Abstract
Background:
Vaccination in patients with multiple sclerosis (MS) treated with immunosuppressive drugs is highly recommended. Regarding COVID-19 vaccination, no specific concern has been raised.
Objectives:
We aimed to evaluate if COVID-19 vaccination or infection increased the risk of disease activity, either radiological or clinical, with conversion to MS in a cohort of people with a radiologically isolated syndrome (RIS).
Methods:
This multicentric observational study analyzed patients in the RIS Consortium cohort during the pandemic between January 2020 and December 2022. We compared the occurrence of disease activity in patients according to their vaccination status. The same analysis was conducted by comparing patients’ history of COVID-19 infection.
Results:
No difference was found concerning clinical conversion to MS in the vaccinated versus unvaccinated group (6.7% vs 8.5%, p > 0.9). The rate of disease activity was not statistically different (13.6% and 7.4%, respectively, p = 0.54). The clinical conversion rate to MS was not significantly different in patients with a documented COVID-19 infection versus non-infected patients.
Conclusion:
Our study suggests that COVID-19 infection or immunization in RIS individuals does not increase the risk of disease activity. Our results support that COVID-19 vaccination can be safely proposed and repeated for these subjects.
Keywords: Radiologically isolated syndrome, COVID-19, vaccines
Introduction
The radiologically isolated syndrome (RIS) was defined in 2009 by Okuda et al. 1 as incidental magnetic resonance imaging (MRI) abnormalities suggestive of multiple sclerosis (MS). These white matter lesions identified on MRI are discovered incidentally and fulfill the criteria for dissemination in space (DIS), with no associated neurological clinical event suggestive of the central nervous system (CNS) demyelinating disease. Postmortem studies estimate RIS prevalence between 0.06% and 0.7%. 2 Overall, the cumulative incidence of RIS is approximately 0.1%. 3 In a multicentric study directed by the RIS Consortium (RISC), the estimated 5- and 10-year risk of developing a first clinical event was 34% and 51.2%, respectively.4,5 These studies identified significant predictors associated with a higher risk of conversion to MS, such as young age, male sex, cerebrospinal fluid (CSF)-restricted oligoclonal bands (OCBs), and spinal cord or infratentorial lesions. The impact of MS-approved disease-modifying therapies (DMTs) within this group recently demonstrated an 80% relative risk reduction of dimethyl fumarate compared to placebo. 6 Another multicentric study investigating the efficacy of teriflunomide in delaying the time to MS conversion is pending. 7
COVID-19 is an infectious disease caused by the SARS-CoV-2 virus. 8 Infections can lead to severe pulmonary affection, sometimes requiring oxygen therapy or other respiratory assistance. Some patients can experience long-term effects of their conditions, with symptoms persisting or appearing many months after the typical recovery. Several vaccines have been designed, including mRNA (Pfizer/BioNTech, Moderna), adenovirus vector vaccines (AstraZeneca, Janssen), inactivated virus vaccines (Sinovac), and recombinant protein vaccines (Sanofi). The vaccination campaign started in January 2021 in France, Turkey, and the United States, being a cornerstone to slow down the pandemic.
The safety of these vaccines in patients with demyelinating diseases has been debated. Concerns about the potential risk of triggering disease activity have been raised, leading to global vaccination hesitancy in this population. Several societies,9–12 including the French MS society (Societé Francophone de la Sclérose en Plaques (SFSEP)), recently published recommendations concerning vaccinations in patients with MS, concluding that the general use of vaccines was safe and efficient. 13 The American Academy of Neurology (AAN) and the ECTRIMS/EAN experts concluded insufficient data to support or refute an association between the development of MS and vaccination. They recommended that people with MS receive vaccines according to standard vaccine guidelines. 14 Regarding COVID-19, no specific warning has been identified, and vaccination of patients with inflammatory diseases of the CNS has thus been recommended.15–19
To date, no study is available about the impact of immunization on the RIS.
The primary objective was to evaluate if COVID-19 vaccination could increase the risk of clinical conversion to MS in RIS. The secondary purpose was to assess if COVID-19 vaccination or infection increases the risk of evidence of disease activity (EDA) in the preclinical stage of MS.
