Abstract
Although solar exposure is necessary for human health, phototoxicology induced by excessive UVB and UVA radiation, which involves sunburns, skin aging and even tumorigenesis, has been widely researched. Sunscreen is one of the most important ways to protect skin from UV phototoxic damage. As well as inorganic and organic UV filters, some natural products or plant extracts with aromatic rings in their structures, such as flavonoids or polyphenols, can absorb UV to reduce sunburn, acting as a natural UV filter; they also show antioxidant or/and anti‐inflammatory activity. This could explain why, although there are no officially approval natural commercial sun‐filters, more and more commercial sunscreen products containing plant extracts are available on the market. Here we summarize articles focusing on natural UV filters from plant published in the last 6 years, selecting the most significant data in order to better understand the photoprotective activity of natural products and extracts from plants, including their major constituents and main biological effects, methods for evaluating UV radiation resistance, anti‐UV radiation experimental models and anti‐UV radiation mechanisms.
Keywords: antioxidants; evaluation method; natural products and extracts from plants, natural UV filters; SPF value
It summarized natural UV filters from plant in the last 6 years in types of major constituent, main biological effects, evaluation methods of UV radiation resistance, anti‐UV radiation experiment models and anti‐UV radiation mechanisms, in order to better understand the role of herbal extracts in exerting their photo protective activity.
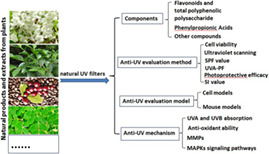
1. INTRODUCTION
The sun is the energy source for living organisms and the earth. Regarding human health, solar radiation exerts most of its positive effects by activating 7‐dehydrocholesterol to synthesize Vitamin D3 in human skin epidermis to prevent osteomalacia. In addition, nitric oxide (NO) production induced by ultraviolet (UV) helps to reduce blood pressure and is anti‐bacterial. Exposure to UV rays can improve one's mood by inducing the release of endorphins.
Solar UV radiation is divided into three categories based on wavelength, namely UVA (400–315 nm), UVB (315–280 nm) and UVC (280–100 nm). 1 UVC rays are dispersed and reduced by the ozone layer and do not reach the ground. About 90%–99% of UVA and 1%–10% of UVB rays reach the earth's surface. The epidermis of the skin serves as a barrier to protect the body from the external environment. 2 Nonetheless, chronic exposure or intermittent over‐exposure of human skin to UV rays leads to various skin diseases, including immunosuppression, irreversible skin photoaging and dermal pathologies, including tumorigenesis. Sunscreen is the most effective way, apart from sun avoidance, to prevent phototoxic damage, including sunburn, skin aging, collagen degradation, wrinkle formation, and pigmentation, to the skin, 3 , 4 and is thus the best way to protect skin against damage from UV rays.
Recently, researchers into skin protection have shown considerable interest in the use of botanicals. Research suggests that natural products are reasonably likely to be the future of cosmetics, and this trend necessarily involves UV filters. 5 Many components are reported to have great potential for use as sunscreen additives in the cosmetics industry. This review presents an overall profile of the botanical extracts or components with anti‐UV radiation identified in the last 6 years, including classification of the components, their main biological effects, methods of evaluation and experimental models of UV radiation resistance, and the mechanisms of anti‐UV activity involved.
2. METHODS
The present review is based on searches of the following electronic databases: Pubmed, Google Scholar, Web of Science, Elsevier and Wiley. The databases were systematically searched for articles published in the last 6 years. Selection criteria were that retrieved articles should include only natural herbal extracts, or pure molecules available from plant sources. The following keywords were used: sunscreen, SPF or Sun Protection Factor, UV filter, natural UV filter. All key words were searched individually and in combination. Only articles written in English were selected.
3. SUNSCREENS—TYPES OF SUN FILTERS
Sun filters are classified as inorganic UV filters, organic UV filters, and plant compounds agent. Inorganic UV filters are classified as physical filters, because their active components are minerals, namely TiO2 and ZnO. They protect skin from solar radiation by scattering and reflecting UV rays. 6 , 7 , 8 , 9 They tend to be opaque and therefore appear white on the skin, which makes them cosmetically less acceptable as sunscreens, and they also have numerous safety challenges that need to be overcome. 10 , 11 , 12
Currently, more than 60 kinds of organic sunscreens have been researched and developed internationally, but there are strict restrictions on their use. There are 14 organic sunscreens approved by the FDA in United States, 20 in the European Union, 27 in Japan, and 25 in China. P‐Aminobenzoic acid (PABA) was the first UVB absorber, patented in 1943 and used by US military during Second World War and then marketed, but was found to be a strong irritant and carcinogenic to the skin. Subsequent research developments include, among others, PABA derivatives, cinnamates, salicylates, benzophenones, benzimidazoles, and camphor derivatives as UVB absorbers, and dibenzoymethanes (such as Parsol 1789) as UVA absorbers. Chemical sunscreens may exhibit phototoxicity and photosensitization, and interactions between UV absorbers and solvents and matrices can cause cross‐sensitization and therefore the amounts used in sunscreens are strictly limited. Since UVB causes immediate and serious sunburn mainly by acting on epidermis layer, and is more genotoxic and about 1000 times more capable of causing sunburn than UVA, 13 most organic UV filter research is focused on the UVB range.
Plant compounds with aromatic rings usually show a broader absorption spectrum covering a wavelength range of 200–400 nm. Botanical ingredients used as UV filters also usually exhibit strong antioxidant properties. Therefore, these natural products are more advantageous as UV filters and are likely to be the future of sunscreens. Based on these properties, the addition of botanical ingredients to skin‐care cosmetics has been a predictable trend. 14
4. PLANT COMPONENTS ACTING AGAINST UV RADIATION
In order to better understand the important role that plant extracts can play in photoprotection, we identified through extensive searches and rigorous screening the most significant data for plant extracts and natural products with photoprotective ability published between 2016 and 2021, which are summarized in Tables 1 and 2. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 The tables include the plant parts used, extraction methods, types of compound, major constituents and main effects. Our work refers to and follows the review by Matteo Radice et al., who summarized research papers published from 2000 to 2015 on herbal extracts and biomolecules as natural photo‐protection alternatives to synthetic UV filters. 54
TABLE 1.
