Abstract
Background
Technological advancements in deep brain stimulation (DBS) require methodological changes in programming. Fractionalization poses significant practical challenges for the most common approach for assessing DBS efficacy, monopolar review (MR).
Objectives
Two DBS programming methods: MR and fixed parameter vertical and horizontal fractionalization (FPF) were compared.
Methods
A two‐phase process of vertical and horizontal FPF was performed. MR was conducted thereafter. After a short wash‐out period, both optimal configurations determined by MR and FPF were tested in a double‐blind randomized manner.
Results
Seven PD patients were enrolled, providing 11 hemispheres to compare the two conditions. In all subjects, the blinded examiner selected a directional or fractionalization configuration. There was no significant difference in clinical benefits between MR and FPF. FPF was the preferred method for initial programming as selected by subject and clinician.
Conclusions
FPF programming is a viable and efficient methodology that may be incorporated into clinical practice.
Keywords: deep brain stimulation, programming, fractionation
The established approach to deep brain stimulation (DBS) initial programming involves a monopolar review (MR) of all contacts. 1 , 2 , 3 With the introduction of segmented (directional) contacts, programming has become more complex. Although these technological innovations have resulted in improved clinical outcomes, exploring the therapeutic window for all contacts is increasingly more time consuming. 4 Many DBS centers are attempting to improve programming efficiency and individualizing DBS therapy to patients’ symptoms by incorporating new technologies, such as multiple independent current control (MICC). 5 , 6 Novel methods for performing initial programming that account for the increased number of contacts are under investigation. 4 In this form of programming, each contact is tested with the same pre‐determined and fixed parameters and stimulation is fractionalized along the electrode, that is, a single parameter with fractionalization (FPF). In this double‐blind study, we compared traditional MR and FPF in terms of clinical efficacy, side effects, and volume of tissue activated (VTA) at initial programming in PD patients who underwent bilateral subthalamic nucleus (STN) DBS.
Methods
Subject Selection
Informed, written consent was obtained from all subjects and approved by Colorado Multiple Institutional Review Board (COMIRB; 20‐0232). Subjects were recruited from the University of Colorado Anschutz Medical Campus, DBS Program. Inclusion criteria were the following: PD diagnosis, recruited prior to implant surgery, implanted DBS system‐Boston Scientific Vercise Gevia™ DBS System (includes Vercise Cartesia™ DB‐2202‐45 lead and Vercise Gevia™ rechargeable implanted pulse generator), prominent motor manifestation of rigidity.
Study Protocol
All subjects underwent bilateral STN‐DBS surgery. 7 , 8 , 9 The study occurred at the initial programming sessions and subjects withheld dopaminergic medication at least 12 hours prior to visit. One hemisphere was tested per study visit. For subjects in which both hemispheres were tested, programming sessions were separated by at least 48 hours and therapeutic stimulation was withheld between sessions. The experimental session was conducted in the following steps: (1) double‐blinded assessment of vertical FPF contact arrangements in randomized order, (2) double‐blinded, randomized assessment of horizontal FPF as determined by the optimal level(s) obtained in step 1, (3) traditional MR, (4) double‐blinded comparison of FPF and MR contact configurations identified from steps 1–3. To ensure a carryover effect did not occur between programming settings, the examiner determined that the subject returned to clinical baseline between tests. All FPF programming was performed with the following parameters: amplitude = 2.0 mA, pulse width = 60 μs, and frequency = 130 Hz. All side effects were recorded including transient (those that resolved within 1 minute) and persistent (those that lasted >1 minute or were discomforting). For both horizontal and vertical FPF conditions, the predetermined sets of percent of stimulation allocated to contacts was selected to result in a great enough change of the stimulation vector that would be clinically observed and to permit efficient programming time. 6
During MR, amplitude was adjusted while pulse width (60 μs) and frequency (130 Hz) where held constant. 2 , 3 The four levels were tested with the middle two levels tested in ring mode. If the ideal level based upon side effects and clinical benefit of rigidity identified was the middle two levels, then the segments of this level were independently tested. Thereafter, all tested conditions, were ranked based upon side effects and clinical benefit providing the optimal MR setting.