Methods
Patients
This multicentric observational study is based on the RISC and the SFSEP (French MS society) cohort (France, the United States, and Turkey). This cohort is registered on the Clinical Trials platform (NCT05388331), in which individuals identified as presenting with an RIS according to the 2009 diagnostic criteria 1 are included.
Baseline demographical, biological, and MRI data are collected. A standardized, prospective follow-up every year, including screening for the occurrence of a first clinical attack associated with CNS demyelination and systematic MRI follow-up, is also performed.
During the COVID-19 vaccination campaign, additional data were collected using a standardized form in each participating center, including the number and administered vaccines and history of documented COVID-19 infection (based on the positivity of an antigenic or PCR nasopharyngeal test). Subjects were excluded if (1) vaccination records were missing or incomplete, (2) if a first clinical event occurred before the COVID-19 vaccination, and (3) if any other vaccine was administered during the study period. Data regarding COVID-19 were collected between January 2021 and December 2022.
Outcome measures
The clinical conversion was defined as a neurological event consistent with MS relapse, lasting > 24 hours without fever or other acute illness. Specialists in neuroimmunology systematically confirmed all clinical events.
EDA was described as a new or enlarged T2-weighted hyperintense lesion or gadolinium-enhancing lesion on a follow-up MRI performed after vaccination.
Statistical analysis
We described the demographical features of the cohort and compared patient groups according to the vaccination status and history of COVID-19 infection.
Complete vaccination (CV) was defined as administering two doses of an mRNA (Pfizer/BioNTech, Moderna) or AstraZeneca vaccine, administration of one dose of Janssen vaccine, or administration of one dose of any vaccine in patients with a history of COVID-19 infection before the onset of vaccination. Other patients were defined as incompletely vaccinated if they received at least one dose of the vaccine without fulfilling the CV definition and unvaccinated (UV) if they did not receive any dose of any vaccine.
We compared the conversion rate to a first event related to CNS demyelination and EDA in both groups of patients.
Means with standard deviations (SD) were calculated to describe the numerical demographical features of the population. Categorical variables were reported as counts and percentages, and continuous variables were declared as median and quartile ranges. A comparison of quantitative measures between patient groups was assessed using the Mann–Whitney U test. Qualitative measures were compared between patient groups using the chi-square test. The p-values less than 0.05 were considered statistically significant. Data analysis was performed using EasyMedStat and R software (R Core Team, 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org).
Sample size
Our study was an exploratory study that aimed to collect data about COVID vaccination and SARS-Cov-2 infections in the entire RISC cohort. Thus, we did not perform a sample size calculation.
Ethical statement
This study was declared on clinical trials (NCT05387395). It complied with reference methodology MR-004 (Commission Nationale de l’Informatique et des Libertés) and was registered on the French Health Data Hub (Registration ID: F20220531152854). Personal data have been collected and processed by the laws and regulations in France and local laws in force in Turkey and in the United States.
