Abstract
Skeletal muscle is a complex and highly adaptable tissue. With aging, there is a progressive loss of muscle mass and function, known as sarcopenia, and a reduced capacity for regeneration and repair following injury. A review of the literature shows that the primary mechanisms underlying the age-related loss of muscle mass and the attenuated growth response are multi-factorial and related to alterations in multiple processes, including proteostasis, mitochondrial function, extracellular matrix remodeling, and neuromuscular junction function. Multiple factors influence the rate of sarcopenia, including acute illness and trauma, followed by incomplete recovery and repair. Regeneration and repair of damaged skeletal muscle involve an orchestrated cross-talk between multiple cell populations, including satellite cells, immune cells, and fibro-adipogenic precursor cells. Proof-of-concept studies in mice have demonstrated that reprogramming of this disrupted orchestration, resulting in the normalization of muscle function, may be possible using small molecules that target muscle macrophages. During aging, as well as in muscular dystrophies, disruptions in multiple signaling pathways and in the cross-talk between different cell populations contribute to the failure to properly repair and maintain muscle mass and function.
Keywords: Macrophages, Neuromuscular junction, Proteostasis, Sarcopenia, Satellite cells
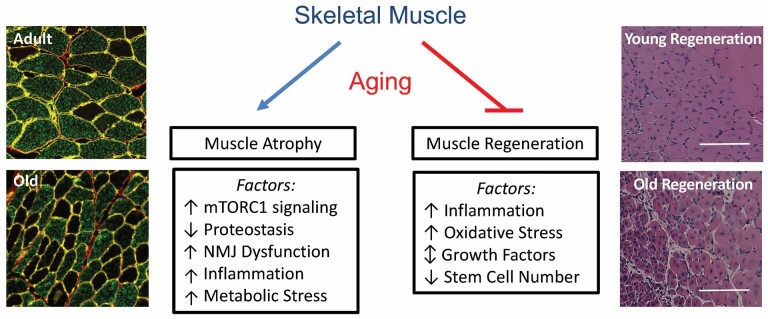
Skeletal muscle performs critical functions related to locomotion, metabolism, and thermoregulation, and thus preservation of muscle mass and strength is important for maintaining an independent, active, and healthy life. Skeletal muscle mass adapts over a lifetime to a variety of stimuli, including growth factors, hormones, satellite cell proliferation and differentiation, external loading, and neural activity. Sarcopenia, or age-associated loss of skeletal muscle mass and function, affects nearly 50% of adults above the age of 80. As such, these individuals are at increased risk for incident disability, all-cause mortality, mobility disability, and loss of independence. This review highlights age-related changes that affect muscle mass and its ability to respond to growth stimuli, as well as repair itself following injury (Figure 1).
Figure 1.
Skeletal muscle aging: Summary of key factors that contribute to the loss of muscle mass and regenerative capacity in aging skeletal muscle. The histological sections on the left illustrate the loss of fiber cross-sectional that occurs with age in rat hindlimb muscles (green fibers: myosin heavy chain type IIb; black fibers: myosin heavy chain type IIx). The histological sections on the right illustrate the decrease in regenerative potential that occurs following injury in old mouse hindlimb muscles.
Age-Related Muscle Atrophy and Attenuated Recovery of Muscle Mass
Skeletal muscle mass and strength will decrease in all individuals as a function of age but at different rates depending on multiple factors, including the level of physical activity, comorbidities, such as diabetes, obesity, and cardiovascular disease, as well as acute illness or injury, which in older individuals may be followed by incomplete recovery. Over the past two decades, research has begun to identify the mechanisms underlying acute atrophy resulting from disuse, denervation, elevated glucocorticoids, inflammation, and other stimuli (1). Sarcopenia differs from many acute atrophy conditions in that it occurs over an extended period and is the result of multiple interacting factors, including decreased physical activity, chronic inflammation, vascular dysfunction, mitochondrial dysfunction, altered proteostasis, and neuromuscular junction instability/denervation (2). The primary mechanisms responsible for sarcopenia are unclear and likely change with advancing age.
