Abstract
Previous clinical trials and systematic reviews on the effects of supplemental vitamin D on musculoskeletal outcomes are conflicting. In this paper, we review the literature and summarize the effects of a high daily dose of 2 000 IU vitamin D on musculoskeletal outcomes in generally healthy adults, in men (≥50 years) and women (≥55 years) in the 5.3-year US VITamin D and OmegA-3 TriaL (VITAL) trial (n = 25 871) and women and men (≥70 years) in the 3-year European DO-HEALTH trial (n = 2 157). These studies found no benefit of 2 000 IU/d of supplemental vitamin D on nonvertebral fractures, falls, functional decline, or frailty. In VITAL, supplementation with 2 000 IU/d of vitamin D did not reduce the risk of total or hip fractures. In a subcohort of VITAL, supplemental vitamin D did not improve bone density or structure (n = 771) or physical performance measures (n = 1 054). In DO-HEALTH, which investigated additive benefits of vitamin D with omega-3 and a simple home exercise program, the 3 treatments combined showed a significant 39% decreased odds of becoming prefrail compared to the control. The mean baseline 25(OH)D levels were 30.7 ± 10 ng/mL in VITAL and 22.4 ± 8.0 ng/mL in DO-HEALTH and increased to 41.2 ng/mL and 37.6 ng/mL in the vitamin D treatment groups, respectively. In generally healthy and vitamin D-replete older adults not preselected for vitamin D deficiency or low bone mass or osteoporosis, 2 000 IU/d of vitamin D had no musculoskeletal health benefits. These findings may not apply to individuals with very low 25(OH)D levels, gastrointestinal disorders causing malabsorption, or those with osteoporosis.
Keywords: Age-related skeletal muscle dysfunction, Body composition, DO-HEALTH trial, Falls, Fractures, Functional decline, Mobility–disability, VITAL trial, Vitamin D
Although vitamin D supplements are generally perceived to improve musculoskeletal health, conclusive data on whether these supplements benefit these outcomes have been inconsistent. Approximately 37% of adults in the United States aged 60 years and older take vitamin D supplements (1). Although some observational studies indicate a positive relationship between 25(OH)D levels and bone mineral density (BMD), meta-analyses of randomized controlled trials (RCTs) have not shown the benefit of supplemental vitamin D in the primary prevention of osteoporosis. Additionally, conflicting results have been found regarding the effect of vitamin D supplementation on physical performance measures. Many RCTs have been limited by bolus dosing, co-administration with calcium, short duration, and small sample sizes. To address these inconsistencies, the recent VITamin D and OmegA-3 TriaL (VITAL) (2–5) and DO-HEALTH (6) studies tested the effects of supplemental vitamin D on clinically important musculoskeletal outcomes.
VITamin D and OmegA-3 TriaL: Effects on Falls, Bone Density/Structure, Body Composition, and Fractures
Falls and fractures increase markedly with age and result in injuries, hospitalizations, and premature death. Vitamin D supplements are widely recommended to benefit bone and musculoskeletal health, but definitive data on whether these supplements prevent falls, improve body composition or BMD, or reduce incident fractures in the general population have been inconsistent. RCTs have been limited by bolus dosing, co-administration with calcium, short duration, and small sample sizes.
To fill these knowledge gaps, we tested in ancillary studies the VITamin D and OmegA-3 TriaL (VITAL) whether daily, supplemental vitamin D3 versus placebo reduced the risk of incident falls, improved body composition (fat and lean tissue), prevented declines in bone density/structure and/or decreased incident fractures. VITAL is a recently completed primary prevention trial that investigated in a 2 × 2 factorial design whether vitamin D3 (cholecalciferol, 2 000 IU/d) and/or omega-3 fatty acid (fish oil, 1 g/d) supplements prevented cancer and cardiovascular disease. From 50 states, 25 871 men (aged ≥50) and women (aged ≥55), including 5 106 Black participants, were enrolled with a median follow-up of 5.3 years. The exclusion criteria included cardiovascular disease, cancer, and hypercalcemia. Participants were not preselected for vitamin D deficiency, low bone mass, high fall risk, or osteoporosis. Participants completed baseline and annual questionnaires evaluating medical history and medications, physical activity, and lifestyle variables.
