Abstract
Background
The association of TEE with all-cause mortality is uncertain, as is the dependence of this association on age.
Objectives
To examine the association between TEE and all-cause mortality, and its age interaction, in a Women’s Health Initiative (WHI) cohort of postmenopausal United States women (1992–present).
Methods
A cohort of 1131 WHI participants having DLW TEE assessment of ∼10.0 y (median) following WHI enrollment with ∼13.7 y (median) of subsequent follow-up, was used to study the EE associations with all-cause mortality. To enhance the comparability of TEE and total EI, key analyses excluded participants having >5% weight change between WHI enrollment and DLW assessment. The influence of participant age on mortality associations was examined, as was the ability of concurrent and earlier weight and height measurements to explain the results.
Results
There were 308 deaths following the TEE assessment through 2021. TEE was unrelated to overall mortality (P = 0.83) in this cohort of generally healthy, older (mean 71 y at TEE assessment) United States women. However, this potential association varied with age (P = 0.003). Higher TEE was associated with a higher mortality rate at the age of 60 y and a lower mortality rate at the age of 80 y. Within the weight-stable subset (532 participants, 129 deaths), TEE was weakly positively related to overall mortality (P = 0.08). This association also varied with age (P = 0.03), with mortality HRs (95% CIs) for a 20% increment in TEE of 2.33 (1.24, 4.36) at the age of 60 y, 1.49 (1.10, 2.02) at 70 y of age, and 0.96 (0.66, 1.38) at 80 y of age. This pattern remained, although was somewhat attenuated, following control for baseline weight and weight changes between WHI enrollment and TEE assessment.
Conclusions
Higher EE is associated with higher all-cause mortality among younger postmenopausal women, only partially explained by weight and weight change.
This study is registered with clinicaltrials.gov identifier: NCT00000611.
Keywords: all-cause mortality, body weight, doubly labeled water, energy expenditure, energy intake
Introduction
There is an immediate need for reliable information on total EI, as a modifiable component of the diet, and chronic disease incidence and mortality. Extensive epidemiologic literature on obesity and chronic disease shows the importance of energy balance in relation to health and disease [1]. The determinants of energy balance, namely, EI and EE, are, however, difficult to measure, and merit continued study as chronic disease risk factors. The nutritional epidemiology literature frequently focuses on dietary composition measures, often defined using ratios of intake of food groups or nutrients to total energy; however, there has been little reporting on total energy itself in relation to chronic disease risk.
The DLW procedure provides a precise and accurate measure of TEE over its (typically) 2-wk protocol period [2]. This DLW measure provides an objective estimate of total EI under the assumption of weight stability. Self-reported EI shows only weak correlations with DLW-measured TEE (hereinafter referred to as TEE) in various populations [3]. Also, self-reported EI exhibits important systematic biases related to BMI, age, and race/ethnicity, as shown in the Women’s Health Initiative (WHI) cohorts, whether using food frequency questionnaires (FFQs), 4-d food records (4DFRs), or 24-h dietary recalls (24HRs) for dietary assessment [4,5].
Our WHI research group has developed calibration equations that use TEE to adjust FFQ EI estimates for measurement error, and we have reported strong positive associations of calibrated total energy with the subsequent incidence of cardiovascular diseases (CVDs), invasive cancers, and type-2 diabetes (T2D) [6]. However, these associations tend to diminish, or completely disappear, when BMI is added to the outcome model. The ability to disentangle the roles of EI and BMI using DLW-calibrated EI is limited by a strong dependence of calibrated energy on BMI, with comparatively weaker dependencies on self-reported energy and other participant characteristics [4,5].
Here, we attempted to circumvent this limitation by considering a prospective cohort of 1131 WHI participants with TEE measured using DLW, for the study of EE associations with subsequent health events. We focused on total mortality (308 deaths), because other clinical outcomes are comparatively less frequent in this small, elderly cohort.
Methods
WHI cohorts
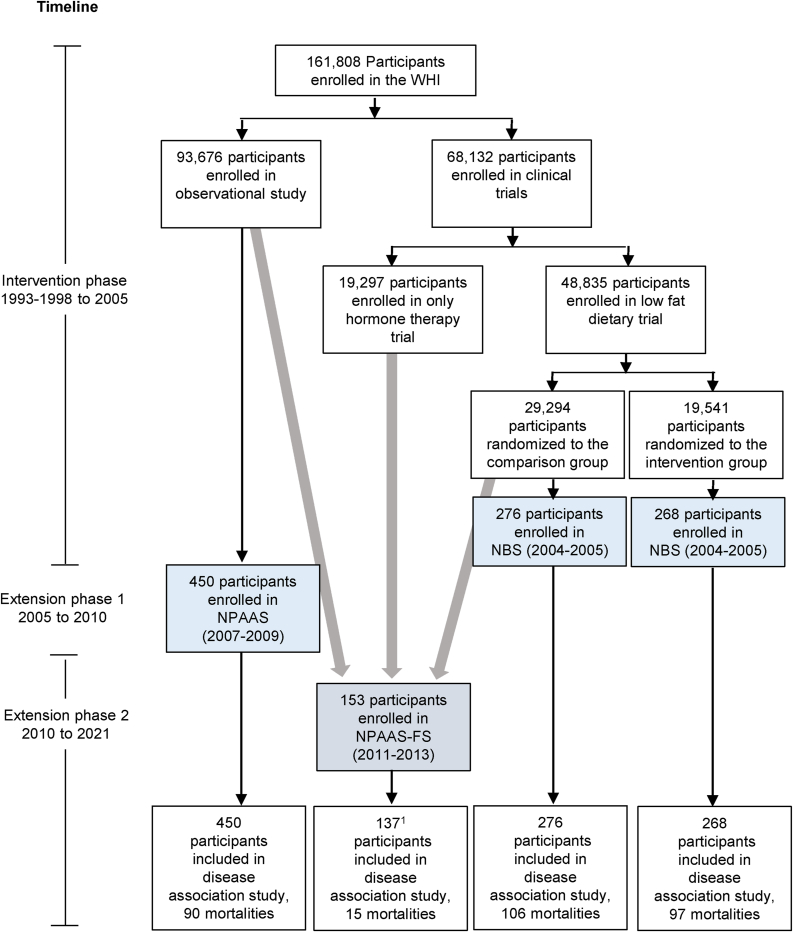
During 1993–1998, 48,835 participants were randomly assigned in the WHI Dietary Modification (DM) trial, with 29,294 assigned to the usual diet comparison group and 19,541 assigned to a low-fat dietary pattern intervention, and 93,676 participants enrolled in the companion prospective WHI observational study (OS) [7]. All participants were postmenopausal women in the age range 50–79 y when enrolled during 1993–1998 at 40 United States clinical centers. The weight and height of the participants were measured by trained clinic personnel at baseline and annually thereafter during the DM trial intervention period (ended 31 March, 2005), and were measured at baseline and year 3 in the OS. The WHI FFQ [8] assessed dietary intake over the preceding 3-mo period, and was administered at baseline, year 1, and thereafter to approximately one-third of participants each year in a rotating sample in the DM trial, and at baseline and year 3 OS. Participants completed core questionnaires at WHI enrollment and subsequently added medical history, reproductive history, family history, personal habits, medications, and dietary supplements, and provided a fasting blood sample [7]. Figure 1 shows participant flow in WHI, and in the nutrition biomarker substudies described in the following section.
