Abstract
The discovery of the growth hormone secretagogues (GHS) and the reverse pharmacology leading to the discovery of GHS receptor which enabled the identification of ghrelin as the natural ligand for the receptor have opened a new horizon in growth hormone (GH) physiology, pathophysiology, and therapeutics. Major progress has been made and we now have orally active GHS which are able to restore optimal pulsatile GH secretion which cannot be overstimulated as insulin-like growth factor feedback regulates the peaks to the optimum level. This enables GH to be restored in the older to levels normally seen in 20- to 30-year-old people; this leads to an increase in fat-free mass and redistribution of fat to the limbs. As these agents are ultimately approved and investigated further, it is likely that they will be shown to restore growth in children with moderate-to-mild GH deficiency; their benefits will be investigated in other indications such as nonalcoholic fatty liver disease, frailty, anemia, osteoporosis, and immune compromise in older subjects. The exquisite regulation of GH secretion reflects the importance of GH pulsatility in the regulation of somatotroph action of GH.
Keywords: Functional limitation, Growth hormone secretagogues, Skeletal muscle dysfunction, Mobility disability, Sarcopenia
Preventing age-associated decline in physical and mental function remains an unmet medical need. In recent years, it has been argued that growth hormone (GH) and insulin-like growth factor (IGF-1) signaling are negatively associated with longevity, but this argument is based on evidence from laboratory studies in short-lived animals such as worms, flies, and mice. These studies were conducted under controlled laboratory conditions free from interference by predators and disease. In general, these animals were also maintained at typical vivarium temperatures which are below thermoneutrality for small rodents; hence, mice must work to maintain their ideal body heat which affects whole-body metabolism. Conclusions derived from work on longevity in inbred model organisms and mammals maintained under artificial conditions should not simply be extrapolated to long-lived outbred species such as humans. This is extensively reviewed by Aguiar-Oliveira and Bartke (1).
A universal feature of aging in humans is a decline in the production of GH and sex steroids; these hormones oppose the negative impact of pro-inflammatory cytokines. Despite the above caveats, major emphasis has been placed on studies in inbred laboratory animals which have concluded that GH and IGF-1 have a negative impact on longevity. Based on this conclusion, it is not surprising that investigators have attempted to extrapolate this hypothesis to humans. However, the issue for aging humans is not longevity but quality of life which is not measured in laboratory animals. It is undeniable that as humans age, they experience a decline in the quality of life that is associated with a reduction in physical, mental, and immune capacity. Quality of life is more important than longevity in humans, yet our simplistic models focus on longevity, which is easy to measure, but not necessarily relevant to function during normal aging. The important question that should be addressed is what physiological changes contribute to the decline in the quality of life.
Discovery of Growth Hormone Secretagogue Ibutamoren, MK-0677 (Renamed LUM-201)
The aging phenotype is associated with attenuated production of GH. GH is released in a pulsatile fashion from somatotroph cells of the anterior pituitary gland. Its release is mainly regulated by 2 hypothalamic hormones, positively by GH-releasing hormone (GHRH) and negatively by somatostatin.
GH has important physiological functions in peripheral tissues and the brain. As we age, the amplitude of the endogenous pulses of GH declines. Therefore, GH replacement by bolus administration does not mimic normal physiology and bypasses negative feedback mechanisms that normally prevent hyperstimulation. True rejuvenation should restore the amplitude of episodic pulses to match that observed in young adults. On this basis, a team at Merck Research Laboratories initiated a project designed to rejuvenate endogenous pulsatile GH release in older subjects.
The objective was to identify a small molecule that when administered orally once daily would restore the amplitude of endogenous peaks of GH in old people to that observed in young adults. A conventional approach for identifying a drug candidate through high-volume screening of chemical libraries was not feasible because the receptor was unknown; hence the development of functional assays was necessary. Following an extensive literature search, we discovered early work from Bowers and Momany describing the synthesis of small peptides based on C-amidated met- and leu- enkephalins. Their studies culminated in the identification of a synthetic hexapeptide, His-d-Trp-Ala-Trp-d-Phe-Lys-NH2 (GHRP-6), that stimulated GH release in vitro and in vivo. However, GHRP-6 had poor oral bioavailability (0.3%) and short in vivo half-life and was therefore unsuitable as a once-daily oral drug. Its mechanism of action was also unknown. Nevertheless, the small size of this peptide was potentially suitable for the design of a peptidomimetic (2).