This study was approved by the following regulatory authorities and ethics committees: Committee on Human Research (University of California, San Francisco, San Francisco, California, USA), Mayo Clinic Institutional Review Board (Mayo Clinic, Rochester, Minnesota, USA), University of Texas Southwestern at Dallas (Dallas, Texas, USA), Istanbul University Cerrahpasa School of Medicine Ethical Committee for Clinical Research and Studies (Istanbul, Turkey), and the Comité de Protection des Personnes (Hôpital Pasteur2, Nice, France). Written informed consent was acquired from all study subjects.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Results
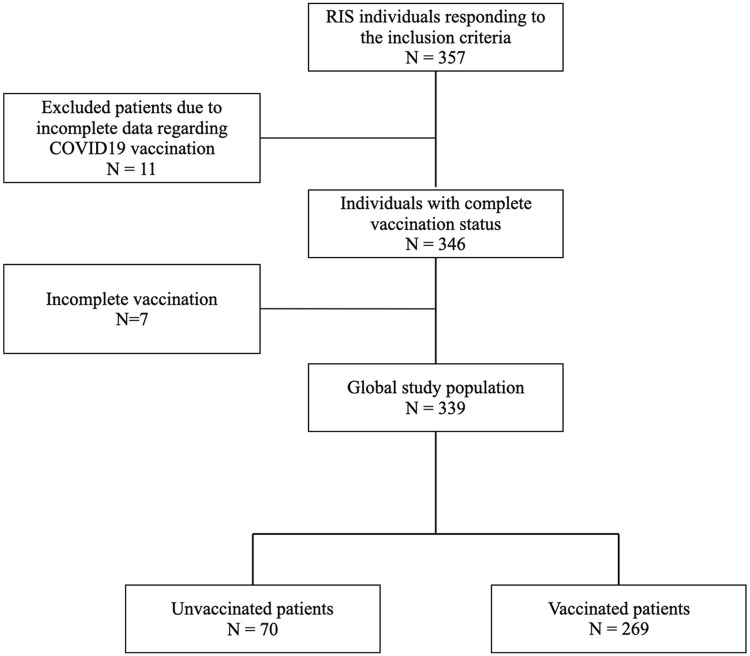
Data regarding COVID-19 vaccination and infection were collected for 346 RIS subjects from 17 centers. Among the whole population, 70 patients (20.2%) were not vaccinated and 269 patients (77.7%) had a CV. The most frequently administrated vaccine was Pfizer/BioNTech (77%), followed by Sinovac (12.5%), Moderna (6.1%), AstraZeneca (3.9%), and Janssen (0.5%). Seven patients (2.1%) were incompletely vaccinated and were excluded from the analysis (cf. Figure 1).
Figure 1.
Study flowchart.
Consequently, the study population included 339 patients. The demographic characteristics are detailed in Table 1. There was no significant difference between the vaccinated and unvaccinated groups concerning demographical characteristics, notably regarding known risk factors of conversion to MS, that is, age, sex, lesions’ location, and the presence of OCB in the CSF.
Table 1.
Demographic characteristics and ancillary findings of CV and incomplete or absent vaccination (IV), and the COVID-19 infected population and no infected.
| Global population (n = 339) |
CV N = 269 |
IV N = 70 |
p | History of COVID-19 N = 84 |
No history of COVID-19 N = 218 |
p | |
|---|---|---|---|---|---|---|---|
| Females, % | 72.8 | 75.5 | 64.9* | 0.09 | 76.2 | 71.1 | 0.46 |
| Age (years, M ± SD) | 38.4 ± 12.4 | 38.7 ± 12.6 | 37 ± 12 | 0.25 | 37.1 ± 12.9 | 38.5 ± 12.2 | 0.27 |
| Caucasian, % | 90.1 | 90.2 | 88.3 | 0.59 | 93.9 | 88.9 | 0.93 |
| Initial MRI | |||||||
| Spinal cord lesion, % | 29.7 | 20.5 | 28.5 | 0.18 | 19 | 20.1 | 0.31 |
| At least one gadolinium-enhancing lesion, % | 15.2 | 16.4 | 10.5 | 0.3 | 19.5 | 13.7 | 0.26 |
| At least one infratentorial lesion, % | 48 | 48.1 | 47.1 | 0.93 | 63.6 | 53 | 0.51 |
| CSF OCBs, % | 63 | 65.9 | 57.1 | 0.55 | 83.3 | 70.3 | 0.51 |
In our cohort, 84 patients (27.8%) had a history of COVID infection, among which 27 were completely vaccinated. The demographic and radiological characteristics of patients with or without a history of COVID-19 disease were comparable.
Primary outcome: vaccination and clinical conversion to MS
Among the population, 38 subjects presented a first clinical event before the first vaccination jab and were excluded from the primary outcome analysis. In the analyzed cohort (n = 312), the first clinical event occurred in 18 subjects (6.7%) of the vaccinated group and 6 subjects (8.5%) of the unvaccinated group. No significant difference was found between these two parameters (p > 0.9). When clinical conversion occurred after vaccination, the mean delay to the first clinical event was 172 days, ranging from 5 to 427 days after the first vaccination.
Secondary outcomes
Brain MRI follow-up data were available for 189 RIS subjects, 162 in the CV and 27 in the IV groups. The demographical data were not statistically different between patients with and without follow-up MRI regarding age, gender, and baseline RIS characteristics (MRI and biological data).