FIGURE 1.
Participant flow from the Women’s Health Initiative (WHI) cohorts of postmenopausal women aged 50–79 y at enrollment during 1993–1998 at 40 United States clinical centers into the DLW cohort (n = 1131). NBS and NPAAS participants (blue) were recruited from geographically dispersed clinical centers, whereas FS participants were exclusively recruited from WA State (gray). 1Does not include overlapping participants (n = 16) who were previously enrolled in NBS (n = 2) or NPAAS (n = 14). NBS, nutritional biomarker study; NPAAS, Nutrition and Physical Activity Assessment Study; FS, feeding study; WA, Washington.
WHI nutrition biomarker studies
TEE assessments were obtained from participants in 3 substudies within the WHI program, including 2 nutrition biomarker studies described here, and a feeding study described in the next subsection.
An initial Nutrition Biomarker Study (NBS) [4] was conducted (2004–2005) among 544 volunteer participants in the DM trial, 268 from the intervention group and 276 from the comparison group, in part to study measurement properties of the WHI FFQ in this trial context. Subsequently, (2007–2009), the Nutrition and Physical Activity Assessment Study (NPAAS) [5] was carried out among 450 OS participants, to examine the measurement properties of FFQs, 4DFRs, and three 24HRs, among other objectives. Weight stability was an eligibility criterion for these 2 studies as well as the subsequent feeding study. Potential participants were excluded who reported trying to gain or lose weight, who gained or lost weight unintentionally, or who experienced a 6.8 kg or larger change in body weight, over the preceding 4 wk. Participants having medical conditions that may interfere with their study participantion were also excluded.
These studies included DLW assessments of TEE over a 2-wk study period, for each participant. NBS staff recruited participants at a representative set of 12 of the 40 WHI clinical centers. NPAAS recruited participants at 9 clinical centers, 8 of which participated in NBS and did so with an overrepresentation of Black and Hispanic women and women having relatively high BMI. The study protocols for both biomarker studies required 2 clinic visits separated by 2 wk and included various at-home activities. Reliability subsamples (20%) repeated the protocol ∼6 mo after their initial biomarker protocol participation. The first clinic visit included eligibility confirmation; measured height and weight; DLW dosing appropriate for each participant’s weight for TEE assessment; completion of FFQ, dietary supplement use, and other questionnaires, and collection of a blood specimen. Participants also received instructions and a kit for 24-h urine collection that is separated from the DLW protocol, for use on the day before the second clinic visit. At the second clinic visit, participants brought 24-h urine specimens and provided a fasting blood specimen as well as an additional spot urine specimen to complete the DLW protocol. Additional details on the DLW protocol and the MS-based TEE assessments at the Stable Isotope Laboratory at the University of Wisconsin are given previously [4]. Baseline characteristics in the NBS and NPAAS cohorts have been presented previously [4,5].
NPAAS feeding study
The NPAAS feeding study (NPAAS-FS) was conducted among 153 WHI participants in the Seattle area during 2011–2013, using essentially the same inclusion/exclusion criteria. Of the 153 participants, 2 had previously participated in NBS, and 14 in NPAAS. The Fred Hutch Human Nutrition Laboratory provided participants with food and beverages over a 2-wk feeding period, with individualized diets that were intended to approximate their usual diets [9], so that blood and urine concentrations would stabilize quickly. The usual diet for a participant was assessed by starting with a 4DFR, then making adjustments based on participant interviews by a study nutritionist and known total EI biases. Specifically, total EI during the feeding period used 4DFR EI together with standard energy estimating equations [10] and estimates from previous WHI calibration equations [4,5] that include a participant’s BMI, race/ethnicity, and age. For the 73% of participants whose 4DFR energy was less than the resulting corrected value, a proportionate increase in provided foods was implemented to yield the corrected intake with a mean (SD) of 335 (220) kcal/d of added energy. For participants having 4DFR energy higher than the corrected value, no change from 4DFR energy was made, to ensure sufficient food intake and to discourage hunger that may lead to supplementation by nonstudy foods [9]. The individualized diet was formulated to retain the variability in dietary habits among participants, using foods similar to the participant’s usual choices with priority given to foods having well-characterized nutrient content. No advice on physical activity was given to these participants. TEE over the 2-wk feeding periods was assessed using the same DLW protocol as for the other 2 nutrition biomarker studies.