List of plant extracts with photo protective properties
| Plant name | Plant part(s) used | Plant extract | Type of compounds | Ref. |
|---|---|---|---|---|
| Achillea biebersteinii Afan. | Flower | Hydroglycolic Extracts | Phenolic acids, coumaroylquinic acid isomers and flavonoids | [15] |
| Achillea millefoliumyarrow | Flower | Hydroglycolic Extracts | Polyphenols, | [15] |
| Aloe vera | Leave | Aloe vera gel was dried | n.r. | [16] |
| Amaranthus viridis | Fabrics | Methanolic and aqueous | Phenolics and flavonoids | [17] |
| Angelica pubescens | Root | Steam distillation | Oils | [18] |
| Aporosa lindleyana | Leaves | 70% methanol | n.r. | [19] |
| Argyreia populifolia | Leaves | 70% methanol | n.r. | [19] |
| Atalantia ceylanica | Leaves | 70% methanol | n.r. | [19] |
| Baccharis antioquensis | Aerial parts | Acidulated acetone | Total anthocyanins, total phenols | [20, 21] |
| Blackberry, raspberry | Fruits | Ethanol | Anthocyanins, flavonoids | [22] |
| Calamagrostis effusa | Rhizome | Acidulated acetone | Total anthocyanins, total phenols | [20] |
| Calea fruticosa | Aerial parts | Extracted successively with n‐hexane, ethyl acetate, and ethanol | Flavonoids and terpenoids, sesquiterpenic lactone, flavonol, glucosylated coumarin | [23] |
| Castilleja fissifolia | n.r. | Acidulated acetone | Total anthocyanins, total phenols | [20] |
| Charthamus tinctorius L. | Seed Oil | Commercial products | Oil | [24] |
| Campomanesia | Leaves | Hydroalcoholic extract | Total of flavonoids, phenolic compounds | [25] |
| Coffea arabica | Coffee | 80% acetone, or hydroalcoholic | Total phenolics | [26, 27] |
| Dalbergia ecastaphyllum | Leaves | Hydroethanol | Carotenoids, chlorophylls, total phenolic and flavonoids | [28] |
| Dirmophandra mollis Benth | Beans | Ethanol | Flavonoid | [29] |
| Disterigma alaternoides | Leaves | Acidulated acetone | Total phenolic; Total monomeric anthocyanin pigment, Total anthocyanins | [21] |
| Drimys granadensis | Leaves | Acidulated acetone | Total phenolic; Total monomeric anthocyanin pigment, Total anthocyanins | [21] |
| Elaeagnus angustifolia | Leaves | 70%MeOH | Flavonoids, Phenols | [30] |
| Erlangea tomentosa | Leaves | Dichloromethane + MeOH, water | n.r. | [31] |
| Ginkgo biloba L. | Purchased extract | Ethanol | Flavonoid | [29] |
| Helianthus annuus L. cv | Leave | Methanolic extract | Hydroxycinnamic acids, cell wall‐bound phenolics | [32] |
| Hibiscus furcatus | Leaves | Methanol | n.r. | [19] |
| Hibiscus roseus | Leaves, flowers | Acidulated ethonal | Phenolic compounds | [14] |
| Hesperomeles ferruginea | n.r. | Acidulated acetone | Total anthocyanins, total phenols | [21] |
| Hylocereus polyrhizus | Peels | Ethanol | Flavonoid, phenolic acids (gallic acid and sinapic acid) and one vitamin B2 | [33] |
| Hypericum juniperinum | n.r. | Acidulated acetone | Total anthocyanins, total phenols | [20] |
| Ipomoea mauritiana | Leaves | Methanol | n.r. | [19] |
| Juglans regia L. | Male flower | Methanolic | Antioxidant fatty acids, flavonoids and other secondary metabolites | [34] |
| Larch | Bark | Ethanol | Larch bark tannin | [35] |
| Lasia spinosa | Leaves | Methanol | n.r. | [19] |
| Leucas zeylanica | Leaves | Methanol | n.r. | [19] |
| Litchi chinensis | Leaves | Ethanolic | n.r. | [36] |
| Lycopodiella alopecuroides | Aerial parts | Acidulated acetone | Total phenolic; Total monomeric anthocyanin pigment; Total anthocyanins | [20, 21] |
| Mentha × villosa | Aerial parts | Ethanol | Polyphenols, flavonoids, and rosmarinic acid | [37] |
| Miscanthus‐sacchariflorus‐ | Aqueous‐dioxane | Lignin | [38] | |
| Mollugo cerviana | Leaves | Methanol | n.r. | [19] |
| Morella parvifolia | Acidulated acetone | Total phenolic, monomeric anthocyanin pigment | [20] | |
| Moringa oleifera | Leaves | Hydroalcoholic, methanolic, and water extract. | Total phenol | [39] |
| Nephelium lappaceum L | Peel | Ethanolic | Tannins and flavonoids | [40] |
| Olax zeylanica | Leaves | Methanol | n.r. | [19] |
| Ophiorrhiza mungos | Leaves | Methanol | n.r. | [19] |
| Pentacalia pulchella | n.r. | Acidulated acetone | Total anthocyanins, total phenols | [20] |
| Perilla, Perilla frutescens | Seed | Cold pressing and filtering | Cold‐pressed perilla oil | [41] |
| peromeles ferruginea | n.r. | Acidulated acetone | Total phenolic, monomeric anthocyanin pigment | [20] |
| Plectranthus amboinicus | Aerial parts | Ethanol | Polyphenols, flavonoids, and rosmarinic acid | [37] |
| Plectranthus caespitosus | Leaves | Dichloromethane + MeOH, or water | n.r. | [31] |
| Plectranthus ecklonii Benth | Aerial parts | Aqueous and ethanol extract | [42] | |
| Plectranthus zeylanicus | Leaves | Methanol | n.r. | [19] |
| Pnus‐densiflora | n.r. | Aqueous‐dioxane | Lignin | [38] |
| Polypodium leucotomos | Leaves | Hydrophilic extract | [43, 44] | |
| Prasiola calophylla | Leaves | Methanol | [45] | |
| Psidium guajava L | Fruit | Hydroalcoholic extract | Flavonoids, tannins | [46] |
| Psorospermum febrifugum | Stem bark | Dichloromethane+MeOH, or water | n.r. | [31] |
| Radix Scutellariae | Root | Ethanol | Flavonoid glycosides | [47] |
| Ruta graveolens L. | Leaves | Ethanol | Flavonoid | [29] |
| Sargassum cristafolium | Leaves | Ethanol | n.r. | [48] |
| Scutellaria radix | Root | Aqueous ethanol | Flavonoids | [49] |
| Silybum marianum L. | Seeds | n.r. | Silymarin, Flavonolignans | [50] |
| Solanum nigrum | Fabrics | Methanolic and aqueous | Phenolics and flavonoids | [17] |
| Sophora japonica L. | Flower | Water | Polysaccharide | [51] |
| Spermacoce princeae | Leaves | Dichloromethane + MeOH, or water | n.r. | [31] |
| theaflavin | Purchased purifed product | n.r. | Polyphenlic | [52] |
| Vitis vinifera L. | Peel or fruits | Hydroalcoholic | Flavonoid enriched extract (FE) | [29, 53] |
TABLE 2.
List of plant: species with major constituent and main biological effects
| plant name | Major constituent(s) | Main effect(s) | Ref. |
|---|---|---|---|
| Achillea biebersteinii | Chlorogenic acids, caffeic acid, cynarin, quinic acid, kaempferol, jaceidin, axillarin, kaempferol | Antioxidant, Tyrosinase Inhibitory Activity, SPF (5%, 11.67) | [15] |
| Achillea millefoliumyarrow | Quinic acid, 5‐caffeoylquinic acid (CQA), 3‐CQA, 4‐CQA, axillarin, jacedin, kaempferol and cynarin | Antioxidant activity, Tyrosinase Inhibitory Activity, SPF (5%, 14.04) | [15] |
| Aloe vera | Aloe gel nanoparticles–chitosan | UV‐protection factor (UPF) on coated fabrics (52.1, 57.2) | [16] |
| Amaranthus viridis | n.r. | UPF (ultraviolet protection factor)↑ | [17] |
| Angelica pubescens | Osthole, eugenol, α‐bisabolol | Anti‐inflammatory, anti‐UV‐B radiation, skin lesions↓, epidermal hyperplasia↓ | [18] |
| Aporosa lindleyana | n.r. | Antioxidant activity, UV absorber, SPF (21.4) | [19] |
| Argyreia populifolia | n.r. | Antioxidant activity, UV absorber, SPF (12.5) | [19] |
| Atalantia ceylanica | n.r. | Antioxidant activity, UV absorber, SPF (26.8) | [19] |
| Baccharis antioquensis | Total phenolic, monomeric anthocyanin pigment | UV absorber, Antioxidant activity, SPF (4.73), Photostability, UVA–UVB absorption coefficient | [20, 21] |
| Blackberry, raspberry | n.r. | Antioxidant activity, SPF (54.57 to blackberry and 37.32 to raspberry) | [22] |
| Calamagrostis effusa | Total phenolic, monomeric anthocyanin pigment | UV absorber, Antioxidant activity; UVA–UVB absorption coefficient | [20] |
| Calea fruticosa | Apigenin‐4′,7‐dimethyl ether, budlein A, quercetin, and cichoriin | Antiproliferative and photoprotective activities; SPF (13.79) | [23] |
| Castilleja fissifolia | Total phenolic, monomeric anthocyanin pigment | UV absorber, Antioxidant activity; UVA–UVB absorption coefficient | [20] |
| Charthamus tinctorius L. | Acacetin | Inhibit UVB‐induced MMP‐1 protein expression | [24] |
| Campomanesia | Myritrine, myricetin, cardamonin, stictane‐3,22‐diol valonic acid, gallic acid, myricitrin, rutin, quercetin pentose, quercetin deox‐yhexoside, quinic acid | UV absorber, SPF (5.58), photoprotective | [25] |
