Abstract
Background
The microbiome-gut-brain-axis (MGBA) is emerging as an important mechanistic link between diet and mental health. The role of significant modifiers of the MGBA, including gut microbial metabolites and systemic inflammation, in individuals comorbid with obesity and mental disorders, is under-investigated.
Objectives
This exploratory analysis examined associations among microbial metabolites—fecal SCFAs, plasma inflammatory cytokines, and diet with depression and anxiety scores in adults comorbid with obesity and depression.
Methods
Stool and blood were obtained from a subsample (n = 34) of participants enrolled in an integrated behavioral intervention for weight loss and depression. Pearson partial correlation and multivariate analyses determined associations among changes in fecal SCFAs (propionic, butyric, acetic, and isovaleric acids), plasma cytokines [C-reactive protein, interleukin 1 beta, interleukin 1 receptor antagonist (IL-1RA), interleukin 6, and TNF-α], and 35 dietary markers over 2 mo, and changes in SCL-20 (Depression Symptom Checklist 20-item) and GAD-7 (Generalized Anxiety Disorder 7-Item) scores over 6 mo.
Results
Changes in the SCFAs and TNF-α at 2 mo were positively associated (standardized coefficients: 0.06–0.40; 0.03–0.34) with changes in depression and anxiety scores at 6 mo, whereas changes in IL-1RA at 2 mo were inversely associated (standardized coefficients: –0.24; –0.05). After 2 mo, changes in 12 dietary markers, including animal protein, were associated with changes in SCFAs, TNF-α, or IL-1RA at 2 mo (standardized coefficients: –0.27 to 0.20). Changes in 11 dietary markers, including animal protein, at 2 mo were associated with changes in depression or anxiety symptom scores at 6 mo (standardized coefficients: –0.24 to 0.20; –0.16 to 0.15).
Conclusions
Gut microbial metabolites and systemic inflammation may be biomarkers of importance within the MGBA, linking dietary markers, such as animal protein intake, to depression and anxiety for individuals with comorbid obesity. These findings are exploratory and warrant replication.
Keywords: microbiome-gut-brain axis, short-chain fatty acids, depression, anxiety, obesity, inflammatory cytokines, animal protein
Introduction
Depression and anxiety are highly comorbid and the most common neuropsychiatric illnesses, affecting approximately 280 and 284 million people worldwide, respectively [1]. Additionally, these disorders are significantly prevalent in individuals with obesity [2]. Unfortunately, effective integrated treatments are lacking, and mechanistic understanding of these neuropsychiatric illnesses, alone or in comorbidities with obesity, are poorly understood.
Research on mechanistic processes involved in depression and anxiety shifted toward understanding diet-related linkages to mental disorders and the microbiome–gut-brain axis (MGBA) [3]. The MGBA is a complex bidirectional communication system involving neural, endocrine, and immune pathways along with gut microbiota and metabolic functions [4]. Specifically, the gut microbiota is implicated in producing neurotransmitters, essential vitamins, amino acid metabolites, secondary bile acids, and SCFAs. In addition, these microbial-produced metabolites modulate brain health and behavior via the immune system [4, 5].
Diet is the predominant factor in gut microbiota establishment, composition, and function [6, 7], and roughly 50% of the variation in the gut microbiome is related to dietary changes [7, 8]. Moreover, gut microbiome dysbiosis is associated with mental disorders, including depression and anxiety [9]. However, mechanistic researches on the linkage between mental disorders, diet-gut microbiota, and inflammation are in its infancy, with studies showing fecal SCFAs, and gut microbiome metabolites produced from fiber fermentation were lower in depressed individuals than in controls [[10], [11], [12]]. Furthermore, SCFAs have beneficial anti-inflammatory and mental health properties by inducing T-cell differentiation, controlling inflammatory cytokine production, and influencing serotonin and other neurotransmitter production [12]. Therefore, microbial fermentation of dietary fiber may play an essential role in the pathophysiology of depression by modulating inflammation and neurotransmitters, thereby regulating mood. However, current research lacks explorations of the relationships between diet, gut microbiome, and depressive and anxiety symptoms in individuals with obesity comorbid with depression and associated anxiety.
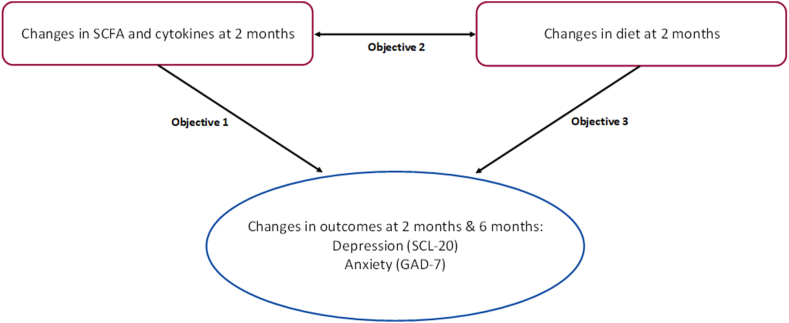
Our recent randomized clinical trial, ENGAGE-2 (Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes in a randomized controlled trial-Phase 2) was designed to elucidate potential multi-system mechanisms underlying a 6-month integrated collaborative care intervention for comorbid obesity and depression [13]. Findings from ENGAGE-2 showed significant treatment effects on depressive and anxiety symptoms but not on BMI after the primary endpoint of 6 mo [14]. Here, we leverage blood and stool samples collected from an ENGAGE-2 subsample (n = 34) to explore the potential mechanistic relationship between dietary behavior change, MGBA, and depression and anxiety symptoms. Based on our conceptual framework (Figure 1), this exploratory study examines if changes in 1) fecal SCFAs and plasma inflammatory cytokines at 2 mo are associated with changes in depressive and anxiety symptoms at 2 and 6 mo; 2) dietary markers at 2 mo are associated with changes in fecal SCFAs and plasma inflammatory cytokines at 2 mo; and 3) dietary markers are associated with depressive and anxiety symptoms at 2 and 6 mo.
FIGURE 1.
Conceptual framework of this study. GAD-7, Generalized Anxiety Disorder 7-Item Scale; SCL-20, Depression Symptom Checklist-20.
Methods
Study design and intervention
This study was approved by the Institutional Review Boards for the University of Illinois at Chicago (UIC) and Stanford University. All the study procedures were conducted in accordance with the Declaration of Helsinki of 1975, as revised in 1983. All participants provided written informed consent. This clinical trial is registered at clinicaltrials.gov (NCT03841682).
This substudy was conducted among a subsample of ENGAGE-2 participants (n = 34) who consented to have their stool and blood specimens collected at baseline and 2 mo (Supplemental Figure 1). The rationale, study design, and main findings for the ENGAGE-2 trial have been described in detail previously [13, 14]. Briefly, the ENGAGE-2 trial was conducted from March 1, 2019, to August 31, 2020, with data assessments at baseline, 2 mo, and 6 mo, with 6 mo as the study’s primary endpoint. Study participants for the parent study were recruited from the Outpatient Care Center at the University of Illinois Hospital and Health Sciences System in Chicago. Eligible participants included patients who were 18 y or older, obese (BMI ≥30, ≥27 if Asian), and depressed [Patient Health Questionnaire-9 (PHQ-9) score ≥10]. Individuals with certain psychiatric (i.e., bipolar disorders or psychotic) or significant medical comorbidities (i.e., cardiovascular disease or diabetes mellitus), or special life circumstances (planned relocation or pregnancy) were ineligible. In addition, patients with magnetic resonance imaging contraindications (i.e., tumor or other known structural abnormality in the brain, traumatic brain injury, or weight >147 kg because of scanner constraints) were ineligible. A total of 106 adults with a mean age of 47.0 y (SD, 11.9) and comorbid obesity and moderate to severe depressive symptoms were enrolled. Participants were randomized using a 2:1 ratio to receive the Integrated Coaching for Better Mood and Weight version 2 (I-CARE2) intervention (n = 71) or usual care (n = 35). Randomization balancing factors included baseline characteristics of age, sex, self-identified race/ethnicity, education, BMI, Depression Symptom Checklist 20-item (SCL-20) score, and current antidepressant medication (Yes/No).
The I-CARE2 intervention combines the Group Lifestyle Balance program [[15], [16], [17]] for weight loss and the PEARLS program [[18], [19]] which is a collaborative stepped depression care approach that utilizes Problem-Solving Therapy (PST) and behavioral activation strategies as the primary therapy, with antidepressant medications as needed. The 6-month I-CARE2 intervention included 6 in-person individual PST sessions in the first 2 mo and 3 additional PST sessions, and 11 home-based self-study Group Lifestyle Balance videos for the following 4 mo [13, 14].
Participants for this substudy were included if they had biomarker and dietary data collected at 2 mo, as the COVID-19 pandemic limited the collection of biospecimen samples at 6 mo. Therefore, participants without 2-month biomarker and dietary data were excluded from this analysis. Additionally, data analysis focused on examining associations with clinical outcome changes after 6 mo (as the primary endpoint of interest) with biomarker and dietary marker changes after 2 mo.
Clinical outcomes
Depression and anxiety were assessed at baseline, 2 mo, and 6 mo. We used SCL-20 as opposed to PHQ-9 for outcome assessment because PHQ-9 was used for screening and for progress monitoring only for the intervention group, whereas SCL-20 was administered at the same time point regardless of randomization. In addition, SCL-20 is a more sensitive assessment tool than PHQ-9, and it measures the perceived impact of symptoms, whereas PHQ-9 measures symptom frequency [20]. The SCL-20 scores range from 0 to 4, with higher scores indicating more severe depressive symptoms [21]. Anxiety symptoms were measured using the self-assessed Generalized Anxiety Disorder 7-Item (GAD-7) Scale. GAD-7 scores range from 0 to 21, with higher scores representing more severe anxiety levels [22]. Biomarkers (SCFAs and cytokines) and dietary markers from baseline to 2 mo were used to predict changes in depression and anxiety outcomes from baseline to 6 mo.