In the final comparison between FPF and MR, the examiner was not present during the stimulation ramp‐up phase to ensure the blind. Following a ~ 2‐minute wash‐in phase, the examiner would perform a unilateral MDS‐UPDRS of the following items: wrist, elbow, ankle and knee rigidity (item 3.3), bradykinesia (items 3.4, 3.5, 3.6, 3.7, 3.8) and tremor (items 3.15, 3.16, 3.17, and 3.18). Upon completion the subject and examiner ranked the test preference independently.
Volume of Tissue Activation Analysis
Reconstruction of the volume of tissue activation (VTA) for each stimulation condition was conducted using LeadDBS v2.515. 10 For each hemisphere, the FastField stimulation model within LeadDBS was used to simulate the optimal FPF and MR VTAs. To quantify the difference in percent overlap with STN between each of the two VTA conditions, we used the Accolla et al., STN atlas within LeadDBS, to visualize the tripartite functional divisions of the STN. 11 To compute volume of overlap, we used an additional custom Matlab script that incorporated mesh and polygon specific functions from the geom3D toolbox. 12 This technique was applied to compute the percent of voxel overlay of each individual VTA and the brain area of interest related to the respective stimulation parameters.
Statistics
A paired t‐test assessed the mean amplitude difference between MR and FPF conditions. A paired t‐test assessed the mean difference in abbreviated MDS‐UPDRS III scores between the conditions. A one‐way ANOVA assessed the mean difference in abbreviated MDS‐UPDRS III sub scores between conditions using Tukey's Honestly Significant Difference. Pair‐wise comparisons were computed for each sub score. A one‐way ANOVA assessed the mean difference in condition generated VTA overlap with STN subregion between the conditions. Pair‐wise comparisons were computed for each STN subregion using Tukey's Honestly Significant Difference. A chi‐square test of independence assessed whether the fraction of clinician or subject preferred selected configurations was greater for one of the programming conditions. The values P < 0.05 were considered statistically significant. Statistical analyses were conducted using both Matlab (v2022a) and Jamovi (v2.2.5; https://www.jamovi.org/).
Results
Seven PD patients (F = 2/7) participated. Three subjects had minimal rigidity and tremor on one side of their body and thus analysis was only performed on the more affected side. Consequently, a total of 11 hemispheres were analyzed. Table S1 supplemental provides the demographics of the subjects. On average, the first study session was conducted 23 days post‐surgery (median; IQR = 8), starting with the left hemisphere in 6/7 subjects. Four subjects were investigated on both hemispheres, 2 subjects right only, 1 subject left hemisphere alone. To compare efficacy between contact configurations, we first assessed current delivered, which was non‐significant between MR and FPF conditions (t = 1.04 (10), P = 0.32, SFP = 2.42 mA (± 1.04), MR = 2.26 (± 0.66); Fig 1A).
FIG. 1.
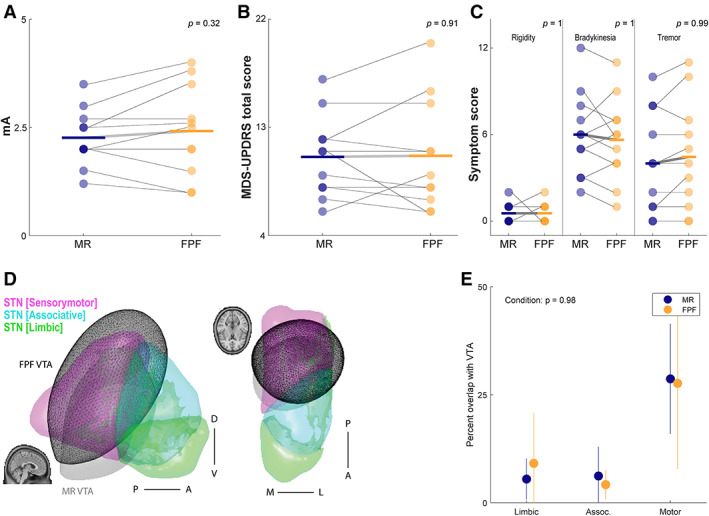
Summary of data of MR to FPF.
