | 20 (2.0) |
| Metabolism and nutrition disorders | |||||||
| Dehydration | 1 (1.2) | 5 (0.7) | 1 (2.1) | 5 (3.0) | 3 (6.3) | 3 (1.8) | 17 (1.7) |
Coding was based on MedDRA, version 20.0.
A participant with multiple events coded to the same preferred term (PT) within a primary system organ class (SOC) was counted only once for the PT within the primary SOC. A participant with multiple events coded to the same SOC was counted only once within the SOC.
CDI, Clostridioides difficile infection; MedDRA, Medical Dictionary for Regulatory Activities; OL, open-label; RBL, fecal microbiota, live-jslm.
Figure 4.
Gastrointestinal disorder system organ class TEAEs in ⩾5% of participants by onset interval. Percentage of Placebo Only and RBL Only participants who reported gastrointestinal adverse events through 26 weeks after blinded treatment administration. TEAEs were assigned to an onset interval according to their start date; the end date was not considered. A participant with multiple TEAEs coded to the same preferred term (e.g. diarrhea) were counted only once for a given interval, but could occur in multiple time intervals, depending on the onset dates of repeat TEAEs for a given participant.
RBL, fecal microbiota, live-jslm; TEAE, treatment-emergent adverse event.
The incidence of serious TEAEs was higher in the RBL Only group [12.3% (94 of 763)] compared with the Placebo Only group [7.2% (6 of 83)] (Supplemental Table 5). Most serious TEAEs were related to CDI [RBL Only, 30 of 763 (3.9%) participants] and preexisting conditions [RBL Only, 73 of 763 (9.6%) participants]. As shown in Supplemental Table 6, there was no clustering of terms or types of serious TEAEs. CDI was the only serious TEAE with ⩾1% frequency in participants who received any RBL; these events were due to CDI recurrences requiring hospitalization.
In PUNCH CD3 and PUNCH CD3-OLS, five participants who received one treatment course with RBL and three participants who received two treatment courses with RBL discontinued due to TEAEs; no participants in the Placebo Only or Placebo + OL RBL groups discontinued the trial because of TEAEs (Supplemental Table 5). None of the TEAEs leading to trial discontinuation in PUNCH CD3 and PUNCH CD3-OLS were considered related to RBL or its administration by the investigators. Four of the TEAEs leading to trial discontinuation were fatal (events of multimorbidity, cardiorespiratory arrest, pulmonary sepsis, and complications of spina bifida); none of these events were considered related to RBL or its administration.
TEAEs leading to death within 6 months after treatment occurred in 1.8% (18 of 978) of Any RBL participants (Supplemental Table 5). TEAEs leading to death spanned various preferred terms across different SOCs, suggesting an implausible biological correlation (Supplemental Table 7). In 17 of the 18 participants, TEAEs were deemed related to preexisting conditions and unrelated to treatment. The death of one participant in PUNCH Open-Label who had severe CDI recurrence on day 21 was considered related to CDI and cardiovascular comorbidities and possibly related to RBL by the investigator. 29 The event was reviewed by the independent medical monitor and determined to not be a product-related safety concern.
Long-term adverse events
Participants in PUNCH CD2 and PUNCH Open-Label who began long-term follow-up are shown by treatment group in Supplemental Table 8. A numerically higher percentage of participants in the RBL Only [56.3% (99 of 176)] than in the Placebo Only group [47.4% (9 of 19)] had at least one TEAE between 6 and 24 months after administration of the first dose of the most recent treatment course, with mild and moderate events accounting for the difference; however, this comparison is limited by the small number of participants in the Placebo Only group who had 24 months of follow-up. Across treatment groups, most TEAEs were related to preexisting conditions; two TEAEs (0.9%) were related to RBL. One event was vertigo, occurring on day 363 in a participant treated with two blinded doses of RBL in PUNCH CD2; the relatedness was not assessed/unknown and was therefore imputed as ‘definitely’ related to the IP. This could not be corrected or further clarified by the source documents. The other TEAE was diarrhea occurring on day 183 after treatment with two doses in PUNCH Open-Label, which was attributed as possibly related to RBL and CDI. One participant in the RBL Only group discontinued the trial because of a TEAE deemed possibly related to a preexisting condition and unrelated to the product, administration procedure, or CDI. None of the potentially life-threatening TEAEs were considered related to RBL.
