Abstract
Decades of evidence across taxa have established the importance of dopamine (DA) signaling in the pFC for successful working memory performance. Genetic and hormonal factors can shape individual differences in prefrontal DA tone. The catechol-o-methyltransferase (COMT) gene regulates basal prefrontal DA, and the sex hormone 17β-estradiol potentiates DA release. E. Jacobs and M. D’Esposito [Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. Journal of Neuroscience, 31, 5286–5293, 2011] investigated the moderating role of estradiol on cognition using the COMT gene and COMT enzymatic activity as a proxy for pFC DA tone. They found that increases in 17β-estradiol within women at two time points during the menstrual cycle influenced working memory performance in a COMT-dependent manner. Here, we aimed to replicate and extend the behavioral findings of Jacobs and D'Esposito by employing an intensive repeated-measures design across a full menstrual cycle. Our results replicated the original investigation. Within-person increases in estradiol were associated with improved performance on 2-back lure trials for participants with low basal levels of DA (Val/Val carriers). The association was in the opposite direction for participants with higher basal levels of DA (Met/Met carriers). Our findings support the role of estrogen in DA-related cognitive functions and further highlight the need to consider gonadal hormones in cognitive science research.
INTRODUCTION
Working memory refers to the ability to store and manipulate mental representations. It is a key function involved in goal-directed behaviors and supports other executive functions. Since the pioneering work of Goldman-Rakic (Brozoski, Brown, Rosvold, & Goldman, 1979), numerous studies have established the critical role of dopamine (DA) signaling within the pFC for regulating delay-period activity and working memory performance. DA's influence on pFC-dependent cognitive processes follows an inverted-U function, such that too little or too much DA can hinder performance, whereas moderate levels contribute to enhanced performance (Arnsten, Wang, & Paspalas, 2015; Cools & D'Esposito, 2011).
In humans, many studies have investigated the association between DA and cognition using the catechol-o-methyltransferase (COMT) gene as a proxy for basal prefrontal DA tone. The COMT gene (codon 158) gained particular interest with respect to pFC-dependent cognitive functions because of its unique role in the metabolization of DA in the mesocortical pathway that innervates pFC. Unlike other dopaminergic projections, such as those innervating the striatum, COMT enzyme accounts for >60% of DA metabolism in pFC (Tunbridge et al., 2019; Yavich, Forsberg, Karayiorgou, Gogos, & Männistö, 2007; Männistö & Kaakkola, 1999). Thus, the COMT gene provides a useful proxy for individual differences in prefrontal DA tone—that is, a person's estimated “baseline” position on the DA-working memory inverted-U curve. Individuals homozygous for the Val allele have enhanced COMT enzyme activity and less basal DA availability in pFC, whereas those homozygous for the Met allele have reduced COMT activity leading to more DA availability (Tunbridge, Harrison, & Weinberger, 2006; Egan et al., 2001). In this way, homozygous Met carriers are thought to have enhanced DA levels under baseline conditions, whereas homozygous Val carriers are thought to have relatively less prefrontal DA. Empirical studies provide support for this classification, such that Met carriers have more COMT enzyme activity (Tunbridge et al., 2019), less D1 binding (Slifstein et al., 2008), enhanced performance, and enhanced cortical efficiency relative to Val carriers (Smith, Swift-Scanlan, & Boettiger, 2014; Cools & D'Esposito, 2011; Mier, Kirsch, & Meyer-Lindenberg, 2010; Meyer-Lindenberg et al., 2006; Egan et al., 2001).
