Abstract
Background
Sestrins (SESN1-3) act as proximal sensors in leucine-induced activation of the protein kinase mechanistic target of rapamycin (mTOR) in complex 1 (mTORC1), a key regulator of cell growth and metabolism.
Objective
In the present study, the hypothesis that SESNs also mediate glucose-induced activation of mTORC1 was tested.
Methods
Rats underwent overnight fasting, and in the morning, either saline or a glucose solution (4 g⋅kg-1 BW/10 mL⋅kg-1) was administered by oral gavage; mTORC1 activation in the tibialis anterior muscle was assessed. To further assess the mechanism through which glucose promotes mTORC1 activation, wild-type (WT) HEK293T and HEK293T cells lacking either all 3 SESNs (SESNTKO) or hexokinase 2 (HK2KO) were deprived of glucose, followed by glucose addback, and mTORC1 activation was assessed. In addition, glucose-induced changes in the association of the SESNs with components of the GAP activity toward the Rags (GATOR2) complex and with hexokinase 2 (HK2) were assessed by co-immunoprecipitation. One- and two-way ANOVA with Tukey post hoc comparisons were used.
Results
Glucose administration to fasted rats promoted mTORC1 activation. Similarly, glucose readdition (GluAB) to the medium of glucose-deprived WT cells also promoted mTORC1 activation. By contrast, SESNTKO cells demonstrated attenuated mTORC1 activation following GluAB compared with WT cells. Interestingly, HK2 associated with all 3 SESNs in a glucose-dependent manner, i.e., HK2 abundance in SESN immunoprecipitates was high in cells deprived of glucose and decreased in response to GluAB. Moreover, similar to SESNTKO cells, the sensitivity of mTORC1 to GluAB was attenuated in HK2KO cells compared with WT cells.
Conclusions
The results of this study demonstrate that the SESNs and HK2 play important roles in glucose-induced mTORC1 activation in HEK293T cells. However, unlike leucine-induced mTORC1 activation, the effect was independent of the changes in SESN-GATOR2 interaction, and instead, it was associated with alterations in the association of SESNs with HK2.
Keywords: skeletal muscle, glucose, metabolism, mTORC1, nutrient signaling
Graphical abstract
Introduction
Glucose is the main energy source for the brain [1] and red blood cells [2]. In addition, glucose is an important source of energy for skeletal muscles, especially during exercise [3]. Indeed, during intense exercise, the contribution of blood glucose to energy production in muscle increases more than four-fold. However, glucose not only is important for energy production but also is an important signaling molecule. For example, glucose stimulates the mechanistic target of rapamycin (mTOR) in complex 1 (mTORC1)—the master regulator of protein synthesis [4]. Traditionally, glucose-induced stimulation of mTORC1 was believed to be an indirect effect of inhibition of AMP-activated protein kinase (AMPK). However, recent findings suggest that glucose also acts through AMPK-independent mechanisms to activate mTORC1 [5,6]. Interestingly, those studies suggest that the signaling pathway through which glucose acts to stimulate mTORC1 may overlap with the one through which leucine activates the kinase.
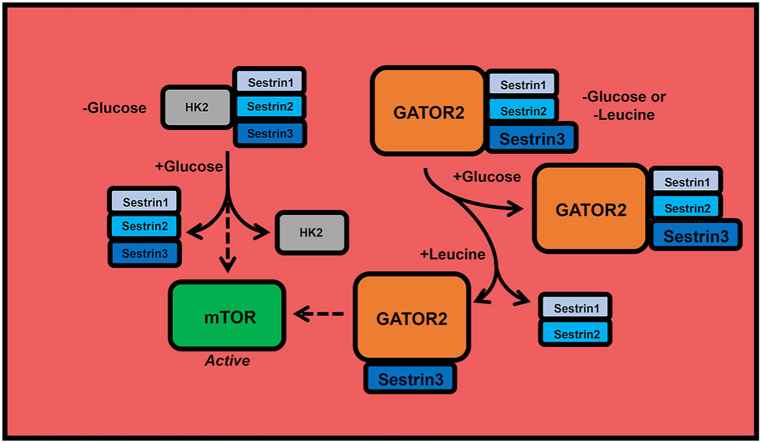
Although much research has been conducted investigating amino acid-induced mTORC1 activation [[7], [8], [9]], little is known about the mechanism through which glucose acts to promote mTORC1 activation. However, recent studies suggest that similar to leucine-induced activation of mTORC1, glucose signaling to mTORC1 involves the Sestrin (SESN1-3) proteins. Thus, in cells that lack all 3 SESN proteins (SESNTKO), mTORC1 activity is relatively insensitive to the changes in glucose availability [10]. The SESNs are a family of stress-sensing proteins [11] that were originally implicated in redox balance [12] and were shown to interact with AMPK [13]. More recently, SESNs 1 and 2 were shown to bind leucine, and leucine activates mTORC1 by promoting dissociation of the SESNs from the mTORC1 repressor complex referred to as GTPase activating protein (GAP) activity toward ras-related GTP-binding proteins (Rags) 2 (GATOR2). However, the mechanism through which glucose might act to promote signaling through the SESN-GATOR2 pathway has not been defined. For example, whether glucose promotes dissociation of the SESNs from GATOR2 as leucine does has not been explored. More importantly, the protein(s) that act upstream of the SESNs to promote glucose-induced mTORC1 activation has not been identified.
The present study tested the hypothesis that SESN3 is a glucose sensor that regulates mTORC1 activation. The hypothesis was based on studies showing that SESN1 and SESN2 appear to function as leucine sensors [10], SESN2 protein content is low in skeletal muscles (14), and SESN3 has been associated with metabolic diseases [15]. We show that glucose-induced activation of mTORC1 is blunted in SESNTKO cells and occurs independent of the changes in SESN association with GATOR2. We also show that glucose addback (GluAB) to glucose-deprived cells disrupts the association of hexokinase 2 (HK2) with all 3 SESNs and that in cells lacking HK2 (HK2KO), GluAB-induced activation of mTORC1 is severely attenuated. Overall, the results are consistent with a model in which HK2 acts to modulate SESN function to alter mTORC1 activation in response to the changes in glucose availability.