Serious TEAEs were reported in 31.6% (6 of 19) of participants in the Placebo Only group and 29.0% (51 of 176) of participants in the RBL Only group (Supplemental Table 8). Across all treatment groups, serious TEAEs were considered related to CDI or preexisting conditions, and none were considered related to RBL or its administration. Between 6 and 24 months, TEAEs leading to death were reported in 7.4% (13 of 176) and 10.5% (2 of 19) of participants in the RBL Only and Placebo Only groups, respectively (Supplemental Table 8). TEAEs leading to death reported in more than one participant in the Any RBL group were cardiac arrest (two), death (three), sepsis (three), and acute kidney injury (three); no TEAE leading to death in the Placebo Only group occurred in more than one participant in any SOC (Supplemental Table 9). There were no clusters of TEAEs leading to death in any SOC; however, characterizations are limited by the small number of events. None of the deaths from 6 to 24 months were considered related to treatment (Supplemental Table 9).
Discussion
The RBL safety database currently comprises final data from four completed trials and preliminary results from the ongoing trial PUNCH CD3-OLS. This integrated analysis, which combines the safety data of over 900 RBL-treated participants through 6 months and nearly 200 RBL-treated participants through 2 years of follow-up, adds to the growing body of evidence that suggests FMT is safe in both the short term and long term. Nearly 50% of RBL-treated participants were over 65 years of age and nearly 80% had three or more prior CDI episodes, underscoring the unmet need and safety concerns for the average CDI patient with age-related comorbidities.
Patients with multiply recurrent CDI have a persistently dysbiotic microbiome, and FMT has been shown to normalize microbial diversity in clinical trials with a low rate of severe or life-threatening AEs. 32 A recent meta-analysis from 20 randomized controlled trials and 109 non-randomized controlled trials between 2000 and 2020 with CDI being the most common indication for FMT reported that 19% of patients had FMT-related TEAEs and 1.4% of patients had FMT-related serious TEAEs. 33 The most commonly reported TEAEs were diarrhea, abdominal discomfort, nausea, vomiting, and flatulence. 33 A similar percentage of RBL Only participants experienced RBL related TEAEs or serious TEAEs. GI events including diarrhea, abdominal pain, and nausea were the most frequently reported TEAEs; most of these events were in close proximity to treatment, and many were related to treatment failure and CDI recurrence. New onset post-infectious IBS can occur in up to one in four patients with rCDI, which could also contribute to the high incidence of gastrointestinal events across all treatment groups in this integrated analysis.34,35 Importantly, most events resolved within a few days.
CDI is a serious illness that can lead to complications, including sepsis, colectomy, and death – the risks of which increase with each subsequent CDI recurrence.18,36 Recent retrospective claims analyses found that approximately 27% of patients aged 18–64 years and 35% of patients aged ⩾65 years with one rCDI event experienced sepsis within 12 months of the initial CDI; these percentages are 20–30 times greater than the less than 1% of participants treated with RBL that experienced sepsis or bacteremia.18,37 Importantly, there were no reported infections for which the causative pathogen was traced to RBL. In a separate retrospective claims analysis, CDI-associated death within 12 months was 16%, 31%, and 39% for patients 65 years of age and older with one, two, and three or more episodes of rCDI, respectively. 38 A study of the 10 US sites within the CDC population- and laboratory-based surveillance program for CDI found a CDI-related mortality rate of 1.3% for community-associated and 9.3% for hospital-associated cases. 4 In light of these statistics, the rate of all-cause death within 6 months and between 6 and 24 months in the Any RBL group was low (2.4% and 7.2%, respectively).4,29,38,39 All but one participant death, due to relapsed CDI on study day 21, were deemed related to CDI and preexisting conditions. Upon review, the independent medical monitor determined this death to be unrelated to RBL. Considering the total treatment exposure time of 978 participants who received any RBL compared with 83 participants who received placebo only (404 patient-years versus 42 patient-years), the observed incidences of death for participants treated with RBL or placebo were low and consistent with the literature.
Because FMT and RBL are manufactured from human fecal matter, they may carry a risk of transmitting infectious agents. Inadequately screened donor stool can potentially lead to pathogen transmission, as documented in FDA safety alerts reporting E. coli transmission and heightened safety concerns around COVID-19 and Mpox.40–44 Although no donor-derived infections have been observed with RBL, a conceptual risk for these transmissions remains. To help address these risks, RBL donor screening and testing continues to be evaluated and iteratively updated through standard operating procedures that monitor public health announcements and threats, and in accordance with FDA requirements. For example, batches produced before the COVID pandemic were used exclusively while the sponsor developed a COVID-19 testing program that met FDA specifications. Stool donors are now required to undergo screening to assess for exposure to SARS-CoV-2 and COVID-19 symptoms and testing for SARS-CoV-2. Testing requirements include at least two negative test results no greater than 14 days before stool donation and two additional negative tests after the date of stool donation. A more recent example is Mpox – prospective donors will be required to complete a questionnaire aimed at identifying those at risk for contracting Mpox or who may have recently been infected with Mpox.