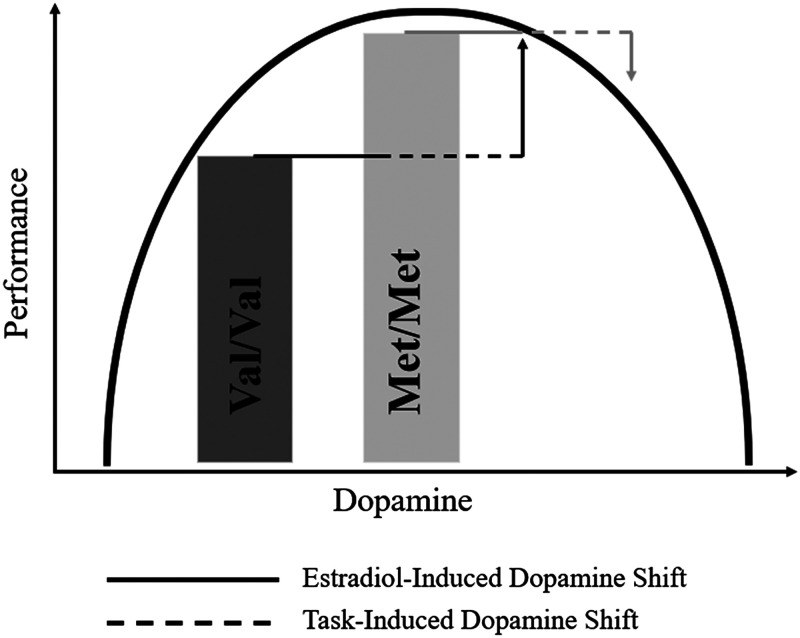
Importantly, however, the COMT gene is not deterministic of cognitive performance. Dopaminergic activity can be influenced by many factors, including sex steroid hormones. Animal studies have shown that 17β-estradiol levels (“estradiol”) stimulate DA release and turnover (Xiao & Becker, 1994; Becker, 1990), leading to greater efficiency within pFC cortical circuits. A recent multimodal PET imaging study revealed that DA synthesis capacity and cognitive flexibility differ between hormonal contraceptive users and non-users (Taylor et al., 2022). Furthermore, estrogen receptors are expressed in regions that receive major projections from midbrain DA neurons, including pFC (Björklund & Dunnett, 2007). Some evidence suggests that circulating estradiol concentrations impact working memory performance in young adult female populations,1 such that increased concentrations are associated with enhanced performance (Hampson & Morley, 2013). Note that one study found that the relationship between estradiol and working memory may follow an inverted-U curve (i.e., a quadratic effect of estradiol) in a young adult female sample; however, the sample size was small (n = 8) and the finding has not been replicated (Rosenberg & Park, 2002). Furthermore, studies examining estradiol's effect on verbal working memory in the ovulatory phase (when estradiol is heightened) are few and mixed (Bernal & Paolieri, 2022). Very few studies have considered Gene × hormone interactions that may shape cognition. Theories posit that estradiol exhibits DA-agonist effects and may therefore modulate a person's putative position on the inverted-U curve between DA and working memory performance (see Figure 1). Indeed, estradiol downregulates COMT enzyme activity by inhibiting COMT gene transcription, which may shift Val carriers to an optimal range for performance on DA-dependent cognitive tasks while hindering Met carriers (Jiang, Xie, Ramsden, & Ho, 2003).
Figure 1. .
Figure showing the modulatory role of estradiol on COMT to affect working memory performance. Increased estradiol levels lead to reductions in COMT enzyme protein expression that result in more dopamine in the pFC. The reduction in COMT enzyme activity moves people along the inverted-U curve, therefore enhancing performance for those with lower basal dopamine levels (Val/Val carriers), while hurting performance for those with higher basal levels (Met/Met carriers). Task difficulty further serves to lessen performance, pushing individuals further along the inverted U.
Although studies have highlighted the powerful role of estradiol in cognition in rodents (Shansky & Lipps, 2013; Shansky et al., 2004; Bimonte & Denenberg, 1999; Luine, Richards, Wu, & Beck, 1998), monkeys (Hara et al., 2014, 2016; Kromrey, Czoty, & Nader, 2015), and midlife women (Dumas, Makarewicz, Bunn, Nickerson, & McGee, 2018; Hampson, 2018; Jacobs & Goldstein, 2018), the literature linking sex hormones and cognition in young adults has yielded inconsistent results. Jacobs and D'Esposito (2011) propose that studies that seek to draw a relationship between menstrual cycle stage and working memory performance fail to replicate because they do not account for individual differences in basal DA levels. To test this, their study followed a within-person design, in which 24 women (13 Val/Val; 8 Met/Met; 3 Met/Val) underwent fMRI scanning at low and high estradiol phases of their menstrual cycle, while completing a verbal n-back task to measure working memory performance. Serum concentrations of 17β-estradiol were determined via liquid chromatography mass-spectrometry. COMT enzyme activity was assessed via serum, providing an individual-level proxy of prefrontal DA tone, in addition to COMT genotype. The authors found that on the 2-back condition of cognitively demanding lure trials, Val carriers had enhanced accuracy when estradiol was high versus low. In contrast, performance for Met carriers was highest when estradiol levels were low, and reduced when estradiol levels were high. These results were also reflected at the neural level. Val carriers at low estradiol levels showed increased neural activity on high demanding trials, which the authors interpreted as reduced neural efficiency. These findings were the first to support the modulating role of estradiol in the association between DA and working memory function in young adult female samples. Subsequent neuroimaging studies have extended their findings, revealing sex-dependent effects of COMT genotype on prefrontal-mediated behaviors and pFC function (Elton, Smith, Parrish, & Boettiger, 2017) as well as interactions between cycle phase and basal DA on executive functions (Hidalgo-Lopez & Pletzer, 2017).