| Coffea arabica | Total phenolics | Against UV‐B, MMP‐1, MMP‐3, MMP‐9, ROS, photo‐protective activity, Xanthine oxidase inhibition activity, antioxidant, SPF (liposome/PHB) particles, 15.13) | [26, 27] |
| Dalbergia ecastaphyllum | Caffeic acid, catechin, epicatechin, naringenin, naringin, protocatechuic acid, quercetin, rutin, sinapic acid, vanillic acid, β‐resorcylic acid | Antioxidant, Tyrosinase inhibitory activity, SPF (~45) | [28] |
| Dirmophandra mollis Benth | Caffeic acid, catechin, epicatechin, naringenin, naringin, protocatechuic acid, quercetin, rutin, sinapic acid, vanillic acid, β‐resorcylic acid | Antioxidant, SPF (4.96) | [29] |
| Disterigma alaternoides | Quercetin, rutin | Antiradical capacity; Antioxidant activity; UVA‐UVB absorption coefficient | [21] |
| Drimys granadensis | Quercetin, rutin | Antiradical capacity, Antioxidant activity, UVA‐UVB absorption coefficient | [21] |
| Elaeagnus angustifolia | n.r. | 8%SPF 21.05 | [30] |
| Erlangea tomentosa | n.r. | Antioxidant, SPF (16.64) | [31] |
| Ginkgo biloba L. | Quercetin;rutin | SPF (7.06), Antioxidant | [29] |
| Helianthus annuus L. cv | n.r. | Anti‐UVA | [32] |
| Hesperomeles ferruginea | Total phenolic, monomeric anthocyanin pigment | UV absorber, Radical scavenging capability, antioxidant activity, UVA–UVB absorption coefficient | [20] |
| Hibiscus furcatus | n.r. | Anti‐UVA, SPF (29.4), antioxidant | [19] |
| Hibiscus roseus | Hydroxycinnamic acid derivatives; flavonoids, Catechin derivatives, dihydrochalcones; anthocyanins, phenolics | SPF (2.6), Collagenase Inhibition Activity, antioxidant | [14] |
| Hylocereus polyrhizus | Rutin, phenolic acids (gallic acid and sinapic acid) and vitamin B2 | UVA/UVB absorber, Antioxidant; photoprotective, SPF (35.02) | [33] |
| Hypericum juniperinum | Phenolic acids | UV absorber, antioxidant, photoprotective | [20] |
| Ipomoea mauritiana | (gallic acid and sinapic acid) and one vitamin B2″ | Antioxidant, SPF (11.3) | [19] |
| Juglans regia L. | 3,7‐Dimethyl‐1,6‐octadiene, pentadecanoic acid, 14‐methyl, methyl ester, 2‐(2,6‐dimethoxy‐benzoylamino)‐propionic acid, ethyl ester, hexadecanoic acid, ethyl ester (palmitic acid), 10‐octadecenoic acid, methyl ester, erucic acid; 1,2,3‐benzothiadiazole; estra‐1,3,5(10),6‐tetraene‐3,17‐diol, (17β)‐; 17‐acetate, 2,2,4‐trimethyl‐3‐(3,8,12,16‐tetramethyl‐heptadeca‐3,7,11,15‐tetraenyl)‐cyclohexanol and oleic acid, trimethylsilyl ester | Antioxidant; anti‐inflammatory, photoprotective, SPF (8.8), UVB absorber | [34] |
| Larch | Tannin | Antioxidant, LBT/PVA composite membranes demonstrated excellent UV protection ability | [35] |
| Lasia spinosa | n.r. | Antioxidant, anti‐inflammatory, anti‐UVB, SPF (8.9) | [19] |
| Leucas zeylanica | n.r. | SPF (39.8); Radical scavenging capability; antioxidant activity | [19] |
| Litchi chinensis | n.r. | The sunscreen formulation with LC showed a photoprotection of 32.9% | [36] |
| Lycopodiella alopecuroides | Total phenolic, Total monomeric anthocyanin pigment, Total anthocyanins | UV absorber, antioxidant, photoprotection | [20, 21] |
| Mentha × villosa | Rosmarinic Acid, total Flavonoids | SPF (13.73); antioxidant, UVA–UVB absorption coefficient | [37] |
| Miscanthus‐sacchariflorus | Lignin | UV blocking activity, SPF (1%, enhanced SPF value of commercial sunscreen 31.6) | [38] |
| Mollugo cerviana | n.r. | Antioxidant, photostability, SPF (29.5) | [19] |
| Morella parvifolia | n.r. | Antioxidant activity; photoprotective | [20] |
| Moringa oleifera | Rutin, Quercetin, Ellagic acid, Chlorogenic acid, Ferulic acid | Antioxidant activity, SPF (4%water extract, 2.01), UVAPF0(1.44), UVA‐UVB absorber | [39] |
| Nephelium lappaceum L | Flavonoids, tannins | UVB absorber, SPF (1% extract increased 7.5% EHMC SPF value from 11.2 to 26.3) | [40] |
| Olax zeylanica | n.r. | SPF (24.5), Radical scavenging capability, antioxidant activity | [19] |
| Ophiorrhiza mungos | n.r. | SPF (39.2); Radical scavenging capability; antioxidant activity | [19] |
| Pentacalia pulchella | n.r. | Antioxidant activity; photoprotective, SPF‐ (10%P. pulchella, 4.36) | [20] |
| Perilla, Perilla frutescens | Linolenic acid, Oleic Acid, Linoleic Acid | Antioxidant, MMP‐1↓, skin wrinkle scores↓, skin aging↓ galactosidase and MMP‐3 mRNA↓ | [41] |
| Plectranthus amboinicus | Rosmarinic Acid, total Flavonoids | SPF (14.79), antioxidant | [37] |
| Plectranthus caespitosus | n.r. | Antioxidant, SPF (37.84) | [31] |
| Plectranthus ecklonii Benth | Rosmarinic acid and parvifloron D | Antioxidant activity; photoprotective ability, The extract association with benzophenone‐4 provided an SPF augmentation of 19.49% | [42] |
| Plectranthus zeylanicus | n.r. | SPF (11.5), Radical scavenging capability, antioxidant activity | [19] |
| Pinus‐densiflora | lignin | UV blocking activity, SPF (1%, enhanced SPF value of commercial sunscreen 11.6) | [38] |
| Polypodium leucotomos | n.r. | The UV Barrier‐Function and Immune Protection‐ Capability | [43, 44] |
| Prasiola calophylla | Prasiolin, mycosporine‐like amino‐acid (MAA) | A new UV‐sunscreen compound, UVA absorber | [45] |
| Psidium guajava L | n.r. | Photoprotective activity, SPF (1.0, improve SPF value of 7.5% 2‐ethyl‐hexyl methoxycinnamate formulation to 8.1) | [46] |
| Psorospermum febrifugum | Antioxidant, SPF (16.67) | [31] | |
| Radix Scutellariae | Baicalin, wogonoside | Antioxidant (ABTS), IL‐6↓, MMP‐1↓, p‐c‐fos, p‐c‐jun↓ p‐ERK /p‐JNK/ p‐‐p‐38↓TGF‐β1, p‐Smad2/3↓Nrf2 (in nuclear), HO‐1, and NQO‐1↑ | [47] |
| Ruta graveolens L. | Quercetin; rutin | SPF (5.34), Antioxidant | [29] |
| Sargassum cristafolium | n.r. | Anti‐UVA; photoprotective;Histological analyze | [48] |
| Scutellaria radix | Baicalin and balcalein | SPF22.7 (sunscreen 5% SR extract BuOH fraction), scavenge Free radical | [49] |
| Silybum marianum L. | Silymarin, flavonolignans | UVA protection factor (PF‐UVA), UVB‐protective | [50] |
| Solanum nigrum | n.r. | Improve UPF (ultraviolet protection factor) value | [17] |
| Sophora japonica L. | Polysaccharide | ROS↓, p‐JNK/ p‐‐p‐38↓ | [51] |
| Spermacoce princeae | n.r. | Antioxidant, SPF (17.05) | [31] |
| theaflavin | Theaflavin | Antityrosinase activity, antioxidation, UV filter, SPF | [52] |
| Vitis vinifera L. | Quercetin; rutin | SPF (3.17); antioxidant activity | [29, 53] |
4.1. Flavonoid and total polyphenolic compounds
As shown in Table 1, total flavonoids and total polyphenolics are the most studied components with photoprotective properties. In the strict sense, flavonoids are a category of polyphenolic compounds synthesized via the phenylpropanoid metabolic pathway in plants and have attracted considerable attention recently in scientific and therapeutic fields. The presence of double bonds or aromatic rings in the molecular structure of flavonoids gives them UV absorption properties in range of 200–400 nm, which makes them suitable for use as sunscreens agents. The flavonoids most often reported to possess photoprotective ability are rutin and quercetin, which have been identified separately or together in Campomanesia, 25 Hylocereus polyrhizus, 33 Moringa oleifera, 39 Dalbergia ecastaphyllum, 28 Disterigma alaternoides, 21 Drimys granadensis, 21 Ginkgo biloba L., 29 Ruta graveolens L., 29 Moringa oleifera 39 and Vitis vinifera L. 29 , 53 These flavinoids showed a significant antioxidant ability, but, interestingly, low SPF values, with the exception of the extract from Hylocereus polyrhizus, which is rich in rutin and phenolic acids (gallic acid and sinapic acid), and showed UVA/UVB absorption, antioxidant and photoprotective effects and an SPF value reaching 35.02. 33
Monomeric anthocyanin pigment, 14 , 20 , 21 ellagitannins 39 and catechin 14 , 29 are also considered to be potent antioxidants that attenuate UV‐induced free radical damage. These botanical compounds, including for example, extracts from rambutan, 40 Pinus densiflora, 38 Psidium guajava L. 46 and Scutellaria radix, 49 may strengthen radiation resistance by stabilizing or reconstituting their chemical structures during UV radiation, but do not show direct photoprotective activity.