EDA occurred in 22 patients (13.6%) in the vaccinated group and in 2 (7.4%) in the unvaccinated group. The observed difference was not statistically significant (p = 0.54).
Impact of COVID-19 infection on RIS course
Among 84 RIS subjects who developed a documented COVID-19 infection, conversion to MS occurred before the onset of infection in 12 individuals excluded from this analysis. In the analyzed population, the first clinical event occurred in 8 patients (12.1%) with a history of COVID-19 infection versus 27 patients (13.5%) without COVID-19 infection. No significant difference was demonstrated (p > 0.999).
MRI follow-up data were available in 62/72 (86.1%) subjects in the infected group and 119/218 (54.5%) in the non-infected group. EDA occurred in 10 patients (16.1%) and 9 patients (7.6%), respectively, with a non-significant difference (p = 0.123)
Discussion
We conducted a prospective study to assess whether COVID-19 vaccines for preventing SARS-Cov-2 infection could impact the risk of conversion to MS in RIS subjects. Our results showed that the COVID-19 infection and vaccination did not lead to a substantial risk increase in clinical conversion or EDA.
This is the first study investigating the safety of COVID-19 vaccines regarding disease activity in RIS subjects. Several studies explored disease activity after COVID-19 vaccination in MS patients.20–28 Most results highlighted that vaccination was not associated with a higher risk of relapse. A multicentric observational study conducted in Italy 29 involving 324 patients with MS showed that Pfizer/BioNTech vaccine did not increase the short-term risk of acute exacerbations 2 months post-exposure. An Israelite study 24 assessed vaccination safety in adult MS patients. Vaccinated MS patients did not show increased clinical disease activity over a median follow-up of 20–38 days. The rate of patients with acute relapses (2.1% after the first dose and 1.6% after the second dose) was like the rate in unvaccinated patients evaluated in the corresponding period in 2017, 2018, 2019, and 2020. Finally, a recent meta-analysis of 19 studies gathering more than 14,000 MS patients concluded that COVID-19 vaccination was not associated with an increased risk of disease activity. 28
Conversely, two reports20,25,27 and one case series 22 of 16 patients described MS relapses shortly after COVID-19 vaccination. Still, the timing between the vaccination and relapse occurrence was often very short, raising the question of a true causality between the two events. One publication reports the clinical experience of a young woman with relapsing–remitting MS (RRMS) treated with rituximab. However, 3 months after the last infusion and 2 days after her first vaccination by Sputnik V, she presented a relapse associated with new lesions on brain MRI. 20 No patient within our cohort received the Sputnik V vaccination, and only a few cases were vaccinated with adenovirus vector vaccines.
In addition, one case reported relapse in a young woman diagnosed with RRMS in 2016 who received two courses of cladribine in 2018 and 2019 without any other disease-modifying treatment later. The relapse occurred in April 2021, 2 days after her first injection of the Pfizer COVID-19 vaccine. MRI showed three new gadolinium-enhancing lesions compared to a previous scan performed 6 months before. 22 Nistri et al. reported data from 16 patients managed in four Italian MS centers referred after COVID-19 vaccination. The most frequently administrated vaccine was Pfizer/BioNTech. Three patients received a new diagnosis of MS after the COVID-19 vaccination, 3–8 days after their first dose. Among other patients, nine were on disease-modifying treatment and presented a relapse between 1 and 21 days after vaccination. All patients had EDA on MRI to support the relapse diagnosis, but the timing of the MRI after symptom onset was very heterogeneous (3–50 days). This latter suggested a temporal association between disease activity and COVID-19 vaccination. 22
Finally, a recent prospective study hypothesized that the AstraZeneca vaccine could be associated with a higher risk of developing myelin oligodendrocyte glycoprotein antibody-associated disease. 30 Still, no relationship with MS onset was recorded. Moreover, only 11 individuals in our study population received this vaccine (3.9%)
In France, the Periodic Safety Update Reports (PSUR), which evaluates the risk–benefit balance of a medicinal product at defined time points after authorization, did not publish concerns about MS relapse after COVID vaccination. 31
Regarding vaccination in general, the evidence of an association between vaccination and MS activity has already been debated.10,32–35 Several societies published recommendations about vaccinations in MS populations. The French MS society SFSEP 13 did not identify an increased risk of MS or relapse after vaccination and recommended comprehensive vaccination in MS patients. The AAN 14 concluded that data are insufficient to support or refute an association between the development of MS or the occurrence of relapse and history of vaccination. Thus, AAN experts recommended that people with MS receive vaccines according to the standard guidelines.
