Abstract
Background
The effect of postoperative delirium on the amyloid cascade of Alzheimer's dementia is poorly understood. Using early postoperative plasma biomarkers, we explored whether surgery and delirium are associated with changes in amyloid pathways.
Methods
We analysed data from 100 participants in the Interventions for Postoperative Delirium: Biomarker-3 (IPOD-B3) cohort study in the USA (NCT03124303 and NCT01980511), which recruited participants aged >65 yr undergoing non-intracranial surgery. We assessed the relationship between the change in plasma amyloid beta ratio (AβR; Aβ42:Aβ40) and delirium incidence (defined by the 3-Minute Diagnostic Confusion Assessment Method) and severity (quantified by the Delirium Rating Scale-Revised-98, the study's primary outcome). We also tested the relationship between plasma amyloid beta and intraoperative variables.
Results
Across all participants, the plasma AβR increased from the preoperative period to postoperative Day 1 (Wilcoxon P<0.001). However, this increase was not associated with delirium incidence (Wilcoxon P=0.22) or peak severity after adjusting for confounders (log[incidence rate ratio]=0.43; P=0.14). Postoperative Day 1 change in plasma AβR was not associated with postoperative Day 1 change in plasma tau, neurofilament light, or inflammatory markers (interleukin [IL]-1β, IL-1Ra, IL-2, IL-4, IL-6, IL-8, IL-10, and IL-12), or with operative time or low intraoperative arterial pressure.
Conclusions
Perioperative changes in plasma amyloid do not appear to be associated with postoperative delirium. Our findings do not support associations of dynamic changes in amyloid with postoperative delirium.
Clinical trial registration
.NCT03124303 and NCT01980511.
Keywords: Alzheimer's disease, amyloid beta, anaesthesia, delirium, dementia, neurocognitive disorders, surgery
Editor's key points.
-
•
A relationship between postoperative delirium and the amyloid cascade of Alzheimer's disease has been suggested but is poorly understood.
-
•
This study used early postoperative plasma biomarkers to explore whether surgery and delirium are associated with changes in amyloid pathways.
-
•
An increase in the plasma amyloid beta ratio from preoperative baseline to postoperative Day 1 was observed but was not associated with the incidence or severity of postoperative delirium.
-
•
These findings do not support associations of changes in plasma amyloid with postoperative delirium; further studies are required to validate other potential biomarkers.
Surgery and anaesthesia have been associated with perioperative neurocognitive disorders (NCDs), including short-term (postoperative delirium and delayed neurocognitive recovery)1 and long-term (postoperative mild or major neurocognitive disorder)2,3 manifestations. Postoperative delirium may play a causal role in accelerating long-term cognitive decline4,5; however, confounding factors in this relationship mean inferences of causation are tenuous. Moreover, not all studies have observed poorer overall long-term cognition in patients with postoperative delirium.6 Investigation of this topic is hampered by uncertainty regarding the neuropathological mechanisms that could underlie this relationship.
The recent amyloid–tau–neurodegeneration (ATN) model provides a pathogenic framework of Alzheimer's disease (AD).7 This model argues that accumulation of toxic amyloid beta (Aβ) species (‘A’) and intracellular hyperphosphorylated tau (‘T’), with accompanying neurodegeneration (‘N’), are pathological hallmarks of AD. Although designed to make no inferences of causality or temporal sequences of these events, the ATN framework implies a deterministic model that relies heavily on the amyloid hypothesis. The amyloid hypothesis posits that the pathophysiological cascade that results in AD is initially triggered by pathogenic Aβ peptide aggregation.8 Preclinical studies have shown that general anaesthetic exposure is associated with increased Aβ production and aggregation,9 possibly implicating general anaesthetics (especially volatile agents10) in the amyloid hypothesis.
Walker and colleagues11 recently highlighted the need for a greater understanding of the biological link between postoperative delirium and long-term cognitive changes, particularly with respect to the amyloid hypothesis as a possible explanation. Previously, we have shown associations of delirium with markers of neurodegeneration (plasma neurofilament light [NfL]12,13 and tau13,14), which have been replicated in other studies.15,16 Herein, we extend our biomarker analyses focusing on the amyloid hypothesis.
Changes in the amyloid cascade that might accompany postoperative delirium are not well understood. Of two small human positron emission tomography (PET) imaging studies, one observed no correlation between cerebral amyloid burden and postoperative delirium,17 whereas our pilot study reported a positive association with delirium severity.18 The pitfall of these neuroimaging studies is that the static ‘snapshot’ of imaging might fail to track important short-term changes, which could influence the pathological trajectory longer term. Although low preoperative CSF Aβ42 has been associated with postoperative delirium in one study,19 another study found no such relationship.20 A recent small study by our group reported that CSF and plasma amyloid beta ratio (AβR; Aβ42:Aβ40) increased from the preoperative to postoperative period, but peak postoperative change in CSF and plasma AβR was not associated with peak delirium severity or delirium incidence.13 However, an adequately powered sample was not obtained, and hence, we undertook a larger sample based on our previous observation13 that CSF and plasma values for change in Aβ40 (Spearman ρ=0.929; P=0.007) and Aβ42 (Pearson r=0.793; P=0.033) on postoperative Day 1 (POD1) were correlated in the 13 patients with CSF samples. We focus on the plasma AβR over Aβ40 or Aβ42 levels, as the former has been shown in meta-analysis to be more closely associated with cognitive decline.21 Our approach is consistent with other studies using plasma AβR in chronic settings.22,23
We hypothesise a causal pathway that anaesthesia/surgery alters the amyloid pathway (detected by fluctuations in plasma Aβ) that is proportional to delirium severity with potential implications for long-term cognition. We contend that a strong correlation between perioperative changes in plasma amyloid and delirium, which is not explained by known confounders, would provide evidence for acceleration of the amyloid cascade, which might explain links between delirium and dementia. Our specific aims were to (i) investigate the relationship between plasma AβR and delirium incidence and severity, (ii) confirm the postoperative increase in plasma AβR in a larger cohort than our previous study, and (iii) establish covariates that could explain such an increase in plasma AβR.