Methods
Cell culture
C2C12 (mouse skeletal muscle; cat. #CRL-1772, ATCC) and wild-type (WT) cells, Hexokinase2 knockout (HK2KO), and SESN triple-knockout (SESNTKO) HEK293T (human embryonic kidney cells expressing the SV40 large T antigen; cat. #CRL-3216, ATCC) cells were cultured in DMEM containing 25 mM glucose (cat. # 11965092; Gibco) and 10% FBS (EqualFETAL, cat. # EF-0500-A, Atlas Biologicals) without antibiotics. SESNTKO cells were a gift from Dr. David Sabatini [16], and knockout was confirmed in our laboratory by using Western blot analysis (Supplemental Figure 1). HK2KO cells were generated by using the pLentiCRISPR v1-shHK2_2 plasmid, which was a gift from Dr. Jason Cantor (Addgene plasmid #163461; http://n2t.net/addgene:163461; RRID:Addgene_163461) with the guide TAAGCGGTTCCGCAAGGAGA. Cells were removed from liquid nitrogen storage, maintained in T150 flasks at 37°C and 5% CO2, passaged twice before use, and plated on CellBIND plates (cat. # 3295, 3335; Corning) to cell densities that resulted in 80% to 90% confluency 48 hours after plating. C2C12 cells were differentiated from myoblasts to myotubes by replacing the media with DMEM supplemented with 0.5% FBS for 5 days.
Cell treatments
Transfected, as well as nontransfected, HEK293T WT, HK2KO, and SESNTKO cells were washed once with their respective treatment and then incubated in either 1) complete media (CM): DMEM, no glucose (cat. # 11966025, Gibco) supplemented with glucose to a final concentration of 10 mM for 3 hours, 2) minus glucose (−Glu): DMEM, no glucose for 3 hours, or 3) Glucose addback (GluAB): DMEM, no glucose for 2.5 hours, and then, glucose was added to the medium to a final concentration of 10 mM for 30 minutes. The glucose concentration and times for deprivation and addback were selected based on preliminary experiments assessing the time course for glucose deprivation and addback and glucose concentration on mTORC1 activation (Supplemental Figure 2). FBS was not present in media with the exception of Figure 1F. For the study depicted in Figure 1F, C2C12 myoblasts and myotubes were incubated in CM (25 mM glucose and 0.8 mM leucine) for 3 hours, glucose (−Glu)- or leucine-deprived (−Leu; custom, R&D Systems) media for 3 hours, or glucose- or leucine-deprived media for 2.5 hours followed by the addition of glucose (GluAB; final concentration 25 mM) or leucine (LeuAB; final concentration 0.8 mM) for 0.5 hours. All conditions in Figure 1F included FBS.
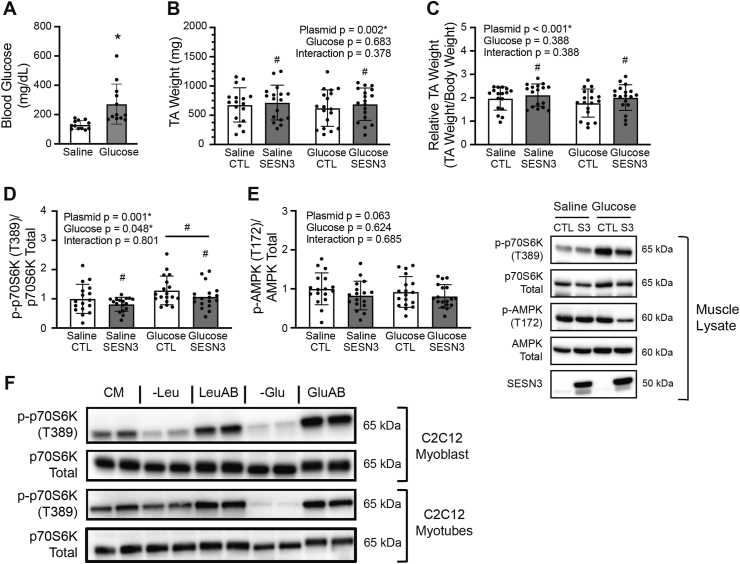
FIGURE 1.
Oral administration of glucose activates mTORC1 in skeletal muscle in vivo and following glucose addback to cells deprived of the nutrient. (A) Rat blood glucose level following saline or glucose oral gavage. (B) Absolute and (C) relative tibialis anterior muscle weight following gavage. (D) Relative phosphorylation of skeletal muscle p70S6K on T389 and (E) AMPK on T172; representative blots are pictured to the right. (F) Nutrient availability effects on mTORC1 activation in C2C12 myoblasts and myotubes. “CTL”—Control, “SESN3/S3”—Sestrin3. Values are presented as mean ± SD. ∗ P < 0.050 between groups. “#” represents a significant main effect (P < 0.050) between 2 conditions. Numerical data, experiment number, replicate number, and n-size are detailed in Supplemental Table 2.
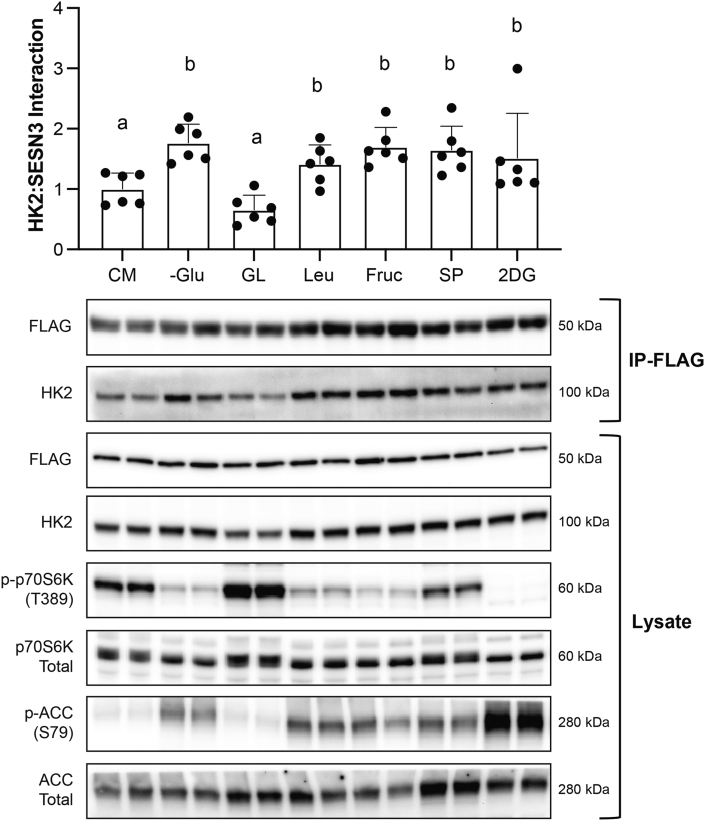
Specific to Figure 4, cells were treated with CM, −Glu, −Glu for 2.5 hours followed by the addition of glucose and leucine (final glucose concentration 10 mM, final leucine concentration 2.8 mM), leucine (Leu; final concentration 2.8 mM), fructose (Fruc; final concentration 10 mM), sodium pyruvate (SP; final concentration 2 mM), or 2-deoxy glucose (2DG; final concentration 10 mM) for 0.5 hour.
