Key Points
-
•
Patients with Asian-type DEL may safely receive D+ red cell transfusion.
-
•
Full-length RHD transcripts were identified in Asian-type DEL erythroblasts and express the complete repertoire of D epitopes like D+ cells.
Visual Abstract
Abstract
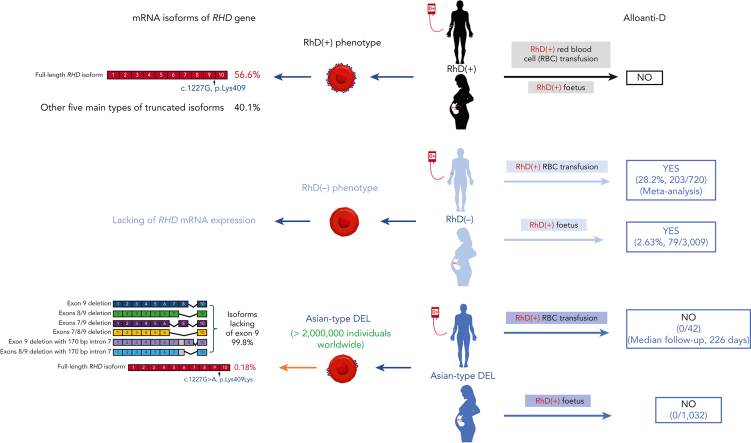
Red blood cells (RBCs) of Asian-type DEL phenotype express few RhD proteins and are typed as serologic RhD-negative (D–) phenotype in routine testing. RhD-positive (D+) RBC transfusion for patients with Asian-type DEL has been proposed but has not been generally adopted because of a lack of direct evidence regarding its safety and the underlying mechanism. We performed a single-arm multicenter clinical trial to document the outcome of D+ RBC transfusion in patients with Asian-type DEL; none of the recipients (0/42; 95% confidence interval, 0-8.40) developed alloanti-D after a median follow-up of 226 days. We conducted a large retrospective study to detect alloanti-D immunization in 4045 serologic D– pregnant women throughout China; alloanti-D was found only in individuals with true D– (2.63%, 79/3009), but not in those with Asian-type DEL (0/1032). We further retrospectively examined 127 serologic D– pregnant women who had developed alloanti-D and found none with Asian-type DEL (0/127). Finally, we analyzed RHD transcripts from Asian-type DEL erythroblasts and examined antigen epitopes expressed by various RHD transcripts in vitro, finding a low abundance of full-length RHD transcripts (0.18% of the total) expressing RhD antigens carrying the entire repertoire of epitopes, which could explain the immune tolerance against D+ RBCs. Our results provide multiple lines of evidence that individuals with Asian-type DEL cannot produce alloanti-D when exposed to D+ RBCs after transfusion or pregnancy. Therefore, we recommend considering D+ RBC transfusion and discontinuing anti-D prophylaxis in patients with Asian-type DEL, including pregnant women. This clinical trial is registered at www.clinicaltrials.gov as #NCT03727230.
Serologically negative RhD (D–) individuals in Asia are predominantly of the RhDEL phenotype, a polymorphism that leads to heterogeneous protein expression including a small amount of full-length RhD insufficient to agglutinate red cells in vitro. These patients have traditionally received D– blood, stressing the blood supply. In a prospective clinical trial of D+ transfusion in 42 patients with RhDEL, Ji and colleagues demonstrated that none of these patients developed antibodies. In a retrospective study of over 4000 serologically D– patients, alloanti-D was found only in true D– individuals and in no patients with RhDEL, strongly suggesting that these individuals can be transfused with D+ blood.
Introduction
RhD is the most immunogenic blood group antigen in transfusion medicine. Alloanti-D immunization against the RhD antigen in RhD-negative (D–) individuals can cause severe transfusion reactions and hemolytic disease of the fetus and newborn (HDFN). The D– phenotype is more common in White people (15%-17%) than in Black people (3%-10%) but is rare in East and Southeast Asian populations (0.3%-0.4%). Hence, D– blood is often in short supply, particularly in Asian regions. Furthermore, many individuals with a serologic D– phenotype in Chinese,1 Korean,2,3 Japanese,4 Thai,5,6 and Myanmar7 populations carry a D-eluate (DEL) phenotype (9%-33%) but not a true D– phenotype. The red blood cells (RBCs) of individuals with the DEL phenotype express a small amount of RhD antigen that cannot generate agglutination in routine RhD typing and is typically typed as the serologic D– phenotype.8 More than 40 distinct genetic variants underlying the DEL phenotype have been recognized thus far. Among them, RHD∗1227A (RHD∗01EL.01) was found predominantly in individuals with DEL (>95%) from East and Southeast Asian populations1, 2, 3, 4, 5 and was thus termed as Asian-type DEL in 2010.9 In China, the United States, and the European Union (EU), ∼96%, 77%, and 9% of the DEL population, respectively, carry the Asian-type DEL allele representing ∼1.7 million people in China,1 90 000 in the United States, and 10 000 in the EU.10
Since the molecular basis was originally identified in 2001, the proper transfusion management method for individuals with Asian-type DEL has been debated.11 As early as 2006,12 it was published that Asian-type DEL recipients may not develop alloanti-D after exposure to RhD-positive (D+) RBCs. Some evidence, namely, that of pregnant women with Asian-type DEL in which alloanti-D immunization was not detected after carrying a D+ fetus, supported this speculation.9,13, 14, 15 Based on these limited data, the transfusion of D+ RBCs to patients with Asian-type DEL was proposed more than 10 years ago.9 Since then, only 2 cases have been reported; both were male patients who received D+ RBCs, one with heart transplantation16 and the other being an emergency case17 and neither patient became immunized to alloanti-D. However, a systematic study concerning the safety of D+ RBC transfusion to patients with Asian-type DEL is still lacking,16 and the underlying molecular mechanism remains elusive. Individuals with the Asian-type DEL phenotype continues to be routinely managed as D– patients in clinics, and these patients are often subjected to a short supply of D– blood,10 although it would be justifiable for them to receive special consideration based on their sheer numbers.
Serologic testing indicates that a low number of D antigens with a deduced complete repertoire of epitopes are expressed on the surfaces of Asian-type DEL RBCs.18,19 These results suggest that patients with Asian-type DEL may not develop alloanti-D immunization after exposure to D+ RBCs because they carry the full repertoire of D epitopes like normal D+ individuals. Although 2 compelling hypotheses have accounted for this,20, 21, 22, 23 neither has been validated by sufficient functional data. Thus, the underlying molecular mechanism, as well as whether and why Asian-type DEL may express a functionally normal RhD protein, remains controversial.