Measurement of age-related muscle loss is difficult given that the rate of change in mass and fiber cross-sectional area varies as a function of age and sex, in addition to varying between upper and lower limbs, across muscle types, and across fiber types. Comparison of the baseline properties of hindlimb muscles from mature adult and old animals has assisted in the identification of potential mechanisms underlying age-related muscle loss. Protein synthesis is often presumed to decrease as a function of age; however, measurement of resting protein synthesis has revealed an increase in the fractional synthesis rate of myofibrillar proteins in old hindlimb muscles, which is consistent with observed increases in mTORC1 (mammalian/mechanistic target of rapamycin complex 1) activation (3). In adult animals that have reached peak lean mass, resting mTORC1 activity in muscle is usually low; however, chronic elevation of mTORC1 activity has been reported in older animals and thought to contribute to sarcopenia (4). Increased mTORC1 activity is often associated with suppression of autophagy through increased phosphorylation of Unc-51-like autophagy activating kinase 1 (ULK1). In old male rats, increases in ULK1 phosphorylation have been reported; however, increased expression of autophagy markers and autophagic flux has also been reported suggesting an mTORC1-independent mechanism for regulating autophagy (5). The extent to which alterations in autophagy occur as a function of aging remains unclear, as does an understanding of whether changes in autophagy are beneficial or harmful. Alterations in autophagy could be associated with changes in other protein degradation pathways or the processing of newly translated proteins. A major protein degradation pathway in skeletal muscle is the ubiquitin-proteasome pathway (UPP). Increased protein degradation through the UPP has been implicated as an underlying mechanism for age-related muscle atrophy; however, measurement of 20S and 26S proteasome subunit activities have revealed either no change or a decrease in activity with age (5). Consistent with suppressed ubiquitin-proteasome activity with age is the observed accumulation of ubiquitin-labeled proteins, an increase in p62 protein expression, and an increase in protein aggregates within the old muscle (5). Altered protein turnover or proteostasis has been highlighted as one of the hallmarks of aging and could contribute to muscle fiber atrophy, as well as to the deterioration of the neuromuscular junction (NMJ) and loss of muscle fiber innervation (6). With respect to the neuromuscular system, aging results in the retraction of terminal axons to specific fibers resulting in a cycle of denervation followed by reinnervation, which over time results in fiber type grouping and ultimately permanent denervation. Neuromuscular junction fragmentation and dysfunction are observed with age and in mice with chronic elevation of mTORC1 (4). The extent to which elevated mTORC1 activation measured in old muscle is responsible for the NMJ dysfunction with aging is unclear; however, treatment of old mice and rats with rapamycin has been shown to decrease the level of expression of these genes, at least in selected muscles (7).
Another aspect of aging that affects muscle mass, and potentially the rate of sarcopenia, is anabolic resistance. Anabolic resistance can be defined as a decrease in the ability to activate protein synthesis in response to anabolic stimuli such as amino acids or increased loading; or as attenuated growth in response to anabolic signals such as increased external loading. In response to a decrease in external loading, such as that which occurs during bed rest or immobilization, skeletal muscle cross-sectional area and contractile force capabilities rapidly decline. Upon reloading, skeletal muscle recovers its mass and force capabilities. In humans, recovery of muscle mass and strength following immobilization is impaired in older subjects even with the addition of resistance exercise (8). The response of young adult and old rats to disuse and their ability to recover muscle mass and strength upon reloading has been studied using the tail suspension model. In response to 14 days of unloading, adult and old male Fisher Brown Norway rats have similar degrees of atrophy in the ankle flexor and extensor muscles (5). Interestingly, while the degree of muscle loss is similar between adult and old rats, loss of ankle extensor isometric torque is significantly greater in old compared to adult rats. Upon reloading, muscle mass and fiber cross-sectional area increase to baseline levels in adult rats by 14 days of recovery. However, recovery of muscle mass and fiber cross-sectional area (especially of the type IIb fiber) is significantly attenuated in old rats. Measurement of strength shows complete recovery in adult rats by 14 days of reloading, while no improvement in isometric torque is measured in old rats, which is comparable with findings in humans following cast immobilization.
