Dear Editor,
Post-COVID-19 syndrome (PCS) refers to new, recurring, or persisting symptoms beyond 3 months after acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. PCS is a multisystem disease that develops regardless of the severity of acute COVID-19. Commonly described symptoms comprise a combination of anosmia, fatigue, cognitive impairment, and symptoms from the cardiovascular, respiratory, gastrointestinal, peripheral, and autonomic nervous system (ANS). Today, the pathophysiology of PCS remains unclear. It has been proposed that some symptoms may be attributed to dysautonomia, due to either direct viral or immune-mediated effects. 1 In turn, dysautonomia may be encountered either as a failure or as an overactivation of either of the ANS branches, the sympathetic or parasympathetic. In a previous study, we demonstrated that ANS dysfunction may contribute to PCS symptoms, 2 but did not include patients with a history of SARS-CoV-2 infection who did not develop PCS (NONPCS).
A case–control study was thus designed to investigate whether ANS dysfunction may distinguish PCS patients (1) from their NONPCS counterparts and (2) from healthy controls without a history of SARS-CoV-2 infection. Adult patients with a history of laboratory-confirmed COVID-19 without hospitalization, with or without PCS symptoms for >3 months from COVID-19 onset, were evaluated at a referral center in Athens, Greece (‘Attikon’ University Hospital) between June 2022 and November 2022. PCS patients with cardiovascular complications or diabetes were excluded. The most commonly reported PCS symptoms were anosmia, fatigue, cognitive impairment (brain fog), arthralgia and myalgia, numbness, mood disorders such as anxiety and depression, breathlessness, chest discomfort, and gastrointestinal symptoms (diarrhea, nausea).
Controls included volunteers without a history of SARS-COV-2 infection, cardiovascular diseases, diabetes, or ANS disorders. All patients and controls were vaccinated for SARS-COV-2. The evaluation of ANS function was performed by sympathetic skin response (SSR) to investigate the sympathetic nervous system (SNS), and the cross-sectional area (CSA) of the vagus nerve (VN) was assessed by ultrasound to investigate the parasympathetic nervous system (PNS). Both investigators who performed SSR and sonography of VN were blinded to clinical data and group allocation of study participants (i.e. PCS, NONPCS, healthy controls). SSR and CSA measurements are described in detail in previously published studies and in the online-only supplement.2–4 The study was approved by the Institutional Research Bioethics Committee (EBD551/04.11.2021). Informed consent was obtained by all participants. Statistical analysis was performed using the Statistical Package for Social Science (SPSS Inc., version 24.0 for Windows; IBM, Armonk, NY, USA). Descriptive statistics are given as mean and standard deviation, frequency, and percentage. Statistical comparisons between groups were performed using the chi-square test for binary outcomes and analysis of variance (ANOVA) or Kruskal–Wallis test for continuous variables as appropriate. Correlations between variables were tested using Spearman’s rank correlation coefficients (r) as appropriate. A two-tailed p value of less than 0.05 was considered significant. Bonferroni correction for multiple comparison was applied as appropriate.