In this study, we combined clinical, epidemiological, molecular, and biochemical data to determine whether individuals with Asian-type DEL should be treated as D+ individuals, as hypothesized for almost 20 years.12 We performed a clinical trial to transfuse patients with Asian-type DEL with D+ RBCs and completed 2 retrospective studies with serologic D– pregnant women to reinforce the epidemiological data. We also investigated the structure of RHD transcript isoforms in primary erythroblasts from individuals with the Asian-type DEL and the D antigen epitope profile expressed by those isoforms to provide molecular data on the underlying mechanism.
Methods
Asian-type DEL typing
Common RhD status was determined using routine serological testing distributions between D+, D–, weak D, and partial D phenotypes. Samples with a weak agglutination reaction (≤2+) compared with D+ controls (4+) were regarded as having a serologic weak D phenotype.24 Samples with discrepant agglutination reactions for anti-D monoclonal antibodies (mAbs) against different D antigen epitopes were regarded as having a partial D phenotype. Samples with a negative agglutination with a set of anti-D mAbs (Clone Rum-1; IgM, Shanghai Hemo-Pharmaceutical & Biological Co, Shanghai, China; Clone TH-28/MS-26; IgM/IgG, Millipore, Livingston, United Kingdom, in this study) indicated a serologic D– phenotype, known to include true D– and DEL samples. Our routine serologic RhD typing was performed to exclude weak D or partial D samples, whereas our adsorption/elution testing19,25,26 was conducted to distinguish DEL from true D– samples (supplemental Methods, available on the Blood website).
To distinguish patients with Asian-type DEL from patients with DEL with other genetic backgrounds, a genotyping method based on high-resolution melting (HRM) was developed. The new method enabled rapid testing of the distinct Asian-type DEL allele (carrying the c.1227A variant of the RHD gene) within 2.5 hours suitable for routine testing (supplemental Methods). The exact RHD genotype was further determined using hybrid rhesus box analysis27 and/or RHD Sanger sequencing combined with multiplex ligation-dependent probe amplification assays,28 as previously described.29
Clinical trial of D+ RBC transfusion in patients with Asian-type DEL
A single-arm multicenter prospective clinical trial (www.clinicaltrials.gov, #NCT03727230) was organized by the Guangzhou Blood Center, which oversees the entire blood supply and RBC reference testing for 209 hospitals serving 15 million inhabitants of Guangzhou city in China. The blood samples of 2011 patients with the serologic D– phenotype from the 58 cooperating hospitals in Guangzhou city were sent to the RBC reference laboratory of the Guangzhou Blood Center for routine Asian-type DEL typing ahead of potential transfusions. Eligible patients were identified using inclusion and exclusion criteria (supplemental Methods). ABO-matched and D+-compatible RBCs were provided to eligible patients with Asian-type DEL by the Guangzhou Blood Center and transfusion was done at their respective hospitals with written informed consent. During transfusion and in the days of hospitalization, the patients were monitored for any adverse events, such as symptoms of hemolysis, when laboratory biochemical measurements were also performed. Antibody screening was performed in all enrolled patients to evaluate alloanti-D formation using untreated and papain-treated cells (ID-DiaCell I-II-III Asia Kit; BIO-RAD, Cressier, Switzerland). The screening was conducted before transfusion and after transfusion during hospitalization and at all follow-up visits.
The clinical trial was approved by the Institutional Review Board of the Guangzhou Blood Center and monitored by the safety monitoring board appointed by the Guangdong Provincial Blood Safety and Quality Committee. The recruitment began in October 2016 and the trial was closed in November 2021.
Alloanti-D immunization observation in pregnant women with Asian-type DEL
Retrospective alloanti-D analysis in pregnant Chinese women with Asian-type DEL at the nationwide level
A nationwide retrospective study was conducted to detect alloanti-D immunization mostly caused by previous incompatible pregnancy or abortion in 5151 Chinese pregnant women typed D– by preliminary screening from October 2016 to November 2021. Blood samples were sent to the RBC reference laboratory of the Guangzhou Blood Center from different regions of China through a third-party cooperative agency (KingMed Diagnostics, Guangzhou, China) for entrusted testing of Asian-type DEL. The study was approved by the Institutional Review Board of the Guangzhou Blood Center. Individual information including age, ethnicity, place of residence, history of gestation, and Rh immune globulin (RhIG) prophylaxis, was collected. RhIG prophylaxis is still not a routine practice throughout China. Hence, we excluded pregnant women who had previously received RhIG prophylaxis.
Investigation of Asian-type DEL in pregnant women who developed alloanti-D
Blood samples from 127 pregnant Chinese women who had a serologic D– phenotype and already developed alloanti-D without RhIG prophylaxis were sent to the RBC reference laboratory of the Guangzhou Blood Center. The study was approved by the Institutional Review Board of the Guangzhou Blood Center. The anti-D titer was determined, and RhD phenotyping and RHD genotyping were conducted as previously described.29
Analysis of RHD transcripts from individuals with Asian-type DEL
RNA extraction and cDNA synthesis
Peripheral blood samples were collected from 8 individuals with Asian-type DEL. Homogenous erythroblasts from these individuals were cultured from isolated peripheral blood mononuclear cells according to the protocol described by van den Akker et al.30 Total RNA was extracted and reverse-transcribed from both peripheral blood and cultured erythroblasts.
PCR amplification and Sanger sequencing analysis of RHD transcripts covering the c.1227A variant and exon 9
The region from RHD exon 9 to the 3’-untranslated regions (UTRs) covering the c.1227A variant of RHD transcripts was amplified and sequenced by the Sanger method (supplemental Methods).
Nanopore sequencing analysis of full-length RHD transcripts
The entire coding region of RHD cDNA was amplified and analyzed using Nanopore sequencing (Oxford Nanopore Technologies, Oxford, England) (supplemental Methods). The A/G ratio of the c.1227 variant of RHD transcripts was analyzed using SAMtools v1.3.1 35.31
Detection of D antigen epitopes from major RHD transcripts of Asian-type DEL
The 7 most common RHD spliced transcripts detected by Nanopore sequencing were cloned and then transiently transfected into HEK 293T cells individually with the wild-type (wt) RHAG construct. After transfection, the surface expression of the RhD antigen was analyzed using a panel of anti-D mAbs for the systematic detection of D epitopes using flow cytometry (supplemental Methods).