Assessment of Fecal SCFAs, Plasma Cytokines, and Dietary Intake Markers
Fecal SCFAs, plasma cytokines, and dietary intake markers were assessed at baseline and 2 mo. At baseline, 59 participants had fecal SCFAs, plasma cytokines, and diet data, except for 1 participant that did not collect a stool sample. At 2 mo, 34 participants had fecal SCFAs and plasma cytokines, and 52 had dietary intake data. A full list of the fecal, blood, and dietary markers examined is shown in Supplemental Table 1.
Fecal SCFAs and Plasma Cytokines
Rectal swabs containing stool samples for the SCFAs assessment were collected by the participants at the UIC Clinical Research Center or home [23] and submitted for processing within 24 h. For home collection, participants stored the collected samples in their freezer until transferred to UIC for processing. The collection kit included 2 rectal swabs, gloves, instructions, and a commode specimen collection system (Minigrip LabGuard). Participants collected samples via rectal swabs or collected stool in the commode specimen system and dipped the swab in the collected stool. Analysis of the swabs for the SCFAs — propionic, acetic, isovaleric, and butyric acid — was performed with liquid chromatography with tandem mass spectrometry, LC-MS/MS, with support from the UIC Mass Spectrometry Core in the UIC Research Resources Center. SCFAs were extracted from swabs using MeOH:H2O (1:1, v/v) (Sigma-Aldrich), derivatized using 200 mM 3-nitrophenylhydrazine hydrochloride in 50% aqueous MeOH with 120 mM ethylene dichloride (Sigma-Aldrich) and ran on an Agilent Single Quad 1290 gas chromatograph-mass spectroscope ultra-high-performance liquid chromatography, UHPLC, system. Standard compounds (propionic, acetic, isovaleric, and butyric acid) were dissolved in H2O:MeOH (1:1, v:v), and standard curves were created with 9 points (0.5, 1, 5, 10, 25, 50, 100, 200, and 500 μM). Internal standards consisting of 13C isotopes of each respective SCFA were used to determine extraction efficiency. Agilent MassHunter, the qualitative analysis software, was used to extract the ion chromatograms. The total amount of each SCFA present in each sample was determined by calculating the area under the curve and comparing it to its respective standard curve. All values were normalized to the total mass of the samples.
Plasma for assessment of cytokines was obtained by venipuncture with participants. Fasting was not required. Commercially available ELISA kits were used (R & D Systems, Inc.) to quantify human CRP, interleukin 1 beta (IL-1β), interleukin 6, interleukin 1 receptor antagonist (IL-1RA), and TNF-α.
Rectal swabs and plasma samples were stored at –80˚C until analysis.
Dietary intake markers
Dietary intake was captured and analyzed using multiple 24-h diet recalls [24] (2 weekdays and 1 weekend day, with the majority within 1–2 wks) with the Windows-based Nutrition Data System for Research software, version 2018 (University of Minnesota). Thirty-five a priori dietary variable, including the DASH score, calories, fats, protein, fruit and vegetable intake, micronutrients, alcohol consumption, fiber, and certain phytochemicals (see the full list in Supplemental Table 1) were selected for inclusion in the analysis based on consultation with team experts in nutrition, gut microbiome, and neuropsychology research. Overall diet quality was assessed using the DASH concordance index [25]. This DASH index consists of 9 nutrient targets (i.e., total fat, saturated fat, cholesterol, total protein, fiber, magnesium, calcium, sodium, and potassium); DASH scores range from 0 to 9 points, with higher scores indicating higher diet quality.
Statistical analysis
The sample size was designed for exploratory analysis of the biospecimen collection from the parent trial with the proposed selection of 60 participants [13], of which 59 participants were included at baseline. However, data from participants in both the intervention and usual care control groups of the parent were eventually combined for the statistical analysis because of the limited number of participants completing biospecimen sample collection at 2 mo (n = 34) because of the COVID-19 pandemic shutdown.
Fecal SCFA and plasma inflammatory cytokine data were log-transformed, and dietary data were standardized per 1000 cal intake. To fulfill each study objective, we conducted 2 sets of analyses using a stepwise variable selection process. First, Pearson partial correlation coefficients were generated to measure the linear relationships between the changes in 1) individual biomarkers (i.e., fecal SCFA and plasma proinflammatory cytokines) at 2 mo and clinical outcomes at 2 and 6 mo, 2) dietary markers and biomarkers at 2 mo, and 3) dietary markers at 2 mo and clinical outcomes at 2 and 6 mo. Correlations were adjusted for age, sex, and treatment group. To aid in transparent interpretation, we reported a false discovery rate (FDR) [26, 27] corrected P value with a cutoff threshold of <0.20, in conjunction with the unadjusted P value, for the Pearson partial correlation. However, due to the exploratory nature of this study, significant correlations were identified by having an unadjusted P < 0.05. Thus, the significant biomarkers and dietary markers were selected as variables for the next step. Second, partial least squares (PLS) analysis was used to further identify linear combinations (factors) of 1) changes in biomarkers at 2 mo as predictors of changes in clinical outcomes at 2 and 6 mo, 2) changes in dietary markers at 2 mo associated with changes in biomarkers at 2 mo, 3) changes in dietary markers at 2 mo as predictors of changes in clinical outcomes at 2 and 6 mo. The factors were used to explain the dependent variable and predictor variation. Variable importance plots and centered and scaled parameter estimates were generated. All significant association results (P < 0.05) are reported, and only larger coefficient loadings that were ≥0.2 or ≤–0.2 for the PLS analysis were discussed.
All analyses were conducted using SAS, version 9.4 (SAS Institute Inc.).
Results
Participant characteristics
A total of 34 (57.6%) participants completed biospecimen collection at 2 mo and were included in this analysis (Supplemental Figure 1). Of the 34 participants, the mean age was 47 y (SD, 11.2), 68% were female, 56% self-identified as African American, 18% as Hispanic, 85% had some college or postcollege education, and 62% reported an annual family income <$55,000 (Table 1). On average, participants had class 2 obesity [mean BMI, 36.6 (SD, 6.2)], moderate depression [mean PHQ-9 score, 12.3 (SD, 2.3); mean SCL-20 score, 1.0 (SD, 0.7)], mild anxiety [mean GAD-7 score 5.0 (SD, 4.0)], and low diet quality [mean DASH score, 1.6 (SD, 0.9)]. Among the 34 participants, 16 and 18 had 2, and 3 diet recalls, respectively, at baseline; 3, 21, and 10 had 1, 2, and 3 diet recalls, respectively, at 2 mo. The mean (SD) days between the first and last diet recalls was 13.53 (8.47) at baseline and 12.68 (13.79) at 2 mo, with 58.82% having at least 2 diet recalls within the same week at baseline and 77.42% having at least 2 diet recalls within the same week at 2 mo. Compared with those that were not included in this analysis because of participant withdrawal and absence of biospecimen samples resulting from the COVID-19 shutdown, participants in this substudy exhibited significantly lower SCL-20 (P = 0.01) and GAD-7 (P = 0.004) scores, and lower use of antidepressant medication (P = 0.03). Out of the 34 participants, 88% were comorbid with moderate depression (PHQ-9 score 10–14); and 88% experienced minimal to mild anxiety (GAD-7 score <10) (Table 1).
TABLE 1.
Characteristics of study participants, collected at baseline1
| Characteristic | Participants included (n = 34) | Participants not included (n = 72) | P |
|---|---|---|---|
| Age, y2 | 46.8 ± 11.2 | 47.0 ± 12.3 | 0.93 |
| Female, n (%)2 | 23 (67.7) | 58 (80.6) | 0.14 |
| Race/ethnicity (self-identified), n (%) | |||
| Non-Hispanic White | 6 (17.7) | 13 (18.1) | 0.74 |
| African American | 19 (55.9) | 39 (54.2) | |
| Asian/Pacific Islander | 0 (0.0) | 2 (2.8) | |
| Hispanic | 6 (17.7) | 15 (20.8) | |
| Other (e.g., decline to state, multirace) | 3 (8.8) | 3 (4.2) | |
| Education, n (%) | |||
| High school/GED or less | 5 (14.7) | 9 (12.5) | 0.78 |
| College - 1 y – 3 y | 13 (38.2) | 30 (41.7) | |
| College - 4 y or more | 11 (32.4) | 18 (25.0) | |
| Postcollege | 5 (14.7) | 15 (20.8) | |
| Income, n (%) | |||
| <$35,000 | 13 (38.2) | 21 (29.2) | 0.36 |
| $35,000 to <$55,000 | 8 (23.5) | 18 (25.0) | |
| $55,000 to <$75,000 | 2 (5.9) | 13 (18.1) | |
| ≥$75,000 | 11 (32.4) | 20 (27.8) | |
| BMI, kg/m2 | 36.6 ± 6.2 | 37.3 ± 6.0 | 0.57 |
| Weight, kg | 101.3 ± 13.3 | 101.6 ± 16.1 | 0.93 |
| Waist circumference, cm | 111.8 ± 12.7 | 113.2 ± 12.6 | 0.61 |
| PHQ-9 score | 12.3 ± 2.3 | 13.1 ± 3.0 | 0.14 |
| 10–14 (moderate depression), n (%) | 30 (88.0) | 51 (70.8) | 0.13 |
| 15–19 (moderately severe depression), n (%) | 3 (8.8) | 18 (25.0) | |
| ≥20 (severe depression), n (%) | 1 (2.9) | 3 (4.2) | |
| SCL-20 score | 1.0 ± 0.7 | 1.3 ± 0.6 | 0.013 |
| GAD-7 score | 5.0 ± 4.0 | 7.9 ± 4.9 | 0.0043 |
| 0–4 (minimal anxiety), n (%) | 15 (44.1) | 28 (38.9) | 0.08 |
| 5–9 (mild anxiety), n (%) | 15 (44.1) | 19 (26.4) | |
| 10–14 (moderate anxiety), n (%) | 3 (8.8) | 20 (27.8) | |
| 15–21 (severe anxiety), n (%) | 1 (2.9) | 5 (6.9) | |
| Current use of ADM, n (%) | 2 (5.9) | 17 (23.6) | 0.033 |
| SBP, mmHg | 124.2 ± 17.9 | 121.8 ± 16.4 | 0.49 |
| DBP, mmHg | 78.9 ± 14.4 | 76.2 ± 8.9 | 0.31 |
| DASH score | 1.6 ± 0.9 | 1.6 ± 1 | 0.95 |
| Intake of fruit and vegetable, servings/d | 2.5 ± 2.0 | 3.1 ± 1.9 | 0.13 |
| Total fat, g/d | 67.6 ± 20.5 | 73.4 ± 34.7 | 0.28 |
| Energy intake, kilocalories/d | 1658.0 ± 550.9 | 1721.8 ± 720.1 | 0.65 |
ADM, antidepressant medication; DBP, diastolic blood pressure; GAD-7, Generalized Anxiety Disorder 7-Item Scale; GED, general educational development; P, P value; PHQ-9, Patient Health Questionnaire-9; SBP, systolic blood pressure; SCL-20, Depression Symptom Checklist-20.
