Abstract
Background
Alzheimer’s disease (AD) is an age-related neurodegenerative disease characterized by amyloid-β (Aβ) plaques. Systemic inflammation and obesity may exacerbate AD pathogenesis. We previously reported anti-inflammatory and anti-obesity effects of EPA in mice.
Objectives
We aimed to determine whether EPA reduces obesity-associated metabolic dysfunctions and Aβ accumulation in AD amyloidogenic mice.
Methods
Two-mo-old APPswe/PS1dE9 transgenic (TG) mice and non-TG littermates were randomly assigned to low fat (LF; 10% kcal fat), high fat (HF; 45% kcal fat), or EPA (36 g/kg)-supplemented HF diets. Body composition, glucose tolerance, and energy expenditure were measured, and serum and brain metabolic markers were tested 38 wk postintervention. Outcomes were statistically analyzed via 3-factor ANOVA, modeling genotype, sex, and diet interactions.
Results
HF-fed males gained more weight than females (Δ = 61 mg; P < 0.001). Compared with LF, HF increased body weights of wild-type (WT) males (Δ = 31 mg; P < 0.001). EPA reduced HF-induced weight gain in WT males (Δ = 24 mg; P = 0.054) but not in females. HF mice showed decreased glucose clearance and respiratory energy compared with LF-fed groups (Δ = −1.31 g/dL; P < 0.001), with no significant effects of EPA. However, EPA conferred metabolic improvements by decreasing serum leptin and insulin (Δ = −2.51 g/mL and Δ = −0.694 ng/mL, respectively compared with HF, P ≤ 0.05) and increasing adiponectin (Δ = 21.6 ng/mL; P < 0.001). As we expected, TG mice expressed higher serum and brain Aβ than WT mice (Δ = 0.131 ng/mL; P < 0.001 and Δ = 0.56%; P < 0.01, respectively), and EPA reduced serum Aβ1-40 in TG males compared with HF (Δ = 0.053 ng/mL; P ≤ 0.05).
Conclusions
To our knowledge, this is the first report that EPA reduces serum Aβ1-40 in obese AD male mice, warranting further investigations into tissue-specific mechanisms of EPA in AD.
Keywords: Alzheimer’s disease, obesity, EPA, APP/PS1 mouse model, amyloid-beta
Introduction
Alzheimer’s disease (AD) is one of the most widespread, chronic, and progressive neurodegenerative disorders, with an estimated 6.2 million Americans living with AD in 2021 [1,2]. AD is characterized by impaired cognitive functions and memory loss, in part because of the accumulation of extracellular aggregated amyloid-beta (Aβ) plaques and intraneuronal neurofibrillary tangles composed of hyperphosphorylated tau [3,4]. The prevalence of AD is positively associated with age, and the majority of patients with AD (95%) are usually diagnosed with this disease after 65 y of age (i.e., late-onset) [5]. Early-onset AD is rare and can be caused by the pathogenic mutation of AD-related genes, including amyloid precursor protein (APP), presenilin 1 (PS1), presenilin 2, and apolipoprotein E genes [6]. Research has documented that AD is a complex disease with multiple potential genetic, environmental, and lifestyle risk factors [6,7].
Considerable evidence suggests that obesity and its comorbidities increase the risk of AD through metabolic dysregulations that include increased inflammation and oxidative stress [[8], [9], [10], [11], [12]]. Accumulation and expansion of white adipose tissue is accompanied by imbalanced levels of several systemic adipokines and metabolic hormones, thereby triggering low-grade systemic inflammation [13]. For example, obesity is associated with increased levels of adipose tissue and circulating proinflammatory adipokines, like leptin and resistin, and decreased anti-inflammatory adipokines, like adiponectin [[13], [14], [15], [16], [17]]. In addition, both systemic inflammation and neuroinflammation [18,19] contribute to cell damage and subsequent neuronal death and also interfere with synapses in the cerebral cortex and hippocampus [20], which further increase the risk of AD initiation and progression. Thus, collectively, obesity is associated with systemic inflammation, neurodegeneration, cognitive decline, and an enhanced risk of AD [21,22].
FDA-approved medications for AD include cholinesterase inhibitors, memantine, N-methyl-D-aspartate receptor modulators [23], and the newly approved drug, aducanumab [24]. For the most part, these medications alleviate mild-to-moderate AD-related dementia yet have limited utility in delaying the progression of AD. As such, there is an urgent need to develop safe, easily accessible, and cost-effective approaches aimed at preventing or reducing AD. Among these, we are interested in dietary interventions that may alleviate AD-related metabolic dysfunctions in obese amyloidogenic models, and we specifically focused on fish oil, which is rich in anti-inflammatory omega-3 (n-3) PUFAs, namely EPA and DHA [25].
Beneficial effects of n-3 PUFAs include reduction of adiposity, inflammation, and increased adiponectin protein levels in adipose tissue of diet-induced obese mice [26]. Moreover, we previously demonstrated that the protective metabolic effects of EPA in male C57BL/6J mice were, in part, mediated by reduced 1) systemic and adipose tissue inflammation, 2) body weight (BW), and 3) insulin resistance [27,28]. Further, another study reported that feeding APPswe/PS1dE9 (APP/PS1) transgenic (TG) AD mice diets containing 5% fat (3% from fish oil and the remaining 2% from corn and coconut oil) for 9 mo inhibited β- and γ-secretase activity and reduced the amyloidogenic cleavage of APP. However, this diet had limited effects on existing Aβ plaques in the brain when compared with mice fed an isocaloric control diet (5% corn, coconut, and soy oil) [29]. In line with these results, an epidemiologic study reported that n-3 PUFA deficiency is associated with mild depression and AD in male and female human subjects (age >75 y) [30] and that supplementation with 1300 mg/d EPA + 880 mg/d DHA improved memory functions in healthy women (age > 60 y) [31]. The above findings suggest that n-3 PUFAs may play an important role in reducing the risk of AD by reducing amyloid deposition. Therefore, in the current study, we determined the potential benefits of EPA supplementation in reducing the metabolic consequences of obesity in an amyloidogenic mouse model of AD. We hypothesized that, compared with high-fat (HF) diets devoid of EPA, supplementation with EPA would reduce HF diet-induced adiposity, increase locomotor activity, improve glucose clearance, and decrease serum and brain Aβ in APP/PS1 TG mice.
Methods
AD mouse model and genotyping
Double TG humanized APP/PS1 mice expressing a chimeric mouse/human APP (APP695swe) and mutant human PS1 (PS1-dE9) were used in this study. These mice typically develop human Aβ deposits in the brain starting ∼5 mo of age. Male APP/PS1 mice were initially obtained from the Jackson Laboratory (Catalog No. 005864-JAX | APP/PS1). Mice used for the current study were bred at Texas Tech University by pairing TG male offspring with non-TG (noncarrier) female offspring. All offspring were genotyped via tail-snip tissue samples using the primers described below. Sequences for the wild-type (WT) forward and reverse primers were 5′-TCGTCATCAATAAGGGGAAAC-3′ and 5′-CTTCTTCCCTGATGCTCCAT-3′, respectively. Forward and reverse primer sequences for APPswe were 5′-TTCCCGTGAATGGAGAGAGTTC-3′ and 5′-ATGAACTTCATATCCTGAGTCATGTCG-3′, respectively. Sequences for the PS1-dE9 forward and reverse primers were 5′-GGTCCACTTCGTATGCTGGT-3′ and 5′-TTCCCATTCCTCACTGAACC-3′, respectively. An alkaline lysis reagent (Sigma-Aldrich) was added to the mice tail snips, followed by a neutralizing reagent to isolate DNA used for PCR. DNA samples were then subjected to agarose gel electrophoresis to determine the genotypes (Figure 1). WT samples showed one specific band, whereas APP and PS1 samples each showed a different band.
FIGURE 1.
Genotyping of APP/PS1 TG and WT mice. Positive/negative PCR products were measured by agarose electrophoresis for genomic DNA from TG and WT using control (A), APP (B) and PS1 (C) primers. APP, amyloid processor protein; PS1, presenilin; TG, transgenic; WT, wild-type.