Methods
We report outcomes from participants in the Interventions for Postoperative Delirium: Biomarker-3 (IPOD-B3) study (NCT03124303 and NCT01980511), which is an ongoing prospective observational cohort study in the USA enrolling patients undergoing non-intracranial surgery ≥65 yr of age. The University of Wisconsin–Madison Institutional Review Board provided ethical approval for the study (2015-374). Further details on this cohort are described elsewhere.14,24 The 14 participants in our previous study13 with data for postoperative change in plasma AβR were also included in this study. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used to report our findings.25
Outcomes
The primary outcome of this study was POD1 change in AβR relative to peak delirium severity, as defined by the Delirium Rating Scale-Revised-98 (DRS-R-98).26 Consistent with our previous work,12 POD1 change was preferred over peak postoperative change, as the latter is biased towards patients with longer postoperative stays.27 Secondary outcomes included POD1 change in AβR based on delirium incidence, which was assessed using the 3-Minute Diagnostic Confusion Assessment Method (3D-CAM)28 or CAM for the ICU (CAM-ICU)29 (if intubated). Assessments were performed twice daily from postoperative Days 1–4, between 05:00–10:00 and 16:00–20:00 supplemented with a chart-based review.30 Preoperative baseline cognitive testing was performed using the Montreal Cognitive Assessment (MoCA),31 Trail Making Test B (TMTB),32 and Controlled Oral Word Association Test (COWAT).33 Intraoperative data, including operation time in minutes, arterial pressure, and blood loss, were obtained from the medical record. Secondary analyses were performed for all outcomes using Aβ40 and Aβ42, and using the peak postoperative change in Aβ40, Aβ42, and AβR. The AβR was calculated by dividing the plasma concentration of Aβ42 by that of Aβ40. We emphasise the results of Aβ42 over Aβ40 because the former is thought to be the more pathogenic amyloid species.34 We also analysed the correlation between postoperative change in amyloid and operative time, intraoperative arterial pressure, and anaesthetic dose to assess the relationship to intraoperative variables. Age-adjusted median sevoflurane (AMS) concentration was calculated by dividing the median sevoflurane concentration (in minimum alveolar concentration [MAC] units) by 2.03∗10(−0.00301∗[age minus 40]).35
Biomarker collection and analysis
Venous blood was collected in ethylenediaminetetraacetic acid tubes before surgery and each morning (04:00–11:00) from POD1 to 4 as close as possible to the time of delirium assessment and stored at –80°C. Samples were also taken at long-term follow-up, at POD90 and POD365. We sent samples to the University of Gothenburg for analysis using an ultrasensitive single-molecule array36 immunoassay for quantification of plasma Aβ40 and Aβ42 according to the manufacturer (Quanterix, Billerica, MA, USA). Blood samples were analysed by laboratory technicians who were blinded to clinical details.
Power analysis
Our primary outcome of POD1 change in AβR and peak delirium severity was adjusted for possible confounders: age, sex, and cognitive baseline. Based on a four-factor general linear model, 99 participants would be required to show a moderate effect size (Cohen F2=0.15) with 90% power at the 0.05 significance level. To ensure this sample size was reached and allow correspondence to the prior analyses of tau and NfL, we sent 114 subject samples for analysis, of which 105 were successfuly assayed for baseline amyloid data.
Statistical analysis
All analyses were performed in R (R Studio 2022.02.1 build 461, base R 4.1.3; https://posit.co/). All biomarker and TMTB data were normalised by log10 transformation. In time course plots, biomarkers were further normalised to baseline. Changes in each biomarker were calculated by subtracting the baseline value from the value on the postoperative day of interest. For all analyses, outliers were identified using Cook's distance with a threshold of >4μ to justify exclusion. The Shapiro–Wilk test, skewness, and visual inspection of histograms and boxplots were used to assess for normality. For unpaired, non-normal data, Spearman correlation methods were used to compare continuous outcomes, and Mann–Whitney/Wilcoxon rank sum tests with continuity correction were used for dichotomous delirium incidence. Fisher's exact test was used to compare proportions. Preoperative to postoperative (paired) change in amyloid was assessed using a Wilcoxon signed rank test. Linear regression with peak DRS-R-98 as the dependent variable used a Poisson distribution family. This was the most suitable model given DRS-R-98 is a count variable with significant rightward skew. We performed a sensitivity analysis using a Gaussian distribution, given this was the method used for our power analysis. Predictors for multivariable models were selected based on previous evidence of their influence on delirium severity or plasma Aβ concentration. Predictors were added using forced entry methods. Bayesian information criteria (BIC) were used to assess model fit. A P-value of <0.05 was used as the threshold for statistical significance. No adjustments were made for multiple comparisons.
Results
A total of 101 participants in the IPOD-B3 study had documented delirium assessments and baseline plasma amyloid levels. One patient experienced acute alcohol withdrawal and was excluded from our analysis, leaving 100 subjects (Supplementary Fig 1). The baseline characteristics of participants in this study are provided in Table 1; 35 of the 100 subjects (35%) were diagnosed with postoperative delirium. Consistent with our prior report on this cohort,12 greater intraoperative blood loss and longer operation times were observed in subjects with delirium. Higher National Surgical Quality Improvement Program-risk of death (NSQIP-D) and -serious complications (NSQIP-SC) scores were also noted in the delirium group. In the delirium group, the mean baseline COWAT scores were lower, and the mean baseline TMTB scores were qualitatively higher than in the no-delirium group. The median length of stay was longer in patients with delirium, and the distribution of length of stay showed a significant rightward skew (Supplementary Fig 2).
Table 1.
Baseline patient characteristics. AUC, area under the curve; CI, confidence interval; COWAT, Controlled Oral Word Association Test; IQR, inter-quartile range; MoCA, Montreal Cognitive Assessment; NSQIP-D, National Surgical Quality Improvement Program-risk of death; NSQIP-SC, National Surgical Quality Improvement Program-serious complications; TMTB, Trail Making Test B. ∗Median (IQR); n (%). †Standardised mean difference.
