Abstract
Rising rates of multidrug-resistant Klebsiella infections necessitate a comprehensive understanding of the major strains and plasmids driving spread of resistance elements. Here, we analysed 540 clinical, screen and environmental Klebsiella isolates recovered from across Wales between 2007 and 2020 using combined short- and long-read sequencing approaches. We identified resistant clones that have spread within and between hospitals including the high-risk strain sequence type (ST)307, which acquired the bla OXA-244 carbapenemase gene on a pOXA-48-like plasmid. We found evidence that this strain, which caused an acute outbreak largely centred on a single hospital in 2019, had been circulating undetected across South Wales for several years prior to the outbreak. In addition to clonal transmission, our analyses revealed evidence for substantial plasmid spread, mostly notably involving bla KPC-2 and bla OXA-48-like (including bla OXA-244) carbapenemase genes that were found among many species and strain backgrounds. Two thirds (20/30) of the bla KPC-2 genes were carried on the Tn4401a transposon and associated with IncF plasmids. These were mostly recovered from patients in North Wales, reflecting an outward expansion of the plasmid-driven outbreak of bla KPC-2-producing Enterobacteriaceae in North-West England. A total of 92.1 % (105/114) of isolates with a bla OXA-48-like carbapenemase carried the gene on a pOXA-48-like plasmid. While this plasmid family is highly conserved, our analyses revealed novel accessory variation including integrations of additional resistance genes. We also identified multiple independent deletions involving the tra gene cluster among pOXA-48-like plasmids in the ST307 outbreak lineage. These resulted in loss of conjugative ability and signal adaptation of the plasmids to carriage by the host strain. Altogether, our study provides, to our knowledge, the first high resolution view of the diversity, transmission and evolutionary dynamics of major resistant clones and plasmids of Klebsiella in Wales, and forms an important basis for ongoing surveillance efforts. This article contains data hosted by Microreact.
Keywords: carbapenem resistance, carbapenemase, genomic surveillance, Klebsiella, pOXA-48-like plasmids, ST307
Data Summary
All raw short-read sequence data and hybrid assemblies are available in the European Nucleotide Archive (ENA) under project accession number PRJEB48990. Individual accession numbers for samples are available in Tables S1 and S2.
Impact Statement.
Multidrug-resistant (MDR) Klebsiella infections are increasing worldwide, especially in healthcare settings where outbreaks are frequently reported. Studies of MDR Klebsiella have largely focused on regions with a high incidence of infections in which the strain diversity is often limited. Using genomic approaches, here we describe the major strains and plasmids driving spread of antibiotic-resistant Klebsiella in Wales, a largely non-endemic setting, between 2007 and 2020. We identified a high diversity of strains carrying numerous different carbapenemase genes, which confer resistance to an important class of antibiotics, the carbapenems. However, several known high-risk clones, including Klebsiella pneumoniae sequence type (ST)307, ST20 and ST15, which carried the carbapenemases bla OXA-244, bla IMP-4 and bla VIM-4, respectively, were responsible for significant spread in hospitals. We also identified healthcare-associated transmission of an ST1788 strain, carrying the extended-spectrum β-lactamase (ESBL) gene, bla CTX-M-15. To our knowledge, this ST has been rarely observed elsewhere yet is continuing to expand its distribution in hospitals across Wales. Finally, we uncovered key plasmid vectors driving the spread of carbapenemases, including the highly dominant pOXA-48-like plasmids carrying bla OXA-48-like genes. Altogether, this study provides a detailed understanding of the diversity and transmission dynamics of MDR Klebsiella in Wales, thereby supporting critical ongoing surveillance efforts.
Introduction
The genus Klebsiella , which belongs to the family Enterobacteriaceae , includes several species that are important opportunistic pathogens of humans. In particular, Klebsiella pneumoniae is a leading cause of healthcare-associated infections globally [1–3]. Klebsiella species cause a range of disease types, including pneumonia, skin and wound infections, urinary tract infections, and sepsis. These occur most frequently in the elderly, neonates and immunocompromised individuals [4].
Klebsiella are commonly carried in the gastrointestinal tract of healthy humans [5]. Analysis using whole-genome sequencing (WGS) performed on paired screening swabs and clinical samples has demonstrated that gastrointestinal carriage is a major reservoir of K. pneumoniae that cause infections [6]. In addition, outbreaks are frequently reported in healthcare settings, driven by transmission via person-to-person contact, contaminated environmental surfaces and medical devices (e.g. ventilators, catheters) [7–9].
Across the world, studies have reported an increasing prevalence of infections with multidrug-resistant (MDR) Klebsiella [10, 11]. A particular concern has been the rise of infections that are resistant to carbapenems, which has been listed as a ‘critical’ threat by the World Health Organization [12]. Carbapenems are a class of antibiotics that are vital for treatment of severe Klebsiella infections due to their broad spectrum of activity [e.g. against extended-spectrum β-lactamase (ESBL) producers] and limited adverse effects. Carbapenem resistance in Klebsiella is usually conferred by plasmid-encoded carbapenemase genes including bla KPC, bla OXA-48-like, bla VIM, bla NDM and bla IMP [13]. These are often associated with mobile genetic elements such as transposons, which commonly insert into plasmid backbones.
A new cohort of drugs has become available in recent years to treat carbapenem-resistant infections including novel β-lactam–β-lactamase inhibitor (BL-BLI) combinations (e.g. ceftazidime/avibactam, meropenem/vaborbactam) and the siderophore cephalosporin, cefiderocol. However, some of these drugs are not effective against certain types of carbapenemase producer, including the BL-BLIs, which have no activity against metallo-β-lactamases (bla VIM, bla NDM and bla IMP). These also may not work reliably against non-carbapenemase-producing carbapenem-resistant strains [14].
As a result, a multi-pronged approach is required for the control of MDR Klebsiella , including new therapeutics, heightened infection control and enhanced surveillance. The latter involves acquiring a detailed understanding of the diversity and transmission routes of different strains and plasmids. Integration of genome sequencing into surveillance systems, which is ongoing across many public-health laboratories, is providing a vital role in achieving this. However, while the current focus is largely on monitoring important strains and detecting clonal transmission, there is still a strong need for bioinformatic workflows that provide data on the role of specific plasmid vectors in resistance spread.
The Specialist Antimicrobial Chemotherapy Unit (SACU) based at Public Health Wales (PHW) Microbiology in Cardiff provides a reference laboratory service for the identification and confirmation of antimicrobial-resistance mechanisms in bacterial isolates including Klebsiella , referred by microbiology laboratories across and beyond Wales. It also collects Klebsiella (and other bacterial) isolates from blood cultures for surveillance purposes and receives isolates from suspected outbreaks as part of the development of PHW’s WGS-based typing service, in partnership with the Pathogen Genomics Unit (PenGU). This study describes the retrospective genomic analysis of a collection of Klebsiella isolates (n=540) gathered by SACU between 2007 and 2020. Using a combination of short- and long-read sequencing, our analysis revealed detailed insights into the evolutionary dynamics of the major K. pneumoniae clones and plasmids driving spread of antibiotic-resistance genes in Wales.
Methods
Sample collection
A total of 540 Klebsiella isolates were included in this study (Tables S1 and S2, available with the online version of this article). Isolates were obtained from clinical, screen or environmental samples between 2007 and 2020 and bead-stored at −80 °C within the SACU culture collection at PHW. They were cultured overnight from beads on blood agar (Oxoid) at 35±1°C prior to sequencing.
Short-read sequencing
Isolates were sub-cultured into 500 µl tryptone soya broth (TSB) (E and O Laboratories) and incubated overnight at 35±1 °C. Growth from 125 µl TSB culture was harvested at 12 000 g for 2 min and the supernatant discarded. A lysis step was performed by adding 190 µl buffer G2 (Qiagen) and 10 µl proteinase K (Qiagen), followed by incubation at 56 °C for 30 min. DNA was extracted from the lysates using a generic 3.0.4 protocol on the EMAG platform (bioMérieux) according to the manufacturer’s instructions and eluted in 100 µl final volume. DNA was quantified using the FLUOstar Omega fluorometer (BMG Labtech) and normalized to approximately 0.4 ng µl−1. Sequencing libraries were prepared using the Nextera XT DNA library preparation kit and combinations of IDT index plates (Illumina). The final libraries were cleaned through AMPure SPRI bead (Beckman Coulter) fragment size selection, and quantified by the Collibri library quantification kit (Invitrogen) before normalizing to approximately 0.5 nM. The normalized final libraries were pooled and loaded onto MiSeq or NextSeq platforms (Illumina).