FIGURE 4.
The Sestrin3 (SESN3):Hexokinase2 (HK2) interaction in HEK293T cells is affected by glucose specifically. HK2 interaction with SESN3 in response to different nutrients. Differing letters denote significant differences (P < 0.050) between groups. Representative images are shown below the figure and in duplicate. Images from immunoprecipitated (IP) samples are listed next to “IP-FLAG,” whereas cell lysate samples are listed next to “Lysate.” All values were made relative to the average of CM and are presented as mean ± SD. Numerical data, experiment number, replicate number, and n-size are detailed in Supplemental Table 2.
Cell transfection
HEK293T cells were transfected by using Lipofectamine 2000 (cat. # 11668019, Invitrogen) with plasmids expressing FLAG-METAP2 (pRK5-FLAG-METAP2; cat. no. 32004, Addgene, a gift from Dr. David Sabatini), FLAG-SESN1 (pRK5-FLAG-SESN1; cat. no. 72594, Addgene; a gift from Dr. David Sabatini), FLAG-SESN2 (pRK5-FLAG-SESN2; cat. no. RC-201386, Origene), FLAG-SESN3 (pRK5-FLAG-SESN3; cat. no. 72592, Addgene; a gift from Dr. David Sabatini), or FLAG-PRK5 Empty Vector. Lipofectamine was mixed with Opti-MEM (cat. # 31985070, Gibco), whereas each plasmid was mixed in a different tube of Opti-MEM according to the manufacturer’s instructions. Each respective tube equilibrated for 5 minutes. Following equilibration, tubes were combined, mixed by gentle inversion, and incubated for 20 minutes at room temperature. During the 20-minute incubation, plating medium was removed from the cells, and the cells received Opti-MEM Reduced Serum Medium (ThermoFisher Scientific) at half the amount required for that specific dish. Following the 20-minute incubation, the other half of the required medium was supplied by the plasmid-lipofectamine mixture in a drop-wise fashion. Cells were returned to the incubator for 6 hours. Thereafter, an equal volume of DMEM containing 20% FBS (final FBS in dish was 10%) was added to the cells and returned to the incubator until the following morning.
Cell lysate preparation
Following treatments, cells were washed once with ice-cold PBS and lysed with CHAPS (3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate) lysis buffer: 40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA·Na2, 10 mM sodium pyrophosphate, 10 mM β-glycerophosphate disodium salt hydrate, 50 mM sodium fluoride (NaF), 0.3% CHAPS, 1 mM Microcystin-LE, 200 mM sodium vanadate, and 10 μL·mL-1 Protease Inhibitor Cocktail (cat. # P-8340, Sigma-Aldrich, St. Louis, MO). The lysate was then centrifuged at 13,400 x g for 10 minutes at 4°C. The supernatant was collected and placed in a new tube. A Bradford protein assay (cat. # 5000006, Bio-Rad) was performed on a dilution of each sample according to the manufacturer’s protocol, and the samples were standardized for protein content. Whole lysate samples were diluted in a 4X SDS sample buffer and boiled for 5 minutes at 100°C.
FLAG immunoprecipitation
Anti-FLAG M2 magnetic beads (cat. # M8823, Millipore) were washed thrice in 5 volumes CHAPS wash buffer: 40 mM HEPES (pH 7.5), 120 mM NaCl, 1 mM EDTA·Na2, 10 mM sodium pyrophosphate, 10 mM β-glycerophosphate disodium salt hydrate, 50 mM NaF, and 0.3% CHAPS. FLAG beads were then rocked in CHAPS lysis buffer for 1 hour at 4°C; beads were captured by using a magnetic stand and resuspended in a volume of CHAPS lysis buffer equal to the bead volume. Lysates standardized for protein content were added to FLAG beads, and the samples were rocked for 2 hours at 4°C. The beads were then washed once in CHAPS lysis buffer followed by 2 washes with CHAPS wash buffer. Beads were then incubated with 3X FLAG Peptide (cat. # F4799, Sigma-Aldrich) in CHAPS lysis buffer for 1 hour at 4°C. During the incubation period, manual vortexing was performed every 10 minutes to agitate the beads. The beads were collected by using a magnetic rack. It was determined that eluting the beads with SDS sample buffer for retrieval of FLAG beads resulted in non-specific bands during Western blot analysis that interfered with the detection of HK2. Eluting the beads with 3X FLAG peptide eliminated the background bands, and this procedure was used in the experiments presented herein. Immunoprecipitated samples were diluted in 4X SDS sample buffer and boiled for 5 minutes at 100°C.
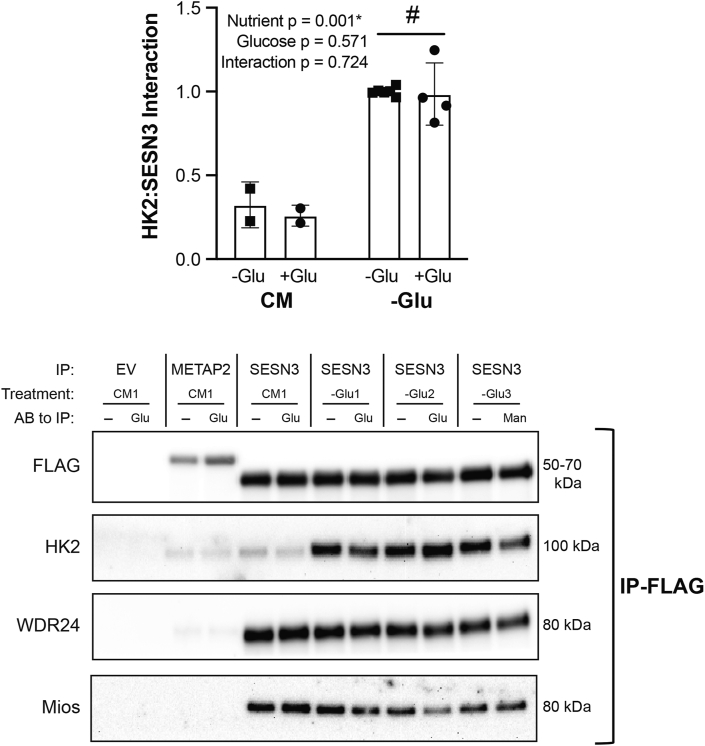
Specific to Figure 5, once lysate samples were standardized for their protein content, an equal amount of protein was placed into 2 new tubes. Both samples were diluted in CHAPS lysis buffer, and one sample received additional CHAPS lysis buffer, whereas the other received 10 mM glucose or 10 mM mannitol. Samples were rocked for 1 hour at 4°C, FLAG beads were added to each sample, and both samples were processed as described in the previous paragraph.