| Characteristic | Overall, N=100∗ | Delirium |
Difference† | 95% CI† | |
|---|---|---|---|---|---|
| Yes, N=35∗ | No, N=65∗ | ||||
| Age (yr) | 71(67–75) | 70 (66–74) | 71 (67–76) | –0.40 | –0.81 to 0.02 |
| Sex, n (%) | 0.20 | –0.21 to 0.61 | |||
| Female | 45 (45) | 18 (51) | 27 (42) | ||
| Male | 55 (55) | 17 (49) | 38 (58) | ||
| NSQIP-D | 1.0 (0.2–3.7) | 3.4 (1.6–5.4) | 0.6 (0.2–1.9) | 0.66 | 0.24–1.1 |
| NSQIP-SC | 14 (7–27) | 27 (19–36) | 10 (7–16) | 1.3 | 0.85–1.7 |
| Operating time (min) | 318 (206–446) | 453 (400–579) | 259 (180–350) | 1.4 | 0.94–1.8 |
| Blood loss (ml) | 500 (150–2350) | 3000 (1000–5675) | 320 (100–700) | 1.1 | 0.62–1.5 |
| Log10 blood pressure AUC 10% | 4.88 (4.54–5.27) | 4.92 (4.46–5.39) | 4.87 (4.56–5.11) | 0.12 | –0.29 to 0.53 |
| Surgery type, n (%) | 0.76 | 0.34–1.2 | |||
| General | 10 (10) | 4 (11) | 6 (9.2) | ||
| Orthopaedic | 36 (36) | 6 (17) | 30 (46) | ||
| Urological | 9 (9.0) | 2 (5.7) | 7 (11) | ||
| Vascular | 45 (45) | 23 (66) | 22 (34) | ||
| Baseline TMTB (s) | 84 (60–121) | 96 (72–150) | 81 (57–114) | 0.41 | –0.05 to 0.87 |
| Baseline MoCA | 24 (23–26) | 24 (23–26) | 24 (23–26) | –0.18 | –0.65 to 0.29 |
| Baseline COWAT | 32 (24–39) | 27 (18–32) | 32 (27–42) | –0.81 | –1.3 to –0.34 |
| Length of hospital stay (days) | 4 (2–9) | 9 (7–14) | 3 (2–5) | 0.79 | 0.36–1.2 |
Time course of plasma Aβ levels
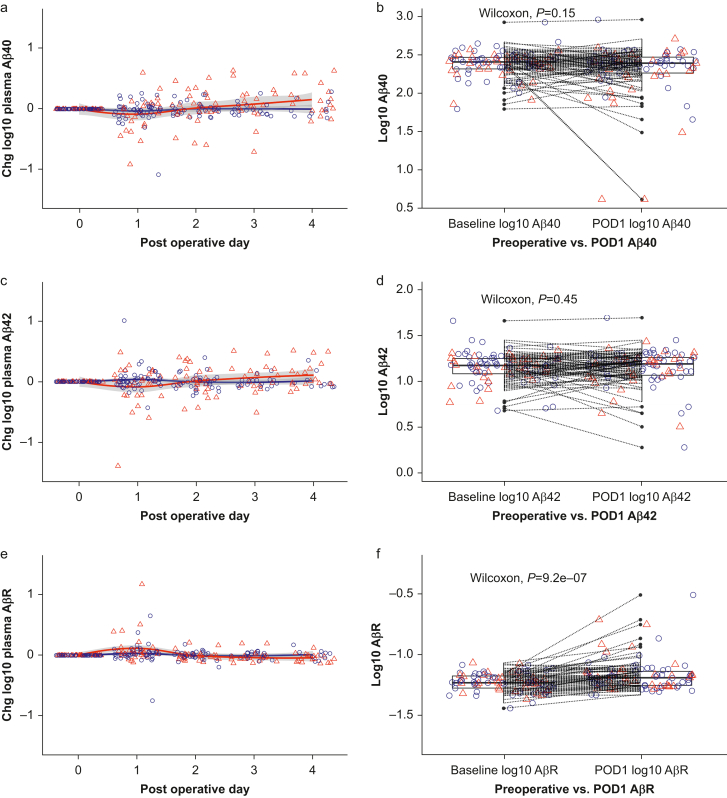
Time course plots of plasma Aβ40, Aβ42, and AβR concentrations (normalised to baseline) over the first 4 postoperative days are shown in Fig 1 with smoothed LOESS (locally estimated scatterplot smoothing) regression curves for subjects with and without delirium. Although there was no significant change in plasma Aβ40 (Wilcoxon P=0.15; Fig 1b) or Aβ42 (Wilcoxon P=0.45; Fig 1d) from baseline to POD1, there was an increase in plasma AβR across this time period (Wilcoxon P<0.001; Fig 1f). This finding remained significant when excluding the 14 participants who overlapped from our previous study (Supplementary Fig 3). Analyses using peak postoperative change in Aβ also showed significant increases in Aβ40, Aβ42, and AβR from baseline (Supplementary Fig 4). Comparison of the plasma CSF time course of Aβ shows strong similarity in trajectory between the two compartments (Supplementary Fig 5), consistent with our previous correlations.13 A generalised linear model was constructed to investigate this postoperative rise in AβR, which suggested that age, sex, NSQIP-D, and area under the curve (AUC) of sevoflurane dose did not explain the POD1 rise in AβR (Table 2).
Fig 1.
(a, c, e) Time plots of Aβ40, Aβ42, and Aβ ratio across postoperative Days 1–4 and (b, d, f) change from baseline on postoperative Day 1. Wilcoxon signed rank test was used to determine the postoperative Day 1 change in each biomarker (paired data). Based on Cook's distances, two, three, and three outliers were excluded for Aβ40, Aβ42, and AβR, respectively. Red triangles indicate participants diagnosed with postoperative delirium, and blue circles represent those without delirium. The red and blue lines represent smoothed LOESS regression curves for participants with and without delirium, respectively. Aβ, amyloid beta; AβR, amyloid beta ratio; DRS, Delirium Rating Scale; LOESS, locally estimated scatterplot smoothing; POD, postoperative day.
Table 2.
Linear regression predicting change in AβR from preoperative to POD1. Two outliers were excluded based on Cook's distances for peak DRS-R-98 ∼ POD1 change in plasma AβR. AβR, amyloid beta ratio; AIC, Akaike's information criteria; AUC, area under the curve; BIC, Bayesian information criteria; CI, confidence interval; df, degrees of freedom; DRS-R-98, Delirium Rating Scale-Revised-98; NSQIP-D, National Surgical Quality Improvement Program-risk of death; POD, postoperative day; se, standard error. Number of observations=65; log-likelihood=36.8; AIC=–61.5; BIC=–48.5; residual df=60. ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001.