Values are mean ± SD unless noted otherwise.
Prognostic factors for randomization: age, sex, and treatment group.
Indicate P values <0.05.
Changes in SCFAs and inflammatory markers predict changes in clinical outcomes
Table 2 shows Pearson partial correlation coefficients and P values for changes in biomarkers at 2 mo and changes in clinical outcomes at 2 and 6 mo. Changes in the clinical outcome scores at 6 mo were positively associated with changes in TNF-α and most of the SCFAs (P < 0.5). Also, these biomarkers exhibited an FDR-corrected P < 0.2. Changes in acetic acid were not associated with changes in GAD-7 scores at 2 or 6 mo but were positively associated with changes in SCL-20 scores at 6 mo. Additionally, changes in IL-1RA were only associated with changes in SCL-20 scores at 2 mo.
TABLE 2.
Pearson partial correlation coefficients (rpartial) and P values for changes in biomarkers at 2 mo and changes in clinical outcomes at 2 and 6 mo1
| Biomarker change at 2 mo | SCL-20 Change |
GAD-7 Change |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| at 2 mo (n = 28) |
at 6 mo (n = 23) |
at 2 mo (n = 28) |
at 6 mo (n = 23) |
|||||||||
| rpartial | P | FDR P | rpartial | P | FDR P | rpartial | P | FDR P | rpartial | P | FDR P | |
| CRP | 0.10 | 0.63 | 0.78 | 0.16 | 0.52 | 0.71 | –0.02 | 0.92 | 0.95 | –0.14 | 0.55 | 0.71 |
| IL-1β | –0.31 | 0.13 | 0.25 | –0.27 | 0.26 | 0.39 | 0.08 | 0.72 | 0.86 | 0.03 | 0.89 | 0.94 |
| IL-1RA | –0.40 | 0.0492 | 0.143 | –0.41 | 0.07 | 0.19 | –0.19 | 0.37 | 0.54 | –0.05 | 0.82 | 0.90 |
| IL-6 | 0.13 | 0.55 | 0.71 | –0.07 | 0.77 | 0.87 | 0.24 | 0.24 | 0.38 | 0.02 | 0.95 | 0.95 |
| TNF-α | 0.07 | 0.74 | 0.86 | 0.50 | 0.032 | 0.123 | 0.33 | 0.11 | 0.24 | 0.48 | 0.032 | 0.123 |
| Propionic acid | 0.32 | 0.11 | 0.24 | 0.59 | 0.012 | 0.113 | 0.45 | 0.022 | 0.123 | 0.48 | 0.032 | 0.123 |
| Butyric acid | 0.31 | 0.13 | 0.25 | 0.68 | <0.012 | 0.043 | 0.43 | 0.032 | 0.123 | 0.50 | 0.022 | 0.123 |
| Isovaleric acid | 0.29 | 0.16 | 0.27 | 0.55 | 0.012 | 0.123 | 0.40 | 0.0462 | 0.143 | 0.49 | 0.032 | 0.123 |
| Acetic acid | 0.24 | 0.24 | 0.38 | 0.45 | 0.0472 | 0.143 | 0.34 | 0.10 | 0.23 | 0.34 | 0.14 | 0.25 |
FDR, false discovery rate; GAD-7, Generalized Anxiety Disorder 7-Item Scale; IL-1β, interleukin 1 beta protein; IL-1RA, interleukin 1 receptor antagonist; IL-6, interleukin 6 protein; P, P value; rpartial, Pearson partial correlation coefficient; SCL-20, Depression Symptom Checklist-20.
Partial correlation adjusted for age, sex, and treatment group.
Indicate P values < 0.05.
Indicate FDR P values < 0.20.
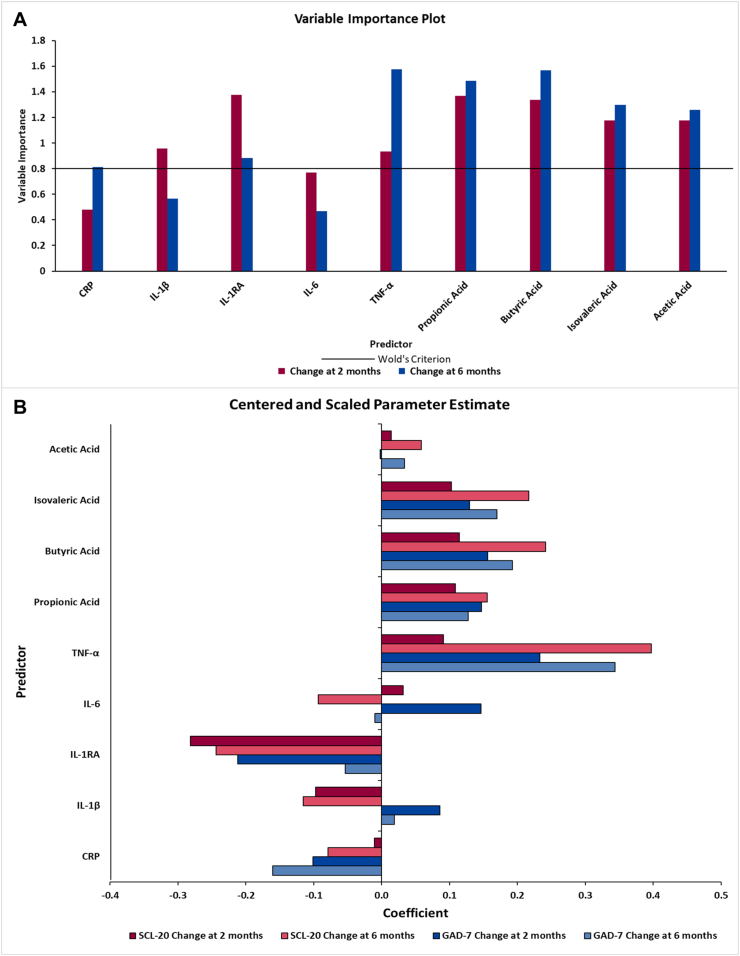
Using the multifactorial PLS analysis, which included all 4 SCFAs and 5 plasma inflammatory cytokines, 3 linear combinations (factors) were extracted, explaining 62.9% of the variation in the dependent variables (changes in SCL-20 and GAD-7 from 0 to 6 mo), whereas 55.0% of the variation in the predictors (changes in the SCFAs and cytokines from 0 to 2 mo) was explained. The variable importance plot and the regression coefficient profile (Figure 2) represent the contribution of changes in each independent variable at 2 mo in predicting the changes in SCL-20 and GAD-7 at 2 and 6 mo. For changes at 6 mo, the 3 factors were driven by the changes in TNF-α, butyric acid, propionic acid, isovaleric acid, acetic acid, IL-1RA, and CRP, with Importance for Projection statistic of Wold >0.8 (Figure 2A). Changes in TNF-α, butyric acid, propionic acid, isovaleric acid, and acetic acid were positively correlated with the changes in SCL-20 (standardized coefficient loadings: 0.05–0.39) and GAD-7 scores (standardized coefficient loadings: 0.03–0.34); and changes in IL-1RA and CRP were negatively correlated with the changes in SCL-20 (standardized coefficient loadings: –0.24 and –0.07, respectively) and GAD-7 scores (standardized coefficient loadings: –0.05 and –0.16, respectively) (Figure 2B). Results for changes in clinical outcomes at 2 mo were consistent with our primary endpoint at 6 mo. IL-1RA, TNF-α, and the 4 SCFAs were selected as significant biomarkers since they were identified as important variables at both 2 and 6 mo.
FIGURE 2.
Variable importance plot (A) and regression parameter profile (B) from PLS analysis (n = 28 for 2 mo; n = 23 for 6 mo). This graph shows the variable important plot (A) and regression parameter profile (B), where in A, several predictors (biomarkers) met or exceeded the Variable Importance for Projection (VIP) statistic Wold of 0.8. The VIP statistic of Wold illustrates the contribution of each predictor in fitting the PLS model for both predictors and clinical outcomes. The most important biomarkers for changes at 6 mo were TNF-α, IL-1RA, and all the SCFAs, whereas changes at 2 mo had similar important biomarkers with the addition of IL-1β. The regression parameter profile shows absolute coefficients for the centered and scaled parameter estimates (B). GAD-7, Generalized Anxiety Disorder 7-Item Scale; IL-1β, interleukin 1 beta; IL-1RA, interleukin 1 receptor agonist; IL-6, interleukin 6; PLS, partial least squares analysis; SCL-20, Depression Symptom Checklist-20.