In our analysis, neither COVID-19 vaccination nor COVID-19 infections were associated with a higher risk of EDA, even if MRI data were unavailable for the whole cohort. We did not find any other data regarding systematic radiological assessment of MS activity after COVID-19 vaccination or infection, except for the few previously cited case reports or series for which MRI data were available in the setting of a relapse. Further studies based on regular MRI follow-ups on larger cohorts of MS patients could support our results.
Our cohort did not show an increased risk of conversion to MS after COVID-19 infection.
To our knowledge, the correlation between infections and RIS conversion to MS has never been studied. In addition, the question of the potential role of conditions in triggering disease activity in MS has been raised for a long time. The French MS society and the ECTRIMS31–33 recently concluded in a set of recommendations about infections and MS that the role of viral, bacterial, or fungal infections in increasing the risk of relapse in MS was not endorsed because of the limited size, retrospective nature, and the lack of MRI information in the available studies. Consequently, the authors stated that infection in MS patients, in general, could lead to a transient worsening of disability in case of fever but were not associated with an increased risk of relapse or permanent worsening of disability. More specifically, a recent publication raised the hypothesis that MS could be triggered by COVID-19 infection in relationship with molecular mimicry between SARS-CoV-2 nucleocapsid and myelin proteolipid protein. 37 However, to date, no clinical data support this hypothesis.
Our study also has several limitations. The experimental design, without sample size calculation, can raise the hypothesis of a lack of study power can be raised. In addition, MRI data were only collected for part of the cohort, mainly because of the lockdowns related to the pandemic, which did not allow patients to perform their annual exams. Moreover, the timing between vaccination and MRI needed to be standardized. However, our findings suggest that the COVID-19 vaccination is safe in subjects with RIS and should be proposed without concern regarding a potential risk of disease evolution.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.C. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Ad Scientiam, Biogen, Merck, Sanofi-Genzyme, Roche, Celgene/Bristol Myers Squibb (BMS), Alexion, Horizon Therapeutics, none related to this study. O.T-R. has nothing to disclose. A.S. reported serving on the steering committee for the ARISE and Teriflunomide in Radiologically Isolated Syndrome (TERIS) studies. D.T.O. received personal compensation for consulting and advisory services from Alexion, Biogen, Celgene/BMS, EMD Serono, Genentech, Genzyme, Janssen Pharmaceuticals, Novartis, Osmotica Pharmaceuticals, RVL Pharmaceuticals, Inc., TG Therapeutics, Viela Bio, Inc., and research support from Biogen and EMD Serono/Merck; he has issued national and international patents and pending patents related to developed technologies; he is the Founder of Revert, a corporation involved in healthcare; he also received royalties for intellectual property licensed by The Board of Regents of The University of Texas System; and he is the Principal Investigator of the ARISE study. R.K., H.E., and M.T. have nothing to disclose. C.C-D. has received travel grants and personal compensation for consulting, serving on a scientific advisory board, and speaking with Biogen, Novartis, Merck, Sanofi-Genzyme, and Roche. F.D-D. and E.T. have nothing to disclose. J.C. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi-Genzyme, Roche, Celgene-BMS, and Alexion, none related to this study. H.Z. has nothing to disclose. B.B. serves on the scientific advisory board and has received funding for travel and research funding from Alexion, BMS, Biogen, Merck, Novartis, Sanofi Roche, and Teva. O.C. has received travel grants, personal compensation for serving on a scientific advisory board, and lecturing fees from Biogen, Novartis, Merck, Sanofi-Genzyme, Roche, and Celgene-BMS. J.D.S. and T.M. have nothing to disclose. J-P.N. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Sanofi-Genzyme, Roche, and Alexion, but none related to this study. D.P. and O.K. reported serving on the steering committee for the ARISE and TERIS studies. Me.T. has nothing to disclose. N.D. reported receiving personal fees from Biogen, Novartis, Merck, and Roche outside the submitted work. C.B. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Alexion, Biogen, BMS-Celgene, Merck, Novartis, Teva, and Sanofi-Genzyme. C.L. has received travel grants and personal compensation for consulting or speaking from Biogen, Novartis, Merck, Sanofi-Genzyme, and Roche, none related to this study. J.B. and C.L-C. have nothing to disclose. C.L-F. reported serving as the Principal Investigator of the TERIS study, on the steering committee for the ARISE study, and as co-president of the Observatoire Français de la Sclérose En Plaques (OFSEP) scientific committee, without honoraria.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Mikael Cohen  https://orcid.org/0000-0002-3985-1297
https://orcid.org/0000-0002-3985-1297
Aksel Siva  https://orcid.org/0000-0002-8340-6641
https://orcid.org/0000-0002-8340-6641
Darin T Okuda  https://orcid.org/0000-0002-6499-1523
https://orcid.org/0000-0002-6499-1523
Clarisse Carra-Dalliere  https://orcid.org/0000-0002-0985-3662
https://orcid.org/0000-0002-0985-3662
Eric Thouvenot  https://orcid.org/0000-0001-8671-7747
https://orcid.org/0000-0001-8671-7747
Jonathan Ciron  https://orcid.org/0000-0002-3386-6308
https://orcid.org/0000-0002-3386-6308
Christine Lebrun-Frenay  https://orcid.org/0000-0002-3713-2416
https://orcid.org/0000-0002-3713-2416
Contributor Information
Mikael Cohen, Department of Neurology, CRC-SEP, CHU de Nice, Pasteur 2 Hospital, Nice, France UR2CA-URRIS, Côte d’Azur University, Nice, France.
Océane Thomel-Rocchi, Department of Neurology, CRC-SEP, CHU de Nice, Pasteur 2 Hospital, Nice, France.
Aksel Siva, Department of Neurology, Cerrahpasa School of Medicine, Istanbul University, Istanbul, Turkey.
Darin T Okuda, The University of Texas Southwestern Medical Center, Dallas, TX, USA.
Rana Karabudak, School of Medicine, Hacettepe University, Ankara, Turkey.
Hüsnü Efendi, School of Medicine, Kocaeli University, Kocaeli, Turkey.
Murat Terzi, School of Medicine, Ondokuz Mayis University, Samsun, Turkey.
Clarisse Carra-Dalliere, Department of Neurology, CRC-SEP, CHU de Montpellier, Gui de Chauliac Hospital, Montpellier, France.
Francoise Durand-Dubief, Service de Neurologie, Sclérose en Plaques, Pathologies de la Myéline et Neuro-Inflammation, Hôpital Neurologique Pierre Wertheimer, Hospices Civils de Lyon, Lyon, France.
Eric Thouvenot, Institut de Génomique Fonctionnelle, Université de Montpellier, Centre National de Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Montpellier, France.
Jonathan Ciron, Department of Neurology, Centre de Ressource et Competence Sclérose En Plaques, Centre Hospitalier Universitaire de Toulouse, Institut Toulousain des Maladies Infectieuses et Inflammatoires (Infinity), Institut National de la Santé et de la Recherche Médicale Unité Mixte de Recherche1291, Centre National de Recherche Scientifique Unité Mixte de Recherche 5051, Université Toulouse III, Toulouse, France.
Helene Zephir, Université de Lille, Inserm Unité Mixte de Recherche-S 1172 LilNcog, Centre Hospitalier Universitaire Lille, Fédération Hospitalo-Universitaire Precise, Lille, France.
Bertrand Bourre, Department of Neurology, CHU Rouen, Rouen, France.
Olivier Casez, Pathologies Inflammatoires du Système Nerveux, Neurologie, CHU Grenoble Alpes, T-RAIG (Translational Research in Autoimmunity and Inflammation Group) TIMC-IMAG, Université de Grenoble-Alpes, CHU Grenoble-Alpes, Grenoble, France.
Jérôme De Seze, Department Clinical Investigation Center, Department of Neurology, Centre Hospitalier Universitaire de Strasbourg, Institut National de la Santé et de la Recherche Médicale, Strasbourg, France.