Sample size calculation and statistical analysis
The primary end point was the alloanti-D occurrence rate of patients with Asian-type DEL after D+ RBC transfusion. In a recent meta-analysis, the alloanti-D rate from patients with true D– with similar indications receiving D+ RBC transfusion was estimated to be 28.2% (203/720; 95% confidence interval [CI], 19.0-32.0).32 The primary objective of this trial was to determine whether the primary end point was lower than the target value of 19.0%; the lower limit of the 95% CI of the alloanti-D occurrence rate from the meta-analysis. The trial was ended when 42 patients with Asian-type DEL had longer alloanti-D follow-up (>1 month), fulfilling the requirement for analysis (≥37, assuming an alloanti-D rate of 5%, 90% power, 2-sided at an alpha significance level of 0.05).
In the statistical analyses, the 95% CI of the alloanti-D rate in the trial was calculated using the Clopper-Pearson exact method. The performance goal was achieved if the lower limit of this 95% CI was lower than the target value of 19%. Hypothetical testing for a single proportion was performed using the exact method. All data analyses were conducted using R software (version 3.6.2).
Results
No production of alloanti-D in patients with Asian-type DEL after D+ RBC transfusion
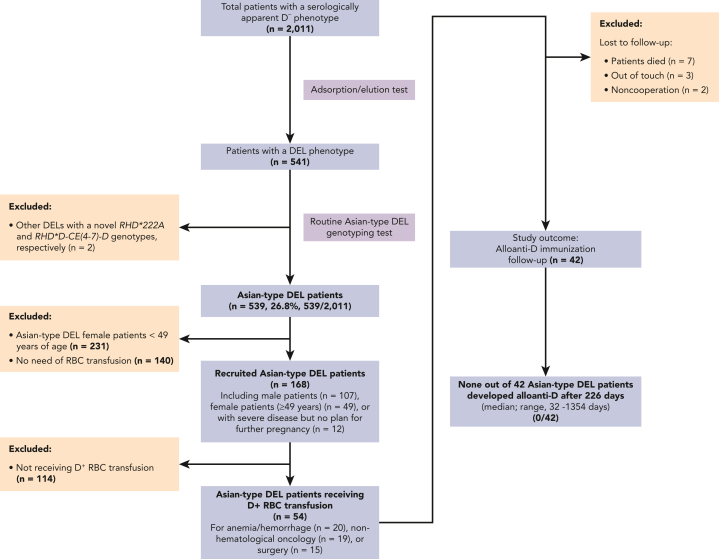
A clinical trial was conducted to address the risk of alloimmunization in patients with Asian-type DEL transfused with D+ RBCs. During the trial, 2011 patients with serologic D– were identified from 58 hospitals in Guangzhou, and 539 patients (26.8%, 539/2011) were identified with Asian-type DEL using the HRM genotyping assay (supplemental Figure 1) and confirmed using genotyping analysis (supplemental Table 1). Before the trial of D+ RBC transfusion, no patients with Asian-type DEL were identified as having alloanti-D (0/539) (supplemental Table 2).
In the trial (Figure 1), a total of 168 patients with Asian-type DEL, including 107 male patients and 61 female patients who met the inclusion criteria, were recruited. Finally, ABO-matched and D+-compatible RBC units were provided by Guangzhou Blood Center and transfused to 54 Asian-type DEL recipients (34 males, mean age, 53.1 ± 25.0 years; 20 females, mean age, 57.3 ± 13.6 years), all of whom were found to be free of alloantibodies before the transfusion (Figure 1; supplemental Table 2). After the D+ RBC transfusion during hospitalization, we did not observe any acute or delayed hemolytic transfusion or nonhemolytic reactions, including allergic or febrile nonhemolytic reactions or circulatory overload. Follow-up of alloantibody detection for longer than one month was completed in 42 of 54 (77.8%) patients with Asian-type DEL (Figure 1). Among these 42 patients (Table 1), 54.8% (23/42) received D+ RBC units once (0.5-8 RBC units/patient), whereas 45.2% (19/42) received D+ RBC units multiple times (2-17 times; in total, 4-28 RBC units/patient).
Figure 1.
Patient enrollment in the clinical trial (NCT03727230). The flow diagram documents the inclusion and exclusion criteria applied and the alloanti-D immunization studied in a cohort of patients with Asian-type DEL receiving D+ RBC transfusions.
Table 1.
Demographics of 42 Asian-type DEL recipients with D+ RBC transfusion and their follow-up examinations
| Characteristic | Data |
|---|---|
| Number of male, female | 30, 12 |
| Male recipient age (y) | |
| Mean ± SD | 51.5 ± 24.9 |
| Median | 58.5 |
| Range | 0.3-86 |
| Female recipient age (y) | |
| Mean ± SD | 52.1 ± 10.8 |
| Median | 51 |
| Range | 30-75 |
| Number of units of D+ RBCs transfused (U) | |
| Mean ± SD | 6.1 ± 5.4 |
| Median | 4 |
| Range | 0.5-28 |
| Transfusion episodes of D+ RBCs (times) | |
| Mean ± SD | 2.6 ± 2.9 |
| Median | 1 |
| Range | 1-17 |
| Number of units of D+ RBCs per episode (U) | |
| Mean ± SD | 2.7 ± 1.4 |
| Median | 2 |
| Range | 0.5-8 |
| Length of serologic follow-up (d) | |
| Mean ± SD | 306.5 ± 264.9 |
| Median | 226 |
| Range | 32-1354 |
| Number of antibody screening test after D+ RBC transfusion | |
| Mean ± SD | 2.2 ± 1.5 |
| Median | 1 |
| Range | 1-7 |
| Transfusion treatment of duration (d) | |
| Mean ± SD | 82.6 ± 238.0 |
| Median | 1 |
| Range | 1-1355 |
| Case of recipients re-exposed to D+ RBCs | 19 |
SD, standard deviation.
Alloanti-D was not detected in these 42 patients (0/42; 95% CI, 0-8.40) who had a median follow-up of 226 days (range, 32-1354 days). The effectiveness of D+ RBC transfusion was recorded in most patients (36/42) with hemoglobin (Hb) value changes or improvements in anemia symptoms (supplemental Table 3).
