Abstract
Background
Current gestational weight change (GWC) recommendations for obese individuals were established with limited evidence of the pattern and timing of weight change across pregnancy. Similarly, the recommendation of 5–9 kg does not differentiate by the severity of obesity.
Objectives
We sought to describe GWC trajectory classes by obesity grade and associated infant outcomes among a large, diverse cohort.
Methods
The study population included 22,355 individuals with singleton pregnancies, obesity (BMI ≥30.0 kg/m2), and normal glucose tolerance who delivered at Kaiser Permanente Northern California between 2008 and 2013. Obesity grade–specific GWC trajectories were modeled at 38 wk using flexible latent class mixed modeling (package lcmm) in R. Multivariable Poisson or linear regression models estimated the associations between the GWC trajectory class and infant outcomes (size-for-gestational age and preterm birth) by obesity grade.
Results
Five GWC trajectory classes were identified for each obesity grade, each with a distinct pattern of weight change before 15 wk (including loss, stability, and gain) followed by weight gain thereafter (low, moderate, and high). Two classes with high overall gain were associated with an increased risk for large for gestational age (LGA) in obesity grade 1 (IRR = 1.27; 95% CI: 1.10, 1.46; IRR = 1.47; 95% CI: 1.24, 1.74). Both high (IRR = 2.02; 95% CI: 1.61, 2.52; IRR = 1.98; 95% CI: 1.52, 2.58) and 2 moderate-gain classes (IRR = 1.40; 95% CI 1.14, 1.71; IRR = 1.51; 95% CI: 1.20, 1.90) were associated with LGA in grade 2, and only early loss/late moderate-gain class 3 (IRR = 1.30; 95% CI: 1.04, 1.62) was associated in grade 3. This class was also associated with preterm birth in grade 2. No associations were detected between GWC and small for gestational age (SGA).
Conclusions
Among the pregnancies affected by obesity, GWC was not linear or uniform. Different patterns of high gain were associated with an increased risk for LGA with the greatest magnitude in obesity grade 2, whereas GWC patterns were not associated with SGA.
Keywords: birthweight, gestational weight gain, GWG, maternal obesity, methods, obesity, pregnancy, prepregnancy BMI, preterm, z-score
Introduction
Evidence-based pregnancy weight gain recommendations are limited for those with obesity, particularly those affected by severe obesity [1]. The prevalence of prepregnancy obesity is rising in the US, with estimates indicating an increase from 26.1% in 2016 to 29.0% in 2019 [2]. These high-risk pregnancies result in disproportionate rates of neonatal complications, including elevated risk for large for gestational age (LGA, >90th percentile weight-for-age) and preterm birth within <37 wk of gestation [3,4]. In addition to excess preconception adiposity, nearly 50% of the pregnancies are affected by weight gain, exceeding the Institute of Medicine (IOM) guidelines, which may lead to weight-related prenatal complications and associated sequelae [3,5]. Excessive pregnancy weight gain is associated with an increased risk of certain adverse infant outcomes, including LGA, macrosomia (birthweight > 4000 g), and preterm birth [1,3,6]. This excess gestational weight change (GWC) among pregnancies with obesity may synergistically increase the risk of weight-related perinatal complications, such as LGA [3,7]. In pregnancies with normal weight BMI, excess GWC is associated with an increased risk of LGA, whereas inadequate GWC is associated with small for gestational age (SGA, <10th percentile weight-for-age); however, the amount of weight change that balances these risks in pregnancies with obesity is less clear [3].
In 2009, the IOM GWC guidelines recommended one weight gain range (5–9 kg) for any BMI of ≥30 kg/m2 due to a lack of existing evidence to support different weight change recommendations based on the severity of obesity [1,8,9]. Furthermore, the IOM highlighted the need for further research examining the impact of GWC pattern stratified by the severity of obesity, particularly in those with low weight gain or loss. More recent evidence supports gaining weight below that per IOM guidelines or prenatal weight loss in those with obesity [[9], [10], [11], [12], [13]]. To date, no studies have examined the associations between the pattern and timing of actual GWC with infant or birth outcomes by the severity of prepregnancy obesity.
In pregnancies characterized by obesity, the effects of pattern, timing, and magnitude of GWC on perinatal and infant outcomes remain unclear. Furthermore, it is unclear if recommendations should differ by the severity of obesity. Therefore, we investigated weight change trajectories across pregnancy by obesity grade and neonatal outcomes in a maternal–infant cohort from Kaiser Permanente Northern California (KPNC). We hypothesized that higher BMI values, specifically grade 3 obesity, and specific patterns of high weight gain would be associated with increased risk for LGA and greater birthweight, as well as decreased risk for SGA and low birthweight (LBW, ≤2500 g).
Methods
Study design and subjects
Data were abstracted from electronic medical records for singleton livebirths delivered between January 1, 2008, and December 31, 2013, at KPNC. KPNC serves the greater San Francisco Bay area and the Central Valley across 14 urban and rural counties representative of the diversity of this area [14]. The inclusion criteria for the present study were as follows: a prepregnancy BMI of ≥30 kg/m2, a singleton index pregnancy, maternal age of ≥18 y, a measured prepregnancy weight within the prior 12 mo or at least one first trimester prenatal weight in <14.0 wk based on the last menstrual period, and a minimum of 3 prenatal weights across pregnancy, including the first trimester weight. Additionally, those with pre-existing diabetes were excluded due to the known association between diabetes, total weight gain, and infant size outcomes (e.g., LGA) [15]. Similarly, we excluded individuals diagnosed with gestational diabetes due to its effect on GWC after diagnosis and treatment.
Data preparation
The measured height, prepregnancy weight, and weights from all prenatal visits were obtained from the entirety of the pregnancy. The height and prepregnancy weight were used to calculate prepregnancy BMI and categorized into obesity grade 1 (≥30.0 to <35 kg/m2), grade 2 (≥35.0 to <40 kg/m2), or grade 3 (≥40.0 kg/m2). Total GWC was calculated as the difference between the final measured prenatal weight and the prepregnancy weight and then classified according to the 2009 IOM guidelines as below, within, or above the recommended range. Observations with potentially spurious prepregnancy weight or BMI, prenatal visit weight, and total GWC were examined by a registered dietitian for clinical feasibility and inclusion in the trajectory analyses. Weight values of >±2 SDs of an individual’s mean weight change across pregnancy were examined for exclusion. Then, individual weight change during the first week, during the first trimester, or at the final prenatal visit that was <5th or >95th percentile of the sample mean was individually examined; dubious values visually identified by individual trajectory scatterplots were excluded (n = 3154 weights; 1.1% of all visits in the analytic sample). Considering that the analytic sample contained 12.3 ± 3.5 visits per pregnancy, the very low rate of biologically implausible values, and the systematic inspection for erroneous values with potential perturbations in shape, timing, and pattern of individual weight change, imputation was deemed unnecessary. We conducted analyses to assess differences in demographic characteristics between those who were included in the sample and those who were excluded (n = 12,671; Supplemental Table 1). For trajectory analyses, prenatal weight change for the final sample was censored at ≤38 wk for base models (mean total GWC in the analytic sample: 38.9 ± 1.9 wk) and at ≤34 wk for preterm models (mean GA at delivery of preterm births: 34.1 ± 2.8 wk) to prevent later weight changes from influencing estimated GWC patterns.