Soto ML et al. have also reported that grape extracts rich in flavonoids and phenolic acids are critical therapeutic components of new after‐sun cosmetics that act by reducing oxidative stress, inflammation and immunosuppression in sunburned skin. 55
4.2. Polysaccharides
Few polysaccharides have been reported to possess anti‐UV ability. The polysaccharide from the flower bud of Sophora japonica L. has shown significant UV‐protectant ability through decreases in UVB radiation‐induced ROS and MAPK signal protein p‐JNK/ phospho p‐38. 51 The superoxide anion radical was initially generated by exposure to UVA, while the generation of the hydroxyl free radical was related to UVB irradiation. The ability of the polysaccharide in the flower bud of S.japonica L to scavenge hydroxyl free radicals and superoxide anion radicals has been confirmed. 56 Fructan from white garlic has also been reported to have a protective effect on UVB‐induced keratinocyte damage. 57
4.3. Lignin
Lignin is a kind of aromatic polymer abundant in plants. It is a natural UV screening agent, but, as reported in a few studies recently, its unfavorable dark color hinders its application as a high value addition to sunscreens. 38 Lee S.C. documented an improved method for separating and purifying lignin to prevent it from darkening during the process of delignification, allowing the purified lignin to be used as a natural sunscreen component for the first time. 38
4.4. Phenylpropionic acids
Phenylpropionic acids are naturally occurring organic acids found in plants. They can combine with sugar or polyols and exist in plants in the form of glycosides or esters, showing strong physiological activity. A lot of phenylpropionic acids such as chlorogenic acids, caffeic acid, quinic acid and cynarin from Achillea biebersteinii 15 and Achillea millefolium (yarrow), caffeic acid, catechin, epicatechin, naringenin, naringin, and protocatechuic acid from Dalbergia ecastaphyllum 28 and Dirmophandra mollis Benth, 29 rosmarinic acid from Plectranthus amboinicus 37 and Plectranthus ecklonii Benth 42 have been reported to show anti‐UV ability through antioxidant and tyrosinase inhibitory activity. This suggests that phenylpropionic acids show anti‐UV property indirectly.
4.5. Other compounds
Besides the compounds mentioned above, some research has shown that osthole—a natural coumarin, 18 α‐bisabolol—a sesquiterpene, 18 carotenoids, 28 and herbal oils, 24 , 41 have also shown significant UV‐protectant abilities.
5. EVALUATION MODELS AND MECHANISMS FOR UV RADIATION RESISTANCE
5.1. Anti‐UV evaluation model in vitro
Skin cell models in vitro are mostly derived from human cell cultures, thereby avoiding the complication of interspecific differences, and have the advantages of short test periods and being easy to operate. HaCaT cells are a non‐tumor‐origin immortalized human normal skin keratinocyte strain that has similar differentiation characteristics to normal in vivo keratinocytes, and has the advantage of being easy to culture and passage multiple times. Therefore, they are widely used in anti‐UV radiation drug screening and mechanism research.
5.2. Cell viability
MTT is coloring agent usually employed to evaluate the viability of epidermal cells. After incubating with a drug, the culture medium needs is removed from the cell plate, and a new culture medium mixed with 5% of MTT is added and incubated for 4 h, followed by addition of DMSO to dissolve formazan crystals. Finally, the absorbance value at 490 nm is detected. 58 Due to a certain level of carcinogenicity and consequent safety risks for operators, MTT has gradually been replaced by CCK‐8 recently. For the CCK‐8 coloring agent assay, the cells are incubated with new culture medium mixed with 10% CCK‐8 for 0.5–1 h, and absorbance is detected at OD450. The easier operation and lack of toxic side effects make CCK‐8 method more acceptable for operators.
5.3. Ultraviolet scanning
UV transmission measurements are performed using a spectrophotometer equipped with an integrating sphere. 59 The ελ value—the absorption characteristics of 1 mole of sunscreen at a thickness of 1 cm at a wavelength of λ—is used to determine the ability of filter to absorb UV in the range of 280–400 nm.
5.4. Determination of the SPF value
In 1934, Friedrich Ellinger first put forward the concept of a minimal erythema dose (MED). 60 MED was defined as the minimal dose or period of UV radiation sufficient needed to produce a minimal perceptible erythema on unprotected skin. F. Creiter introduced the term sun protection factor (SPF) in 1974. 61 The SPF was defined as the UV energy required to generate a MED on protected skin divided by the UV energy required to generate a MED on unprotected skin. It is usually used to evaluate how well a sunscreen protects skin from UVB rays.
Studies have shown that extracts from natural plants enhance the SPF of sunscreen formulations (Table 2). G.B. Katarzyna et al. reported that a water: polyethylene glycol (4:1) extract of Achillea millefolium (yarrow) provided a maximum SPF value of 14.04 ± 0.17, while a 5% hydroglycolic extract of Achillea biebersteinii provided a maximum SPF value of 11.67; the extracts also showed cytotoxicity for A375 melanoma and human keratinocyte HaCaT cells. 15 T.N. Mayuri and co‐researchers reported that 1 mg/ml methanol extracts of Hibiscus furcatus, Atalantia ceylanica, Mollugo cerviana, Leucas zeylanica, Ophiorrhiza mungos and Olax zeylanica leaves displayed SPF values ≥25, with the value for Atalantia ceylanica extract being 26.8, while a 1% methanol extract of Miscanthus sacchariflorus showed good UV blocking activity and anti‐UVB ability, and enhanced the SPF value of commercial sunscreen to 31.6. 19 Milleno Dantas Mota and co‐researchers reported that a 1% ethanol extract of Nephelium lappaceum L. peel improved the SPF value of a sunscreen formulation containing 7.5% of ethylhexyl metoxycinnamate (EHMC) from 11.2 to 26.3. 40 Nephelium lappaceum L. also displayed a higher total phenolic content (151 mg/g), which could explain the reported SPF values. Another study by Jane Namukobe et al. reported the photoprotective effect of plants in Uganda; 2 mg/mL of aqueous extracts from Spermacoce princeae and Plectranthus caespitosus provided SPF values of 37.84 and 17.05, respectively, and 2 mg/ml of organic extracts from Erlangea tomentosa and Psorospermum febrifugum provided SPF values of 16.46 and 16.67, respectively. 31 Pinus densiflora extract also showed good UV blocking activity, with a 1% extract increasing the SPF value of commercial sunscreen by 11.6. 38 Plectranthus ecklonii Benth extract combined with benzophenone‐4 provided an SPF augmentation of 19.49%. 42
5.5. UVA‐protection factor (PF)
UVA (320–400 nm) does not primarily lead to reddening or pain, but could cause invisible skin damage and skin photoaging. The COLIPA in vitro UVA method, a standard and reproducible measure of sunscreen UVA protection, was published in 2010. 62 The L'Oréal company reported a revised COLIPA in vitro UVA method in 2013 63 and this is currently the most authoritative method for determination of UVA protection in the world.
