This study attempts to update estimates of diabetic retinopathy and vision-threatening diabetic retinopathy prevalence by demographic factors and US county and state.
Key Points
Question
What was the 2021 US prevalence of diabetic retinopathy and vision-threatening diabetic retinopathy?
Findings
The study team estimated that 9.60 million people in the US (26.43% of those with diabetes) had diabetic retinopathy and 1.84 million people (5.06% of those with diabetes) had vision-threatening diabetic retinopathy in 2021. There was marked variation in prevalence across states and the number of people living with diabetes-related eye disease grew substantially since prevalence was last estimated in 2004.
Meaning
The US prevalence of diabetes-related eye disease remains high and may grow in the coming decades due to the increasing burden of diabetes among youth and adults.
Abstract
Importance
Diabetic retinopathy (DR) is a common microvascular complication of diabetes and a leading cause of blindness among working-age adults in the US.
Objective
To update estimates of DR and vision-threatening diabetic retinopathy (VTDR) prevalence by demographic factors and US county and state.
Data Sources
The study team included data from the National Health and Nutrition Examination Survey (2005 to 2008 and 2017 to March 2020), Medicare fee-for-service claims (2018), IBM MarketScan commercial insurance claims (2016), population-based studies of adult eye disease (2001 to 2016), 2 studies of diabetes in youth (2021 and 2023), and a previously published analysis of diabetes by county (2012). The study team used population estimates from the US Census Bureau.
Study Selection
The study team included relevant data from the US Centers for Disease Control and Prevention’s Vision and Eye Health Surveillance System.
Data Extraction and Synthesis
Using bayesian meta-regression methods, the study team estimated the prevalence of DR and VTDR stratified by age, a nondifferentiated sex and gender measure, race, ethnicity, and US county and state.
Main Outcomes and Measures
The study team defined individuals with diabetes as those who had a hemoglobin A1c level at 6.5% or more, took insulin, or reported ever having been told by a physician or health care professional that they have diabetes. The study team defined DR as any retinopathy in the presence of diabetes, including nonproliferative retinopathy (mild, moderate, or severe), proliferative retinopathy, or macular edema. The study team defined VTDR as having, in the presence of diabetes, severe nonproliferative retinopathy, proliferative retinopathy, panretinal photocoagulation scars, or macular edema.
Results
This study used data from nationally representative and local population-based studies that represent the populations in which they were conducted. For 2021, the study team estimated 9.60 million people (95% uncertainty interval [UI], 7.90-11.55) living with DR, corresponding to a prevalence rate of 26.43% (95% UI, 21.95-31.60) among people with diabetes. The study team estimated 1.84 million people (95% UI, 1.41-2.40) living with VTDR, corresponding to a prevalence rate of 5.06% (95% UI, 3.90-6.57) among people with diabetes. Prevalence of DR and VTDR varied by demographic characteristics and geography.
Conclusions and Relevance
US prevalence of diabetes-related eye disease remains high. These updated estimates on the burden and geographic distribution of diabetes-related eye disease can be used to inform the allocation of public health resources and interventions to communities and populations at highest risk.
Introduction
More than 37 million people in the US have diabetes and are at risk for serious macrovascular and microvascular complications.1 Diabetic retinopathy (DR) is a common microvascular diabetes complication and a leading cause of blindness among working-age US adults.2 Non–vision-threatening stages of DR include mild and moderate nonproliferative DR. Vision-threatening DR (VTDR) includes severe nonproliferative DR, proliferative DR, and diabetic macular edema that can occur at any DR stage. Diabetes duration and glycemic control influence risk of VTDR.3,4,5,6,7
In 2004, the Eye Diseases Prevalence Research Group (EDPRG) published meta-analytic estimates of the crude US prevalence of DR and VTDR for adults 40 years or older.8 They found that 40.3% of adults with diabetes had DR and 8.2% had VTDR, corresponding to 4.1 million and 899 000 individuals, respectively. Among adults with diabetes, DR and VTDR prevalence did not vary substantially by age or sex. The prevalence of DR was higher among Hispanic individuals than non-Hispanic Black individuals.8 The EDPRG DR prevalence estimates are based on older data derived from population-based studies (PBS) with limited geographic representation that were not nationally representative of the US. Additionally, their estimates were only for the US population aged 40 years or older.
There are several single-source estimates of the national DR prevalence on the US Centers for Disease Control and Prevention’s Vision and Eye Health Surveillance System (VEHSS). Eye examinations were conducted in the 2005 to 2008 National Health and Nutrition Examination Survey (NHANES), an ongoing nationally representative, examination-based survey to assess the health and nutritional status of community-dwelling people in the US.9,10 Based on the 2005 to 2008 NHANES, the prevalence of DR and VTDR was 28.5% and 4.4%, respectively, among adults 40 years or older with diabetes.11 VEHSS also provides diagnosed DR documented in claims for commercial insurance and Medicare and Medicaid fee-for-service insurance.