Statistical methods
Our analyses were conducted sequentially: 1) We stratified the sample by obesity grade, 2) we fit latent class mixed models with 4–6 identified classes of weight change for each obesity grade as outlined in the Supplemental Methods [[16], [17], [18], [19], [20], [21]], and 3) we conducted multivariable Poisson regression with robust standard errors [22,23] or linear regression to assess associations between identified latent classes and infant outcomes.
We modeled GWC trajectories using latent class mixed models (R, package lcmm function hlme) that were censored to <38 wk or <34 wk in preterm models to prevent later weight change from driving latent class predictions or skewing trajectory patterns. The five-class model for each obesity grade was ultimately selected for analysis (Supplemental Table 2) based on the best overall fit statistics as outlined in the Supplemental Methods.
Multivariable Poisson or linear regression models estimated the associations between the GWC trajectory latent class and infant outcomes stratified by obesity grade. Potential confounders were identified by literature search and based on the data available in the electronic medical records. Maternal age at delivery, height, parity, race and ethnicity, gestational age at delivery, pre-existing or gestational hypertension, smoking status, drug or alcohol use, and infant’s sex were examined and retained in the model by evaluating the change in effect size (≥10%). The primary models by obesity grade were adjusted for maternal age at delivery, race or ethnicity, gestational age at delivery (except in preterm models) [[24], [25], [26]], infant’s sex [27], and maternal height since greater torso volume in taller individuals may affect fetal growth and gestational length [[28], [29], [30]]. Parity was excluded due to definition imprecision (per individual compared with per medical record). Models included race and ethnicity as a marker of social experiences, not as a measure of biological differences. Primary infant outcomes included birthweight (in g), gestational age at delivery (preterm <37 wk), and LGA (>90th percentile) or SGA (<10th percentile), and continuous size-for-gestational age z-scores were calculated for each infant [31]. Secondary outcomes include birthweight (in g) and macrosomia (birthweight ≥4000 g). To determine whether the effects of GWC varied by prepregnancy BMI within each obesity grade, we assessed effect modification by including interaction terms between the GWC latent class and continuous prepregnancy BMI. For comparison of these novel latent class models to the existing methods, we replaced the GWC latent class with calculated GWC z-scores in our models and compared the association of the GWC z-score with that of the GWC latent class with our outcome measures [25,32]. As a second sensitivity analysis, we examined term deliveries by restricting analyses with base models to pregnancies with delivery in ≥37 wk (n = 1586 excluded). All analyses were performed using Stata version 14.2 (StataCorp LLC).
Ethics
This data-only project was approved by the KPNC Institutional Review Board that waived the requirement for informed consent from participants.
Results
Sample characteristics
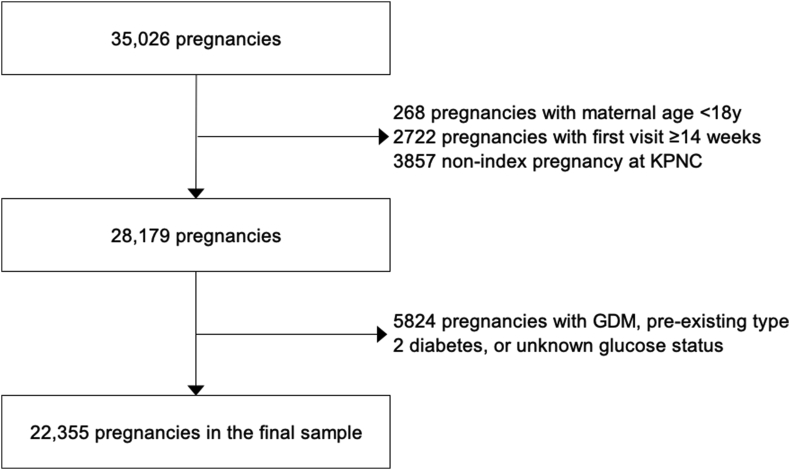
Of the 36,093 pregnancies identified, 22,355 pregnancies met the inclusion criteria (Figure 1). Grade 1 obesity comprised the greatest portion of our sample (59.0%), followed by grade 2 (25.9%) and grade 3 (15.2%) obesity (Table 1). The majority of this sample self-identified as White (37.2%) or Hispanic (36.4%), followed by Black (12.5%) or Asian (8.3%) (Table 1). Maternal age, height, parity, and gestational age at delivery did not differ meaningfully by obesity grade. Compared with those excluded from the analysis, those in the analytic sample were younger (29.8 ± 5.5 compared with 31.0 ± 5.8 y), were more likely to be classified as grade 1 obesity (59.0 compared with 47.4%), and had GWC above the IOM guidelines (60.5 compared with 44.1%), and were less likely to have a preterm delivery (7.1 compared with 10.8%); infants tended to be smaller, with lower birthweight for gestational age (GA) z-score (0.34 ± 1.10 compared with 0.48 ± 1.18 SD) and reduced rates of macrosomia (16.5 compared with 17.0%), LGA (14.3 compared with 18.9%), and LBW (4.5 compared with 6.1%; Supplemental Table 1).
FIGURE 1.
Flow chart of participants. GDM, gestational diabetes; KPNC, Kaiser Permanente Northern California.
TABLE 1.