Alloanti-D was identified only in pregnant women with true D– but not in pregnant women with Asian-type DEL
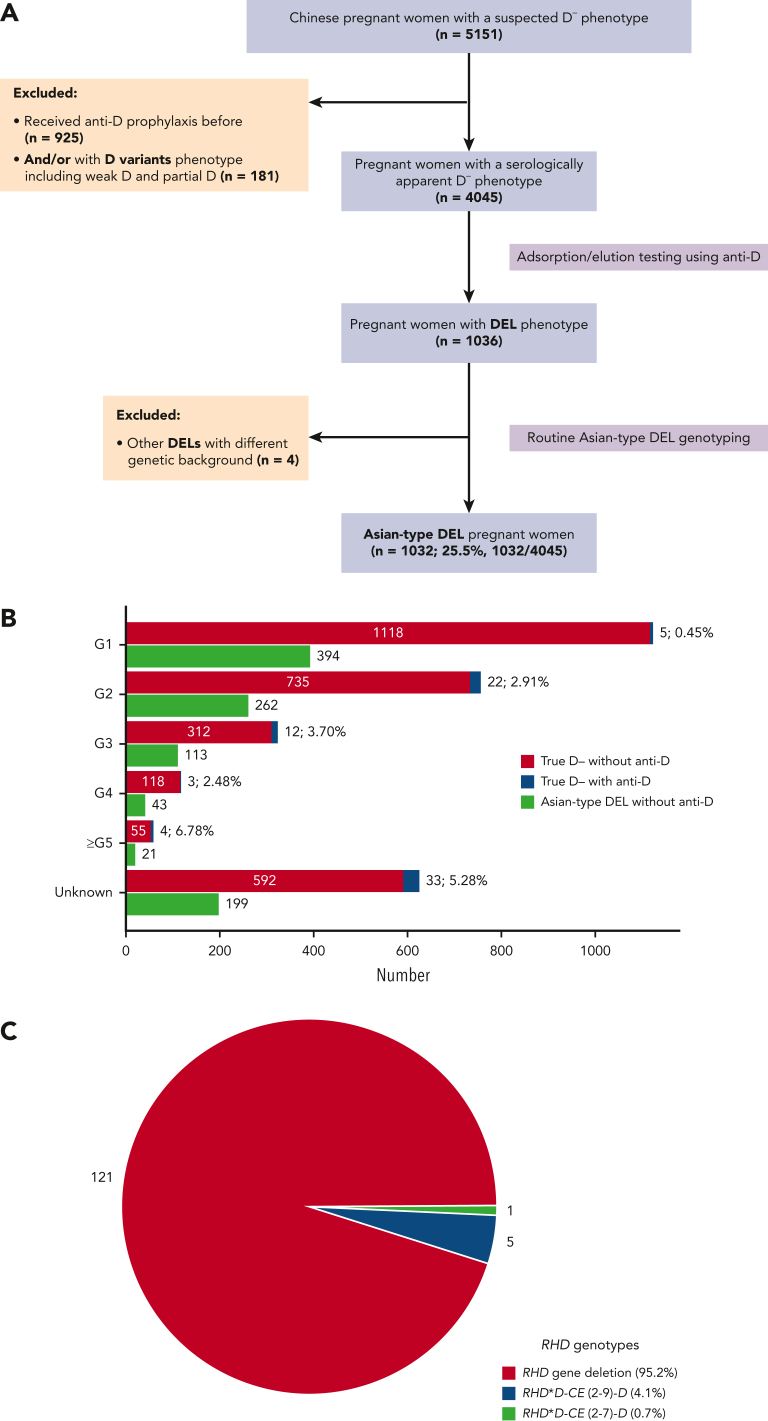
To confirm that there was no alloanti-D immunization in pregnant women with Asian-type DEL, we conducted a large-sample-size retrospective study throughout China (Figure 2A). A total of 4045 pregnant women with a serologic D– phenotype (mean age, 29.4 ± 4.3 years) were recruited from more than 400 medical settings distributed across 30 of 34 administrative regions of China. The majority (99.0%, 4004/4045) were Han Chinese and only 41 represented ethnic minorities (1.0%, 41/4045). A cohort of 1032 cases of pregnancy with Asian-type DEL (25.5%, 1032/4045) were detected, and the exact genotypes were further determined (supplemental Table 1). The alloantibodies against RBC antigens identified in pregnant women with true D– and Asian-type DEL phenotypes are summarized and compared according to gestation time (Figure 2B; details in supplemental Table 2). Overall, 79/3009 cases of alloanti-D (2.63%) were identified in the true D– group, but none were found in the Asian-type DEL group (0/1032). The frequencies were significantly different between the 2 groups (P <.001; χ2 test, 2-sided). The D+ type of the newborn was not confirmed in all serologic D– women; however, there was a high probability of carrying a D+ fetus (>95%) because of the known low frequency of RHD heterozygotes among D+ fathers (less than 7%) in the Chinese population. We concluded that pregnant women with Asian-type DEL are not or are very infrequently immunized by fetal D+ RBCs during pregnancy at a nationwide level in China.
Figure 2.
Detection of alloanti-D in the pregnant women with true D– and Asian-type DEL phenotypes. (A) Flow diagram of enrollment of the pregnant women with serologic D– phenotype. (B) The numbers and percentages of pregnant women, according to the gestational time (G1 to ≥G5), were identified with alloanti-D in the true D– and Asian-type DEL groups. (C) The RHD genotypes of 127 patients who developed alloanti-D. The percentages of RHD gene variants, including deletion and 2 nonfunctional alleles accounting for the true D– phenotype rather than Asian-type DEL, were shown. The gestations with D+ fetus were not prospectively confirmed because >95% of Chinese women with true D– or women with Asian-type DEL carry a D+ fetus.
Absence of Asian-type DEL among pregnant Chinese women who had developed alloanti-D
To further investigate whether anti-D immunization occurred only in patients with true D– but not in patients with Asian-type DEL, the RHD genotype was analyzed in an independent set of 127 pregnant women with alloanti-D immunization (mean age, 32.0 ± 4.9 years). The alloanti-D titers in these pregnant women were between 1:2 and 1:4096 (≤1:16, n = 54; >1:16, 72; not tested, 1). No woman was found to carry the c.1227A variant (Asian-type DEL), and only women lacking the functional RHD gene with a true D– phenotype was identified (Figure 2C). Alternatively, these results confirm that pregnant women with Asian-type DEL carrying D+ fetuses do not develop alloanti-D.