Thibault Moreau, Department of Neurology, Centre Hospitalier Universitaire de Dijon, Dijon, France.
Jean-Philippe Neau, Department of Neurology, Centre Hospitalier Universitaire de Poitiers, Poitiers, France.
Daniel Pelletier, University of Southern California, Los Angeles, CA, USA.
Orhun Kantarci, Mayo Clinic, Rochester, MN, USA.
Melih Tutuncu, Department of Neurology, Cerrahpasa School of Medicine, Istanbul University, Istanbul, Turkey.
Nathalie Derache, Department of Neurology, Centre Hospitalier Universitaire de Caen Normandie, Caen, France.
Caroline Bensa, Neurology Department, CRC-SEP, Hopital Fondation Adolphe de Rothschild, Paris, France.
Celine Louapre, CIC Neurosciences, Department of Neurology, Sorbonne University, Assistance Publique des Hôpitaux de Paris, Pitié-Salpêtrière Hospital, Paris, France.
Jeanne Benoit, Department of Neurology, CRC-SEP, CHU de Nice, Pasteur 2 Hospital, Nice, France UR2CA-URRIS, Côte d’Azur University, Nice, France.
Cassandre Landes-Chateau, Department of Neurology, CRC-SEP, CHU de Nice, Pasteur 2 Hospital, Nice, France UR2CA-URRIS, Côte d’Azur University, Nice, France.
Christine Lebrun-Frenay, Department of Neurology, CRC-SEP, CHU de Nice, Pasteur 2 Hospital, Nice, France UR2CA-URRIS, Côte d’Azur University, Nice, France.
References
- 1.Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: The radiologically isolated syndrome. Neurology 2009; 72(9): 800–805. [DOI] [PubMed] [Google Scholar]
- 2.Granberg T, Martola J, Kristoffersen-Wiberg M, et al. Radiologically isolated syndrome—Incidental magnetic resonance imaging findings suggestive of multiple sclerosis, a systematic review. Mult Scler J 2013; 19(3): 271–280. [DOI] [PubMed] [Google Scholar]
- 3.Forslin Y, Granberg T, Jumah AA, et al. Incidence of radiologically isolated syndrome: A population-based study. Am J Neuroradiol 2016; 37(6): 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS ONE 2014; 9(3): e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebrun-Frénay C, Kantarci O, Siva A, et al. Radiologically isolated syndrome: 10-year risk estimate of a clinical event. Ann Neurol 2020; 88(2): 407–417. [DOI] [PubMed] [Google Scholar]
- 6.Okuda DT, Kantarci O, Lebrun-Frénay C, et al. Dimethyl fumarate delays multiple sclerosis in radiologically isolated syndrome. Ann Neurol 2023; 93(3): 604–614. [DOI] [PubMed] [Google Scholar]
- 7.Lebrun-Frénay C, Rollot F, Mondot L, et al. Risk factors and time to clinical symptoms of multiple sclerosis among patients with radiologically isolated syndrome. JAMA Netw Open 2021; 4(10): e2128271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muralidar S, Ambi SV, Sekaran S, et al. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020; 179: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otero-Romero S, Rodríguez-García J, Vilella A, et al. Recommendations for vaccination in patients with multiple sclerosis who are eligible for immunosuppressive therapies: Spanish consensus statement. Neurol Engl Ed 2021; 36(1): 50–60. [DOI] [PubMed] [Google Scholar]
- 10.Riva A, Barcella V, Benatti SV, et al. Vaccinations in patients with multiple sclerosis: A Delphi consensus statement. Mult Scler J 2021; 27(3): 347–359. [DOI] [PubMed] [Google Scholar]
- 11.Multiple sclerosis and (non-COVID) vaccinations: Consensus recommendations, http://www.medscape.com/viewarticle/961117
- 12.Vaccini e sclerosi multipla, https://www.aism.it/vaccini_e_sclerosi_multipla
- 13.Lebrun-Frénay C, Vukusic S. Immunization and multiple sclerosis: Recommendations from the French Multiple Sclerosis Society. Mult Scler Relat Disord 2019; 31: 173–188. [DOI] [PubMed] [Google Scholar]
- 14.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2019; 93(13): 584–594. [DOI] [PubMed] [Google Scholar]
- 15.ECTRIMS/EAN statement on COVID-19 vaccination in MS patients, http://www.medscape.com/viewarticle/961005