The mechanisms underlying attenuated growth and lack of functional recovery in old animals are unclear and likely related to multiple factors. Potential mechanisms responsible for the attenuated growth response to reloading include suppression of protein synthesis, enhanced activation of protein degradation pathways, injury of muscle fibers, metabolic stress related to a diminished capacity for adenosine triphosphate production, and NMJ destabilization and denervation. Measurement of protein synthesis and protein degradation during recovery from disuse suggests an imbalance between synthesis and degradation, which is associated with an increase in the unfolded protein response and endoplasmic reticulum (ER) stress (5). Although protein translation is significantly increased upon reloading in old animals, the specific proteins being translated have not been identified, and increases in protein misfolding may be occurring given the significant rise in p62 protein, ER stress, and protein aggregates within the fibers. Thus, even though the synthesis of myofibrillar proteins is increased upon reloading, the contractile proteins may not be properly assembled into functional sarcomeres.
The significantly greater loss of strength in response to unloading in old compared to young rats suggests the possibility of NMJ instability or denervation, which could also contribute to the attenuated growth response. Examination of the expression of a subset of genes (myogenin, HDAC4, Runx1, Musk, NCAM1, AChRα, and AChRγ) associated with NMJ instability/inactivity shows increased expression of these genes in old muscle at baseline and further increases in their expression during unloading and upon reloading suggestive of NMJ dysfunction and possible denervation. Recent data suggest that this response may also be occurring in human subjects in response to bedrest (9). These data reveal significant differences in the response of multiple pathways involved in NMJ structure and function, protein turnover, metabolism, and extracellular matrix remodeling between old and young animals to both disuse and reloading that require further investigation.
In sum, loss of skeletal muscle mass with advancing age is multi-factorial and occurs over a prolonged period making it difficult to identify the primary drivers underlying the atrophy process. Longitudinal studies are needed that examine the time course of changes in muscle mass and identify the primary mechanisms, which may change over time and be complicated by co-morbidities, such as diabetes, obesity, cardiovascular disease, and inactivity. The primary target for therapeutics to treat sarcopenia has been anabolic agents that increase protein synthesis. However, increasing evidence suggests that protein synthesis is not suppressed but may be increased due to increased mTORC1 activity. Suppression of mTORC1 by low doses of rapamycin and other rapalogs is being investigated as a strategy to reduce the effects of aging on muscle and other organs, thus extending health span and life span (7); however, further study is required to investigate the potential negative effects on hypertrophy and adaptation. Additional research is also needed to identify the specific proteins undergoing increased translation as well as degradation with aging and in response to acute atrophy-inducing conditions and recovery from atrophy. Treatment strategies for age-related muscle atrophy, as well as the recovery of muscle mass following atrophy-inducing conditions such as bedrest, immobilization, and trauma need to look beyond anabolic agents and may require the use of a combination of strategies that target multiple pathways associated with inflammation, metabolic and oxidative stress, extracellular matrix remodeling, and protein degradation.
Restoring muSC Myogenic Potential During Aging
Recent studies suggest that loss of skeletal muscle regeneration, which declines substantially with aging, may contribute to the development of sarcopenia. Skeletal muscle regeneration depends on resident skeletal muscle stem cells (muSCs), which are further necessary for postnatal growth in response to loading and homeostatic maintenance of muscle fibers (10). Importantly, this loss of muSC myogenic potential is reversible, as exposure of old muSCs to a young systemic environment, as done using heterochronic parabiosis experiments, rejuvenates their regenerative capacity (11). As such, therapeutic strategies to target muSC function, either through the delivery of exogenous factors or cells, may potentially be of benefit to the older adults’ population suffering from skeletal muscle loss.
Loss of skeletal muscle regenerative potential with aging involves both intrinsic changes in muSCs as well as extrinsic changes in the stem cell niche. In preclinical (murine) models, muSCs are most commonly present in a quiescent state in healthy skeletal muscle but proliferate rapidly in response to injury, and next differentiate to form new muscle fibers or fuse to existing fibers to promote fiber hypertrophy. Intrinsic, or cell-autonomous factors limiting muSC function include DNA damage, altered metabolism, and oxidative stress, all of which limit muSC activation in response to injury or loading. A combination of these factors can also lead to cellular senescence or apoptosis, thus reducing the number of available muSCs. Extrinsic factors regulating muSC function, which are differentially regulated in aging, include inflammation, growth factor signaling, and loss of extracellular matrix cues. Identification of changes in both muSCs and the stem cell niche with aging has elucidated pharmacologic and cellular targets to restore skeletal muscle regeneration.