Identification of the full-length RHD transcripts carrying the c.1227A variant in individuals with Asian-type DEL
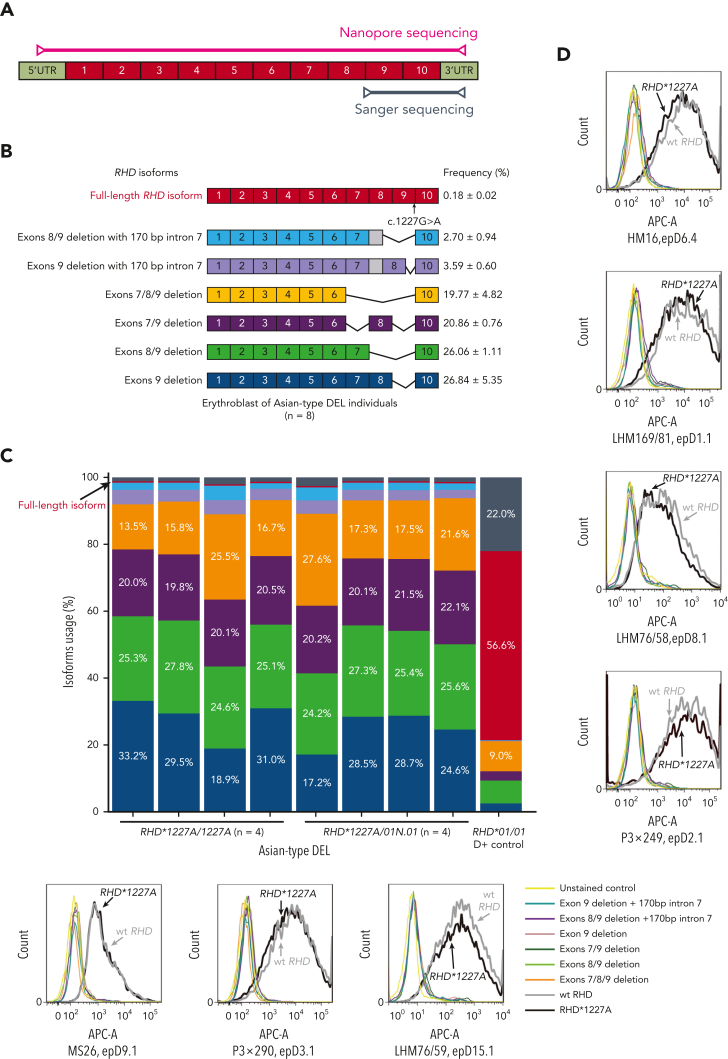
To elucidate the specific serologic and clinical features of individuals carrying the c.1227A variant, we analyzed RHD transcript expression in individuals with Asian-type DEL at the molecular level (Figure 3). First, an RT-PCR product ranging from exon 9 to the 3’-UTR (Figure 3A) could be amplified from the cultured erythroblasts but not in the whole blood samples of individuals with Asian-type DEL (n = 8). Interestingly, the c.1227A variant of RHD was identified in this product using Sanger sequencing (supplemental Figure 2), confirming the observation made previously in a minigene model.22 This result suggested the low abundance of RHD transcripts containing exon 9. Next, the whole structure of the RHD transcript isoforms, ranging from the 5’-UTR to the 3’-UTR, was explored in cultured erythroblasts from individuals with Asian-type DEL (n = 8) using a long-read sequencing approach (Figure 3A). Approximately 200 000 reads were obtained from each sample with an average mapping rate of 97.6% aligned with RHD transcripts (supplemental Table 4). The original Nanopore sequencing data have been deposited at the China National GeneBank DataBase (CNGBdb)33 in its CNGB Sequence Archive (China National Space Administration [CNSA]; accession number CNP0002612).34 We found that the 6 most abundant transcripts lacked exon 9 and accounted for 98.6% of all RHD transcripts (Figure 3B and 3C), which is consistent with previous observations.20,21,23 Importantly, for the first time in primary cells, a full-length RHD transcript isoform carrying the c.1227A variant was detected in all samples at a low abundance (Figure 3B; mean frequency, 0.18% of the total). We determined the sequences of all RHD transcripts including the full-length transcript (supplemental Figure 3) in individuals with Asian-type DEL using Nanopore technology, which aligned with different reference RHD transcripts (supplemental Figure 4; and supplemental Figure 5). The read lengths for the sequences and their accuracy rates in the Nanopore technology platform were documented (supplemental Figure 6; and supplemental Figure 7, respectively). The result was a critical observation suggesting that a normal RhD protein expressing all D epitopes can be translated from this full-length isoform.
Figure 3.
Analysis of RHD transcripts from individuals with Asian-type DEL. (A) The schematic diagram of full-length RHD transcript and the primer positions for Sanger sequencing and Nanopore sequencing analyses. Specifically, the primers for fragment amplification of RHD transcripts and Sanger sequencing located on exon 8 to 9 and 3’-UTRs to cover the c.1227A mutation of RHD and the primers for whole coding region amplification of RHD transcripts and Nanopore sequencing located on 5’-UTRs and 3’-UTRs. (B) Schematic representation of RHD transcripts identified in individuals with Asian-type DEL. The RT-PCR products of full-length RHD coding region, which were amplified using cDNA from the cultured erythroblast of individuals with Asian-type DEL, were analyzed using Nanopore sequencing. The top 7 major types of RHD transcripts identified in individuals with Asian-type DEL are presented. The structural schematic diagram and frequency of RHD transcripts are indicated in the right panel. Different types of transcripts are indicated by various colors. (C) Barplot showing the frequencies for major types of RHD transcripts in each individual with Asian-type DEL (n = 8) or D+ control sample. The color codes for different types of transcripts are identical to those in panel B. The red bar indicates the full-length transcripts, which was identified in all individuals with Asian-type DEL with lower abundance (mean, 0.18%) but higher abundance in D+ control (56.6%). The gray bar indicates other types of transcripts. In the D+ control, 3 major transcripts including the full-length RHD transcript in the red bar, the truncated transcripts with exon 7 deletion (19.0% of total, shown in the gray bar accompanied by several other low-frequency transcripts), or exons 7/8/9 deletion (9.0%) in the orange bar, were identified as shown in one previous study.23 (D) Recognition of RhD antigenic epitopes using anti-D mAbs in vitro. The top 7 types of RHD transcripts identified in Asian-type DEL erythroblasts were cloned into an expression vector and cotransfected into HEK 293T cells with wt RHAG construct. RhD antigen on the transfected cell surface was measured compared with unstained cell negative control and wt RHD-positive control using flow cytometry with 7 different anti-D mAbs.
Expression of complete D antigen by a full-length RHD transcript carrying the c.1227A variant
To determine the expression of the D antigen resulting from the product translated from these isoforms and their respective D epitope profile, the 7 most frequent RHD transcript isoforms (its alignment to reference RHD mRNA shown in supplemental Figure 8) identified using Nanopore sequencing were separately overexpressed in a human cell model. D+ cells were detected only when the full-length RHD∗1227A construct was overexpressed at a level comparable with the wild-type construct (Figure 3D). This result suggests that only the full-length isoform can produce an RhD protein detectable using flow cytometry. Moreover, these cells were all positive for the D epitopes investigated by the anti-D mAb panel (Figure 3D). Our results suggested that the biosynthesized RhD protein carried the full repertoire or at least a large part of the D epitopes expressed by the normal RhD protein, thus representing a full D antigen.