At baseline, VITAL participants had a mean ± standard deviation [SD] age of 67.1 ± 7.1 years and mean ± SD body mass index (BMI) of 28.1 (5.7) kg/m2 (7). Of the 25 871 participants, 51% were female and 20% were Black. At baseline, 42.6% of participants took nonstudy vitamin D supplements limited to ≤800 IU/d and 20.0% of participants took calcium supplements limited to ≤1 200 mg/d. Fasting blood samples were collected in 16 956 participants at baseline and approximately 6 000 at follow-up (7–9). The mean ± SD baseline 25(OH)D level was 30.7 ± 10.0 ng/mL (n = 16 757). In a subgroup of participants randomized to supplemental vitamin D (n = 1 347), mean 25(OH)D levels increased from 29.2 ng/mL at baseline to 41.2 ng/mL at a 2-year follow-up. Adherence to the study interventions was 87.3% at 2 years and 85.4% at 5 years. More information can be found at the parent trial’s website at vitalstudy.org (7).
Although 10.3% of participants had a history of fragility fracture at baseline, only 4.8% were taking osteoporosis medications. In total, 2.4% of VITAL participants had 25(OH)D levels <12 ng/mL, which is similar to 2.9% of the U.S. population 60 years and older with 25(OH)D <12 ng/mL, as measured by the National Health and Nutrition Examination Survey (NHANES) (10). In VITAL, 12.9% of participants had 25(OH)D levels less than 20 ng/mL in comparison to 15.2% of persons 60 years and older examined in NHANES (10).
Incident and Injurious Falls
In the United States, falls among older adults are a leading cause of injuries, fractures, and death (11). Individuals who fall more than once are twice as likely to be admitted to nursing homes. Systematic reviews and meta-analyses have shown conflicting results on the effects of supplemental vitamin D for the prevention of falls in the aging U.S. population.
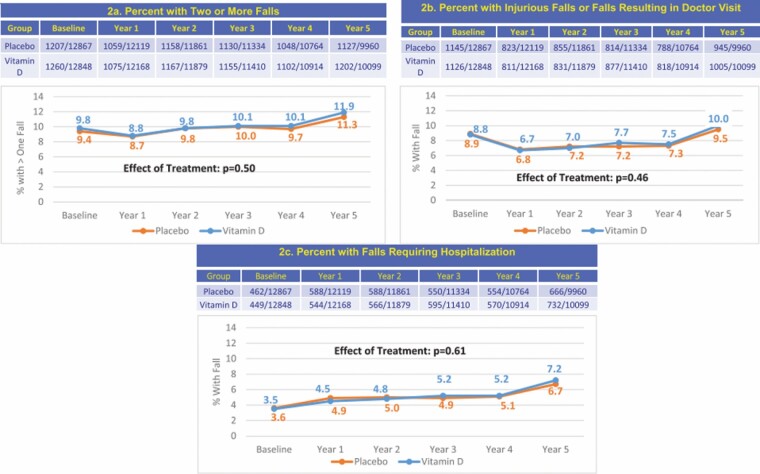
In a VITAL ancillary study, we examined the effects of supplemental vitamin D3 (2 000 IU/d) versus placebo on incident falls and those requiring health care utilization. Primary outcomes were 2 or more falls and injurious falls resulting in a doctor or hospital visit, which were self-reported on annual questionnaires. The mean age ± SD of the participants was 67.1 ± 7.1 years (50.6% females); 33.3% had ≥1 fall(s) in the year prior to the trial. Baseline characteristics were balanced between groups. Participants recorded the number of falls (0, 1, 2, 3+); a number of injurious falls or falls resulting in a doctor visit (0, 1, 2, 3+); and falls resulting in a hospital visit (yes or no), as well as risk factors for falls and medications (2).
Over a median follow-up duration of 5.3 years, 15 161 participants reported a total of “51 260 falls.” In the vitamin D versus placebo groups, there were no differences in the proportion of participants having 2 or more falls (odds ratio [OR] = 0.97; 95% CI: 0.90–1.05, p = .50), falls resulting in a doctor visit (OR = 1.03; 95% CI: 0.94–1.13, p = .46), or falls resulting in a hospital visit (OR = 1.04; 95% CI: 0.90–1.19, p = .61; Figure 1). Results did not differ by baseline age, sex, race, BMI, or quintiles of 25(OH)D levels (2).
Figure 1.
Incident fall outcomes with 5.3 years of follow-up in the vitamin D and placebo groups, adjusted for age, race, sex, and randomization group in VITamin D and OmegA-3 TriaL (2).
Participants also reported physical activity on annual questionnaires, including the metabolic equivalent of task (MET) hours/week, activities of daily living (ADLs; such as feeding or dressing oneself), and instrumental activities of daily living (IADLs; such as carrying groceries or walking more than 1 mile) (2). As assessed at 3 years, we found no differences in MET hours between the vitamin D and placebo groups. We also found no differences in ADLs or IADLs, assessed at years 3, 4, and 5 (2). Finally, supplemental vitamin D had no effect on the incidence or rate of change of frailty indices in VITAL (12).