With regards to muSC rejuvenation, many preclinical studies have focused on aging-associated changes in the muSC niche. Previous heterochronic parabiosis experiments identified the restoration of Notch as a central transcription factor in rejuvenating skeletal muscle. To date, however, no trials activating the Notch signaling pathway in patients with skeletal muscle loss have been attempted, which may partially be due to concerns that Notch activation may lead to tumorogenesis or rapid tumor growth. As such, other growth factors and signaling pathways which are differentially regulated in the aging niche have been targeted. Local increases in transforming growth factor-β and fibroblast growth factor have been further identified as impairing skeletal muscle activation, but as these factors are necessary for a multitude of other functions within the body, clinical trials with systemic inhibition of these factors have not been attempted. Similarly, the Wingless/Integrated (Wnt) pathway is differentially regulated in skeletal muscle with aging in preclinical models, but its role in promoting muSC regenerative potential remains unclear. Some studies have suggested that an increase in canonical Wnt signaling promotes loss of regenerative capacity and promotes fibrosis, whereas others suggest that activation of Wnt signaling can maintain muSC myogenic potential. Growth differentiation factor (GDF)-11, which was also identified by heterochronic parabiosis experiments and is reduced in old mice, remains controversial as well with preclinical studies offering conflicting evidence in its role modulating skeletal muscle regeneration. Inhibition of chronic inflammation that occurs with aging in skeletal muscle may be another option by which to improve skeletal muscle regenerative potential (12), although clinical studies remain lacking. Collectively, further research is necessary regarding extrinsic factors presented here prior to clinical trials.
Another proposed method to improve muscle regeneration with regards to aging has been to replenish the pool of available muSCs. In preclinical models, both allotransplantation and autotransplantation of either muSCs or myoblasts have been attempted. Other cell types which may influence muSC proliferation and differentiation within skeletal muscle, such as fibrogenic adipogenic precursors (FAPs) and pericytes have similarly been evaluated in preclinical models and supplementing these cell types in sarcopenia may also potentially be of benefit, either alone or in conjunction with muSCs and myoblasts. However, although potential exists for transplantation of these cell types to induce and participate in myogenesis, overarching limitations include poor engraftment and the potential requirement for serial injections. Culture techniques to increase myoblast number face concerns with differentiation within media and cost as a clinical treatment. More recently, induced pluripotent stem cells (iPSC) have emerged as a possible stem cell source that can be differentiated into muscle and obviates any risk of immune rejection if performed as an autotransplantation. Preclinical studies suggest iPSC transplantation may be beneficial to muscle strength in a model of muscular dystrophy, but studies regarding aging and muscle loss remain necessary (13). To date, no clinical studies regarding stem cell transplantation have been performed.
It is important to note that as research continues to proceed on the restoration of skeletal muscle myogenic potential to limit sarcopenia, there remains controversy over the potential therapeutic effectiveness of this strategy. Preclinical studies in genetically modified mice in which muSCs are deleted do not exhibit the earlier onset of sarcopenia, but muscle hypertrophy and strength in response to loading and resistance are diminished with the loss of muSCs. It remains to be determined, as such, whether aging-associated loss of muSC number and myogenic potential plays a causal role in sarcopenia or whether loss of myogenic potential with sarcopenia is a correlation instead. Furthermore, it remains unclear whether human muSCs and murine muSCs behave similarly with regards to aging. Although human skeletal muscle decreases in muSC number with aging, muSCs collected from young and old skeletal muscle in humans appear to exhibit similar rates of differentiation and proliferation (14).
In sum, preclinical models firmly establish a loss of both muSC number and myogenic potential decrease with aging. Given that multiple studies have shown that this loss of myogenic potential is potentially reversible, potential therapies to improve skeletal muscle regeneration with aging is under active investigation. Pilot clinical studies will be necessary to determine which therapies, utilizing either pharmacologic agents, cells, or a combination of the two, may be most beneficial to older patients suffering from loss of muscle mass.