Jacobs and D'Esposito's findings were specific to the 2-back load condition of the task, and not the 3-back condition. They posit that this is because of a “task-induced dopamine shift” related to the increased cognitive demand of the 3-back condition (see Figure 1). Higher levels of cognitive demand often place stress on the individual and cognitive system, which can also increase dopaminergic signaling (Arnsten, 2015; Shanmugan & Epperson, 2014; Shansky & Lipps, 2013; Williams & Castner, 2006). Thus, interactive effects of estradiol and DA are likely not to be consistent across levels of cognitive load and, relatedly, stress. For this reason, understanding the COMT and estradiol interaction at various difficulty levels may be important for specifying the contexts in which their interactive effects are evident.
The current study aimed to examine whether the behavioral findings reported by Jacobs and D'Esposito (2011) would replicate in a larger sample (n = 74) using the same cognitive task. The current study built upon the prior investigation by having up to four repeated assessments within a person. This allowed us to test our effects with more statistical power and use estradiol as a continuous measure to examine its predictive value more precisely for cognitive performance. In addition, our design allowed us to assess whether an individual's average estradiol levels across the menstrual cycle would impact the association between COMT and cognitive performance. The inclusion of between-centered estradiol levels allowed us to delineate between- and within-person estradiol effects more clearly. We predicted that within-person effects of estradiol would follow those reported in Jacobs and D'Esposito (2011), such that Val carriers would show improved performance when estradiol is high, whereas Met carriers would show the opposite effect. Jacobs and D'Esposito reported that this effect is specific to the 2-back condition, as the 3-back condition results in a “task-induced dopamine shift” because of increased cognitive demand and hinders performance irrespective of changes in estradiol levels. Therefore, we did not predict that there would be a COMT × Estradiol interaction on the 3-back condition.
To our knowledge, no study has examined whether between-persons levels of estradiol (i.e., average estradiol levels between people) would impact the association between COMT and cognitive performance in a young adult sample. One study in postmenopausal women did not find a COMT × Estradiol Level (between-groups) interaction (Dumas et al., 2018) on n-back performance. Another study completed a between-groups analysis investigating the effect of COMT on women pre- and postmenopause (which they defined as hormonal status). They found that hormonal status moderated the effects of COMT, such that there was no effect of COMT in women premenopause, whereas there was a distinct effect of COMT in the expected direction in women postmenopause (Papaleo, Sannino, Piras, & Spalletta, 2015). Critically, these findings did not consider menstrual cycle phase in the premenopausal women. Combined, these findings suggest that between-persons levels of estradiol may not evidence the expected COMT × estradiol interactive effect in a young adult sample. In addition, because of research indicating that within-person changes in estradiol in particular influence DA tone, we did not expect that between-persons levels of estradiol would moderate the association between COMT and cognitive performance. Examining this hypothesis allowed us to further specify whether the estradiol's modulating effect is specific to within-person changes.