Sample characteristics by prepregnancy obesity grade (n = 22,355)
| Grade 1 | Grade 2 | Grade 3 | All | |
|---|---|---|---|---|
| n (%) | 13,180 (59.0) | 5787 (25.9) | 3388 (15.2) | 22,355 |
| Age, y | 29.9 ± 5.5 | 29.6 ± 5.4 | 29.7 ± 5.4 | 29.8 ± 5.5 |
| Height, cm | 162.6 ± 7.3 | 163.4 ± 7.2 | 163.7 ± 7.1 | 163.0 ± 7.3 |
| Parity <3 | 11,802 (89.5) | 5116 (88.4) | 2993 (88.3) | 19,911 (89.1) |
| GA,1 wk | 38.9 ± 1.8 | 38.9 ± 2.0 | 38.8 ± 2.0 | 38.9 ± 1.9 |
| BMI, kg/m2 | 32.2 ± 1.4 | 37.1 ± 1.4 | 44.4 ± 4.4 | 35.3 ± 4.9 |
| Ethnicity | ||||
| White | 4829 (36.6) | 2203 (38.1) | 1276 (37.7) | 8308 (37.2) |
| Hispanic | 4859 (36.9) | 2108 (36.4) | 1166 (34.4) | 8133 (36.4) |
| Black | 1393 (10.6) | 751 (13.0) | 647 (19.1) | 2791 (12.5) |
| Asian | 1394 (10.6) | 370 (6.4) | 96 (2.8) | 1860 (8.3) |
| Other2 | 705 (5.4) | 355 (6.1) | 203 (6.0) | 1263 (5.7) |
| Total GWC,3 kg | 12.0 ± 6.5 | 10.3 ± 7.0 | 8.7 ± 7.2 | 11.1 ± 6.9 |
| GWC z-score | −0.14 ± 0.83 | 0.01 ± 0.76 | 0.12 ± 0.67 | −0.06 ± 0.80 |
| Comparison to IOM4 | ||||
| Above IOM | 8743 (66.3) | 3203 (55.4) | 1569 (46.3) | 13,515 (60.5) |
| Within IOM | 2788 (21.2) | 1319 (22.8) | 767 (22.6) | 4874 (21.8) |
| Below IOM | 1649 (12.5) | 1265 (21.9) | 1052 (31.1) | 3966 (17.7) |
| Sex, m | 6682 (50.7) | 2971 (51.3) | 1700 (50.2) | 11,353 (50.8) |
| Preterm5 | 892 (6.8) | 429 (7.4) | 265 (7.8) | 1586 (7.1) |
| Birthweight, g | 3469.5 ± 556.6 | 3480.5 ± 601.8 | 3508.2 ± 629.0 | 3478.2 ± 580.1 |
| Birthweight GA z-score | 0.31 ± 1.08 | 0.36 ± 1.10 | 0.45 ± 1.15 | 0.34 ± 1.10 |
| Macrosomia6 | 2005 (15.2) | 1024 (17.7) | 650 (19.2) | 3679 (16.5) |
| Low birthweight7 | 554 (4.2) | 281 (4.9) | 171 (5.1) | 1006 (4.5) |
| LGA8 | 1757 (13.3) | 860 (14.9) | 583 (17.2) | 3200 (14.3) |
| SGA9 | 1013 (7.7) | 430 (7.4) | 225 (6.6) | 1668 (7.5) |
Values are expressed as n (%) or mean ± SD.
Gestational age.
Includes unknown/not reported.
Gestational weight change
IOM guidelines: 5–9 kg.
Delivery in <37 wk.
Birthweight ≥4000 g.
Birthweight ≤2500 g.
Large for gestational age: birthweight >90th percentile.
Small for gestational age: birthweight <10th percentile.
Maternal weight change
The overall mean total GWC at delivery was 11.1 ± 6.9 kg (range: −25.0 to 46.2 kg), above the IOM guideline of 5–9 kg. A majority of individuals (60.5%) gained weight above the IOM recommendation, whereas 21.8% met the recommendation and 17.7% gained weight below that per recommendation or lost weight (n = 824; 3.7%). Total GWC decreased across BMI categories from obesity grade 1 to obesity grade 3 (Table 1). Accordingly, the incidence of excessive GWC decreased across the BMI categories from obesity grade 1 to obesity grade 3 (66.3% to 46.3%), whereas the likelihood of meeting the IOM recommendations was similar between groups (P = 0.395). Although many lost weight at various timepoints during pregnancy, only 756 (3.3%) maintained weight from prepregnancy to their final pregnancy weight (±1 kg) and 824 (3.7%) lost weight (<0 kg).
Incidence of infant outcomes
The rate of preterm delivery within 37 wk of gestation increased from obesity grade 1 (6.8%) to grade 3 (7.8%; Table 1). Birthweight and birthweight for GA z-score markedly increased from grade 1 obesity (z-score: 0.31 ± 1.08 SD) to grade 3 obesity (0.45 ± 1.15 SD), paralleled by similar trends in macrosomia and LGA. The incidence of LBW increased from obesity grade 1 (4.2%) to grade 3 (5.1%), whereas the rate of SGA decreased across the BMI categories from obesity grade 1 to grade 3.
GWC trajectories and infant outcomes by obesity grade
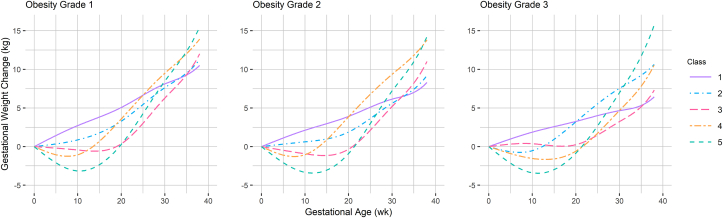
The latent class trajectory models identified five gestational weight change classes with notable similarities across obesity grades (Figure 2). Class 1 (IOM), the reference class, exhibited steady gain that followed a pattern most similar to the IOM recommendations; class 2 (slow moderate gain) demonstrated slower early weight gain than class 1, but with a similar total weight gain at 38 wk. Class 3 (moderate gain) was characterized by weight maintenance or a small loss in the first half of pregnancy, followed by rapid, moderately high gain in the second half of pregnancy. Generally, classes 4 and 5 exhibited high weight gain. Class 4 (rapid high gain) exhibited early, rapid weight gain; class 5 (high gain) exhibited the greatest weight loss followed by the fastest and highest weight gain, but only during the second half of pregnancy.
FIGURE 2.
Predicted gestational weight change from 0 to 38 wk of gestation by obesity grade. Obesity grade 1 – class 1: n = 2264; class 2: n = 5090; class 3: n = 2303; class 4: n = 2450; and class 5: n = 1073. Obesity grade 2 – class 1: n = 1067; class 2: n = 2368; class 3: n = 1104; class 4: n = 836; and class 5: n = 412. Obesity grade 3 – class 1: n = 729; class 2: n = 1172; class 3: n = 725; class 4: n = 606; and class 5: n = 156.
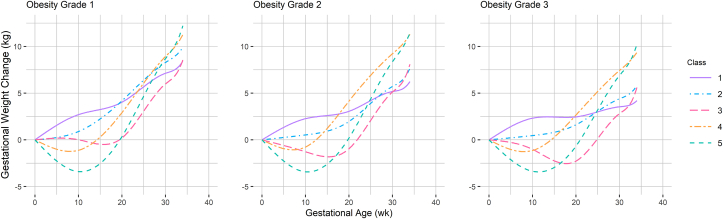
There are also noteworthy differences in GWC patterns across obesity grades. The patterns identified in obesity grade 1 demonstrate excessive weight gain with similar weight at 38 wk for classes 1–3 and classes 4–5. The slopes for all classes in obesity grade 3 are shallower, and the range of predicted final weight change is quite wide (Table 2). The predicted weight change at 38 wk for all classes was above the IOM recommendation in obesity grade 1, whereas the model for obesity grade 3 estimated the lowest GWC (6.4 kg) for reference class 1 and the greatest GWC in high-gain class 5 (15.8 kg) of all the models. Figure 3 depicts GWC latent class trajectory classes up to 34 wk for assessing preterm delivery associations by obesity grade.