The mechanism of delivery is an important safety consideration for FMT and other microbiota-based therapies. Delivery routes include esophagogastroduodenoscopy (EGD), colonoscopy, enema, and oral capsule. While the specific complications associated with EGD and colonoscopy – cardiopulmonary AEs, aspiration, perforation, bleeding, infection, and death – have been rarely reported, they must be considered for each patient, in particular for medically complex patients.45–48 RBL was developed for rectal administration without the need for bowel preparation or sedation to avoid more invasive procedures and increase accessibility. This mechanism of delivery can also benefit patients at higher risk of anesthetic complications associated with sedation and those unable or unwilling to swallow pills.49,50
This analysis is limited in that patients with fulminant CDI and life expectancy <12 months were excluded from all RBL trials; patients with IBD were also excluded from all trials except PUNCH CD3-OLS. The safety of RBL in these populations remains to be assessed.
This integrated safety analysis of RBL, which is the largest safety evaluation of any microbiota-based live biotherapeutic product reported to date, demonstrates that RBL was optimized for patient safety through rigorous donor screening, standardized manufacturing protocols, and a noninvasive delivery mechanism. The integrated data from five prospective clinical trials provide further evidence RBL is a safe and well-tolerated treatment for patients with rCDI.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231174277 for Safety of fecal microbiota, live-jslm (REBYOTA™) in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical trials by Christine Lee, Thomas Louie, Lindy Bancke, Beth Guthmueller, Adam Harvey, Paul Feuerstadt, Sahil Khanna, Robert Orenstein and Erik R. Dubberke in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors thank the investigators, site staff, participants, and their families and caregivers. Medical writing support, under the guidance of the authors, was provided by Michelle Boland, PhD (ApotheCom, Yardley, PA, USA), and was funded by Ferring Pharmaceuticals, Parsippany, NJ, USA.
Footnotes
ORCID iDs: Paul Feuerstadt  https://orcid.org/0000-0002-7643-9576
https://orcid.org/0000-0002-7643-9576
Sahil Khanna  https://orcid.org/0000-0002-7619-8338
https://orcid.org/0000-0002-7619-8338
Robert Orenstein  https://orcid.org/0000-0001-5210-1258
https://orcid.org/0000-0001-5210-1258
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Christine Lee, Medical Microbiologist and Researcher, Island Health, Clinical Professor, Department of Pathology and Laboratory Medicine, The University of British Columbia Faculty of Medicine, Vancouver, BC V6T 1Z3, Canada; Island Medical Program University of British Columbia, University of Victoria, Victoria, BC, Canada.
Thomas Louie, University of Calgary and Foothills Medical Center, Calgary, AB, Canada.
Lindy Bancke, Rebiotix Inc., a Ferring Company, Roseville, MN, USA.
Beth Guthmueller, Rebiotix Inc., a Ferring Company, Roseville, MN, USA.
Adam Harvey, Rebiotix Inc., a Ferring Company, Roseville, MN, USA.
Paul Feuerstadt, Yale School of Medicine, New Haven, CT, USA.
Sahil Khanna, Mayo Clinic, Rochester, MN, USA.
Robert Orenstein, Mayo Clinic, Phoenix, AZ, USA.
Erik R. Dubberke, Washington University School of Medicine, St. Louis, MO, USA
Declarations
Ethics approval and consent to participate: All trials were conducted in the United States and Canada according to the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines, principles of informed consent, and requirements of publicly registered clinical trials. All participants provided written informed consent and all protocols received institutional review board/research ethics board approval before commencement.
Consent for publication: All participants provided written informed consent for publication.
Author contribution(s): Christine Lee: Investigation; Writing – original draft; Writing – review & editing.
Thomas Louie: Investigation; Writing – original draft; Writing – review & editing.
Lindy Bancke: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Beth Guthmueller: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Adam Harvey: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Paul Feuerstadt: Investigation; Writing – original draft; Writing – review & editing.
Sahil Khanna: Investigation; Writing – original draft; Writing – review & editing.
Robert Orenstein: Investigation; Writing – original draft; Writing – review & editing.