Merck scientists elucidated the mechanism of action of GHRP-6 based on functional assays in primary cultures of rat pituitary cells. The Merck team showed that GHRP-6 stimulated GH release from pituitary somatotrophs by amplifying GHRH signaling and by antagonizing somatostatin action (3). This mechanism and the knowledge that benzodiazepine-like structures could mimic small peptides led to the discovery of the benzolactam L-163,429 (4). Using the concept of privileged structures, Merck medicinal chemists developed a series of non-peptides and named them GH secretagogues (GHS) to differentiate them from GHRH. Elaboration of these privileged structures led to the identification of the spiropiperidine, MK-0677 (now named LUM-201), which has high oral bioavailability and pharmacokinetics suitable for once-daily administration (5). By application of expression cloning in xenopus oocytes, MK-0677 was used to isolate a new orphan G-protein coupled receptor. The receptor was named the GHS receptor (GHSR1a) (6,7). Subsequently, the discovery of ghrelin in stomach extracts led to the recognition that ghrelin was an endogenous ligand for GHSR1a (8).
GHS Trials in Older Adults
The reason the pharmaceutical companies developed GHS was that they believed these compounds would have promised to treat and ultimately prevent sarcopenia and the frailty of aging. This was an ambitious project since the Food and Drug Administration (FDA) typically approves drugs to treat disease and not normal physiological changes such as aging. However, both academic and pharmaceutical investigators pursued this endeavor. Two studies evaluated the effects of GHS in older adults, one performed by Thorner and colleagues at the University of Virginia using MK-0677 in healthy adults, aged 60–81 years, which was funded by the National Institutes of Health (NIH) (9), and the second study performed by Pfizer that was stopped early because the interim analysis did not show the prespecified increase in percent lean body mass at 6 months. As both lean body mass (LBM) and weight increased, the drug was not pursued (10).
Two secretagogues, ibutamoren (9) and capromorelin (10), have been studied. Ibutamoren (MK-0677 now LUM-201) is an orally active GHS that has a long biologic effect so it can be administered once daily at a dose of 25 mg. Sixty-five healthy adults (men, women receiving hormone replacement therapy, and women not receiving hormone replacement therapy) ranging from 60 to 81 years of age were enrolled in a 2-year, double-blind, randomized, placebo-controlled, modified-crossover clinical trial. The MK-0677 study demonstrated an increase in pulsatile GH secretion for as long as the medications were given for up to 2 years and levels returned to baseline after the medication was stopped (9) (See Figure 1). Capromorelin is also orally active but has a shorter half-life than ibutamoren. A total of 395 men and women aged 65–84 years were randomized for an intended 2 years of treatment to 4 dosing groups of capromorelin (10 mg 3 times/wk, 3 mg twice a day, 10 mg each night, and 10 mg twice a day capromorelin) or placebo. The study was terminated early according to predetermined treatment effect on an interim analysis performed after 265 subjects completed 6 months of treatment. Although absolute LBM increased, participants also gained weight, and therefore the increase in %LBM was not significant. The study was terminated early according to predetermined criteria; 315 subjects completed 6 months of treatment and 284 completed 12 months (10).