FIGURE 5.
Glucose does not directly modify the Sestrin3 (SESN3):Hexokinase2 (HK2) interaction. HK2 interaction with SESN3 in response to glucose addition to cell lysates from HEK293T cells. Representative images are below the figure and in duplicate. Images from immunoprecipitated (IP) samples are listed next to “IP-FLAG.” Two separate experiments were completed, and each experiment contained one CM culture dish and 3 −Glu dishes. Each dish was split, and one duplicate received no glucose (−Glu), whereas the other received 10 mM glucose (+Glu). The −Glu/+Glu condition only contains 4 samples as the other 2 samples received mannitol. All values were made relative to the average of −Glu and are presented as mean ± SD. # represents a significant main effect (P < 0.05) between 2 conditions. Numerical data, experiment number, replicate number, and n-size are detailed in Supplemental Table 2.
Animal research design and in vivo electroporation
The animal facilities and experimental protocols were reviewed and approved by the Institutional Animal Care and Use committee of the Penn State College of Medicine (approval number PRAMS200746928). The following 3 different cohorts of male Sprague–Dawley rats were used: an initial cohort of 12 rats in which the tibialis anterior muscle (TA) was transfected with a plasmid expressing pmaxGFP (cat. no. VCA-1003, Lonza), a second cohort of 16 rats were transfected with a plasmid expressing FLAG-METAP2, and a third cohort of 7 rats were transfected with a FLAG-empty vector. In all 3 cohorts, the TA in the contralateral leg was transfected with a plasmid expressing FLAG-SESN3 as described below. Given that there was no statistical difference between the cohorts for p70S6K activation in the control muscle (P > 0.1), the cohorts were combined as shown in Figure 1. Notably, Figure 1 depicts the results combining all animals, and the control plasmids are noted as “Control/CTL.”
Animals were maintained on a 12:12 hours light:dark cycle with a standard diet (Rodent Chow 8604 (calories from protein—32%, fat—14%, and carbohydrate—54%); Harlan-Tekland) and water, provided ad libitum for 2 weeks. Male Sprague–Dawley rats aged 3 to 4 months weighing 250 to 550 g body weight (Charles River Laboratories) were anesthetized by using isoflurane and then underwent in vivo electroporation, as previously described [14,17]. Briefly, both lower hind limbs were shaved and cleaned with 70% ethanol, and a control plasmid (FLAG-empty vector, FLAG-METAP2, or pmaxGFP) was injected into the TA of one hind limb, and a plasmid expressing FLAG-SESN3 was injected into the TA of the contralateral limb. Plasmids were prepared by using ZymoPURE II Plasmid Gigaprep Kit (cat. # D4204, Zymo Research) or ZymoPURE II Plasmid Maxiprep Kit (cat. # D4203, Zymo Research), and 100 μL of plasmid concentrated at 2 mg·mL-1 in manufacturer-supplied elution buffer was injected into the TA. Once the plasmid was injected, an electroporator (model 830, BTX) delivered square-wave impulses through caliper electrodes (model 384, BTX). The electrodes were coated in conductive gel, and one electrode was applied to the skin, superficial to the TA, whereas the other was placed to the skin, superficial to the gastrocnemius. Pulse trains were delivered at 200 V·cm-1 (8 pulses, 20 ms·pulse-1, 1 Hz, 1 second delay between pulses). If the animal’s limb moved out of the electrodes during the process, then the process was repeated. After recovery from anesthesia, animals were returned to their housing for 3 days. However, 12 hours before the experimentation, animals were transferred to cages without food. Following fasting, rats were given either a glucose gavage (4 g.kg-1 body weight, 10 mL⋅kg-1 in 0.9% saline) or 0.9% saline gavage of equal volume. 30 minutes after gavage, animals were anesthetized by using isoflurane; both TA muscles were removed, blood was collected for glucose analysis, and the animal was killed by excision of the diaphragm. Muscles were immediately homogenized in CHAPS lysis buffer and prepared as described under “Cell Lysate Preparation.”
Western blot analysis
Samples were prepared and analyzed similarly as described in the previous publications [18,19]. Briefly, samples underwent SDS-PAGE on 10% Criterion Tris-HCl Precast Gels (Bio-Rad) for 70 minutes at 150 V by using 1X Tris/glycine/SDS buffer (cat. # 1610732, Bio-Rad). Gels were then transferred for 2 hours at 40 V by using ice-cold CAPS buffer (pH 11.0) to a polyvinylidene difluoride (PVDF) membrane. If applicable, membranes were stained with Ponceau S to quantify the total protein. Membranes were then blocked in 5% non-fat milk powder in Tris-buffered saline with 0.1% Tween-20 (TBST). Following blocking, membranes were washed 3x for 5 minutes each, with TBST and incubated in primary antibody diluted in TBST with 0.1% sodium azide. Blots were incubated in primary antibodies overnight at 4°C. Details about the antibodies used are presented in Supplementary Table 1, and the specificity of the SESN and HK2 antibodies is shown in Supplemental Figure 1. The following day, membranes were washed 3 x 5 minutes in TBST and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibody in 5% non-fat milk powder for 1 hour at room temperature. Following secondary antibody incubation, another 3 x 5 minutes wash in TBST was performed, and membranes were visualized with Clarity Western ECL blotting substrate (cat. 1705061, Bio-Rad) by using a Fluorchem M (Bio-Techne–Protein Simple). Blots were quantified by using ImageJ software (National Institutes of Health), whereby each band was outlined with a box; the same size box was used for all bands on the blot, and the background was then determined for each band by quantifying the same kDa area above or below each band with the same sized box. The band and background values for each sample/lane were inverted by deducting 255 from the obtained value, and the background was deducted from each band to give a final result.
Statistical analyses
An a-priori power analysis was completed and suggested that 6 animals per group was adequate by utilizing α = 0.05 and β = 0.80. This was based on preliminary data collected in house by using phosphorylated p70S6K because this was our primary outcome variable. Post hoc power analysis demonstrated that we were well-powered to detect an effect. By using the effect of glucose on phosphorylated p70S6K (Figure 1), there was a partial 2 = 0.414 that gave a post hoc power of 1.000. In conjunction, by using the effect of glucose on the HK2-SESN3 interaction (Figure 3), there was a 2 = 0.390 that gave a post hoc power of 0.787.