Animal study design
Male and female APP/PS1 TG and WT (2-mo-old) mice were randomized into 3 dietary intervention groups, as described below. Given the length of the study and the number of mice required, the study was conducted in 11 cohorts (a total of 8–12 mice in each cohort). Cohort 3 was excluded from the study because we started the diet interventions for this cohort 2 wk later than the other 10 cohorts. Also, 7 mice were lost due to natural death, leaving an overall number of 7–11 mice per group. Mice were fed a low fat (LF) diet (10% kcal fat, 20% kcal protein, and 70% kcal carbohydrates), a HF diet (45%, 20%, and 35% of energy from fat, protein, and carbohydrates, respectively), or a HF diet supplemented with 36 g/kg EPA ethyl ester-enriched fish oil (45% fat, 20% protein, and 35% carbohydrates). Alaskomega EPA-enriched fish oil contained 700 mg EPA/g fish oil and was kindly provided to us by Wiley Companies. Diets were prepared by Research Diets, Inc. (V10001, AIN-76A Vitamin Mix and S10001 Mineral Mix) according to our specifications above. Detailed diet information is provided in Supplemental Table 1. Mice were housed individually in our accredited animal facility with a 12:12 h light-dark cycle at 22–23 °C with free access to water and measured amounts of food. Mice were monitored regularly to ensure that they had sufficient water and food. In a few cases in which all or most of the food was consumed before the next measurement time, food was replenished as needed and amounts recorded accordingly. We also ensured that the diet formulation had adequate amounts of micronutrients and macronutrients, even for those mice who consumed the lowest amounts of food. BW and food intake were recorded weekly for 32 wk after starting the intervention. Data for BW and food intake are shown for 30 wk; however, the last 2 wk are not included because mice were in metabolic cages toward the end of the intervention. Body composition was assessed every 8 wk using Echo-MRI 3-in-1 instrument (EchoMRI LLC). Glucose tolerance tests (GTTs) and energy expenditure (EE) measurements were conducted at 12 wk and 30 wk, respectively, of the dietary interventions. These interventions were conducted from age 8 wk and lasted 32 wk, mice were deprived of feed for 5 h, then killed by CO2 inhalation, and tissues were harvested. Blood was collected by cardiac puncture into microtainer tubes (BD Biosciences) and then centrifuged for serum separation and stored at −80 °C until further analyses. Serum samples were thawed for the first time to measure the serum biomarkers reported. All animal protocols were approved by the Institutional Animal Care and Use Committee of Texas Tech University (protocol number: T19040). This study was part of a larger research project in which other various stress and behavioral outcomes were determined, which will be presented elsewhere.
GTT
The GTT was conducted on wk 12 after starting the intervention (mice aged ≥5 mo). Mice were deprived of food for 5 h before the GTT, previously established as an appropriate duration for starving rodents for insulin resistance studies [32]. Blood glucose levels were measured in the basal state and 15, 30, 60, 90, and 120 min following the intraperitoneal glucose injection (2 g/kg BW) using OneTouch Ultra Glucose Meter (AlphaTrack).
EE
The metabolic activity of the mice was measured using the PhenoMaster system (TSE Systems). This instrument is equipped to detect indirect calorimetry and monitor activity. Exhaust air from individual cages was sampled at 48-min intervals for 3 min. Sample air was passed through sensors to determine VO2 and volume of carbon dioxide production. The respiratory exchange ratio (RER) was calculated as the ratio of carbon dioxide produced to oxygen consumed (fuel preference 1.0: carbohydrates, 0.7: fat) [33]. The PhenoMaster system allows measuring of both cumulative (increasing distance with time) and total locomotor activity (K and H, respectively) calculation based on the animal’s actual weight. Home-cage locomotor activity was determined by an ActiMot infrared light beam system integrated into the calorimetry system. Mice were given 3 d to acclimate to the metabolic cage to beginning data collection, which took place over another 3-d period. At the age of 38 wk, mice were housed individually and maintained on a 12:12 h light-dark cycle and were continued to be fed with the same diet (LF, HF, or EPA) with water ad libitum.
Serum measurements
Serum leptin, resistin, adiponectin, and insulin concentrations were measured using multiplex kits (Catalog No. MMHE-44K, for metabolic hormones, and MADPNMAG-70K-01 for adiponectin; EMD Millipore Corporation) in a multiplexing system (Luminex xMAP, Luminex Corporation), as we previously reported [34]. For Aβ1-40 and Aβ1-42, a human multiplex kit (Catalog No. HNABTMAG-68K, EMD Millipore Corporation) was used. Samples were run according to manufacturer’s protocol, using internal controls and standards; both measured within <10% CV.
Immunohistochemistry
Brains were embedded, frozen, and fixed in O.C.T. cryostat-embedding compound (Tissue-Tek) and cut into 12-μm thick sections on a cryostat at 4 °C. The tissue slides were stained with primary antibody against Aβ1-16 (803014, Biolegend Inc, dilution 1:1000) overnight at 4 °C, followed by secondary antibody Cy3-conjugated AffiniPure donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc; dilution 1:100) for 2–4 h the following day at room temperature. Images were taken under 20× magnification on an EVOS FL Auto Imaging System (Thermo Fisher Scientific). To exclude the area of interest (AOI) in the brain image slides, the fluorescent stained images were first separated into red-green-blue channels. The Aβ plaque (AOI) staining appeared as very bright green spots in the green channel. This AOI was separated and measured from the green background in the green channel using the proper thresholding level. The green channel images were filtered using a 2D-convolution kernel in OpenCV libraries using C++ codes to get a sharper image. Sharpening the dataset images helped to distinguish between the AOI and the green background better. To analyze the amount of Aβ plaque (AOI) in the brain slides, AdipoGauge software was used for quantification of the Aβ plaque area in the right hemisphere [35]. Using this method, the AOI for the samples calculated were converted to μm units, if required, by multiplying this value by the image size. In some brain images in which the image size was too large, we used C++ scratch codes to calculate the AOI using the same method. In C++, OpenCV libraries were utilized to find the pixels with green values of >70. In the last step toward final output images, all pixels with green values that were <70 thresholds were put to black color, so the Aβ plaque was excluded from the background. The AOI pixel values were not changed, so the final output images had the original values in the AOI areas.
Statistical analyses
All data were analyzed using the R statistical software (version 3.5.3). Data were visually examined to ensure normality and homogeneity of variance when grouped by genotype (i.e., non-TG compared with TG), dietary intervention (i.e., LF compared with HF compared with HF diet enriched with EPA), and sex (i.e., male compared with female). Missing data (<10%) were imputed via multiple preseeded imputations implemented using the mice package (version 3.6.0) in the R statistical software (50 imputed datasets and 50 maximum iterations per imputation). A series of a 3-factor ANOVA tests were performed on the imputed data to predict each outcome variable using the main effects and interactions of genotype, diet, and sex. Subsequent pairwise comparisons were performed using Tukey distributions, which maintained a type I error rate for each examined outcome variable at 0.05. The AUC was computed to assess the changes in fat mass, lean mass, food intake, BW, and GTT. These changes were examined using 3-factor ANOVA models and subsequent pairwise comparisons as described above.
Furthermore, to examine the temporal trends of fat mass, lean mass, food intake, and BW changes, the time series of these outcome variables were analyzed using the ArfimaMLM package in the R statistical software (version 1.3). The time series were examined in 2-level models that showed the regression of each outcome variable (genotype, diet, sex, and time) and their interactions, accounting for the nested nature of the time-series data within-subjects and while modeling random slopes for time. WT mice, HF, and female sex were included in the models as reference categories. Degrees of freedom for each regression coefficient were determined using the Satterthwaite method to compute P values. P value of ≤0.05 was considered statistically significant, β and Δ for the mean differences in time-series analysis and 3-factor ANOVA, respectively. F value indicates analysis of variances. All graphs were created based on mean ±SEM using GraphPad Prism (Prism version 9.1.0).
Results
Effects of HF diet and EPA on BW, adiposity, circulating adipokines, and glucose tolerance
BW and food intake
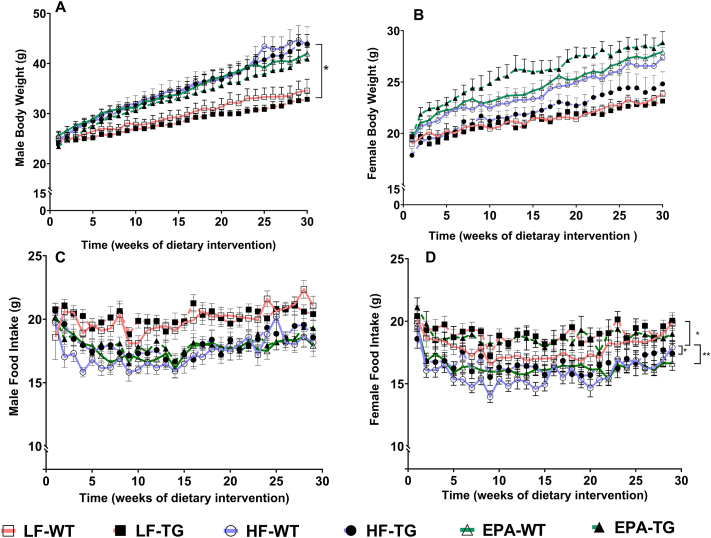
The time-series analyses that examined the effects of genotype and diet revealed no significant differences in the starting weights of TG compared with WT mice (males and females) or between mice assigned to the 3 diet groups (Figure 2 and Supplemental Table 2). The significant interactions were as follows: at baseline, WT male mice were heavier than WT females (β = 4.975 g; P < 0.001). On average, WT male mice fed HF gained ∼61 mg more weight daily than HF-fed WT females (P < 0.001). As expected, EPA supplementation decreased daily weight gain in WT males by ∼24 mg, compared with HF-fed WT males, and this difference approached significance (P = 0.054). However, this effect of EPA was not observed in WT females, or in male and female TG mice (P = 0.436, P = 0.583, and P = 0.943, respectively). LF-fed WT males had a significantly lower rate of daily weight gain than HF-fed WT males (β = −31 mg; P = 0.015). This effect was not seen in LF-fed female WT mice (P = 0.135) or in LF-fed male TG mice (P = 0.985). Moreover, there were no significant differences among male and female LF-fed mice compared with respective EPA-fed groups in both genotypes.