5.6. Photoprotective efficacy in vitro
Photoprotective efficacy in vitro is calculated according to the following formulas:
UVB efficacy is estimated by SPF after and before UV radiation; UVA efficacy is estimated by UVA‐PF after and before UV radiation. 20 Six parallel tests are performed for each sample.
5.7. SI value
The selectivity index (SI) is defined as the value of the toxic concentration divided by bioactive concentration for a drug or sample. 64 An effective drug should have a relatively high toxicity concentration but a low active concentration. For any study of a botanical drug and/or isolated plant component, estimation of the SI value is crucially important to determine whether to continue the study. The index has also been used by researchers in Japan to evaluate anti‐UV activity, defined as the value of the concentration that reduces the viable cell number by 50% divided by the concentration that increases the viability of UV‐irradiated cells to 50% (Table 3). 65 , 66 , 67 , 68
TABLE 3.
Selectivity index (SI) value of herbal constituents
| Category | Cell line | SI value | References |
|---|---|---|---|
| Shosoikoto | HaCaT | 34 | [62] |
| Hangesyashinto | HaCaT | >28 | [62] |
| Uniseiin | HaCaT | >23 | [62] |
| Saireito | HaCaT | >19 | [62] |
| Ninjinyotiyo | HaCaT | 23 | [62] |
| Scurellaria root | HaCaT | 38 | [62] |
| Polyporus sclerotium | HaCaT | >26 | [62] |
| Gardenia fruit | HaCaT | >23 | [62] |
| Japanese Gentian | HaCaT | >20 | [62] |
| Saposhnikovia root | HaCaT | >20 | [62] |
| Glycyrrhizin | HaCaT | 36 | [62] |
| Vitamin C (Positive control) | HaCaT | 200 | [62] |
| Luteolin 6‐C‐β‐D‐glucoside in leaves of Sasa senanensis Rehder | HSC‐2 | >2 | [63] |
| Luteolin 7‐O‐β‐D‐glucoside in leaves of Sasa senanensis Rehder | HSC‐2 | 7 | [63] |
| Luteolin 6‐C‐α‐L‐arabinoside in leaves of Sasa senanensis Rehder | HSC‐2 | >7 | [63] |
| Tricin in leaves of Sasa senanensis Rehder | HSC‐2 | 27 | [63] |
| Alkaline extracts in leaves of Sasa senanensis Rehder | HSC‐2 | 40 | [63] |
| Lentinus edodes mycelia extract (LEM) | HSC‐2 | 13.9 | [64] |
| lignin−carbohydrate complex (LCC) from pine cones and seed shells | HSC‐2 | 7.6–38 | [64] |
| Vanillin | 63.8 | [64] | |
| Gallic acid | 5.4 | [64] | |
| EGCG | >2.6 | [64] | |
| EGCG | HSC‐2 | 7.7 | [65] |
| Gallic acid | HSC‐2 | 17.1 | [65] |
| sodium ascorbate (Vc) | HSC‐2 | 42.4 | [65] |
| Lentinus edodes Mycelia Extract (LEM) | 41.9 | [65] |
6. ANTI‐UV EVALUATION MODEL IN VIVO
HR‐1 hairless mice and C57BL/6 mice are the current strains used to establish animal models in vivo to evaluate the anti‐UV ability of plant extracts. Choi Sun‐Il et al. used HR‐1 hairless mice to investigate the anti‐photoaging properties of soybean cake, sesame seed cake and fermented rice bran induced by UVB irradiation. 69 Nan et al used C57BL/6 mice to evaluate the anti‐UV ability of quercetin‐loaded chitosan nanoparticles. 70
For animal models in vivo, the HE and Masson methods are used to evaluate pathological and histological changes.
7. ANTI‐UV MECHANISMS OF NATURAL PLANT SUBSTANCES USED AS SUNSCREEN FILTERS
7.1. UVA and UVB absorption
A perfect botanical UV filter can absorb UV rays, convert the electrons to an excited state, and then convert them back to the original state through ultra‐fast photoisomerization, effectively transferring the UV energy to the environment to avoid DNA damage. Therefore, evaluation of whether botanical extracts exhibit photo‐protection after UV irradiation is crucial for their application as UV filters in sunscreen formulas.
UVA can be subdivided into UVA‐2 (320–340 nm) and UVA‐1 (340–400 nm). UVA‐1 has the strongest penetrating power and can reach the dermis layer of the skin and tan the skin. It is radiation most damaging to the skin, but it is also the easiest to ignore. Although the intensity of UVA‐1 is weak, especially in the non‐summer seasons, it still causes skin damage due to long‐term accumulation of the radiation dose. UVA‐2 can reach the epidermis, like UVB, and can cause sunburn, redness and pain, solar keratosis, and loss of transparency.
Botanicals rich in flavonoids and total phenolonic compounds, such as ellagic acid, corilagin, geraniin, apigenin, quercetin, catechin, and anthocycanins in rambutan extract, always show a maximum absorption range of 280–400 nm. 40 All of the botanical components have conjugated bonds and chemical groups responsible for absorbing UV radiation at different wavelengths. Quercetin and anthocyanins directly absorb UV radiation to inhibit UVB‐induced skin damage. Thus we find that the substances from plants showing photoprotective activities almost always belong to total phenolic and flavonoid groups, as summarized in Table 2.
On the other hand, most of chemical sunscreen filters, such as PEG‐25 PABA, ethylhexyl salicylate, octocrylene, and 4‐oxymethylcinnamate‐2‐ethylhexyl, are focused on UVB absorption. Parsol 1789 is one of the most effective UVA absorbers in chemical sunscreen filters, but is difficult to synthesize, and has poor photostability and high sensitization. Therefore, natural substances from plants have unique advantages in developing sunscreen filters compared to chemical sunscreen filters.
7.2. Anti‐oxidant activity
Most of the herbal extracts with anti‐UV ability have shown antioxidant activity, as shown in Table 2. Antioxidant activity is a crucial mechanism by which botanical extracts exert photo‐protective activity.
7.2.1. Anti‐oxidant activity in vitro
Up to now, a number of analytical methods for evaluating the antioxidant activity of plant extracts have been developed, including evaluation of the ability to eliminate radicals, ABTS, 71 , 72 DPPH, 73 oxygen radical absorbance capacity (ORAC), 74 photochemiluminescence (PCL) or reduction potentials of compounds (ferric reducing antioxidant potential, FRAP). Among these, evaluation of the eliminating ability of ABTS and DPPH ability is efficient and has been adopted in most cases for primary determination of antioxidant activity in vitro of herbal extracts; see Table 2 for details. Using these methods, many researchers have reported significant antioxidant activity of various plant extracts. 15 , 19 , 20 , 21 , 26 , 28 , 29 , 31 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 43 , 47 , 48 , 52 , 53 , 54
7.2.2. ROS assay
Reactive oxygen species (ROS) are highly reactive chemicals derived from O2, including hydroxyl radicals, singlet oxygen molecules, peroxides, superoxides and alpha‐oxygen. ROS are the normal metabolism byproducts of oxygen at low levels in organisms and play an important role in cell signaling and homeostasis. 3 , 4 When organisms are under environmental stress (e.g. UV or heat exposure), ROS levels can increase dramatically. If the excessive ROS are not eliminated quickly, significant damage to cell structure may result. Excessive UV radiation‐induced ROS in skin cells further lead to DNA damage, membrane lipid‐peroxidation, skin aging and tissue damage.