METHODS
Participants
Data from a subset of these participants have been reported elsewhere to answer a different research question involving anxiety and not estradiol (Louis, D'Esposito, & Moser, 2021). The findings below are, therefore, results from a novel set of research aims and analyses. The sample consisted of individuals with a consistent pattern of menstrual cycles between 22 and 32 days (considered the typical cycle length). In addition, participants could not be using hormonal contraceptives (including pill, patch, or intrauterine device), as they affect the fluctuation of sex steroid hormones across the menstrual cycle. Furthermore, participants could not have a previous diagnosis that affected the neuroendocrine system (e.g., polycystic ovary syndrome, endometriosis) or be on any psychotropic medication. One hundred thirty-nine individuals were genotyped for the COMT Val158/Met (rs4680) polymorphism. Of these, 74 of them were homozygous allele carriers (33 Met/Met and 41 Val/Val carriers), which comprised the final sample included in the analyses.
The mean age was 20.63 (SD = 1.60). The sample mostly consisted of individuals who identified as White (63%), followed by individuals who identified as Black (24%), Asian (8%), and those who identified as more than one race (5%). For gender identity, one participant identified as nonbinary, and the remainder of the sample identified as women. Most of the sample consisted of those who completed partial college (55%); however, many had a college level education (32%), completed a high school level of education (12%), or had a graduate level education (1%). For income levels, 49% of the sample reported an annual household income of $50,000 or less, whereas 51% of the sample reported an annual household income > $50,000. Most of the sample consisted of students enrolled full or part time (78%), and most of the sample reported being financially supported by someone else in the past year (71%).
Materials
n-back
Working memory was measured with the verbal n-back task (Jacobs & D'Esposito, 2011; Kirchner, 1958). For each trial, letters were presented sequentially for 1000 msec. Participants were tasked with responding to each letter by identifying whether the letter was presented n trials back. The task consisted of three conditions—0-, 2-, and 3-back load. The n-back consisted of 320 trials. The 0-back load condition consisted of 160 trials (targets: 128; nontargets: 32), and 2- and 3-back conditions consisted of 80 trials each (targets: 52; nontargets: 16; lures: 12. For the 0-back load condition, participants were asked to identify the letter “X” as a “target” (left button press) when it appeared on the screen, and respond to any other letters as nontargets (right button press). On 2- and 3-back conditions, memory load was manipulated by asking participants to respond to a letter based on whether the letter presented n-trials prior. For instance, on a 2-back load condition, a “target” (i.e., the correct response) is a letter that was presented two trials prior, whereas a “nontarget” (i.e., incorrect response) would be a letter that was not presented two trials back. Furthermore, 2- and 3-back conditions included lure trials. Lure trials are those in which a familiar letter is presented an incorrect number of trials back. Lure trials add an additional “load” complexity, as they require participants to not only remember the sequence of letters that were presented prior, but also require them to inhibit a prepotent response to seeing a previously presented letter. The analyses included RTs for correct responses only. We excluded trials with RTs that were below 200, and we excluded observations in which participants had an accuracy of less than 30% across all trial types.
COMT and Estradiol Analysis
As part of the larger investigation, participants provided daily assays of 1.8 mL of saliva using the passive drool method across the full length of their menstrual cycle within 30 min of waking. Participants were asked to keep completed samples in their own personal freezer. During in-person lab visits, participants provided their saliva samples which were then transferred to a -80F degree freezer. All the samples were sent to Salimetrics LLC (State College, PA) to assay estradiol levels. On a separate occasion, one saliva sample for each participant was shipped to CD Genomics (Shirley, NY) to extract COMT Val158/Met (rs4680) polymorphisms using SNaPshot Multiplex System for SNP Genotyping. All participants' observed genotype frequencies were in Hardy-Weinberg equilibrium (χ2= .53, df = 1 ns), indicating no significant difference from the expected frequencies. Of the 139 participants, 74 participants were homozygous allele carriers (33 Met/Met, 41 Val/Val).
Procedure and Data Analysis
As part of the larger investigation, participants provided saliva samples daily to capture changes in hormones across 35 days to capture the full length of the menstrual cycle. Participants also completed four in-person laboratory visits that were meant to correspond with different phases of their menstrual cycle to complete the n-back task. In the analyses reported below, estradiol levels from the four laboratory visits were included in the analyses.