DLW procedure
TEE was estimated using a standard DLW protocol for each participant [2,4]. DLW is considered the best validation standard for assessing short-term energy turnover [2]. NBS and NPAAS participants were asked to retain their usual dietary and activity patterns during the biomarker study protocol periods. For the DLW procedure, there was a 6.8% quality control failure rate for the 3 studies combined, approximately half of which were due to low tracer enrichments or lack of equilibration, whereas the others were due to 2H compared with 18O dilution space or other internal reproducibility issues.
DLW cohort
The number of distinct participants with TEE measured in the 3 biomarker studies was 1131. These participants have measures of weight and height (and hence BMI, kg/m2), both at the time of TEE assessment and at preceding WHI enrollment ∼10 y (median) earlier. Waist circumference was also measured at WHI enrollment. Because weight stability is required for the TEE assessment to be regarded as estimating EI, we also considered the associations of TEE with mortality among participants having <5% weight change between the WHI baseline and DLW assessment as key analyses for results interpretation.
Outcome ascertainment and follow-up
Clinical outcomes were reported biannually in the DM trial and annually in the OS by self-administered questionnaire [11] throughout the time from enrollment in 1993–1998 to the end of the WHI intervention period, and annually thereafter in both these cohorts. All deaths were centrally reviewed by expert physician investigator committees, and these data were supplemented by periodic National Death Index [12] matching.
Following the WHI intervention period, WHI participants had the opportunity to enroll in additional follow-up until 30 September, 2010, and subsequently for an additional open-ended annual follow-up that continues, with 99% and 90% of the DLW cohort doing so on these two occasions. All-cause mortality outcomes through 31 December, 2021 were essentially complete, and are included here. Nearly all of the follow-up considered here was after the clinical trial intervention period had ended. Accordingly, the TEE measures in NBS were obtained essentially at the end of the DM trial intervention period. Also, these TEE values did not differ significantly between the DM randomization groups [4]. Self-reported recreational physical activity was assessed using a WHI Personal Habits Questionnaire [13] as a part of each of the nutritional biomarker study protocols.
Statistical methods
In preliminary analyses, log-TEE was studied for association with log-weight, log-height, and age at the time of the TEE assessment, using linear regression.
Mortality data were analyzed using Cox regression HR methods [14], stratified on age at DLW assessment in 5-y categories, and on study cohort (NBS, NPAAS, NPAAS-FS). The log-HR was modeled as a linear function of log-TEE so that the HR for a percentage increment in TEE does not depend on the TEE value. A 20% increment in TEE was used for HR displays. Additionally, the modeled regression variables included indicator variables for self-reported race and ethnicity (Black, Hispanic, all other, and mixed compared with White), indicator variables for education (high school/general equivalency qualification or less, post–high school, compared with college degree or higher), current cigarette smoking indicator, and age in years (linear) at DLW assessment.
To allow TEE-related HRs to vary with age at DLW assessment, we added a product term between age at DLW assessment centered at 70 y and log-TEE, with a corresponding 1 degree of freedom test for interaction. This product term allows the mortality HR for the TEE to depend on age in a smooth fashion. HRs with corresponding 95% CIs are displayed at 60, 70, and 80 y of age.
To examine the influence of body anthropometrics on TEE HRs, the analyses described in the previous paragraphs were augmented by including log-transformed weight and height, both at DLW assessment and at earlier WHI enrollment in the regression model. The covariates spanned by these log-transformed variables encompass log-BMI as a special case at each time point. To control more fully for these weight and height measures, a quadratic term in log-weight at DLW assessment was added also to the HR model. Additional analyses that included measured waist circumference at WHI enrollment were also carried out, as were additional analyses that control for an additional aspect of body composition by including a % body fat assessment defined as 1 − (weight of total body water/total body weight)/0.73, with total body water assessed by isotope dilution as a part of the DLW procedure [15].
Participants having missing data on variables needed for regression modeling were excluded from primary analyses. In further analyses, multiple imputations were used to allow a contribution from these excluded participants. Additional sensitivity analyses excluded the first 2 y of follow-up after the TEE assessment and excluded current or former cigarette smokers.
We defined disease occurrence time for a “case” developing a study outcome as days from DLW assessment to outcome occurrence. We defined censoring time for “non-cases” as days from DLW assessment to the earliest date of last contact, or 31 December, 2021. The median (IQR) follow-up duration (y) of post–TEE measurement was 13.7 (11.3, 16.6).
Statistical analyses were conducted using SAS, version 9.4 (SAS Institute).
Ethics
The WHI is funded primarily by the National Heart, Lung, and Blood Institute. Participants provided written informed consent for their overall WHI, NPAAS, and NPAAS-FS activities. Related protocols were approved by the Institutional Review Boards at the Fred Hutchinson Cancer Research Center and at each participating clinical center (clinicaltrials.gov identifier: NCT00000611).
Results
Figure 1 shows participant flow in WHI leading to the DLW cohort and related number of deaths in this cohort.
Table 1 shows participant characteristics, at enrollment and the time of (initial) DLW measurement, for the DLW cohort (n = 1131), stratified by tertiles of measured TEE. Participants mostly reported good health and a high level of physical functioning. Most of them were White (80%), highly educated, and nonsmokers. The median (IQR) for the TEE in the combined biomarker studies was 2032 (1815, 2240) kcal/d. Compared with the bottom TEE tertile, participants in the top tertile tended to be younger (mean age 68.3 compared with73.6 y) and more overweight (median BMI 30.9 compared with 24.8 kg/m2) but had similar self-reported leisure physical activity (median 11.8 compared with 11.8 metabolic equivalent unit-h/wk). Participant characteristics summarized separately for the 3 biomarker studies are given in Supplemental Table 1. Because of the planned participant recruitment procedures, NPAAS participants were more racially and ethnically diverse than the other 2 groups.
TABLE 1.