Changes in SCFAs and inflammatory markers are associated with changes in certain dietary markers at 2 mo
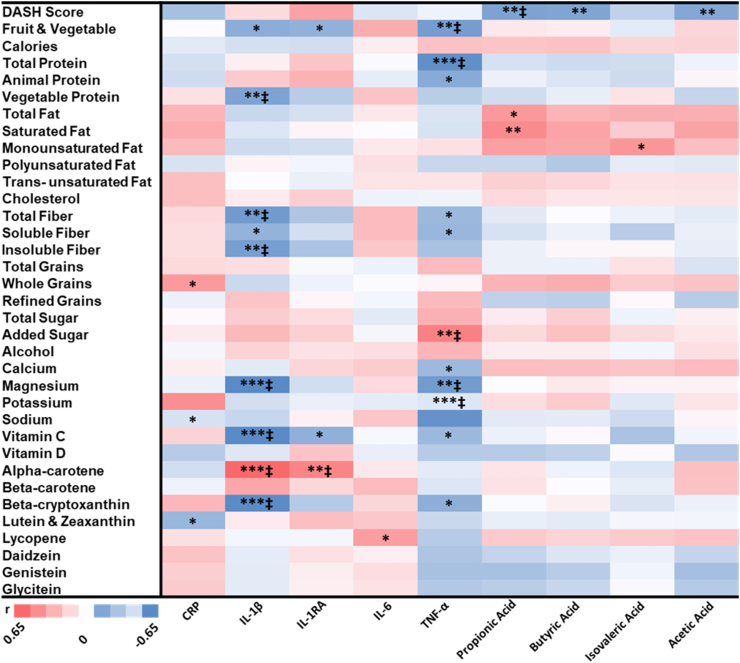
Based on the Pearson partial correlation results (Figure 3) showing associations between dietary markers and the above 6 significant biomarkers, IL-1RA, TNF-α, and the 4 SCFAs, and between dietary marker changes at 2 mo and clinical outcome changes at 2 or 6 mo (Table 3), 14 dietary markers were selected for PLS analysis which included DASH score, fruits and vegetables, calories, animal protein, monounsaturated fat, soluble fiber, added sugar, vitamin C, vitamin D, beta-cryptoxanthin, alpha-carotene, daidzein, genistein, and glycitein. These 14 dietary markers were significantly associated (P < 0.05) with the changes in the 6 significant biomarkers at 2 mo or the clinical outcomes at 2 or 6 mo. Among these 14 dietary markers, 4 had FDR-corrected P < 0.2 for the correlations with the 6 significant biomarkers (Figure 3), and 6 had FDR-corrected P < 0.2 for the correlations with the clinical outcomes (Table 3). Components of the DASH score calculation (total fat, saturated fat, total protein, total fiber, calcium, magnesium, and potassium) that were significant in the Pearson partial correlation between dietary markers and biomarkers were excluded in the PLS analysis to limit the number of variables as predictors. In this PLS analysis, 3 linear combinations (factors) were extracted, explaining 41.3% of the variation in the dependent variables (changes in TNF-α, butyric acid, propionic acid, isovaleric acid, acetic acid, and IL-1RA from 0 to 2 mo), whereas 41.7% of the variation in the predictors (changes in the 14 dietary markers from 0 to 2 mo) was explained.
FIGURE 3.
Heatmap of Pearson partial correlation coefficients, P values, and FDR-corrected P values for changes in dietary markers at 2 mo and changes in biomarkers at 2 mo (n = 28). P values are as follows: ∗P < 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001. ‡FDR values < 0.2. FDR, false discovery rate; IL-1β, interleukin 1 beta; IL-1RA, interleukin 1 receptor agonist.
TABLE 3.
Pearson partial correlation coefficients (rpartial) and P values for changes in dietary markers at 2 mo and changes in clinical outcomes at 2 and 6 mo1
| Dietary marker change at 2 mo | SCL-20 change |
GAD-7 change |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| at 2 mo (n = 34) |
at 6 mo (n = 26) |
at 2 mo (n = 34) |
at 6 mo (n = 26) |
|||||||||
| rpartial | P | FDR P | rpartial | P | FDR P | rpartial | P | FDR P | rpartial | P | FDR P | |
| DASH score | –0.16 | 0.39 | 0.76 | –0.43 | 0.042 | 0.23 | –0.13 | 0.50 | 0.84 | –0.12 | 0.57 | 0.82 |
| Fruit and vegetable | 0.08 | 0.65 | 0.85 | –0.08 | 0.73 | 0.81 | 0.04 | 0.85 | 0.96 | –0.03 | 0.88 | 0.92 |
| Calories | 0.08 | 0.68 | 0.85 | 0.42 | 0.0452 | 0.23 | 0.18 | 0.32 | 0.84 | 0.40 | 0.06 | 0.51 |
| Total protein | –0.24 | 0.20 | 0.63 | –0.55 | 0.012 | 0.143 | –0.26 | 0.16 | 0.64 | –0.34 | 0.11 | 0.69 |
| Animal protein | –0.23 | 0.21 | 0.63 | –0.53 | 0.012 | 0.143 | –0.21 | 0.26 | 0.84 | –0.29 | 0.18 | 0.82 |
| Vegetable protein | 0.05 | 0.77 | 0.85 | 0.07 | 0.74 | 0.81 | –0.06 | 0.75 | 0.94 | –0.08 | 0.73 | 0.85 |
| Total fat | 0.39 | 0.032 | 0.63 | 0.34 | 0.12 | 0.33 | 0.35 | 0.05 | 0.37 | 0.07 | 0.75 | 0.85 |
| Saturated fat | 0.22 | 0.24 | 0.63 | 0.27 | 0.21 | 0.42 | 0.13 | 0.48 | 0.84 | 0.12 | 0.59 | 0.82 |
| Monounsaturated fat | 0.28 | 0.13 | 0.63 | 0.49 | 0.022 | 0.163 | 0.32 | 0.08 | 0.48 | 0.14 | 0.51 | 0.82 |
| Polyunsaturated fat | 0.20 | 0.28 | 0.70 | –0.25 | 0.24 | 0.45 | 0.18 | 0.34 | 0.84 | –0.24 | 0.28 | 0.82 |
| Trans-unsaturated fat | 0.01 | 0.95 | 0.95 | 0.12 | 0.57 | 0.81 | 0.04 | 0.82 | 0.96 | –0.09 | 0.68 | 0.85 |
| Cholesterol | –0.05 | 0.78 | 0.85 | –0.08 | 0.72 | 0.81 | –0.16 | 0.40 | 0.84 | –0.13 | 0.55 | 0.82 |
| Total fiber | –0.14 | 0.45 | 0.82 | 0.00 | 0.99 | 0.99 | –0.15 | 0.41 | 0.84 | –0.21 | 0.33 | 0.82 |
| Soluble fiber | –0.25 | 0.17 | 0.63 | –0.28 | 0.19 | 0.42 | –0.29 | 0.12 | 0.58 | –0.27 | 0.21 | 0.82 |
| Insoluble fiber | –0.05 | 0.78 | 0.85 | 0.13 | 0.54 | 0.81 | –0.07 | 0.72 | 0.93 | –0.16 | 0.48 | 0.82 |
| Total grains | –0.08 | 0.67 | 0.85 | –0.09 | 0.68 | 0.81 | 0.11 | 0.57 | 0.84 | 0.15 | 0.49 | 0.82 |
| Whole grains | 0.13 | 0.47 | 0.82 | 0.23 | 0.29 | 0.51 | 0.20 | 0.27 | 0.84 | –0.08 | 0.71 | 0.85 |
| Refined grains | –0.17 | 0.36 | 0.76 | –0.20 | 0.36 | 0.59 | –0.01 | 0.94 | 0.98 | 0.18 | 0.41 | 0.82 |
| Total sugar | –0.09 | 0.65 | 0.85 | 0.27 | 0.22 | 0.42 | –0.11 | 0.56 | 0.84 | 0.15 | 0.48 | 0.82 |
| Added sugar | –0.03 | 0.87 | 0.90 | 0.36 | 0.09 | 0.30 | –0.01 | 0.96 | 0.98 | 0.16 | 0.46 | 0.82 |
| Alcohol | 0.08 | 0.65 | 0.85 | –0.02 | 0.92 | 0.94 | 0.10 | 0.60 | 0.84 | 0.06 | 0.80 | 0.87 |
| Calcium | 0.07 | 0.72 | 0.85 | –0.09 | 0.68 | 0.81 | –0.14 | 0.45 | 0.84 | –0.15 | 0.51 | 0.82 |
| Magnesium | 0.04 | 0.84 | 0.89 | –0.03 | 0.90 | 0.94 | –0.15 | 0.43 | 0.84 | –0.20 | 0.36 | 0.82 |
| Potassium | –0.30 | 0.10 | 0.63 | –0.37 | 0.08 | 0.29 | –0.27 | 0.13 | 0.59 | –0.24 | 0.26 | 0.82 |
| Sodium | –0.05 | 0.78 | 0.85 | –0.08 | 0.73 | 0.81 | 0.04 | 0.82 | 0.96 | –0.08 | 0.71 | 0.85 |
| Vitamin C | 0.16 | 0.39 | 0.76 | 0.08 | 0.72 | 0.81 | –0.12 | 0.54 | 0.84 | –0.02 | 0.92 | 0.92 |
| Vitamin D | –0.16 | 0.38 | 0.76 | –0.31 | 0.15 | 0.36 | –0.40 | 0.032 | 0.24 | –0.33 | 0.12 | 0.69 |
| Alpha-carotene | –0.30 | 0.10 | 0.63 | –0.51 | 0.012 | 0.143 | 0.00 | 0.99 | 0.99 | –0.09 | 0.67 | 0.85 |
| Beta-carotene | –0.23 | 0.22 | 0.63 | –0.33 | 0.12 | 0.33 | 0.08 | 0.66 | 0.89 | –0.02 | 0.92 | 0.92 |
| Beta-cryptoxanthin | 0.13 | 0.50 | 0.83 | 0.10 | 0.64 | 0.81 | –0.13 | 0.50 | 0.84 | –0.15 | 0.49 | 0.82 |
| Lutein and zeaxanthin | –0.29 | 0.12 | 0.63 | –0.32 | 0.14 | 0.35 | –0.11 | 0.56 | 0.84 | –0.13 | 0.54 | 0.82 |
| Lycopene | –0.06 | 0.77 | 0.85 | 0.12 | 0.60 | 0.81 | –0.03 | 0.89 | 0.97 | –0.16 | 0.46 | 0.82 |
| Daidzein | –0.31 | 0.09 | 0.63 | –0.37 | 0.08 | 0.29 | –0.54 | 0.0022 | 0.023 | –0.54 | 0.012 | 0.093 |
| Genistein | –0.29 | 0.11 | 0.63 | –0.42 | 0.042 | 0.23 | –0.58 | <0.0012 | 0.023 | –0.55 | 0.012 | 0.093 |
| Glycitein | –0.23 | 0.22 | 0.63 | –0.39 | 0.06 | 0.28 | –0.54 | 0.0022 | 0.023 | –0.57 | 0.012 | 0.093 |
FDR, false discovery rate; GAD-7, Generalized Anxiety Disorder 7-Item Scale; P, P value; rpartial, Pearson partial correlation coefficient; SCL-20, Depression Symptom Checklist-20.