Erik R. Dubberke: Investigation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These trials were sponsored by Ferring Pharmaceuticals.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CL received grants from Rebiotix Inc., a Ferring Company, Seres Therapeutics, and Summit Therapeutics for clinical trials. TL received grants from Rebiotix Inc, Seres Therapeutics, Summit Therapeutics and Finch Therapeutics. RO and ERD serve on the Rebiotix Physician Advisory Board. ERD received grants from Synthetic Biologics and Pfizer, and is a consultant for Pfizer, Merck, Seres Therapeutics, Summit Therapeutics, Ferring Pharmaceuticals and Abbott. PF is a consultant/advisory board member and speaker for Ferring Pharmaceuticals, Seres Therapeutics, and Takeda Pharmaceuticals and consultant for Merck & Co., Inc. LB, BG, and AH are employees of Rebiotix Inc., a Ferring Company. SK is a consultant/advisory board member for Ferring Pharmaceuticals, Takeda Pharmaceuticals, Niche Pharmaceuticals, and Immuron Ltd.
Availability of data and materials: The datasets generated and/or analyzed during the current study are not publicly available but may be available from the corresponding author on reasonable request.
References
- 1.Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care–associated infections in U.S. Hospitals. New Engl J Med 2018; 379: 1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Antibiotics resistance threats in the United States 2019. Vol. 2021. Washington, DC: US Department of Health and Human Services, 2019. [Google Scholar]
- 3.Balsells E, Shi T, Leese C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health 2019; 9: 010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. New Engl J Med 2015; 372: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J 2017; 474: 1823–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits WK, Lyras D, Lacy DB, et al. Clostridium difficile infection. Nat Rev Dis Primers 2016; 2: 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seekatz AM, Safdar N, Khanna S. The role of the gut microbiome in colonization resistance and recurrent Clostridioides difficile infection. Therap Adv Gastroenterol 2022; 15: 17562848221134396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract 2013; 26: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 2014; 16: 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly CR, Fischer M, Allegretti JR. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol 2021; 116: 1124–1147. [DOI] [PubMed] [Google Scholar]
- 12.Johnson S, Lavergne V, Skinner AM, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73: e1029–e1044. [DOI] [PubMed] [Google Scholar]
- 13.Chaar A, Feuerstadt P. Evolution of clinical guidelines for antimicrobial management of Clostridioides difficile infection. Therap Adv Gastroenterol 2021; 14: 17562848211011953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox MA, Hernandez LD, Gupta P, et al. Chapter 5: Assays for measuring C. difficile toxin activity and inhibition in mammalian cells. In: Enany S. (ed.) Clostridium difficile – a comprehensive overview. London: IntechOpen, 2017, pp. 111–127. [Google Scholar]
- 15.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 2012; 18 Suppl6: 21–27. [DOI] [PubMed] [Google Scholar]
- 16.McDonald LC, Coignard B, Dubberke E, et al. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 2007; 28: 140–145. [DOI] [PubMed] [Google Scholar]
- 17.Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55 Suppl2: S154–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med 2021; 9: 2050312120986733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Ananthakrishnan AN. Economic burden and cost-effectiveness of therapies for Clostridiodes difficile infection: a narrative review. Therap Adv Gastroenterol 2021; 14: 17562848211018654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tariq R, Pardi DS, Bartlett MG, et al. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis 2019; 68: 1351–1358. [DOI] [PubMed] [Google Scholar]
- 21.van Prehn J, Reigadas E, Vogelzang EH, et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect 2021; 27 Suppl2: S1–s21. [DOI] [PubMed] [Google Scholar]
- 22.Sandhu A, Chopra T. Fecal microbiota transplantation for recurrent Clostridioides difficile, safety, and pitfalls. Therap Adv Gastroenterol 2021; 14: 17562848211053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Department of Health and Human Services. Guidance for industry. Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information. Vol. 2022. Washington, DC: US Department of Health and Human Services, 2016. [Google Scholar]
- 24.REBYOTATM (fecal microbiota, live - jslm) suspension, for rectal use [prescribing information]. Roseville, MN: Ferring Pharmaceuticals, 2022. [Google Scholar]
- 25.Orenstein R, Dubberke E, Hardi R, et al. Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection: results of the PUNCH CD Study. Clin Infect Dis 2016; 62: 596–602. [DOI] [PubMed] [Google Scholar]