TABLE 2.
Predicted total, trimester, and weekly weight change (kg) to 38 wk for each gestational weight change latent class by prepregnancy obesity grade (n = 22,355)
| Week | Obesity grade 1 |
Obesity grade 2 |
Obesity grade 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Trimester rate | Weekly rate | Total | Trimester rate | Weekly rate | Total | Trimester rate | Weekly rate | ||
| Class 1 | 14 | 3.61 | 0.26 | 2.80 | 0.20 | 2.45 | 0.17 | |||
| 28 | 7.59 | 3.99 | 0.28 | 5.78 | 2.97 | 0.21 | 4.42 | 1.98 | 0.14 | |
| 38 | 10.51 | 2.92 | 0.29 | 8.29 | 2.52 | 0.25 | 6.44 | 2.02 | 0.20 | |
| Class 2 | 14 | 1.56 | 0.11 | 0.89 | 0.06 | 0.14 | 0.01 | |||
| 28 | 6.81 | 5.26 | 0.38 | 4.85 | 3.96 | 0.28 | 2.54 | 2.40 | 0.17 | |
| 38 | 11.28 | 4.46 | 0.45 | 9.20 | 4.35 | 0.44 | 7.31 | 4.78 | 0.48 | |
| Class 3 | 14 | −0.59 | −0.04 | −1.16 | −0.08 | −1.65 | −0.12 | |||
| 28 | 5.01 | 5.60 | 0.40 | 3.98 | 5.14 | 0.37 | 3.48 | 5.12 | 0.37 | |
| 38 | 12.01 | 7.00 | 0.70 | 11.02 | 7.04 | 0.70 | 10.56 | 7.09 | 0.71 | |
| Class 4 | 14 | 0.23 | 0.02 | 0.38 | 0.03 | 0.56 | 0.04 | |||
| 28 | 8.42 | 8.20 | 0.59 | 8.37 | 7.99 | 0.57 | 6.80 | 6.24 | 0.45 | |
| 38 | 13.92 | 5.50 | 0.55 | 13.81 | 5.44 | 0.54 | 10.63 | 3.83 | 0.38 | |
| Class 5 | 14 | −2.65 | −0.19 | −3.24 | −0.23 | −3.20 | −0.23 | |||
| 28 | 6.85 | 9.51 | 0.68 | 5.59 | 8.83 | 0.63 | 5.23 | 8.43 | 0.60 | |
| 38 | 15.48 | 8.63 | 0.86 | 14.25 | 8.66 | 0.87 | 15.75 | 10.52 | 1.05 | |
Values are estimates from latent class mixed model analysis. Class 1 serves as the reference group for each prepregnancy obesity grade.
FIGURE 3.
Predicted gestational weight change from 0 to 34 wk of gestation by obesity grade. Obesity grade 1 – class 1: n = 2359; class 2: n = 4423; class 3: n = 2317; class 4: n = 3177; and class 5: n = 904. Obesity grade 2 – class 1: n = 1068; class 2: n = 2526; class 3: n = 971; class 4: n = 835; and class 5: n = 387. Obesity grade 3 – class 1: n = 497; class 2: n = 1530; class 3: n = 498; class 4: n = 653; and class 5: n = 210.
Obesity grade 1 GWC class and infant outcomes
In those with obesity grade 1, trajectory classes 2 and 4 showed gain above the prepregnancy weight in the first 10 wk of pregnancy, while moderate-gain class 3 and high-gain class 5 exhibited accelerated weight gain above the prepregnancy weight, but only in the second half of pregnancy; the estimated weight change was above the IOM recommendation for all latent classes (Figure 2). However, in infants of pregnancies with grade 1 obesity, only classes 2, 4, and 5 were associated with an increased risk of adverse outcomes (Table 3). In high-gain classes 4 and 5, the risks for LGA were 27% and 47% higher, respectively, compared to reference class 1; the predicted birthweight for GA z-score was analogously elevated. Interestingly, slow moderate-gain in class 2 was associated with a decreased risk of LBW. In the preterm model for grade 1 obesity, no associations were observed between the GWC latent class and preterm delivery.
TABLE 3.
Adjusted associations between gestational weight change latent class and infant outcomes by prepregnancy obesity grade (n = 22,355)
| LGA1 IRR (95% CI) | SGA2 IRR (95% CI) | Birthweight GA z-score β (95% CI) | Low birthweight3 IRR (95% CI) | Preterm delivery4 IRR (95% CI) | |
|---|---|---|---|---|---|
| Grade 1 (n = 13,180) | |||||
| Class 1 | Referent | ||||
| Class 2 | 1.00 (0.88, 1.14) | 1.01 (0.86, 1.20) | 0.05 (−0.00, 0.10) | 0.76 (0.60, 0.97)5 | 0.87 (0.73, 1.05) |
| Class 3 | 1.02 (0.87, 1.19) | 1.18 (0.97, 1.43) | −0.02 (−0.08, 0.04) | 0.99 (0.73, 1.35) | 1.13 (0.92, 1.39) |
| Class 4 | 1.27 (1.10, 1.46)5 | 0.81 (0.66, 1.00) | 0.18 (0.12, 0.24)5 | 0.77 (0.58, 1.01) | 1.04 (0.86, 1.27) |
| Class 5 | 1.47 (1.24, 1.74)5 | 0.82 (0.62, 1.08) | 0.18 (0.10, 0.26)5 | 0.93 (0.67, 1.29) | 1.05 (0.79, 1.39) |
| Grade 2 (n = 5787) | |||||
| Class 1 | Referent | ||||
| Class 2 | 1.40 (1.14, 1.71)5 | 1.14 (0.88, 1.47) | 0.08 (−0.00, 0.15) | 1.05 (0.72, 1.53) | 1.04 (0.80, 1.36) |
| Class 3 | 1.51 (1.20, 1.90)5 | 1.06 (0.78, 1.45) | 0.07 (−0.02, 0.17) | 1.55 (1.05, 2.28)5 | 1.44 (1.06, 1.95)5 |
| Class 4 | 2.02 (1.61, 2.52)5 | 0.87 (0.61, 1.23) | 0.27 (0.17, 0.36)5 | 0.68 (0.39, 1.16) | 0.92 (0.65, 1.30) |
| Class 5 | 1.98 (1.52, 2.58)5 | 1.18 (0.79, 1.78) | 0.28 (0.15, 0.40)5 | 1.12 (0.68, 1.85) | 1.48 (1.00, 2.19) |
| Grade 3 (n = 3388) | |||||
| Class 1 | Referent | ||||
| Class 2 | 0.83 (0.67, 1.04) | 1.12 (0.79, 1.58) | −0.11 (−0.22, −0.01)5 | 1.14 (0.76, 1.72) | 0.95 (0.67, 1.35) |
| Class 3 | 1.30 (1.04, 1.62)5 | 1.23 (0.84, 1.78) | 0.07 (−0.05, 0.19) | 1.27 (0.78, 2.06) | 1.43 (0.94, 2.16) |
| Class 4 | 1.18 (0.94, 1.48) | 0.72 (0.45, 1.14) | 0.18 (0.06, 0.30)5 | 0.81 (0.47, 1.39) | 0.93 (0.61, 1.41) |
| Class 5 | 1.20 (0.82, 1.73) | 1.11 (0.60, 2.05) | 0.13 (−0.07, 0.32) | 1.56 (0.87, 2.80) | 1.61 (0.98, 2.66) |
Values are estimated incidence-rate ratios for multivariable Poisson regression with robust standard errors or β-coefficients for multivariable linear regression models adjusted for maternal age, ethnicity, height (cm), gestational age at the time of delivery (except in preterm models), and infant’s sex. Weight trajectories modeled to ≤38 wk in base models and to ≤34 wk in preterm models. Class 1 serves as the reference group for each prepregnancy obesity grade.