Reprogramming Muscles to MaintainNormal Function
Normal repair and regeneration of skeletal muscle following injury involves an interplay between the innate immune system and resident stem cells within the muscle (15). Cross-talk between the cells allows a progression from an initial inflammatory phase, which allows the removal of damaged tissue and temporary extracellular matrix remodeling, to a repair phase in which the satellite cells (muscle stem cells) proliferate, differentiate and fuse with damaged muscle fibers, in coordination with signals from FAPs and macrophages (15). In a number of degenerative muscular dystrophies, including Duchenne muscular dystrophy (DMD), chronic activation of inflammation leads to inappropriate signaling from macrophages and FAPs and a failure of regeneration. During aging, there is also an impairment of skeletal muscle repair and regeneration.
A number of factors are perturbed within the skeletal muscle and the circulation that may contribute to this, including decreased levels of insulin-like growth factor-1 (IGF-I) (16,17) and increased levels of GDF-15, which is correlated with inflammation and muscle weakness (18). Increased activation of the innate immune system also leads to low level, chronic inflammation, which may both directly and indirectly contribute to skeletal muscle dysfunction. These factors combine to produce sarcopenia, characterized by muscle weakness, loss of muscle mass, and decreased muscle repair capacity. By targeting the inappropriate signaling derived from the macrophages and FAPs, as well as the muscle precursor cells (satellite cells), it may be possible to improve muscle repair in dystrophic muscle and at least partially abrogate sarcopenia during aging.
Parabiosis experiments involving young and old mice demonstrated that there are circulating factors in old animals that drive sarcopenia (19). This likely is derived, at least in part, from the systemic inflammation associated with aging (18). In an analogous fashion, chronic activation of the inflammatory response in dystrophic muscle interferes with muscle regeneration and drives continuous deposition of fibrosis from fibroblasts as well as differentiation of fFAPs into an adipocytic fate (15). Intriguingly, both in mouse models of sarcopenia and DMD, chronic, local over-expression of IGF-1 in muscle was able to largely prevent the muscle functional losses associated with aging and dystrophy (16,18,20). These studies were an early indication that the local environment of a muscle could be shifted from a pro-inflammatory environment to a regenerative environment by reprograming the resident cells, including the satellite cells, myofibers, FAPs and inflammatory cells. IGF-1 is secreted by a number of cell populations within the muscle niche, including satellite cells, myofibers, fibroblasts, and inflammatory cells to facilitate muscle regeneration. While increasing IGF-1 levels in a muscle appears to help promote the regenerative behaviors of all cell types and can overcome even systemic inflammatory signals during aging (16), increasing IGF-1 levels only in skeletal muscles currently can only be achieved via targeted gene therapy approaches, as was done in the initial proof-of-concept study (16).
IGF-1 promotes the inflammatory (M1) to regenerative (M2) macrophage conversion and is produced in M2 macrophages to provide signals that further drive muscle regeneration. Small molecule approaches to drive this conversion have been described and applied to sarcopenia (21,22). Using peroxisome proliferator-activated receptor gamma activators to drive M1 to M2 conversion of macrophages resulted in an amelioration of sarcopenia, and enhanced muscle regeneration (21). The treatment increased GDF3 levels, which is known to target satellite cells to facilitate regeneration (21), as well as likely increasing IGF-1 and other regenerative signaling molecules released from M2 macrophages. Another macrophage-secreted factor, prostaglandin E2 (PGE2), stimulates muscle satellite cells and is involved in muscle repair. Increasing PGE2 levels in sarcopenic muscles using small molecule inhibition of the prostaglandin-degrading enzyme, 15-PGDH, was able to restore muscle mass and function in old mice (22).
In sum, while the initial demonstrations that skeletal muscles in old mice, as well as in dystrophic mice, can be reprogrammed to return to a state that promotes regeneration and repair, thus improving both mass and function, there have only been recent demonstrations that small molecule approaches may be able to achieve similar results. If these approaches prove applicable to humans as well as to mice, then there may be therapies on the horizon that can ameliorate the functional loss of muscle due to aging (sarcopenia) as well as slow the progression of muscle degenerative diseases, such as DMD. Whether these approaches will be efficacious as monotherapies, or whether it will require modulation of multiple targets in multiple cell types using a combination of therapies to fully restore muscle functionality remains to be explored.