Participant characteristics1 at WHI enrollment and DLW assessment, overall and stratified by tertiles of TEE (n = 11312)
| Participant characteristics at WHI enrollment | Tertiles of TEE, kcal/d2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 1131) | <1895 (n = 351) | 1895 to <2167 (n = 352) | ≥2167 (n = 351) | |||||
| Age, mean (SD), y | 59.8 | (6.2) | 61.6 | (6.5) | 60.1 | (6.0) | 57.6 | (5.4) |
| Hispanic/Latina3 | 95 | 8.4 | 35 | 10.0 | 24 | 6.8 | 26 | 7.4 |
| Race3 | ||||||||
| American Indian/Alaska Native | 3 | 0.3 | 1 | 0.3 | 1 | 0.3 | 1 | 0.3 |
| Asian/Pacific Islander | 13 | 1.1 | 10 | 2.8 | 2 | 0.6 | 0 | 0.0 |
| Black/African-American | 142 | 12.6 | 32 | 9.1 | 46 | 13.1 | 57 | 16.2 |
| White | 911 | 80.5 | 289 | 82.3 | 288 | 81.8 | 273 | 77.8 |
| Multiracial/other/unknown or not reported | 62 | 5.5 | 19 | 5.4 | 15 | 4.3 | 20 | 5.7 |
| Education | ||||||||
| High school/GED or less | 176 | 15.6 | 59 | 17.0 | 45 | 12.8 | 56 | 16.0 |
| Post–high school | 416 | 37.0 | 130 | 37.5 | 120 | 34.2 | 142 | 40.5 |
| College degree or higher | 533 | 47.4 | 158 | 45.5 | 186 | 53.0 | 153 | 43.6 |
| Smoking status | ||||||||
| Never | 602 | 53.8 | 197 | 56.6 | 190 | 54.8 | 187 | 53.7 |
| Past | 455 | 40.6 | 132 | 37.9 | 138 | 39.8 | 141 | 40.5 |
| Current | 63 | 5.6 | 19 | 5.5 | 19 | 5.5 | 20 | 5.7 |
| Had hysterectomy | 448 | 39.6 | 141 | 40.2 | 131 | 37.2 | 150 | 42.7 |
| Self-rated health | ||||||||
| Excellent | 222 | 19.7 | 69 | 19.7 | 83 | 23.7 | 55 | 15.8 |
| Very good | 519 | 46.1 | 167 | 47.7 | 161 | 46.0 | 152 | 43.6 |
| Good | 337 | 29.9 | 101 | 28.9 | 90 | 25.7 | 126 | 36.1 |
| Fair/poor | 48 | 4.3 | 13 | 3.7 | 16 | 4.6 | 16 | 4.6 |
| Height, mean (SD), cm | 162.2 | (6.3) | 160.1 | (6.1) | 162.1 | (6.3) | 164.4 | (6.0) |
| Weight, median [Q1, Q3], kg | 70.9 | [62.3, 81.2] | 64.3 | [57.2, 72.0] | 70.1 | [62.7, 79.0] | 79.6 | [70.9, 91.6] |
| Waist, mean (SD), cm | 84.5 | 12.6 | 79.4 | 10.6 | 84.4 | 12.2 | 90.3 | 12.8 |
| BMI, median [Q1, Q3], kg/m2 | 26.8 | [23.7, 31.1] | 25.1 | [22.5, 28.1] | 26.6 | [23.9, 30.6] | 29.8 | [26.0, 34.0] |
| Physical functioning score4, median [Q1, Q3] | 90 | [80, 100] | 90 | [80, 95] | 92.5 | [80, 100] | 90 | [80, 95] |
| Participant characteristic at DLW collection | ||||||||
| Years from enrollment to DLW Collection, median [Q1, Q3] | 10.0 | [8.2, 11.8] | 10.4 | [8.9, 12.4] | 10.0 | [8.1, 11.8] | 9.7 | [8.1, 11.3] |
| Age, mean (SD), y | 71.0 | (6.1) | 73.6 | (6.1) | 71.3 | (5.9) | 68.3 | (5.1) |
| Current smoker | 32 | 2.9 | 10 | 2.9 | 10 | 2.9 | 9 | 2.6 |
| Self-rated health5 | ||||||||
| Excellent | 177 | 16.0 | 53 | 15.3 | 62 | 18.0 | 47 | 13.7 |
| Very good | 499 | 45.0 | 148 | 42.8 | 162 | 47.0 | 161 | 47.1 |
| Good | 369 | 33.3 | 126 | 36.4 | 101 | 29.3 | 115 | 33.6 |
| Fair/poor | 63 | 5.7 | 19 | 5.5 | 20 | 5.8 | 19 | 5.6 |
| Height, mean (SD), cm | 160.9 | (6.4) | 158.7 | (6.3) | 161.0 | (6.3) | 163.2 | (6.1) |
| Weight, median [Q1, Q3], kg | 70.5 | [61.9, 81.4] | 63.1 | [55.8, 70.3] | 70.1 | [62.9, 77.9] | 82.0 | [72.3, 93.7] |
| BMI, median [Q1, Q3], kg/m2 | 27.1 | [23.9, 31.7] | 24.8 | [22.4, 27.9] | 27.0 | [24.2, 30.5] | 30.9 | [26.6, 35.5] |
| Physical functioning score4,5, median [Q1, Q3] | 85 | [70, 95] | 90 | [70, 95] | 85 | [70, 95] | 85 | [70, 95] |
| Leisure physical activity, median [Q1, Q3], MET-h/wk | 11.7 | [4.2, 21.8] | 11.8 | [3.8, 21.0] | 11.5 | [4.3, 22.0] | 11.8 | [5.0, 21.8] |
| Estimated body fat from DLW, % | 42.6 | (6.6) | 41.4 | 6.5 | 42.1 | 6.1 | 44.3 | 6.7 |
| Estimated energy from DLW, median [Q1, Q3], kcal/d | 2032 | [1815, 2240] | 1723 | [1620, 1815] | 2032 | [1961, 2094] | 2334 | [2240, 2477] |
GED, general equivalency qualification; MET, metabolic equivalent unit; WHI, Women’s Health Initiative.
Categorical characteristics summarized by n (%). Continuous variables summarized by mean (SD), unless data were skewed (|skew| > 1), then median [Q1, Q3] was used.