Long-read sequencing
Isolates were sub-cultured into 2 ml LB broth (Becton Dickinson) and incubated overnight at 35±1 °C. DNA was extracted using the automated QIAcube (Qiagen) platform with an additional RNase step and then quantified using the Qubit 4.0 instrument (ThermoFisher) using HS and BR dsDNA kits as appropriate. DNA was purified and concentrated using SPRI beads (Mag-Bind TotalPure; Omega) at a 1 : 1 ratio with a final 15 µl elution in nuclease-free water. Genomic libraries were prepared using the Rapid Barcoding kit (SQK-RBK004; Oxford Nanopore Technologies). Eight to twelve isolates were loaded onto a FLO-MIN106 R9.4 flow cell and sequenced on a MinION device (Oxford Nanopore Technologies). Sequencing was performed on an Intel i7-6700 desktop computer over a 72 h period and basecalling was performed within MinKnow using Guppy v4.5.4 (Oxford Nanopore Technologies).
Short-read and hybrid assembly
Short sequence reads from all isolates were assembled using SPAdes v3.10.0 [15] using the ‘--careful’ flag and the ‘--cov-cutoff’ flag set to ‘auto’. Long sequence reads were de-multiplexed within Minknow and assembled with the corresponding short reads using Unicycler v0.4.7 [16] with default parameters. Assembly statistics are available in Tables S1 and S2. Assemblies were annotated using Prokka v1.14.5 [17].
Characterization of Klebsiella genomes
Kleborate v2.0 [18] was used to determine the species and sequence type (ST) (for species with available schemes) from the assemblies, and identify virulence and resistance genes. Kaptive [19], integrated into Kleborate, was used to type the capsule and O antigen biosynthesis loci.
Species-wide phylogenetic analyses
Core genes (i.e. those present in ≥99 % of isolates) were identified among assemblies of each Klebsiella species using Panaroo v1.2.4 [20] in the ‘sensitive’ mode (in species with ≥5 isolates only). Variable positions were extracted from each core-gene alignment using SNP-sites [21]. These were used to reconstruct a maximum-likelihood phylogenetic tree for each species using iq-tree v1.6.10 [22].
Phylogenetic analyses of individual STs
Additional public genomes from K. pneumoniae ST307 (n=964) and ST1788 (n=2), the two most frequent STs in the PHW collection, were identified using the Pathogenwatch database [23]. Using the available accession numbers, all corresponding short-read data were downloaded from the European Nucleotide Archive (ENA) while the associated metadata were obtained from Pathogenwatch (https://pathogen.watch/).
Short-read sequencing data from ST307 and ST1788 isolates from both the PHW and public collections (including also single-locus variants of ST307 from the PHW collection) were mapped to ST-specific reference genomes. For ST307, this was the complete chromosome from the hybrid assembly of ARGID_32304, while the short-read assembly of ARGID_33748 (62 contigs) was used for ST1788. Mapping was performed using Burrows Wheeler Aligner [24] with SNPs identified using SAMtools v1.2 mpileup and BCFtools v1.2 [25]. Gubbins v2.4.1 [26] was used to remove recombined regions from the resulting pseudo-genome alignments and generate maximum-likelihood phylogenies based on the remaining variable sites. Pairwise SNP differences between isolates were determined from the pseudo-genome sequences using pairsnp (https://github.com/gtonkinhill/pairsnp).
Visualization of trees and metadata
Phylogenetic trees were visualized together with metadata using the Interactive Tree of Life tool v6 [27] and further annotated using Adobe Illustrator v2017.1.0. Interactive visualizations were also created using Microreact [28].
Characterization of plasmids and mobile genetic elements
Plasmid replicon types carried by all isolates were determined from the short-read sequence data using Ariba v2.14.6 with the PlasmidFinder database [29]. The PlasmidFinder tool (https://bitbucket.org/genomicepidemiology/plasmidfinder/src/master/) [29] was used to identify plasmid replicons in completely sequenced plasmids. oriTfinder v1.1 [30] was used to identify the origin of transfer site (oriT) among complete pOXA-48-like plasmid sequences.
Linear comparisons between multiple pairs of complete plasmids were generated using EasyFig [31] and further annotated using Adobe Illustrator v2017.1.0. The percentage nucleotide similarities between individual plasmid genes and those from a reference plasmid were determined using blastn v2.6.0 [32]. The number of SNPs between pairs of aligned plasmids were determined using NUCmer v3.1 from the MUMmer package [33]. The presence of the Tn4401 transposon among bla KPC-encoding isolates, together with the Tn4401 variant type, was determined from the short sequence reads using TETyper v1.1 [34].
Identification and comparison of pOXA-48-like plasmid sequences from short-read data
Sequence reads from all isolates were mapped to the 61.8 kb IncL plasmid, pOXA48a [35] (GenBank accession no. JN626286), using Burrows Wheeler Aligner [24] with SNPs identified using SAMtools v1.2 mpileup and BCFtools v1.2 [25]. The binary alignment map (BAM) file from each isolate was used to determine the length of the reference plasmid that was mapped by at least one sequence read. A SNP matrix, calculated from pseudo-plasmid sequences of isolates with ≥70 % mapping to the reference, was used to reconstruct a minimum spanning tree using the standalone version of GrapeTree v1.5.0 [36]. Pairwise SNP differences between all plasmid pseudo-sequences were also determined using pairsnp (https://github.com/gtonkinhill/pairsnp).
To determine the short-read plasmid coverage over the pOXA48a reference relative to the chromosomal coverage, we mapped all isolates to the NTUH-K2044 chromosome (GenBank accession no. AP006725.1), as described above. We used the ‘genomecov’ function within BEDTools v2.29.0 [37] to determine the chromosomal coverage using sorted BAM files as input, and determined the median value for each isolate. We then calculated the plasmid coverage, relative to the median chromosomal coverage, across 100 bp windows of each mapped plasmid sequence (sliding every 20 bp) and visualized the resulting matrix using the ‘heatmap.2’ function in R v.4.0.2.
Bacterial conjugation
Transfer of the pOXA-48-like plasmid-encoded bla OXA-244 gene by six K. pneumoniae ST307 isolates was determined using a liquid-mating conjugation method. All mating experiments were performed using both the Escherichia coli J53 strain (200 mg sodium azide l−1) and a K. pneumoniae strain, 71.1 (passaged over 10 days to generate a rifampicin minimum inhibitory concentration of 4096 mg l−1), as separate recipients. Two mating culture ratios were tested, 1 : 3 and 5 : 1, for both donor and recipient, respectively. Briefly, donor and recipient isolates were grown to log phase in LB broth and mixed according to the appropriate mating-culture ratio, then incubated at 37 °C for 16–20 h. Following serial dilution of the mating cultures, transconjugant colonies were selected for using appropriate chromogenic agar (Sigma Aldrich) and antibiotic combination (J53, 200 mg sodium azide l−1 and 4 mg meropenem l−1; 71.1, 256 mg rifampicin l−1). To differentiate donor–recipient in the K. pneumoniae–K. pneumoniae experiment, the ST307 donor colonies were selected for using the chromogenic agar antibiotic combination (4 mg meropenem l−1 and at 32 mg ampicillin l−1) as 71.1 was sensitive to ampicillin. Transfer of the bla OXA-244 was confirmed via PCR, and transconjugates from the K. pneumoniae–K. pneumoniae experiments were further confirmed by short-read (Illumina) sequencing. Briefly, genomic DNA (gDNA) from transconjugates was extracted using the automated QIAcube (Qiagen). gDNA libraries were prepared using the Nextera XT v2 kit with bead-based normalization, and sequenced using V3 chemistry on an Illumina MiSeq. The conjugation frequency was calculated as a ratio of the c.f.u. transconjugate ml−1 to c.f.u. donor isolate ml−1.
Results
High diversity of carbapenemase-producing Klebsiella among clinical isolates in Wales
We first used short-read sequencing to analyse 540 Klebsiella isolates collected between 2007 and 2020 in Wales that had largely been referred to SACU at PHW for investigation of antimicrobial resistance or obtained from blood cultures (regardless of the resistance phenotype) (Table S1). They also included three unrelated isolates recovered in the wider UK region (from UK NEQAS) that were sequenced for quality-control purposes. Overall, 387/540 (71.7 %) isolates were from clinical samples (including 238 from blood, 93 from urine, 23 from a wound site, 33 other/unknown), 143/540 (26.5 %) from screen samples (from a variety of sources including rectal, urine and wound swabs), 6/540 (1.1 %) from environmental samples and 4/540 (0.7 %) from an unknown source. The clinical and screen isolates were recovered from 405 patients in hospitals and GP surgeries across Wales. A total of 72/405 (17.8 %) of the patients contributed two or more isolates (up to eight), which included those obtained from different sample types and cultured at different time points according to clinical need.