- 26.Dubberke ER, Orenstein R, Khanna S, et al. Final results from a phase 2b randomized, placebo-controlled clinical trial of RBX2660: a microbiota-based drug for the prevention of recurrent Clostridioides difficile infection. Infect Dis Ther 2023; 12: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blount KF, Shannon WD, Deych E, et al. Restoration of bacterial microbiome composition and diversity among treatment responders in a phase 2 trial of RBX2660: an investigational microbiome restoration therapeutic. Open Forum Infect Dis 2019; 6: ofz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langdon A, Schwartz DJ, Bulow C, et al. Microbiota restoration reduces antibiotic-resistant bacteria gut colonization in patients with recurrent Clostridioides difficile infection from the open-label PUNCH CD Study. Genome Med 2021; 13: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein R, Dubberke ER, Khanna S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis 2022; 22: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cammarota G, Ianiro G, Kelly CR, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut2019; 68: 2111–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller JJ, Ooijevaar RE, Hvas CL, et al. A standardised model for stool banking for faecal microbiota transplantation: a consensus report from a multidisciplinary UEG Working Group. United European Gastroenterol J 2021; 9: 229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. New Engl J Med 2013; 368: 407–415. [DOI] [PubMed] [Google Scholar]
- 33.Marcella C, Cui B, Kelly CR, et al. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther 2021; 53: 33–42. [DOI] [PubMed] [Google Scholar]
- 34.Dayananda P, Wilcox MH. Irritable bowel syndrome following Clostridium difficile infection. Curr Opin Gastroenterol 2019; 35: 1–5. [DOI] [PubMed] [Google Scholar]
- 35.Wadhwa A, Al Nahhas MF, Dierkhising RA, et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther 2016; 44: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin A, Nelson WW, Dreyfus J, et al. Mortality, healthcare resource utilization, and cost among medicare beneficiaries with Clostridioides difficile infection with and without sepsis. Ther Adv Infect Dis 2022; 9: 20499361221095679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feuerstadt P, Nelson WW, Teigland C, et al. Clinical burden of recurrent Clostridioides difficile infection in the medicare population: a real-world claims analysis. Antimicrob Steward Healthc Epidemiol 2022; 2: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerstadt P, Nelson WW, Drozd EM, et al. Mortality, health care use, and costs of Clostridioides difficile infections in older adults. J Am Med Dir Assoc 2022; 23: 1721–1728.e19. [DOI] [PubMed] [Google Scholar]
- 39.Reveles KR, Lawson KA, Mortensen EM, et al. National epidemiology of initial and recurrent Clostridium difficile infection in the Veterans Health Administration from 2003 to 2014. PLoS One 2017; 12: e0189227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms. Vol. 2022. Silver Spring, MD: US Food and Drug Administration, 2020. [Google Scholar]
- 41.US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse events likely due to transmission of pathogenic organisms. Vol. 2022. Silver Spring, MD: US Food and Drug Administration, 2020. [Google Scholar]
- 42.US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and additional safety protections pertaining to SARS-CoV-2 and COVID-19. Vol. 2021. Silver Spring, MD: US Food and Drug Administration, 2020. [Google Scholar]
- 43.US Food and Drug Administration. Safety alert regarding use of fecal microbiota for transplantation and additional safety protections pertaining to monkeypox virus, vol. 2023. Silver Spring, MD: US Food and Drug Administration, 2022. [Google Scholar]
- 44.Ianiro G, Mullish BH, Iqbal TH, et al. Minimising the risk of monkeypox virus transmission during faecal microbiota transplantation: recommendations from a European expert panel. Lancet Gastroenterol Hepatol 2022; 7: 979–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol 2016; 30: 705–718. [DOI] [PubMed] [Google Scholar]
- 46.Friedman-Korn T, Livovsky DM, Maharshak N, et al. Fecal transplantation for treatment of Clostridium difficile infection in elderly and debilitated patients. Dig Dis Sci 2018; 63: 198–203. [DOI] [PubMed] [Google Scholar]
- 47.Fretheim H, Chung BK, Didriksen H, et al. Fecal microbiota transplantation in systemic sclerosis: a double-blind, placebo-controlled randomized pilot trial. PLoS One 2020; 15: e0232739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev 2018; 11: Cd012774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman WK, Gibbons RJ. Perioperative cardiovascular assessment of patients undergoing noncardiac surgery. Mayo Clin Proc 2009; 84: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yancey R. Anesthetic management of the hypertensive patient: Part I. Anesth Prog 2018; 65: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231174277 for Safety of fecal microbiota, live-jslm (REBYOTA™) in individuals with recurrent Clostridioides difficile infection: data from five prospective clinical trials by Christine Lee, Thomas Louie, Lindy Bancke, Beth Guthmueller, Adam Harvey, Paul Feuerstadt, Sahil Khanna, Robert Orenstein and Erik R. Dubberke in Therapeutic Advances in Gastroenterology