In midlife and older adults, not selected for high fall risk, supplemental vitamin D3 versus placebo did not prevent recurrent falls or falls requiring healthcare utilization with 5.3 years of follow-up in participants. Additionally, in exploratory analyses, vitamin D supplementation did not improve ADLs or IADLs.
Body Composition in a Subcohort
In the United States, a third of the older adult population is obese. Obesity is associated with an increased risk of cancer, cardiovascular disease, and fractures. Observational studies have provided evidence for an association between low 25(OH)D levels and high body weight and fat mass. Although some RCTs have shown evidence for a beneficial effect of supplemental vitamin D on weight and adiposity measures, most RCTs on these outcomes have found no effects of supplemental vitamin D on the measure of body composition.
A VITAL subcohort of 771 participants from the New England area, not on osteoporosis medications, had assessments of weight, BMI, waist circumference, and dual X-ray absorptiometry (DXA) scans to measure total and/or regional fat and lean tissue measures at baseline (n = 771) and 2-year follow-up (n = 687; 89% retention) (13,14). Supplemental vitamin D3 versus placebo had no effect on weight, BMI, or measures of adiposity and lean tissue. Effects did not vary according to age, sex, race, baseline BMI, fat mass index, or total 25(OH)D levels. In subgroup analyses, vitamin D3 supplementation did slightly improve body fat percentage in participants with normal BMI at baseline, but not in the overweight or obese participants (p = .04) (3). Further research is needed to determine whether supplemental vitamin D affects body composition and whether the effects of supplemental vitamin D are modified by baseline BMI (eg, in individuals with normal BMI).
Bone Density in a Subcohort
Supplemental vitamin D is widely prescribed for bone health and observational studies suggest a positive relationship between BMD and 25(OH)D levels. However, recent meta-analyses of RCTs do not support the use of vitamin D supplements for the primary prevention of osteoporosis in the general population. In a study of monthly doses of supplemental vitamin D, exploratory analyses suggested a small benefit of vitamin D on BMD when baseline 25(OH)D levels were <12 ng/mL (15).
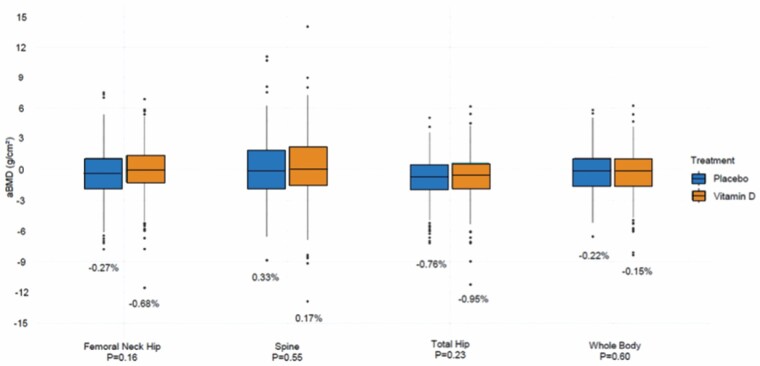
To investigate the effects of supplemental vitamin D on bone health, a VITAL subcohort of 771 participants who were not on bone-active medications, had BMD measured at the spine, hip, and whole body by DXA at baseline and Year 2. Of the 771 participants, 677 had bone structure assessed at the radius and tibia by peripheral quantitative computed tomography at baseline and Year 2 (4,14). Mean ± SD age of the participants was 63.8 ± 6.1 years (46.7% females) and mean ± SD BMI was 27.2 ± 4.8. Baseline characteristics were balanced between groups (13).
We found no effect of supplemental vitamin D3 versus placebo on absolute or percent changes in areal BMD between baseline and 2 years of follow-up. There was also no effect of supplemental vitamin D3 versus placebo on changes in areal BMD using baseline 25(OH)D thresholds (<12, <20, or <30 ng/mL). Additionally, we found no effect of supplemental vitamin D3 versus placebo on 2-year changes in volumetric BMD (Figure 2) (4).
Figure 2.
Mean absolute and percent changes in a BMD between baseline and year 2 of follow-up in the vitamin D3 and placebo groups, adjusted for age, sex, and race in VITamin D and OmegA-3 TriaL (4). BMD = bone mineral density.