Developing new multisource composite estimates of the US prevalence of DR and VTDR is important, since previously documented estimates are older and do not reflect recent changes in population demographics or diabetes prevalence over the last 2 decades. Studies using NHANES data have documented an increase in diabetes prevalence among US adults within the last 2 decades12,13 and this increase in diabetes prevalence has likely influenced the number of people living with DR. Additionally, efforts to focus investments for public health and clinical interventions to prevent vision loss from DR could benefit from estimates at the state and county level that show the geographic distribution of DR. Herein, we use bayesian meta-analytic methods to create composite estimates of the prevalence of DR and VTDR using multiple data sources. We provide these prevalence estimates by age, nondifferentiated sex and gender, race, ethnicity, state, and county.
Methods
DR and VTDR Prevalence
We used bayesian meta-regression methods developed by the Global Burden of Disease Study14 to separately estimate national prevalence of DR and VTDR among those with diabetes. The statistical models are extensively described in eAppendix 2 and eAppendix 5 of Supplement 1. This study was determined not to be human subjects research by NORC’s institutional review board because it is based on secondary analysis of deidentified data sources. The study adhered to Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines. We estimated prevalence by 5-year age group, a nondifferentiated sex and gender measure, and a composite indicator of race and ethnicity. Nondifferentiated sex and gender (hereafter “sex and gender”) is a composite of sex- and gender-related variables in our data sources. No data sources measured sex and gender separately or included values besides “male” and “female,” preventing us from estimating prevalence in gender minority populations. Race and ethnicity (hereafter “race”) has 4 categories: Hispanic; non-Hispanic Black; non-Hispanic White; and all other races and ethnicities, including individuals who are Asian, American Indian, Alaska Native, Pacific Islander, multiracial, and other groups not otherwise classified. Hereafter, these 4 categories are referred to as “Black,” “Hispanic,” “White,” and “other races”; more detailed categories could not be analyzed due to data availability (eAppendix 1 in Supplement 1).
Our meta-regression incorporates: (1) published results of PBS in adults and youth that ascertained DR using retinal fundus photographs,15,16,17,18,19,20,21,22,23 (2) NHANES data from 2005 through 2008 (years for which retinal fundus photographs were available),9,10 (3) 2019 Medicare Part B fee-for-service claims,24 and (4) 2016 IBM MarketScan Commercial Research Database of medical claims to commercial insurers.25 We extracted each source’s sample size and DR and VTDR prevalence among those with diabetes by age group, sex and gender, and race, where available (eAppendix 1 and eAppendix 4 in Supplement 1). All race information was self-reported. In NHANES, 20.1% and 20.4% of participants with diabetes aged 40 years or older were missing DR and VTDR status information, respectively; we imputed those values (methods in eAppendix 3 in Supplement 1). The model assumes that the stratified prevalence rate has not changed substantially since it was measured in our data sources.
We included PBS published after 2000 that were representative of the target population and presented primary results. We identified 5 such studies in adults: (1) the Chinese American Eye Study (2010 to 2013),15 (2) the Los Angeles Latino Eye Study (2000 to 2003),7 (3) Proyecto Vision and Eye Research (1997 to 1999),16 (4) the Multi-Ethnic Study of Atherosclerosis (2002 to 2004),17 and (5) the Diabetic Retinopathy Inpatient Study (2011 to 2012).18 We included 2 such studies in youth: (1) the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study (2010 to 2018), a multicenter clinical trial of youth aged 10 to 17 years with type 2 diabetes and its observational follow-up study19,20,21 and (2) the SEARCH for Diabetes in Youth registry study (2010 to 2019),22,23 a multicenter observational cohort of youth with type 1 or type 2 diabetes.
We estimated county-level random effects using 2018 Medicaid claims26 and 2017 to 2019 claims in the Medicare Part B fee-for-service program (eAppendix 2 in Supplement 1).24 We used a mixed-effects regression model with 5% trimming to avoid being unduly influenced by outliers.27 Outliers may be related to nonepidemiological causes, such as local billing or practice patterns that lead to overdiagnosis or underdiagnosis. We controlled for county-level number of ophthalmologists per capita, which could affect access to diagnosis.28 Due to complex differences in Medicaid inclusion and administration between states, we controlled for US state in Medicaid data. To account for differential inclusion in Medicare fee-for-service claims, we controlled for the percentage of Medicare beneficiaries enrolled in Medicare Advantage and the state-level percentage of the working-age population 18 to 65 years covered by Medicare through Social Security Disability Insurance, applied to the younger than 65 years age groups only.29,30 This approach assumes that, among those with diabetes, the prevalence of DR or VTDR has the same county pattern as the DR or VTDR claims rate in the Medicare fee-for-service and Medicaid populations, after adjusting for the aforementioned confounding variables.
We derived county-level estimates from national estimates and county-level random effects. We estimated standardized rates of DR and VTDR by sex and gender and race as the expected prevalence for each stratum assuming the national distribution for age, and sex and gender or race. We estimated standardized rates by county as the expected prevalence for that county assuming the national distribution of age, sex and gender, and race.