| Characteristic | Beta | se | 95% CI | P-value |
|---|---|---|---|---|
| (Intercept) | 0.1003 | 0.266 | –0.4220 to 0.6227 | 0.71 |
| Age | –0.0008 | 0.004 | –0.0078 to 0.0061 | 0.81 |
| Sex: male | 0.0383 | 0.036 | –0.0332 to 0.1097 | 0.30 |
| NSQIP-D | 0.0063 | 0.007 | –0.0071 to 0.0196 | 0.36 |
| Log10 AUC sevoflurane | –0.0077 | 0.028 | –0.0626 to 0.0471 | 0.78 |
Primary outcome: association of AβR with postoperative peak delirium severity
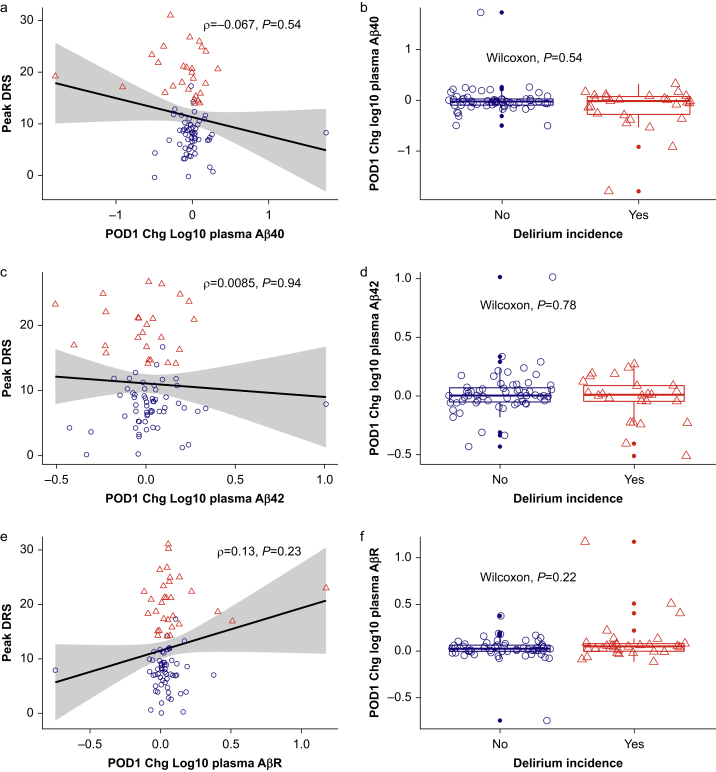
There was no statistically significant correlation between peak DRS-R-98 and POD1 change in Aβ40 (Spearman's ρ=–0.067; P=0.54; Fig 2a), Aβ42 (ρ=0.0085; P=0.94; Fig 2c), or the primary outcome, AβR (ρ=0.13; P=0.23; Fig 2e). Similarly, there was no difference in patients with and without delirium in terms of POD1 change in Aβ40 (Wilcoxon P=0.54; Fig 2b), Aβ42 (Wilcoxon P=0.78; Fig 2d), or AβR (Wilcoxon P=0.22; Fig 2f).
Fig 2.
Peak DRS-R-98 to POD1 change in plasma amyloid univariate (a, c, e) correlation plots and (b, d, f) delirium boxplots. Based on Cook's distances for peak DRS-R-98 ∼ amyloid univariate regression, six, seven, and three outliers were excluded for Aβ40, Aβ42, and AβR, respectively. Spearman methods were used for univariate correlation. Red triangles indicate participants diagnosed with postoperative delirium, and blue circles represent those without delirium. Black lines represent linear regression smoothing for all participants. Aβ, amyloid beta; AβR, amyloid beta ratio; DRS, Delirium Rating Scale-Revised-98; POD, postoperative day.
Poisson regression of peak DRS-R-98 with POD1 plasma Aβ change after adjusting for age, sex, and baseline TMTB is described in Table 3. A decrease in plasma Aβ40 on POD1 was associated with more severe delirium in unadjusted (log[incidence rate ratio {IRR}]=–0.31; P<0.001) and adjusted (log[IRR]=–0.22; P=0.023) models. Conversely, POD1 change in plasma Aβ42 was not associated with peak delirium severity in unadjusted (log[IRR]=–0.19; P=0.29) or adjusted (log[IRR]=–0.13; P=0.49) models. An increase in POD1 plasma AβR was associated with higher delirium severity in unadjusted (log[IRR]=0.57; P<0.001) but not adjusted (log[IRR]=0.43; P=0.14) models. In the sensitivity analysis using a Gaussian distribution, POD1 change in plasma AβR was not associated with peak DRS-R-98 in unadjusted or adjusted models (Supplementary Table 1).
Table 3.
Poisson regression of peak DRS ∼ POD1 change in plasma amyloid. Based on Cook's distances for univariate peak DRS ∼ amyloid regression, six, seven, and three outliers were excluded for Aβ40, Aβ42, and AβR, respectively. Aβ, amyloid beta; AβR, amyloid beta ratio; AIC, Akaike's information criteria; BIC, Bayesian information criteria; CI, confidence interval; df, degrees of freedom; DRS, Delirium Rating Scale; IRR, incidence rate ratio; POD, postoperative day; se, standard error; TMTB, Trail Making Test B. Aβ40: AIC=594; BIC=606. Aβ42: AIC=596; BIC=607. AβR: AIC=665; BIC=677. ∗P<0.05; ∗∗P<0.01; ∗∗∗P<0.001.
| Characteristic | Aβ40 |
Aβ42 |
AβR |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Log(IRR) | se | 95% CI | P-value | N | Log(IRR) | se | 95% CI | P-value | N | Log(IRR) | se | 95% CI | P-value | |
| Unadjusted analysis | |||||||||||||||
| (Intercept) | 85 | 2.4 | 0.033 | 2.4–2.5 | <0.001∗∗∗ | 84 | 2.4 | 0.033 | 2.3–2.5 | <0.001∗∗∗ | 88 | 2.4 | 0.033 | 2.4–2.5 | <0.001∗∗∗ |
| POD1 change in plasma amyloid | 85 | –0.31 | 0.092 | –0.48 to –0.13 | <0.001∗∗∗ | 84 | –0.19 | 0.18 | –0.55 to 0.16 | 0.29 | 88 | 0.57 | 0.152 | 0.26–0.86 | <0.001∗∗∗ |
| Adjusted analysis | |||||||||||||||
| (Intercept) | 77 | 2.97 | 0.559 | 1.88–4.07 | <0.001∗∗∗ | 77 | 3.14 | 0.56 | 2.049–4.24 | <0.001∗∗∗ | 79 | 3.36 | 0.540 | 2.30–4.42 | <0.001∗∗∗ |
| POD1 change in plasma amyloid | 77 | –0.22 | 0.100 | –0.42 to –0.029 | 0.023∗ | 77 | –0.13 | 0.20 | –0.523 to 0.24 | 0.49 | 79 | 0.43 | 0.294 | –0.13 to 1.02 | 0.14 |
| Age | 77 | –0.010 | 0.008 | –0.026 to 0.0057 | 0.21 | 77 | –0.013 | 0.008 | –0.029 to 0.0028 | 0.11 | 79 | –0.016 | 0.008 | –0.031 to –0.0007 | 0.042∗ |
| Sex: male | 77 | –0.18 | 0.070 | –0.32 to –0.042 | 0.010∗ | 77 | –0.15 | 0.070 | –0.287 to –0.012 | 0.034∗ | 79 | –0.11 | 0.068 | –0.25 to 0.020 | 0.094 |
| TMTB baseline | 77 | 0.0023 | 0.001 | 0.0009–0.004 | <0.001∗∗∗ | 77 | 0.0025 | 0.001 | 0.001–0.004 | <0.001∗∗∗ | 79 | 0.0026 | 0.001 | 0.0013–0.0039 | <0.001∗∗∗ |
Secondary analysis: association of peak change and baseline amyloid with delirium severity
Analyses using peak change in plasma Aβ showed a statistically significant association of change in plasma Aβ40 with peak DRS-R-98 (ρ=0.26; P=0.013), and the change in Aβ40 was higher in delirious patients (Wilcoxon P=0.0024). There were no statistically significant associations between peak plasma Aβ42 or AβR and delirium incidence or severity (Supplementary Fig 6). Peak DRS-R-98 was not correlated with preoperative plasma Aβ40, Aβ42, or AβR, and preoperative concentrations of these biomarkers were not different in subjects with postoperative delirium compared with those without delirium (Supplementary Fig 7).