The antioxidant properties of natural products and extracts from plants may provide new possibilities to treat and prevent skin from UV‐induced damage. Polyphenolic and flavonoid compounds are important sources of natural antioxidant extracts. Furthermore, ROS play a central role in initiating and driving various signaling pathways that lead to a cellular response to UVA and UVB irradiation. Therefore ROS detection has been used to evaluate the antioxidant activity of natural products or extracts. CGA isolated from beans of Coffea arabica dose‐dependently inhibited intracellular ROS production in CCRF cells stimulated by UV radiation. 26 Pretreatment of HaCaT cells with a methanol extract of the male flower of Juglans regia L. for 0.5 h before UVB‐irradiation inhibited ROS generation and lipid peroxidation, and restored antioxidant activity in cells. 34 Cold‐pressed perilla oil extracted from Perilla frutescens reduced UV‐induced ROS production in NHDF cells, 41 and 1.0 mg/ml of polysaccharide from the flower bud of Sophora japonica L. significantly decreased the level of ROS induced by UVB (120 mJ/cm2 and 240 mJ/cm2). 51
7.3. MMPs
Matrix metalloproteinases (MMPs), a family of zinc‐containing proteinases, are responsible for degradation of extracellular matrix (ECM) proteins. 6 Previous studies have revealed that UV radiation promoted the expression level of interstitial collagenase (MMP‐1), stromelysin‐1 (MMP‐3), and gelatinase (MMP‐9) in human skin in vivo. 7 Via this mechanism, UV irradiation can rapidly induce and activate transcription factor AP‐1, and the latter could strongly regulated the expressions of MMP‐1, MMP‐3 and MMP‐9 in human skin in vivo. On the other hand, UV directly induces an increase in ROS, which provokes secretion of inflammatory cytokines and enhances levels of MMPs in dermal fibroblasts, furthermore reducing the synthesis of procollagen, collagen, elastin fibers, fibronectin, and laminin, and thus having adverse effects on skin elasticity resulting in wrinkle formation. 75 Therefore, MMPs are thought to be responsible for dermal photoaging in human skin.
Evaluation of MMP‐1 expression along with collagen synthesis is therefore a preliminary screening process for anti‐aging agents due to its significant ability to initiate collagen cleavage. 76 Safflower seed oil and its purified compound acacetin significantly inhibited UVB‐induced MMP‐1 protein expression in HaCaT cells and human dermal fibroblasts (HDF). 24 Chlorogenic acid isolated from beans of Coffea arabica effectively suppressed the expression of the MMP‐1, 3, and 9 and increased synthesis of type‐I procollagen in UVB‐stimulated CCRF cells. 26 Cold‐pressed perilla oil (CPO) from Perilla frutescens, mainly consisting of linolenic acid and oleic acid, markedly suppressed UV‐induced MMP‐1 protein levels in NHDF cells. 41 Wogonin, baicalin and baicalein isolated from Radix Scutellariae diminished the increase in MMP‐1 mRNA levels caused by UVB radiation, and 100 μg/ml of enzyme‐treated Radix Scutellariae reduced UVB‐induced MMP‐1 expression by 68.35%. 47 MMP‐1 expression is also related to the activation of mitogen‐activated protein kinase (MAPK)/AP‐1 pathway. 9
7.4. MAPK signaling pathways
MAPKs are a large family of protein kinases that phosphorylate and sequentially activate one another in a series of distinct cascades in response to extraordinarily diverse sets of stimuli involved in the regulation of development, growth, differentiation, inflammation and cell death. The family includes Jun‐N‐terminal kinase (JNK), extracellular‐regulated protein kinase (ERK), and p38 kinase. The MAPK pathway is a well‐known ROS‐sensitive signaling pathway. 77 , 78 Activation of MAPKs by UV radiation is one of the early cellular responses and depends strictly on time, dosage and wavelength. 79 Wang et al verified that enzyme‐treated Radix Scutellariae significantly inhibited the excessive UVB‐induced expressions of MMP‐1 and IL‐6 by inactivating the MAPK/AP‐1 and NF‐κB/IκB‐α signaling pathways, and their compounds baicalin and wogonoside significantly decreased p‐ERK, p‐JNK, and p–p‐38 levels. 47 Our research reported that polysaccharide from the flower bud of Sophora japonica L. decreased ROS generation, and down‐regulated the expression of phosphor‐JNK and phosphor‐p38 MAPK proteins significantly in UVB‐irradiated cells. 52 Evidently therefore, the MAPK signaling pathways form a core unit of UV‐induced stress responses in human keratinocytes, which had been summarized by Zerihun Assefa et al. 80
8. CONCLUSION
More and more commercial sunscreen products with natural products or extracts from plants are available on the market due to the many benefits they provide that traditional sunscreens do not have. Here we have summarized the articles on anti‐UV activity published in the last 6 years and selected the most significant data to better understand the photoprotective activity of natural products and extracts from plants, including their major constituents and main biological effects, methods for evaluating UV radiation resistance, anti‐UV radiation experimental models, and anti‐UV radiation mechanisms. This review will provide a strong foundation for evaluating the status and potential use of natural UV filters.
AUTHOR CONTRIBUTIONS
Liyan Li and Hui Ding designed and supervised the manuscript. Liyan Li, Chong Lan wrote the manuscript. Tao Huang, Yunge Ma and Yingyan Li revised the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This study was supported by Henan Provincial Department of Education (No. 21B350001) and Zhengzhou Science and Technology Department (No. ZZSZX202109 and ZZSZX202108).
CONFLICT OF INTEREST
The authors declared no conflict of interest for this article.
ACKNOWLEDGMENTS
This work was supported by Henan Provincial Department of Education (No. 21B350001) and Zhengzhou science and technology department (No. ZZSZX202109 and ZZSZX202108).
Li L, Chong L, Huang T, Ma Y, Li Y, Ding H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim Models Exp Med. 2023;6:183‐195. doi: 10.1002/ame2.12295
Contributor Information
Liyan Li, Email: liyanli0921@163.com.
Hui Ding, Email: snnu_dh@126.com.
REFERENCES
- 1. Battistin M, Dissette V, Bonetto A, et al. A new approach to UV protection by direct surface functionalization of TiO2 with the antioxidant polyphenol dihydroxyphenyl benzimidazole carboxylic acid. Nanomaterials (Basel). 2020;10(2):231‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu X, Li N, Wang Y, et al. Protective effects of quercetin on UVB irradiation‐induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol Rep. 2017;37:209‐218. [DOI] [PubMed] [Google Scholar]
- 3. Duale N, Olsen AK, Christensen T, Butt ST, Brunborg G. Octyl methoxycinnamate modulates gene expression and prevents cyclobutane pyrimidine dimer formation but not oxidative DNA damage in UV‐exposed human cell lines. Toxicol Sci. 2010;114:272‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seité S, Colige A, Piquemal‐Vivenot P, et al. A full‐UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol Photo. 2000;16:147‐155. [DOI] [PubMed] [Google Scholar]
- 5. Menguzzato P, Ziosi P, Vertuani S, et al. Naturale e innovazione sostenibile: il futuro della cosmesi? Natural. 2015;3:44‐52. [Google Scholar]
- 6. Wang SK, Balagula I, Osterwalder U. Photoprotection: a review of the current and future technologies. Dermato Ther. 2010;23:31‐47. [DOI] [PubMed] [Google Scholar]
- 7. Antoniou C, Kosmadaki MG, Stratigos AJ, Katsambas AD. Sunscreens – what's important to know. J Eur Acad Dermatol Venereol. 2008;22:1110‐1118. [DOI] [PubMed] [Google Scholar]
- 8. Jin CY, Zhu BS, Wang XF, Lu QH. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem Res Toxicol. 2008;21:1871‐1877. [DOI] [PubMed] [Google Scholar]