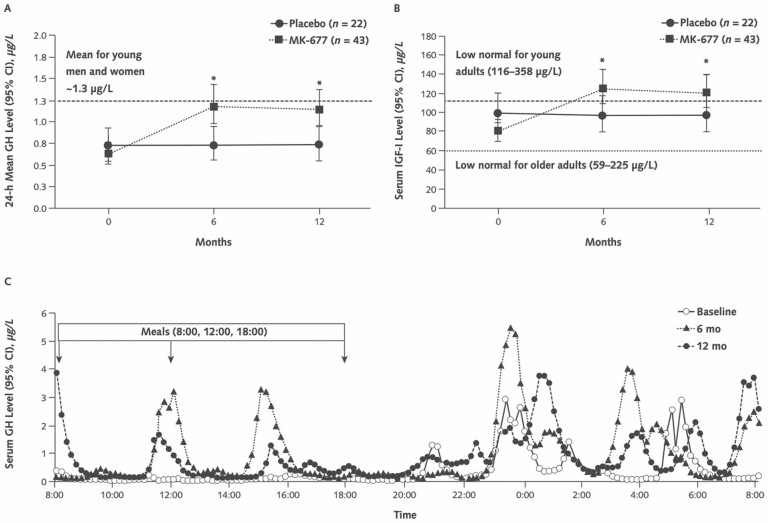
Figure 1.
(A) Mean 24-hour GH levels. The dashed line indicates 24-hour mean GH level for young men and women combined (~1.3 µg/L). *p < .001 for MK-677 versus placebo. (B) Serum IGF-I levels. The lower dotted line indicates the lower limit of the IGF-I normal range for older adults (59–225 µg/L), and the upper dashed line indicates the lower limit in adults aged 21–25 years (116–358 µg/L). (C) Representative 24-hour GH profile in a 70-year-old man with a body mass index of 23.2 kg/m2 who received MK-677 for 1 year. His 24-hour mean GH levels were 0.37, 1.0, and 0.86 µg/L at baseline, 6 months, and 12 months, respectively. The pulsatile pattern of GH secretion at baseline is maintained and enhanced at 6 and 12 months, primarily because of increased secretion per peak rather than peak frequency. Reproduced with permission from Nass et al. (9). GH = growth hormone; IGF-1 = insulin-like growth factor.
The capromorelin studies were very similar to those with MK-0677 in terms of an increase in lean body mass, serum IGF-1, and a very mild increase in insulin resistance which is not thought to be clinically significant. The administration of the orally active GHS capromorelin for 1 year can improve physical performance in generally healthy older adults with mild functional decline.
The studies demonstrated an increase in serum IGF-1, compared to single daily doses of ibutamoren higher doses of capromoerlin or repeated daily dosing of capromorelin, was needed to sustain elevations of IGF-1, which was also necessary to prevent stimulation of cortisol release.
There was 1 important difference between the 2 studies. The MK-0677 study involved healthy older adults, while the capromorelin study involved participants, who were at risk of functional decline.
The results of 1 year of therapy with MK-0677 (Table 1) and capromorelin (Table 2) are summarized and demonstrate that the results are similar in increasing IGF-1 and lean body mass and weight. Capromorelin increased stair climb power and tandem walking speed with nonsignificant effects on other functional measures. These effects were not observed with ibutamoren treatment but the 2 studies differed in the selection of the study participants; the ibutamoren study selected healthy older adults who were interested in preserving their health status while the capromorelin study selected older adults at risk of functional decline.
Table 1.
MK-0677 Study in Healthy Older Adults
| Change Versus Baseline at 12 Months | ||
|---|---|---|
| Placebo (kg) | MK-0677 (kg) | |
| FFM | −0.5 (CI, −1.1 to 0.2) | 1.1 (CI, 0.7 to 1.5)*** |
| Total appendicular FFM | −0.3 (CI, −0.6 to 0.1) | 0.5 (CI, 0.3 to 0.8)*** |
| Increase total appendicular fat on MK-0677 versus placebo | 0.24 | 1.1** |
| Body weight | 0.8 (CI, −0.3 to 1.8) | 2.7 (CI, 2.0 to 3.5)* |
Notes: GH 24-h mean GH increased 1.8-fold (CI, 1.56- to 2.0-fold) from baseline (p < .001). IGF-1 Serum IGF-I levels also increased by 1.5-fold (CI, 1.4- to 1.6-fold; p < .001). FFM = fat-free mass; GH = growth hormone; IGF-1 = insulin-like growth factor.
*** p < .001.
** p = .001.
*p = .003.
Table 2.