Poisson regression showed that an increase in peak plasma Aβ40 and Aβ42 explained peak delirium severity in adjusted and unadjusted models. AβR was significant in the unadjusted model but not in the model adjusted for age, sex, and baseline TMTB (Supplementary Table 2).
Secondary outcome: association of perioperative variables with amyloid
There was no relationship between AUC low arterial pressure (<10% of baseline) and plasma Aβ40, Aβ42, and AβR on either POD1 or peak change analyses (Supplementary Fig 8). Similarly, there was no correlation between operation time and peak or POD1 change in plasma Aβ40, Aβ42, and AβR, with the exception of peak change in Aβ40, which was positively correlated with operation time (ρ=0.26; P=0.012; Supplementary Fig 9). There was no significant correlation between POD1 change in plasma AβR and POD1 changes in plasma inflammatory markers (interleukin [IL]-1β, IL-1Ra, IL-2, IL-4, IL-6, IL-8, IL-10, and IL-12) (Supplementary Fig 10). Plasma Aβ40 was negatively correlated with POD1 change in plasma IL-2 and IL-4 (Supplementary Fig 11), whereas POD1 change in Aβ42 was negatively correlated with POD1 change in plasma IL-1β, IL-2, IL-4, IL-6, and IL-12 (Supplementary Fig 12).
A total of 70 participants received sevoflurane for maintenance for anaesthesia, whereas the remaining 30 did not receive a volatile agent. There was no difference in POD1 change in plasma Aβ40, Aβ42, and AβR in those with an AMS concentration of 0.2–1.0 MAC compared with those with AMS >1.0 MAC (Supplementary Fig 13). Similarly, excluding those with AMS <0.2, there was no correlation between AUC of sevoflurane and delirium incidence or severity (Supplementary Fig 14).
The POD1 change in both plasma Aβ40 and Aβ42 was positively correlated with the POD1 change in plasma tau, whereas there was no correlation between AβR and tau. The POD1 change in plasma NfL was not correlated with the POD1 change in any plasma amyloid biomarker (Supplementary Fig 15).
Discussion
We explored the short-term relationship between surgery, postoperative delirium, and plasma Aβ levels. As in our previous study,13 plasma AβR increased from baseline to POD1. However, after adjusting for covariates, this increase did not appear to be associated with delirium incidence or peak severity. Postoperative plasma amyloid concentrations do not appear to correlate sufficiently with delirium to implicate amyloid changes in the link between delirium and accelerated cognitive decline that has been reported in other studies. Hence, despite no long-term cognitive outcomes being reported, our results do not support a pathophysiological chain linking postoperative delirium with long-term postoperative cognitive decline involving the amyloid hypothesis of AD.
The absence of a significant change in plasma Aβ42 from before surgery to postoperative Day 1 supports other studies without delirium analyses showing no change in CSF Aβ42 postoperatively.37, 38, 39 One study showed no change in CSF Aβ42 from baseline to up to 24 h postoperatively in 39 participants,37 which was supported by another study with 103 participants showing no change in CSF Aβ42 out to 6 weeks postoperatively.38 Furthermore, a PET study of 40 patients undergoing cardiac surgery showed no association between cortical amyloid burden and cognitive function at 6 weeks postoperatively, no difference in cortical amyloid burden between patients undergoing surgery and controls not undergoing surgery at 6 weeks postoperatively, and no association between cortical amyloid burden at 6 weeks or 1 yr postoperatively and cognitive function at 1 or 3 yr postoperatively.40 Another PET study in 313 participants showed no differences in the odds of elevated brain amyloid between participants with previous surgical hospitalisation compared with those without.41 Although no direct inferences about delirium can be made, the normal distribution of data in the aforementioned studies provides evidence against a subpopulation (including those with postoperative delirium) that might have larger fluctuations in CSF Aβ42 in the perioperative period. However, these studies do not report the changes in CSF AβR with surgery, which have been shown to be more strongly associated with CSF tau and cognitive decline than Aβ4242; it was for this reason that we focused on the AβR. Furthermore, our prior analyses of PET18 and CSF13 focused on continuous relationships with delirium severity that are not dependent on subgroups, so we believe that further investigations are required to elucidate links of amyloid disease, delirium, and longer-term cognitive changes.
Our observation that the plasma AβR increases after surgery suggests a small increase in Aβ42 and decrease in Aβ40 that were not large enough on their own to reach statistical significance. It is unclear for how long the short-term increase in plasma AβR that we observed persists and if there is any impact on long-term trends in plasma AβR, brain amyloid deposition, or cognitive function. Longitudinal studies have shown that plasma AβR decreases proportionally with age and increasing cerebral amyloid deposition,22,43 and this decrease is associated with greater cognitive decline in many44,45 but not all46 studies. It is possible that the increase in plasma AβR relates to worsening AD pathology that is independent of any effect on postoperative delirium, although the links drawn here would be speculative. Moreover, if general anaesthesia were increasing accumulation of the more pathogenic Aβ42 species in the brain, we would expect a decrease, not an increase, in the plasma AβR after surgery.