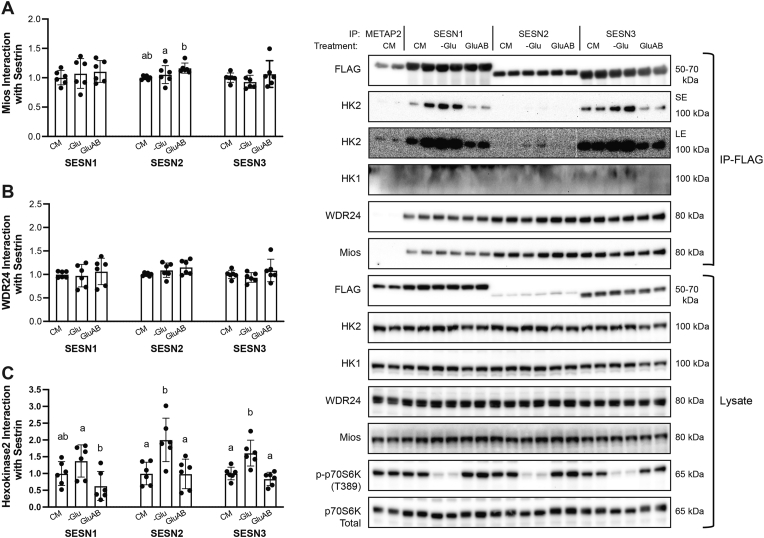
FIGURE 3.
Sestrin (SESN) interaction with Hexokinase2, but not GATOR2, is influenced by glucose availability in HEK293T cells. Interaction of the GATOR2 proteins (A) Mios and (B) WDR24, respectively, and (C) hexokinase 2 (HK2) with FLAG-tagged SESNs in response to glucose deprivation and readdition. Differing letters denote significant differences (P < 0.050) between groups within a SESN. Representative images for all conditions are adjacent to the panels and are in duplicate. Images from immunoprecipitated (IP) samples are listed next to “IP-FLAG,” whereas cell lysate samples are listed next to “Lysate.” All values were made relative to the average of each respective SESN’s CM and are presented as mean ± SD. “SE”, short exposure. “LE”, long exposure. Numerical data, experiment number, replicate number, and n-size are detailed in Supplemental Table 2.
Statistics were performed by using SPSS version 28.0 (IBM) or GraphPad Prism 9.2 (GraphPad Software). Shapiro-Wilk tests were used to determine the normality for all dependent variables. Bartlett’s tests for equality of variances were used to ensure equal variances. Outliers were determined by using the ROUT method with a Q value set to 0.5% and when the value was also ± 2 SD from the mean. n sizes and technical and biological replicates are summarized in Supplementary Table 2.
If 2 separate groups were being compared (e.g., rats gavaged with saline vs glucose), an independent samples t-test was utilized. In FIGURE 2, FIGURE 6 comparing WT and SESNTKO or HK2KO cells, a two-way ANOVA was utilized comparing cell line (WT vs. SESNTKO or HK2KO) and time point (−Glu, 1, 5, 10, 20, and 30 minutes). If a significant interaction was found, the cell lines were compared at each time point by using a Tukey post hoc comparison. These statistics are listed under the significant interaction and denote where the interaction existed. For glucose addition to cell lysate experiments, a two-way ANOVA was used comparing nutrient (CM, −Glu) and glucose addition (−Glu, +Glu). For glucose gavage experiments comparing transfected legs, a repeated measures ANOVA was utilized comparing glucose as a between factor (saline, glucose) and plasmid/leg as a within factor (CTL, SESN3). In FIGURE 3, FIGURE 4, a one-way ANOVA was used to test the molecular interactions within a plasmid on immunoprecipitated samples. If a significant ANOVA was found, then a Tukey post hoc test was used to determine significant group differences. All data are presented as means ± SD, and the α-level was set at P < 0.050. If blots are present with no accompanying figure, then statistics were not computed, and the blots are stand-alone.
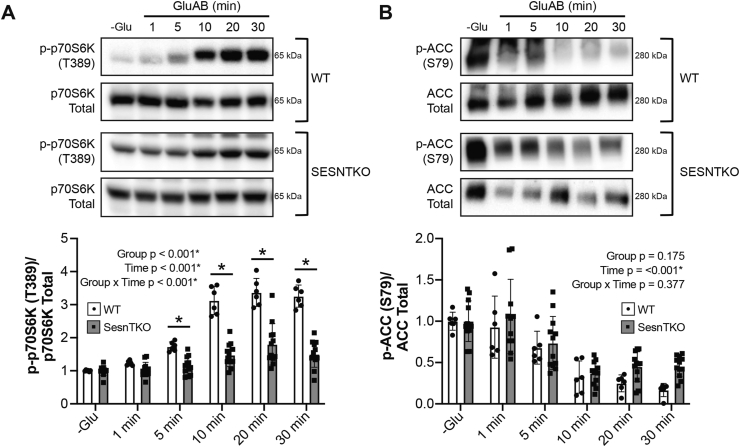
FIGURE 2.
Sestrin-deficient HEK293T cells demonstrate blunted mTORC1 activation in response to glucose. (A) Relative phosphorylation of p70S6K on T389 and (B) ACC on S79 in response to glucose readdition following glucose deprivation in wild-type and Sestrin triple-knockout cells. Representative blots accompany each figure. Values are presented as mean ± SD. ∗ P < 0.050 between groups. Numerical data, experiment number, replicate number, and n-size are detailed in Supplemental Table 2.
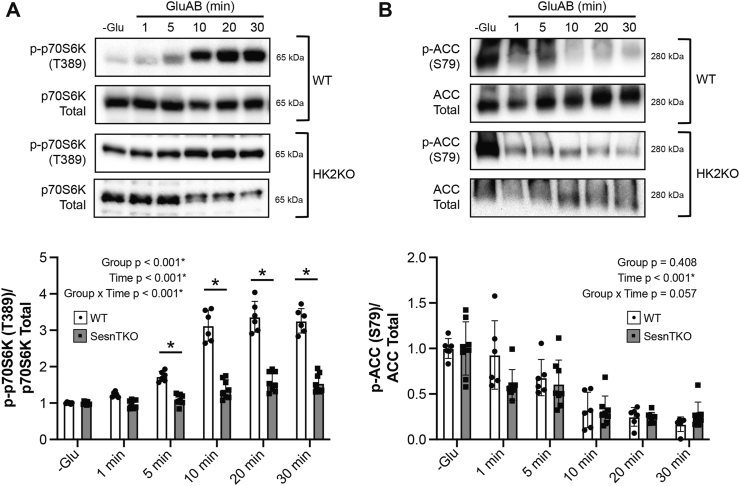
FIGURE 6.