TTE was missing on n = 77 (6.8%) participants.
Ethnic group and race were self-reported by participants. Multiracial participants self-identified with >1 race. Participants of other race or unknown race self-identified with those categories.
Physical function was assessed using RAND36, scored 0–100, with higher scores reflecting better functional status.
Self-rated health and physical functioning were not exactly concurrently collected with DLW. Instead, NBS utilized data from study close-out, collected median [Q1, Q3] = 0.4 [0.3, 0.5] years after DLW. NPAAS, and NPAAS-FS utilized the most proximal annual report preceding DLW, assessed 0.5 [0.3, 0.8] years before DLW.
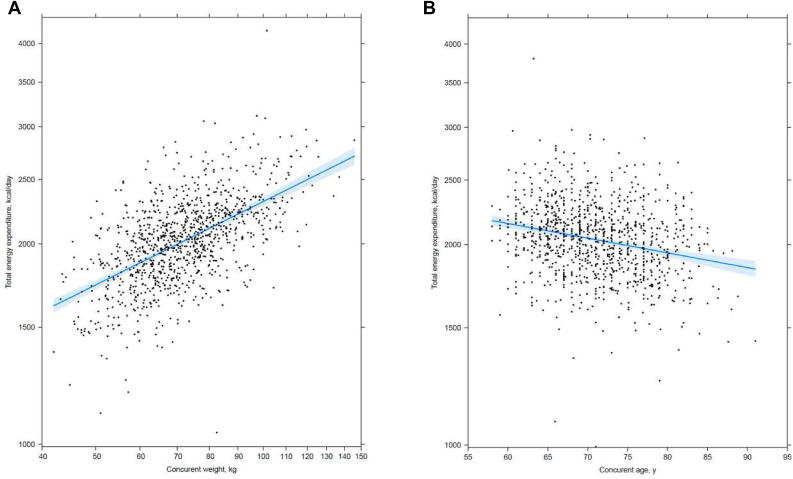
Log-TEE was approximately linearly related to log-weight and age at the time of DLW assessment. From the fitted lines in Figure 2, one can, for example, calculate that a 20% higher weight corresponds to an estimated 7.9% higher TEE (A), and a 10-y younger age corresponds to an estimated 5.2% higher TEE (B). The TEE association with weight did not depend significantly on age (P = 0.14), but older women tended to weigh less and have lower TEE (Supplemental Figure 1).
FIGURE 2.
Multivariable mean (95% CI) associations between TEE with concurrent weight and age [n = 1131 (n = 92 participants excluded due to missing TEE and covariate data, leaving 1039 participants in each graph)]. Mean (95% CI) from a multivariable regression model that included baseline variables race/ethnicity and education, and variables collected concurrently with DLW assessment: study, log-transformed weight, log-transformed height, age, and smoking. Adjusted R-squared = 0.42; 20% higher concurrent weight was associated with mean (95% CI) =7.9 (7.1, 8.7) % higher total daily EE (A). Ten years younger age was associated with mean (95% CI) = 5.2 (3.8, 6.6) % higher total daily EE (B). Fitted means (95% CI) for concurrent measures were computed at the average value for the remaining covariates.
Table 2 shows HRs for 20% increments (95% CIs) and P values (model 1) for the association of TEE with all-cause mortality. Overall, TEE was not significantly associated with mortality. However, a significant interaction was observed between log-TEE and age at DLW assessment (P = 0.003), with 55% higher mortality rates for a 20% TEE increment at the age of 60 y, compared with ∼20% reduction at the age of 80 y. For context on the number of deaths across the age distribution at DLW assessment, the left side of Table 2 also shows the number of deaths and annualized death rates for <65, 65 to <75, and ≥75 y of age.
TABLE 2.
All-cause mortality HRs for a 20% increment in TEE (n = 1131)
| Subgroup | Number of events (annualized rate, %) | Model 11 |
P4 | Model 22 |
P4 |
|---|---|---|---|---|---|
| HR (95% CI)3 | HR (95% CI)3 | ||||
| Overall | 308 (2.06) | 0.98 (0.85, 1.14) | 0.83 | 0.91 (0.76, 1.09) | 0.28 |
| Age at DLW, y | 0.003 | 0.04 | |||
| <65 | 24 (0.86) | 1.55 (1.11, 2.17) | 1.25 (0.87, 1.80) | ||
| 65 to <75 | 128 (1.53) | 1.12 (0.94, 1.33) | 0.99 (0.81, 1.22) | ||
| ≥75 | 156 (4.11) | 0.80 (0.66, 0.98) | 0.79 (0.63, 0.99) |
Summary statistics from Cox regression: For model 1, baseline hazard functions were stratified by 5-y age groups at DLW assessment and study cohort (NBS, NPAAS, and NPAAS-FS), with baseline covariates self-reported race/ethnicity, education, smoking status, and age (linear) at DLW assessment; n = 92 participants excluded for missing TEE and covariate data.
Summary statistics from Cox regression: For model 2, the model 1 covariate list was expanded to included height and weight at DLW assessment and height and weight at WHI enrollment; these covariates were log-transformed (linear) with an additional quadratic term for log-weight at DLW assessment; n = 96 participants excluded for missing TEE and covariate data.
Risk estimates correspond to a 20% increment in TEE. Age-specific HR were estimated by including a product (interaction) term between log-TEE × age (linear) in models 1 and 2, and evaluating the corresponding estimate at ages 60, 70, and 80 y.
P values correspond to a score-test for the main effect for log-TEE or interaction term between log-TEE × age (linear).