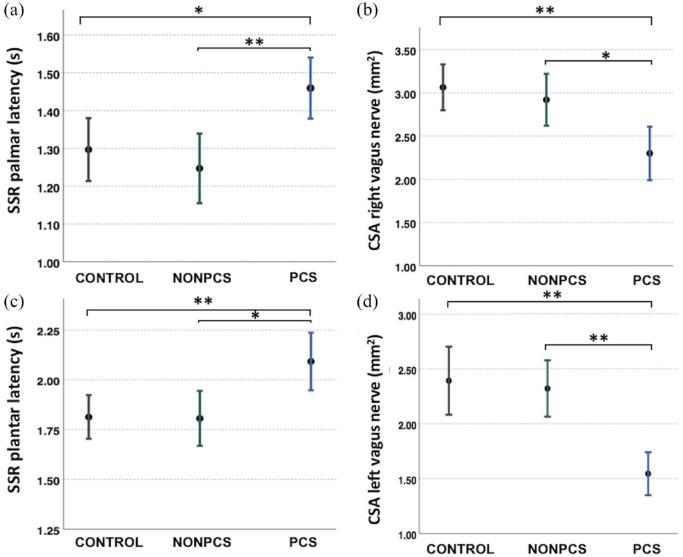
A total of 40 healthy subjects (24 women, 16 men), 20 NONPCS patients (13 women, 7 men), and 20 PCS (16 women, 4 men) were included. The mean age of controls was 43.3 ± 2 years (range: 23–65 years), of NONPCS was 36.8 ± 2 years (range: 30–59), and of PCS was 38.55 ± 3 years (range: 18–66 years). The PCS group did not differ in age from NONPCS (p = 1.000) nor from controls (p = 0.387). The three groups did not differ in sex (p = 0.322, Fisher’s exact). Among the study participants, 32 (80%) and 8 (20%) healthy controls had received two and three COVID-19 vaccine doses, respectively. Among NONPCS, 15 (75%) and 5 (25%) had received two and three vaccine doses, respectively. Among the PCS patients, 14 had received two vaccine doses (70%) and 6 had received three vaccine doses (30%), while none of the study participants had received a fourth vaccination at the time of the study. SSR amplitudes were similar between groups. However, palmar SSR latencies differed significantly between PCS patients (mean ± standard deviation: 1.45 ± 0.17 s), NONPCS patients (1.24 ± 0.19 s), and healthy controls (1.32 ± 0.24 s) (p = 0.007). Similarly, plantar SSR latencies differed significantly between PCS patients (2.09 ± 0.31 s), NONPCS patients (1.80 ± 0.29 s), and healthy controls (1.80 ± 0.31 s) (p = 0.004). CSA of both the right and left VN was significantly smaller in PCS compared with NONPCS patients and healthy controls. CSA of the right VN was 2.97 ± 0.73 mm2 (controls), 2.92 ± 0.64 mm2 (NONPCS), and 2.30 ± 0.66 mm2 (PCS) (p = 0.001); CSA of the left VN was 2.24 ± 0.93 mm2 (controls), 2.32 ± 0.54 mm2 (NONPCS), and 1.54 ± 0.41 mm2 (PCS) (p < 0.001). Post hoc analysis revealed that (1) controls and NONPCS did not differ in SSR latencies nor in VN CSA, (2) PCS patients had longer palmar latencies compared with controls (p = 0.027) and NONPCS (p = 0.009), (3) PCS patients had longer plantar latencies compared with controls (p = 0.006) and NONPCS (p = 0.013), (4) PCS patients had smaller right CSA VN compared with controls (p = 0.001) and NONPCS (p = 0.029), and (5) PCS patients had smaller left CSA VN compared with controls (p < 0.001) and NONPCS (p = 0.006) (Figure 1). No correlation was observed between SSR latencies and CSA of VN (Table 1). Similar findings were observed in a previous published study in amyotrophic lateral sclerosis (ALS) patients. 4
Figure 1.
Longer sympathetic skin response (SSR) latencies and smaller cross-sectional area (CSA) of the vagus nerve are noted in post-COVID syndrome (PCS) patients compared with healthy controls and non-post-COVID patients (NONPCS): (a) SSR palmar latencies, (b) SSR plantar latencies, (c) CSA of the right vagus nerve, and (d) CSA of the left vagus nerve.
One asterisk (*) indicates statistically significant differences (p < 0.05) and two asterisks (**) indicate statistically significant differences (p < 0.01).
Table 1.
Correlation analysis between sympathetic skin response (SSR) latencies and cross-sectional area (CSA) of the vagus nerve.
| Variable | Spearman’s correlation (r) | p value | Spearman’s correlation (r) | p value | |
|---|---|---|---|---|---|
| Right | Left | ||||
| CONTROLS | SSR palmar latencies versus CSA VN | 0.338 | −0.226 | 0.4 | −0.199 |
| SSR plantar latencies versus CSA VN | 0.185 | −0.309 | 0.366 | −0.214 | |
| NONPCS | SSR palmar latencies versus CSA VN | 0.338 | −0.226 | 0.400 | −0.199 |
| SSR plantar latencies versus CSA VN | 0.185 | −0.309 | 0.366 | −0.214 | |
| PCS | SSR palmar latencies versus CSA VN | 0.312 | −0.238 | 0.507 | −0.158 |
| SSR plantar latencies versus CSA VN | 0.050 | −0.444 | 0.502 | −0.159 | |
| NONPCS | SSR palmar latencies versus CSA VN | 0.338 | −0.226 | 0.400 | −0.199 |
| SSR plantar latencies versus CSA VN | 0.185 | −0.309 | 0.366 | −0.214 |
CSA, cross-sectional area; NONPCS, non-post-COVID; PCS, post-COVID syndrome; SSR, sympathetic skin response; VN, vagus nerve.