- 9. van der Pols JC, Williams GM, Pandeya N, Logan V, Green ÀC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546‐2548. [DOI] [PubMed] [Google Scholar]
- 10. Grande F, Tucci P. Titanium dioxide nanoparticles: a risk for human health? Mini Rev med Chem. 2016;16(9):762‐769. [DOI] [PubMed] [Google Scholar]
- 11. González S, Fernández‐Lorente M, Gilaberte‐Calzada Y. The latest on skin photoprotection. Clin Dermatol. 2008;26:614‐626. [DOI] [PubMed] [Google Scholar]
- 12. Nelson DL and Cox MM. Metabolismo do DNA. In: Lehninger, A.L , ed. Princípios de Bioquímica do Lehninger. Sarvier; 2006:940–984. [Google Scholar]
- 13. Svobodová A, Psotová J, Walterová D, et al. Natural phenolics in the prevention of UV‐induced skin damage. A review. Biomed Pap med Fac. 2003;147:137‐145. [PubMed] [Google Scholar]
- 14. Beatriz L, dos Nascimento S, Gori A, Raffaelli A, et al. Phenolic compounds from leaves and flowers of Hibiscus roseus: potential skin cosmetic applications of an under‐investigated species. Plan Theory. 2021;10(3):522. doi: 10.3390/plants10030522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katarzyna Gaweł B˛ e, Marcelina Strz˛ e‐G, Marcin C, et al. Achillea millefolium L. and Achillea biebersteinii Afan. Hydroglycolic extracts–bioactive ingredients for cosmetic use. Molecule. 2020;25:3368‐3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subramani K, Shanmugam BK, Rangaraj S, et al. Screening the UV‐blocking and antimicrobial properties of herbal nanoparticles prepared from Aloe vera leaves for textile applications. IET Nanobiotechnol. 2018;12(4):459‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asad SM, Ahsan N, Faiza N, et al. Comparison of UV protection properties of cotton fabrics treated with aqueous and methanolic extracts of solanum nigrum and Amaranthus Viridis plants. Photodermatol Photoimmunol Photomed. 2019;35(2):93‐99. [DOI] [PubMed] [Google Scholar]
- 18. Chen DK, Du ZY, Lin ZR, et al. The chemical compositions of Angelica pubescens oil and its prevention of UV‐B radiation‐induced cutaneous photoaging. Chem Biodivers. 2018;15(10):e1800235. doi: 10.1002/cbdv.201800235 [DOI] [PubMed] [Google Scholar]
- 19. Napagoda MT, Malkanthi BMAS, Abayawardana SAK, Qader MM, Jayasinghe L. Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complement Altern med. 2016;16(1):479‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juan CMG, Kelly HZ, Cecilia G, et al. Novel in vitro antioxidant and photoprotection capacity of plants from high altitude ecosystems of Colombia. Photochem Photobiol. 2016;92:150‐157. [DOI] [PubMed] [Google Scholar]
- 21. Juan CMG, Cecilia G, Miguel APM. Selected extracts from high mountain plants as potential sunscreens with antioxidant capacity. Photochem Photobiol. 2022;98(1):211‐219. [DOI] [PubMed] [Google Scholar]
- 22. Cefali LC, Franco JG, Nicolini GF, Ataide JA, Mazzola PG. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J Cosmet Dermatol. 2019;18(2):539‐544. [DOI] [PubMed] [Google Scholar]
- 23. Seregheti TMQ, Pinto APR, Goncalves MD, et al. Antiproliferative and photoprotective activities of the extracts and compounds from Calea fruticose . Braz J Med Biol Res. 2020;53(9):e9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeong EH, Yang H, Kim JE, Lee KW. Safflower seed oil and its active compound acacetin inhibit UVB‐induced skin photoaging. J Microbiol Biotechnol. 2020;30(10):1567‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Catelan TBS, Gaiola L, Duarte BF, Cardoso CAL. Evaluation of the in vitro photoprotective potential of ethanolic extracts of four species of the genus Campomanesia . J Photochem Photobiol B: Biol. 2019;197:111500. [DOI] [PubMed] [Google Scholar]
- 26. Cho YH, Kim ABHH, Kim DI, et al. Potential effect of compounds isolated from Coffea Arabica against UV‐B induced skin damage by protecting fibroblast cells. J Photochem Photobiol B: Biol. 2017;174:323‐332. [DOI] [PubMed] [Google Scholar]
- 27. Pavelkova R, Matouskova P, Porizka JHJ, et al. Preparation and characterization of organic UV filters based on combined PHB/liposomes with natural phenolic compounds. J Biotechnol. 2020;324 S:100021. [DOI] [PubMed] [Google Scholar]
- 28. Morais DV, Costa MAPC, Bárbara MFS, et al. Antioxidant, photoprotective and inhibitory activity of tyrosinase in extracts of Dalbergia ecastaphyllum . PLoS One. 2018;13(11):e0207510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cefali LC, Ataide JA, Fernandes AR, et al. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo Biloba L, Ruta Graveolens L, and Vitis vinífera L. Plants. 2019;8(11):453‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahmady A, Amini MH, Zhakfar AM, et al. Sun protective potential and physical stability of herbal sunscreen developed from afghan medicinal plants. Turk J Pharm Sci. 2020;17(3):285‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Namukobe J, Sekandi P, Byamukama R, et al. Antibacterial, antioxidant, and sun protection potential of selected ethno medicinal plants used for skin infections in Uganda. Trop Med Health. 2021;49(1):49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stelzner J, Roemhild R, Adriana GH, et al. Hydroxycinnamic acids in sunflower leaves serve as UV‐A screening pigments. Photochem Photobiol Sci. 2019;18(7):1649‐1660. [DOI] [PubMed] [Google Scholar]
- 33. Vijayakumar R, Gani SSA, Zaidan UH, et al. Exploring the potential use of Hylocereus polyrhizus peels as a source of cosmeceutical sunscreen agent for its antioxidant and photoprotective properties. Evid Based Complement Alternat Med. 2020;2020:7520736. doi: 10.1155/2020/7520736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muzaffer U, Paul VI, Prasad NR, et al. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine. 2018;42:100‐111. [DOI] [PubMed] [Google Scholar]
- 35. Zhai YX, Wang JT, Wang H, Song T, Hu W, Li S. Preparation and characterization of antioxidative and UV‐protective larch bark tannin/PVA composite membranes. Molecules. 2018;23:2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thiesen LC, Bretzke PE, Bittencourt CMS, et al. Litchi chinensis leaf extract provides high in vitro photoprotection associated to a natural mineral clay. Photodermatol Photoimmunol Photomed. 2019;00:1‐2. [DOI] [PubMed] [Google Scholar]
- 37. de Medeiros Gomes J, Cahino Terto MV, Golzio do Santos S, Sobral da Silva M, Fechine Tavares J. Seasonal variations of polyphenols content, sun protection factor and antioxidant activity of two Lamiaceae species. Pharmaceutics. 2021;13:110‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SC, Tran TMT, Choi JW, Won K. Lignin for white natural sunscreens. Int J Biol Macromol. 2019;122:549‐554. [DOI] [PubMed] [Google Scholar]
- 39. Baldisserotto A, Buso P, Radice M, et al. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules. 2018;23:664‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mota MD, Nascimento A, da Morte B, Cerqueira e Silva LCR, et al. Sunscreen protection factor enhancement through supplementation with rambutan (Nephelium lappaceum L) ethanolic extract. Int J Biol Macromol. 2020;205:111837‐111843. [DOI] [PubMed] [Google Scholar]
- 41. Choi HJ, Song BR, Kim JE, et al. Therapeutic effects of cold‐pressed perilla oil mainly consisting of linolenic acid, oleic acid and linoleic acid on UV‐induced photoaging in NHDF cells and SKH‐1 hairless mice. Molecules. 2020;25:989‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nicolai M, Mota J, Fernandes AS, et al. Assessment of the potential skin application of Plectranthus ecklonii Benth. Pharmaceuticals. 2020;13:120‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aguilera J, Vicente‐Manzanares M, De Gálvez MV, Herrera‐Ceballos E, Rodríguez‐Luna A, González S. Booster effect of a natural extract of polypodium Leucotomos that improves the UV barrier function and immune protection capability of sunscreen formulations. Front Med (Lausanne). 2021;8:684665‐684674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pourang A, Dourra M, Ezekwe N, Kohli I, Hamzavi I, Lim HW. The potential effect of polypodium leucotomos extract on ultraviolet‐and visible light‐induced photoaging. Photochem Photobiol Sci. 2021;20:1229‐1238. [DOI] [PubMed] [Google Scholar]