A similar age-dependent pattern of mortality with TEE remained evident but was somewhat attenuated (P = 0.04) following the inclusion of log-weight and log-height at TEE assessment and at earlier WHI enrollment, as well as the square of log-weight at TEE assessment, in the HR model (model 2). In these analyses, there was a strong dependence of mortality on weight at WHI enrollment with HR (95%CI) = 1.54 (1.20, 1.98) for a 20% higher weight, in conjunction with an inverse dependence on weight at DLW assessment in the presence of the enrollment weight. Height at either time point was not significantly related to mortality. As expected, annualized mortality rates increased with the age group with 86, 153, and 411 deaths per 10,000 person-years for women <65, 65 to <75, and ≥75 y of age, respectively. Table 2 mortality patterns were similar, but somewhat attenuated, following the exclusion of the first 2 y of mortality data post–TEE assessment.
Table 2 analyses were repeated while using multiple imputations to include the 8.5% of the DLW cohort having missing data for 1 or more data items. The missing data rates were 6.8% for the TEE, 1.0% for smoking status, 0.5% for education, and 0.2% for baseline weight and height. As shown in Supplemental Table 2, this inclusion had little impact on HRs or 95% CIs.
Table 3 presents a similar summary following the exclusion of participants having a 5% or greater weight change between the WHI baseline and DLW assessment. A total of 312 (27.6%) of DLW cohort participants had a weight loss of ≥5%, whereas 285 (25.2%) had a weight gain of 5% or more, leaving 532 participants in a “weight-stable” cohort. These exclusions were somewhat age-dependent with a 37.4% weight loss exclusion rate among women 75 y or older compared with 12.2% among those younger than 65 y, and with only an 18.5% weight gain exclusion rate for 75 y or older compared with 36.7% for those younger than 65 y.
TABLE 3.
All-cause mortality HRs for a 20% increment in TEE among participants who were weight stable1 (n = 532)
| Subgroup | Number of events (annualized rate, %) | Model 12 |
P5 | Model 23 |
P5 |
|---|---|---|---|---|---|
| HR (95% CI)4 | HR (95% CI)4 | ||||
| Overall | 129 (1.79) | 1.26 (0.97, 1.63) | 0.08 | 1.15 (0.84, 1.58) | 0.38 |
| Age at DLW, y | 0.03 | 0.06 | |||
| <65 | 12 (0.85) | 2.33 (1.24, 4.36) | 2.02 (1.03, 3.94) | ||
| 65 to <75 | 55 (1.37) | 1.49 (1.10, 2.02) | 1.35 (0.94, 1.94) | ||
| ≥75 | 62 (3.49) | 0.96 (0.66, 1.38) | 0.90 (0.60, 1.36) |
Participants whose weight changed <5% from WHI enrollment to DLW assessment.
Summary statistics from Cox regression: For model 1 baseline hazard functions were stratified by 5-y age groups at DLW assessment and study cohort (NBS, NPAAS, NPAAS-FS), with baseline covariates self-reported race/ethnicity and education, and smoking status and age (linear) at DLW assessment; n = 45 participants excluded for missing TEE and covariate data.
Summary statistics from Cox regression: For model 2 the model 1 list of covariates was expanded to included height and weight at DLW assessment, and height and weight at WHI enrollment; these covariates were log-transformed (linear) with an additional quadratic term for log-weight at DLW assessment; n = 47 participants excluded for missing TEE and covariate data.
Risk estimates correspond to a 20% increment in TEE. Age-specific HR were estimated by including a product (interaction) term between log-TEE × age (linear) in models 1 and 2, and evaluating the corresponding estimate at 60, 70, and 80 y of age.
P values correspond to a score-test for the main effect for log-TEE, or interaction term between log-TEE × age (linear).
Following these weight change exclusions, there is a stronger justification for regarding TEE values as estimating EI, and there is a suggestion of higher total mortality rates overall with higher TEE (P = 0.08), as well as corresponding evidence (P = 0.03) of a dependence of the TEE and mortality HR on age at DLW assessment. The estimated HRs for a 20% increase in TEE are 2.33 at age of 60 y and 1.49 at age of 70 y, and 0.96 at age of 80 y.
The further inclusion of baseline log-waist circumference in the model 2 analyses of TABLE 2, TABLE 3 had minimal impact on the estimated HRs (Supplemental Table 3). Similarly, the inclusion of percentage of body fat from the DLW procedure had little impact on these same analyses (Supplemental Table 4). The exclusion of the first 2 y of post-DLW follow-up likewise had little influence on the TEE mortality patterns, among these weight-stable (Table 3) participants.
Because of the substantial association of cigarette smoking with mortality, analyses similar to those reported in Table 3 were carried out among never-smokers, of which there were 289 in the weight-stable group, of whom 65 died during follow-up. Despite of the small number of deaths, there was a nominally significant (P = 0.05) positive TEE association with mortality in this subgroup with HR (95% CI) of 1.43 (1.00, 2.05) for a 20% increment in TEE. There was also a nominally significant (P = 0.04) dependence of this HR on age at DLW assessment with HRs (95% CIs) for a 20% TEE increment of 3.40 (1.34, 8.62) at 60 y of age, 1.92 (1.21, 3.06) at 70 y of age, and 1.09 (0.68, 1.74) at 80 y of age.
Sensitivity analyses that censored the follow-up at the time of death due to accidents, homicide, suicide, or other injuries led to a minimal change in the results shown in Table 2 (n = 8 such deaths) or Table 3 (n = 3 such deaths).
Discussion
There is a wealth of evidence that energy restriction can reduce chronic disease incidence and mortality in animals; review and discussion of mechanisms were described previously [16]. However, there are a few data on the effects of energy restriction, and EI variation within the range of typical intake, on human health. As an exception, the CALERIE randomized trial among 218 healthy, nonobese young and middle-aged healthy United States men and women were able to achieve modestly lower (∼13%) EI in their intervention group and demonstrated substantial weight loss, especially fat mass reduction, as well as improvement in multiple CVD risk factors, over a 2-y study period [17]. However, the Look AHEAD trial of a composite intervention of dietary change and physical activity increase among adults with obesity and T2D also observed weight loss and improvement in CVD risk factors but was stopped based on a lack of evidence in support of actual CVD outcome rate reduction [18]. Also, the Health, Aging, and Body Composition Study among 298 older adults used DLW energy in conjunction with REE measured by indirect calorimetry, to define an objective measure of activity-related EE, and reported clinical outcome benefits at higher activity levels [19] and among those with a “good appetite” [20], but results concerning mortality in relation to TEE do not appear to have been reported.