Diabetes Prevalence
We analyzed the 2017 to March 2020 cycle of the NHANES survey to estimate diabetes prevalence by age, sex and gender, and race.31 To address sample size issues, we used logistic regression with covariates based on past research32 to borrow strength between similar demographic strata. We applied sex-specific county patterns of age-standardized diabetes prevalence estimated by Dwyer-Lindgren et al.33 NHANES examination weights were applied to account for complex sample design. Additional methods are described (eAppendix 2 in Supplement 1).
Population
To estimate prevalence counts of DR and VTDR, we used 2021 US Census Bureau population estimates stratified by age, sex, race, and county.34 These estimates top-code age at 85 years; we used county-specific and race-specific age distributions from the 2010 US Census35 to disaggregate this oldest group. We aggregated our county-level prevalence estimates to create state-level estimates.
Results
Diabetic Retinopathy
The study team estimated 9.60 million people (95% uncertainty interval [UI], 7.90-11.55) living with DR in the US in 2021, corresponding to a prevalence rate of 26.43% (95% UI, 21.95-31.60) among people with diabetes (Table). The study team estimated 5.56 million individuals with male sex and gender (95% UI, 4.38-6.98) and 4.04 million individuals with female sex and gender (95% UI, 3.24-5.01) living with DR. The age- and race-standardized rate of DR was 15.7% (95% UI, −7.0 to 45.2) higher for males at 28.10% (95% UI, 22.47-34.74) than for females at 24.43% (95% UI, 19.82-29.97); however, the UI of this percent difference indicates a high degree of uncertainty. Age- and sex and gender-standardized rates of DR among Hispanic individuals, 29.21% (95% UI, 22.13-38.26) and Black individuals, 34.39% (95% UI, 26.09-43.75) were higher than among White individuals, 24.40% (95% UI, 18.38-31.56). The standardized DR prevalence was 21.90% (95% UI, −17.00 to 79.30) higher among Hispanic individuals than among White individuals and it was 43.40% (95% UI, −0.90 to 107.40) higher among Black individuals than among White individuals; however, these differences were highly uncertain. The standardized DR prevalence for individuals in the other race category was lower at 21.65% (95% UI, 13.25-32.65) than among White individuals; however, this percent difference was highly uncertain (−9.90% [95% UI, −46.70 to 42.20]).
Table. Estimated Prevalence of Diabetic Retinopathy and Vision-Threatening Diabetic Retinopathy, Stratified by Nondifferentiated Sex and Gender and Race and Ethnicity.
| Characteristic | Prevalence count, in millions (95% UI) | Standardized prevalence rate, % (95% UI)a |
|---|---|---|
| Diabetic retinopathy | ||
| Total | 9.60 (7.90-11.55) | 26.43 (21.95-31.60) |
| Nondifferentiated sex and genderb | ||
| Female | 4.04 (3.24-5.01) | 24.43 (19.82-29.97) |
| Male | 5.56 (4.38-6.98) | 28.10 (22.47-34.74) |
| Ethnicityc | ||
| Hispanic | 1.85 (1.34-2.49) | 29.21 (22.13-38.26) |
| Non-Hispanic | ||
| Racec | ||
| Black | 1.83 (1.34-2.39) | 34.39 (26.09-43.75) |
| White | 5.19 (3.84-6.89) | 24.40 (18.38-31.56) |
| Other | 0.73 (0.44-1.12) | 21.65 (13.25-32.65) |
| Vision-threatening diabetic retinopathy | ||
| Total | 1.84 (1.41-2.40) | 5.06 (3.90-6.57) |
| Nondifferentiated sex and genderb | ||
| Female | 0.78 (0.59-1.03) | 4.72 (3.55-6.18) |
| Male | 1.05 (0.78-1.41) | 5.34 (3.97-7.07) |
| Ethnicityc | ||
| Hispanic | 0.46 (0.29-0.66) | 7.14 (4.86-10.32) |
| Non-Hispanic | ||
| Racec | ||
| Black | 0.46 (0.31-0.67) | 8.66 (5.91-12.17) |
| White | 0.75 (0.46-1.17) | 3.55 (2.22-5.41) |
| Other | 0.16 (0.07-0.30) | 4.86 (2.18-9.21) |
Abbreviation: UI, uncertainty interval.
Prevalence rates in this Table were calculated using a denominator of persons with diabetes. Prevalence rates stratified by sex and gender were standardized for age and for race and ethnicity. Prevalence rates stratified by race and ethnicity were standardized for age and sex and gender.
Nondifferentiated sex and gender is a composite of sex- and gender-related variables in our data sources. No data sources measured sex and gender separately or included values besides “male” and “female,” preventing us from estimating prevalence in gender minority populations.
Ethnicity and race were self-reported. “Other” includes Alaska Native individuals, American Indian individuals, Asian individuals of any origin, multiracial individuals, Pacific Islander individuals, and other groups not otherwise classified.