- 45. Hartmann A, Holzinger A, Ganzera M, Karsten U. Prasiolin, a new UV‐sunscreen compound in the terrestrial green macroalga Prasiola calophylla (Carmichael ex Greville) Ku¨tzing (Trebouxiophyceae, Chlorophyta). Planta. 2016;243:161‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mota MD, Costa RYS, Silva Guedes A, et al. Guava‐fruit extract can improve the UV‐protection efficiency of synthetic filters in sun cream formulations. J Photochem Photobiol B: Biol. 2019;201:111639. [DOI] [PubMed] [Google Scholar]
- 47. Wang YS, Cho JG, Hwang ES, et al. Enhancement of protective effects of radix Scutellariae on UVB‐induced photo damage in human HaCaT keratinocytes. Appl Biochem Biotechnol. 2018;184(4):1073‐1093. [DOI] [PubMed] [Google Scholar]
- 48. Prasedya ES, Syafitri SM, Geraldine BAFD, et al. UVA photoprotective activity of Brown macroalgae sargassum cristafolium . Biomedicine. 2019;7:77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seok JK, Kwak JY, Choi GW, et al. Scutellaria radix extract as a natural UV protectant for human skin. Phytother Res. 2016;30:374‐379. [DOI] [PubMed] [Google Scholar]
- 50. Vostálová J, Tinková E, Biedermann D, Kosina P, Ulrichová J, Rajnochová Svobodová A. Skin protective activity of silymarin and its flavonolignans. Molecules. 2019;24:1022‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li LY, Huang T, Lan C, Ding H, Yan C, Dou Y. Protective effect of polysaccharide from Sophora japonica L. flower buds against UVB radiation in a human keratinocyte cell line (HaCaT cells). J Photochem Photobio, B: Biol. 2019;191:135‐142. [DOI] [PubMed] [Google Scholar]
- 52. Chen JM, Ran MN, Wang MX, et al. Evaluation of antityrosinase activity and mechanism, antioxidation, and UV filter properties of theaflavin. Biotechnol Appl Biochem. 2021;69:1‐12. doi: 10.1002/bab.2166 [DOI] [PubMed] [Google Scholar]
- 53. Cefali LC, Ataide JA, Oliveria Sousa IM. In vitro solar protection factor, antioxidant activity, and stability of a topical formulation containing Benitaka grape (Vitis vinifera L.) peel extract. Nat Prod Res. 2020;34(18):2677‐2682. [DOI] [PubMed] [Google Scholar]
- 54. Radice M, Manfredini S, Ziosi P. Herbal extracts, lichens and biomolecules as natural photo‐protection alternatives to synthetic UV filters. A systematic review. Fitoterapia. 2016;114:144‐162. [DOI] [PubMed] [Google Scholar]
- 55. Soto ML, Falqu é E, Dominguez H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics. 2015;2:259‐276. [Google Scholar]
- 56. Zhang YW, Li LY, Gao YJ, et al. Primary research on protective effect of Houttuynia cordata flavonoid and Sophora japonica L.polysaccharide as anti‐UV sun‐screening supplement. Agric Biotechnol. 2019;8(2):63‐67. [Google Scholar]
- 57. Chen JL, Cheong KL, Song ZL, et al. Structure and protective effect on UVB‐induced keratinocyte damage of fructan from white garlic. Carbohyd Polym. 2013;92:200‐205. [DOI] [PubMed] [Google Scholar]
- 58. Seidl K, Zinkernagel AS. The MTT assay is a rapid and reliable quantitative method to assess Staphylococcus aureus induced endothelial cell damage. J Microbiol Methods. 2013;92(3):307‐309. [DOI] [PubMed] [Google Scholar]
- 59. Hoffmann K, Kaspar K, von Kobyletzki G, et al. UV transmission and UV protection factor (UPF) measured on split skin following exposure to UVB radiation—correlation with the minimal erythema dose (MED). Photodermatol Photoimmunol Photomed. 1999;15(3–4):133‐139. [DOI] [PubMed] [Google Scholar]
- 60. Henne W. In vivo determination of the sunscreen factor of cosmetic preparations, history and the present state of art, Parf. Kosm. 1983;64:415‐423. [Google Scholar]
- 61. Greiter F. Sun protection factor‐development methods, Parf. Kosm. 1974;55:70‐75. [Google Scholar]
- 62. Matts PJ, Alard V, Brown MW, et al. The COLIPA in vitro UVA method: a standard and reproducible measure of sunscreen UVA protection. Int J Cosmet Sci. 2010;32(1):35‐46. [DOI] [PubMed] [Google Scholar]
- 63. Moyal D, Alard V, Bertin C, et al. The revised COLIPA in vitro UVA method. Int J Cosmet Sci. 2013;35(1):35‐40. [DOI] [PubMed] [Google Scholar]
- 64. Pritchetta JC, Naesens L, Montoya J. Treating HHV‐6 infections, the laboratory efficacy and clinical use of anti‐HHV‐6 agents. In: Flamand L, Lautenschlager I, Krueger G, Ablashi D, eds. Human Herpes Viruses HHV‐6A, HHV‐6B, and HHV‐7. Diagnosis and Clinical Management. 3rd ed. Elsevier; 2014:311‐331. [Google Scholar]
- 65. Kato T, HINO S, HORIE N, et al. Anti‐UV activity of Kampo medicines and constituent plant extracts: Re‐evaluation with skin keratinocyte system. In Vivo. 2014;28:571‐578. [PubMed] [Google Scholar]
- 66. Matsuta T, Sakagami H, Ssatoh K, et al. Biological activity of luteolin glycosides and tricin from Sasa senanensis Rehder. In Vivo. 2011;25:757‐762. [PubMed] [Google Scholar]
- 67. Nanbu T, Shimada J, Kobayashi M, et al. Anti‐UV activity of lignin–carbohydrate complex and related compounds. In Vivo. 2013;27:133‐140. [PubMed] [Google Scholar]
- 68. Nanbu T, Matsuta T, Sakagami H, Shimada J, Maki J, Makino T. Anti‐UV activity of Lentinus edodes mycelia extract (LEM). In Vivo. 2011;25:733‐740. [PubMed] [Google Scholar]
- 69. Choi SL, Jung TD, Cho BY, et al. Anti photoaging effect of fermented agricultural by‐products on ultraviolet B irradiated hairless mouse skin. Int J Mol Med. 2019;44:559‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nan WN, Ding L, Chen HJ, et al. Topical use of quercetin‐loaded chitosan nanoparticles against ultraviolet B radiation. Front Pharmacol. 2018;9:826‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice‐Evans C. Antioxidant activity applying improved ABTS redical cation decolorization assay. Free Radical Biol Med. 1999;26:1231‐1237. [DOI] [PubMed] [Google Scholar]
- 72. Sharma OP, Bhat TK. DPPH antioxidant assay revisited. Food Chem. 2009;113:1202‐1205. [Google Scholar]
- 73. Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48(4):412‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ou B, Hampsch‐Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescence probe. J Agric Food Chem. 2001;49(10):4619‐4626. [DOI] [PubMed] [Google Scholar]
- 75. Kohl E, Steinbauer J, Landthaler M, Szeimies RM. Skin ageing. J Eur Acad Dermatol Venereol. 2011;25(8):873‐884. [DOI] [PubMed] [Google Scholar]
- 76. Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix‐degrading metalloproteinases in photoaging. J Invest Dermatol. 2009;14:20‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee CW, Na Y, Park NH, et al. Amentoflavone inhibits UVB‐induced matrix metalloproteinase‐1 expression through the modulation of AP‐1 components in normal human fibroblasts. Appl Biochem Biotech. 2012;166:1137‐1147. [DOI] [PubMed] [Google Scholar]
- 78. Son Y, Kim S, Chung HT, et al. Reactive oxygen species in the activation of MAP kinases. Method Enzymol. 2013;528:27‐48. [DOI] [PubMed] [Google Scholar]
- 79. Kabuyama Y, Homma MK, Sekimata M, Homma Y. Wavelength‐specific activation of MAP kinase family proteins by monochromatic UV irradiation. Photochem Photobiol. 2001;73:147‐152. [DOI] [PubMed] [Google Scholar]
- 80. Assefa Z, Laethem AV, Garmyn M, et al. Ultraviolet radiation‐induced apoptosis in keratinocytes: on the role of cytosolic factors. Biochim Biophys Acta. 2005;1755:90‐106. [DOI] [PubMed] [Google Scholar]