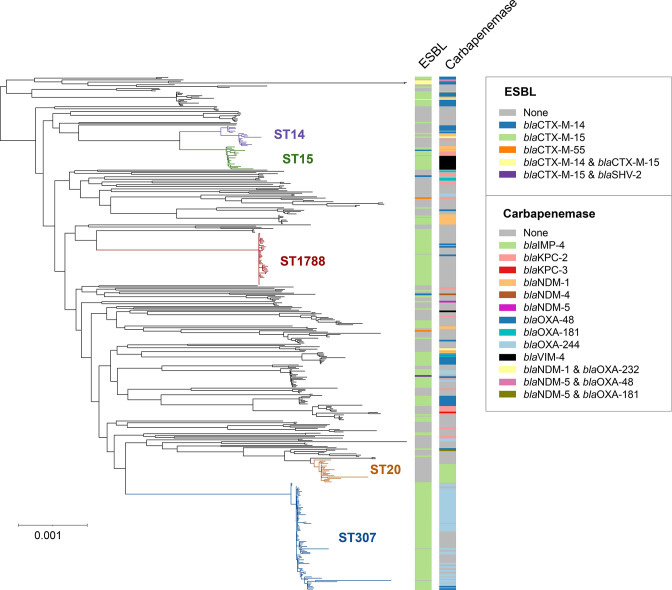
Among our collection, K. pneumoniae was the dominant species, accounting for 421/540 (78.0 %) isolates. A further eight species were also identified: Klebsiella aerogenes (33/540; 6.1 %), Klebsiella michiganensis (27/540; 5.0 %), Klebsiella variicola (23/540; 4.3 %), Klebsiella oxytoca (17/540; 3.1 %), Klebsiella quasipneumoniae (11/540; 2.0 %), Klebsiella grimontii (5/540; 0.9 %), Klebsiella planticola (2/540; 0.4 %) and Klebsiella pasteurii (1/540; 0.2 %). A phylogenetic tree of the 421 K . pneumoniae , reconstructed from an alignment of 4034 core genes, demonstrated a high number of distinct lineages (Fig. 1; https://microreact.org/project/aSJqpc9MZVZcurTWtoadW8-k-pneumoniae-phw-n421). We identified 130 STs across this species, with 98 (75.4 %) of these restricted to a single patient. However, some STs were observed frequently, including ST307 (88 isolates; 55 patients), ST1788 (43 isolates; 30 patients), ST20 (19 isolates; 10 patients), ST15 (19 isolates; 16 patients) and ST14 (17 isolates; 12 patients). Phylogenetic analyses of all other Klebsiella species also revealed a high diversity among each, with most isolates occurring as singletons or in small clusters (see Table S3 for Microreact URLs).
Fig. 1.
Maximum-likelihood phylogenetic tree of 421 K . pneumoniae isolates demonstrates high diversity. The tree was reconstructed from an alignment of 4034 core genes that were present in ≥99 % of K. pneumoniae isolates referred to the SACU laboratory at PHW. The most frequently observed STs are highlighted. Metadata columns show the ESBL and carbapenemase variants found among the isolates (if applicable). The scale indicates the number of SNPs per variable site. The tree is available to view interactively and with additional metadata at https://microreact.org/project/aSJqpc9MZVZcurTWtoadW8-k-pneumoniae-phw-n421.
Inspection of the Klebsiella genomes for antimicrobial-resistance markers revealed that 282/540 (52.2 %) isolates carried an ESBL gene, which was largely bla CTX-M-15 (228/282; 80.9 %) (Fig. 1). A total of 207/540 (38.3 %) isolates carried a carbapenemase gene. The most frequently observed carbapenemases were bla OXA-244 (58/207; 28.0 %), bla OXA-48 (46/207; 22.2 %), bla KPC-2 (31/207; 15.0 %), bla NDM-1 (29/207; 14.0 %), bla IMP-4 (16/207; 7.7 %) and bla VIM-4 (12/207; 5.8 %). Carbapenemase-encoding isolates were found in six of the nine observed species, although K. pneumoniae accounted for the vast majority (181/207; 87.4 %). Among K. pneumoniae , isolates carrying carbapenemase genes were found in 52 STs. However, bla OXA-244 was mostly carried by ST307 (49/58; 84.5 %), bla IMP-4 by ST20 (15/16; 93.8 %) and bla VIM-4 by ST15 (11/12; 91.7 %) or their related variants. Other carbapenemases such as bla KPC-2 and bla OXA-48 were more widely dispersed across different species and STs.
Persistent spread of globally and locally important clones of K. pneumoniae
Despite the overall high diversity among isolates, our phylogenetic analyses revealed numerous small to large clusters, representing clonal transmission of Klebsiella within and between healthcare facilities in Wales. Below, we discuss features of the two largest clusters involving K. pneumoniae ST307 and ST1788, as well as two additionally important clones, ST20 and ST15.
ST307
The most frequently observed ST in our collection, ST307, is an important clone that has been identified in diverse locations worldwide and associated with the ESBL gene bla CTX-M-15 [38]. In 2019, an outbreak involving ST307 was identified in an acute tertiary hospital in South-West Wales (Hospital A), based on epidemiological and resistance profiling data. The isolates showed a MDR phenotype (resistance to third-generation cephalosporins, gentamicin, ciprofloxacin ± ertapenem). The carbapenemase gene, bla OXA-244, was detected in the subset of isolates that exhibited carbapenem resistance (ertapenem ± meropenem ± imipenem). Recognition of the outbreak prompted regular faecal screening for carbapenemase-producing Enterobacteriaceae (CPE) carriage in the initial affected ward, and this was extended to additional wards where clinical isolates of bla OXA-244-producing K. pneumoniae were later identified. Here, we analysed genome data of 88 isolates from ST307 (or single-locus variants) collected between 2017 and 2020 from ten hospitals and additional GP surgeries in Wales, with particular emphasis on understanding the emergence, evolution and transmission of the outbreak strain.
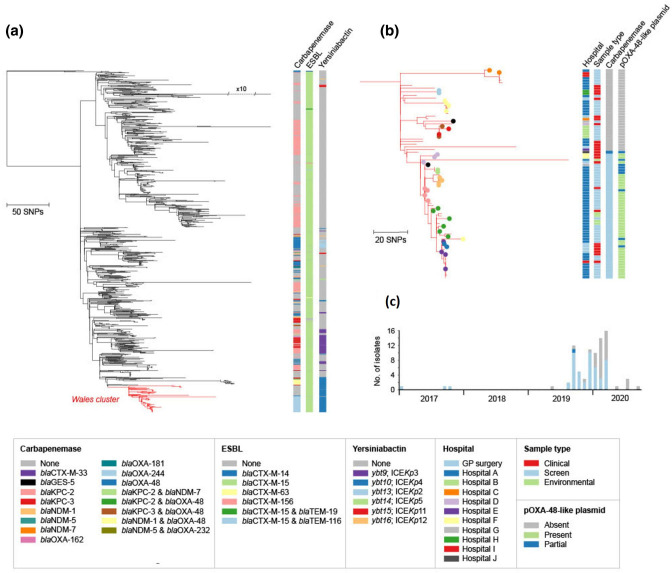
We reconstructed a phylogenetic tree of these 88 isolates, also including a further 964 geographically diverse public ST307 genomes to aid contextualization. The phylogeny demonstrated that 82/88 (93.2 %) of the ST307s from Wales formed a monophyletic cluster (Fig. 2a; https://microreact.org/project/1ZRxgto9HVsaV6qRHAuf6P-st307s-phw-n1052). This is indicative of a single major introduction of this strain into Wales, followed by local spread. The cluster incorporated clinical isolates from 19 patients (of whom 7 had bloodstream infections), as well as screen isolates from a further 31 patients.
Fig. 2.
(a) Maximum-likelihood phylogenetic tree of 1052 K . pneumoniae ST307 isolates demonstrates a main introduction of ST307 into Wales. The tree includes 964 ST307 isolates from public archives (from diverse international locations) and 88 isolates from the PHW collection. The tree was reconstructed from an alignment generated by mapping all short sequence reads to an ST307 reference genome (ARGID_32304) and after the removal of recombined regions. A total of 82 ST307 isolates from the PHW collection form a monophyletic cluster highlighted in red. Metadata columns show the carbapenemase, ESBL and yersiniabactin variants among all isolates (if applicable). The tree is available to view interactively and with additional metadata at https://microreact.org/project/1ZRxgto9HVsaV6qRHAuf6P-st307s-phw-n1052. (b) Cluster of 82 ST307 isolates from the PHW collection, as zoomed in from (a). Filled circles of the same colour on the tree tips represent isolates from the same patient. Metadata columns show the sampling hospital, sample type, carbapenemase variant and the presence/absence of the pOXA-48-like plasmid. For the latter, the plasmid was scored as ‘present’ if sequence reads mapped to ≥90 % of the pOXA48a reference plasmid (GenBank accession no. JN626286), ‘partial’ if between 70 and 90%, and ‘absent’ if <70 %. The scale bars in (a) and (b) represent the number of SNPs. (c) Timeline showing the isolation dates of the 82 clustered ST307 isolates from the PHW collection. Bars are coloured by the carbapenemase variant (if applicable).