Partial correlation adjusted for age, sex, and treatment group.
Indicate P values < 0.05.
Indicate FDR P values < 0.20.
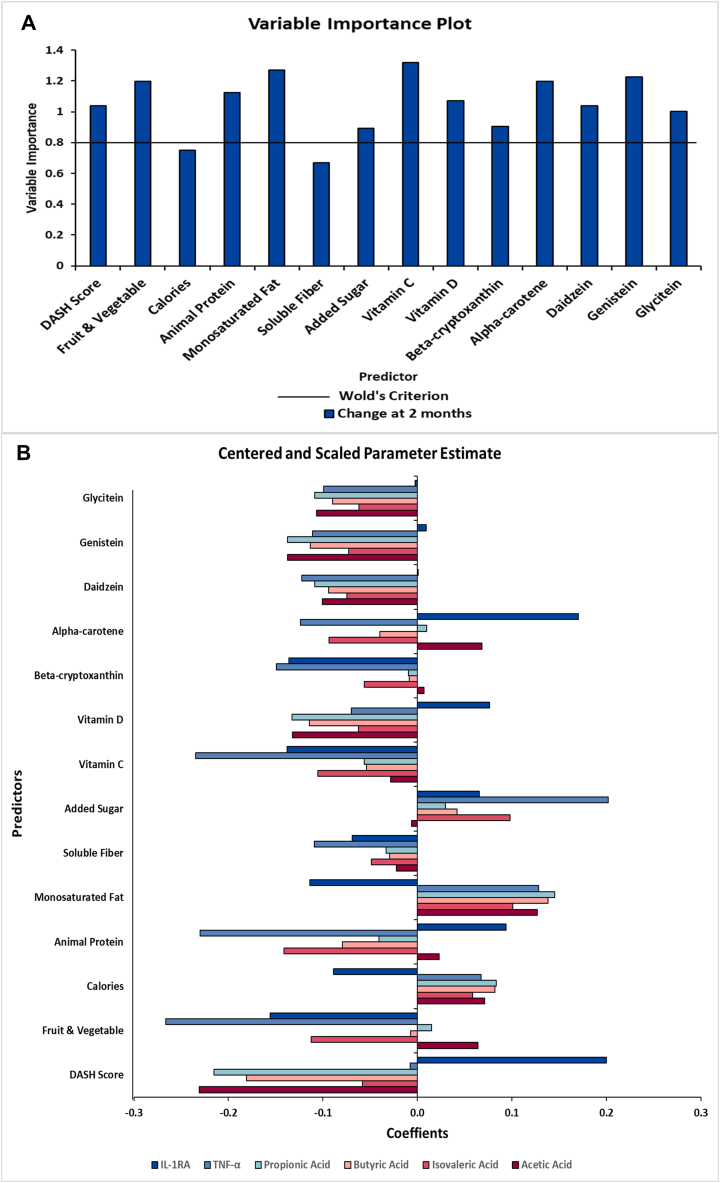
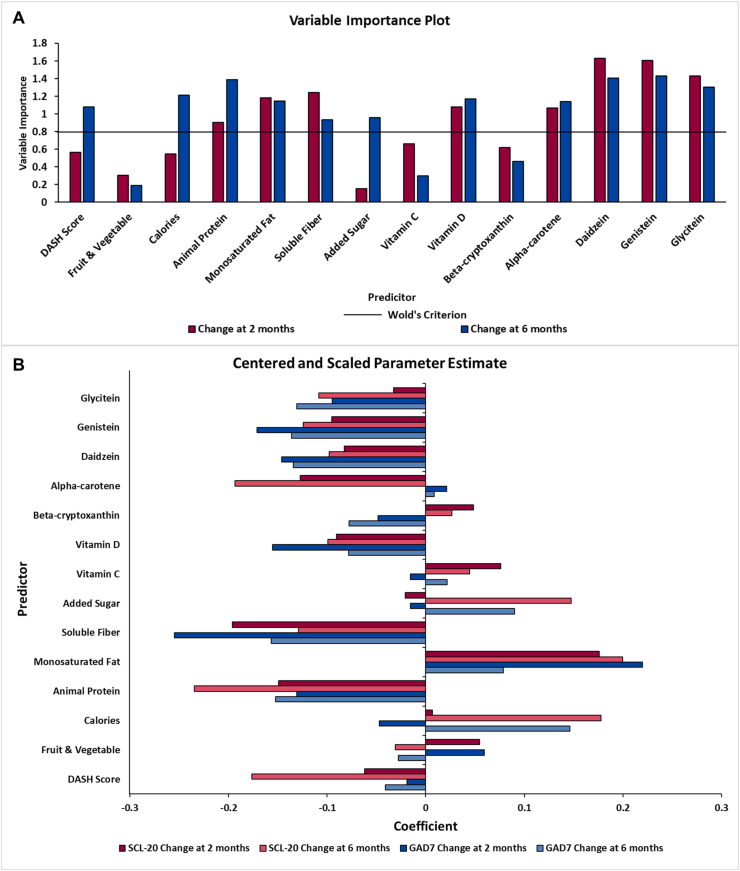
Based on the variable importance plot and the regression coefficient profile (Figure 4), the 3 factors were mainly driven by changes in 12 of the 14 dietary markers, including DASH score, fruit and vegetable intake, animal protein, monounsaturated fat, added sugar, vitamin C, vitamin D, beta-cryptoxanthin, alpha-carotene, daidzein, genistein, and glycitein from baseline to 2 mo (Figure 4A). Changes in these 12 dietary variables were mainly negatively correlated with the changes in TNF-α, butyric acid, propionic acid, isovaleric acid, and acetic acid (standardized loading coefficients: -0.24 to –0.01), except for monounsaturated fat and added sugar which was mainly positively associated (standardized loading coefficients: 0.03–0.20) with these 5 biomarkers (Figure 4B). For correlations with changes in IL-1RA (Figure 4B), changes in monounsaturated fat, fruit and vegetable intake, vitamin C, beta-cryptoxanthin, and glycitein were negatively associated (standardized loading coefficients: –0.16 to –0.00), whereas DASH score, animal protein, vitamin D, added sugar, alpha-carotene, daidzein, and genistein were positively associated (standardized loading coefficients: 0.001–0.20). The most significant correlations (coefficients ≥0.2 or ≤–0.2) were between DASH score, fruit and vegetable intake, animal protein, added sugar, vitamin C, and alpha-carotene with the biomarkers.
FIGURE 4.
Variable importance plot (A) and regression parameter profile (B) from PLS analysis (n = 29). This graph shows the variable important plot (A) and regression parameter profile (B), where in A, several predictors (dietary markers) met or exceeded the Variable Importance for Projection (VIP) statistic Wold of 0.8. The VIP statistic of Wold illustrates the contribution of each predictor in fitting the PLS model for both predictors and biomarkers. The majority of the examined dietary markers were important except calories and soluble fiber. The regression parameter profile shows absolute coefficients for the centered and scaled parameter estimates (B). IL-1RA, interleukin 1 receptor agonist; PLS, partial least squares analysis.
Diet changes within the first 2 mo were associated with anxiety and depression scores at 6 mo
In this PLS analysis including the same 14 dietary markers, 3 linear combinations (factors) were extracted, explaining 62.2% of the variation in the dependent variables (changes in SCL-20 and GAD-7 from 0 to 6 mo), whereas 44.3% of the variation in the predictors (changes in dietary markers from 0 to 2 mo) was explained.
Based on the variable importance plot and the regression coefficient profile (Figure 5), the 3 factors were driven by changes in 11 out of the 14 dietary markers, including DASH score, calories, animal protein, monounsaturated fat, soluble fiber, added sugar, vitamin D, alpha-carotene, daidzein, genistein, and glycitein from baseline to 2 mo (Figure 5A). Changes in DASH score, animal protein, soluble fiber, vitamin D, daidzein, genistein, and glycitein at 2 mo were negatively correlated with the changes in SCL-20 and GAD-7 (standardized coefficient loadings: –0.24 to –0.10 and –0.16 to –0.04, respectively) at 6 mo. Changes in calories, monounsaturated fat, and added sugar at 2 mo were positively correlated with changes in SCL-20 and GAD-7 (standardized coefficient loadings: 0.15–0.20 and 0.08–0.15, respectively) at 6 mo (Figure 5B). The most significant correlations (coefficients ≥0.2 or ≤–0.2) were between animal protein and monounsaturated fat with the clinical outcomes.
FIGURE 5.