Capromorelin Study of Older Subjects at Risk of Functional Decline
| Change Versus Baseline at 12 Months | |||
|---|---|---|---|
| Placebo (kg) | Capromorelin (kg) | ||
| FFM | 0.6 + 1.4 | 1.6 + 3.2 | p = .02 |
| Body weight | −0.1 + 2.2 | 1.2 + 5.2 | p = .06 |
Notes: IGF-I levels in capromerlin- versus placebo-treated subjects increased by ~1.5-fold p = .001. FFM = fat-free mass; IGF-1 = insulin-like growth factor
No change in muscle strength or function or quality of life was seen with MK-0677. Shoulder flexion total work decreased in the placebo group but not in the MK-677 group. When corrected for baseline arm appendicular skeletal muscle mass, shoulder flexion total work decreased more in the placebo group than the ibutamoren group (change, −5.7 N·m/kg [CI, −10.1 to −1.4 N·m/kg] vs −1.0 N·m/kg [CI, −4.1 to 2.1 N·m/kg]; p = .073). In contrast, in the capromorelin study power stair climb improved significantly and improvements were also seen in tandem walk. Changes in the 6-minute walk and 5-chair stands approached statistical significance.
In both studies, there was a mild increase at 1 year in insulin resistance and an increase in HbA1C: ibutamoren versus placebo: change, 0.2% (CI, 0.1%–0.3%), versus −0.1% [CI, −0.2%–1.0%], respectively; p = .002; capromorelin versus placebo: change, 0.07% ± 0.45 versus −0.14% ± 0.29, p = .0008. This increase in insulin resistance is unlikely to be of clinical significance.
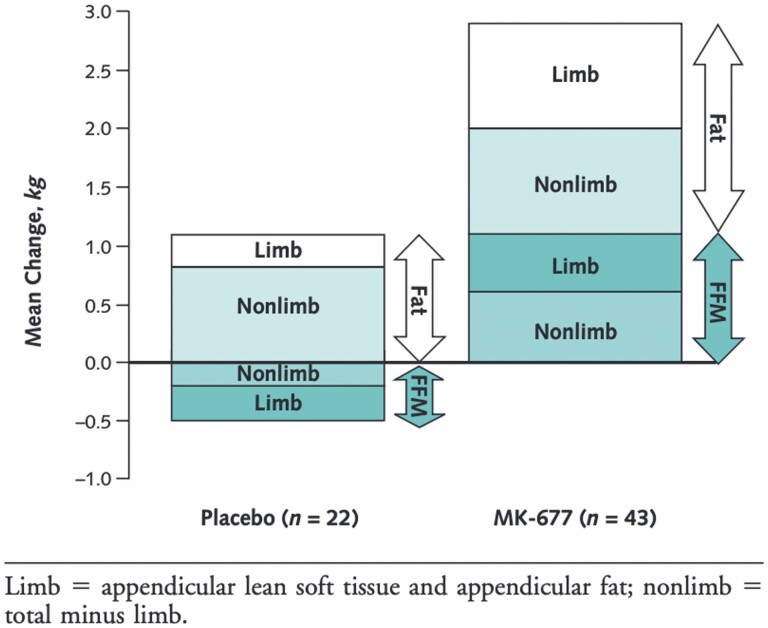
These 2 studies demonstrate that GHS increased GH levels in older adults to those observed in 20- to 30-year-old subjects and that the increase in GH levels was associated with an increase in fat-free mass (Figure 2). The ibutamoren study also demonstrated that subcutaneous fat is redistributed to the limbs which may be beneficial in older adults. Functional improvement was only observed in sarcopenic individuals in the ibutamoren study and was observed in the subjects at risk of decline in the capromorelin study. Future studies will need to build on these proof-of-concept studies by defining the population who likely will benefit and add exercise and possibly nutritional components. As discussed later, it is possible that this therapeutic approach might have beneficial effects on other age-associated morbidities including osteoporosis, impaired immune function, nonalcoholic fatty liver disease, memory loss, and anemia. Another GHSR agonist, anamorelin, has been studied in cancer cachexia. It is approved for this indication in Japan (11).
Figure 2.
Mean changes in fat and FFM at 12 months. FFM = fat-free mass.