Almost all of the 82 clustered isolates carried bla CTX-M-15 (80/82; 97.6%), while a descendent subclade of the cluster later acquired bla OXA-244 (49/82; 59.8%). A single isolate also carried the combination of bla CTX-M-15 and bla OXA-48, the latter of which has a single amino acid substitution relative to bla OXA-244. We searched for alterations (including truncations) in major outer membrane porin genes that have been shown to reduce susceptibility to carbapenems when acting in concert with ESBL, AmpC or carbapenemase enzymes [39, 40]. We found that all 82 isolates carried a truncated ompK35 gene, while 12/82 (14.6 %) also possessed a truncated ompK36 gene.
Isolates in the bla OXA-244 subclade were characterized by low pairwise SNP differences (range 0–116; mean 15.5) and mostly collected from patients (both clinical and screen) or environmental samples in Hospital A (40/49; 81.6%) over the outbreak period (Fig. 2b, c). This is consistent with heightened within-hospital transmission. The remaining nine bla OXA-244-encoding isolates were recovered from other nearby hospitals and GP surgeries in the South Wales region. Notably, the first sampled isolates from this subclade were obtained from a single patient in 2017 (outside of Hospital A). This demonstrates the acquisition of bla OXA-244 by this strain and emergence of this subclade at least 2 years prior to its later expansion within Hospital A.
The non-bla OXA-244-encoding isolates in the Welsh ST307 cluster had been referred to SACU from 2019 onwards, largely prompted by the similarity of their phenotypic profiles to the bla OXA-244-encoding outbreak isolates rather than by epidemiological links. Despite their collection over a limited timeframe, we found that they were considerably more divergent than the bla OXA-244-encoding isolates (pairwise SNP differences from 0 to 134; mean 54.4) (Fig. 2b). They were also recovered from a wider number of hospitals in South Wales (nine in total), although included isolates from Hospital A. These findings are further consistent with the undetected circulation of this ST307 strain across South Wales over several years prior to the emergence and expansion of the bla OXA-244-encoding outbreak subclade in Hospital A in 2019. The bla OXA-244-encoding ST307 outbreak was declared formally closed in May 2020 with no further isolates of this subclade identified since March 2020.
ST1788
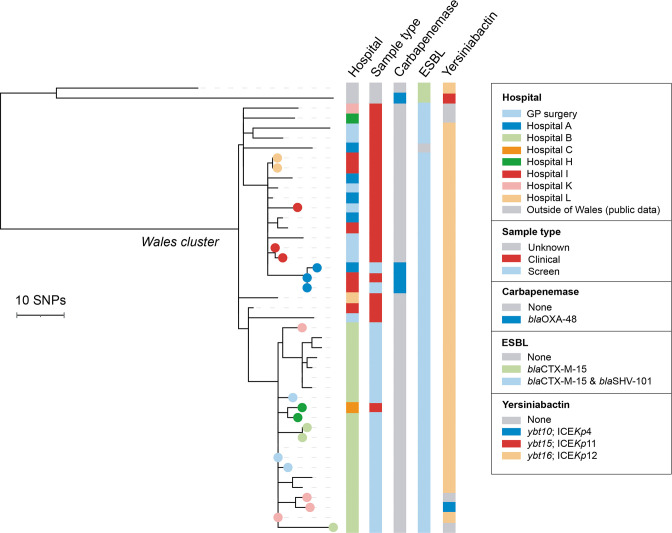
ST1788 is the second most common ST of K. pneumoniae in our collection. In contrast to ST307, this has rarely been observed elsewhere to our knowledge. SACU first detected ST1788 during the 2019 ST307 outbreak investigation and it continued to be recovered across South Wales up until the end of the study period (November 2020). The 43 ST1788 isolates in this sequenced collection are derived from 30 patients (including 18 with clinical isolates and 3 with blood isolates) across seven hospitals and additional GP surgeries. They include 20 isolates from 13 patients obtained from a rehabilitation unit (Hospital B) in South-East Wales from early 2020 onwards.
To investigate the evolution and spread of this strain, we reconstructed a phylogenetic tree of the 43 ST1788 isolates from Wales, together with 2 publicly available ST1788 genomes from Nigeria. This revealed that the Welsh isolates formed a distinct cluster (Fig. 3; https://microreact.org/project/rMY2fakRxkNKXqqBPs3is6-st1788-phw-n45). Although additional contextual data are required to draw strong conclusions on the routes of strain spread, these findings are consistent with a single introduction into South Wales, followed by local transmission within and between healthcare facilities. This is further supported by the low SNP differences between our isolate pairs (0–36 SNPs, mean 17.9), calculated after recombination removal, suggestive of a recent clonal expansion.
Fig. 3.
Maximum-likelihood phylogenetic tree of 45 ST1788 isolates demonstrates high clonality among isolates from Wales. The tree includes 2 isolates from public archives (both from Nigeria) and 43 from the PHW collection. It was reconstructed from an alignment generated by mapping all short sequence reads to an ST1788 reference genome (ARGID_33748) and after the removal of recombined regions. The 43 ST1788 isolates from the PHW collection form a monophyletic cluster. Filled circles of the same colour on the tree tips represent isolates from the same patient. Metadata columns show the sampling hospital, sample type, and carbapenemase, ESBL and yersiniabactin variants (if applicable). The scale bar represents the number of SNPs. The tree is available to view interactively and with additional metadata at https://microreact.org/project/rMY2fakRxkNKXqqBPs3is6-st1788-phw-n45.
Our phylogenetic analysis also revealed that the 20 isolates obtained from patients in Hospital B clustered together, also with a bloodstream isolate from an inpatient at an acute tertiary care hospital in South-East Wales (Hospital C) who was transferred from Hospital B (Fig. 3). This demonstrated the occurrence of a single introduction of the strain into Hospital B followed by within-hospital spread. The maximum number of SNPs between any of these isolate pairs was 20. Apart from the isolate obtained from the Hospital C patient, all isolates obtained from Hospital B were screen isolates, obtained via an enhanced screening programme at this site. Furthermore, we also detected a cluster of 14 isolates harbouring up to 13 pairwise SNP differences that were recovered from two hospitals in the same city, Hospitals A and I, as well as from several GP surgeries from varied locations across South Wales.
Almost all ST1788 isolates from Wales carried bla CTX-M-15 (42/43; 97.7 %) (Fig. 3). Long-read sequencing of one of these isolates, ARGID_33072, revealed that the ESBL gene was carried on a 240.5 kb plasmid with IncFIB(K) and IncFII(K) replicons. Three isolates from one patient also carried bla OXA-48, while the majority (39/43; 90.7 %) had intact ompK35 and ompK36 genes. Notably, all ST1788 isolates possessed the KL2 capsule type, which has been associated with increased virulence [41], and most (39/43; 90.7 %) also possessed a yersiniabactin locus.
At the time of writing, transmission of ST1788 remains an ongoing concern, in particular in Hospital B. We also have evidence of ongoing secondary cases at nearby hospitals within the health board, linked by epidemiology and genomic typing, and have recently recovered ST1788 isolates from patients in other regions of Wales.
Other STs
Using the species-wide phylogeny of K. pneumoniae isolates, we detected other clusters also suggestive of localized transmission. For example, we found that 15 isolates belonging to ST20 (and other closely-related STs) carrying bla IMP-4 clustered together, and were phylogenetically distinct from another 4 ST20 isolates lacking this carbapenemase. The 15 isolates were recovered from a total of six patients in Hospital Y (9 isolates, 4 patients), Hospital C (1 isolate, 1 patient) and a GP surgery (5 isolates, 1 patient), all in South Wales, during a 2 year period (August 2017 to October 2019). All 15 isolates shared a truncated ompK35 gene as well as an mcr-9 gene, the latter of which belongs to the mobile colistin resistance (mcr) gene family. Of note, some of these ST20 isolates harbouring mcr-9 underwent colistin broth microdilution as part of SACU's reference susceptibility testing service and all isolates were susceptible. This is in keeping with other authors' findings, although some publications suggest that mcr-9 may be responsible for inducible colistin resistance in some isolates [42, 43].
Furthermore, among the 19 isolates from ST15 (and other nested STs) in our collection, we detected a cluster of 11 isolates that carried the bla VIM-4 carbapenemase. The 11 isolates were recovered from a total of ten patients located in Hospital N in North Wales (9 isolates, 8 patients), the nearby Hospital R (1 isolate, 1 patient) and a GP surgery (1 isolate, 1 patient). All 11 isolates possessed a bla CTX-M-15 gene and a truncated ompK35 gene, while one isolate had a truncated ompK36 gene. A total of 10/11 isolates also carried a yersiniabactin locus (ybt 10).
Plasmid epidemiology of bla KPC-2-producing Klebsiella reflects distinct hospital networks and referral patterns between North and South Wales
We next aimed to elucidate the spread of particular carbapenemase genes in Wales where we could not identify clonal transmission as a key driver. We first investigated bla KPC-2 genes, which were found in 30 isolates from five different species among our collection (excluding a NEQAS isolate with bla KPC-2). While the majority were from K. pneumoniae (21/30; 70.0 %), 13 different STs from this species were represented and no single ST accounted for more than four isolates.