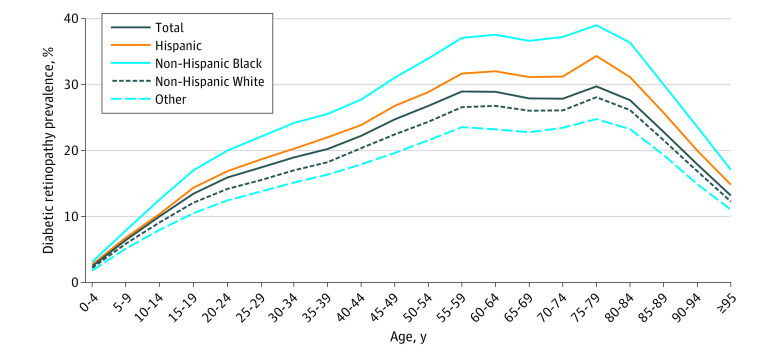
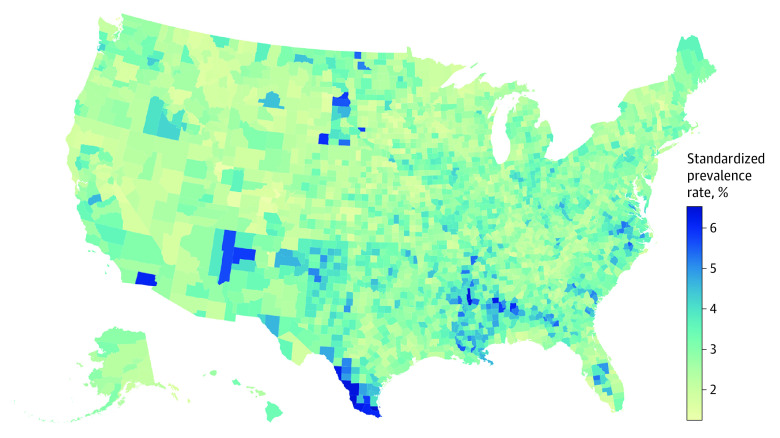
The prevalence of DR increased as a function of age before declining in the oldest age groups (Figure 1); prevalence among those with diabetes was 13.01% (95% UI, 7.83-25.68) among those younger than 25 years, 19.34% (95% UI, 14.73-25.36) among those 25 to 39 years, 27.15% (95% UI, 21.71-33.65) among those 40 to 64 years, 28.38% (95% UI, 21.29-37.08) among those 65 to 79 years, and 24.81% (95% UI, 17.41-33.62) among those 80 years or older. Estimated crude and standardized prevalence rates for each state are provided in the eTable in Supplement 1. Crude prevalence rates of DR among those with diabetes ranged from 20.84% (95% UI, 17.45-24.81) in Nevada to 31.31% (95% UI, 25.14-38.30) in Massachusetts. Age-, sex and gender-, and race-standardized prevalence among those with diabetes ranged from a low of 21.17% (95% UI, 17.61-25.34) in Nevada to a high of 34.22% (95% UI, 28.27-40.78) in Hawaii (eTable and eFigure 1 in Supplement 1). Standardized rates of DR among the total population varied by US county (Figure 2). An interactive version of this county-level map is available on the CDC VEHSS website.36
Figure 1. US Prevalence of Diabetic Retinopathy Among People With Diabetes by Age, Race, and Ethnicity in 2021.
Figure 2. Age–, Sex and Gender–, Race and Ethnicity–Standardized Diabetic Retinopathy Prevalence Among the Total Population by County in 2021.
We used a nondifferentiated sex and gender indicator for standardization, which is a composite of sex- and gender–related variables in our data sources. No data sources measured sex and gender separately or included values besides “male” and “female.”
Vision-Threatening Diabetic Retinopathy
The study team estimated 1.84 million people (95% UI, 1.41-2.40) living with VTDR in the US in 2021, corresponding to a prevalence rate of 5.06% (95% UI, 3.90-6.57) among people with diabetes (Table). The study team estimated 1.05 million individuals with male sex and gender (95% UI, 0.78-1.41) and 0.78 million individuals with female sex and gender (95% UI, 0.59-1.03) living with VTDR. The age- and race-standardized rate of VTDR was 13.9% (95% UI, −10.1 to 42.3) higher for males at 5.34% (95% UI, 3.97-7.07) than for females at 4.72% (95% UI, 3.55-6.18); however, this percent difference was uncertain. Age- and sex and gender-standardized rates of VTDR among Hispanic individuals at 7.14% (95% UI, 4.86-10.32) and Black individuals at 8.66% (95% UI, 5.91-12.17) were higher than among White individuals at 3.55% (95% UI, 2.22-5.41). This equated to a 155.50% (95% UI, 45.90-338.30) higher prevalence of VTDR among Black individuals compared with White individuals and a 111.30% (95% UI, 17.70-251.70) higher prevalence among Hispanic individuals compared with White individuals. The age- and sex and gender-standardized rate for individuals in the other race group at 4.86% (95% UI, 2.18-9.21) was 43.30% (95% UI, −42.50 to 206.60 [highly uncertain]) higher than that of White individuals.