MK-0677 and Bone Density
GH administration in older adults increases bone turnover and stimulates osteoblast activity. These effects are likely mediated by the GH-induced increase in circulating and locally produced IGF-1. To test for the effects of enhancing endogenous pulsatile GH release on IGF-1 and markers of bone formation, chronic administration of the GHS, MK-677, was tested in 3 randomized, double-blind, placebo-controlled clinical studies. Older adults (65 years or older) were treated with MK-0677 for 2–9 weeks to test for the effects on serum IGF-I and markers of bone turnover (12). The biologic effects of MK-677 were evaluated in 105 older subjects that met objective criteria for functional impairment. The effects of MK-0677 on changes from baseline in serum osteocalcin levels correlated with a significant increase in serum IGF-I (p < .01). Oral dosing with the MK-677 was associated with an increase in the marker of bone resorption N-telopeptide cross-links (NTXs) in the urine, and specific bone-formation markers, serum osteocalcin, and bone-specific alkaline phosphatase (BSAP) in urine.
Based on these findings, it was hypothesized that administration of MK-677, alone or in combination with alendronate, an inhibitor of bone resorption, would enhance bone formation and bone mineral density (BMD) in women with postmenopausal osteoporosis. BMD; IGF-1; markers of bone formation, osteocalcin, BSAP; and resorption markers, urinary NTx were measured in a randomized, double-blind, placebo-controlled study in 292 women (64–85 years old) with low BMD at the femoral neck treated for 12 and 18 months (13). At 12 months, MK-677, with or without alendronate, markedly increased IGF-1 (39% and 45%; p < .05), respectively. MK-677 increased osteocalcin (+22%) and urinary NTx (+41%), respectively (p < .05 vs placebo). The combination of MK-677 and alendronate further prevented a reduction in bone formation and increased BMD at the femoral neck compared to alendronate alone. However, because the combination did not increase BMD at the lumbar spine, total hip, or total body over that seen with alendronate alone, clinical development of the MK-0677 plus alendronate combination was not pursued.
Aging and Immune-Enhancing Effects of GHSR1a Agonism
During aging, immune responses decline and the thymus regresses. GH and IGF-1 are important stimulators of immune responses. Young (5–6 weeks) and old (16–24 months) mice were treated for 3–5 weeks with L-163,255, a close analog of MK-0677. The GHS treatment of young mice increased peripheral lymphocytes and the treatment of old mice increased thymic cellularity and differentiation, and this was statistically significant (14). Antibody responses were also enhanced by the GHS treatment of old mice. Furthermore, when inoculated with a transplantable lymphoma cell line, GHS-treated old mice were more resistant to tumor initiation and metastases and had lower mortality compared with untreated mice. GHS administration also enhanced the cytotoxic T lymphocyte response to transplantable lymphoma cell line (EL4) cells, and in severe combined immunodeficient (SCID) mice, GHS improved thymic engraftment of bone marrow cells. The spleens of GHS-treated mice contained increased numbers of cycling cells suggesting immune enhancement by promoting cell division of lymphoid cells. These findings are consistent with immune-enhancing properties of the GHS suggesting that treatment with MK-0677 may prove useful in people whose immune functions are compromised, such as in older adults.
Aging is associated with a decline in adaptive immunity, T lymphocyte output, and lower T-cell receptor repertoire that appear to be attributable to thymic involution. This progressive loss of thymic function during aging is likely a result of diminished progenitor cells, critical cytokines, and hormonal changes in the thymic microenvironment. Ghrelin, the endogenous ligand of GHSR1a, is expressed in immune cells and regulates T-cell activation and inflammation. Ghrelin and GHSR1a are also expressed in thymic stromal cells and their expression declines during normal aging and adipocytes become the major cell type contributing to the thymic microenvironment in older animals. The age-dependent changes in thymic architecture are inhibited when ghrelin is infused into 14-month-old mice to compensate for reduced endogenous ghrelin signaling. Indeed, ghrelin infusion induced thymopoiesis in old mice increasing the numbers of early thymocyte progenitors and bone marrow–derived cells (15).