Using our short-read data with the TETyper tool [34], we first determined whether the bla KPC-2 genes were carried on the Tn4401 transposon, which has previously been associated with bla KPC gene spread [44, 45]. In ten isolates (10/30; 33.3 %), TETyper did not identify any known Tn4401 variant. However, the remaining isolates (20/30; 66.7 %), from a variety of species and STs, carried bla KPC-2 on the Tn4401a variant. These were recovered from 16 patients between 2013 and 2020. Tn4401a is the same variant associated with endemic spread of bla KPC-2-encoding Enterobacteriaceae in North-West England recognized from 2008 onwards [46]. Notably, the majority (17/20; 85.0 %) of our isolates with bla KPC-2 carried on Tn4401a were sampled from patients in North Wales. These findings likely reflect acquisition of bla KPC-2-encoding strains and/or plasmids in North-West England hospitals, consistent with the typical referral of patients in North Wales to this region for specialist treatment.
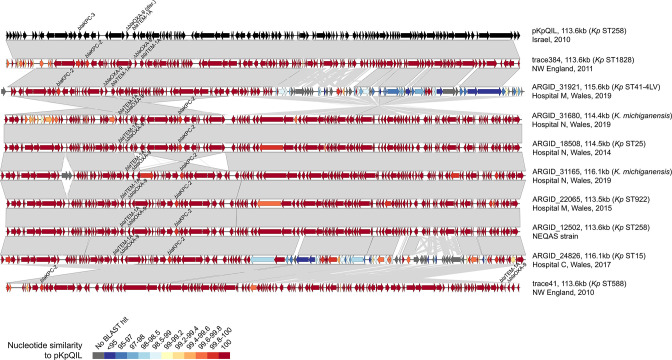
To further elucidate the diversity of plasmids carrying bla KPC-2 on Tn4401a, we long-read sequenced 13 of these isolates (including the NEQAS isolate) (Table S2). These were selected to represent the diversity of species and STs observed. We obtained complete plasmids carrying bla KPC-2 from hybrid (combined short- and long-read) assemblies in all 13 isolates. One K. pneumoniae ST11 isolate (ARGID_32407) also carried an additional copy of bla KPC-2 on the chromosome. Two of the 13 bla KPC-2 plasmids possessed a single IncF replicon, while the remainder were multi-replicon plasmids with either two IncF replicons or an IncF and IncR replicon. They ranged in size from 113.5 to 210.3 kb. In particular, we found that seven had a similar structure and size to pKpQIL-like plasmids, a family of plasmids represented by pKpQIL [47].
A visual comparison of pKpQIL-like plasmids from our collection, together with two published pKpQIL-like plasmids from North-West England [46] and pKpQIL [47], showed the high structural conservation overall (Fig. 4). However, two plasmids (from ARGID_31921 and ARGID_24826) had lower nucleotide similarity with pKpQIL over one half, likely as a result of recombination. All others showed high nucleotide similarity to pKpQIL, differing by 11–32 SNPs. We also found some support for recent common ancestry between plasmids from North Wales and North-West England. For example, the plasmids from ARGID_18508 and ARGID_31165 (both North Wales) differed from trace384 (North-West England) by only four SNPs.
Fig. 4.
Pairwise comparisons between complete bla KPC-encoding pKpQIL-like plasmids demonstrate high structural and sequence conservation. The plasmids include the original pKpQIL sequence (GenBank accession no. GU595196) [47], seven from the PHW collection and two previously sequenced plasmids recovered in North-West England hospitals [46]. ORFs are depicted as arrows with resistance genes (including bla KPC) labelled. Grey blocks between plasmid pairs indicate regions of homology. The colour of the ORFs represents the percentage nucleotide similarity to pKpQIL via blastn.
We next assessed the remaining ten bla KPC-2-carrying isolates with no known Tn4401 variant. These isolates were from five patients and one environmental sample, and from a mixture of species and STs. They were recovered between 2017 and 2020 from three hospitals in South Wales, with seven isolates from Hospital A. Inspection of the short-read genome assemblies demonstrated a shared genetic context around bla KPC-2. We also found that all ten isolates had an IncN replicon in common. To investigate the possibility of bla KPC-2-IncN plasmid spread, we long-read sequenced six of these isolates from different species and/or STs (Table S2).
We obtained a complete IncN plasmid from the hybrid assemblies of all six isolates, and confirmed carriage of bla KPC-2 in a conserved genetic environment (next to bla TEM-1) on these plasmids. The IncN plasmids varied considerably in size from 48.2 to 137.4 kb, and the largest also carried an additional IncFII replicon. Visual comparison of the plasmids showed that they shared high nucleotide similarity across shared core regions (Fig. 5). This is despite frequent recombination events leading to alternate structural arrangements and a large variation in accessory sequence. Using blastn, we also found numerous plasmids in the NCBI non-redundant nucleotide database harbouring bla KPC-2 with >99 % nucleotide identity to the Welsh IncN plasmids. These belonged to a variety of Enterobacteriaceae species including Klebsiella spp., Escherichia coli , Citrobacter freundii , Enterobacter spp. and Salmonella enterica , and were recovered (or submitted) from a variety of international locations. They included a previously described IncN plasmid from an ST307 isolate recovered in the UK (GenBank accession no. KY271414) [48] (Fig. 5). Altogether these findings suggest that this plasmid backbone has disseminated widely, including over the South Wales region, and become an important vector of bla KPC-2 genes.
Fig. 5.
(a) Pairwise comparisons between complete bla KPC-2-encoding IncN plasmids demonstrate substantial plasticity despite high nucleotide similarity among shared backbone regions. The plasmids include a previously published sequence (GenBank accession no. KY271414) [48] and six from the PHW collection. ORFs are depicted as arrows with resistance genes (including bla KPC-2) labelled. Grey blocks between plasmid pairs indicate regions of homology. The colour of the ORFs represents the percentage nucleotide similarity to the plasmid from ARGID_28504 via blastn. (b) Conserved genetic environment of bla KPC-2 among the IncN plasmids.
Frequent carriage and horizontal dissemination of bla OXA-48-like genes via pOXA-48-like plasmids and related variants
The most common type of carbapenemase genes among our Klebsiella collection were bla OXA-48-like genes, which comprised bla OXA-244 (n=58), bla OXA-48 (n=46), bla OXA-181 (n=7) and bla OXA-232 (n=3) variants. While most bla OXA-244-encoding isolates (49/58; 84.5%) belonged to the ST307 lineage, other bla OXA-48-like genes were identified in diverse species and STs. In particular, bla OXA-48 genes were found in three species ( K. pneumoniae , K. quasipneumoniae , K. oxytoca ) and 15 different STs of K. pneumoniae , and recovered between 2013 and 2020.
We investigated a possible association of bla OXA-48-like genes with pOXA-48-like plasmids, which are highly conserved ~61–63 kb IncL plasmids shown previously to frequently carry these carbapenemase genes [35, 49]. To do this, we mapped the short reads of all isolates in our collection (including those without bla OXA-48-like genes) to the 61.8 kb pOXA48a reference plasmid [35]. A total of 113/540 (20.9 %) isolates mapped to ≥70 % of the plasmid length (including 95 that mapped to ≥90 %), suggesting that they harboured the same or a similar plasmid. Most of the remaining isolates mapped to ≤10 %. The 113 isolates with ≥70 % plasmid mapping were from 61 different patients in 15 hospitals and additional GP surgeries, largely from across South Wales. They accounted for most isolates with bla OXA-48-like genes, including all of those with bla OXA-244 (58/58) and bla OXA-48 (46/46), and 1/3 (33.3 %) of those with bla OXA-232. Only 8/113 (7.1 %) possessed no bla OXA-48-like gene. Among the 113 isolates, 94 (83.2 %) possessed an IncL replicon (the same as pOXA48a). We did not find an IncL replicon in the remaining 19/113 (16.8 %) isolates, but instead found either an IncM1 (n=18) or IncM2 (n=1) replicon. IncL and IncM plasmids are known to be closely related and indeed were previously classified in the same family, IncL/M [50]. However, the latter have not been implicated as vectors of bla OXA-48-like genes to date.