Incident Fractures in 25 871 Participants Followed for a Median of 5.3 Years
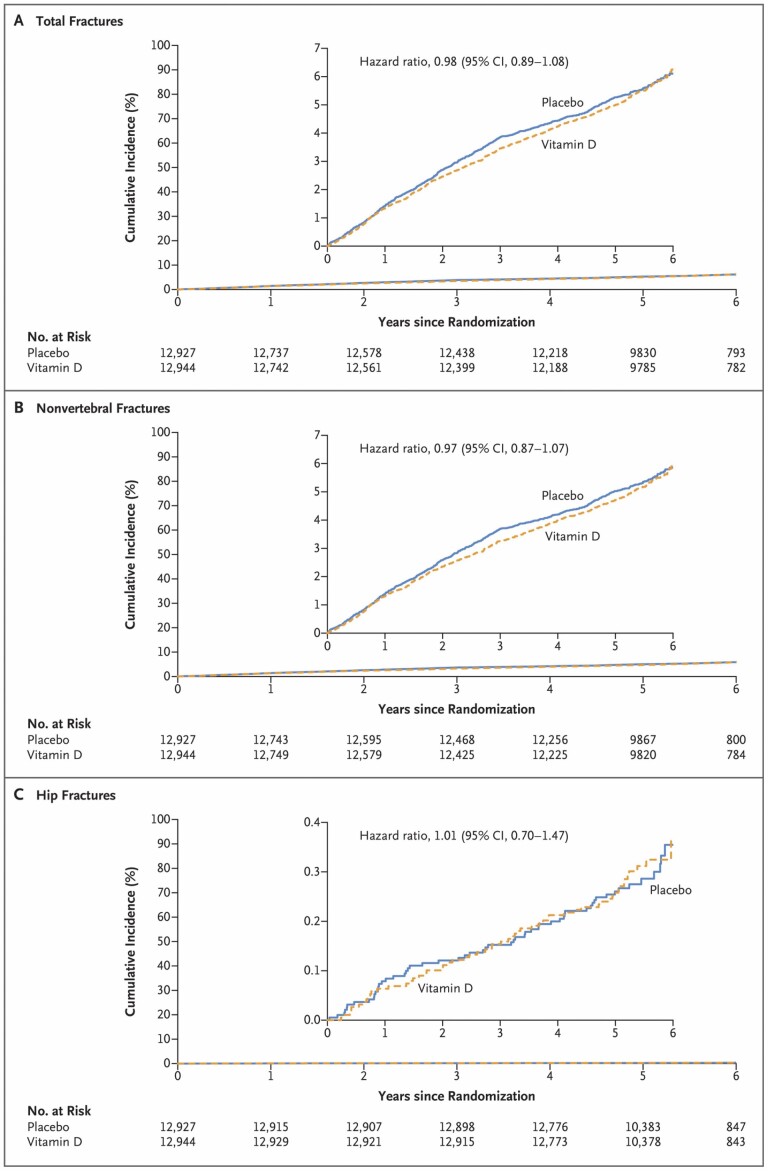
As the population ages, there is an exponential rise in osteoporotic fractures that lead to excess morbidity and mortality. Although vitamin D supplements are widely used to prevent fractures in the general population, results from RCTs investigating the effects of supplemental vitamin D on fracture outcomes have been inconsistent. We recently completed studies testing whether supplemental vitamin D3 versus placebo decreased total, nonvertebral, and hip fractures during the interventions (median follow-up of 5.3 years) (5). Incident fractures were reported on annual questionnaires and adjudicated by medical record review, including examination of hip/femur radiographs with a bone radiologist (14). There were 2 133 self-reported total fractures in the VITAL cohort, and 1 991 fractures were confirmed by adjudication in 1 551 participants.
In this VITAL ancillary study, the largest study of supplemental vitamin D3 (2 000 IU/d) versus placebo, vitamin D supplements had no effect on incident total fractures (hazard ratio [HR]: 0.98; 95% CI: 0.89–1.08; p = .70), nonvertebral fractures (HR: 0.97; 95% CI: 0.87–1.07; p = .50), or hip fractures (HR: 1.01; 95% CI: 0.70–1.47; p = .96; Figure 3). There was no effect modification by age, sex, race, BMI, or baseline 25(OH)D quartiles or 25(OH) D levels (<12, <20, <30, or ≥50 ng/mL). Statistical power was limited in participants with baseline 25(OH)D levels <12 ng/mL. Results did not change in sensitivity analyses for adherence.
Figure 3.
Cumulative incident total (A), nonvertebral (B), and hip (C). Fractures in vitamin D and placebo groups: median 5.3 years of follow-up in VITamin D and OmegA-3 TriaL (5).
Vitamin D Supplementation: DO-HEALTH
DO-HEALTH: What Is the Issue?