Large for gestational age: birthweight >90th percentile.
Small for gestational age: birthweight <10th percentile.
Birthweight ≤2500 g.
Delivery in <37 wk of gestation.
CI does not contain 0 (β-coefficient) or 1 (IRR).
Obesity grade 2 GWC class and infant outcomes
In those with obesity grade 2, the predicted weight change was only within the IOM recommendation for reference class 1 (8.3 kg at 38 wk). In adjusted infant outcome models, all 4 GWC patterns were associated with increased risk for LGA compared to the reference class. This was most pronounced in the high-gain classes (4 and 5) that were each associated with a 2-fold increased risk for LGA and nearly 30% higher estimated birthweight for GA z-score. Only the class 3 GWC pattern of weight maintenance before 20 wk followed by rapid, moderate gain was associated with a 55% increased risk for LBW and 44% increased risk for preterm birth.
Obesity grade 3 GWC class and infant outcomes
The trajectory classes identified in those with grade 3 obesity exhibited the highest (class 5) and lowest (class 1) predicted total weight change at 38 wk of gestation (Figure 2, Table 2); however, obesity grade 3 trajectories were associated with few infant outcomes. Rapid high-gain class 4 exhibited weight gain only slightly higher than moderate-gain class 3 at term (Table 2), but only class 3 was associated with an increased risk of LGA; in contrast, class 4 was associated with increased birthweight for GA z-score (Table 3). Weight gain in slow-gain class 2 was depressed through the first half of pregnancy compared with that in class 2 in the other obesity grades, and the estimated birthweight for GA z-score was reduced for this trajectory in obesity grade 3. No associations were detected between the GWC classes and preterm delivery for any class of weight changes. Additional results for birthweight and macrosomia are presented in Supplemental Table 3.
GWC z-scores and infant outcomes by obesity grade
Similar patterns of risk for infant outcomes were observed across GWC z-score and obesity grades (Table 4), as observed in the trajectory class analyses (Table 3). Compared to the reference z-score of −1 to 1 corresponding to the expected weight gain based on the IOM guidelines [32], those with a GWC z-score of <−1 (lower GWC) were associated with a decreased risk of LGA across obesity grades, whereas those with a GWC z-score of >1 (higher GWC) were associated with an increased risk of LGA. Lower GWC z-scores were associated with a greater risk of SGA in obesity grades 1 and 2, whereas higher GWC z-scores were associated with a decreased risk of SGA only in grade 1 obesity. For birthweight for GA z-score, the patterns were similar to those observed with LGA: the predicted birthweight for GA z-score was lower for those with lower GWC z-scores and higher for those with higher GWC z-scores across obesity grades. Increased risk for preterm delivery was only associated with a higher GWC z-score for grade 3 obesity, but no associations were detected between the GWC class and LBW. Additional outcome data are presented in Supplemental Table 4.
TABLE 4.
Adjusted associations between gestational weight change z-score and infant outcomes by prepregnancy obesity grade (n = 22,353)
| LGA1 IRR (95% CI) | SGA2 IRR (95% CI) | Birthweight GA z-score β (95% CI) | Low birthweight3 IRR (95% CI) | Preterm delivery4 IRR (95% CI) | |
|---|---|---|---|---|---|
| Grade 1 (n = 13,180) | |||||
| >1 | 1.64 (1.45, 1.86)5 | 0.57 (0.41, 0.79)5 | 0.27 (0.20, 0.34)5 | 0.82 (0.58, 1.15) | 1.15 (0.91, 1.47) |
| −1 to 1 | Referent | ||||
| <−1 | 0.48 (0.40, 0.57)5 | 1.62 (1.41, 1.87)5 | −0.36 (−0.41, −0.31)5 | 1.21 (0.93, 1.56) | 1.07 (0.89, 1.28) |
| Grade 2 (n = 5787) | |||||
| >1 | 1.82 (1.55, 2.13)5 | 0.66 (0.43, 1.02) | 0.31 (0.20, 0.41)5 | 0.90 (0.60, 1.37) | 1.33 (0.98, 1.79) |
| −1 to 1 | Referent | ||||
| <−1 | 0.47 (0.34, 0.65)5 | 1.74 (1.36, 2.22)5 | −0.41 (−0.51, −0.32)5 | 1.16 (0.76, 1.78) | 0.98 (0.71, 1.36) |
| Grade 3 (n = 3388) | |||||
| >1 | 1.53 (1.19, 1.96)5 | 0.51 (0.26, 1.01) | 0.40 (0.25, 0.56)5 | 1.12 (0.71, 1.77) | 2.19 (1.55, 3.09)5 |
| −1 to 1 | Referent | ||||
| <−1 | 0.46 (0.27, 0.79)5 | 1.65 (1.01, 2.68)5 | −0.41 (−0.59, −0.23)5 | 0.91 (0.48, 1.71) | 1.54 (0.98, 2.43) |
Values are estimated incidence-rate ratios for multivariable Poisson regression or β-coefficients for multivariable linear regression models adjusted for maternal age, ethnicity, height (cm), prepregnancy BMI, gestational age at delivery, and infant’s sex. GWC z-scores of −1 to 1 serve as the reference group for each prepregnancy obesity grade.