This study provides further evidence of autonomic dysfunction in PCS, with VN atrophy and prolonged SSR latencies accounting for parasympathetic and sympathetic nervous system involvement, respectively. On the contrary, NONPCS patients did not show evidence of subclinical ANS dysfunction, as reflected by the normal neurophysiological and neurosonography findings, and did not differ from healthy controls.
Dysautonomia has been associated in the past with post-viral conditions—that is, following Epstein-Barr virus infection 5 —while recent studies have associated the broad constellation of PCS symptoms with ANS dysfunction following SARS-CoV-2 infection.1,5,6 Cardiovascular and respiratory PCS symptoms, including postural orthostatic tachycardia syndrome (POTS) and persisting dyspnea, have been related to the involvement of chemoreceptors and mechanoreceptors or dysfunction of brainstem neurons. 6 In addition, neuropsychiatric PCS symptoms, such as fatigue, myalgias, anxiety, dizziness, and anosmia, have been linked to impaired ANS function.1,5
Although the pathophysiology underlying PCS still remains largely enigmatic, the correlation between PCS and ANS dysfunction has been gaining ground with growing evidence. According to the angiotensin-converting enzyme-2 (ACE2) hypothesis, ANS dysfunction may be mediated by ACE2 receptors that are abundantly expressed in ANS neurons and facilitate via binding to the spike protein of SARS-CoV-2 the viral cell entry in acute COVID-19. Within the ANS, ACE2 receptors are predominantly expressed in the nucleus tractus solitarius, a brainstem nucleus related to the dorsal vagal nucleus, the involvement of which may account for prolonged ANS impairment in PCS.5,6
From an epidemiological perspective, it is also noteworthy that women are more susceptible to PCS than men.1,5 Interestingly, chronic fatigue syndrome, another disease with clinical similarities to PCS, also exhibits female sex predominance and clinical features of dysautonomia, which could suggest that ANS dysfunction comprises a common pathophysiological denominator of the two disorders. 7
It should be stressed here, however, that the pathophysiology of PCS still remains to be untangled. Besides ANS dysfunction, pathways implicated in PCS comprise (1) sustained neuroinflammation via microglia activation or auto-immune responses, (2) altered microcirculation due to hypercoagulation or mitochondrial failure, and (3) structural alterations and hypometabolic activity in certain brain regions. 6 In addition, psychiatric comorbidities and also psychosomatic factors including enhanced somatization have been linked to increased PCS risk.8,9 Interestingly, as psychological triggers may also correlate with dysautonomia (e.g. increased anxiety may trigger dysautonomia and vice versa), the extent to which ANS dysfunction may contribute to PCS remains to be established.
The evidence provided by this study, and the fact that not all infected patients present with PCS nor do they present subclinical dysautonomia, could indicate an increased predisposition to ANS dysfunction in PCS. Further studies are, thus, direly needed to elucidate why some patients may be more prone to ANS dysfunction and PCS following SARS-CoV-2 infection.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864231180711 for Autonomic dysfunction entwined with post-COVID but absent in non-post-COVID patients: a neurophysiological and neurosonology study by Marianna Papadopoulou, Eleni Bakola, Apostolos Papapostolou, Maria-Ioanna Stefanou, Elisabeth Andreadou, Vasiliki Zouvelou, Maria Stefanatou, Mina Gaga, Ioannis Michopoulos and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iDs: Marianna Papadopoulou  https://orcid.org/0000-0002-0163-7455
https://orcid.org/0000-0002-0163-7455
Maria-Ioanna Stefanou  https://orcid.org/0000-0002-2305-6627
https://orcid.org/0000-0002-2305-6627
Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marianna Papadopoulou, Laboratory of Neuromuscular and Cardiovascular Study of Motion, Department of Physiotherapy, University of West Attica, Athens, Greece; Second Department of Neurology, School of Medicine and Attikon University General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Eleni Bakola, Second Department of Neurology, School of Medicine and Attikon University General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Apostolos Papapostolou, Second Department of Neurology, School of Medicine and Attikon University General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Maria-Ioanna Stefanou, Second Department of Neurology, School of Medicine and Attikon University General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Elisabeth Andreadou, First Department of Neurology, School of Medicine and Eginition University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Vasiliki Zouvelou, First Department of Neurology, School of Medicine and Eginition University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Maria Stefanatou, First Department of Neurosurgery, National and Kapodistrian University of Athens, Athens, Greece.