Genetic ablation of ghrelin and Ghsr leads to loss of thymic epithelial cells. Reduced ghrelin signaling during aging is associated with an increase in adipogenic fibroblasts in the thymus and impaired T-cell production. Youm et al. showed that loss of interactions between GHSR1a and ghrelin facilitates epithelial–mesenchymal transitions (EMT) leading to thymic adipogenesis (16). The lack of GHSR1a activation compromises the thymic stromal microenvironment and is associated with reduced numbers of naive T cells. Collectively, these results suggest GHSR1a agonists are regulators of EMT and that their administration may preserve the thymic cell microenvironment by inhibiting age-related adipocyte development. Reconstituting thymic function by administering a GHSR1a agonist has the potential to provide therapeutic benefits in older subjects.
Ghrelin and GHSR1a are expressed in mouse and human T cells. Administration of ghrelin in old mice lowers the production of pro-inflammatory cytokines. RNAi-mediated “knock-down” of ghrelin in primary cultures of human T cells activated IkB, and increased the secretion of Th1 cytokines and IL-17. Hence, ghrelin acts in an autocrine and paracrine capacity as a regulator of pro-inflammatory cytokine expression in both mouse and human T cells (17).
Endotoxemia in older adults can prove lethal. Since ghrelin has anti-inflammatory properties, its potential for renal protection during endotoxemia-induced acute kidney injury (AKI) was evaluated. Normotensive endotoxemia was induced in AKI mice by intraperitoneal injection of LPS. Endotoxemia was accompanied by increases in serum TNF-α, IL-1β, and IL-6. Increases in ghrelin concentrations were also observed as well as increased concentration of GHSR1a in the kidney. Although the increase in endogenous ghrelin did not prevent inflammation, the addition of exogenous ghrelin suppressed the LPS-induced increases in serum TNF-α, IL-1β, IL-6, NO, and renal-inducible NO synthase. Ghrelin pretreatment prior to LPS protected from a decrease in glomerular filtration rate and renal blood flow caused by LPS treatment (18). Intriguingly, it appears that a physiological response to certain pathological challenges is associated with pro-inflammatory cytokine production, which triggers an increase in the production of endogenous ghrelin, yet in extreme situations, this is insufficient to overcome pathology. We hypothesize that in certain pathological situations, where pro-inflammatory cytokines are highly elevated that therapeutic benefit can only be achieved by treatment with pharmacological doses of ghrelin.
Ghrelin and GHSR1a are expressed in murine macrophages. Ghrelin dose dependently inhibited the production of the pro-inflammatory cytokines IL-1β and TNF-α in LPS-stimulated murine macrophages. Pretreatment with ghrelin also reduced LPS-induced NFkB activation and enhanced the release of anti-inflammatory cytokine IL-10 by activation of MAPK independent of NFkB. These effects were blocked by a specific GHSR1a antagonist. Hence, ghrelin exhibits anti-inflammatory properties by regulating the secretion of pro-inflammatory and anti-inflammatory cytokines (19).
GH and Brain
To test for an association between reduced GH production during aging with changes in memory and cognitive function, the number of neurons in the hilus of the dentate gyrus was compared with Wistar rats at ages 3–24 months. At ages 22 and 24 months, neuronal loss was observed in both male and female mice. Chronic treatment between ages 22 and 24 months with GH for 10 weeks resulted in an increase in neurons in the hilus in both sexes indicating GH may prevent neuronal loss associated with aging. These results support the notion that GH is neuroprotective during aging (20). Merck performed a large, double-blind, placebo-controlled MK-0677 trial in patients with Alzheimer’s disease and it was found to be ineffective (21).
GH, Aging, and Anemia
Christ et al. showed that erythropoiesis is impaired in adults with GH deficiency which may be rescued by GH treatment (22). GH treatment in these adults also increases total blood and plasma volume. These benefits may contribute to increased exercise performance observed in these patients.
The Importance of Pulsatile GH Secretion and Modest Changes Have Profound Biologic Effects
GHS stimulate GH secretion for as long as they are administered. The use of GHS has opened up a new approach to augment the somatotrophic axis.