Variable importance plot (A) and regression parameter profile (B) from PLS analysis (n = 34 for 2 mo; n = 26 for 6 mo). This graph shows the variable important plot (A) and regression parameter profile (B), where in A, several predictors (dietary markers) met or exceeded the Variable Importance for Projection (VIP) statistic Wold of 0.8. The VIP statistic of Wold illustrates the contribution of each predictor in fitting the PLS model for both predictors and clinical outcomes. The most important dietary markers for changes at 6 mo were monounsaturated fat, soluble fiber, and animal protein. Changes at 2 mo had similar important dietary markers. The regression parameter profile shows absolute coefficients for the centered and scaled parameter estimates (B). GAD-7, Generalized Anxiety Disorder 7-Item Scale; PLS, partial least squares analysis; SCL-20, Depression Symptom Checklist-20.
PLS analysis on the associations between changes in dietary markers at 2 mo and changes in clinical outcomes at 2 mo showed similar relationships to the results for changes in clinical outcomes at 6 mo. However, added sugar, calories, and DASH score were not associated with the clinical outcomes at 2 mo (Figure 4A). The most significant correlations (coefficients ≥0.2 or ≤–0.2) were between monounsaturated fat and soluble fiber with the clinical outcomes at 2 mo.
Discussion
To our knowledge, this is the first study exploring longitudinal relationships within the MGBA in individuals with obesity and depression. Our data indicate that after 2 mo, changes in fecal SCFAs and TNF-α positively correlated with changes in depressive scores at 6 mo, whereas changes in IL-1RA negatively correlated. In addition, at 2 mo, these biomarkers were associated with changes in dietary markers, including animal protein, which also correlated with depressive scores at 6 mo. Together, these results are hypothesis-generating, supporting future studies examining microbial fermentation and inflammation as potential mediators in diet effects on depression and anxiety in obesity.
SCFAs, cytokines, and depression and anxiety
In this study, changes in fecal SCFAs were positively associated with changes in depressive and anxiety symptoms. Gut-derived SCFAs maintain central nervous system homeostasis and impact the hippocampus and striatum by modulating reward-associated behaviors, cognition, and learning [[28], [29], [30], [31]]. Evidence on SCFA supplementation in obese mice decreased anxiety and depressive-like behavior, improved Hypothalamic-Pituitary-Adrenal (HPA) axis hyperactivity and intestinal permeability, and altered anhedonia whereas showing increased fecal SCFAs levels caused by stress [29]. Interestingly, this mice study, cross-sectional studies in individuals with hypertension [[32], [33], [34]] and obesity [[35], [36], [37]], and our findings support the hypothesis that higher SCFA excretion and lower circulating SCFAs may indicate impaired gut health, and higher stress and inflammation, thus representing a potential pathway within MGBA. However, studies assessing circulating and fecal SCFAs are needed to confirm this hypothesis. Furthermore, other cross-sectional studies demonstrated no association, or either a positive [11, 38] or negative [10, 11, 38] association, between fecal SCFAs and mood disorders, warranting further investigations.
Our data showed that after 2 mo, an increase in proinflammatory TNF-α and a decrease in anti-inflammatory IL-1RA could predict an increase in depressive and anxiety symptoms at 6 mo, supporting the “cytokine” hypothesis of depression [39, 40]. Proinflammatory cytokines may stimulate serotonin uptake, regulate neuronal serotonin transporter activity, and activate indolamine-2,3-dioxygenase and tryptophan pathways that reduce serotonin in depression [40]. For anxiety, activation of stress response via HPA axis dysregulation and diminishing actions of glucocorticoids inhibit proinflammatory activity through nuclear factor kappa B [41]. Anti-inflammatory IL-1RA is an antagonist to proinflammatory interleukin 1 alpha and IL-1β [42] and prevents their effect on serotonin homeostasis and stress response. Nonetheless, longitudinal studies on associations of cytokines with depression and anxiety symptoms are conflicting, showing no association [43] or higher TNF-α in depressed subjects versus healthy controls [[43], [44], [45], [46]]. Although for IL-1RA, showing positive [[47], [48], [49]] and negative associations [50]. For anxiety, results showed TNF-α positively correlating with anxiety symptoms compared with controls [51, 52], and to our knowledge, longitudinal investigations examining IL-1RA for anxiety are lacking. In contrast to previous studies [44, 45, 56], our data did not show any associations between changes in IL-1β and interleukin 6, and a modest inverse association between CRP at 2 mo with changes in depressive and anxiety symptoms at 6 mo, meriting future studies.
Animal protein, biomarkers, and depression and anxiety
In this study, changes in 12 dietary markers significantly correlated with the SCFAs, TNF-α, and IL-1RA, with DASH score, fruit and vegetable intake, added sugar, vitamin C, and animal protein, exhibiting larger coefficient loading standards (≥0.2 or ≤–0.2). In addition, for depression and anxiety scores, 10 dietary markers significantly correlated with SCL-20 and GAD-7 scores at 2 or 6 mo, with monounsaturated fat, soluble fiber, and animal protein displaying larger coefficient loading standards. Interestingly, animal protein was the only dietary marker associating significantly with the biomarkers and a clinical outcome at 6 mo, highlighting its potential MGBA importance.
Our findings showed changes in animal protein intake inversely associated with changes in SCFAs at 2 mo. Mechanistically, fecal SCFA production involves carbohydrate fermentation, such as fiber, from plant-based food consumption and nutrient bioavailability [53]. Animal protein can be high in fat, reducing plant carbohydrate metabolizing bacteria, whereas plant-based protein increases beneficial bacteria production [53]. Contrary to our findings, a previous cohort study in individuals with obesity showed no association between fecal SCFAs and animal protein [54]. Since SCFAs are produced by microbial fiber fermentation [53], surprisingly, our data lacked any associations between fiber and SCFAs, and other studies show similar results [55, 56].
Animal protein intake inversely correlated with TNF-α and positively correlated with IL-1RA at 2 mo. The underlying mechanisms between animal protein and inflammation are unclear [57, 58]. However, in a cross-over [59] and parallel-arm [60] randomized clinical trial with healthy individuals, kangaroo, and wagyu beef were associated with increased TNF-α levels, whereas salmon and herring fish were associated with lower TNF-α levels [60]. Additionally, in individuals with metabolic syndrome and obesity, there were no associations between changes in TNF-α from baseline and between control and high protein diets; however, overall inflammatory scores (which included TNF-α levels) were associated with increased meat intake [61]. For our study, we did not differentiate between the type and levels of animal protein which may explain the deviation from previous studies, thus warranting further analysis. Additionally, to our knowledge, no previous studies examined animal protein and IL-1RA associations.
Our findings determined that after 2 mo, animal protein significantly and inversely correlated with SCL-20 scores at 6 mo. The mechanisms involved in the relationship between diet and mental disorders are complex and poorly understood, acting on inflammation, epigenetics, mitochondrial dysfunction, gut microbiome, obesity, the HPA axis, and neurogenesis pathways [62]. Several systematic reviews and cross-sectional studies suggest that certain animal protein intake is associated with higher depression prevalence and incidence [[63], [64], [65], [66], [67]], which complements our data. Interestingly, other reviews and cross-sectional studies show that fish and white meat consumption is associated with lower depression incidence [[67], [68], [69]]. Because we did not differentiate between animal protein types, possibly explaining differences in our data versus previous studies, more investigations in this area.
Potential Mechanisms within the MGBA
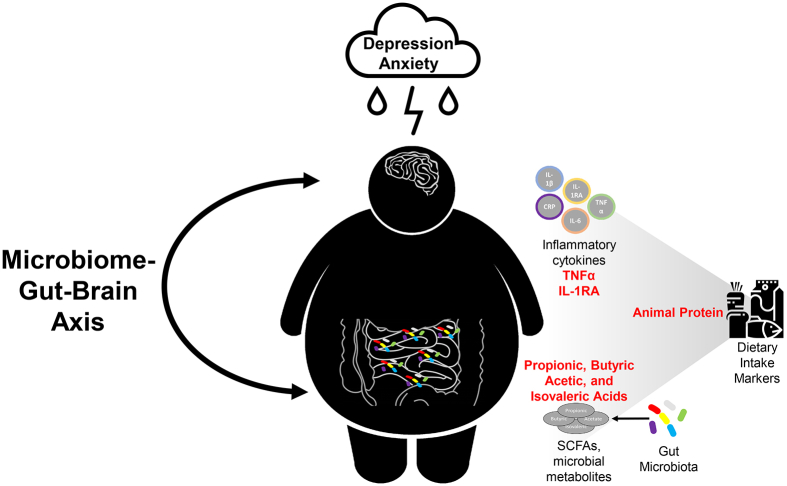
This study does not infer causality or define the clinical impact of the observed associations between fecal SCFAs, plasma cytokines, dietary markers, and depression and anxiety symptoms. However, the data presented suggest a potential mechanistic model within the MGBA in individuals with obesity, depression, and anxiety. In this sample population, inflammation and gut microbial fermentation may be potential mediators in the dietary effects (specifically animal protein) on depression and anxiety (Figure 6). Since our sample size was limited, we could not perform a mediation analysis. However, this data informs future mediation studies that examine these biomarkers in relation to diet within the MGBA.
FIGURE 6.
Potential microbiome-gut-brain axis relationships depicted in obesity comorbid with depression and associated anxiety involving diet, fecal SCFAs, and inflammatory cytokines. The linkage between diet and mental health may rely on the mediating relationship of biomarkers such as inflammatory cytokines and gut microbial metabolites, SCFAs. For this sample population, biomarkers at 2 mo, including 2 plasma cytokines, TNF-α and IL-1RA, and 4 SCFAs, propionic, butyric, acetic, and isovaleric acids, were associated with depression and anxiety scores after 6 mo. These biomarkers at 2 mo were also associated with dietary intake markers such as animal protein at 2 mo and animal protein associated with depression at 6 mo. IL-1RA, interleukin 1 receptor agonist;.