DO-HEALTH was designed as a “healthy longevity trial and cohort” funded by the European Commission and designed to be the European partner RCT to VITAL. DO-HEALTH tested 2 of the same nutritional supplements as VITAL (2 000 IU vitamin D and 1 g omega-3 fatty acids daily) plus a simple home exercise program ([SHEP]; strength training versus control exercise focused on joint mobility) in a 2 × 2 × 2 factorial design RCT. The trial enrolled 2 157 White and generally healthy community-dwelling adults, 70 years and older, from 5 European countries (Switzerland, Germany, Austria, France, and Portugal). Inclusion criteria were the absence of major health events in the 5 years prior to enrollment, sufficient mobility, and good cognitive status.
Participants were randomized to receive 2 000 IU/d vitamin D3, and/or 1 g/d omega-3s, and/or a simple strength-training exercise program (SHEP) for 3 years (vitamin D3 and omega-3s and SHEP: n = 264; vitamin D3 and omega-3s: n = 265; vitamin D3 and SHEP: n = 275; vitamin D3: n = 272; omega-3s and exercise: n = 275; omega-3s: n = 269; SHEP: n = 267; placebo: n = 270).
All participants were followed in standardized yearly clinical visits at baseline, 12, 24, and 36 months with complete phenotyping of multiple organ functions, detailed physical and cognitive function, medication use and health care utilization, as well as lifestyle factors (detailed nutritional assessment with DO-HEALTH food frequency questionnaire, physical activity, education, living status, and mental health). In between the 4 clinical visits at baseline, 12, 24, and 36 months, all participants were contacted in person every 3 months for the prospective assessment of falls and other adverse events. This review summarizes the individual and additive effects of the 3 interventions on the function-related outcomes of DO-HEALTH, including lower extremity function, falls, and frailty.
DO-HEALTH: What Are the Research/Clinical Gaps?
Similar to VITAL, but enrolling somewhat older adults, DO-HEALTH was designed as a primary prevention trial in older adults. As addressed in the National Institutes of Health (NIH) workshop in March 2022, effective public health interventions on the prevention of functional decline, targeted at generally healthy and community-dwelling older adults, are urgently needed.
Meta-analyses of double-blind RCTs suggest a benefit of daily 800–1 000 IU of vitamin D on fall prevention in vulnerable older adults at risk of vitamin D deficiency and falls (16,17). This is supported by the demonstrated effects of vitamin D supplementation on skeletal muscle fiber number and size in vitamin D-deficient adults (18).
Mechanistically, this has been explained by the suggested presence of the VDR in muscle tissue, and muscle weakness observed in vitamin-D-deficient older adults (18). However, more recent meta-analyses of clinical trials have questioned the benefit of vitamin D for primary fall and fracture prevention among noninstitutionalized older adults unselected for vitamin D deficiency or osteoporosis (19); and prior trials have consistently documented that vitamin D in very high intermittent dosing is detrimental for fall and fracture prevention (19). “With regard to omega-3 fatty acids (omega-3s),” several intervention studies among older adults suggested that supplemental omega-3s may have beneficial effects due to their anti-inflammatory properties (20). However, clinical trials on the effect of supplemental omega-3s on falls are lacking. “With regard to exercise,” consistent benefits have been reported by meta-analyses of clinical trials in the prevention of falls among older adults, both for primary (21) and secondary prevention (21). However, there is limited evidence for unsupervised and long-term home-based exercise interventions among relatively healthy and active community-dwelling older adults.
DO-HEALTH: What Are the Findings for Function-Related Outcomes?
In DO-HEALTH, among 2 157 randomized participants (mean age 74.9 years; 61.7% women), 1 900 (88%) completed the study. The mean age was 74.9 years, 61.7% were women, and at baseline, 40.7% had serum 25(OH)D concentration <20 ng/mL, and 83% were at least moderately physically active. The median follow-up was 2.99 years.
“Lower extremity function” (6) was measured using the short physical performance battery (SPPB); there were no statistically significant improvements in lower extremity function with any intervention individually or in combination. However, participants had good lower extremity function at baseline (median baseline SPPB 11.0, IQR 10.0–12.0), and 82.6% engaged in moderate to high physical activity based on the Nurses’ Health Study (NHS) questionnaire. On average across the 3-year follow-up, all participants’ SPPB declined minimally (−0.07, 95% CI: −0.12, −0.01; p = .001). These findings suggest that with 83% of participants already engaging in moderate to high physical activity at baseline, there may have been little potential for a benefit of the 3 interventions with regard to lower extremity function assessed by the SPPB.