FIGURE 2.
Body weight (A, B) and food intake (C, D) of male and female, WT, and TG mice fed LF, HF, or a HF-EPA diets, measured weekly for 30 wk of the dietary interventions. Data are given as mean ± SEM, n = 10/group. Pairwise comparisons with significant differences were presented as ∗P ≤ 0.05 (A; LF-WT vs. HF-WT, D; HF-TG vs. HF-WT and EPA-TG), ∗∗P ≤ 0.01 (D; HF-TG vs. LF-TG). Although male and female data are graphed here separately, they were analyzed together to assess sex differences. Complete statistical test results are reported in Supplemental Tables 2 and 3. F, female; HF, high fat; HF-EPA, high-fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
HF-fed female TG mice ate more than HF-fed WT female mice (β = 1.536 g; P = 0.036). WT females fed the LF and EPA diets ate significantly more than HF-fed WT female mice (β = 2.338 g; P = 0.002 and β = 1.585 g; P = 0.030, respectively). The rate of increase in daily food intake of EPA-fed WT female mice was significantly lower by ∼10 mg/d than the respective intake in HF-fed WT female mice (P = 0.011). Diet or sex did not significantly affect the rate of increase in food intake in the TG genotype or in the male mice (Figure 2 and Supplemental Table 3).
Body composition
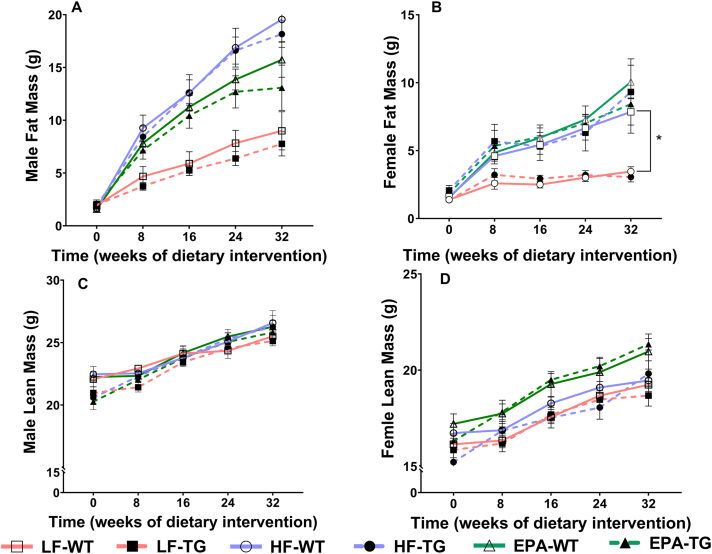
The results of the time-series analyses to examine the effects of the 3 diets, genotypes, and sex on fat mass are summarized in Figure 3. At the baseline, fat mass was not significantly different based on genotype or diet in females or males. However, HF-fed WT male mice had ∼2.45 g higher fat mass than HF-fed WT female mice at baseline (P = 0.026). In addition, in HF-fed WT mice, females gained ∼19 mg of fat mass/d (P < 0.001), and males gained an additional 38 mg of fat/d compared with females (P < 0.001). The rate of fat mass gain was ∼14 mg lower among LF-fed WT female mice than HF-fed WT female mice (P = 0.043). No significant differences in the rate of fat mass gain were seen between WT compared with TG male or in female mice. Similarly, diet did not significantly affect the rate of fat mass gain among male WT or TG mice. Overall, EPA had no significant effects on the rate of fat mass gain, but there were trends toward reduced fat mass in EPA-fed WT and TG male mice, compared with HF-fed groups (P = 0.054) (Supplemental Table 4).
FIGURE 3.
Fat mass (A, B) and lean mass (C, D) of male and female, WT and TG mice fed LF, HF, or a HF-EPA diets measured bimonthly for 32 wk of the dietary interventions. Data are given as mean ± SEM, n = 10/group. Pairwise comparisons with significant differences were presented as ∗P ≤ 0.05 (B: LF-WT vs. HF-WT). Although male and female data are graphed here separately, they were analyzed together to assess sex differences. Complete statistical test results are reported in Supplemental Tables 4 and 5. F, female; HF, high fat; HF-EPA, high-fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
Like fat mass, the lean mass of female or male mice at baseline was comparable between the 2 genotypes and the different diets and is summarized in Figure 3. HF-fed WT male mice had a higher lean mass at baseline than corresponding females (β = 4.393 g; P < 0.001). The lean mass gain of HF-fed WT females was ∼15 mg/d (P < 0.001), and the rate of lean mass gain for HF-fed WT male mice was greater by ∼10 mg/d than HF-fed WT females (P < 0.001) in 30 wk. The genotype or diet type did not significantly affect lean mass (Supplemental Table 5).
EE
The effects of genotype, diet, and sex on VO2 were examined in 3-factor ANOVA models, and the main effects for each genotype (F1,120 = 4.922; P = 0.028), diet (F2,120 = 4.964; P = 0.008), and sex (F1,120 = 91.544; P < 0.001) were significant. However, none of the examined 2-factor or 3-factor interactions were significant. The significant main effect of genotype revealed that TG mice had higher VO2 than WT mice regardless of diet or sex (Δ = 158 mL/[h.g]; P = 0.028). Similarly, female mice had higher VO2 than males, regardless of diet or genotype (Δ = 681 mL/[h.g]; P < 0.001). Moreover, post hoc pairwise comparisons for diet effects revealed that, in general, HF-fed mice had higher VO2 than LF-fed mice (Δ = 260 mL/[h.g]; P = 0.008), but did not differ from EPA-fed mice (P = 0.071); EPA and LF diet groups did not differ from one another (P = 0.712) (Figure 4).
FIGURE 4.
Metabolic cage analyses for males and females, WT, and TG mice fed LF, HF, or a HF-EPA diets at wk 30 of the dietary interventions. (A) VO2 (B) RER; VCO2/VO2, (C) cumulative distance, and (D) total distance was measured. Data are given as mean ± SEM, n = 10/group. Pairwise comparisons are significant at P values of ∗∗∗≤0.001 (B; LF vs. HF and EPA, main effect of diet); pairwise comparisons are nonsignificant (P > 0.05) if not marked. D, total distance; F, female; HF, high fat; HF-EPA, high-fat diet supplemented with EPA; K, cumulative distance; LF, low fat; M, male; RER, respiratory exchange ratio; TG, transgenic; VCO2, carbon dioxide consumption; WT, wild-type.
A 3-factor ANOVA model examined the effects of genotype, diet, and sex on RER and revealed a significant main effect for diet (F2,120 = 223.130; P < 0.001); however, the main effects for genotype and sex were not significant (F1,120 = 1.078; P = 0.301 and F1,120 = 1.856; P = 0.175, respectively). Also, significant genotype × sex and diet × sex interactions were observed for RER (F1,120 = 4.172; P = 0.043 and F2,120 = 3.467; P = 0.034, respectively). The genotype × diet interaction and the 3-factor interaction were not significant. In subsequent pairwise comparisons, the significant main effect for diet type was found to be driven by higher RER in the LF-fed mice than that in HF- or EPA-fed mice (Δ = 0.10; P < 0.001 and Δ = 0.097; P < 0.001, respectively). No significant differences were observed between HF- compared with EPA-fed mice (Δ = 0.002; P = 0.945). The significant genotype × sex interaction was possibly driven by a higher RER among TG male mice than TG female mice, which approached statistical significance after correcting for multiple comparisons using Tukey distributions (Δ = 0.01; P = 0.082). Male compared with female differences were substantially small among WT mice (Δ = 0.003; P = 0.960). The significant diet × sex interaction was driven primarily by the significant male compared with female differences in RER among LF-fed mice (Δ = 0.02; P = 0.048). This difference was not observed among males compared with females fed a HF or an EPA diet (P = 0.998 and P = 0.999, respectively) (Figure 4).