Strengths and limitations
This exploratory study has several strengths. To our knowledge, this is the first human study to provide insight into changes in SCFAs, inflammatory cytokines, and dietary markers with depression and anxiety with respect to the MGBA in adults with comorbid obesity and depression. Also, this study expands and extends current epidemiological studies by addressing gaps in longitudinal associations of fecal SCFAs and plasma inflammatory cytokines with depression and anxiety, a diet with these biomarkers, and a diet with these neuropsychological disorders, highlighting animal protein as a possible significant MGBA dietary marker.
This study is not without limitations. By combining the data, we explored relationships for adults with obesity comorbid with depression and associated anxiety; however, we cannot infer the relationship differences based on the treatment effect of the intervention. We only examined associations at 2 and 6 mo; therefore, a longer study could provide insights into long-term associations. The gut microbiota composition, structure, and functional metabolism could provide an extensive understanding of gut microbiome relationships to diet and mental health, which our future study includes. We did not measure dietary supplement intake, which could affect depression and anxiety scores and their associations with diet, SCFAs, and inflammatory cytokines. We observed associations between animal protein; however, the type of animal protein was not examined, which may show different results. Lastly, depression and anxiety were not validated by a provider and based on self-reported assessments, the majority of this substudy participants experienced lower mood symptoms. Thus, further work is needed to determine if our findings extend to those with higher depression and anxiety levels.
In conclusion, in this study, after 2 mo, increased fecal SCFAs were associated with worse depressive and anxiety symptoms after 6 mo. Additionally, increased proinflammatory TNF-α and decreased anti-inflammatory IL-1RA after 2 mo were associated with worse depression and anxiety symptoms after 6 mo. Animal protein is significantly associated with biomarkers and depression, displaying its potential impact within the MGBA. These findings are data-driven and hypothesis-generating, highlighting the potential of implementing dietary changes and using anti-inflammatory medication to treat depression, anxiety, and obesity simultaneously.
Funding
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant numbers UH2HL132368 and UH3HL132368, and the National Institute of Mental Health R61MH119237. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. TCJB was supported by the NHLBI grant number T32HL134634 and is currently supported as a University of Illinois Chicago Bridge to Faculty and Ford Foundation Postdoctoral Fellowships. BPB was supported by the K-12 BIRCWH Award grant number K12HD101373.
Author disclosures
TCJB was supported by the NHLBI grant number T32HL134634 and is currently supported as a University of Illinois Chicago Bridge to Faculty and Ford Foundation Postdoctoral Fellowships. BPB was supported by the K-12 BIRCWH Award grant number K12HD101373. JM is a paid scientific consultant for Health Mentor, Inc. (San Jose, CA). OAA is the co-founder of Keywise AI and serves on the advisory boards of Sage Therapeutics, Blueprint Health, and Embodied Labs. All other authors report no conflicts of interest.
Acknowledgments
We thank the Mass Spectrometry Core in the Research Resources Center of the University of Illinois at Chicago for performing the quantitative analysis of the SCFAs. We also thank the ENGAGE-2 participants and their families, who made this study possible.
Footnotes
This trial was registered at clinicaltrials.gov (NCT03841682).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.01.018.
Author contributions
The authors’ responsibilities were as follows: JM and OAA designed the research project (project conception, development of overall research plan, and study oversight). TCJB, LX, and NL analyzed data or performed statistical analysis. TCJB, NL, and PT wrote the manuscript (only authors who made a major contribution). OAA and JM had primary responsibility for the final content. TCJB, NL, PT, YW, LT-H, LX, BPB, OAA, and JM performed data interpretations and edited the manuscript. All authors, except GNP, who passed on October 15, 2022, critically revised, read, and approved the final manuscript.
Data Availability
To ensure compliance with the study’s informed consent process, we would share de-identified data and associated data dictionary only under a formalized data sharing and use agreement that provides the commitment to the following: 1) using the data only for research purposes and not identifying any individual participant; 2) securing the data using appropriate computer technology, which needs to be specified, 3) destroying or returning the data after analyses are completed, 4) accepting reporting responsibilities, 5) abiding by restrictions on redistribution of the data for commercial purposes or to third parties, and 6) proper acknowledgment of the data resource. Additionally, appropriate fees may be assessed upon mutual agreement on requests for information in a format other than that we intend to provide. We will not be responsible for providing any analytical support.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.S. Dattani, H. Ritchie, M. Roser, Mental Health – Our World in Data, Mental Health, 2020 [Internet]. Available from: https://ourworldindata.org/mental-health [April 4, 2022].
- 2.Pereira-Miranda E., Costa P.R.F., Queiroz V.A.O., Pereira-Santos M., Santana M.L.P. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36(3):223–233. doi: 10.1080/07315724.2016.1261053. [DOI] [PubMed] [Google Scholar]
- 3.Bear T.L.K., Dalziel J.E., Coad J., Roy N.C., Butts C.A., K Gopal P. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv Nutr. 2020;11(4):890–907. doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutsch A., Kantsjö J.B., Ronchi F. Front, Immunol. 2020;11:604179–604180. doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspani G., Kennedy S., Foster J.A., Swann J. Gut microbial metabolites in depression: understanding the biochemical mechanisms. Microb Cel. 2019;6(10):454–481. doi: 10.15698/mic2019.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lankelma J.M., Nieuwdorp M., de Vos W.M., Wiersinga W.J. The gut microbiota in internal medicine: implications for health and disease. Neth J Med. 2015;73(2):61–68. [PubMed] [Google Scholar]
- 7.Oriach C.S., Robertson R.C., Stanton C., Cryan J.F., Dinan T.G. Food for thought: the role of nutrition in the microbiota-gut-brain axis. Clin Nutr Exp. 2016;6:25–38. [Google Scholar]
- 8.Zhang C., Zhang M., Wang S., Han R., Cao Y., Hua W., et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4(2):232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 9.Capuco A., Urits I., Hasoon J., Chun R., Gerald B., Wang J.K., et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37(4):1328–1346. doi: 10.1007/s12325-020-01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grochans E., Maciejewska D., Szkup M., Schneider-Matyka D., Jurczak A., et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. 2018;10(12):1939–1940. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szczesniak O., Hestad K.A., Hanssen J.F. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. 2016;19(7):279–283. doi: 10.1179/1476830515Y.0000000007. [DOI] [PubMed] [Google Scholar]
- 12.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020;11:25–26. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv N., Ajilore O.A., Ronneberg C.R., Venditti E.M., Snowden M.B., Lavori P.W., et al. The ENGAGE-2 study: engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes in a randomized controlled trial (Phase 2) Contemp Clin Trials. 2020;95:106072–106073. doi: 10.1016/j.cct.2020.106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv N., Ajilore O.A., Xiao L., Venditti E.M., Lavori P.W., Gerber B.S., et al. Mediating effects of neural targets on depression, weight and anxiety outcomes of an integrated collaborative care intervention: the ENGAGE-2 mechanistic pilot randomized clinical trial. Biol Psychiatry Glob Open Sci. 2023 doi: 10.1016/j.bpsgos.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J., Yank V., Xiao L., Lavori P.W., Wilson S.R., Rosas L.G., S R. Translating the diabetes prevention program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J., King A.C., Wilson S.R., Xiao L., Stafford R.S. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract. 2009;10:71–72. doi: 10.1186/1471-2296-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer M.K., Kriska A.M., Venditti E.M., Miller R.G., Brooks M.M., Burke L.E., et al. Translating the diabetes prevention program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Ciechanowski P., Wagner E., Schmaling K., Schwartz S., Williams B., Diehr P., et al. Community-integrated home-based depression treatment in older adults: A randomized controlled trial. JAMA. 2004;291(13):1569–1577. doi: 10.1001/jama.291.13.1569. [DOI] [PubMed] [Google Scholar]
- 19.Ciechanowski P., Chaytor N., Miller J., Fraser R., Russo J., Unutzer J., Gilliam F. PEARLS depression treatment for individuals with epilepsy: a randomized controlled trial. Epilepsy Behav. 2010;19(3):225–231. doi: 10.1016/j.yebeh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Majd M., Smyth J.M., Lv N., Xiao L., Snowden M.B., Venditti E.M., et al. The factor structure of depressive symptoms in patients with obesity enrolled in the RAINBOW clinical trial. J Affect Disord. 2021;281:367–375. doi: 10.1016/j.jad.2020.11.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glass R.M., Allan A.T., Uhlenhuth E.H., Kimball C.P., Borinstein D.I. Psychiatric screening in a Medical Clinic. An evaluation of a self-report inventory. Arch Gen Psychiatry. 1978;35(10):1189–1195. doi: 10.1001/archpsyc.1978.01770340039003. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 23.Abrahamson M., Hooker E., Ajami N.J., Petrosino J.F., Orwoll E.S. Successful collection of stool samples for microbiome analyses from a large community-based population of elderly men. Contemp Clin Trials Commun. 2017;7:158–162. doi: 10.1016/j.conctc.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moshfegh A.J., Rhodes D.G., Baer D.J., Murayi T., Clemens J.C., Rumpler W.V., et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 25.Mellen P.B., Gao S.K., Vitolins M.Z., Goff D.C. Deteriorating dietary habits among adults with hypertension: dash dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 26.Sorkin J.D., Manary M., Smeets P.A.M., Macfarlane A.J., Astrup A., Prigeon R.L., et al. A guide for authors and readers of the American Society for Nutrition Journals on the proper use of P values and strategies that promote transparency and improve research reproducibility. Am J Clin Nutr. 2021;114(4):1280–1285. doi: 10.1093/ajcn/nqab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yekutieli Y. Benjamini D. The control of the false discovery rate in multiple testing under dependency. Ann Statist. 2001;29(4):1165–1188. [Google Scholar]