Large for gestational age: birthweight >90th percentile.
Small for gestational age: birthweight <10th percentile.
Birthweight ≤2500 g.
Delivery within 37 wk of gestation.
CI does not contain 0 (β-coefficient) or 1 (IRR).
Sensitivity analyses
In our sensitivity analyses restricted to the term sample (Supplemental Table 5), the observed associations were comparable with our findings in the larger analytic sample. However, for obesity grade 2, the association between slow gain class 2 and LBW was fully attenuated in the term sample.
Discussion
The 2009 IOM gestational weight gain recommendations are still widely used despite a lack of evidence to distinguish optimal weight change by obesity severity [1]. Although prepregnancy obesity has been associated with LGA and elevated birthweight in prior studies, our results indicate that the risk for adverse infant outcomes, particularly LGA and elevated birthweight, may vary by obesity severity, pattern, and timing of GWC—especially in obesity grades 1 and 2 with higher gain in the second half of pregnancy, resulting in total GWC above that recommended by the IOM guidelines.
Five analogous GWC patterns were identified by obesity grade using latent class trajectory analysis. Overall, the associations between the GWC pattern and SGA were not detected for any obesity grade, but LGA was associated with several patterns: high-gain classes 4 and 5 for grade 1, all classes for grade 2, and delayed moderate-gain class 3 for grade 3. Of particular note in obesity grade 3, the estimated GWC for class 3 was equal to high-gain class 4 at 38 wk, suggesting that the delayed rapid weight gain may be driving the LGA association rather than the total GWC. In parallel, increased birthweight for GA z-score was estimated for high-gain classes 4 and 5 for grades 1 and 2, but only in class 4 for obesity grade 3; indeed, moderate-gain class 2 in grade 3 was associated with a z-score decrease of −0.11. When compared to the term sample in sensitivity analyses, our findings were comparable except for LBW of class 2 in grade 2 obesity.
For LBW or preterm delivery, moderate-gain class 2 in obesity grade 1 was associated with a decreased risk of LBW, whereas the associated risk increased for delayed moderate-gain class 3 in obesity grade 2. However, in our term sensitivity analyses, LBW was not associated with any GWC class, suggesting that earlier births were driving these results. Additionally, delayed moderate-gain class 3 in obesity grade 2 was the only trajectory independently associated with an increased risk (44%) of preterm delivery, despite total gain being above the IOM recommendations at 38 wk. Previous evidence between obesity and preterm birth is conflicting; some findings point to the rising incidence of preterm birth across obesity grades and the increasing risk with increasing weight gain [33], or increased risk with an average of 0.5–1.5 lb/wk [34] or total gain above the IOM guidelines [4]. Furthermore, previous methodologies investigating modifiable risk factors for preterm birth are complicated by increasing GWC as the pregnancy duration increases. Thus, some studies have relied on weight gain for GA z-score charts for gestational age–dependent outcomes [[35], [36], [37]]. In our analyses, a GWC z-score of >1 in grade 3 obesity was associated with twice the risk of a preterm delivery; however, when differentiated by trajectory, only the grade 2 association with preterm birth was preserved (Table 4). Thus, a pattern of delayed moderate GWC, rather than total gain, may be a stronger predictor of preterm birth in obesity grade 2. The mechanism underlying this finding is currently unknown, but should be confirmed in future studies.
GWC patterns may help determine when it is important to monitor weight change. Although some trials have developed standardized weight gain for gestational age charts [[35], [36], [37], [38]], few studies have examined the patterns of actual weight change across pregnancy [[39], [40], [41], [42], [43]], only one trajectory study was adequately powered to stratify by obesity grade [43], and none have reported on how patterns impact pregnancy and birth outcomes. Santos et al. [43] used a generalized additive model to summarize GWC distributions that distinguished 5 patterns varying by prepregnancy BMI in a large pooled analysis of 218,216 pregnancies from 33 countries primarily in Northern Europe. Compared to our study, that sample included only a small portion of pregnancies with obesity (8.1%), particularly grades 2 (1.6%) and 3 (0.5%), and used 3 weights across pregnancy despite the availability of more, and weights between conception and 8 wk of gestation were not available. Additionally, the majority of the included cohorts relied on self-reported prepregnancy weight and height, and some used self-reported prenatal weights and gestational age. Unlike our results, at least one identified pattern in each obesity grade resulted in weight maintenance or loss, but associations with infant outcomes were not examined.
Our GWC trajectory results demonstrate that excessive weight gain declined with the increase in prepregnancy BMI [24,33,36], consistent with prior studies, and support prior evidence that excessive total gain is associated with larger infant size [24,33,44]—but only for specific GWC patterns. In a systematic review of 10 studies, Faucher et al. [44] found that GWC varied by obesity grade with most individuals gaining above that recommended by the IOM guidelines; the lowest combined risks for LGA, SGA, and cesarean delivery were with gains of 5–9 kg for grade 1, 1–5 kg for grade 2, and 0 kg for grade 3 obesity. This contradicts our trajectory findings. When the total GWC was ≤12 kg, grade 1 pregnancies were not at risk for LGA; the risk for LGA was only elevated for obesity grade 3 with delayed moderate gain (class 3) despite predicted GWC being above 0 kg in all GWC classes. In the LifeCycle Project [33], the optimal GWC to reduce the risk for a composite of adverse outcomes was 2.0–6.0 kg for obesity grades 1 and 3 and 0–4.0 kg for grade 2; however, 7 outcomes were equally weighted such that more common, less severe outcomes may have insensitively affected GWC ranges. In our analyses, despite excessive weight gain in all GWC classes for obesity grade 1, classes 2–5 for grade 2, and 3–5 for grade 3, not all of these trajectories were associated with an increased risk of LGA or greater birthweight. Thus, latent class models provide insights that analyses based on the total weight change cannot provide – the nuances of the timing and pattern and how these might affect birth outcomes. Further work in this area, including more outcomes and weighing them based on clinical importance, is needed to better inform potential recommendations based on trajectories.
This study has many strengths, including the application of novel nonlinear methods of GWC trajectory analysis that surpass limitations associated with total and rate of change that assume linear gain across pregnancy. Similarly, although some findings were supported in GWC z-score sensitivity analyses, estimation of trajectories provided nuances between patterns and revealed specific associations with infant outcomes. We used a large, socioeconomically and ethnically diverse US sample adequately powered to stratify by obesity severity. The use of measured weight and height provided evidence of very early weight change and limited misclassification of BMI and GWC compared with the approaches using self-reported measures or calculated BMI from the first prenatal visit weight. This study also has limitations. Because we excluded cardiometabolic conditions known to affect GWC, individuals in the included sample tended to have lower BMI and rates of infant outcomes. Furthermore, our models did not identify trajectories with low weight gain (13.6%) or loss (3.7%) due to small proportions in our sample. In addition, while our findings could reflect naturally occurring patterns in adipose tissue changes during pregnancy with those with higher stores needing to gain less weight [43,44], we could also be observing regression to the mean or a combination of these phenomena and regression to the mean. Finally, our health record data lacked evidence of preconception or prenatal diet, activity, socioeconomic status, food security, and other social determinants of health that may affect weight and pregnancy outcomes.