A 3-factor ANOVA model constructed to examine the effects of diet, genotype, and sex on H (total distance; distance completed in 72 h) revealed only significant main effects for the diet and sex parameters (F2,120 = 3.720; P = 0.027 and F1,120 = 8.865; P = 0.003, respectively). None of the interactions were significant. This significant main effect for diet was driven by a higher H of the EPA-fed mice than that of LF-fed mice (Δ = 142 m; P = 0.033). Other pairwise diet group comparisons were not significant. The significant main effect for sex was driven by a greater H achieved by the female than that by male mice (Δ = 136 m; P = 0.003). A similar ANOVA model that examined the effects of diet, sex, and genotype on K (cumulative distance; increasing distance with time and adding that to previous days) revealed only a significant main effect for sex (F1120 = 8.325; P = 0.004), and this was driven by a higher cumulative distance; the effects and interactions were not significant (Figure 4).
GTT
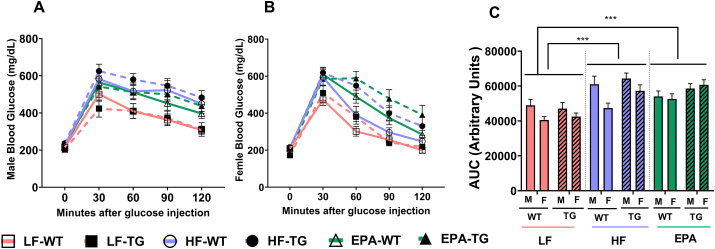
The effects of genotype, diet, and sex on the area under the curve of GTT were examined in a 3-factor ANOVA model. The main effects for diet and sex were significant (F2,120 = 23.591; P < 0.001 and F1,120 = 12.601; P < 0.001, respectively). The significant main effect of diet revealed that LF-fed mice cleared glucose more rapidly from the bloodstream compared with HF and EPA-fed mice (Δ = −1.31 g/dL; P < 0.001: Δ = −1.27 g/dL; P < 0.001, respectively). However, no significant differences in glucose clearance were observed between HF and EPA groups. Moreover, the significant main effect for sex indicated that the overall glucose tolerance of male mice was lower than that of female mice, irrespective of the genotype or diet type (Δ = 6.29 g/dL; P < 0.001). None of the examined interactions were significant (Figure 5).
FIGURE 5.
Glucose tolerance tests (GTT) of (A) male and (B) female and (C) GTT area under the curve of WT and TG mice fed LF, HF, or HF-EPA diets measured at wk 12 of the dietary interventions. Data are given as mean ± SEM, n = 10/group. Pairwise comparisons are significant at P values of ∗∗∗<0.001 (C; LF vs. HF and EPA, main effect of diet); pairwise comparisons are nonsignificant (P > 0.05) if not marked. Although male and female data are graphed here separately, they were analyzed together to assess sex differences. F, female; GTT, glucose tolerance test; HF, high fat; HF-EPA, high-fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
Circulating metabolic hormones in serum
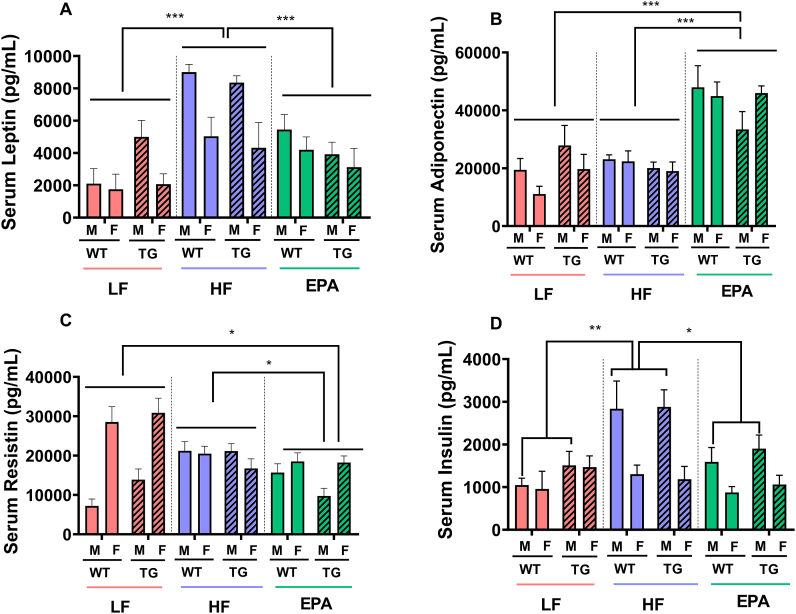
Leptin. Significant main effects were observed for sex and diet (F1,64 = 16.163; P < 0.001 and F2,64 = 17.913, P < 0.001, respectively), but not the genotype for serum leptin (F1,64 = 0.078; P = 0.781). None of the 2-factor or 3-factor interactions were significant for leptin. The main effect for sex was driven by a significantly higher fasting leptin level among male than female mice (Δ = 2.18 ng/mL; P < 0.001). The main effect of diet was found to be driven by significantly higher leptin levels in the HF-fed mice than that in LF-fed mice (Δ = 3.95 ng/mL; P < 0.001) or EPA-fed mice (Δ = 2.51 g/mL; P < 0.001). Furthermore, leptin levels were not significantly different between LF-fed compared with EPA-fed mice (Figure 5).
Adiponectin. A similar 3-factor ANOVA model constructed to examine the effects of diet, sex and genotype on serum adiponectin revealed only a significant main effect for diet (F2,64 = 32.613; P < 0.001). This main effect was the result of significantly greater fasting adiponectin levels in EPA-fed mice than that in LF- or HF-fed mice (Δ = 23.2 ng/mL; P < 0.001 and Δ = 21.6 ng/mL; P < 0.001, respectively). However, adiponectin levels were not significantly different between HF- compared with LF-fed mice (Δ = 1.63 ng/mL; P = 0.872) (Figure 6).
FIGURE 6.
Serum concentrations of metabolic hormones and adipokines in male and female, WT and TG mice fed LF, HF, or a HF-EPA diets after 32 wk of the dietary interventions. (A) leptin, (B) adiponectin, (C) resistin, and (D) insulin in serum were measured. Data are given as mean ± SEM, n = 6–7/group. Pairwise comparisons are significant P values of ∗≤0.05 (C; EPA vs. LF and HF, main effect of diet. D; EPA-M vs. HF-M effect of diet x sex interaction), ∗∗≤0.01 (D; HF-M vs. LF-M effect of diet x sex interaction), ∗∗∗≤0.001 (A; HF vs. LF and EPA, B; EPA vs. LF and HF, main effect of diet); pairwise comparisons are nonsignificant (P > 0.05) if not marked. F, female; HF, high fat; HF-EPA, high fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
Insulin. Significant main effects for serum insulin were observed for sex and diet (F1,64 = 19.235; P < 0.001 and F2,64 = 6.981; P = 0.002, respectively). In addition, a significant diet × sex interaction was observed (F2,64 = 5.178; P = 0.008) for this hormone. There was no significant main effect for genotype or for the other examined interactions. The main effect for sex was because of significantly higher fasting insulin levels in males than that in females (Δ = 0.845 ng/mL; P < 0.001), whereas the main effect for diet was because of significantly higher fasting insulin levels in HF-fed mice than that in EPA-fed or LF-fed mice (Δ = 0.694 ng/mL; P = 0.011 and Δ = 0.809 ng/mL; P = 0.003, respectively). In addition, no significant differences were observed between EPA and LF groups. The significant diet × sex interactions were driven by significantly higher fasting insulin levels in male mice fed a HF diet than male mice fed a LF diet or an EPA diet (Δ = 1.57 ng/mL; P < 0.001 and Δ = 1.1 ng/mL; P = 0.017, respectively). The fasting insulin levels were not significantly different between female mice fed with HF compared with LF or EPA diets (Figure 6).