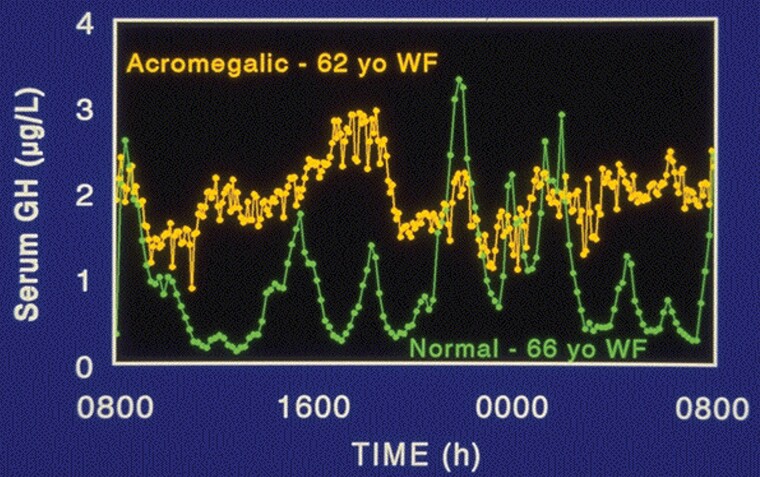
The significance of the exquisite regulation of pulsatile GH secretion comes from several lines of evidence. When GHS are chronically administered, they increase the amplitude of GH pulses by approximately 70%–100%. But the serum GH levels remain quite low when compared to levels observed with injections of recombinant GH. Despite these apparently modest changes in GH secretion, GHS mimic the effects of exogenous GH given by daily injections. This is true both in terms of body composition changes in older adults and in terms of growth. A small proportion of patients with acromegaly have GH levels that never rise above 2 or 5 υg/L, but their levels never fall to the low levels seen in normal subjects (Figure 3). Their serum IGF-1 is elevated and these patients have signs and symptoms of acromegaly. If the profile does not return to normal with normal low interpulse GH levels following surgical removal of the GH-secreting tumor, the clinical signs and symptoms will typically persist, including acral enlargement, thickened skin, tight rings, osteoarthritis, sleep apnea, hyperhidrosis, and insulin resistance (23).
Figure 3.
GH levels in an acromegalic woman and a normal woman. GH = growth hormone.
Summary
The discovery of the GHS receptor (GHSR1a) has provided fundamentally important new knowledge to our understanding of the physiology-regulating endogenous pulsatile GH release. We have shown that agonists of this receptor by regulating the activity of downstream mediators, GHRH, and somatostatin provide a unique approach for restoring the amplitude of GH in older subjects to that seen in young adults. In contrast to pharmacologic treatment with exogenous GH, which bypasses regulatory feedback pathways, the normalization of GH secretion in older subjects through a physiologically relevant pathway has the potential to show benefits in the broad spectrum of age-dependent disorders summarized earlier that are not commonly observed in young adults. Long-term use of agonists of the GHSR1a will have to be balanced against safety in the older subjects. The immunological effects have only been studied in animal models and are yet to be determined in humans.
Contributor Information
Roy G Smith, Department of Molecular Medicine, Scripps Research Institute, La Jolla, California, USA; Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, Texas, USA.
Michael O Thorner, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA; Lumos Pharma, Austin, Texas, USA.
Funding
No funding was provided for this manuscript. Each of the studies is referenced with the appropriate funding being acknowledged.
This supplement is sponsored by the National Institute on Aging (NIA) at the National Institutes of Health (NIH).
Conflict of Interest
R.G.S. and M.O.T. are consultants to Lumos Pharma, Inc. and have equity in Lumos Pharma, Inc.