Consequently, our findings concerning TEE, measured using the DLW procedure, and subsequent mortality risk are novel. The cohort of postmenopausal women available for this purpose had a mean age of 71 y at the time of DLW assessment, with generally good health and physical function and had sufficient follow-up to reveal a pattern of mortality dependence on TEE that varied with age. There is a wealth of epidemiologic data indicating increased mortality and morbidity among women or men having relatively high weight except possibly among very old persons [[21], [22], [23], [24]], as well as data indicating unfavorable mortality implications among persons having unintended weight loss [25,26]. Accordingly, despite the smaller cohort size and a smaller number of outcomes, we view our Table 3 findings among weight-stable participants as more useful for information on EE in relation to clinical outcomes among healthy participants, than are those for all participants in the larger DLW cohort (Table 2). Although not examined further here, participants experiencing >5% weight gain between WHI baseline and DLW assessment may have been somewhat out of energy balance, although meeting biomarker study inclusion criteria, or they may have reduced activity levels, perhaps due to health conditions, without compensatory reduction in EI. Similarly, participants with >5% weight loss during the 10-y (median) preceding time period may have experienced chronic disease-induced weight loss, perhaps with appetite reduction related to such disease.
With our weight-stability restriction, one may be able to regard the TEE as estimating EI. If so, for the interpretation of Table 3 results, one sees quite a major estimated adverse influence of higher EI among postmenopausal women at 60 and 70 y of age with little or no association at 80 y of age when issues related to nutritional adequacy may be important. An approximate doubling of the estimated mortality risk at the age of 60 y, based on a limited number of deaths, and ∼50% mortality risk elevation at 70 y of age, for a 20% increment in energy consumption, suggests considerable public health importance to the avoidance of high energy consumption in this population of postmenopausal United States women. These mortality rate increases may be partially explained by (log-transformed) weight and height at TEE assessment and especially at WHI enrollment a decade (median) earlier, and alternatively by weight and BMI values and their changes over the preceding decade, much of the mortality association remains. It then follows that the estimated higher energy consumption among younger postmenopausal United States women relates positively to higher mortality, even after making an allowance for mediating effects through related body composition changes. The mechanisms for such elevation deserve a high priority in the future nutritional epidemiology research agenda. For example, these mortality rate elevations could simply reflect the need to metabolize and process a large intake volume regardless of dietary composition or could relate to dietary composition if, for example, participants having a relatively high-energy diet do so with intake of saturated fats that are more than proportionately higher, or with fiber intake that is less than proportionately lower.
The need to rely on expensive DLW measurements for the estimation of EI is a limitation for epidemiologic purposes. Other approaches, such as the interesting use of frequent body weight measurements [27], or the possibility of combining objective measures of REE and activity-related EE, merit further development. Because of our weight-stability constraints here, we were able to avoid the explicit use of activity measures. The leisure physical activity was measured by self-report in the WHI cohorts. REE, as measured using indirect calorimetry, is available only in the NPAAS component of the DLW cohort studied here.
Strengths of this study include the use of an established objective measure of TEE, and the potential to regard the TEE as also estimating EI among weight-stable participants, in the context of nutrition biomarker studies embedded in WHI cohorts of United States postmenopausal women. WHI cohorts are well characterized, with careful outcome ascertainment over >2 decades.
Limitations include a cohort of only 1131, reduced to 532 following the exclusion of those having weight instability over a 10-y (median) period before biomarker study enrollment, with modest number of deaths during post-DLW follow-up. Also, the TEE assessment pertains to only 2 wk at the time of biomarker study conduct, so only short-term EE is assessed. Our analyses included only relatively healthy postmenopausal United States women, with a mean age of 71 y. The results obtained may not apply to other populations in the United States or elsewhere. For example, recent reports [28,29] indicate that men have considerably greater variation in DLW-measured TEE than women.
In summary, upon acknowledging an interaction with age, DLW-measured TEE is positively and substantially related to total mortality among weight-stable, midlife-to-older (age < 75 y) postmenopausal women, with little indication of association at ≥75 y of age. Body weight and height over time can help to explain some, but not all, of the higher mortality rates. Further research on EE and intake as well as on energy reduction and energy balance, in relation to disease incidence and mortality across the lifespan, deserves a high priority in the future nutrition and public health research agendas.
Acknowledgement
The authors acknowledge the following investigators in the Women’s Health Initiative (WHI) Program:
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; and (University of Nevada, Reno, NV) Robert Brunner.
WHI Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland.
For a list of all the investigators who have contributed to WHI science, please visit: https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Long-List.pdf
The views expressed are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.02.023.
Funding
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, United States Department of Health and Human Services (contracts HHSN268202100046C, HHSN268202100001C, HHSN268202100002C, HHSN268202100003C, HHSN268202100004C, and HHSN271202100004C); and National Cancer Institute, National Institutes of Health, United States Department of Health and Human Services grant R01 CA119171.
Conflicts of interest
The authors’ responsibilities were as follows–RLP, DAS, LFT, JWL, and MLN: designed the research; RLP, AA, JEM, DAS, LFT, JLW, and MLN: conducted the research and drafted the manuscript; all authors: participated actively in critical evaluation of the manuscript and read and approved the final manuscript; and RLP: had primary responsibility for the final content.
Decisions concerning study design, data collection, and analysis, interpretation of the results, the preparation of the manuscript, and the decision to submit the manuscript for publication resided with committees comprised WHI investigators that included NHLBI representatives. The contents of the article are solely the responsibility of the authors.
The authors report no conflicts of interest.