Resistin. Three-factor ANOVA of fasting resistin levels also revealed significant main effects for sex and diet (F1,64 = 26.327; P < 0.001 and F2,64 = 4.794; P = 0.012, respectively) and a significant diet × sex interaction in serum resistin (F2,64 = 19.472; P < 0.001). Other main effects and interactions were not significant. The main effect for sex was because of significantly higher resistin levels in female than that in male mice (Δ = 7.24 ng/mL; P < 0.001), whereas the main effect for diet was because of significantly lower resistin levels in EPA-fed mice than that in HF-fed or LF-fed mice (Δ = 4.53 ng/mL; P = 0.027 and Δ = 4.68 ng/mL; P = 0.024, respectively). There were no significant differences between LF- and HF-fed groups in male mice. Furthermore, the significant diet × sex interaction was driven by significantly higher resistin levels in female mice fed LF diet than that in female mice fed HF diet or EPA diet (Δ = 8.52 ng/mL; P = 0.012 and Δ = 1.13 ng/mL; P < 0.001, respectively), and there were no significant differences between HF- and EPA-fed groups of female mice. By contrast, male WT mice fed HF diet had significantly higher levels of resistin than male WT mice fed LF or EPA diet (Δ = 1.06 ng/mL; P < 0.001 and Δ = 8.68 ng/mL; P = 0.008, respectively), further contributing to the significant diet × sex interactions for serum resistin (Figure 6).
Serum amyloids
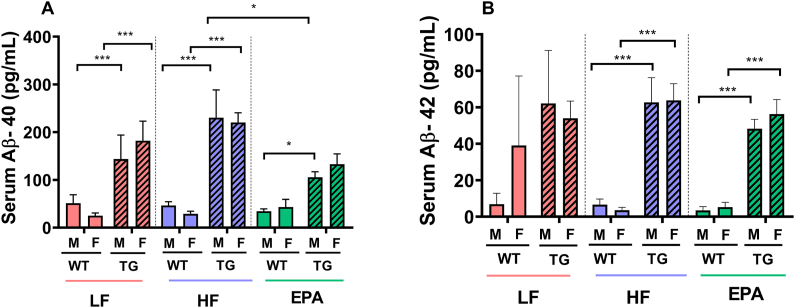
Aβ1-40and Aβ1-42. Three-factor ANOVA examining the effects of sex, diet, and genotype on serum Aβ1-40 revealed significant main effects for both the genotype and diet type (F1,64 = 64.169; P < 0.001 and F2,64 = 3.584; P = 0.034, respectively). We also observed a significant diet × genotype interaction for serum Aβ1-40 (F1,64 = 3.693; P = 0.030). As expected, the main effect for genotype was because of significantly higher serum Aβ1-40 levels in the TG mice than that in WT mice (Δ = 0.131 ng/mL; P < 0.001). Moreover, the significant main effect for diet was because of significantly higher serum Aβ1-40 levels in the HF-fed mice than that in EPA-fed mice (Δ = 0.053 ng/mL; P = 0.026). The significant diet × genotype interaction was driven by a significant HF compared with EPA group difference among TG mice (Δ = 0.106 ng/mL; P = 0.004). These differences in response to HF compared with EPA diets were not observed among WT mice (Δ = −0.63 X 103 ng/mL; P > 0.05) and there were no significant differences among HF- compared with LF- or EPA- compared with LF-fed groups in either genotype. Serum Aβ1-40 levels were not significantly different between LF- compared with HF- and LF- compared with EPA-fed in both WT and TG mice. For serum Aβ1-42 levels, the 3-factor ANOVA model revealed only a significant main effect for the genotype (F1,60 = 64.169; P < 0.001), because of significantly higher Aβ1-42 levels in the TG mice than that in WT mice (Δ = 0.047 ng/mL; P < 0.001); no other interactions were significant (Figure 7).
FIGURE 7.
(A) Aβ1-40 and (B) Aβ1-42 serum concentrations in male and female TG and WT mice fed LF, HF, or a HF-EPA diets, after 32 wk of the dietary interventions. Data are given as mean ± SEM, n = 6–7/group. Pairwise comparisons are significant at P values of ∗≤0.05 (A; EPA-TG vs. HF-TG diet × sex × genotype interaction, EPA-M-TG vs. EPA-M-WT, genotype effect) ∗∗∗≤0.001(A; LF-M-TG vs. LF-M-WT, LF-F-TG vs. LF-F-WT, HF-M-TG vs. HF-M-WT, HF-F-TG vs. HF-F-WT and B HF-M-TG vs. HF-M-WT, HF-F-TG vs. HF-F-WT, EPA-M-TG vs. EPA-M-WT, EPA-F-TG vs. EPA-F-WT, genotype effect); pairwise comparisons are nonsignificant (P > 0.05) if not marked. Aβ, amyloid-beta; F, female; HF, high fat; HF-EPA, high-fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
Brain amyloids (using Aβ1-16 antibody)
The 3-factor ANOVA analysis of quantitative immunohistochemistry (IHC) data showed that genotype had a significant main effect on Aβ plaques in the brain (F1,29 = 10.12; P = 0.001), as staining for Aβ1-16 (green color in Figure 8) in the brains of TG mice was significantly higher than in their WT non-TG littermates in both males and females (Δ = 0.56 %; P = 0.0021). As we expected, no Aβ plaques were visualized in WT male and female mice (Figure 9). Surprisingly, LF-treated TG mice had the highest area of Aβ plaques among all the treatments in both males and females (LF compared with EPA, Δ = 0.84 %; P = 0.039), with no significant differences between males and females.
FIGURE 8.
Brain Immunohistochemistry of Aβ 1-16 in male and female TG and WT mice fed LF, HF, or a HF-EPA diets after 32 wk of the dietary interventions. Brain sections were used for amyloids immunohistochemical analyses, using Aβ1-16 antibodies, shown in the green channel. F, female; HF, high fat; HF-EPA, high fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
FIGURE 9.
Brain area fluorescent density percentage by immunohistochemistry of Aβ1-16 in male and female TG and WT mice fed LF, HF, or a HF-EPA diets after 32 wk of the dietary interventions. Fluorescent area density (green channel) of amyloids in mice was quantified in immunohistochemical images from Figure 8. Data are given as mean ± SEM, n = 2–4/group, pairwise comparisons are significant at P values of ∗<0.05 (LF-F-TG vs. EPA-F-TG, diet effect), ∗∗≤0.01 (LF-M-TG vs. LF-M-WT, LF-F-TG vs. LF-F-WT, HF-M-TG vs. HF-M-WT, HF-F-TG vs. HF-F-WT and B HF-M-TG vs. HF-M-WT, HF-F-TG vs. HF-F-WT, EPA-M-TG vs. EPA-M-WT, EPA-F-TG vs. EPA-F-WT, genotype effect); pairwise comparisons are nonsignificant (P > 0.05) if not marked. F, female; HF, high fat; HF-EPA, high fat diet supplemented with EPA; LF, low fat; M, male; TG, transgenic; WT, wild-type.
Discussion
We previously demonstrated protective effects of EPA on HF diet-induced obesity, inflammation, and glucose intolerance using C57BL/6J mice [36]. Here we hypothesized that EPA would exert protective metabolic effects in an obese Alzheimer’s amyloidogenic model and would reduce serum and brain amyloid levels. To our knowledge, the current study reports for the first time some potential protective metabolic effects of EPA, not only in attenuating HF diet-induced metabolic dysfunction, but also in reducing circulating and brain amyloids (especially serum Aβ1-40) in an amyloidogenic model of AD, the APP/PS1 mice.
BW, food intake, and EE
In the current study, male mice gained more weight and fat mass than females. As expected, HF diet increased BW and fat mass significantly in WT male mice compared with LF diet. However, EPA trended toward lowering BW in WT male mice compared with the HF-fed group. We previously reported that male mice fed a HF diet supplemented with 36 g/kg EPA for 12 wk exhibited significantly reduced BW, fat mass, and glucose intolerance, compared with the HF-fed group [27,28,34].
Consistent with our current findings, recent studies reported that obesity increased amyloid deposition in male APP/PS1 mice [[37], [38], [39]]. Moreover, Bracko et al. [40] reported that the HF diet in APP/PS1 mice caused comparable weight gain between WT and TG mice, and female mice had a slower weight gain than males on both unpurified and HF diet; these findings are in agreement with our results. Kohjima et al. [41] fed Tg2576 AD female mice with an HF diet for 12 wk and observed increased BW, food intake, and Aβ in the brain compared with the LF group. Very limited data are available on female APP/PS1 mice to allow for comprehensive comparisons; but overall, male mice were more susceptible to weight gain than females [42], possibly because of the protective effects of estrogen in females [43]. However, APP/PS1 male mice appear to be more susceptible to weight gain in short HF feeding durations (8 wk), but not when fed a regular diet during longitudinal aging experiments [44].