The observed negative correlation between plasma Aβ40 and delirium severity could represent greater CNS Aβ40 deposition in patients with more severe delirium. However, this is unlikely to carry pathological significance. Despite the concentration of Aβ40 in the CNS being several-fold higher than of Aβ42,47 the latter forms the major (and sometimes only) component of brain amyloid plaques,48 and even small increases in the AβR induce greater neurotoxicity.49 Perhaps a more parsimonious explanation is that plasma Aβ40 has shown an inverse correlation with IL-1β in other studies50 (although it did not reach statistical significance in our cohort [P=0.079]), which suggests the association of Aβ40 with delirium severity merely reflects its inverse relationship with inflammation, a putative driver of delirium severity.
The increase in CSF and plasma AβR observed in our study could represent increased amyloid clearance from the CNS by microglia; expression of IL-1β is increased in surgery, and stimulation by IL-1β has been shown to promote microglial clearance of Aβ.51 Although this hypothesis and other provocative neuropathological explanations are attractive, they are difficult to support. First, we observed no correlation between plasma inflammatory markers and POD1 change in plasma AβR. Second, plasma Aβ is a less reliable indicator of AD compared with CSF Aβ,52,53 as Aβ is also produced by non-CNS organs (e.g. liver and kidney),54 in which homeostasis is often perturbed by surgery, and other unknown factors contributing to the production, clearance, and equilibration of Aβ might also play a role. The relationship between CSF and plasma Aβ is much more poorly understood in the perioperative setting compared with non-perioperative settings. As such, inferences regarding CNS amyloid based on plasma amyloid measurements should be drawn with caution until more is known about the relationship between amyloid concentrations in the two compartments. Changes in plasma Aβ have also been associated with white matter hyperintensities, lacunar infarcts, and hypoxic brain injury after cardiac arrest,55, 56, 57 meaning perioperative plasma Aβ could be influenced by ischaemia. Together, the aforementioned observations suggest plasma Aβ could fluctuate in the perioperative period independent of any change in cerebral amyloid burden that is of pathophysiological significance to delirium and AD. Third, preoperative Aβ was not correlated with delirium incidence or severity in our cohort; however, our study was likely underpowered for this secondary outcome. Finally, we are not aware of a rationale for the relative increase in plasma Aβ42 over Aβ40 in the perioperative period. Although differential cleavage of the amyloid precursor protein in central or peripheral tissues could be induced by anaesthesia or surgery, or Aβ42 could be preferentially cleared from plaques in the brain, preclinical studies have shown no obvious signal for this.9,10,58
We did not find evidence to support Aβ as an explanatory link for any causative relationship between short-term and long-term postoperative cognitive decline. These findings are supported by a recent cross-sectional study that observed an association between past surgery and cortical thinning on PET imaging in areas typically implicated in AD but no association between past surgery and cerebral amyloid deposition.59 Considering the reported association between surgery and accelerated cognitive decline in some59,60 but not all61 high-quality studies, current evidence suggests a harmful effect of surgery on neurodegeneration via a mechanism that is independent of cerebral Aβ deposition. This is supported by a study of CSF biomarkers showing no impact of surgery on CSF Aβ42 at 6 weeks postoperatively and no association between changes in Aβ42 and postoperative cognitive function, both of which were also true for CSF tau and pTau181.38 However, these findings stand in contrast to our own observations, where there were strong associations for CSF and plasma tau and pTau181.13 Further research is required to reconcile these differences.
Support for the role of tau in the clinical progression of AD appears to be greater than that for Aβ,62 and we have recently associated plasma tau with postoperative delirium incidence and severity.14,63 Furthermore, tau resolved in parallel with resolution of delirium symptoms. We observed a positive correlation of change in plasma tau with Aβ40 and Aβ42, and no correlation with AβR. Given the POD1 change in plasma Aβ40 and Aβ42 was not related to delirium incidence, and there was no correlation between amyloid and NfL, the relationship between tau and amyloid likely arose from processes separate to those occurring in delirium. Aside from tau, the recently proposed probabilistic model of AD also stresses the increased importance of stochastic factors and decreased importance of the amyloid cascade in the pathogenesis of apolipoprotein E (APOE) ε4-unrelated AD (62% of AD cases64).65 This suggests that future studies could consider assessing a broader range of potential pathophysiological pathways. For example, neuroinflammation driven by the surgery-induced peripheral inflammatory response has also emerged as a potentially critical mediator of delirium and postoperative cognitive decline,66 and inflammation was unrelated to amyloid in our data.
Our study had some limitations. We analysed only one component of the ATN framework, and we did not consider the many other factors implicated in AD pathogenesis. Moreover, the amyloid hypothesis, which was the focus of this study, implies a linear causal chain that begins with an amyloid trigger, an assumption that has come under significant scrutiny in recent years.67 Another possible reason for our negative finding is missing data in our primary outcome (n=82 prior to outlier exclusion): not all participants with amyloid data underwent baseline cognitive testing. However, it is worth reflecting that we retained 83% power to detect our primary outcome. We also did not report long-term cognitive data; hence, we cannot be certain that delirium was associated with long-term cognitive decline in our cohort. Future adequately powered studies should investigate the link with long-term cognitive decline and AβR; however, with advances in plasma phosphorylated tau assays, AβR could be superseded by a superior marker.13 We did not control for APOE ε4 status, which is known to affect plasma Aβ concentrations.68 However, APOE status has been found to not be associated with delirium.69 Our attrition rate for biomarker collection from POD1 onwards was an expected consequence of patient discharge from hospital (with a median length of stay of 4 days). We focused on POD1 in an effort to reduce bias, the pitfall being that we are limited in commenting on important fluctuations in Aβ that could occur in subsequent days and weeks.
Conclusions
We observed an increase in the plasma AβR from the preoperative to postoperative period; however, this increase was not associated with the incidence or severity of postoperative delirium. Perioperative fluctuations in the plasma AβR therefore appear to be unrelated to severe perioperative changes in cognition. Our findings do not support associations of dynamic changes in amyloid with postoperative delirium.
Authors’ contributions
Study design: RDS, RCL, RAP (in consultation with MP, CC, and DK).
Supply of assays: HZ, KB
Management of biofluid analysis: HZ, KB
Data analysis: TP
Drafting of paper: TP (with input from JT).
All authors provided critical feedback on the paper.
Declarations of interest
HZ has served at scientific advisory boards or as a consultant for AbbVie, Acumen, Alector, ALZpath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, AlzeCure, Biogen, and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KB has served as a consultant at advisory boards or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. The other authors declare no competing interests that may be relevant to this work.