- 28.Byrne C.S., Chambers E.S., Alhabeeb H., Chhina N., Morrison D.J., Preston T., et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104(1):5–14. doi: 10.3945/ajcn.115.126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Wouw M., Boehme M., Lyte J.M., Wiley N., Strain C., O’Sullivan O., et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol. 2018;596(20):4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Val-Laillet D., Guérin S., Coquery N., Nogret I., Formal M., Romé V., et al. Oral sodium butyrate impacts brain metabolism and hippocampal neurogenesis, with limited effects on gut anatomy and function in pigs. FASEB J. 2018;32(4):2160–2171. doi: 10.1096/fj.201700547RR. [DOI] [PubMed] [Google Scholar]
- 31.Cryan J.F., O’Riordan K.J., Cowan C.S.M., v Shu K.V., Bastiaanssen T.F.S., Boehme M., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877. doi: 10.1152/physrev.00018.2018. 13. [DOI] [PubMed] [Google Scholar]
- 32.Meurs M., Groenewold N.A., Roest A.M., van der Wee N.J., Veltman D.J., van Tol M.J., de Jonge P. The associations of depression and hypertension with brain volumes: independent or interactive? Neuroimage Clin. 2015;8:79–86. doi: 10.1016/j.nicl.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuesta-Zuluaga J., Mueller N.T., Álvarez-Quintero R., Velásquez-Mejía E.P., Sierra J.A., Corrales-Agudelo V., et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2019;11(1):51–52. doi: 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderón-Pérez L., Gosalbes M.J., Yuste S., Valls R.M., Pedret A., Llauradó E., et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep. 2020;10(1):6436–6437. doi: 10.1038/s41598-020-63475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez-Cuesta M.C., del Campo R., Garriga-García M., Peláez C., Requena T. Taxonomic characterization and short-chain fatty acids production of the obese microbiota. Front Cell Infect Microbiol. 2021;11(June):598093–598094. doi: 10.3389/fcimb.2021.598093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K.N., Yao Y., Ju S.Y. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. 2019;11(10):2512–2513. doi: 10.3390/nu11102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teixeira T.F.S., Grześkowiak Ł., Franceschini S.C.C., Bressan J., Ferreira C.L.L.F., Peluzio M.C.G. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr. 2013;109(5):914–919. doi: 10.1017/S0007114512002723. [DOI] [PubMed] [Google Scholar]
- 38.Müller B., Rasmusson A.J., Just D., Jayarathna S., Moazzami A., Novicic Z.K., Cunningham J.L. Fecal short chain fatty acid ratios are related to both depressive and gastrointestinal symptoms in young adults. Psychosom Med. 2021;83(7):693–699. doi: 10.1097/PSY.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maes M. The cytokine hypothesis of depression: inflammation, oxidative and nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29(3):287–291. [PubMed] [Google Scholar]
- 40.Yao L., Pan L.H., Qian M., Sun W., Gu C.H., Chen L.H., et al. Tumor necrosis factor-α variations in patients with major depressive disorder before and after antidepressant treatment. Front Psychiatry. 2020;11(December):518837–518838. doi: 10.3389/fpsyt.2020.518837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michopoulos V., Powers A., Gillespie C.F., Ressler K.J., Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42(1):254–270. doi: 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milaneschi Y., Corsi A.M., Penninx B.W., Binelli S., Guralnik J.M., Ferrucci L. Vol. 65. 2009. Interleukin-1 receptor antagonist and incident depressive symptoms over 6 years in older persons: the InCHIANTI study; pp. 973–978. (Biol Psychiatry). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mac Giollabhui N., Ng T.H., Ellman L.M., Alloy L.B. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. 2021;26(7):3302–3314. doi: 10.1038/s41380-020-00867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osimo E.F., Pillinger T., Rodriguez I.M., Khaker G.M., Pariante C.M., Howes O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:901–909. doi: 10.1016/j.bbi.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dowlati N. Herrmann, Swardfager W., Liu H., Sham L., Reim E.K., et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Ho R.C.M., Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Martínez-Cengotitabengoa M., Carrascón L., O’Brien J.T., Díaz-Gutiérrez M.J., Bermúdez-Ampudia C., Sanada K., et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):2022–2023. doi: 10.3390/ijms17122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herder C., Schmitt A., Budden F., Reimer A., Kulzer B., Roden M., et al. Longitudinal associations between biomarkers of inflammation and changes in depressive symptoms in patients with type 1 and type 2 diabetes. Psychoneuroendocrinology. 2018;91:216–225. doi: 10.1016/j.psyneuen.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 49.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 50.van den Biggelaar A.H.J., Gussekloo J., de Craen A.J.M., Frölich M., Stek M.L., van der Mast R.C., et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42(7):693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Arranz L., Guayerbas N., De la Fuente M. Impairment of several immune functions in anxious women. J Psychosom Res. 2007;62(1):1–8. doi: 10.1016/j.jpsychores.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 52.Salim S., Chugh G., Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 53.Tomova A., Bukovsky I., Rembert E., Yonas W., Alwarith J., Barnard N.D., et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47–48. doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Møller G., ersen J.R., Jalo E., Ritz C., Br-Miller J., Larsen T.M., et al. The association of dietary animal and plant protein with putative risk markers of colorectal cancer in overweight pre-diabetic individuals during a weight-reducing programme: a PREVIEW sub-study. Eur J Nutr. 2020;59(4):1517–1527. doi: 10.1007/s00394-019-02008-2. [DOI] [PubMed] [Google Scholar]
- 55.Oliver A., Chase A.B., Weihe C., Orchanian S.B., Riedel S.F., Hendrickson C.L., et al. High-Fiber, whole-food dietary intervention alters the human gut microbiome but not fecal short-chain fatty acids. mSystems. 2021;6(2) doi: 10.1128/mSystems.00115-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.So D., Whelan K., Rossi M., Morrison M., Holtmann G., Kelly J.T., et al. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am J Clin Nutr. 2018;107(6):965–983. doi: 10.1093/ajcn/nqy041. [DOI] [PubMed] [Google Scholar]
- 57.Schwedhelm C., Pischon T., Rohrmann S., Himmerich H., Linseisen J., Nimptsch K. Plasma inflammation markers of the tumor necrosis factor pathway but not C-reactive protein are associated with processed meat and unprocessed red meat consumption in Bavarian adults. J Nutr. 2017;147(1):78–85. doi: 10.3945/jn.116.237180. [DOI] [PubMed] [Google Scholar]
- 58.Montonen J., Boeing H., Fritsche A., Schleicher E., Joost H.G., Schulze M.B., et al. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur J Nutr. 2013;52(1):337–345. doi: 10.1007/s00394-012-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arya F., Egger S., Colquhoun D., Sullivan D., Pal S., Egger G. Differences in postprandial inflammatory responses to a ‘modern’ v. traditional meat meal: A preliminary study. Br J Nutr. 2010;104(5):724–728. doi: 10.1017/S0007114510001042. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J., Wang C., Li L., Man Q., Meng L., Song P., et al. Dietary inclusion of salmon, herring and pompano as oily fish reduces CVD risk markers in dyslipidaemic middle-aged and elderly Chinese women. Br J Nutr. 2012;108(8):1455–1465. doi: 10.1017/S0007114511006866. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Legarrea P., de la Iglesia R., Abete I., Navas-Carretero S., Martinez J.A., Zulet M.A. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition. 2014;30(4):424–429. doi: 10.1016/j.nut.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Marx W., Lane M., Hockey M., Aslam H., Berk M., Walder K., et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 2021;26(1):134–150. doi: 10.1038/s41380-020-00925-x. [DOI] [PubMed] [Google Scholar]
- 63.D’Cunha N.M., Foscolou A., Tyrovolas S., Chrysohoou C., Rallidis L., Polychronopoulos E., et al. The association between protein consumption from animal and plant sources with psychological distress in older people in the Mediterranean region. Nutr Healthy Aging. 2020;5(4):273–285. [Google Scholar]
- 64.Zhang Y., Yang Y., Xie M.S., Ding X., Li H., Liu Z.C., Peng S.F. Is meat consumption associated with depression? A meta-analysis of observational studies. BMC Psychiatry. 2017;17(1):409–410. doi: 10.1186/s12888-017-1540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nucci D., Fatigoni C., Amerio A., Odone A. Gianfredi v, Red and Processed Meat Consumption and Risk of Depression: a Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2020;17(18):1–20. doi: 10.3390/ijerph17186686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Darooghegi Mofrad M.M., Mozaffari H., Sheikhi A., Zamani B., Azadbakht L. The association of red meat consumption and mental health in women: a cross-sectional study. Complement Ther Med. 2021;56:102588–102589. doi: 10.1016/j.ctim.2020.102588. [DOI] [PubMed] [Google Scholar]
- 67.Kazemi S., Keshteli A.H., Saneei P., Afshar H., Esmaillzadeh A., Adibi P. Red and white meat intake in relation to mental disorders in Iranian adults. Front Nutr. 2021:450–451. doi: 10.3389/fnut.2021.710555. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y., Zeng L., Zou K., Shan S., Wang X., Xiong J., et al. Role of dietary factors in the prevention and treatment for depression: an umbrella review of meta-analyses of prospective studies. Transl Psychiatry. 2021;11(1):478–479. doi: 10.1038/s41398-021-01590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shab-Bidar A. Jayedi S. Fish consumption and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Adv Nutr. 2020;11(5):1123–1133. doi: 10.1093/advances/nmaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To ensure compliance with the study’s informed consent process, we would share de-identified data and associated data dictionary only under a formalized data sharing and use agreement that provides the commitment to the following: 1) using the data only for research purposes and not identifying any individual participant; 2) securing the data using appropriate computer technology, which needs to be specified, 3) destroying or returning the data after analyses are completed, 4) accepting reporting responsibilities, 5) abiding by restrictions on redistribution of the data for commercial purposes or to third parties, and 6) proper acknowledgment of the data resource. Additionally, appropriate fees may be assessed upon mutual agreement on requests for information in a format other than that we intend to provide. We will not be responsible for providing any analytical support.