Data Availability
Data, codebook, and analytic code used in this report may be accessed in a collaborative mode, as described on the Women’s Health Initiative website (www.whi.org).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hu F. Oxford University Press Inc; New York: 2008. Obesity epidemiology. [Google Scholar]
- 2.Schoeller D.A. Recent advances from application of doubly labeled water to measurement of human energy expenditure. J. Nutr. 1999;129:1765–1768. doi: 10.1093/jn/129.10.1765. [DOI] [PubMed] [Google Scholar]
- 3.Freedman L.S., Commins J.M., Moler J.E., Arab L., Baer D.J., Kipnis V., et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am. J. Epidemiol. 2014;180:172–188. doi: 10.1093/aje/kwu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhouser M.L., Tinker L., Shaw P.A., Schoeller D., Bingham S.A., Van Horn L., et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the women’s health initiative. Am. J. Epidemiol. 2008;167:1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 5.Prentice R.L., Mossavar-Rahmani Y., Huang Y., Van Horn L., Beresford S.A., Caan B., et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment using recovery biomarkers. Am. J. Epidemiol. 2011;174:591–603. doi: 10.1093/aje/kwr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng C., Beresford S.A.A., Van Horn L., Tinker L.F., Thomson C.A., Neuhouser M.L., et al. Simultaneous association of total energy consumption and activity-related energy expenditure with risks of cardiovascular disease, cancer, and diabetes risk among postmenopausal women. Am. J. Epidemiol. 2014;180:526–535. doi: 10.1093/aje/kwu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Women’s Health Initiative Study Group Design of the Women’s Health Initiative Clinical Trial clinical trial and observational study. Control Clin. Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Patterson R.E., Kristal A.R., Tinker L.F., Carter R.A., Bolton M.P., Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann. Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 9.Lampe J.W., Huang Y., Neuhouser M.L., Tinker L.F., Song X., Schoeller D.A., et al. Dietary biomarker evaluation in a controlled feeding study in women from the Women’s Health Initiative cohort. Am. J. Clin. Nutr. 2017;105:466–475. doi: 10.3945/ajcn.116.144840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mifflin M.D., St Jeor S.T., Hill L.A., Scott B.J., Daugherty S.A., Koh Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Curb J.D., McTiernan A., Heckbert S.R., Kooperberg C., Stanford J., Nevitt M., et al. Outcomes ascertainment and adjudication methods in the women’s health initiative. Ann. Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics . 2013. National Death Index user’s guide. Hyattsville, MD. [Google Scholar]
- 13.Johnson-Kozlow M., Rock C.L., Gilpin E.A., Hollenbach K.A., Pierce J.P. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am. J. Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 14.Cox D.R. Regression analysis and life tables (with discussion) J. R. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- 15.Wang Z., Deurenberg P., Wang W., Pietrobelli A., Baumgartner R.N., Heymsfield S.B. Hydration of fat-free body mass: a new physiological modeling approach. Am. J. Physiol. 1999;276:E995–E1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]
- 16.Bordone L., Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 17.Kraus W.E., Bhapkar M., Huffman K.M., Pieper C.F., Das S.K., Redman L.M., et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicenter, phase 2, randomized controlled trial. Lancet Diabetes Endocrinol. 2019;7:673–683. doi: 10.1016/S2213-8587(19)30151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Look AHEAD Research Group. Wing R.R., Bolin P., Brancati F.L., Bray G.A., Clark J.M., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manini T.M., Everhart J.E., Patel K.V., Schoeller D.A., Colbert L.H., Visser M., et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006;296:171–179. doi: 10.1001/jama.296.2.171. [DOI] [PubMed] [Google Scholar]
- 20.Shahar D.R., Yu B., Houston D.K., Kritchevsky S.B., Lee J.-S., Rubin S.M., et al. Dietary factors in relation to daily activity energy expenditure and mortality among older adults. J. Nutr. Health Aging. 2009;13:414–420. doi: 10.1007/s12603-009-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manson J.E., Stampfer M.J., Hennekens C.H., Willett W.C. Body weight and longevity: a reassessment. JAMA. 1987;257:353–358. doi: 10.1001/jama.1987.03390030083026. [DOI] [PubMed] [Google Scholar]
- 22.Manson J.E., Willett W.C., Stampfer M.J., Colditz G.A., Hunter D.J., Hankinson S.E., et al. Body weight and mortality among women. N. Engl. J. Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 23.Anaji U.A., Lotufo P.A., Gaziano J.M., Lee I.M., Spelsberg A., Buring J.E., et al. Body mass index and mortality among US male physicians. Ann. Epidemiol. 2004;14:731–739. doi: 10.1016/j.annepidem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Park S.-Y., Wilkens L.R., Maskarinec G., Haiman C.A., Kolonel L.N., Marchand L.L. Weight change in older adults and mortality: the multiethnic cohort study. Int. J. Obes (Lond). 2018;42:205–212. doi: 10.1038/ijo.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Stefani F.D.C., Pietraroia P.S., Fernandes-Silva M.M., Faria-Neto J., Baena C.P. Observational evidence for unintentional weight loss in all-cause mortality and major cardiovascular events: a systematic review and meta-analysis. Sci. Rep. 2018;8:15447. doi: 10.1038/s41598-018-33563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wannamethee S.G., Shaper A.G., Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch. Intern. Med. 2005;165:1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 27.Sanghvi A., Redman L.M., Martin C.K., Ravussin E., Hall K.D. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am. J. Clin. Nutr. 2015;102:353–358. doi: 10.3945/ajcn.115.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pontzer H., Yamada Y., Sagayama H., Ainslie P.N., Andersen L.F., Anderson L.J., et al. Daily energy expenditure through the human life course. Science. 2021;373:808–812. doi: 10.1126/science.abe5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halsey L.G., Careau V., Pontzer H., Ainslie P.N., Andersen L.F., Andersen L.J., et al. Variability in energy expenditure is much larger in males than females. J. Human Evol. 2022;171 doi: 10.1016/j.jhevol.2022.103229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, codebook, and analytic code used in this report may be accessed in a collaborative mode, as described on the Women’s Health Initiative website (www.whi.org).