Funding
US National Institutes of Health (NIH) (R01 AG063849-01) to RDS, RCL, and RAP; US National Institutes of Health (K23 AG055700) to RDS; Swedish Research Council (#2018-02532) to HZ (who is a Wallenberg Scholar); European Union's Horizon Europe research and innovation programme (No. 101053962) to HZ; Swedish State Support for Clinical Research (#ALFGBG-71320) to HZ; Alzheimer's Drug Discovery Foundation (ADDF), USA (#201809-2016862) to HZ; Alzheimer's Disease Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C) to HZ; Bluefield Project to HZ, Olav Thon Foundation to HZ, Erling Persson Family Foundation to HZ, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270) to HZ, European Union's Horizon 2020 research and innovation programme (Marie Skłodowska-Curie grant agreement no. 860197) (MIRIADE) to HZ; European Union Joint Programme–Neurodegenerative Disease Research (JPND2021-00694) to HZ; UK Dementia Research Institute at UCL (UKDRI-1003) to HZ; Swedish Research Council (#2017-00915) to KB; Alzheimer's Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615) to KB; Swedish Alzheimer's Foundation (#AF-930351, #AF-939721, and #AF-968270) to KB; Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006) to KB; Swedish state under the agreement between the Swedish government and the County Councils to KB; ALF agreement (#ALFGBG-715986 and #ALFGBG-965240) to KB; European Union Joint Programme for Neurodegenerative Disease Research (JPND2019-466-236) to KB; US National Institutes of Health (NIH), (1R01AG068398-01) to KB; Alzheimer's Association 2021 Zenith Award (ZEN-21-848495) to KB.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2023.01.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Rudolph J.L., Marcantonio E.R. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112:1202–1211. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause B.M., Sabia S., Manning H.J., Singh-Manoux A., Sanders R.D. Association between major surgical admissions and the cognitive trajectory: 19 year follow-up of Whitehall II cohort study. BMJ. 2019;366:l4466. doi: 10.1136/bmj.l4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulte P.J., Roberts R.O., Knopman D.S., et al. Association between exposure to anaesthesia and surgery and long-term cognitive trajectories in older adults: report from the Mayo Clinic Study of Aging. Br J Anaesth. 2018;121:398–405. doi: 10.1016/j.bja.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saczynski J.S., Marcantonio E.R., Quach L., et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inouye S.K., Marcantonio E.R., Kosar C.M., et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. doi: 10.1016/j.jalz.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown C.H.I.V., Probert J., Healy R., et al. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology. 2018;129:406–416. doi: 10.1097/ALN.0000000000002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack C.R., Jr., Bennett D.A., Blennow K., et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan Y., Xu J., Meng F., et al. Cognitive decline following major surgery is associated with gliosis, β-amyloid accumulation, and τ phosphorylation in old mice. Crit Care Med. 2010;38:2190–2198. doi: 10.1097/CCM.0b013e3181f17bcb. [DOI] [PubMed] [Google Scholar]
- 10.Eckenhoff R.G., Johansson J.S., Wei H., et al. Inhaled anesthetic enhancement of amyloid-β oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Walker K.A., Eckenhoff R.G., Brown C.H. Untangling anaesthesia and amyloid. Br J Anaesth. 2020;125:232–235. doi: 10.1016/j.bja.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Casey C.P., Lindroth H., Mohanty R., et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2019;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker M., White M., Casey C., et al. Cohort analysis of the association of delirium severity with cerebrospinal fluid amyloid–tau–neurodegeneration pathologies. J Gerontol A Biol Sci Med Sci. 2022;77:494–501. doi: 10.1093/gerona/glab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballweg T., White M., Parker M., et al. The association of plasma tau and postoperative delirium. Br J Anaesth. 2021;126:458–466. doi: 10.1016/j.bja.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fong T.G., Vasunilashorn S.M., Ngo L., et al. Association of plasma neurofilament light with postoperative delirium. Ann Neurol. 2020;88:984–994. doi: 10.1002/ana.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page V.J., Watne L.O., Heslegrave A., et al. Plasma neurofilament light chain protein as a predictor of days in delirium and deep sedation, mortality and length of stay in critically ill patients. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolandi E., Cavedo E., Pievani M., et al. Association of postoperative delirium with markers of neurodegeneration and brain amyloidosis: a pilot study. Neurobiol Aging. 2018;61:93–101. doi: 10.1016/j.neurobiolaging.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Velázquez M., Parker M., Bo A., et al. Amyloid deposition on positron emission tomography correlates with severity of perioperative delirium: a case-control pilot study. Br J Anaesth. 2022;128:e226–e228. doi: 10.1016/j.bja.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham E.L., McGuinness B., McAuley D.F., et al. CSF beta-amyloid 1-42 concentration predicts delirium following elective arthroplasty surgery in an observational cohort study. Ann Surg. 2019;269:1200–1205. doi: 10.1097/SLA.0000000000002684. [DOI] [PubMed] [Google Scholar]
- 20.Witlox J., Kalisvaart K.J., de Jonghe J.F., et al. Cerebrospinal fluid β-amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. J Am Geriatr Soc. 2011;59:1260–1267. doi: 10.1111/j.1532-5415.2011.03482.x. [DOI] [PubMed] [Google Scholar]
- 21.Koyama A., Okereke O.I., Yang T., Blacker D., Selkoe D.J., Grodstein F. Plasma amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fandos N., Pérez-Grijalba V., Pesini P., et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst) 2017;8:179–187. doi: 10.1016/j.dadm.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giudici K.V., de Souto Barreto P., Guyonnet S., et al. Assessment of plasma amyloid-β42/40 and cognitive decline among community-dwelling older adults. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.28634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanabe S., Mohanty R., Lindroth H., et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. doi: 10.1016/j.bja.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Trzepacz P.T., Mittal D., Torres R., Kanary K., Norton J., Jimerson N. Validation of the Delirium Rating Scale-Revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 27.Baek H., Cho M., Kim S., Hwang H., Song M., Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcantonio E.R., Ngo L.H., O’Connor M., et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ely E.W., Margolin R., Francis J., et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Inouye S.K., Leo-Summers L., Zhang Y., Bogardus S.T., Jr., Leslie D.L., Agostini J.V. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 31.Freitas S., Simões M.R., Alves L., Santana I. Montreal Cognitive Assessment: validation study for mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2013;27:37–43. doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- 32.Giovagnoli A.R., Del Pesce M., Mascheroni S., Simoncelli M., Laiacona M., Capitani E. Trail Making Test: normative values from 287 normal adult controls. Ital J Neurol Sci. 1996;17:305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- 33.Benton A.L., Hamsher K.D., Sivan A.B. Psychological Assessment Resources; Lutz, FL: 1994. Multilingual Aphasia Examination. [Google Scholar]