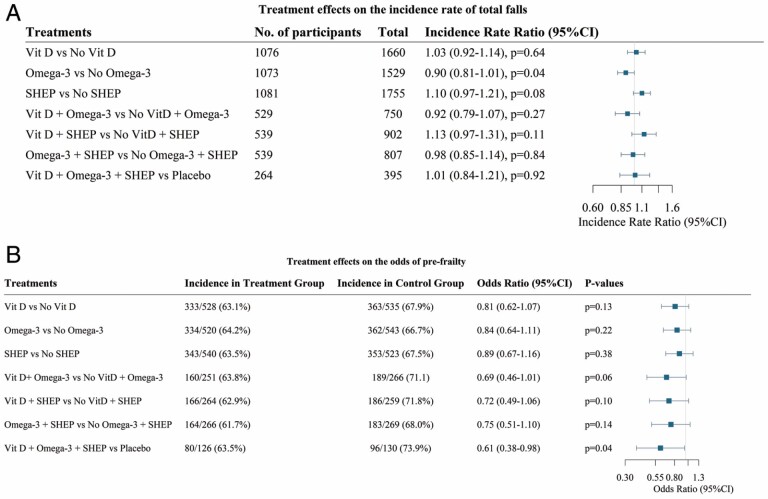
“With regard to falls” (22), in total, 3 333 falls were recorded over a median follow-up of 2.99 years. The overall incidence of falls was 0.56 (95% CI: 0.53, 0.59) per person per year, and 1 311 participants reported at least 1 fall. Overall, vitamin D and the SHEP had no effect on total falls, while omega-3s compared to no omega-3s reduced total falls by 10% (incidence rate ratio [IRR] = 0.90, 95% CI: 0.81, 1.00, p = .04). Benefits were found among women (IRR = 0.88, 95% CI: 0.77, 1.00), among older participants (age 75+; IRR = 0.81, 95% CI: 0.69, 0.95), among participants with higher omega-3 PUFA (poly-unsaturated fatty acid) levels at baseline (IRR = 0.83, 95% CI: 0.71, 0.97), and among participants who were physically more active at baseline (IRR = 0.84, 95% CI: 0.72, 0.97). These findings suggest that among healthy, active, and vitamin-D-replete older adults, supplemental omega-3s may have a modest benefit on the incidence of total falls, whereas daily high-dose vitamin D or SHEP had no individual or additive benefit.
“With regard to prefrailty and frailty” (23) at baseline, 1 137 out of 2 157 DO-HEALTH participants were robust (mean age 74.3 years, 56.5% women, mean gait speed 1.18 m/s). Participants with 0 positive items were classified as robust, and participants with 1 or 2 positive items were classified as being prefrail, while participants with 3 or more positive items were classified as frail according to the definition by Fried et al. (24). Frailty status was assessed at baseline and annually over 3 years of follow-up according to the 5 domains of the Fried physical frailty phenotype. Over a median follow-up time of 2.9 years, 696 (61.2%) became prefrail and 29 (2.6%) became frail. ORs for becoming prefrail were not significantly lower for vitamin D3, or omega 3-s, or SHEP (which focused on the strength), individually, compared to control (placebo for the supplements and control exercise which focused on joint mobility). However, the 3 treatments combined showed a significant 39% decreased odds (OR = 0.61; 95% CI: 0.38–0.98]; p = .04) of becoming prefrail compared to the control. None of the individual treatments or their combination significantly reduced the odds of becoming frail, but the numbers of incident frailty cases were very low.
These findings suggest that robust, healthy and active, and largely vitamin D-replete older adults may benefit from a combination of high-dose, supplemental vitamin D3, marine omega-3s, and SHEP with regard to the risk of becoming prefrail over 3 years.
DO-HEALTH: What Is the Relevance and What Are the Recommendations for Future Research?
In largely vitamin-D-replete, active, and generally healthy adults age 70 years and older, there was no benefit of high-dose daily vitamin D (2 000 IU/d) on SPPB or fall reduction (Figure 4) (23). The results for DO-HEALTH were not corrected for multiple hypothesis testing and secondary, tertiary, and subgroup analyses should be interpreted with caution.
Figure 4.
DO-HEALTH functional outcomes. Falls (A): estimates from negative binomial regression model with an offset of the log of person years in the study controlling for study site, sex, age, previous fall, baseline body mass index, and baseline use of walking aids. Pre-frailty (B): estimates are from logistic regression model comparing pre-frail versus robust participants. Models are controlled for the randomization stratification variables: age, sex, low-trauma fall during the 12 months preceding the randomization day (yes/no) and study site. (A) DO-HEALTH falls (22). (B) DO-HEALTH prefrailty (23).