References
- 1. Aguiar-Oliveira MH, Bartke A. Growth hormone deficiency: health and longevity. Endocr Rev. 2019;40:575–601. doi: 10.1210/er.2018-00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114:1537–1545. doi: 10.1210/endo-114-5-1537 [DOI] [PubMed] [Google Scholar]
- 3. Cheng K, Chan WW, BarretoA, Jr, Convey EM, Smith RG. The synergistic effects of His-D-Trp-Ala-Trp-D-Phe-Lys-NH2 on growth hormone (GH)-releasing factor-stimulated GH release and intracellular adenosine 3’,5’-monophosphate accumulation in rat primary pituitary cell culture. Endocrinology. 1989;124:2791–2798. doi: 10.1210/endo-124-6-2791 [DOI] [PubMed] [Google Scholar]
- 4. Smith RG, Cheng K, Schoen WR, et al. . A nonpeptidyl growth hormone secretagogue. Science. 1993;260:1640–1643. doi: 10.1126/science.8503009 [DOI] [PubMed] [Google Scholar]
- 5. Patchett AA, Nargund RP, Tata JR, et al. . Design and biological activities of L-163,191 (MK-0677): a potent, orally active growth hormone secretagogue. Proc Natl Acad Sci U S A. 1995;92:7001–7005. doi: 10.1073/pnas.92.15.7001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard AD, Feighner SD, Cully DF, et al. . A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974 [DOI] [PubMed] [Google Scholar]
- 7. Smith RG, Van der Ploeg LH, Howard AD, et al. . Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316 [DOI] [PubMed] [Google Scholar]
- 8. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230 [DOI] [PubMed] [Google Scholar]
- 9. Nass R, Pezzoli SS, Oliveri MC, et al. . Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–611. doi: 10.7326/0003-4819-149-9-200811040-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White HK, Petrie CD, Landschulz W, et al. . Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab. 2009;94:1198–1206. doi: 10.1210/jc.2008-0632 [DOI] [PubMed] [Google Scholar]
- 11. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12(1):14–16. doi: 10.1002/jcsm.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy MG, Bach MA, Plotkin D, et al. . Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults: the MK-677 Study Group. J Bone Miner Res. 1999;14:1182–1188. doi: 10.1359/jbmr.1999.14.7.1182 [DOI] [PubMed] [Google Scholar]
- 13. Murphy MG, Weiss S, McClung M, et al. . Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2001;86:1116–1125. doi: 10.1210/jcem.86.3.7294 [DOI] [PubMed] [Google Scholar]
- 14. Koo GC, Huang C, Camacho R, et al. . Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166:4195–4201. doi: 10.4049/jimmunol.166.6.4195 [DOI] [PubMed] [Google Scholar]
- 15. Dixit VD, Yang H, Sun Y, et al. . Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Youm YH, Yang H, Sun Y, et al. . Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem. 2009;284:7068–7077. doi: 10.1074/jbc.M808302200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009;113:5202–5205. doi: 10.1182/blood-2008-09-181255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F1032–F1037. doi: 10.1152/ajprenal.00044.2009 [DOI] [PubMed] [Google Scholar]
- 19. Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. l. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143:334–342. doi: 10.1016/j.surg.2007.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Azcoitia I, Perez-Martin M, Salazar V, et al. . Growth hormone prevents neuronal loss in the aged rat hippocampus. Neurobiol Aging. 2005;26:697–703. doi: 10.1016/j.neurobiolaging.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 21. Sevigny JJ, Ryan JM, van Dyck CH, et al. . Growth hormone secretagogue MK-677: no clinical effect on AD progression in a randomized trial. Neurology. 2008;71:1702–1708. doi: 10.1212/01.wnl.0000335163.88054.e7 [DOI] [PubMed] [Google Scholar]
- 22. Christ ER, Cummings MH, Westwood NB, et al. . The importance of growth hormone in the regulation of erythropoiesis, red cell mass, and plasma volume in adults with growth hormone deficiency. J Clin Endocrinol Metab. 1997;82:2985–2990. doi: 10.1210/jcem.82.9.4199 [DOI] [PubMed] [Google Scholar]
- 23. Hartman ML, Veldhuis JD, Vance ML, Faria AC, Furlanetto RW, Thorner MO. Somatotropin pulse frequency and basal concentrations are increased in acromegaly and are reduced by successful therapy. J Clin Endocrinol Metab. 1990;70:1375–1384. doi: 10.1210/jcem-70-5-1375 [DOI] [PubMed] [Google Scholar]