There was no significant difference in unilateral MDS‐UPDRS overall scores between MR and FPF (t = 0.12 (10), P = 0.907, SFP = 10.6 (± 4.57), MR = 10.5 (± 3.39); Fig 1B) and no significant effect of subscore analysis performed by an ANOVA (F = 0.123 (2,65), P = 0.884; Fig 1C). The examiner preference was based on clinical features extrapolated from the MDS‐UPDRS, whereas the subject preference may have included subjective experience. For the examiner, a greater number of FPF configurations were selected in comparison to MR across hemispheres (FPF = 7/MR = 4; X2 = 0.818, df = 1, P‐value = 0.3657); similarly for the subject, a greater number of FPF configurations were selected over MR (FPF = 7/MR = 2; X2 = 2.77, df = 1, P‐value = 0.095). In no condition did the examiner select a ring configuration (eg, 2–3‐4‐) (Table 1).
TABLE 1.
Summary of optimal MR and FPF stimulation conditions
| Sub # Hemisphere | MR | FPF | Levels MR|FPF | Patient preference | Clinician preference |
|---|---|---|---|---|---|
| 1 R | 7 (100%) | 5 (25%), 6 (75%) | 2|2 | – | FPF |
| 2 L | 5 (100%) | 6 (50%), 8 (50%) | 2|1 & 2 | – | FPF |
| 2 R | 5 (100%) | 6 (38%), 7 (12%), 8 (50%) | 2|1 & 2 | MR | MR |
| 3 L | 5‐(100%) | 6 (50%), 7 (50%) | 2|2 | FPF | FPF |
| 3 R | 7‐(100%) | 5 (75%), 6 (25%) | 2|2 | FPF | FPF |
| 4 L | 5‐ (100%) | 6 (20%), 7 (60%), 8 (20%) | 2|1 & 2 | FPF | FPF |
| 4 R | 6‐ (100%) | 3 (20%), 6 (80%) | 2|2 & 3 | FPF | MR |
| 5 R | 6‐ (100%) | 3 (10%), 4 (10%), 6 (40%), 7 (40%) | 2|2 & 3 | FPF | FPF |
| 6 L | 7‐ (100%) | 3 (38%), 4 (12%), 6 (38%), 7 (12%) | 2|2 & 3 | MR | MR |
| 7 L | 7‐ (100%) | 7 (25%), 5 (25%), 8 (50%) | 2|1 & 2 | FPF | MR |
| 7 R | 5‐ (100%) | 1 (50%), 2 (38%), 3 (12%) | 2|3 & 4 | FPF | FPF |
| Ratio FPF | 7/9 | 7/11 |
Finally, we compared the estimated VTA as a percentage of the functional sub‐regions of STN (Fig. 1D). An ANOVA comparing percentage of VTA overlap for each of the three functional sub‐regions of STN by condition found no significant effect (F = 0.259 (2,48), P = 0.772; Fig 1E).
Discussion
Overall, the acute outcomes were comparable between MR and FPF. MDS‐UPDRS analysis for unilateral overall signs and sub scores were equivalent within patient and across patients. The greatest benefit was in rigidity for both methods consistent with known benefits of STN DBS. 13 , 14 Interestingly, in evaluating patient and clinician preferences there was a greater selection for the FPF settings, patient preference 7/9 and clinician preference 7/11. Furthermore, there was agreement as to the optimal programming settings between the subject and clinician in 7 of 9 test conditions. The instances when there was not agreement were due to subjective sensations of the patient that the examiner was not privileged to observe or discuss. In clinical practice, these subjective assessments would have been evaluated and further adjustments to stimulation would have been made.
In 10 of 11 conditions, the contact selected by MR was at the same level as the FPF, vertical fractionalization in one, horizontal fractionalization in two, and combination of vertical and horizontal fractionalization in seven conditions. This is remarkable given that 25 test conditions were evaluated in the FPF methodology. The overlap is additionally exemplified in the VTA analysis. Furthermore, in none of the test conditions did the examiner select a single level (eg, ring 2‐3‐4‐) with 100% cathodic stimulation. Although the clinical benefits of directionality defined as cathodic stimulation of a single segmental contact has been well reported, the applicability of fractionalization has had minimal investigations. 2 , 5 , 6 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
The strengths of the study included that FPF was performed in a blinded and randomized manner for each parameter adjustment. Furthermore, allotted time was permitted to ensure an adequate washout period. It may take minutes upon cessation of stimulation for the individual to return to baseline rigidity. 13 , 14 Without allowing for the subject to return to baseline, the subsequently assessment may have a carry‐over effect. While FPF programming as performed in this study was conducted to prevent unwanted bias essential for interpretation of results, this methodology was time consuming. As we demonstrated that FPF is at least as effective to MR, a modification of this form of programming may be employed to improve time efficiency. 4
Limitations include that the amplitude of stimulation had to be adjusted, which was anticipated per the protocol. In three of eleven test conditions the stimulation had to be reduced due to unwanted side effects, while in six of the eleven the stimulation had to be increased to appreciate a distinction in clinical outcomes. Additionally, the long‐term outcomes of FPF were not evaluated, which could include effects of mood that may not be appreciated in the acute setting. Finally, the maximum amplitude to induce side effects was not evaluated with FPF.