The DO-HEALTH results on function and falls are in line with recent meta-analyses and clinical trials suggesting that vitamin D does not reduce falls among adults who are not vitamin D deficient and not at high risk of osteoporosis and falling (19). Fifty-nine percent of participants were vitamin D replete at baseline, and about 30% reported vitamin D supplement use of up to 800 IU daily throughout the 3-year trial period (6). Thus, our results do not challenge the clinical trial findings of daily 800 to 1 000 IU vitamin D for older adults at increased risk for falls, osteoporosis, and vitamin D deficiency (16,25).
DO-HEALTH trial found no benefit of “omega-3” on SPPB, but a significant individual benefit on fall reduction and omega-3 supplementation contributed to the combined benefit of all 3 treatments in the prevention of prefrailty. To the best of our knowledge, DO-HEALTH is the first clinical trial reporting the effect of supplemental marine omega-3s on fall prevention. Although the overall 10% reduction in the rate of total falls is modest, its consistency across subgroups of the generally healthy and active study population aged 70 years and older, may be relevant in this population. These findings are held mechanistically by the anti-inflammatory and thereby muscle preserving benefits of omega-3s and suggested direct anabolic effects on muscle by activation of mTOR signaling (26).
DO-HEALTH found no benefit of SHEP on physical function or falls. In contrast, in the pilot trial among 173 frail older adults with acute hip fracture, the same exercise program improved function and reduced the rate of falling by 25% (95% CI: −44, −1) over 12 months (27). Also, a 2019 Cochrane meta-analysis that included 108 RCTs found that exercise reduced the rate of falls by 24% among community-dwelling older adults (21). Our simple exercise program may have lacked intensity for the already active participants, or an adequate balance or supervision component. We cannot exclude a possible harmful effect of SHEP on falls among men, younger participants, and the physically most active older adults. We can only speculate why the SHEP, which 60% of participants performed 3 times a week over the 3-year trial duration (6), had a detrimental effect with a significant increase in the risk of falling among men, younger participants, and those with the highest physical activity. This may in part be explained by observations that the relationship between physical activity and falling is complex, with some studies suggesting a lower risk of falls with higher physical activity (28).
Conclusion
Overall, the results of the VITAL and DO-HEALTH trials do not support the use of high daily dose vitamin D supplements (2 000 IU/d) for the primary prevention of fractures or to improve a variety of musculoskeletal outcomes in generally healthy midlife and older adults, who were not vitamin D deficient at baseline and who were not preselected for low bone mass or osteoporosis.
It is possible that the current thresholds for 25(OH)D levels are too high. Only modest amounts of vitamin D may be needed in this population for optimal musculoskeletal health, which has likely already been provided through sun exposure and the fortification of food sources with vitamin D. The results of both VITAL and DO-HEALTH apply to generally healthy middle-aged and older adults in the United States and Europe, respectively, and are not generalizable to individuals with osteoporosis, high fall risk, low bone mass, vitamin D deficiency, or those living in residential communities. These findings have implications for public health guidelines on the use of vitamin D supplements to support musculoskeletal health in generally healthy U.S. and European women and men. Notably, however, as both trials have tested a higher dose of vitamin D (2 000 IU/d) than currently recommended (600–800 IU/d) in older adults, and as the trials were conducted in unselected generally healthy and vitamin D-replete older adults, their findings do not question current guidelines in the US or Europe for the prevention of vitamin D deficiency in vulnerable older adults.
Contributor Information
Meryl S LeBoff, Calcium and Bone Section, Skeletal Health and Osteoporosis Center and Bone Density Unit, Harvard Medical School, Boston, Massachusetts, USA; Endocrinology, Diabetes and Hypertension Division, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Heike A Bischoff-Ferrari, Department of Geriatrics and Aging Research, University of Zurich and University Hospital of Zurich, Zurich, Switzerland.
Funding
The VITAL ancillary studies are supported by grants (R01 AR060574, R01 AR070854, and R01 AR059775; PI M.S.L.) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases by grants (U01 CA138962, R01 CA138962, and R01AT011729, PI Drs. Manson and Buring) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The DO-HEALTH study was funded by the Seventh framework program of the European Commission (Grant Agreement n°278588; PI H.A.B.), the University of Zurich (Chair for Geriatric Medicine and Aging Research), DNP, Roche, NESTEC, Pfizer and Streuli.
This supplement is sponsored by the National Institute on Aging (NIA) at the National Institutes of Health (NIH).
Any opinions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the NIA, National Institutes of Health, or the U.S. Department of Health and Human Services.
Conflict of Interest
None declared.