Similarly, in our study, male mice gained more weight than females, and female mice had higher VO2, and both WT and TG females completed a greater locomotor distance than WT and TG males. Higher activity in females could be another reason, besides sex steroid hormone levels, that may explain reduced weight gain in females compared with males. On the other hand, EPA-fed mice exhibited trends toward reduced BW and fat mass in males, consistent with one of our recent studies using a shorter duration of EPA supplementation (14 wk lowered BW and fat mass in male mice) [34]. Furthermore, a fish oil-enriched diet was previously shown to increase EE and oxygen consumption in WT male mice at 4 mo of age [45]. In agreement with these reports, in our current study, EPA-fed groups also had significantly higher locomotor activity in both genotypes and sexes when compared with LF groups; but no differences were observed when EPA groups were compared with HF groups.
Glucose homeostasis: serum insulin and GTT
We also demonstrated that HF diet-fed groups were more glucose intolerant and had higher serum insulin levels than LF in both sex and genotype, which is in line with similar findings by others in both APP/PS1 and WT mice fed HF diet [27,46,47]. In addition, male WT mice treated with EPA cleared glucose faster than the HF group, as indicated in the time-series analyses of GTT in this current study and as we previously reported in younger mice with shorter EPA feeding (11–14 wk) [27,34]. Similar to our previous reports in WT mice [48], there were no improvements in glucose clearance in the EPA-fed WT compared with HF-fed females in this study, which may be because of the longer duration of HF feeding in the current work.
Further, Denver et al. [49] showed that in APP/PS1 male mice fed with a regular unpurified diet, the genotype was comparable for peripheral glucose tolerance at 15–18 mo of age after overnight fasting compared with WT mice. Macklin et al. [50] demonstrated impaired glucose intolerance after 12 h of fasting in unpurified-fed APP/PS1 male mice at 3 different ages, compared with WT mice on the identical diets, consistent with the trends observed in our data for genotype differences, with consideration to different dietary interventions. In the current study, we did not observe any improvements in glucose clearance with EPA in male and female TG mice. This may have contributed to EPA’s inability to reverse the effects of long-term HF feeding in TG mice because APP/PS1 mice are more susceptible to glucose intolerance [50]. Some of the differences in our results with other studies could also be attributed to shortening the length of fasting before GTT (4–6 h in our studies compared with overnight in others, as discussed above).
Adiposity markers
A critical feature of obesity is decreased circulating adiponectin and increased leptin, both of which were previously linked to negative effects on the brain and on dementia [51,52]. Leptin effects included reduced β-secretase, increased apolipoprotein E-dependent Aβ uptake in neuroblastoma cells, and changes in Aβ turnover [53]. In addition, 14 mo of fish oil supplementation reduced serum leptin significantly in patients with spinal cord injury [54]. Moreover, we have previously shown that, compared with HF, EPA reduced leptin in WT males [34], which is in line with findings in our current study. As expected, compared with LF groups, HF increased serum leptin in the WT group and in TG male mice fed with HF. Leptin was significantly attenuated by EPA in TG males, consistent with reduced adiposity. In agreement with our findings, EPA also reduced plasma leptin levels in WT males but not in females [34].
Interestingly, leptin levels trended to higher amounts in LF-TG male than that in WT mice fed LF diet, which could contribute to leptin resistance because of higher Aβ and which, in turn, modulates leptin receptors. Furthermore, 20-wk HF feeding increased hypothalamic leptin resistance in the APP/PS1 mice, leading to higher leptin, Aβ, and inflammatory markers, in TG, compared with HF-fed WT mice [46]. Thus, HF feeding to AD mice may reduce leptin’s efficacy in suppressing food intake and subsequently contribute to leptin resistance [55]. Moreover, sustained levels of Aβ attenuated the response of a marker of leptin signaling, the signal transducer and activator of transcription 3, by leptin in HF-fed WT mice, suggesting that Aβ may promote hypothalamic leptin resistance [46].
Several studies showed that EPA [28,56] or combined EPA and DHA [57] increased adiponectin levels in obese B6 mice, consistent with our findings in WT and APP/PS1 male and female mice. A few studies also demonstrated associations between lower adiponectin and increased AD pathology. One such study showed that aged adiponectin-knockout mice exhibited increased Aβ1-42 levels and plaques, protein levels of inflammatory markers in the brain, and tau hyperphosphorylation in mice and humans [58]. Thus, the role of changes in adiponectin by EPA in AD pathogenesis in our amyloidogenic TG mice merits further investigation.
In male mice of both genotypes, serum resistin was lower in the EPA-fed group than HF. Female LF-fed mice had higher resistin than HF-fed groups. In patients with AD, serum resistin levels were significantly increased and correlated with inflammatory markers at 65–85 y [59]. However, we observed no significant differences among WT and TG groups. This may be due to age differences, as these patients with AD had an average age >75 y and severe symptoms compared to this AD mouse model. Therefore, we may have detected more differences if mice were aged for a longer time (1.5–2 y) to match the age of these patients with AD. In Studzinski et al. [60] study, resistin was elevated after 4 wk of HF diet (40% fat) intervention starting at one mo of age in male and female mice compared to regular diet in WT and APP/PS1. Moreover, resistin was reduced in EPA-fed WT male mice compared to HF-fed WT males, but not in TG mice, consistent with downregulation of resistin by EPA in diet-induced obese mice fed with HF diet [61].
Resistin levels were significantly elevated in LF-fed females compared to LF-fed males and EPA-fed groups in both sexes. In line with our data, Gui et al. [62] reported that resistin in perigonadal white fat WT mice was higher in female compared to male mice. Also, human studies showed higher resistin levels in women [63].
Aβ
As expected, serum Aβ1-40 and Aβ1-42 levels were higher in the TG group compared to WT groups in both males and females. Aβ1-40 and Aβ1-42 showed only trending increases in the HF-fed group compared to LF, which may be linked to low-grade inflammation caused by obesity, which may increase Aβ aggregation [34,64]. It is possible that longer feeding periods are required in our studies to see more significant effects of EPA on amyloids. Further, Aβ1-40 was significantly decreased by EPA in TG males but not in females compared to HF-fed mice. Based on previous research, fish oil inhibited β- and γ-secretase activity, thereby reducing amyloidogenic cleavage of APP and decreasing Aβ in female APP/PS1 TG mice [29]. Thus, it is plausible that EPA may help reduce the risk for AD through reduction of inflammation and adiposity [65]. The above mentioned beneficial effect of EPA in reducing the Aβ depositions was supported by the quantitative IHC data, reported by observed trends toward lower area of Aβ plaques in brains of TG mice treated with EPA compared to LF- and HF-fed groups. Unexpectedly, the Aβ density in LF-fed TG groups was remarkably higher than that of HF-fed groups, which may be because of the higher carbohydrates (70%) in LF diet than the HF and HF + EPA diet groups (35% carbohydrate). Indeed, Yeh et al. [66] reported that compared to a normal unpurified diet containing 50% of kcal from carbohydrates, the high carbohydrate diets (67.3% carbohydrates) significantly increased cortical protein levels of Aβ in APP/PS1 TG mice. The other reason could be that, in our IHC experiments, we used antibodies against Aβ1-16, which is the non-toxic form of Aβ [67], and further brain analyses are required using different type of antibodies against Aβ peptides to confirm differences among dietary interventions.
In summary, the current study focused on metabolic effects of obesity and EPA in WT and an AD amyloidogenic mouse model. However, the exact mechanisms by which fish oil reduces AD-related pathologies are not entirely understood and merit further investigation. This study’s major strength is being the first to show the effects of EPA on markers of BW, adiposity, glucose homeostasis, and energy balance in the TG APP/PS1 mouse model. Our main finding that EPA decreased serum Aβ1-40 in TG male mice compared to the HF-fed group is a novel finding; however, our study also has limitations. The length of study could have been increased up to 18 mo of age in this model to see additional effects of Aβ on metabolic features. In addition, conducting GTT at different time points would have been helpful to investigate the progress of AD and related effects on glucose tolerance. Also, using APP/PS1/Tau TG mice is worth investigating because of the high impact of tau phosphorylation on metabolic changes of patients with AD; our model was only an amyloidogenic APP/PS1 model. Because our primary focus was on metabolic dysfunctions related to diet-induced obesity and inflammation in AD, one limitation was not testing the effects of EPA in LF-fed mice or using lower percentages of carbohydrates in the LF diet. Moreover, different types of Aβ should be measured in the brain to validate the effects of HF and EPA on brain amyloid in AD. Here, we tested only Aβ1-16, which unexpectedly showed higher levels in LF-fed group compared to HF- and EPA-fed mice; thus, further investigation is needed to measure the Aβ1-42, specifically as a more toxic peptide in the brain, and to determine the exact effects of HF and EPA in AD and Aβ toxicity.