Discussion
Asian-type DEL is the most prevalent DEL variant worldwide.1,10,35 The proper treatment of transfusion recipients and pregnant women carrying the Asian-type DEL phenotype has been a matter of debate since the molecular basis and diversity was discovered more than 20 years ago.11
Early on, D+ RBC transfusion was proposed for all patients with Asian-type DEL,9,12 gaining support when no alloanti-D was detected in a limited sample of pregnant women with Asian-type DEL carrying a D+ fetus.9,13, 14, 15 Later on, there was 1 male patient with Asian-type DEL (transfused with 5, 40, 12, 6, and 15 units of D+ RBCs, single donor platelets, platelet concentrates, fresh-frozen plasma, and cryoprecipitate, respectively, after heart transplantation)16 and another male patient who received 2 units of D+ RBCs in an emergency17; both received no RhIG, and neither showed anti-D immunization on day 348 or 3 months after the D+ RBC transfusions, respectively. Because of this limited clinical evidence, the recommendation to transfuse D+ RBCs into patients with Asian-type DEL has not been widely adopted.
Here, we provide several lines of clinical evidence in strong support that patients with Asian-type DEL may be safely managed as any common D+ recipients. We report a clinical trial with a statistically adequate sample size to address the safety of D+ RBC management in Asian-type DEL transfusion recipients. The upper limit of the 95% CI for alloanti-D incidence (8.40%) in the trial group was far lower than the lower limit of the 95% CI (19.0%) in the true D– control group, as previously estimated in our meta-analysis.32 In a large nationwide retrospective study, we found no anti-D immunization in any pregnant women with Asian-type DEL (n = 1032), despite their high probability of carrying D+ fetuses (>95%) owing to the low prevalence of D heterozygotes (7%) in D+ fathers.29,36 In line with this, no Asian-type DEL but only individuals with true D– were identified in another group of pregnant women who had developed alloanti-D (0/127), which is in agreement with 2 previous independent studies (0/165).9,13 Therefore, our results indicate that patients with Asian-type DEL, perhaps including pregnant women, can safely be transfused with D+ RBCs without the risk of becoming immunized to alloanti-D. Thus, our proposal will considerably simplify the transfusion management of patients with Asian-type DEL to receive the common D+ RBC products in Western countries and East and Southeast Asian countries with a limited supply of D– blood.
Our findings in patients with Asian-type DEL, including pregnant women, support the discontinuation of RhIG prophylaxis and alloanti-D monitoring in women with Asian-type DEL during pregnancy. The implementation of this possible new strategy can be promoted by the routine diagnosis of the Asian-type DEL variant. The new recommendation when applied to pregnant women with Asian-type DEL (∼9000 cases a year in China, commonly 2 doses per individual) would not only save at least 18 000 doses of RhIG per year ($700 per dose, 12.6 million dollars per year)1 but also avoid frequent antibody screening testing for anti-D monitoring ($10 per testing, 10 tests during pregnancy, 900 000 dollars per year) in China alone. This approach might also be implemented in other East and Southeast Asian countries with a dense population and high frequency of Asian-type DEL, such as Korea,2,3 Japan,4 Thailand,5,6 and Myanmar,7 which together include ∼300 000 individuals with Asian-type DEL. The cost–benefit of using Asian-type DEL genotyping to guide RhIG prophylaxis among pregnant women with a serologic D– phenotype is much greater in these countries.1 Implementation can also be explored in countries with large populations of East Asian background, such as the USA with at least 90 000 individuals with Asian-type DEL.10
The mechanism by which individuals with Asian-type DEL tolerate D+ RBCs is still elusive. Previous serologic results suggested that a few D antigens with a complete repertoire of epitopes were expressed on the surface of Asian-type DEL RBCs.18,19 This serologic feature may explain the immune tolerance of patients with Asian-type DEL against D+ RBCs because they carry a normal D antigen albeit at extremely low quantity. To investigate the underlying molecular mechanism, RHD transcript isoforms were analyzed. For D+ control individuals, the majority of full-length RHD transcripts and lesser alternative splicing transcripts were identified.23,37, 38, 39 For individuals with Asian-type DEL carrying the synonymous c.1227G>A variant, located at the last nucleotide position of exon 9 of RHD and known to disrupt splicing drastically, numerous variant RHD mRNA isoforms lacking exon 9 were identified as described in previous studies.20,21,23 However, which transcript could express the complete D antigen in Asian-type DEL RBCs remains controversial. One hypothesis suggested that some truncated RHD transcripts might be translated into RhD proteins with weak antigenicity.20,23
However, in a previous study using a minigene splicing assay in vitro,22 we identified traces of mRNA transcripts that included a full-length RHD exon 9 in the presence of the c.1227A substitution. Based on this result, we hypothesized that a minimal amount of RHD mRNA transcript containing exon 9, which could be the full-length transcript carrying the c.1227A variant, may be biosynthesized and sufficient to be translated into a minimal amount of the normal RhD protein,22 thereby resulting in the Asian-type DEL phenotype. In this study, a full-length RHD transcript containing the c.1227A variant with a low abundance (0.18% of the total) was indeed detected in the erythroblasts of individuals with Asian-type DEL, confirming our hypothesis.22 This full transcript expressed the functional RhD antigen epitopes recognized with 7 different anti-D mAbs in an expression study. Thus, a low level of full-length RHD transcript can contribute to expressing RhD antigen with a complete repertoire of epitopes in individuals with Asian-type DEL in sufficient quantity to prevent alloanti-D immunization after D+ RBC immune stimulation, thereby bridging the gap between clinical and molecular mechanisms.
Our study also has some limitations. Concerning the follow-up time of alloanti-D in patients with Asian-type DEL after D+ RBC transfusion, not all patients had regular and long-term follow-up visits. Primary alloanti-D immunization in D– recipients commonly occurs between 2 and 3 months after D+ RBC transfusion, and secondary alloanti-D immunization generally occurs within 1 month.32 Once alloanti-D is produced, disappearance is unlikely.40 Hence, we chose to follow the patients for more than 3 months to avoid false negative results if the anti-D immunization was delayed. In our study, antibody screening was performed after less than 3 months in 5 patients, which may have missed potential anti-D immunization in these cases. However, the analysis requirements were still satisfied, even after the exclusion of these cases.