References
- 1. Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary supplement use among adults: United States, 2017–2018. NCHS Data Brief. 2021(399):1–8. [PubMed] [Google Scholar]
- 2. LeBoff MM, Cook NR, Cawthon PM, et al. . VITamin D and OmegA-3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab. 2020;105(9):2929–2938. doi: 10.1210/clinem/dgaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou SH, Murata EM, Yu C, et al. . Effects of Vitamin D3 supplementation on body composition in the VITamin D and OmegA-3 TriaL (VITAL). J Clin Endocrinol Metab. 2021. doi: 10.1210/clinem/dgaa981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LeBoff MS, Chou SH, Murata EM, et al. . Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res. 2020;35(5):883–893. doi: 10.1002/jbmr.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LeBoff MS, Chou SH, Ratliff KA, et al. . Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299–309. doi: 10.1056/nejmoa2202106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. . Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LeBoff MS, Chou SH, Ratliff KA, et al. . Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387(4):299–309. doi: 10.1056/NEJMoa2202106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassuk SS, Manson JE, Lee IM, et al. . Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2016;47:235–243. doi: 10.1016/j.cct.2015.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manson JE, Cook NR, Lee IM, et al. . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrick KA, Storandt RJ, Afful J, et al. . Vitamin D status in the United States, 2011–2014. Am J Clin Nutr. 2019. doi: 10.1093/ajcn/nqz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged >/=65 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(37):993–998. doi: 10.15585/mmwr.mm6537a2. [DOI] [PubMed] [Google Scholar]
- 12. Orkaby AR, Dushkes R, Ward R, et al. . Effect of vitamin D3 and omega-3 fatty acid supplementation on risk of frailty: an ancillary study of a randomized clinical trial. JAMA Netw Open. 2022;5(9):e2231206–e2231206. doi: 10.1001/jamanetworkopen.2022.31206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donlon CM, LeBoff MS, Chou SH, et al. . Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL): effects on bone structure and architecture. Contemp Clin Trials. 2018;67:56–67. doi: 10.1016/j.cct.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-bone health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2015;41(C):259–268. doi: 10.1016/j.cct.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reid IR, Horne AM, Mihov B, et al. . Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J Intern Med. 2017;282(5):452–460. doi: 10.1111/joim.12651 [DOI] [PubMed] [Google Scholar]
- 16. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. . Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339(7725):1–11. doi: 10.1136/bmj.b3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. . A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49. doi: 10.1056/NEJMoa1109617 [DOI] [PubMed] [Google Scholar]
- 18. Ceglia L, Niramitmahapanya S, da Silva Morais M, et al. . A randomized study on the effect of vitamin D(3) supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98(12):E1927–E1935. doi: 10.1210/jc.2013-2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazess RB, Bischoff-Ferrari HA, Dawson-Hughes B. Vitamin D: bolus is bogus-a narrative review. JBMR Plus. 2021;5(12):e10567. doi: 10.1002/jbm4.10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(Suppl 6):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16841861) [DOI] [PubMed] [Google Scholar]
- 21. Sherrington C, Fairhall NJ, Wallbank GK, et al. . Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;( 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bischoff-Ferrari HA, Freystätter G, Vellas B, et al. . Effects of vitamin D, omega-3 fatty acids and a simple home strength exercise program on fall prevention: the DO-HEALTH randomized clinical trial. Am J Clin Nutr. 2022. doi: 10.1093/ajcn/nqac022 [DOI] [PubMed] [Google Scholar]
- 23. Gagesch M, Wieczorek M, Vellas B, et al. . Effects of vitamin D, Omega-3 fatty acids and a home exercise program on prevention of pre-frailty in older adults: the DO-HEALTH randomized clinical trial. J Frailty Aging. 2022. doi: 10.14283/jfa.2022.48 [DOI] [PubMed] [Google Scholar]
- 24. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 25. Bischoff-Ferrari HA, Orav EJ, Abderhalden L, Dawson-Hughes B, Willett WC. Vitamin D supplementation and musculoskeletal health. Lancet Diabetes Endocrinol. 2019;7(2):85. doi: 10.1016/S2213-8587(18)30347-4 [DOI] [PubMed] [Google Scholar]
- 26. Dupont J, Dedeyne L, Dalle S, Koppo K, Gielen E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019;31(6):825–836. doi: 10.1007/s40520-019-01146-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bischoff-Ferrari HA, Dawson-Hughes B, Platz A, et al. . Effect of high-dosage cholecalciferol and extended physiotherapy on complications after hip fracture: a randomized controlled trial. Arch Intern Med. 2010;170(9):813–820. doi: 10.1001/archinternmed.2010.67 [DOI] [PubMed] [Google Scholar]
- 28. Mertz KJ, Lee DC, Sui X, Powell KE, Blair SN. Falls among adults: the association of cardiorespiratory fitness and physical activity with walking-related falls. Am J Prev Med. 2010;39(1):15–24. doi: 10.1016/j.amepre.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]