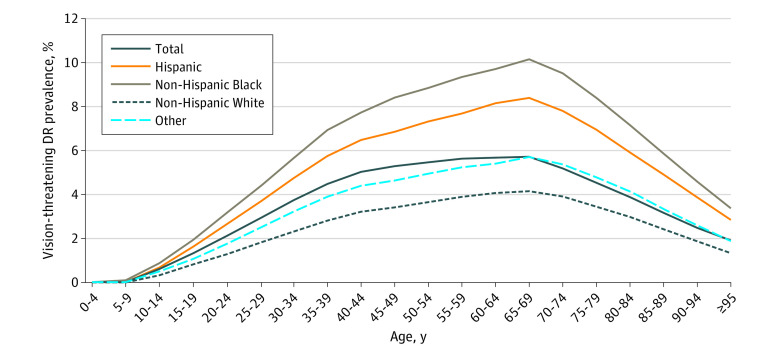
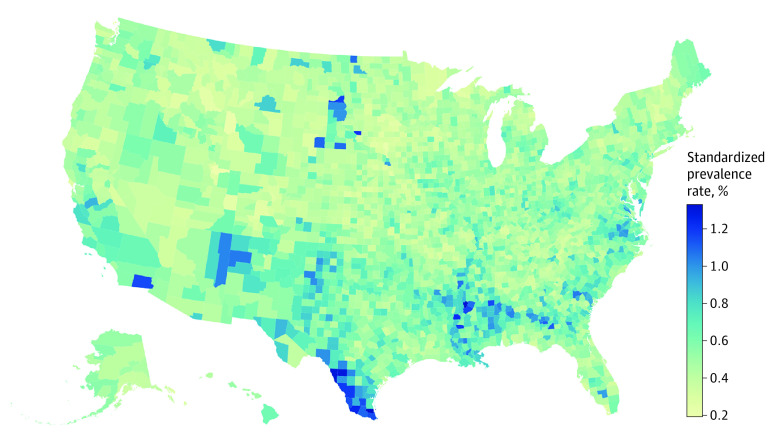
The prevalence of VTDR increased as a function of age until 65 to 69 years, after which it declined (Figure 3); prevalence was 1.41% (95% UI, 0.80-2.23) among those younger than 25 years, 3.96% (95% UI, 2.84-5.47) among those 25 to 39 years, 5.53% (95% UI, 4.06-7.39) among those 40 to 64 years, 5.27% (95% UI, 3.69-7.41) among those 65 to 79 years, and 3.48% (95% UI, 2.17-5.20) among those 80 years or older. Crude prevalence rates of VTDR among those with diabetes ranged from 3.29% (95% UI, 2.27-4.69) in South Dakota to 7.25% (95% UI, 5.51-9.62) in Washington, DC (eTable in Supplement 1). Age-, sex and gender-, and race-standardized prevalence among those with diabetes ranged from a low of 4.07% (95% UI, 3.12-5.29) in South Dakota to a high of 6.02% (95% UI, 4.62-7.83) in Hawaii (eTable and eFigure 2 in Supplement 1). Standardized prevalence of VTDR in the total population varied by US county (Figure 4).
Figure 3. US Prevalence of Vision-Threatening Diabetic Retinopathy (DR) Among People With Diabetes by Age, Race, and Ethnicity in 2021.
Figure 4. Age-, Sex and Gender–, and Race and Ethnicity–Standardized Vision-Threatening Diabetic Retinopathy Prevalence Among the Total Population by County in 2021.
We used a nondifferentiated sex and gender indicator for standardization, which is a composite of sex- and gender–related variables in our data sources. No data sources measured sex and gender separately or included values besides “male” and “female.”
Discussion
This analysis updates estimates on the US prevalence of DR and VTDR. We found that 9.60 million people (26.43% of those with diabetes) have DR. Of those, 1.84 million people (5.06% of those with diabetes) have VTDR. Black and Hispanic individuals have a higher standardized prevalence of VTDR (8.66% and 7.14%, respectively) than White individuals (3.55%). Prevalence of DR and VTDR among people with diabetes increased substantially with age but decreased in older age groups, likely because DR and VTDR are markers for more severe diabetes, which can lead to early mortality.
Our estimates of the number of people in the US living with DR and VTDR are higher than previously estimated by the EDPRG which found that 4.1 million individuals had DR and 899 000 individuals had VTDR; however, the EDPRG estimate was based on much older data.8 In the intervening period, studies have documented an increase in diabetes prevalence among US adults 18 years or older from 9.8% in 1999 to 2000 to 14.3% in 2017 to 2018.12,13 Additionally, glycemic control has worsened in recent years with the prevalence of hemoglobin A1c less than 7% among US adults 20 years or older with diabetes decreasing from 57.4% in 2007 to 2010 to 50.5% in 2015 to 2018.37 These trends would be expected to result in a larger number of people living with DR and VTDR in the US.
The EDPRG estimates for the crude prevalence of DR and VTDR among people with diabetes (40.3% and 8.2%, respectively) are higher than our estimates. However, the EDPRG estimates were for those older than 40 years, whereas our estimates are for all ages and used new data from the TODAY and SEARCH studies. Additionally, the EDPRG results were impacted by the inclusion of the Wisconsin Epidemiologic Study of Diabetic Retinopathy, which covered a largely White population with unusually high rates of DR. After excluding Wisconsin Epidemiologic Study of Diabetic Retinopathy data, the EDPRG prevalence was 35.8% for DR and 7.3% for VTDR.