Another limitation in translating our findings to humans is that we fed our mice a high dose of EPA-enriched fish oil (36 g/kg diet). Our primary aim was to identify mechanisms mediating the effects of EPA on AD; thus, we initially used a high dose to first detect marked EPA effects. The dose we used translates to a daily human intake of 10–12 g of EPA or fish oil, which is double the therapeutic amounts of fish oil prescribed clinically to treat hypertriglyceridemia [68]. While the current study was ongoing, we also conducted a dose response study in obese B6 mice. Although all doses of EPA used (9, 18, and 36 g/kg) exerted some metabolic benefits and reduced inflammation, it was only in mice fed the highest dose of 36 g/kg of EPA that we observed significant reductions in BW and adiposity when compared to HF-fed mice [34]. Future studies in the AD model are warranted using doses that are consistent with recommended human intakes and therapeutic doses that are used to treat hypertriglyceridemia.
Taken together, our results demonstrate that the HF diet increased metabolic risk factors compared with the LF diet and showed trends in increasing serum Aβ. Male mice gained more weight and fat compared to females and had higher serum leptin and insulin. EPA reduced Aβ1-40 and altered adipokines, including reducing leptin and increasing adiponectin in serum. Our findings provide evidence that dietary approaches, such as omega-3 fatty acids may effectively protect against obesity-related AD in amyloidogenic models. Our studies warrant further investigations of mechanisms underlying associations between EPA, Aβ, and adipokines in AD and tissue-specific differences. Given the current use of high doses of fish oil and EPA in hypertriglyceridemia, our findings support future clinical studies to determine the potential benefits of EPA and fish oil in AD.
Author disclosures
NM-M, MYa, LR, BNH, CNK, AC, CB, LM, KAM, SS, YZ, NSK, MYo, and HM, report no conflicts of interest.
Acknowledgments
The authors’ contributions were as follows – NMM, LR, BH: conceptualized and designed study and created the methodology of the study; MYa: investigated, formally analyzed, curated the data, visualized, wrote the original draft; MYo, HM: developed software, conducted image analyses, and quantification; NMM, LR, BH: investigated, curated the data; MYa, LR, BH: managed the animal studies, timeline, and curated the data; CNK, MYa: formally analyzed, curated the data; CNK: conducted statistical analyses and coding/programming; AC, CB, LM, KAM, SS: assisted with animal investigations; NMM, LR, BH, CNK, YZ, and NSK: wrote, reviewed, and edited and reviewed critically; NMM: supervised, administered the project; and all authors: read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.01.030.
Funding
The research leading to these results was funded by the NIH National Center for Complementary and Integrated Health (NCCIH) and a Supplement from the National Institute on Aging (NIA) (R15 AT008879-01A1S1).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tan J.Z.A., Gleeson P.A. The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer’s disease. Biochim. Biophys. Acta. Biomembr. 2019;1861(4):697–712. doi: 10.1016/j.bbamem.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 2.2021 Alzheimer’s disease facts and figures. Alzheimers. Dement. 2021;17(3):327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 3.2020 Alzheimer’s disease facts and figures. Alzheimer. Dement. 2020;16(3):391–460. doi: 10.1002/alz.12068. [DOI] [PubMed] [Google Scholar]
- 4.Bhatti G.K., Reddy A.P., Reddy P.H., Bhatti J.S. Lifestyle modifications and nutritional interventions in aging-associated cognitive decline and Alzheimer’s disease. Front. Aging. Neurosci. 2019;11:369. doi: 10.3389/fnagi.2019.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellechea P., Pujol N., Esteve-Belloch P., Echeveste B., García-Eulate M.R., Arbizu J., et al. Early-and late-onset Alzheimer disease: are they the same entity? Neurología (Engl Ed). 2018;33(4):244–253. doi: 10.1016/j.nrl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacace R., Sleegers K., Van Broeckhoven C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer. Dement. 2016;12(6):733–748. doi: 10.1016/j.jalz.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Cao D., Lu H., Lewis T.L., Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2007;282(50):36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 9.Ho L., Qin W., Pompl P.N., Xiang Z., Wang J., Zhao Z., et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB. J. 2004;18(7):902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 10.Shie F.S., Jin L.W., Cook D.G., Leverenz J.B., LeBoeuf R.C. Diet-induced hypercholesterolemia enhances brain Aβ accumulation in transgenic mice. Neuroreport. 2002;13(4):455–459. doi: 10.1097/00001756-200203250-00019. [DOI] [PubMed] [Google Scholar]
- 11.Levin-Allerhand J.A., Lominska C.E., Smith J.D. Increased amyloid- levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J. Nutr. Health. Aging. 2002;6(5):315–319. [PubMed] [Google Scholar]
- 12.Nuzzo D., Picone P., Baldassano S., Caruana L., Messina E., Marino Gammazza A., et al. Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr. Alzheimer. Res. 2015;12(8):723–735. doi: 10.2174/1567205012666150710115506. [DOI] [PubMed] [Google Scholar]
- 13.Forny-Germano L., De Felice F.G., Vieira M.N.D.N. The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer’s disease. Front. Neurosci. 2019;12:1027. doi: 10.3389/fnins.2018.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRM. Inflamm. 2013 doi: 10.1155/2013/139239. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fröhlich M., Imhof A., Berg G., Hutchinson W.L., Pepys M.B., Boeing H., et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes. Care. 2000;23(12):1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez-Hernández H., Simental-Mendía L.E., Rodríguez-Ramírez G., Reyes-Romero M.A. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013 doi: 10.1155/2013/678159. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 18.Deng Y., Li B., Liu Y., Iqbal K., Grundke-Iqbal I., Gong C.X. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: implication for Alzheimer’s disease. Am. J. Pathol. 2009;175(5):2089–2098. doi: 10.2353/ajpath.2009.090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckman L.B., Hasty A.H., Flaherty D.K., Buckman C.T., Thompson M.M., Matlock B.K., et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain. Behav. Immun. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colom-Cadena M., Spires-Jones T., Zetterberg H., Blennow K., Caggiano A., DeKosky S.T., et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers. Res. Ther. 2020;12(1):21. doi: 10.1186/s13195-020-00588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow V.W., Mattson M.P., Wong P.C., Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular. Med. 2010;12(1):1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Sarasúa S., Fernández-Pérez I., Espinosa-Fernández V., Sánchez-Pérez A.M., Ledesma J.C. Can we treat neuroinflammation in Alzheimer’s disease? Int. J. Mol. Sci. 2020;21(22):8751. doi: 10.3390/ijms21228751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long J.M., Holtzman D.M. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Drug Administration . 2021. Aducanumab (marketed as Aduhelm) information.https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/aducanumab-marketed-aduhelm-information [Internet] Available from: [Google Scholar]
- 25.Cleland L.G., James M.J., Proudman S.M. Fish oil: what the prescriber needs to know. Arthritis. Res. Ther. 2006;8(1):202. doi: 10.1186/ar1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalupahana N.S., Claycombe K.J., Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv. Nutr. 2011;2(4):304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalupahana N.S., Claycombe K., Newman S.J., Stewart T., Siriwardhana N., Matthan N., et al. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. J. Nutr. 2010;140(11):1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 28.LeMieux M.J., Kalupahana N.S., Scoggin S., Moustaid-Moussa N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J. Nutr. 2015;145(3):411–417. doi: 10.3945/jn.114.202952. [DOI] [PubMed] [Google Scholar]
- 29.Koivisto H., Grimm M.O., Rothhaar T.L., Berkecz R., Lütjohann D., Giniatullina R., et al. Special lipid-based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer's disease independent of brain amyloid deposition. J. Nutr. Biochem. 2014;25(2):157–169. doi: 10.1016/j.jnutbio.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Conquer J.A., Tierney M.C., Zecevic J., Bettger W.J., Fisher R.H. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids. 2000;35(12):1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 31.Külzow N., Witte A.V., Kerti L., Grittner U., Schuchardt J.P., Hahn A., et al. Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J. Alzheimers. Dis. 2016;51(3):713–725. doi: 10.3233/JAD-150886. [DOI] [PubMed] [Google Scholar]