Contributor Information
Sue C Bodine, Department of Internal Medicine, Division of Endocrinology and Metabolism, University of Iowa Carver College of Medicine, and Iowa City VA Health Care System, Iowa City, Iowa, USA.
Indranil Sinha, Department of Surgery, Division of Plastic Surgery, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Hugh Lee Sweeney, University of Florida Myology Institute and Department of Pharmacology & Therapeutics, University of Florida College of Medicine, Gainesville, Florida, USA.
Funding
S.C.B. is supported by VA Merit 1I01BX005626, I.S. is supported by K76AG059996 from NIA, and H.L.S. is supported by a Wellstone Muscular Dystrophy Cooperative Center grant (P50-AR-052646) from NIAMS.
This supplement is sponsored by the National Institute on Aging (NIA) at the National Institutes of Health (NIH).
Conflict of Interest
None declared.
References
- 1. Baehr LM, Hughes DC, Waddell DS, et al. Snapshop: skeletal muscle atrophy. Cell. 2022;85(9):1618–1618.e1. doi: 10.1016/j.cell.2022.03.028 [DOI] [PubMed] [Google Scholar]
- 2. Larsson L, Degens H, Li M, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2017;99:427–511. doi: 10.1152/physrev.00061.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller BF, Baehr LM, Musci RV, et al. Muscle specific changes in protein synthesis with aging and reloading after disuse atrophy. J. Cachexia, Sarcopenia and Muscle. 2019;10:1195–1209. doi: 10.1002/jcsm.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tintignac LA, Brenner H-R, Ruegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev. 2015;95:809–852. doi: 10.1152/physrev.00033.2014 [DOI] [PubMed] [Google Scholar]
- 5. Baehr LM, West DWD, Marcotte G, et al. Age-related deficits in skeletal muscle recovery following disuse atrophy are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Milano). 2016;8:127–146. doi: 10.18632/aging.100879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joseph GA, Wang SX, Jacobs CE, et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol. 2019;39(19):e00141–e00119. doi: 10.1128/MCB.00141-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suetta C, Frandsen U, Mackey AL, et al. Ageing is assocaited with diminshed muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in himan skeletal muscle. J Physiol. 2013;591:3789–3804. doi: 10.1113/jphysiol.2013.257121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monti E, Reggiani C, Franchi M, et al. Neuomuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J Physiol. 2021;599:3037–3061. doi: 10.1113/JP281365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blau HM, Cosgrove BD, Ho ATV. The central role of muscle stem cells in regenerative failure with aging. Nat Med. 2015;21:854–862. doi: 10.1038/nm.3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- 12. Oh J, Sinha I, Tan KY, et al. Age-associated NF-κB signaling in myofibers alters the satellite cell niche and re-strains muscle stem cell function. Aging (Milano). 2016;8(11):2871–2896. doi: 10.18632/aging.101098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Darabi R, Arpke RW, Irion S, et al. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10(5):610–619. doi: 10.1016/j.stem.2012.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alsharidah M, Lazarus NR, George TE, Agley CC, Velloso CP, Harridge SDR. Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell. 2013;12:333–344. doi: 10.1111/acel.12051 [DOI] [PubMed] [Google Scholar]
- 15. Wosczyna MN, Rando TA. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev Cell. 2018;46:135–143. doi: 10.1016/j.devcel.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musarò A, McCullagh K, Paul A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839 [DOI] [PubMed] [Google Scholar]
- 18. Conte M, Martucci M, Mosconi G, et al. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol. 2020;11:915. doi: 10.3389/fimmu.2020.00915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260 [DOI] [PubMed] [Google Scholar]
- 20. Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varga T, Mounier R, Patsalos A, et al. Macrophage PPARγ, a lipid activated transcription factor controls a growth factor GDF3 and skeletal muscle regeneration. Immunity. 2016;45:1038–1051. doi: 10.1016/j.immuni.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palla AR, Ravichandran M, Wang YX, et al. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. 2021;371(6528):eabc8059. doi: 10.1126/science.abc8059 [DOI] [PMC free article] [PubMed] [Google Scholar]