We estimated that the crude prevalence of DR and VTDR among people with diabetes younger than 25 years was 13.01% (95% UI, 7.83-25.68) and 1.41% (95% UI, 0.80-2.23), respectively, or 97 000 (95% UI, 52 000-195 000) and 10 000 (95% UI, 6000-18 000) individuals. However, given the lack of nationally representative data for this age group, there is a high degree of uncertainty in our estimates that are based on data from 2 PBS, TODAY and SEARCH, and MarketScan claims. Both TODAY and SEARCH found that nearly 1 in 2 youth and young adults with type 2 diabetes have DR.20,21,22,23 Additional research on ocular sequelae in youth with diabetes will help reduce the uncertainty of estimates in this important and growing population. Like previous studies,3,8,11,38,39 we documented disparities by race and ethnicity with the rate of VTDR higher among Hispanic and Black individuals with diabetes compared with White individuals. Reasons for this disparity could include poorer glycemic control among Black and Hispanic individuals with diabetes compared with White individuals,40,41,42 the earlier age at diabetes diagnosis and longer disease duration among Black and Hispanic patients,43 or disparities in the quality of diabetes care experienced by Black and Hispanic patients.44
It has been projected that, by 2060, 60.6 million US adults (17.9%) will have diabetes,32 increasing the number of people living with diabetes complications, such as vision loss from DR. Forecasting studies project a nearly 3-fold increase in the number of individuals in the US with DR (from 5.5 million to 16.0 million) and vision-threatening DR (from 1.2 million to 3.4 million) between 2005 and 2050.45 Preventing vision loss from this diabetes complication requires long-term glycemic control and routine dilated eye examinations to ensure timely DR detection and treatment. Left untreated, proliferative DR confers a 50% chance of becoming blind within 5 years.46,47,48 The past 2 decades have seen the expansion of effective but costly new anti–vascular endothelial growth factor intravitreal injections. In 2018, treatment of DR cost an estimated $753 million for Medicare Part B fee-for-service.49
Limitations
This study is subject to limitations. First, we chose nationally representative NHANES data as the basis for our estimates; however, these were last collected during 2005 to 2008. Some PBS data used are even older. Future NHANES eye health data are needed to better understand US trends in DR. Second, for NHANES participants with diabetes who were missing DR and VTDR status information, we imputed those values, yielding 3.6% higher estimates for DR and 11.0% higher estimates for VTDR in this population compared with complete case analysis. Third, we used Medicaid and Medicare Part B fee-for-service claims indicating diagnosed DR and VTDR to estimate county variation in DR and VTDR prevalence. This assumes that diagnosed prevalence from claims is correlated with total population prevalence after model adjustments. Claims diagnoses are likely influenced by access to ophthalmologists and local variation in the Medicare and Medicaid covered population. Therefore, we adjusted for the per capita number of ophthalmologists and Medicare Advantage penetration at the county level, state differences in Medicaid DR and VTDR diagnosis rates, and state variation in Social Security Disability Insurance coverage for Medicare beneficiaries younger than 65 years. However, to the extent that these variables do not fully capture access to diagnosis and sampling bias of insured populations, our model may mistake these factors for true variation in prevalence. Fourth, most data sources in our meta-analysis diagnosed DR using retinal fundus photography. Therefore, our figures may underestimate the prevalence of VTDR because newer optical coherence tomography is better at detecting diabetic macular edema (particularly noncentral diabetic macular edema ). Lastly, in the absence of studies on the prevalence of DR and VTDR among older adults living in institutional settings, such as assisted living or nursing homes, we assumed that the prevalence derived from NHANES, a noninstitutionalized population, reflects the prevalence among all older adults.
Conclusions
We estimated that 9.60 million people in the US have DR (26.43% of those with diabetes) and 1.84 million people have VTDR (5.06% of those with diabetes) with substantial variation in prevalence across US states and counties. These estimates can inform the allocation of public health resources and interventions to communities and populations at highest risk, such as expanding teleretinal imaging to improve DR screening in counties with the highest prevalence.