- 32.Ayala J.E., Samuel V.T., Morton G.J., Obici S., Croniger C.M., Shulman G.I., et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010;3(9–10):525–534. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith R.L., Soeters M.R., Wüst R.C.I., Houtkooper R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018;39(4):489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pahlavani M., Ramalingam L., Miller E.K., Davis H., Scoggin S., Moustaid-Moussa N. Discordant dose-dependent metabolic effects of eicosapentanoic acid in diet-induced obese mice. Nutrients. 2020;12(5):1342. doi: 10.3390/nu12051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yosofvand M., Liyanage S., Kalupahana N.S., Scoggin S., Moustaid-Moussa N., Moussa H. AdipoGauge software for analysis of biological microscopic images. Adipocyte. 2020;9(1):360–373. doi: 10.1080/21623945.2020.1787583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahlavani M., Razafimanjato F., Ramalingam L., Kalupahana N.S., Moussa H., Scoggin S., et al. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J. Nutr. Biochem. 2017;39:101–109. doi: 10.1016/j.jnutbio.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Walker J.M., Dixit S., Saulsberry A.C., May J.M., Harrison F.E. Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, β-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2017;100:87–98. doi: 10.1016/j.nbd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thériault P., ElAli A., Rivest S. High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget. 2016;7(42):67808–67827. doi: 10.18632/oncotarget.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rollins C.P.E., Gallino D., Kong V., Ayranci G., Devenyi G.A., Germann J., et al. Contributions of a high-fat diet to Alzheimer's disease-related decline: a longitudinal behavioural and structural neuroimaging study in mouse models. Neuroimage. Clin. 2019;21 doi: 10.1016/j.nicl.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bracko O., Vinarcsik L.K., Cruz Hernández J.C., Ruiz-Uribe N.E., Haft-Javaherian M., Falkenhain K., et al. High fat diet worsens Alzheimer's disease-related behavioral abnormalities and neuropathology in APP/PS1 mice, but not by synergistically decreasing cerebral blood flow. Sci. Rep. 2020;10(1):9884. doi: 10.1038/s41598-020-65908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohjima M., Sun Y., Chan L. Increased food intake leads to obesity and insulin resistance in the tg2576 Alzheimer’s disease mouse model. Endocrinology. 2010;151(4):1532–1540. doi: 10.1210/en.2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong J., Stubbins R.E., Smith R.R., Harvey A.E., Núñez N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stubbins R.E., Holcomb V.B., Hong J., Núñez N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012;51(7):861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 44.Mody N., Agouni A., McIlroy G.D., Platt B., Delibegovic M. Susceptibility to diet-induced obesity and glucose intolerance in the APP (SWE)/PSEN1 (A246E) mouse model of Alzheimer’s disease is associated with increased brain levels of protein tyrosine phosphatase 1B (PTP1B) and retinol-binding protein 4 (RBP4), and basal phosphorylation of S6 ribosomal protein. Diabetologia. 2011;54(8):2143–2151. doi: 10.1007/s00125-011-2160-2. [DOI] [PubMed] [Google Scholar]
- 45.Kim M., Goto T., Yu R., Uchida K., Tominaga M., Kano Y., et al. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci. Rep. 2015;5 doi: 10.1038/srep18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y.H., Hsu H.C., Kao P.C., Shiao Y.J., Yeh S.H., Shie F.S., et al. Augmented insulin and leptin resistance of high fat diet-fed APPswe/PS1dE9 transgenic mice exacerbate obesity and glycemic dysregulation. Int. J. Mol. Sci. 2018;19(8):2333. doi: 10.3390/ijms19082333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Y., Ma X., Li P., Dong S., Huang X., Ren X., et al. High-fat diet induced discrepant peripheral and central nervous systems insulin resistance in APPswe/PS1dE9 and wild-type C57BL/6J mice. Aging. 2020;13(1):1236–1250. doi: 10.18632/aging.202262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller E.K., Pahlavani M., Ramalingam L., Scoggin S., Moustaid-Moussa N. Uncoupling protein 1-independent effects of eicosapentaenoic acid in brown adipose tissue of diet-induced obese female mice. J. Nutr. Biochem. 2021;98 doi: 10.1016/j.jnutbio.2021.108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denver P., English A., McClean P.L. Inflammation, insulin signaling and cognitive function in aged APP/PS1 mice. Brain. Behav. Immun. 2018;70:423–434. doi: 10.1016/j.bbi.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 50.Macklin L., Griffith C.M., Cai Y., Rose G.M., Yan X.X., Patrylo P.R. Glucose tolerance and insulin sensitivity are impaired in APP/PS1 transgenic mice prior to amyloid plaque pathogenesis and cognitive decline. Exp. Gerontol. 2017;88:9–18. doi: 10.1016/j.exger.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., et al. Neuroinflammation in Alzheimer’s disease. Lancet. Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ishii M., Iadecola C. Adipocyte-derived factors in age-related dementia and their contribution to vascular and Alzheimer pathology. Biochim. Biophys. Acta. 2016;1862(5):966–974. doi: 10.1016/j.bbadis.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fewlass D.C., Noboa K., Pi-Sunyer F.X., Johnston J.M., Yan S.D., Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. FASEB. J. 2004;18(15):1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 54.Sabour H., Norouzi Javidan A., Latifi S., Shidfar F., Heshmat R., Emami Razavi S.H., et al. Omega-3 fatty acids’ effect on leptin and adiponectin concentrations in patients with spinal cord injury: a double-blinded randomized clinical trial. J. Spinal. Cord. Med. 2015;38(5):599–606. doi: 10.1179/2045772314Y.0000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussain Z., Khan J.A. Food intake regulation by leptin: mechanisms mediating gluconeogenesis and energy expenditure. Asian. Pac. J. Trop. Med. 2017;10(10):940–944. doi: 10.1016/j.apjtm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Itoh M., Suganami T., Satoh N., Tanimoto-Koyama K., Yuan X., Tanaka M., et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler. Thromb. Vasc. Biol. 2007;27(9):1918–1925. doi: 10.1161/ATVBAHA.106.136853. [DOI] [PubMed] [Google Scholar]
- 57.Flachs P., Mohamed-Ali V., Horakova O., Rossmeisl M., Hosseinzadeh-Attar M.J., Hensler M., et al. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia. 2006;49(2):394–397. doi: 10.1007/s00125-005-0053-y. [DOI] [PubMed] [Google Scholar]
- 58.Ng R.C., Cheng O.Y., Jian M., Kwan J.S., Ho P.W., Cheng K.K., et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016;11(1):71. doi: 10.1186/s13024-016-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demirci S., Aynalı A., Demirci K., Demirci S., Arıdoğan B.C. The serum levels of resistin and its relationship with other proinflammatory cytokines in patients with Alzheimer's disease. Clin. Psychopharmacol. Neurosci. 2017;15(1):59–63. doi: 10.9758/cpn.2017.15.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Studzinski C.M., Li F., Bruce-Keller A.J., Fernandez-Kim S.O., Zhang L., Weidner A.M., et al. Effects of short-term Western diet on cerebral oxidative stress and diabetes related factors in APP x PS1 knock-in mice. J. Neurochem. 2009;108(4):860–866. doi: 10.1111/j.1471-4159.2008.05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramalingam L., Menikdiwela K.R., Spainhour S., Eboh T., Moustaid-Moussa N. Sex differences in early programming by maternal high fat diet induced-obesity and fish oil supplementation in mice. Nutrients. 2021;13(11):3703. doi: 10.3390/nu13113703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gui Y., Silha J.V., Murphy L.J. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes. Res. 2004;12(9):1481–1491. doi: 10.1038/oby.2004.185. [DOI] [PubMed] [Google Scholar]
- 63.Yannakoulia M., Yiannakouris N., Blüher S., Matalas A.L., Klimis-Zacas D., Mantzoros C.S. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J. Clin. Endocrinol. Metab. 2003;88(4):1730–1736. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]
- 64.Julien C., Tremblay C., Phivilay A., Berthiaume L., Emond V., Julien P., et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol. Aging. 2010;31(9):1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 65.Fotuhi M., Mohassel P., Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat. Clin. Pract. Neurol. 2009;5(3):140–152. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- 66.Yeh S.H., Shie F.S., Liu H.K., Yao H.H., Kao P.C., Lee Y.H., et al. A high-sucrose diet aggravates Alzheimer's disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice. Neurobiol. Aging. 2020;90:60–74. doi: 10.1016/j.neurobiolaging.2019.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Moore B.D., Martin J., de Mena L., Sanchez J., Cruz P.E., Ceballos-Diaz C., et al. Short Aβ peptides attenuate Aβ42 toxicity in vivo. J. Exp. Med. 2018;215(1):283–301. doi: 10.1084/jem.20170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Keefe E.L., Harris W.S., DiNicolantonio J.J., Elagizi A., Milani R.V., Lavie C.J., et al. Sea change for marine omega-3s: randomized trials show fish oil reduces cardiovascular events. Mayo. Clin. Proc. 2019;94(12):2524–2533. doi: 10.1016/j.mayocp.2019.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.