The analyses were completed using the “lme4” package (Bates, Mächler, Bolker, & Walker, 2014) in R Version 3.5.1. To examine whether changes in estradiol levels moderated the association between COMT and performance, estradiol was within-person centered. To do this, a mean was computed for each participant and subtracted from each of their own observations (Hoffman & Stawski, 2009). We also examined the effect of average levels of estradiol between people, by computing a mean for each participant, which was used to compute an overall mean (i.e., mean of means). The overall mean was subtracted from each participant's mean value. This approach provides an estimate of between-persons average differences in estradiol levels. In the final models, COMT was included as an effects-coded predictor, estradiol levels within-person centered and between-persons centered as main effects, as well as a COMT × Within-Person Estradiol and COMT × Between-Persons Estradiol interactions. We did not include the three-way interaction. All models also included time (up to four laboratory visits) as an effect-coded predictor to control for practice effects, and 0-back performance to control for their accuracy on the task. The final models did not include random slopes and only included a random intercept, which calculated a mean score (either RT or accuracy) for each participant. To break down effects, we dummy-coded COMT. Because we had a precise directional hypothesis for Met and Val carriers, when breaking down interactions, we also report the p values for a one-tailed simple slope test for the effect of interest. For all models, assumptions were examined, and Cook's distance was computed to assess for leverage.
To determine whether our sample size was adequate to answer the question of interest, we computed the effect size for the behavioral effects reported in Jacobs and D'Esposito (2011), which revealed a large effect for the behavioral findings on 2-back lures (η2 = .19). Using G*Power, we determined that for models examining a COMT × Within-Person Centered Estradiol interaction, we were powered to detect small effects of RTs and accuracy on 2- and 3-back lure conditions (ηp2's ranging between .02 and .03). For models examining a COMT × Between-Persons Estradiol interaction, we were powered to detect small-to-medium effects of RTs and accuracy on 2- and 3-back load conditions (ηp2's ranging between .05 and .07). We proceeded with the knowledge that we were able to detect a small COMT × Estradiol interaction with this sample size, which is more than adequate for the size of the effect reported in Jacobs and D'Esposito (2011). Below, partial eta squared ηp2 is reported to estimate effect size with .05 representing a small effect, .1 a medium effect, and .2 a large effect (Cohen & Taylor, 1973).
RESULTS
There was no difference in average estradiol levels between Met (M = 1.29 pg/mL, SE = .09) and Val (M = 1.51 pg/mL, SE = .08) carriers (p = ns).
As previously reported with this sample (Louis et al., 2021), participants' behavioral results replicated those found in Jacobs and D'Esposito (2011) for load and trial type manipulations. Load effects on RT (ηp2 = .28) revealed that participants were significantly faster on 0-back than all other load conditions (ps < .001), whereas 2- and 3-back were not significantly different from each other (p = .62). Load effects for accuracy (ηp2 = .16) demonstrated that participants were significantly more accurate on 0-back than other load conditions (ps < .001). In addition, participants were more accurate on 2-back than they were on 3-back trials (p < .001). Results for trial type (ηp2 = .29) revealed participants were slower on lures than nontargets and targets (ps < .001). In addition, participants responded more slowly to targets than nontargets (p < .001). Similarly, accuracy was significantly different across trial types (ηp2 = .44). Participants were significantly less accurate on lures than nontargets and targets (ps < .001). They were also significantly less accurate on targets compared with nontargets (p < .001). In summary, participants responded more slowly and were less accurate on lure trials, replicating that this task condition may have been more cognitively demanding. Therefore, the analyses below solely focus on lure trials.