The management of Asian-type DEL donors and their RBC donations is another important topic for blood transfusion services, especially in East Asian countries. Asian-type DEL RBCs can elicit primary or secondary alloanti-D immunization in true D– recipients, albeit rarely with fewer than 10 case reports to date.41 If we label DEL RBCs as D+ RBCs, as is practiced by several blood services in Europe, the United States, and Brazil, all alloanti-D immunization possibly caused by Asian-type DEL RBCs will be consistently avoided.10 For Asian countries, this expedient approach would absolutely exacerbate the shortage of D– blood. For the decision process, properly powered prospective studies are needed to determine the exact incidence of alloanti-D immunization caused by Asian-type DEL RBC transfusions in true D– patients.
In summary, we have provided several lines of strong clinical, epidemiological, and molecular functional evidence that individuals with Asian-type DEL do not produce alloanti-D when exposed to D+ RBCs during pregnancy or transfusion. Based on our findings, at least in China, we propose an amendment to the recommendations and that the use of common D+ blood for transfusions in all patients with Asian-type DEL be considered, that RhIG immunoprophylaxis in women with Asian-type DEL is dropped, and that anti-D immunization monitoring during any Asian-type DEL pregnancy is stopped.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors thank the participating patients and their families for their dedication and the doctors working at the local hospitals for their support, without whom this research would not have been possible. Clinical trial NCT03727230 was initiated, planned, conducted, analyzed, and published by the investigators without financial support from any commercial entities. The main recruitment strategy was to guarantee the blood supply of participants with Asian-type DEL, especially with a need for massive or long-term transfusion rather than using the rare D– blood, which is often in short supply in East and Southeast Asian countries including China. The authors also thank Erwei Song (Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China), Jean-Pierre Allain (Department of Hematology, University of Cambridge, Cambridge, UK), Tobias J. Legler (Department of Transfusion Medicine, University Medical Center Göttingen, Göttingen, Germany), Limin Zheng (School of Life Sciences, Sun Yat-sen University, Guangzhou, China), and Li Xu (Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China) for their valuable suggestions and careful revision in the preparation of this manuscript.
This work was supported by funding from the National Natural Science Foundation of China (grants 81900184 and 82070198); the Key Medical Disciplines and Specialties Program of Guangzhou (2021-2023), Guangdong, China; and the Intramural Research Program (ZIC CL002128) of the National Institutes of Health Clinical Center.
Authorship
Contribution: Y.F., R.Z., C.L., Y.J., J.W., Y.F., C.S., and W.A.F. designed the study; C.O. and J.C. performed the statistical analysis; Y.J., Y.L., and J.W. drafted the manuscript; Y.F., R.Z., C.L., Y.F., S.S., and W.A.F. performed critical revisions of the manuscript; and all authors contributed to the manuscript and interpretation of the data, and approved the final version of the manuscript.
Footnotes
∗Y.J., Y.L., and J.W. are joint first authors.
†Y. Fu, R.Z., and C.L. are joint senior authors.
Part of retrospective result of alloanti-D immunization observation in Asian-type DEL pregnant women was presented in abstract form at the virtual meeting of the 36th International ISBT Congress, 12 December 2020 to 16 December 2020.
Data are available on request from the corresponding author, Yongshui Fu (fuyongshui@gzbc.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Yin Q, Flegel WA. DEL in China: the D antigen among serologic RhD-negative individuals. J Transl Med. 2021;19(1):439. doi: 10.1186/s12967-021-03116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JY, Kim SY, Kim CA, Yon GS, Park SS. Molecular characterization of D- Korean persons: development of a diagnostic strategy. Transfusion. 2005;45(3):345–352. doi: 10.1111/j.1537-2995.2005.04311.x. [DOI] [PubMed] [Google Scholar]
- 3.Luettringhaus TA, Cho D, Ryang DW, Flegel WA. An easy RHD genotyping strategy for D- East Asian persons applied to Korean blood donors. Transfusion. 2006;46(12):2128–2137. doi: 10.1111/j.1537-2995.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogasawara K, Suzuki Y, Sasaki K, et al. Molecular basis for D- Japanese: identification of novel DEL and D- alleles. Vox Sang. 2015;109(4):359–365. doi: 10.1111/vox.12290. [DOI] [PubMed] [Google Scholar]
- 5.Srijinda S, Suwanasophon C, Visawapoka U, Pongsavee M. RhC phenotyping, adsorption/elution test, and SSP-PCR: the combined test for d-elute phenotype screening in Thai RhD-negative blood donors. ISRN Hematol. 2012;2012:358316. doi: 10.5402/2012/358316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thongbut J, Laengsri V, Raud L, et al. Nation-wide investigation of RHD variants in Thai blood donors: impact for molecular diagnostics. Transfusion. 2021;61(3):931–938. doi: 10.1111/trf.16242. [DOI] [PubMed] [Google Scholar]
- 7.Wah ST, Chi SN, Kyaing KK, Khin AA, Aung T. Serological detection of Rh-Del phenotype among Rh-negative blood donors at National Blood Center, Yangon, Myanmar. Adv Hematol. 2020;2020:3482124. doi: 10.1155/2020/3482124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniels G. Variants of RhD--current testing and clinical consequences. Br J Haematol. 2013;161(4):461–470. doi: 10.1111/bjh.12275. [DOI] [PubMed] [Google Scholar]
- 9.Shao CP. Transfusion of RhD-positive blood in “Asia type” DEL recipients. N Engl J Med. 2010;362(5):472–473. doi: 10.1056/NEJMc0909552. [DOI] [PubMed] [Google Scholar]