GWC is neither linear nor consistent across or between pregnancies, and the optimal pattern that balances perinatal risks may vary by obesity severity. Informing evidence-based recommendations for pregnancy weight change requires robust methods and clinically significant applications at the individual level. This may include assessing weight histories and values, as well as support for achieving health goals while optimizing nutrition prior to and during pregnancy [45]. Prenatal weight change charts informed by our analyses would provide a useful instrument for monitoring pregnancy health. This is the first study to use a clinically translatable approach to generate weight trajectories and associated risk patterns for a considerable population of pregnancies in reproductive science—those affected by obesity. Indeed, for individuals with obesity, optimizing GWC during pregnancy may aid risk reduction of certain outcomes while respecting the lived experience.
Acknowledgements
We thank Paul J. Rathouz from The University of Texas at Austin for his valuable insights, contributions, and critical review of this paper. We also acknowledge the contributions of Emily M. Merriman and Julia Smith during preprocessing and development of this work.
Author contribution
The authors’ responsibilities were as follows – ARN: designed and conceived the study and was responsible for data acquisition, management, analysis, and interpretation and composition of the manuscript; ARN, MMH, EMW: were responsible for the conception and design of the study, data acquisition and interpretation, and drafting and critical review of the manuscript; EMW: supervised data management; FX: supported data acquisition and database management, and provided critical feedback on the manuscript; NB: supported data analysis and interpretation, and provided critical feedback on the manuscript; SFF, RR: supported data analysis and interpretation, and provided critical feedback on the manuscript; RR: supported management of the data; and all authors have read and approved the final manuscript. EW reports financial support from the National Institute of Health. MH reports financial support from Kaiser Permanente and the National Institutes of Health. The remaining authors ARN, NB, FX, SFF, and RR report no conflicts of interest.
Data Availability
Data described in the manuscript, code book, and analytic code will not be made available because of the electronic health record data and confidential nature of the data collected.
Funding
Research activities conducted by ARN were supported by a fellowship from The University of Texas at Austin. EW was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NIH/NICHD K99/R00 HD086304). MH was supported by a Kaiser Permanente Community Benefit Grant and by the The National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK R01DK118455). Supporting sources had no involvement or restrictions regarding publication. The authors have no disclaimers to report.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.03.001.
Contributor Information
Monique M. Hedderson, Email: monique.m.hedderson@kp.org.
Elizabeth M. Widen, Email: elizabeth.widen@austin.utexas.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Institute of Medicine . In: Weight gain during pregnancy: reexamining the guidelines. Rasmussen K.M., Yaktine A.L., editors. National Academies Press, National Academy of Sciences; Washington (DC): 2009. The National Academies collection: reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 2.Driscoll A.K., Gregory E.C.W. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020;392:1–8. [PubMed] [Google Scholar]
- 3.Goldstein R.F., Abell S.K., Ranasinha S., Misso M., Boyle J.A., Black M.H., et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faucher M.A., Hastings-Tolsma M., Song J.J., Willoughby D.S., Bader S.G. Gestational weight gain and preterm birth in obese women: a systematic review and meta-analysis. BJOG. 2016;123(2):199–206. doi: 10.1111/1471-0528.13797. [DOI] [PubMed] [Google Scholar]
- 5.Deputy N.P., Sharma A.J., Kim S.Y., Hinkle S.N. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet. Gynecol. 2015;125(4):773–781. doi: 10.1097/AOG.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaillard R., Durmus B., Hofman A., Mackenbach J.P., Steegers E.A., Jaddoe V.W. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21(5):1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 7.Blomberg M. Maternal obesity, mode of delivery, and neonatal outcome. Obstet. Gynecol. 2013;122(1):50–55. doi: 10.1097/aog.0b013e318295657f. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists, ACOG Committee opinion no 548: weight gain during pregnancy. Obstet. Gynecol. 2013;121(1):210–212. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 9.Bodnar L.M., Siega-Riz A.M., Simhan H.N., Himes K.P., Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am. J. Clin. Nutr. 2010;91(6):1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nohr E.A., Vaeth M., Baker J.L., Sorensen T., Olsen J., Rasmussen K.M. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am. J. Clin. Nutr. 2008;87(6):1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 11.Beyerlein A., Schiessl B., Lack N., von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am. J. Clin. Nutr. 2009;90(6):1552–1558. doi: 10.3945/ajcn.2009.28026. [DOI] [PubMed] [Google Scholar]
- 12.Siega-Riz A.M., Viswanathan M., Moos M.K., Deierlein A., Mumford S., Knaack J., et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am. J. Obstet. Gynecol. 2009;201(4):339.e1–339.e14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Gavard J.A., Artal R. The association of gestational weight gain with birth weight in obese pregnant women by obesity class and diabetic status: a population-based historical cohort study. Matern. Child Health J. 2014;18(4):1038–1047. doi: 10.1007/s10995-013-1356-0. [DOI] [PubMed] [Google Scholar]
- 14.Bornstein S. An integrated EHR at Northern California Kaiser Permanente: pitfalls, challenges, and benefits experienced in transitioning. Appl. Clin. Inform. 2012;3(3):318–325. doi: 10.4338/aci-2012-03-ra-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahavandi S., Price S., Sumithran P., Ekinci E.I. Exploration of the shared pathophysiological mechanisms of gestational diabetes and large for gestational age offspring. World J. Diabetes. 2019;10(6):333–340. doi: 10.4239/wjd.v10.i6.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proust-Lima C., Philipps V., Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J. Stat. Softw. 2017;78(2):1–56. doi: 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- 17.Biernacki C., Celeux G., Govaert G. Choosing starting values for the EM algorithm for getting the highest likelihood in multivariate Gaussian mixture models. Comput. Stat. Data Anal. 2003;41(3):561–575. doi: 10.1016/s0167-9473(02)00163-9. [DOI] [Google Scholar]