Accuracy
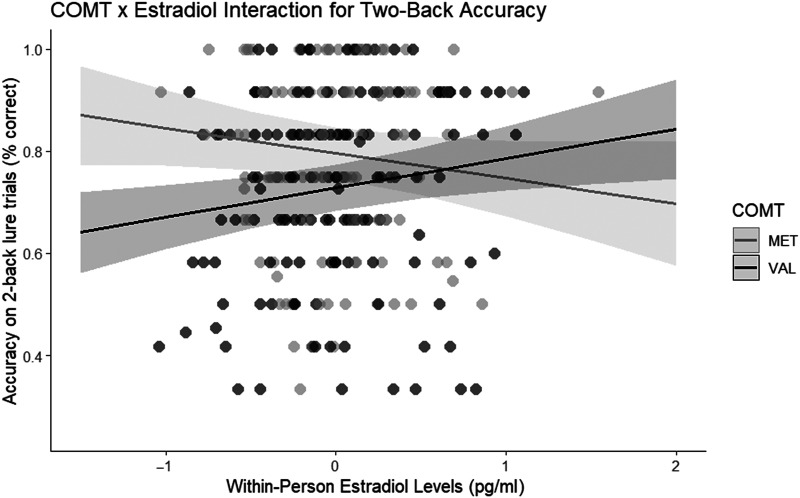
The expected direction of COMT effects emerged (p = .04, ηp2 = .06) such that Val carriers (M = .73, SE = .02) were less accurate than Met carriers on 2-back lure trials (M = .80, SE = .03). Importantly, and consistent with hypotheses, there was a significant COMT × Within-Person Estradiol Level interaction on 2-back lures (p = .003, ηp2 = .05).2 This was probed further by examining the effect of estradiol for Met and Val carriers separately. For Val carriers, higher estradiol levels predicted higher accuracy (b = .06, two-tailed p = .01; one-tailed p = .005; see Figure 2). For Met carriers, on the other hand, the effect was in the opposite direction (b = −.05, two-tailed p = .08; one-tailed p = .04), similar to the findings in Jacobs and D'Esposito (2011). Importantly, there was no effect of between-centered levels of estradiol (p = .37) nor did it interact with COMT (p = .29). For the model examining these effects for three back lures, no effects reached significance (see Table 1).
Figure 2. .
Line graph demonstrating the interaction between COMT and estradiol, such that Val carriers have a positive association between estradiol and accuracy and Met carriers have a negative association. The shaded region around the fitted line depicts the 95% confidence interval.
Table 1. .
Model Results Examining the Effect of COMT, Estradiol Levels (within- and between-centered), and their Interaction on 2- and 3-back Lure Accuracy
| Dependent Variable | Fixed Effect | Estimate | Standard Error | t | p (two-tailed) |
|---|---|---|---|---|---|
| Two-back accuracy | – | – | – | – | – |
| Intercept | .73 | .02 | 32.12 | .000* | |
| Estradiol levels (within-centered) | .06 | .02 | 2.64 | .01 | |
| Estradiol levels (between-centered) | −.03 | .04 | −.90 | .37 | |
| COMT | .07 | .03 | 2.05 | .04* | |
| COMT × Estradiol Levels (within-centered) | −.11 | .03 | −3.03 | .003* | |
| COMT × Estradiol Levels (between-centered) | .07 | .07 | 1.06 | .29 | |
| Zero-back accuracy (within-centered) | −.95 | .51 | −1.87 | .06 | |
| Variance Components | Variance | Standard Deviation | – | – | |
| Intercept | .02 | .13 | |||
| Residual | .01 | .12 | |||
| Dependent Variable | Fixed Effect | Estimate | Standard Error | t | p (two-tailed) |
| Three-back accuracy | – | – | – | – | – |
| Intercept | .66 | .02 | 33.52 | .000* | |
| Estradiol levels (within-centered) | .04 | .03 | 1.36 | .18 | |
| Estradiol levels (between-centered) | −.06 | .03 | −1.94 | .06 | |
| COMT | .02 | .03 | .52 | .60 | |
| COMT × Estradiol Levels (within-centered) | −.05 | .04 | −1.14 | .26 | |
| COMT × Estradiol Levels (between-centered) | .08 | .06 | 1.43 | .16 | |
| Zero-back accuracy (within-centered) | −.54 | .61 | −.89 | .37 | |
| Variance Components | Variance | Standard Deviation | – | – | |
| Intercept | .009 | .10 | |||
| Residual | .02 | .14 | |||
COMT was included as a dummy-coded variable, and Val is the base in the models presented in the table. Laboratory visits (i.e., time) were also included in this model as an effects-coded predictor to control for practice effects (p < .001).