Hexokinase2 knockout HEK293T cells demonstrate blunted mTORC1 activation in response to glucose in HEK293T cells. (A) Relative phosphorylation of p70S6K on T389 and (B) ACC on S79 in response to glucose readdition in wild-type (WT) and hexokinase2 knockout cells (HK2KO). The data obtained from WT cells in Figure 2A, B were reused for Figure 6A, B, respectively. For WT cells, 3 separate experiments containing 2 experimental replicates for each experiment were utilized. For the HK2KO cells, 2 separate experiments containing 4 experimental replicates for each experiment were utilized. Values were made relative to the average of the −Glu condition for each cell line (WT or SesnTKO) and are presented as mean ± SD. Representative blots accompany each figure. ∗ P < 0.050 between groups. Numerical data, experiment number, replicate number, and n-size are detailed in Supplemental Table 2.
Results
To assess a possible role for SESN3 in glucose-induced mTORC1 activation in skeletal muscle, the TA in one rat hindlimb was transfected with a plasmid expressing SESN3 and the TA in the contralateral hindlimb was transfected with a control plasmid. Three days later, the animals were administered either a solution containing or lacking glucose by oral gavage. As expected, blood glucose levels were higher in rats administered glucose compared with control animals (Figure 1A). Both absolute (Figure 1B) and relative (Figure 1C) TA weights were slightly but notably greater in the SESN3-transfected legs compared with the contralateral control muscles. Glucose administration increased mTORC1 activation (Figure 1D) but had no significant effect on AMPK activation (Figure 1E). Interestingly, overexpression of SESN3 was associated with reduced mTORC1 activation and a trend toward decreased AMPK activation (P = 0.063).
The activation of mTORC1 that occurred in response to glucose gavage may have, in part, been because of the glucose-induced increases in the insulin levels. In an attempt to prevent glucose-induced insulin release from pancreatic β-cells, rats were administered somatostatin before glucose gavage. However, in agreement with the results of a previous study [20], somatostatin was ineffective at preventing glucose-induced increases in insulin levels in rats (Supplemental Figure 3). Therefore, to assess insulin-independent glucose-induced changes in mTORC1 activation, C2C12 myoblasts and myotubes were deprived of glucose (−Glu) followed by glucose resupplementation. Deprivation (–Leu) or readdition of leucine served as a positive control. Deprivation of either leucine or glucose downregulated mTORC1 activation, whereas readdition of the deprived nutrient activated mTORC1 (Figure 1F). The effects of glucose deprivation and readdition on mTORC1 activation were independent of the cell type because changes in glucose availability had the same effect on mTORC1 activation in HEK293T cells (Supplemental Figure 2) as it did in C2C12 myoblasts and myotubes (Figure 1F).
To delineate the role of the SESNs in mediating glucose-induced changes in mTORC1 activation, the time course of mTORC1 activation by glucose was assessed in WT and SESNTKO cells. Glucose addback to glucose-deprived cells rapidly (within 5 minutes) activated mTORC1 in WT cells, but not in SESNTKO cells (Figure 2A). GluAB repressed AMPK activation, as assessed by phosphorylation of its substrate acetyl-CoA carboxylase (ACC) on S79, with similar kinetics in both WT and SESNTKO cells (Figure 2B).
Leucine activates mTORC1 by promoting the release of SESNs 1 and 2 from GATOR2 in association with dephosphorylation of SESN2 [10,21]. In the present study, in contrast to leucine, glucose had no effect on SESN phosphorylation as assessed by changes in electrophoretic mobility in either HEK293T cells or C2C12 myotubes (Supplemental Figure 1). Furthermore, glucose deprivation and addback had no effect on the association of any of the SESNs with GATOR2, as assessed by co-immunoprecipitation of 2 different GATOR2 subunits, Mios and WDR24, with exogenously expressed FLAG-tagged SESNs, other than a small but statistically significant increase in Mios association with SESN2 in response to GluAB (Figure 3A, B). Considering that changes in glucose availability had no effect on SESN2 binding to WDR24 and that SESN association with GATOR2 has been linked to suppression rather than activation of mTORC1 [10, 22], it seems likely that the change in SESN2-Mios interaction has little, if any, functional significance regarding mTORC1 activation.
To identify other possible SESN-interacting proteins that might contribute to glucose signaling to mTORC1, HK2 was examined because it has been previously linked to glucose-induced regulation of mTORC1 [23]. Interestingly, HK2, but not HK1, was found to associate with all 3 SESNs, and this interaction was influenced by glucose availability (Figure 3C). Thus, HK2 bound most avidly to SESNs during glucose deprivation, and glucose readdition to the media resulted in dissociation of the complex. Mannitol addition to glucose-deprived cells had no effect on HK2 association with any of the SESNs, suggesting that the glucose effect was not a consequence of changes in osmolarity (unpublished observations). The HK2-SESN interaction also inversely coincided with mTORC1 activation, as assessed by the changes in phosphorylation of the mTORC1 substrate, p70S6K.
Given that SESN3 has been implicated in metabolic disorders [11,15,24,25] and that glucose alters the interaction of HK2 with all 3 SESNs, we chose to focus on SESN3 for the remainder of the studies. Notably, the glucose effect on SESN3-HK2 interaction was specific to glucose as neither leucine, fructose, sodium pyruvate, nor 2-deoxy-glucose affected the interaction (Figure 4).
To determine whether glucose directly affects the association of SESN3 with HK2, FLAG-tagged SESN3 was expressed in HEK293T WT cells; the cells were incubated in either complete medium or glucose-free medium, and then, FLAG-tagged proteins were immunoprecipitated. The immunoprecipitated sample was then incubated with or without glucose. In agreement with the results shown in FIGURE 3, FIGURE 4, glucose deprivation of the cells promoted HK2 association with SESN 3 (Figure 5). However, glucose addition to the immunoprecipitated sample had no effect on the interaction.
To further assess the role of HK2 in mediating glucose-induced mTORC1 activation, glucose activation time course experiments were completed in HK2 knockout cells (HK2KO). In contrast to WT cells, mTORC1 activation in HK2KO cells was blunted in response to GluAB (Figure 6A). Similar to SESNTKO cells, ACC phosphorylation was not different between WT and HK2KO cells, suggesting that the attenuation in mTORC1 activation was independent of AMPK activation (Figure 6B).