- 18.Proust-Lima C., Dartigues J.F., Jacqmin-Gadda H. Misuse of the linear mixed model when evaluating risk factors of cognitive decline. Am. J. Epidemiol. 2011;174(9):1077–1088. doi: 10.1093/aje/kwr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widen E.M., Burns N., Daniels M., Backlund G., Rickman R., Foster S., et al. Gestational weight change and childhood body composition trajectories from pregnancy to early adolescence. Obesity (Silver Spring) 2022;30(3):707–717. doi: 10.1002/oby.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widen E.M., Burns N., Kahn L.G., Grewal J., Backlund G., Nichols A.R., et al. Prenatal weight and regional body composition trajectories and neonatal body composition: the NICHD Foetal Growth Studies. Pediatr. Obes. 2023;18(3) doi: 10.1111/ijpo.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crainiceanu C.M., Ruppert D., Wand M.P. Bayesian analysis for penalized spline regression using WinBUGS. J. Stat. Softw. 2005;1(14):1–24. doi: 10.18637/jss.v014.i14. [DOI] [Google Scholar]
- 22.Cummings P. Methods for estimating adjusted risk ratios. Stata J. 2009;9(2):175–196. doi: 10.1177/1536867X0900900201. [DOI] [Google Scholar]
- 23.Zou G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 24.Bodnar L.M., Pugh S.J., Lash T.L., Hutcheon J.A., Himes K.P., Parisi S.M., et al. Low gestational weight gain and risk of adverse perinatal outcomes in obese and severely obese women. Epidemiology. 2016;27(6):894–902. doi: 10.1097/ede.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leonard S.A., Hutcheon J.A., Bodnar L.M., Petito L.C., Abrams B. Gestational weight gain-for-gestational age z-score charts applied across U.S. populations. Paediatr. Perinat. Epidemiol. 2018;32(2):161–171. doi: 10.1111/ppe.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutcheon J.A., Bodnar L.M. A systematic approach for establishing the range of recommended weight gain in pregnancy. Am. J. Clin. Nutr. 2014;100(2):701–707. doi: 10.3945/ajcn.114.085258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson J.G., Kajantie E., Osmond C., Thornburg K., Barker D.J. Boys live dangerously in the womb. Am. J. Hum. Biol. 2010;22(3):330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polzlberger E., Hartmann B., Hafner E., Stumpflein I., Kirchengast S. Maternal height and pre-pregnancy weight status are associated with fetal growth patterns and newborn size. J. Biosoc. Sci. 2017;49(3):392–407. doi: 10.1017/s0021932016000493. [DOI] [PubMed] [Google Scholar]
- 29.Addo O.Y., Stein A.D., Fall C.H., Gigante D.P., Guntupalli A.M., Horta B.L., et al. Maternal height and child growth patterns. J. Pediatr. 2013;163(2):549–554. doi: 10.1016/j.jpeds.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myklestad K., Vatten L.J., Magnussen E.B., Salvesen K.Å., Romundstad P.R. Do parental heights influence pregnancy length?: a population-based prospective study, HUNT 2. BMC Pregnancy Childbirth. 2013;13:33. doi: 10.1186/1471-2393-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aris I.M., Kleinman K.P., Belfort M.B., Kaimal A., Oken E. A 2017 US reference for singleton birth weight percentiles using obstetric estimates of gestation. Pediatrics. 2019;144(1) doi: 10.1542/peds.2019-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutcheon J.A., Platt R.W., Abrams B., Himes K.P., Simhan H.N., Bodnar L.M. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am. J. Clin. Nutr. 2013;97(5):1062–1067. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group, E. Voerman. Santos S., Inskip H., Amiano P., Barros H., et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702–1715. doi: 10.1001/jama.2019.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schieve L.A., Cogswell M.E., Scanlon K.S., Perry G., Ferre C., Blackmore-Prince C., et al. Prepregnancy body mass index and pregnancy weight gain: associations with preterm delivery. Obstet. Gynecol. 2000;96(2):194–200. doi: 10.1016/S0029-7844(00)00883-8. [DOI] [PubMed] [Google Scholar]
- 35.Catov J.M., Abatemarco D., Althouse A., Davis E.M., Hubel C. Patterns of gestational weight gain related to fetal growth among women with overweight and obesity. Obesity (Silver Spring) 2015;23(5):1071–1078. doi: 10.1002/oby.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutcheon J.A., Platt R.W., Abrams B., Himes K.P., Simhan H.N., Bodnar L.M. Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring) 2015;23(3):532–535. doi: 10.1002/oby.21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang A., Xiao Y., Hu H., Zhao W., Yang Q., Ma W., et al. Gestational weight gain charts by gestational age and body mass index for Chinese women: a population-based follow-up study. J. Epidemiol. 2020;30(8):345–353. doi: 10.2188/jea.je20180238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson K., Hutcheon J.A., Stephansson O., Cnattingius S. Pregnancy weight gain by gestational age and BMI in Sweden: a population-based cohort study. Am. J. Clin. Nutr. 2016;103(5):1278–1284. doi: 10.3945/ajcn.115.110197. [DOI] [PubMed] [Google Scholar]
- 39.Kac G., Carilho T.R.B., Rasmussen K.M., Reichenheim M.E., Farias D.R., Hutcheon J.A., et al. Gestational weight gain charts: results from the Brazilian Maternal and Child Nutrition Consortium. Am. J. Clin. Nutr. 2021;113(5):1351–1360. doi: 10.1093/ajcn/nqaa402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riddell C.A., Platt R.W., Bodnar L.M., Hutcheon J.A. Classifying gestational weight gain trajectories using the SITAR growth model. Paediatr. Perinat. Epidemiol. 2017;31(2):116–125. doi: 10.1111/ppe.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galjaard S., Pexsters A., Devlieger R., Guelinckx I., Abdallah Y., Lewis C., et al. The influence of weight gain patterns in pregnancy on fetal growth using cluster analysis in an obese and nonobese population. Obesity (Silver Spring) 2013;21(7):1416–1422. doi: 10.1002/oby.20348. [DOI] [PubMed] [Google Scholar]
- 42.Pugh S.J., Albert P.S., Kim S., Grobman W., Hinkle S.N., Newman R.B., Wing D.A., Grantz K.L. Patterns of gestational weight gain and birthweight outcomes in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Fetal Growth Studies-Singletons: a prospective study. Am. J. Obstet. Gynecol. 2017;217(3):346.e1–346.e11. doi: 10.1016/j.ajog.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santos S., Eekhout I., Voerman E., Gaillard R., Barros H., Charles M.A., et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. 2018;16(1):201. doi: 10.1186/s12916-018-1189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faucher M.A., Barger M.K. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: a systematic review. Women Birth. 2015;28(3):e70–e79. doi: 10.1016/j.wombi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Siega-Riz A.M., Bodnar L.M., Stotland N.E., Stang J. The current understanding of gestational weight gain among women with obesity and the need for future research. NAM Perspect. 2020;2020 doi: 10.31478/202001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available because of the electronic health record data and confidential nature of the data collected.