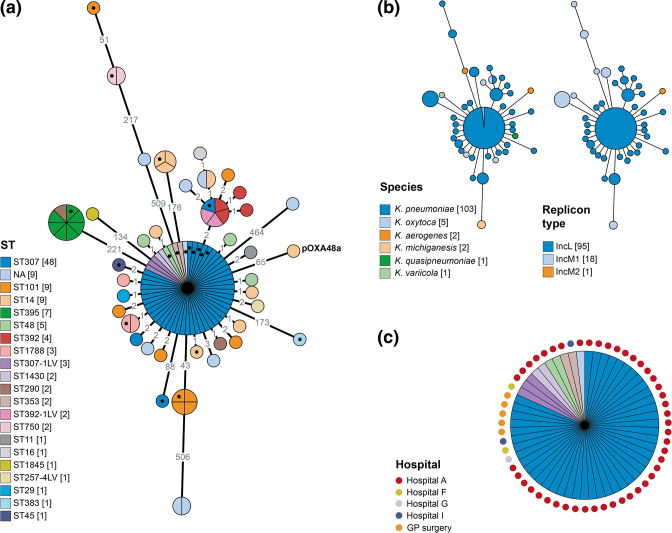
Analysis of SNP diversity among the 113 mapped plasmid sequences demonstrated 0–5 differences among many (≥75 %) of the sequences, despite their recovery from diverse locations and times. This high similarity was reflected in a minimum spanning tree (Fig. 6a). Only some sequences were considerably more divergent, including those associated with IncM replicons (Fig. 6b). The minimum spanning tree showed that some STs harboured divergent sequences (e.g. ST101, ST14), indicative of multiple acquisitions of the plasmid. It also demonstrated several instances of plasmid sequences with 0 SNP differences carried by isolates of the same ST, suggestive of clonal transmission. Furthermore, while the overall low amount of SNP diversity among these plasmid sequences makes inference of transmission events difficult, we found identical plasmid sequences shared among different species and STs, suggestive of recent horizontal spread. For example, we found 0 SNPs among 55 sequences (all associated with bla OXA-244), which included 48 from the K. pneumoniae ST307 outbreak isolates, 2 from ST1430, 2 from ST48, 2 from ST353 and 1 from K. oxytoca (Fig. 6c). The vast majority of these, including the non-ST307 isolates, were recovered from Hospital A, supporting the possibility of horizontal transmission. Notably, five of these non-ST307 isolates were recovered in 2017–2018, implying local circulation of this plasmid variant prior to the expansion of bla OXA-244-encoding ST307 in 2019.
Fig. 6.
(a) Minimum spanning tree of 113 pOXA-48-like plasmid sequences demonstrates high nucleotide conservation, as well as some divergent sequences. Plasmid sequences included in the tree, which were obtained by mapping short reads to pOXA48a (GenBank accession no. JN626286) [35], are from isolates with ≥70 % mapping to this reference sequence. The tree was reconstructed from a SNP distance matrix. The branch lengths are displayed using a log scale and indicated on the branches. The nodes are partitioned by (and their sizes proportional to) the number of represented isolates. Isolates are coloured by ST ( K. pneumoniae species complex only) with the number of isolates from each ST indicated in square brackets. Isolates marked with a black filled circle were selected for long-read sequencing to enable complete plasmid sequencing. (b) The same tree as in (a) coloured by species of the host isolate or replicon type identified from the genome. (c) The major node from the tree in (a) coloured by ST, with annotations marking the sampling hospitals.
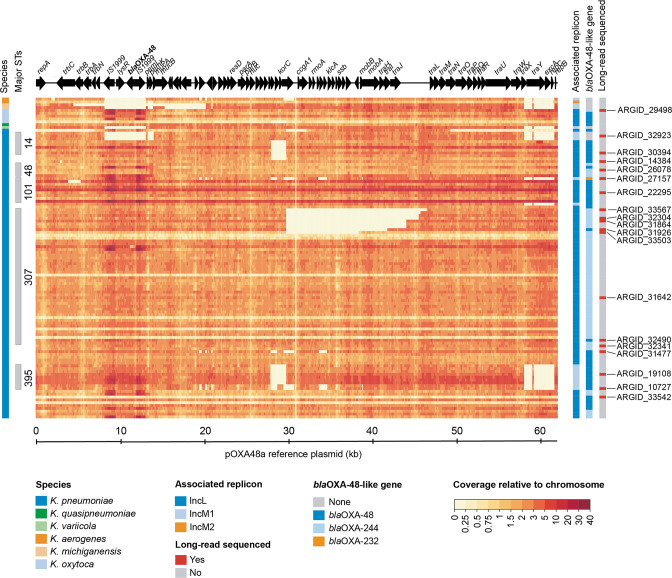
Finally, we used the short-read data to investigate structural differences among the 113 mapped plasmid sequences. To do this, we calculated and visualized the short-read coverage across the length of pOXA48a, relative to the median chromosomal coverage of the host strain (Fig. 7). This revealed that nine of the bla OXA-244-encoding ST307 outbreak isolates lacked coverage across parts of the plasmid (8.6–16.6 kb) that include the tra region in most cases, with a conserved breakpoint at one end of the deletion. This suggests that these plasmids may have lost the ability to self-conjugate and/or be mobilized. Notably, they did not cluster into a single clade in the core genome-based phylogenetic tree, suggesting that the deletions occurred on multiple independent occasions (Fig. 2).
Fig. 7.
Heatmap showing the short-read coverage across the pOXA48a reference sequence indicates deletions among some isolates, including several involving the tra region in ST307. The plasmid coverage is shown relative to the median chromosomal coverage for 113 isolates with ≥70 % mapping to pOXA48a (GenBank accession no. JN626286) [35]. ORFs from the pOXA48a plasmid are depicted at the top, including bla OXA-48. Metadata columns indicate the host species, those belonging to frequently observed STs, and replicon and bla OXA-48-like variants identified from the isolate genomes. The far-right column indicates isolates selected for long-read sequencing to enable complete plasmid assembly.
Structural evolution of pOXA-48-like plasmids including repeated loss of conjugative ability in K. pneumoniae ST307
We next used our short-read analysis of the SNP and structural diversity among pOXA48-like plasmids to guide selection of 19 isolates for further investigation with long-read sequencing (Table S2). These were chosen to investigate: (a) the possible carriage of bla OXA-48-like genes on IncM plasmids and their structural differences with IncL plasmids; (b) other divergent variants of pOXA-48-like plasmids; and (c) the evolution of pOXA-48-like plasmids in the ST307 outbreak lineage. We constructed hybrid assemblies and obtained complete IncL or IncM1 plasmids in all 19 isolates, which ranged in size from 46.8 to 73.0 kb.
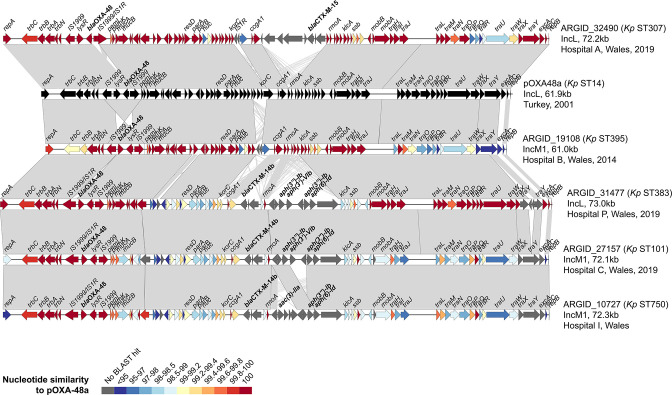
Among the 19 complete plasmids, we obtained 3 with an IncM1 replicon from bla OXA-48-encoding isolates and confirmed carriage of this carbapenemase on these plasmids. Overall, they showed high structural homology to the IncL plasmids, albeit with key differences (Fig. 8). These included a section with the replicon typing determinants that lacks homology, as well as lower sequence similarity over the tra gene cluster region. However, large parts of the IncL and IncM1 plasmids shared 100 % nucleotide similarity, including the region containing bla OXA-48, suggestive of recent recombination. We also obtained two additional complete IncM1 plasmids that lacked any bla OXA-48-like gene from ARGID_32776 and ARGID_32923. We found that the IncM1 plasmid from ARGID_32776 instead carried the ESBL gene bla SHV-5, while that from ARGID_32923 carried no known carbapenemase or other β-lactamase gene.
Fig. 8.
Pairwise comparisons between complete pOXA-48-like plasmids show structural differences to the classic backbone of pOXA48a, including insertion of islands carrying resistance genes. The plasmids include pOXA48a (GenBank accession no. JN626286) [35] and five from the PHW collection. They are from a range of STs and include those with both IncL and IncM1 replicon types. ORFs are depicted as arrows with resistance genes (including bla OXA-48) labelled. Grey blocks between plasmid pairs indicate regions of homology. The colour of the ORFs represents the percentage nucleotide similarity to pOXA48a via blastn.
Among other isolates selected for long-read sequencing based on the high structural and/or SNP divergence of their pOXA-48-like plasmids from pOXA48a, we found several with novel integrations (Fig. 8). These included an island harbouring bla CTX-M-15 in one plasmid recovered from a K. pneumoniae ST307 isolate (unrelated to the ST307 outbreak strain). Furthermore, a genetic island with another ESBL gene, bla CTX-M-14b, as well as aminoglycoside-resistance genes, was found in both IncL and IncM1 plasmids recovered from K. pneumoniae ST101, ST383 and ST750 isolates.