- 34.Murphy M.P., LeVine H., 3rd Alzheimer’s disease and the amyloid-beta peptide. J Alzheimers Dis. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooter M., Ni K., Thomas J., et al. Age-dependent decrease in minimum alveolar concentration of inhaled anaesthetics: a systematic search of published studies and meta-regression analysis. Br J Anaesth. 2020;124:e4–e7. doi: 10.1016/j.bja.2019.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rissin D.M., Kan C.W., Campbell T.G., et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger M., Nadler J.W., Friedman A., et al. The effect of propofol versus isoflurane anesthesia on human cerebrospinal fluid markers of Alzheimer’s disease: results of a randomized trial. J Alzheimers Dis. 2016;52:1299–1310. doi: 10.3233/JAD-151190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger M., Browndyke J.N., Cooter Wright M., et al. Postoperative changes in cognition and cerebrospinal fluid neurodegenerative disease biomarkers. Ann Clin Transl Neurol. 2022;9:155–170. doi: 10.1002/acn3.51499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Browndyke J.N., Wright M.C., Yang R., et al. Perioperative neurocognitive and functional neuroimaging trajectories in older APOE4 carriers compared with non-carriers: secondary analysis of a prospective cohort study. Br J Anaesth. 2021;127:917–928. doi: 10.1016/j.bja.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klinger R.Y., James O.G., Borges-Neto S., et al. 18F-florbetapir positron emission tomography-determined cerebral β-amyloid deposition and neurocognitive performance after cardiac surgery. Anesthesiology. 2018;128:728–744. doi: 10.1097/ALN.0000000000002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker K.A., Gottesman R.F., Coresh J., et al. Association of surgical hospitalization with brain amyloid deposition: the Atherosclerosis Risk in Communities-Positron Emission Tomography (ARIC-PET) study. Anesthesiology. 2020;132:1407–1418. doi: 10.1097/ALN.0000000000003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delaby C., Estellés T., Zhu N., et al. The Aβ1–42/Aβ1–40 ratio in CSF is more strongly associated to tau markers and clinical progression than Aβ1–42 alone. Alzheimers Res Ther. 2022;14:20. doi: 10.1186/s13195-022-00967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnham S.C., Fandos N., Fowler C., et al. Longitudinal evaluation of the natural history of amyloid-β in plasma and brain. Brain Commun. 2020;2:fcaa041. doi: 10.1093/braincomms/fcaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seppälä T.T., Herukka S.K., Hänninen T., et al. Plasma Aβ42 and Aβ40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. 2010;81:1123–1127. doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schupf N., Tang M.X., Fukuyama H., et al. Peripheral Aβ subspecies as risk biomarkers of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toledo J.B., Vanderstichele H., Figurski M., et al. Factors affecting Aβ plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol. 2011;122:401–413. doi: 10.1007/s00401-011-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta P.D., Pirttilä T., Mehta S.P., Sersen E.A., Aisen P.S., Wisniewski H.M. Plasma and cerebrospinal fluid levels of amyloid β proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 48.Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 49.Kuperstein I., Broersen K., Benilova I., et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahdavi M., Karima S., Rajaei S., et al. Plasma cytokines profile in subjects with Alzheimer’s disease: interleukin 1 alpha as a candidate for target therapy. Galen Med J. 2021;10 doi: 10.31661/gmj.v10i0.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rivera-Escalera F., Pinney J.J., Owlett L., et al. IL-1β-driven amyloid plaque clearance is associated with an expansion of transcriptionally reprogrammed microglia. J Neuroinflammation. 2019;16:261. doi: 10.1186/s12974-019-1645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rissman R.A., Trojanowski J.Q., Shaw L.M., Aisen P.S. Longitudinal plasma amyloid beta as a biomarker of Alzheimer’s disease. J Neural Transm (Vienna) 2012;119:843–850. doi: 10.1007/s00702-012-0772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toledo J.B., Shaw L.M., Trojanowski J.Q. Plasma amyloid beta measurements—a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res Ther. 2013;5:8. doi: 10.1186/alzrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selkoe D.J., Podlisny M.B., Joachim C.L., et al. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988;85:7341–7345. doi: 10.1073/pnas.85.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Dijk E.J., Prins N.D., Vermeer S.E., et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55:570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 56.Gurol M.E., Irizarry M.C., Smith E.E., et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 57.Zetterberg H., Mörtberg E., Song L., et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid β levels in humans. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie Z., Xu Z. General anesthetics and β-amyloid protein. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:140–146. doi: 10.1016/j.pnpbp.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sprung J., Warner D.O., Knopman D.S., et al. Exposure to surgery with general anaesthesia during adult life is not associated with increased brain amyloid deposition in older adults. Br J Anaesth. 2020;124:594–602. doi: 10.1016/j.bja.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eriksson L.I., Lundholm C., Narasimhalu K., et al. Hospitalization, surgery, and incident dementia. Alzheimers Dement. 2019;15:534–542. doi: 10.1016/j.jalz.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprung J., Jankowski C.J., Roberts R.O., et al. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc. 2013;88:552–561. doi: 10.1016/j.mayocp.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brier M.R., Gordon B., Friedrichsen K., et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med. 2016;8:338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McKay T.B., Qu J., Liang F., et al. Tau as a serum biomarker of delirium after major cardiac surgery: a single centre case-control study. Br J Anaesth. 2022;129:e13–e16. doi: 10.1016/j.bja.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamazaki Y., Zhao N., Caulfield T.R., Liu C.C., Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frisoni G.B., Altomare D., Thal D.R., et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat Rev Neurosci. 2022;23:53–66. doi: 10.1038/s41583-021-00533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortese G.P., Burger C. Neuroinflammatory challenges compromise neuronal function in the aging brain: postoperative cognitive delirium and Alzheimer’s disease. Behav Brain Res. 2017;322:269–279. doi: 10.1016/j.bbr.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makin S. The amyloid hypothesis on trial. Nature. 2018;559:S4–S7. doi: 10.1038/d41586-018-05719-4. [DOI] [PubMed] [Google Scholar]
- 68.Swaminathan S., Risacher S.L., Yoder K.K., et al. Association of plasma and cortical amyloid beta is modulated by APOE ε4 status. Alzheimers Dement. 2014;10 doi: 10.1016/j.jalz.2013.01.007. e9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vasunilashorn S., Ngo L., Kosar C.M., et al. Does apolipoprotein E genotype increase risk of postoperative delirium? Am J Geriatr Psychiatry. 2015;23:1029–1037. doi: 10.1016/j.jagp.2014.12.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.