Reaction Time
For two back lures, there were no significant effects of estradiol levels, COMT, or interactions (all ps > .14). However, there was a significant effect of RT on 0-back trials (b = .97, p < .001), indicating a positive association between RTs on 0-back trials and 2-back lures. For 3-back lures, there was a significant effect of within-person estradiol levels (b = −40.94, p = .02), revealing that higher within-person estradiol levels predicted faster RTs. The effect of 0-back RTs remained for 3-back lures (b = 1.17, p < .001). However, COMT did not interact with within-person or between-persons estradiol levels to predict 3-back lure-RTs (both ps > .19).
DISCUSSION
The main aim of the current study was to determine whether the behavioral findings from Jacobs and D'Esposito (2011), that estradiol moderates the effect of COMT on working memory performance, replicate in another young adult sample. The original study tracked women at two timepoints, whereas our study design consisted of up to four repeated assessments across the menstrual cycle that allowed for testing these effects with more statistical power, investigating the predictive value of estradiol by using it as a continuous predictor, and examining both within- and between-persons differences of estradiol on behavioral performance. Our results replicated Jacobs and D'Esposito (2011) such that on 2-back lure trials, Val carriers had enhanced performance when endogenous estradiol levels were elevated. We found an effect in the opposite direction for Met carriers, as hypothesized. Furthermore, consistent with the original investigation, we did not find this moderation effect at higher loads. These findings add to additional reports of Cycle × COMT interactions on behavior (Wu et al., 2019; Smith, Sierra, Oppler, & Boettiger, 2014).
These findings strengthen the case that rhythmic changes in sex hormone production within a person over time shape cognitive functions that are sensitive to dopaminergic signaling in the pFC. In line with the signal-to-noise hypothesis, DA plays a critical role in “sculpting” mental representations in pFC and dampening noise or distraction (Arnsten et al., 2015). Val/Val individuals typically show enhanced COMT activity and reduced prefrontal DA relative to Met homozygotes. Some of estradiol's “pro-dopaminergic” effects likely arise via the hormone's ability to downregulate COMT activity. At the behavioral level, across both studies, Val carriers exhibited a relative boost in working memory performance when endogenous estradiol levels are elevated, perhaps because of changes in their ability to gate distractions. Similarly, at the neural level, Jacobs and D'Esposito found that individuals with low pFC DA levels (indexed by high COMT enzyme activity) displayed exaggerated working memory-related BOLD responses in the dorsolateral pFC. Individuals with elevated pFC DA had reduced working memory-related pFC activity sustained across task blocks, a putative marker of heightened cortical efficiency (Green, Kraemer, DeYoung, Fossella, & Gray, 2013; Gray, Chabris, & Braver, 2003). This pattern of results is consistent with the broader COMT literature, in which Val carriers consistently show exaggerated task-evoked BOLD responses on 2-back conditions relative to Met carriers (Egan et al., 2001). The specificity of this effect to moderate working memory loads (e.g., 2-back conditions) suggests that there might be a particular context in which estradiol's moderating effect can be seen. When cognitive demands exceed this level (e.g., 3-back load), participants may experience additional task-induced DA release that results in a further shift along the inverted-U (see Figure 2). Our findings suggest that estradiol's modulating role may depend on task parameters, such as cognitive load. The heightened cognitive demand on the 3-back condition may be similar to an increase in acute stress. Therefore, heightened estradiol and stress may increase DA signaling on 3-back conditions, pushing individuals further along the “inverted U.”
On the other hand, we did not find that between-persons differences in estradiol influenced the COMT-working memory performance association on 2-back lure trials. Few studies have investigated the differential effects of within- versus between-persons effects of hormones on cognition. It is unknown whether individuals with higher average levels of estradiol across the menstrual cycle also exhibit elevated dopaminergic tone. Our test of between-persons estradiol levels and COMT is a between-subjects test, examining how individuals may perform in comparison to each other across the length of the cycle. Perhaps regardless of average estradiol levels, individuals adapt to task demands in ways that result in comparable levels of performance. Although our findings did not examine imaging data, it would be useful for future studies to examine whether there are COMT × between-estradiol interactive effects on pFC activity. Importantly, our findings imply that relative increases in estradiol may acutely alter performance because of changes in dopaminergic action. This has important implications for future study designs, disaggregating within- and between-persons estrogenic effects.