Mina Gaga, Seventh Respiratory Medicine Department and Asthma Center, Athens Chest Hospital ‘Sotiria’, Athens, Greece.
Ioannis Michopoulos, Second Department of Psychiatry, School of Medicine and Attikon University General Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Georgios Tsivgoulis, Second Department of Neurology, School of Medicine and Attikon University General Hospital, National and Kapodistrian University of Athens, Iras 39, Gerakas Attikis, Athens 15344, Greece.
Declarations
Ethics approval and consent to participate: The study was approved by the Institutional Research Bioethics Committee (EBD551/04.11.2021). Informed consent was obtained from all study participants.
Consent for publication: No identifiable patient data are included in this study. All participants consent to publication.
Author contributions: Marianna Papadopoulou: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft.
Eleni Bakola: Data curation; Investigation; Methodology; Writing – original draft.
Apostolos Papapostolou: Investigation; Writing – review & editing.
Maria-Ioanna Stefanou: Visualization; Writing – review & editing.
Elisabeth Andreadou: Writing – review & editing.
Vasiliki Zouvelou: Writing – review & editing.
Maria Stefanatou: Writing – review & editing.
Mina Gaga: Validation; Writing – review & editing.
Ioannis Michopoulos: Formal analysis; Writing – review & editing.
Georgios Tsivgoulis: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Validation; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Anonymized data used in this article will be made available on reasonable request from the corresponding author.
References
- 1.Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med 2021; 21: e63–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulou M, Bakola E, Papapostolou A, et al. Autonomic dysfunction in long-COVID syndrome: a neurophysiological and neurosonology study. J Neurol 2022; 269: 4611–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadopoulou M, Papapostolou A, Bakola E, et al. Neurophysiological and ultrasonographic comparative study of autonomous nervous system in patients suffering from fibromyalgia and generalized anxiety disorder. Neurol Sci 2021; 43: 2813–2821. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulou M, Bakola E, Papapostolou A, et al. Autonomic dysfunction in amyotrophic lateral sclerosis: a neurophysiological and neurosonology study. J Neuroimaging 2022; 32: 710–719. [DOI] [PubMed] [Google Scholar]
- 5.Vallée A.Dysautonomia and Implications for anosmia in long COVID-19 disease. J Clin Med 2021; 10: 5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022; 54: 1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth KJ, Scheibenbogen C.Pathophysiology of skeletal muscle disturbances in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 2021; 19: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischer M, Szepanowski F, Tovar M, et al. Post-COVID-19 syndrome is rarely associated with damage of the nervous system: findings from a prospective observational cohort study in 171 patients. Neurol Ther 2022; 11: 1637–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudroff T, Fietsam AC, Deters JR, et al. Post-COVID-19 fatigue: potential contributing factors. Brain Sci 2020; 10: 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864231180711 for Autonomic dysfunction entwined with post-COVID but absent in non-post-COVID patients: a neurophysiological and neurosonology study by Marianna Papadopoulou, Eleni Bakola, Apostolos Papapostolou, Maria-Ioanna Stefanou, Elisabeth Andreadou, Vasiliki Zouvelou, Maria Stefanatou, Mina Gaga, Ioannis Michopoulos and Georgios Tsivgoulis in Therapeutic Advances in Neurological Disorders