eAppendix 1. Description of Source Data Processing
eAppendix 2. Description of Statistical Models
eAppendix 3. Imputation of Missing Data
eAppendix 4. ICD-10 Diagnosis Codes
eAppendix 5. Description of Validation and Verification
eTable. Diabetic Retinopathy (DR) and Vision-Threatening Diabetic Retinopathy (VTDR) Prevalence Rates (%) Among Those with Diabetes by US State in 2021
eFigure 1. Age-, Sex/Gender-, and Race and Ethnicity-Standardized Diabetic Retinopathy Prevalence Among Those with Diabetes by US State in 2021, with 95% Uncertainty Intervals
eFigure 2. Age-, Sex/Gender-, and Race and Ethnicity-Standardized Vision-Threatening Diabetic Retinopathy Prevalence Among Those with Diabetes by US State in 2021, with 95% Uncertainty Intervals
eReferences
Data sharing statement
References
- 1.US Centers for Disease Control and Prevention . National diabetes statistics report website. Accessed May 9, 2023. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 2.Klein R, Klein BEK. Vision disorders in diabetes. [Chapter 14] In: Diabetes in America. 2nd ed. 1995:293-338. [Google Scholar]
- 3.Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340. doi: 10.1001/jamaophthalmol.2014.2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64(2):631-642. doi: 10.2337/db14-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jampol LM, Glassman AR, Sun J. Evaluation and care of patients with diabetic retinopathy. N Engl J Med. 2020;382(17):1629-1637. doi: 10.1056/NEJMra1909637 [DOI] [PubMed] [Google Scholar]
- 6.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902-916. doi: 10.1001/jama.298.8.902 [DOI] [PubMed] [Google Scholar]
- 7.Varma R, Torres M, Peña F, Klein R, Azen SP; Los Angeles Latino Eye Study Group . Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(7):1298-1306. doi: 10.1016/j.ophtha.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Kempen JH, O’Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Ophthalmol. Arch Ophthalmol. 2004;122(4):552-563. doi: 10.1001/archopht.122.4.552 [DOI] [PubMed] [Google Scholar]
- 9.US Centers for Disease Control and Prevention (CDC) , National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) 2005-2006. Accessed September 6, 2022. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2005
- 10.Centers for Disease Control and Prevention (CDC) . National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) 2007-2008. Accessed September 6, 2022. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2007
- 11.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649-656. doi: 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. 2021;326(8):704-716. doi: 10.1001/jama.2021.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaxman AD, Vos DT, Murray CJ. An Integrative Metaregression Framework for Descriptive Epidemiology. University of Washington Press; 2015. [Google Scholar]
- 15.Varma R, Wen G, Jiang X, et al. ; Chinese American Eye Study Group . Prevalence of diabetic retinopathy in adult Chinese American individuals: The Chinese American Eye Study. JAMA Ophthalmol. 2016;134(5):563-569. doi: 10.1001/jamaophthalmol.2016.0445 [DOI] [PubMed] [Google Scholar]
- 16.West SK, Klein R, Rodriguez J, et al. ; Proyecto VER . Diabetes and diabetic retinopathy in a Mexican-American population: Proyecto VER. Diabetes Care. 2001;24(7):1204-1209. doi: 10.2337/diacare.24.7.1204 [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446-455. doi: 10.1016/j.ajo.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovarik JJ, Eller AW, Willard LA, Ding J, Johnston JM, Waxman EL. Prevalence of undiagnosed diabetic retinopathy among inpatients with diabetes: the diabetic retinopathy inpatient study (DRIPS). BMJ Open Diabetes Res Care. 2016;4(1):e000164. doi: 10.1136/bmjdrc-2015-000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitsky LL, Danis RP, Drews KL, et al. ; TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36(6):1772-1774. doi: 10.2337/dc12-2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjornstad P, Drews KL, Caprio S, et al. ; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021;385(5):416-426. doi: 10.1056/NEJMoa2100165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubitosi-Klug R, Libman I, Drews KL, et al. ; TODAY Study Group . Development and progression of diabetic retinopathy in adolescents and young adults with type 2 diabetes: results from the TODAY study. Diabetes Care. 2021;45(5):1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer-Davis EJ, Davis C, Saadine J, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med. 2012;29(9):1148-1152. doi: 10.1111/j.1464-5491.2012.03591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen ET, Rigdon J, Rezaei KA, et al. Prevalence, progression and modifiable risk factors for diabetic retinopathy in youth and young adults with youth-onset type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2023:dc222503. doi: 10.2337/dc22-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Centers for Disease Control and Prevention . Vision and eye health surveillance system. Accessed May 9, 2023. https://www.cdc.gov/visionhealth/vehss/data/claims/medicare.html
- 25.US Centers for Disease Control and Prevention . Vision and eye health surveillance system: MarketScan. Accessed May 9, 2023. https://www.cdc.gov/visionhealth/vehss/data/claims/marketscan.html
- 26.US Centers for Disease Control and Prevention . Vision and eye health surveillance system: Medicaid. Accessed May 9, 2023. https://www.cdc.gov/visionhealth/vehss/data/claims/medicaid.html
- 27.Zheng P, Barber R, Sorensen RJ, Murray CJ, Aravkin AY. Trimmed constrained mixed effects models: formulations and algorithms. Accessed May 9, 2023. https://arxiv.org/abs/1909.10700