In this study, FPF has equivalent benefit to that of MR. Furthermore, there appeared to be a preference for fractionalized stimulation based upon subject and clinician blinded preference. Although FPF programming as tested in the most rigorous manner in this study demonstrated clear benefits, optimal programming may best be performed by incorporating a combination of FPF and MR.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
J.A.T.: 1A, 1B, 1C, 2A, 2B, 3A
L.H.: 1C, 2C, 3C
P.D‐G.: 1C, 2C, 3C
A.F.: 1B, 2C, 3B
D.R.K.: 1C, 2C, 3C
S.G.O.: 1C, 2C, 3C
D.S.K.: 1A, 1B, 1C, 2A, 2B, 3A.
Disclosures
Ethical Compliance Statement: As noted in the first line of the Methods Section, “Informed, written consent was obtained from all subjects and approved by Colorado Multiple Institutional Review Board (COMIRB; 20‐0232).” We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: Boston Scientific Investigator Initiative Grant was awarded to DSK and JAT. A mentorship award through the Parkinson Study Group was awarded to DSK and AF.
Financial Disclosures for the Previous 12 Months: JAT: Grant support provided from Boston Scientific, Medtronic. He has received compensation as a consultant/scientific advisory board member from Medtronic. LH: Reports no financial disclosures. PD‐G: Reports no financial disclosures. AF: Grant support provided from Abbvie, Boston Scientific, Dystonia Medical Research Foundation, University of Toronto, Michael J. Fox Foundation, Medtronic, MSA coalition. He has received compensation as a consultant/scientific advisory board member for Abbvie, Abbott, Boston Scientific, Ceregate, Inbrain, Ipsen, Medtronic, Sunovion; and publishing royalties from Springer; and honoraria from Abbvie, Abbott, American Academy of Neurology, Boston Scientific, Brainlab, Ipsen, Medtronic, Merz, Movement Disorders Society, Sunovion, Paladin Labs, UCB pharma. DRK: Reports no financial disclosures. SGO: Reports received educational grant support from Boston Scientific, Medtronic, and Abbott. DSK: Reports grant support from Boston Scientific, Medtronic, and the Parkinson's Foundation. He has received compensation as a consultant/scientific advisory board member for Colorado Clinical and Translational Sciences Institute (CCTSI), Boston Scientific, Medtronic, Abbott and AbbVie Pharmaceutics; and honoraria from Abbott.
Supporting information
TABLE S1. Supplemental: Subject demographics
Acknowledgment
Special thanks to Andres M. Hurtado, PhD for review of the manuscript.