- 10.Flegel WA, Wagner FF., DEL Blood Transfus. 2020;18(3):159–162. doi: 10.2450/2020.0296-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner FF, Frohmajer A, Flegel WA. RHD positive haplotypes in D negative Europeans. BMC Genet. 2001;2:10. doi: 10.1186/1471-2156-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegel WA. Response to: are weak D RBCs really immunogenic? Transfusion. 2006;46(6):1063–1064. doi: 10.1111/j.1537-2995.2006.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QP, Dong GT, Wang XD, et al. An investigation of secondary anti-D immunisation among phenotypically RhD-negative individuals in the Chinese population. Blood Transfus. 2014;12(2):238–243. doi: 10.2450/2013.0184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Wang BL, Xu W, et al. Anti-D alloimmunisation in pregnant women with DEL phenotype in China. Transfus Med. 2015;25(3):163–169. doi: 10.1111/tme.12211. [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Zhu M, Wang BL, Su H, Wang M. Prospective evaluation of a transfusion policy of RhD-positive red blood cells into DEL patients in China. Transfus Med Hemother. 2015;42(1):15–21. doi: 10.1159/000370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Chun S, Seo JY, Yang JH, Cho D. Planned transfusion of D-positive blood components in an Asia type DEL patient: proposed modification of the Korean National Guidelines for Blood Transfusion. Ann Lab Med. 2019;39(1):102–104. doi: 10.3343/alm.2019.39.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JY, Cho D, Choi HW, et al. A patient with RhDel (1227G>A) failed to produce detectable anti-D after transfusion of RhD positive red blood cells. Korean J Blood Transfus (in Korean) 2006;17(2):153–158. [Google Scholar]
- 18.Kormoczi GF, Gassner C, Shao CP, Uchikawa M, Legler TJ. A comprehensive analysis of DEL types: partial DEL individuals are prone to anti-D alloimmunization. Transfusion. 2005;45(10):1561–1567. doi: 10.1111/j.1537-2995.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 19.Gu J, Sun AY, Wang XD, et al. Analysis of density and epitopes of D antigen on the surface of erythrocytes from DEL phenotypic individuals carrying the RHD1227A allele. Blood Transfus. 2014;12(2):244–249. doi: 10.2450/2013.0091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao CP, Xiong W, Zhou YY. Multiple isoforms excluding normal RhD mRNA detected in Rh blood group Del phenotype with RHD1227A allele. Transfus Apher Sci. 2006;34(2):145–152. doi: 10.1016/j.transci.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Liu HC, Eng HL, Yang YF, et al. Aberrant RNA splicing in RHD 7-9 exons of DEL individuals in Taiwan: a mechanism study. Biochim Biophys Acta. 2010;1800(6):565–573. doi: 10.1016/j.bbagen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Fichou Y, Gehannin P, Corre M, et al. Extensive functional analyses of RHD splice site variants: insights into the potential role of splicing in the physiology of Rh. Transfusion. 2015;55(6 Pt 2):1432–1443. doi: 10.1111/trf.13083. [DOI] [PubMed] [Google Scholar]
- 23.Chen DP, Sun CF, Ning HC, Wang WT, Tseng CP. Comprehensive analysis of RHD splicing transcripts reveals the molecular basis for the weak anti-D reactivity of Del -red blood cells. Transfus Med. 2016;26(2):123–129. doi: 10.1111/tme.12270. [DOI] [PubMed] [Google Scholar]
- 24.Flegel WA, Denomme GA, Queenan JT, et al. It’s time to phase out “serologic weak D phenotype” and resolve D types with RHD genotyping including weak D type 4. Transfusion. 2020;60(4):855–859. doi: 10.1111/trf.15741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Hou L, Guo ZH, Ye LY, Yue DQ, Zhu ZY. Molecular basis of the RHD gene in blood donors with DEL phenotypes in Shanghai. Vox Sang. 2009;97(2):139–146. doi: 10.1111/j.1423-0410.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 26.Flegel WA, von Zabern I, Wagner FF. Six years’ experience performing RHD genotyping to confirm D- red blood cell units in Germany for preventing anti-D immunizations. Transfusion. 2009;49(3):465–471. doi: 10.1111/j.1537-2995.2008.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner FF, Flegel WA. RHD gene deletion occurred in the Rhesus box. Blood. 2000;95(12):3662–3668. [PubMed] [Google Scholar]
- 28.Haer-Wigman L, Veldhuisen B, Jonkers R, et al. RHD and RHCE variant and zygosity genotyping via multiplex ligation-dependent probe amplification. Transfusion. 2013;53(7):1559–1574. doi: 10.1111/j.1537-2995.2012.03919.x. [DOI] [PubMed] [Google Scholar]
- 29.Ji YL, Luo H, Wen JZ, et al. RHD genotype and zygosity analysis in the Chinese Southern Han D+, D- and D variant donors using the multiplex ligation-dependent probe amplification assay. Vox Sang. 2017;112(7):660–670. doi: 10.1111/vox.12554. [DOI] [PubMed] [Google Scholar]
- 30.van den Akker E, Satchwell TJ, Pellegrin S, Daniels G, Toye AM. The majority of the in vitro erythroid expansion potential resides in CD34(-) cells, outweighing the contribution of CD34(+) cells and significantly increasing the erythroblast yield from peripheral blood samples. Haematologica. 2010;95(9):1594–1598. doi: 10.3324/haematol.2009.019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Y, Luo G, Fu Y. Incidence of anti-D alloimmunization in D-negative individuals receiving D-positive red blood cell transfusion: a systematic review and meta-analysis. Vox Sang. 2022;117(5):633–640. doi: 10.1111/vox.13232. [DOI] [PubMed] [Google Scholar]
- 33.Chen FZ, You LJ, Yang F, et al. CNGBdb: China National GeneBank DataBase. Hereditas. 2020;42(8):799–809. doi: 10.16288/j.yczz.20-080. [DOI] [PubMed] [Google Scholar]
- 34.Guo X, Chen F, Gao F, et al. CNSA: a data repository for archiving omics data. Database (Oxford) 2020;2020 doi: 10.1093/database/baaa055. article ID baaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao CP, Maas JH, Su YQ, Kohler M, Legler TJ. Molecular background of Rh D-positive, D-negative, D(el) and weak D phenotypes in Chinese. Vox Sang. 2002;83(2):156–161. doi: 10.1046/j.1423-0410.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 36.Shao CP, Qin JJ, Sun GD, Xu HX, Ma LF. An analysis of RHD zygosity of Rh(D)-positive Chinese Han population. Chin J Med Genet (in Chinese) 2011;28(1):29–32. doi: 10.3760/cma.j.issn.1003-9406.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Arce MA, Thompson ES, Wagner S, Coyne KE, Ferdman BA, Lublin DM. Molecular cloning of RhD cDNA derived from a gene present in RhD-positive, but not RhD-negative individuals. Blood. 1993;82(2):651–655. [PubMed] [Google Scholar]
- 38.Suyama K, Lunn R, Haller S, Goldstein J. Rh(D) antigen expression and isolation of a new Rh(D) cDNA isoform in human erythroleukemic K562 cells. Blood. 1994;84(6):1975–1981. [PubMed] [Google Scholar]
- 39.Westhoff CM, Wylie DE. Identification of a new RhD-specific mRNA from K562 cells. Blood. 1994;83(10):3098–3100. [PubMed] [Google Scholar]
- 40.Tormey CA, Stack G. The persistence and evanescence of blood group alloantibodies in men. Transfusion. 2009;49(3):505–512. doi: 10.1111/j.1537-2995.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 41.Sandler SG, Flegel WA. Does transfusion of Asian-type DEL red blood cells to D-recipients cause D alloimmunization? Transfusion. 2019;59(7):2455–2458. doi: 10.1111/trf.15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.