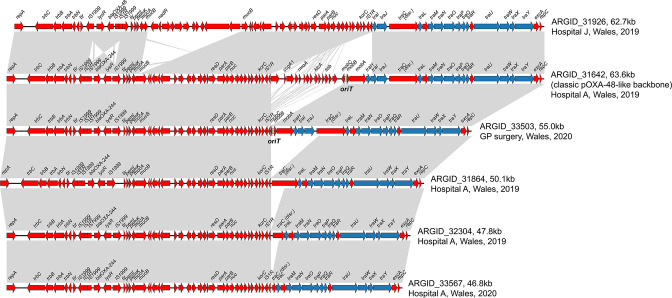
Finally, we analysed complete pOXA-48-like plasmids from six ST307 outbreak-associated isolates to investigate the putative deletions involving the tra region. These included ARGID_31642, in which no deletion had been observed, and five isolates with putative deletions. As expected, we found that the plasmid from ARGID_31642 had a similar structure and size to pOXA48a (Fig. 9). All others possessed a deletion with a conserved breakpoint at one end adjacent to an IS1R element. The variable size and nature of these deletions further confirmed that they had occurred on multiple independent occasions. This suggests that the deletions confer a fitness advantage. The deletions involved one or more tra genes and the oriT region in four of the five cases, with one plasmid (from ARGID_33503) retaining these regions despite a deletion. We used conjugation experiments to test the ability of the six isolates with complete pOXA-48-like sequences to transfer the plasmid to both E. coli (J53 strain) and K. pneumoniae (71.1 strain), using two different ratios of donor/recipient (1 : 3 and 5 : 1). These confirmed that both ARGID_31642 and ARGID_33503 could transfer the plasmid, while no transfer of plasmids with deletions in the tra/oriT regions was observed (Table S4).
Fig. 9.
Pairwise comparisons between complete pOXA-48-like plasmids from ST307 demonstrate multiple independent deletions of regions adjacent to an IS1R element. One plasmid (from ARGID_31642), which possesses the classic pOXA-48-like backbone and which lacks these deletions, is included. ORFs are depicted as arrows with resistance genes (including bla OXA-48/bla OXA-244) labelled. Grey blocks between plasmid pairs indicate regions of homology. ORFs coloured blue are predicted to be involved in conjugation, while those in red have other predicted functions. The oriT region is indicated if identified in the plasmid.
Discussion
In line with other geographical regions worldwide, Klebsiella infections represent a substantial healthcare burden in Wales and are the third most common cause of bloodstream infections [51]. While rates of resistance to carbapenems have been low (<1 % among bloodstream isolates in 2017) [51], the rapid global rise of resistance necessitates vigilant monitoring [10, 11]. Here, we describe the major strains and plasmids driving spread of MDR Klebsiella in Wales between 2007 and 2020, providing a detailed view of resistance dynamics in a largely non-endemic setting.
We found that more than half of carbapenemase-producing Klebsiella isolates (124/207; 59.9 %) referred to our laboratory at PHW belonged to just nine STs from K. pneumoniae . This finding echoes numerous other studies reporting a concentration of resistant Klebsiella in high-risk STs [52–54]. The major carbapenemase-producing clones detected in our region include the globally important K. pneumoniae strains ST307, ST20 and ST15, which carried bla OXA-244, bla IMP-4 and bla VIM-4, respectively. We also identified spread of a strain that has rarely been reported elsewhere, ST1788, carrying bla CTX-M-15 and which also acquired bla OXA-48 in one patient. Detailed phylogenetic analyses of the ST307 and ST1788 clones revealed that a single major introduction of each into Wales, followed by local spread within and between hospitals, accounted for the dominance of these clones among patient isolates.
Our phylogenetic analyses also suggested that the ST307 strain had been circulating in Wales for several years prior to its acquisition of bla OXA-244 and an outbreak involving the bla OXA-244-encoding subclade centred on Hospital A in 2019. The lack of recognition of ST307 by SACU until the 2019 outbreak reflects the lack of active surveillance or typing of third-generation cephalosporin-resistant Klebsiella infections in Wales over this time period, and the absence of clear epidemiological links between infections. Since the outbreak involving the bla OXA-244-encoding subclade was declared closed, it is possible that the ST307 strain (without bla OXA-244) continues to disseminate and be responsible for a significant proportion of invasive infections. However, in the absence of epidemiological links and active surveillance of all isolates, this once more remains undetected. Yet past reports demonstrate the ability of ST307 to readily acquire additional resistance determinants and expand rapidly [55, 56]. Thus, as our laboratory and others move towards using WGS data for routine surveillance of Klebsiella , this experience demonstrates the importance of urgently tailoring our systems to detect and control the spread of high-risk clones, ideally prior to acquisition of additional resistance determinants.
Our analysis also revealed the dominant plasmid vectors driving spread of bla KPC-2 and bla OXA-48-like genes in Wales, which were found among diverse species and strain backgrounds. We found that bla KPC-2 genes from North and South Wales were largely encoded on different plasmid backgrounds, consistent with the referral of patients from these regions to different hospitals for their tertiary medical care (i.e. to North-West England and South Wales, respectively). Notably, bla KPC-2 genes from isolates in North Wales collected from 2013 onwards were typically encoded within the Tn4401a transposon on IncF plasmids, the same genetic background identified among bla KPC-2 isolates from North-West England. This region has experienced endemic spread of bla KPC-2-encoding Enterobacteriaceae since 2008, driven largely by horizontal exchange of plasmids and plasmid fragments between strains and species [46]. Thus, our findings likely reflect the outward expansion of this complex, multi-species, plasmid-driven outbreak to Wales via hospital networks.
By contrast, bla KPC-2 genes found among isolates from South Wales since 2017 were typically harboured by IncN plasmids. Despite the large variation in size and structure in these plasmids, the high nucleotide similarity found among their shared regions strongly suggests the existence of a recent common ancestor and thereby the occurrence of local plasmid transmission events between different species and STs in Welsh hospitals. This genomic evidence is further supported by the spatial clustering of these plasmids (i.e. 7/10 were recovered from a single hospital). As exemplified with these IncN plasmids, the large structural variability among some plasmid types (despite recent common ancestry) heightens the challenge of routinely identifying plasmid transmissions, even with complete plasmid sequences. We propose that calculating nucleotide similarity across individual segments (or sliding windows) of plasmid sequences, rather than determining an average over the entire sequence, is crucial for detecting transmissions involving plasmids that can rapidly acquire or lose accessory regions, and/or exchange modular regions with other backbone types.
We found that most bla OXA-48-like genes (105/114; 92.1%) encoded by isolates referred to our laboratory were harboured by pOXA-48-like plasmids. These accounted for the clonal spread of bla OXA-244 by the ST307 outbreak strain and also contributed to the horizontal spread of both bla OXA-48 and bla OXA-244 variants across numerous species and STs. Other countries and regions have reported a similar dominance of these plasmids, which have been found to be remarkably conserved over time and space [49, 57]. Given their importance, we propose that the identification and typing of pOXA-48-like plasmids by genomic surveillance systems would have large gains for detection and control of resistance spread. Due to the high structural conservation of this plasmid family, this is largely achievable using short-read approaches (e.g. mapping or plasmid multilocus sequence typing) [58].
Our analysis did, however, reveal a small proportion of novel divergent variants of pOXA-48-like plasmids. These included those carrying additional resistance genes, as well as those associated with IncM, rather than IncL, replicons. We also detected deletions among pOXA-48-like plasmids in the ST307 outbreak lineage which extended to the oriT and tra regions in most cases. Experiments confirmed that the plasmids lacking these regions could no longer be transferred via conjugation to a recipient K. pneumoniae or E. coli strain. Since these deletions occurred multiple times independently, we propose that they represent adaptation of the pOXA-48-like plasmid to carriage by ST307 strains, likely via a reduction in the fitness cost imposed on the host.
Altogether, this study provides a comprehensive picture of resistance-gene spread among Klebsiella species in Wales, mediated via both clonal and horizontal transmission. It provides important contextual data for ongoing surveillance, as well as key considerations for the design and optimization of routine genomic surveillance systems in public-health laboratories.
Supplementary Data
Funding information
S.D. and D.M.A. are supported by funding from the Centre for Genomic Pathogen Surveillance and the Li Ka Shing Foundation. Illumina sequencing was funded as part of the Antimicrobial Resistance and Genomic Typing Project (ARGENT) by the Welsh Assembly Government. MinION sequencing was funded by the PHW R and D Pump-Priming Fund – 2018/2019.
Acknowledgements
We thank the Pathogen Informatics team at the Wellcome Sanger Institute for informatics support, the Pathogen Genomics Unit laboratory and bioinformatics teams at PHW, the team at the SACU at PHW, the Infection Prevention and Control departments for Health Boards, especially those teams that commented on the local outbreaks reported herein, and referring Microbiology Laboratories from across Wales and their host Health Boards.