Discussion
In the present study, overexpression of SESN3 in the TA was associated with decreased mTORC1 activation and increased muscle weight. The decrease in mTORC1 activation was anticipated based on previous studies showing that mTORC1 activity is repressed in cells overexpressing SESN2 [10,26]. Moreover, in the liver and skeletal muscles of mice lacking both SESN2 and SESN3, mTORC1 is constitutively activated [25], suggesting that SESNs act to repress mTORC1 activity. A possible explanation for this finding is that as previously shown for SESN1 and 2 [13], overexpression of SESN3 activated AMPK and subsequently inhibited mTORC1. However, in the present study, SESN3-induced downregulation of mTORC1 activation appeared to be independent of AMPK activation because this marker trended to be repressed in the SESN3-transfected muscle. These data suggest that glucose can activate mTORC1, independent of AMPK activity. The finding that the SESN3-transfected muscles weighed more than the control-transfected muscles was unexpected because a reduction in mTORC1 activity would be expected to decrease, not increase, muscle weight. However, it is possible that overexpression affected metabolic properties to influence water retention. Although this notion is speculative, the cause of the weight discrepancy is unknown and will need to be further examined in future studies.
Compared with amino acids, few studies have focused on defining the signaling pathway through which glucose alters mTORC1 activity. One reason for this paucity of data is the assumption that suppression of AMPK is responsible for most, if not all, of the glucose-induced alterations in mTORC1 activation. However, recent studies have shown that glucose signaling to mTORC1 does not differ in cells lacking both catalytic subunits of AMPK, i.e., AMPKα1 and AMPKα2 double knockout cells, compared with wild-type cells [5,27]. Moreover, cells overexpressing the GLUT1 transporter exhibit increased glucose uptake and upregulation of mTORC1 independent of the changes in AMPK activation [28]. The results of the present study agree with the previous research and show that although GluAB represses AMPK activation with similar kinetics in WT cells and SESNTKO or HK2KO cells, the activation of mTORC1 is blunted in SESNTKO and HK2KO cells compared with WT cells. Overall, the available data strongly suggest that glucose signals to mTORC1 at least in part through an AMPK-independent mechanism(s).
Glucose availability has been shown to promote mTORC1 activation [10]; however, the mechanism through which this occurs remains relatively unexplored. Chantranupong et al. demonstrated that mTORC1 activity in SESNTKO cells was unaffected by glucose readdition, following glucose deprivation [10]. The data presented herein expand on their findings by showing when SESNTKO glucose-deprived cells are supplemented with glucose, activation of mTORC1 activity is notably attenuated. The blunted glucose effect in SESNTKO cells is unlikely to be due to a delayed response, considering that maximal activation was achieved within 10 minutes after GluAB in WT cells, and little mTORC1 activation occurred in 30 minutes in SESNTKO cells. Moreover, the attenuated mTORC1 response appears independent of AMPK activation because ACC phosphorylation was not different between WT and SESNTKO cells. Likewise, similar results were found in HK2KO cells, suggesting that the temporal response of mTORC1 to activation by glucose was maximal within 10 minutes. The results also support the conclusion that both HK2 and SESNs are required for a maximal glucose-induced mTORC1 response. Furthermore, both HK2 and SESNs affect mTORC1 activation, independent of AMPK activation, demonstrating unique mechanisms for the proteins in the activation of mTORC1.
To further assess the role that HK2 plays in glucose-induced mTORC1 activation, immunoprecipitation experiments were performed for each SESN. When cells were deprived of glucose, the binding of HK2 to each SESN increased. By contrast, GluAB led to HK2-SESN complex dissociation. Importantly, this malleable relationship was specific to HK2 because HK1 was not found in immunoprecipitated samples. HK2 contains a mTOR signaling motif (TOS motif), and this motif binds to mTORC1 [23]. Roberts et al. demonstrated that HK2 associates with mTORC1 when cells and ex-vivo tissue are deprived of glucose, and the interaction dissociates with 2-deoxy-glucose addition that stimulates mTORC1 [23]. These data, along with others [29], demonstrate HK2 as a signaling protein. The data presented herein expand on these previous findings by showing the potential involvement of SESNs in HK2 signaling. Indeed, SESNs bind to mTOR [30], and this interaction was not influenced by glucose availability or 2-deoxy-glucose in the present study (unpublished observations), suggesting that mTOR is not an intermediary between SESNs and HK2. The interaction of the GATOR2 components Mios and WDR24 with SESNs was unaltered with glucose availability as well. Previous studies have demonstrated that mTORC1 senses glucose concentration through a mechanism involving the Rag GTPases, a downstream target of GATOR2 [31]. A prominent function of SESNs is inhibiting mTORC1 through the GATOR complexes and the downstream Rags [10,22,26]. Given that SESN interaction with GATOR2 was unaffected by glucose availability, it is possible that other mechanisms exist besides signaling through the Rags or that glucose-induced alterations in GATOR2 function do not require dissociation of SESNs from the complex.
In an attempt to test the relative importance of the 3 SESNs in glucose signaling to mTORC1, shRNAs were used to individually knockdown their expression. Unfortunately, knockdown of an individual SESN was associated with changes in expression of one or more of the other SESNs (Supplemental Figure 4). For example, in SESN1 knockdown cells, SESN2 expression was reduced, whereas SESN3 expression was increased. Future studies using alternative methods to assess the role of the individual SESNs in mediating glucose signaling to mTORC1 are warranted.
In the present study, of the substrates/metabolites tested, it was found that the HK2-SESN3 interaction is only influenced by glucose. This finding potentially links the connection between metabolic disorders and SESNs. When glucose-deprived cells were treated with leucine, fructose, sodium pyruvate, or 2-deoxy-glucose, the HK2-SESN3 interaction was unaffected. Fructose and sodium pyruvate are alternative metabolic substrates that did not affect the interaction. Leucine is a potent signaling molecule and also did not affect the interaction. Although leucine affects the SESN1/2 interaction with GATOR2, it did not affect the SESN3 interaction with HK2, demonstrating a unique difference between glucose and leucine signaling to mTORC1. Notably, sodium pyruvate activated mTORC1 and 2-deoxy-glucose inhibited mTORC1; meanwhile, neither treatment affected the HK2-SESN3 interaction. Future studies are needed to delineate the magnitude of the effect the SESN3:HK2 has on mTORC1 activation.