- 16.COVID-19 vaccine guidance for people living with MS, https://www.nationalmssociety.org/coronavirus-covid-19-information/covid-19-vaccine-guidance
- 17.American Academy of Neurology position statement on COVID-19 vaccination, https://www.aan.com/advocacy/covid-19-vaccination-position-statement
- 18.Recommandations vaccinales anti-COVID – 25 Octobre 2021, https://sfsep.org/recommandations-vaccinales-anti-covid-25-octobre-2021/
- 19.MS, COVID—19 and vaccines—updated global advice, https://www.msif.org/news/2020/02/10/the-coronavirus-and-ms-what-you-need-to-know/
- 20.Etemadifar M, Sigari AA, Sedaghat N, et al. Acute relapse and poor immunization following COVID-19 vaccination in a rituximab-treated multiple sclerosis patient. Hum Vaccines Immunother 2021; 17(10): 3481–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol 2022; 362: 577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nistri R, Barbuti E, Rinaldi V, et al. Case report: Multiple sclerosis relapses after vaccination against SARS-CoV2: A series of clinical cases. Front Neurol 2021; 12: 765954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quintanilla-Bordás C, Gascón-Gimenez F, Alcalá C, et al. Case report: Exacerbation of relapses following mRNA COVID-19 vaccination in multiple sclerosis: A case series. Front Neurol 2022; 13: 897275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler J 2021; 27(6): 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilimuri S, Mantri N, Gongati S, et al. COVID-19 vaccine failure in a patient with multiple sclerosis on ocrelizumab. Vaccines 2021; 9(3): 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fragoso YD, Gomes S, Gonçalves MVM, et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult Scler Relat Disord 2022; 57: 103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniscalco GT, Manzo V, Di Battista ME, et al. Severe multiple sclerosis relapse after COVID-19 vaccination: A case report. Front Neurol 2021; 12: 721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanou MI, Palaiodimou L, Theodorou A, et al. Safety of COVID-19 vaccines in multiple sclerosis: A systematic review and meta-analysis. Mult Scler J 2023; 29(4–5): 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis, https://jnnp.bmj.com/content/93/4/448 [DOI] [PubMed]
- 30.Francis AG, Elhadd K, Camera V, et al. Acute inflammatory diseases of the central nervous system after SARS-CoV-2 vaccination. Neurol Neuroimmunol Neuroinflam 2023; 10(1): e200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Point de situation sur la surveillance des vaccins contre la COVID—19—Période du 22/04/2022 au 05/05/2022, https://ansm.sante.fr/actualites/point-de-situation-sur-la-surveillance-des-vaccins-contre-la-covid-19-periode-du-22-04-2022-au-05-05-2022
- 32.Zrzavy T, Kollaritsch H, Rommer PS, et al. Vaccination in multiple sclerosis: Friend or foe? Front Immunol 2019; 10: 1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeStefano F, Verstraeten T, Jackson LA, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol 2003; 60(4): 504–509. [DOI] [PubMed] [Google Scholar]
- 34.Confavreux C, Suissa S, Saddier P, et al. Vaccinations and the risk of relapse in multiple sclerosis. N Engl J Med 2001; 344(5): 319–326. [DOI] [PubMed] [Google Scholar]
- 35.Langer-Gould A, Qian L, Tartof SY, et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol 2014; 71(12): 1506–1513. [DOI] [PubMed] [Google Scholar]
- 36.Papeix C, Donze C, Lebrun-Frénay C, et al. Infections and multiple sclerosis: Recommendations from the French Multiple Sclerosis Society. Rev Neurol 2021; 177(8): 980–994. [DOI] [PubMed] [Google Scholar]
- 37.Lake CM, Breen JJ. Sequence similarity between SARS-CoV-2 nucleocapsid and multiple sclerosis-associated proteins provides insight into viral neuropathogenesis following infection. Sci Rep 2023; 13(1): 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.