Author contributions
S.D., M.M., K.S., M.W. and L.J. conceived the study. S.D. performed the bioinformatic data analysis and drafted the manuscript. M.M., K.S., E.P., L.G. and J.W. performed the sequencing, laboratory experiments and data analysis. O.B.S., D.M.A., M.W. and L.J. supervised the study. All authors reviewed and edited the manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ESBL, extended-spectrum β-lactamase; MDR, multidrug resistant; PHW, Public Health Wales; SACU, Specialist Antimicrobial Chemotherapy Unit; ST, sequence type; WGS, whole-genome sequencing.
All supporting data, code and protocols have been provided within the article or through supplementary data files. Four supplementary tables are available with the online version of this article.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling ML, Apisarnthanarak A, Madriaga G. The burden of healthcare-associated infections in Southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis. 2015;60:1690–1699. doi: 10.1093/cid/civ095. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J-L, Sakr Y, Singer M, Martin-Loeches I, Machado FR, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–1487. doi: 10.1001/jama.2020.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meatherall BL, Gregson D, Ross T, Pitout JDD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122:866–873. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, et al. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis. 2017;65:208–215. doi: 10.1093/cid/cix270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, et al. NISC Comparative Sequencing Program Group Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu D, Dong N, Zheng Z, Lin D, Huang M, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18:37–46. doi: 10.1016/S1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 9.Tavoschi L, Forni S, Porretta A, Righi L, Pieralli F, et al. Prolonged outbreak of New Delhi metallo-beta-lactamase-producing carbapenem-resistant Enterobacterales (NDM-CRE), Tuscany, Italy, 2018 to 2019. Euro Surveill. 2020;25:2000085. doi: 10.2807/1560-7917.ES.2020.25.6.2000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castanheira M, Deshpande LM, Mendes RE, Canton R, Sader HS, et al. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis. 2019;6:S23–S33. doi: 10.1093/ofid/ofy347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: World Health Organization; 2017. [Google Scholar]
- 13.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley N, Lee Y. Practical implications of new antibiotic agents for the treatment of carbapenem-resistant enterobacteriaceae. Microbiol Insights. 2019;12:1178636119840367. doi: 10.1177/1178636119840367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 18.Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, et al. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun. 2021;12:4188. doi: 10.1038/s41467-021-24448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyres KL, Wick RR, Gorrie C, Jenney A, Follador R, et al. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom. 2016;2:e000102. doi: 10.1099/mgen.0.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonkin-Hill G, MacAlasdair N, Ruis C, Weimann A, Horesh G, et al. Producing polished prokaryotic pangenomes with the Panaroo pipeline. Genome Biol. 2020;21:180. doi: 10.1186/s13059-020-02090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom. 2016;2:000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argimón S, David S, Underwood A, Abrudan M, Wheeler NE, et al. Rapid genomic characterization and global surveillance of Klebsiella using pathogenwatch. Clin Infect Dis. 2021;73:S325–S335. doi: 10.1093/cid/ciab784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argimón S, Abudahab K, Goater RJE, Fedosejev A, Bhai J, et al. Microreact: visualizing and sharing data for genomic epidemiology and phylogeography. Microb Genom. 2016;2:e000093. doi: 10.1099/mgen.0.000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Xie Y, Liu M, Tai C, Sun J, et al. oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 2018;46:W229–W234. doi: 10.1093/nar/gky352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard AE, Stoesser N, German-Mesner I, Vegesana K, Walker AS, et al. TETyper: a bioinformatic pipeline for classifying variation and genetic contexts of transposable elements from short-read whole-genome sequencing data. Microb Genom. 2018;4:000232. doi: 10.1099/mgen.0.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poirel L, Bonnin RA, Nordmann P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother. 2012;56:559–562. doi: 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z, Alikhan N-F, Sergeant MJ, Luhmann N, Vaz C, et al. GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;28:1395–1404. doi: 10.1101/gr.232397.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peirano G, Chen L, Kreiswirth BN, Pitout JDD. Emerging antimicrobial-resistant high-risk Klebsiella pneumoniae clones ST307 and ST147. Antimicrob Agents Chemother. 2020;64:10. doi: 10.1128/AAC.01148-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-Martínez L, Pascual A, Hernández-Allés S, Alvarez-Díaz D, Suárez AI, et al. Roles of beta-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae . Antimicrob Agents Chemother. 1999;43:1669–1673. doi: 10.1128/AAC.43.7.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamzaoui Z, Ocampo-Sosa A, Maamar E, Fernandez Martinez M, Ferjani S, et al. An outbreak of NDM-1-producing Klebsiella pneumoniae, associated with OmpK35 and OmpK36 porin loss in Tunisia. Microb Drug Resist. 2018;24:1137–1147. doi: 10.1089/mdr.2017.0165. [DOI] [PubMed] [Google Scholar]
- 41.Shon AS, Bajwa RPS, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babiker A, Bower C, Lutgring JD, Petit RA, Howard-Anderson J, et al. Clinical and genomic epidemiology of mcr-9-carrying carbapenem-resistant enterobacterales isolates in Metropolitan Atlanta, 2012 to 2017. Microbiol Spectr. 2022;10:e0252221. doi: 10.1128/spectrum.02522-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kieffer N, Royer G, Decousser J-W, Bourrel A-S, Palmieri M, et al. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob Agents Chemother. 2019;63:e00965-19. doi: 10.1128/AAC.00965-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, et al. Genetic structures at the origin of acquisition of the beta-lactamase blaKPC gene. Antimicrob Agents Chemother. 2008;52:1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuzon G, Naas T, Nordmann P. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother. 2011;55:5370–5373. doi: 10.1128/AAC.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoesser N, Phan HTT, Seale AC, Aiken Z, Thomas S, et al. Genomic epidemiology of complex, multi-species, plasmid-borne blaKPC carbapenemase in Enterobacterales in the United Kingdom from 2009 to 2014. Antimicrob Agents Chemother. 2020;64:e02244-19. doi: 10.1128/AAC.02244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leavitt A, Chmelnitsky I, Carmeli Y, Navon-Venezia S. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob Agents Chemother. 2010;54:4493–4496. doi: 10.1128/AAC.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villa L, Feudi C, Fortini D, Brisse S, Passet V, et al. Diversity, virulence, and antimicrobial resistance of the KPC-producing Klebsiella pneumoniae ST307 clone. Microb Genom. 2017;3:e000110. doi: 10.1099/mgen.0.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.David S, Cohen V, Reuter S, Sheppard AE, Giani T, et al. Integrated chromosomal and plasmid sequence analyses reveal diverse modes of carbapenemase gene spread among Klebsiella pneumoniae . Proc Natl Acad Sci. 2020;117:25043–25054. doi: 10.1073/pnas.2003407117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carattoli A, Seiffert SN, Schwendener S, Perreten V, Endimiani A. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS One. 2015;10:e0123063. doi: 10.1371/journal.pone.0123063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Public Health Wales Antibacterial Resistance in Wales 2008–2017. Cardiff: Public Health Wales; 2018. [Google Scholar]
- 52.David S, Reuter S, Harris SR, Glasner C, Feltwell T, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4:1919–1929. doi: 10.1038/s41564-019-0492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnin RA, Jousset AB, Chiarelli A, Emeraud C, Glaser P, et al. Emergence of new non-clonal group 258 high-risk clones among Klebsiella pneumoniae carbapenemase-producing K. pneumoniae isolates, France. Emerg Infect Dis. 2020;26:1212–1220. doi: 10.3201/eid2606.191517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Pilato V, Errico G, Monaco M, Giani T, Del Grosso M, et al. The changing epidemiology of carbapenemase-producing Klebsiella pneumoniae in Italy: toward polyclonal evolution with emergence of high-risk lineages. J Antimicrob Chemother. 2021;76:355–361. doi: 10.1093/jac/dkaa431. [DOI] [PubMed] [Google Scholar]
- 55.Lowe M, Kock MM, Coetzee J, Hoosien E, Peirano G, et al. Klebsiella pneumoniae ST307 with blaOXA-181, South Africa, 2014-2016. Emerg Infect Dis. 2019;25:739–747. doi: 10.3201/eid2504.181482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haller S, Kramer R, Becker K, Bohnert JA, Eckmanns T, et al. Extensively drug-resistant Klebsiella pneumoniae ST307 outbreak, north-eastern Germany, June to October 2019. Euro Surveill. 2019;24:1900734. doi: 10.2807/1560-7917.ES.2019.24.50.1900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skalova A, Chudejova K, Rotova V, Medvecky M, Studentova V, et al. Molecular characterization of OXA-48-like-producing enterobacteriaceae in the Czech Republic and evidence for horizontal transfer of pOXA-48-like plasmids. Antimicrob Agents Chemother. 2017;61:e01889-16. doi: 10.1128/AAC.01889-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brehony C, McGrath E, Brennan W, Tuohy A, Whyte T, et al. An MLST approach to support tracking of plasmids carrying OXA-48-like carbapenemase. J Antimicrob Chemother. 2019;74:1856–1862. doi: 10.1093/jac/dkz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.