Overall, the results of the present study are consistent with a model in which glucose deprivation downregulates mTORC1 activity by promoting HK2 association with the SESNs. How association of these proteins might lead to the downregulation of mTORC1 activity is unknown, but the effect appears to be at least partially independent of changes in AMPK activity. In future studies, the mechanism through which glucose acts to alter HK2 association with the SESNs needs to be delineated. In addition, how changes in HK2-SESN interaction modulate mTORC1 activation is a topic for further investigation.
Funding
This work is supported by NIH grants R01DK015658(SRK), R37AA011290(CHL), and F32DK126312(PAR).
Author disclosures
SRK is an editor on The Journal of Nutrition and played no role in the Journal’s evaluation of the manuscript.
Acknowledgments
The authors thank David L. Waning, Brian A. Hain, Michael D. Dennis, Siddharth Sunilkumar, William P. Miller, Ashley M. VanCleave, Shaunaci A. Stevens, Allyson L. Toro, Esma I. Yerlikaya, Christopher M. McCurry, Anne M. Pruznak, and Abigale L. Whitsell for insightful feedback and technical assistance.
The authors’ responsibilities were as follows – PAR and SRK conceived and designed research. PAR analyzed the data and prepared figures. PAR and SRK interpreted the results of experiments. PAR drafted the manuscript. All authors edited and revised the manuscript, approved final version, and assisted with data collection and performed experiments.
Footnotes
See corresponding article on page 915.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2022.11.021.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mergenthaler P., Lindauer U., Dienel G.A., Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Wijk R., van Solinge W.W. The energy-less red blood cell is lost: erythrocyte enzyme abnormalities of glycolysis. Blood. 2005;106:4034–4042. doi: 10.1182/blood-2005-04-1622. [DOI] [PubMed] [Google Scholar]
- 3.Hargreaves M., Spriet L.L. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020;2:817–828. doi: 10.1038/s42255-020-0251-4. [DOI] [PubMed] [Google Scholar]
- 4.Shao D., Villet O., Zhang Z., Choi S.W., Yan J., Ritterhoff J., et al. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat Commun. 2018;9:2935. doi: 10.1038/s41467-018-05362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orozco J.M., Krawczyk P.A., Scaria S.M., Cangelosi A.L., Chan S.H., Kunchok T., et al. Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat Metab. 2022;2:893–901. doi: 10.1038/s42255-020-0250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simcox J., Lamming D.W. The central moTOR of metabolism. Dev Cell. 2022;57:691–706. doi: 10.1016/j.devcel.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condon K.J., Sabatini D.M. Nutrient regulation of mTORC1 at a glance. J Cell Sci. 2019;132:jcs222570. doi: 10.1242/jcs.222570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony J.C., Yoshizawa F., Anthony T.G., Vary T.C., Jefferson L.S., Kimball S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 9.Mobley C.B., Haun C.T., Roberson P.A., Mumford P.W., Romero M.A., Kephart W.C., et al. Effects of whey, soy or leucine supplementation with 12 weeks of resistance training on strength, body composition, and skeletal muscle and adipose tissue histological attributes in college-aged males. Nutrients. 2017;9:972. doi: 10.3390/nu9090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chantranupong L., Wolfson R.L., Orozco J.M., Saxton R.A., Scaria S.M., Bar-Peled L., et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell Rep. 2014;9:1–8. doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.H., Budanov A.V., Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budanov A.V. Stress-responsive Sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxid Redox Signal. 2011;15:1679–1690. doi: 10.1089/ars.2010.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budanov A.V., Karin M. p53 target genes Sestrin1 and Sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D., Shimkus K.L., Lacko H.A., Kutzler L., Jefferson L.S., Kimball S.R. Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2019;316:E817–E828. doi: 10.1152/ajpendo.00522.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nascimento E.B., Osler M.E., Zierath J.R. Sestrin 3 regulation in type 2 diabetic patients and its influence on metabolism and differentiation in skeletal muscle. Am J Physiol Endocrinol Metab. 2013;305:E1408–E1414. doi: 10.1152/ajpendo.00212.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfson R.L., Chantranupong L., Saxton R.A., Shen K., Scaria S.M., Cantor J.R., et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuckow A.P., Vary T.C., Kimball S.R., Jefferson L.S. Ectopic expression of eIF2Bepsilon in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab. 2010;299:E241–E248. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberson P.A., Mobley C.B., Romero M.A., Haun C.T., Osburn S.C., Mumford P.W., et al. LAT1 protein content increases following 12 weeks of resistance exercise training in human skeletal muscle. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.628405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberson P.A., Romero M.A., Osburn S.C., Mumford P.W., Vann C.G., Fox C.D., et al. Skeletal muscle LINE-1 ORF1 mRNA is higher in older humans but decreases with endurance exercise and is negatively associated with higher physical activity. J Appl Physiol. 2019;127:895–904. doi: 10.1152/japplphysiol.00352.2019. [DOI] [PubMed] [Google Scholar]
- 20.Lang C.H., Dobrescu C. Gram-negative infection increases noninsulin-mediated glucose disposal. Endocrinology. 1991;128:645–653. doi: 10.1210/endo-128-2-645. [DOI] [PubMed] [Google Scholar]
- 21.Kimball S.R., Gordon B.S., Moyer J.E., Dennis M.D., Jefferson L.S. Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation. Cell Signal. 2016;28:896–906. doi: 10.1016/j.cellsig.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.S., Ro S.H., Kim M., Park H.W., Semple I.A., Park H., et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci Rep. 2015;5:9502. doi: 10.1038/srep09502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts D.J., Tan-Sah V.P., Ding E.Y., Smith J.M., Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521–523. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T., Niu Y., Liu S., Yuan H., Liu X., Fu L. Exercise improves glucose uptake in murine myotubes through the AMPKalpha2-mediated induction of Sestrins. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3368–3377. doi: 10.1016/j.bbadis.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.H., Budanov A.V., Talukdar S., Park E.J., Park H.L., Park H.W., et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parmigiani A., Nourbakhsh A., Ding B., Wang W., Kim Y.C., Akopiants K., et al. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014;9:1281–1291. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalender A., Selvaraj A., Kim S.Y., Gulati P., Brule S., Viollet B., et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buller C.L., Heilig C.W., Brosius F.C. GLUT1 enhances mTOR activity independently of TSC2 and AMPK. Am J Physiol Renal Physiol. 2011;301:F588–F596. doi: 10.1152/ajprenal.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts D.J., Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao R., Xiong X., Liangpunsakul S., Dong X.C. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211–1223. doi: 10.2337/db14-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efeyan A., Zoncu R., Chang S., Gumper I., Snitkin H., Wolfson R.L., et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.