References
- 1. Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A. Programming deep brain stimulation for Parkinson's disease: the Toronto Western hospital algorithms. Brain Stimul 2016;9(3):425–437. [DOI] [PubMed] [Google Scholar]
- 2. Dembek TA, Reker P, Visser‐Vandewalle V, et al. Directional DBS increases side‐effect thresholds‐a prospective, double‐blind trial. Mov Disord 2017;32(10):1380–1388. [DOI] [PubMed] [Google Scholar]
- 3. Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. Mov Disord 2006;21(Suppl 14):S284–S289. [DOI] [PubMed] [Google Scholar]
- 4. Steigerwald F, Matthies C, Volkmann J. Directional deep brain stimulation. Neurotherapeutics 2019;16(1):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juárez‐Paz LM. In silico accuracy and energy efficiency of two steering paradigms in directional deep brain stimulation. Front Neurol 2020;11:593798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang S, Silburn P, Pouratian N, Cheeran B, Venkatesan L, Kent A, Schnitzler A. Comparing current steering Technologies for Directional Deep Brain Stimulation Using a computational model that incorporates heterogeneous tissue properties. Neuromodulation 2020;23(4):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson JA, Oukal S, Bergman H, et al. Semi‐automated application for estimating subthalamic nucleus boundaries and optimal target selection for deep brain stimulation implantation surgery. J Neurosurg 2018;130:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Kern DS, Uy D, Rhoades R, Ojemann S, Abosch A, Thompson JA. Discrete changes in brain volume after deep brain stimulation in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2020;91(9):928–937. [DOI] [PubMed] [Google Scholar]
- 9. Abosch A, Timmermann L, Bartley S, et al. An international survey of deep brain stimulation procedural steps. Stereotact Funct Neurosurg 2013;91(1):1–11. [DOI] [PubMed] [Google Scholar]
- 10. Horn A, Li N, Dembek TA, et al. Lead‐DBS v2: towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Accolla EA, Dukart J, Helms G, et al. Brain tissue properties differentiate between motor and limbic basal ganglia circuits. Hum Brain Mapp 2014. Oct;35(10):5083–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Legland D. geom3d; (https://www.mathworks.com/matlabcentral/fileexchange/24484‐geom3d) MATLAB Central File Exchange Retrieved October 1, 2022.
- 13. Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K. Deep brain stimulation programming for movement disorders: current concepts and evidence‐based strategies. Front Neurol 2019;10:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin J, Krafczyk S, Valkovic P, Eggert T, Claassen J, Bötzel K. Objective measurement of muscle rigidity in parkinsonian patients treated with subthalamic stimulation. Mov Disord 2009;24(1):57–63. [DOI] [PubMed] [Google Scholar]
- 15. Kramme J, Dembek TA, Treuer H, Dafsari HS, Barbe MT, Wirths J, Visser‐Vandewalle V. Potentials and limitations of directional deep brain stimulation: a simulation approach. Stereotact Funct Neurosurg 2021;99(1):65–74. [DOI] [PubMed] [Google Scholar]
- 16. Schnitzler A, Mir P, Brodsky MA, et al. Vesper J; PROGRESS study investigators. Directional deep brain stimulation for Parkinson's disease: results of an international crossover study with randomized, double‐blind primary endpoint. Neuromodulation. 2022;25(6):817–828. [DOI] [PubMed] [Google Scholar]
- 17. Maciel R, Soh D, Munhoz RP, et al. Programming directional deep brain stimulation in Parkinson's disease: a randomized prospective trial comparing early versus delayed stimulation steering. Stereotact Funct Neurosurg 2021;99(6):484–490. [DOI] [PubMed] [Google Scholar]
- 18. Timmermann L, Jain R, Chen L, et al. Multiple‐source current steering in subthalamic nucleus deep brain stimulation for Parkinson's disease (the VANTAGE study): a non‐randomised, prospective, multicentre, open‐label study. Lancet Neurol 2015;14(7):693–701. [DOI] [PubMed] [Google Scholar]
- 19. Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current‐controlled device in Parkinson's disease (INTREPID): a multicentre, double‐blind, randomised, sham‐controlled study. Lancet Neurol 2020;19(6):491–501. [DOI] [PubMed] [Google Scholar]
- 20. Ojukwu DI, Wang AR, Hornbeck TS, et al. Conversion to hybrid deep brain stimulation system to enable multi‐contact fractionation can be therapeutic. Mov Disord 2022;37(6):1321–1323. [DOI] [PubMed] [Google Scholar]
- 21. Pollo C, Kaelin‐Lang A, Oertel MF, et al. Directional deep brain stimulation: an intraoperative double‐blind pilot study. Brain 2014;137(Pt 7):2015–2026. [DOI] [PubMed] [Google Scholar]
- 22. Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J. Directional deep brain stimulation of the subthalamic nucleus: a pilot study using a novel neurostimulation device. Mov Disord 2016;31(8):1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soh D, Maciel R, Algarni M, et al. Flexible vs. standard subthalamic stimulation in Parkinson disease: a double‐blind proof‐of‐concept cross‐over trial. Parkinsonism Relat Disord 2021;89:93–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Supplemental: Subject demographics