- 28.Health Services Resources Administration . Area health resources files: 2019-2020 county-level data. Accessed May 9, 2013. https://data.hrsa.gov/data/download?data=AHRF
- 29.US Centers for Medicare & Medicaid Services . MA state/county penetration 2018. Accessed May 9, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MCRAdvPartDEnrolData/MA-State-County-Penetration-Items/MA-State-County-Penetration-2018-06
- 30.Kaiser Family Foundation . Total disabled social security disability insurance (SSDI) beneficiaries, Ages 18-64. Accessed May 9, 2023. https://www.kff.org/medicare/state-indicator/total-disabled-social-security-disability-insurance-ssdi-beneficiaries-ages-18-64/?currentTimeframe=2&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
- 31.US Centers for Disease Control and Prevention (CDC) . National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) 2017-March 2020 Pre-pandemic. Accessed September 6, 2022. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020
- 32.Lin J, Thompson TJ, Cheng YJ, et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr. 2018;16(1):9. doi: 10.1186/s12963-018-0166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, Flaxman AD, Mokdad AH. Diagnosed and undiagnosed diabetes prevalence by county in the US, 1999–2012. Diabetes Care. 2016;39(9):1556-1562. doi: 10.2337/dc16-0678 [DOI] [PubMed] [Google Scholar]
- 34.US Census Bureau . County population by characteristics: 2021. [CSV data file]. Published online 2022. Accessed August 15, 2022. https://www2.census.gov/programs-surveys/popest/datasets/2020-2021/counties/asrh/
- 35.US Census Bureau . Decennial census 2010. Accessed May 9, 2023. https://data.census.gov/cedsci/table?q=PCT12%2a&g=0100000US%240500000&d=DEC%20Summary%20File%201&tid=DECENNIALSF12010.PCT12A
- 36.US Centers for Disease Control and Prevention . Vision and Eye Health Surveillance System composite prevalence estimates. Accessed May 22, 2023. https://www.cdc.gov/visionhealth/vehss/estimates/
- 37.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med. 2021;384(23):2219-2228. doi: 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundeen EA, Andes LJ, Rein DB, et al. Trends in prevalence and treatment of diabetic macular edema and vision-threatening diabetic retinopathy among Medicare part B fee-for-service beneficiaries. JAMA Ophthalmol. 2022;140(4):345-353. doi: 10.1001/jamaophthalmol.2022.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez JM, Bailey RA, Rupnow MF. Demographic disparities among Medicare beneficiaries with type 2 diabetes mellitus in 2011: diabetes prevalence, comorbidities, and hypoglycemia events. Popul Health Manag. 2015;18(4):283-289. doi: 10.1089/pop.2014.0115 [DOI] [PubMed] [Google Scholar]
- 40.Saydah S, Cowie C, Eberhardt MS, De Rekeneire N, Narayan KM. Race and ethnic differences in glycemic control among adults with diagnosed diabetes in the United States. Ethn Dis. 2007;17(3):529-535. [PubMed] [Google Scholar]
- 41.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167(17):1853-1860. doi: 10.1001/archinte.167.17.1853 [DOI] [PubMed] [Google Scholar]
- 42.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22(3):403-408. doi: 10.2337/diacare.22.3.403 [DOI] [PubMed] [Google Scholar]
- 43.Wang MC, Shah NS, Carnethon MR, O’Brien MJ, Khan SS. Age at diagnosis of diabetes by race and ethnicity in the United States from 2011 to 2018. JAMA Intern Med. 2021;181(11):1537-1539. doi: 10.1001/jamainternmed.2021.4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canedo JR, Miller ST, Schlundt D, Fadden MK, Sanderson M. Racial/ethnic disparities in diabetes quality of care: the role of healthcare access and socioeconomic status. J Racial Ethn Health Disparities. 2018;5(1):7-14. doi: 10.1007/s40615-016-0335-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saaddine JB, Honeycutt AA, Narayan KMV, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126(12):1740-1747. doi: 10.1001/archopht.126.12.1740 [DOI] [PubMed] [Google Scholar]
- 46.Ferris FL III, Davis MD, Aiello LM. Treatment of diabetic retinopathy. N Engl J Med. 1999;341(9):667-678. doi: 10.1056/NEJM199908263410907 [DOI] [PubMed] [Google Scholar]
- 47.Caird FI, Burditt AF, Draper GJ. Diabetic retinopathy. a further study of prognosis for vision. Diabetes. 1968;17(3):121-123. doi: 10.2337/diab.17.3.121 [DOI] [PubMed] [Google Scholar]
- 48.Deckert T, Simonsen SE, Poulsen JE. Prognosis of proliferative retinopathy in juvenile diabetics. Diabetes. 1967;16(10):728-733. doi: 10.2337/diab.16.10.728 [DOI] [PubMed] [Google Scholar]
- 49.Wittenborn JS, Gu Q, Erdem E, et al. The prevalence of diagnosis of major eye diseases and their associated payments in the Medicare fee-for-service program. Ophthalmic Epidemiol. 2021;16:1-13. doi: 10.1080/09286586.2021.1968006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Description of Source Data Processing
eAppendix 2. Description of Statistical Models
eAppendix 3. Imputation of Missing Data
eAppendix 4. ICD-10 Diagnosis Codes
eAppendix 5. Description of Validation and Verification
eTable. Diabetic Retinopathy (DR) and Vision-Threatening Diabetic Retinopathy (VTDR) Prevalence Rates (%) Among Those with Diabetes by US State in 2021
eFigure 1. Age-, Sex/Gender-, and Race and Ethnicity-Standardized Diabetic Retinopathy Prevalence Among Those with Diabetes by US State in 2021, with 95% Uncertainty Intervals
eFigure 2. Age-, Sex/Gender-, and Race and Ethnicity-Standardized Vision-Threatening Diabetic Retinopathy Prevalence Among Those with Diabetes by US State in 2021, with 95% Uncertainty Intervals
eReferences
Data sharing statement