In contrast to the original findings on 3-back lure trials, however, we found that within-person increases in estradiol levels negatively predicted RT overall, such that participants responded more quickly when estradiol levels were higher. The emergence of this finding could be because of differences in our study design and analytic approach. Importantly, this finding also coincides with previous studies demonstrating that estradiol may lead to faster RTs (Amunts, Camilleri, Eickhoff, Heim, & Weis, 2020; Ho, Gilger, & Brink, 1986), and work indicating that processing speed declines during perimenopause (Greendale et al., 2009). Such a finding does not exclude a role for DA but suggests it may not depend on where people start at baseline (i.e., COMT). Typically, when individuals have improved processing speed without changes in accuracy, it is interpreted as enhanced processing efficiency. Indeed, findings suggest that estradiol may facilitate faster performance in high load contexts, perhaps because of its DA-agonist effects that lead to efficient neural activation and synaptic transmission (Del Río et al., 2018; Barth, Villringer, & Sacher, 2015; Jacobs & D'Esposito, 2011). Importantly however, individuals still performed less favorably on 3-back lures than 2-back lure conditions. Therefore, although participants may be more efficient during this context, it is not resulting in better performance overall.
Our study should also be interpreted with some limitations. Although our behavioral study had a larger sample than the initial investigation, more efforts to replicate in larger samples would be of great utility. In addition, whereas Jacobs and D'Esposito (2011) found a large effect for 2-back accuracy (η2 =.19), the effect was small in our study. The discrepancy in effect size may be because of methodological differences in our study design, including using saliva (instead of serum), and having more repeated measures. We also conducted a multilevel model to examine our interactions of interest, which accounts for multiple sources of variance. In addition, we used estradiol as a continuous predictor, in contrast to the 2011 investigation that compared group differences in accuracy scores. Therefore, methodological and analytic differences could explain the discrepancy in effect sizes.
Replication of the findings in Jacobs and D'Esposito (2011) offers additional support for the importance of considering estradiol's influence on dopamine-dependent cognitive functions. Indeed, recent work has called for a more holistic understanding of cognition by including the sex hormone milieu (Taylor, Pritschet, & Jacobs, 2021; Beltz & Moser, 2020). Our study supports the notion that empirical efforts probing DA's influence on cognition should include a role for estradiol (Colzato & Hommel, 2014). Importantly, our results indicate that the role of estradiol may depend on task conditions, including overall difficulty, which has implications for how we broadly understand estradiol's role in cognitive performance. The current findings, therefore, have important implications for the generalizability of our knowledge of working memory, in that we must consider for whom, and in what contexts, certain effects may or may not be seen.
Acknowledgments
The authors would like to thank Courtney Callahan, Megan Wright, Teona Velehorschi, and Alycia Winters for their invaluable role in the administration of this study, as well as all the participants for their vital contribution to this research.
Reprint requests should be sent to Courtney C. Louis, Department of Psychology, Michigan State University, 157F Psychology Building, East Lansing, Michigan 48824, United States, or via e-mail: louiscou@msu.edu.
Data Availability Statement
Data and code can be made available upon request.
Author Contributions
Courtney C. Louis: Data curation; Formal analysis; Funding acquisition; Investigation; Project administration; Visualization; Writing—Original draft; Writing—Review & editing. Emily Jacobs: Conceptualization; Funding acquisition; Methodology; Supervision; Writing—Review & editing. Mark D'Esposito: Conceptualization; Funding acquisition; Methodology; Supervision; Writing—Review & editing. Jason Moser: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Visualization; Writing—Review & editing.
Funding Information
National Institute of Mental Health (https://dx.doi.org/10.13039/100000025), grant numbers: 1R01MH108511 and 1F31MH125604-01.
Diversity in Citation Practices
Retrospective analysis of the citations in every article published in this journal from 2010 to 2021 reveals a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .407, W(oman)/M = .32, M/W = .115, and W/W = .159, the comparable proportions for the articles that these authorship teams cited were M/M = .549, W/M = .257, M/W = .109, and W/W = .085 (Postle and Fulvio, JoCN, 34:1, pp. 1–3). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance. The authors of this article report its proportions of citations by gender category to be as follows: M/M = .261; W/M = .348; M/W = .087; W/W = .304.
Notes
It is critical to make the distinction between sex and gender; neither of which are binary, nor do they have to overlap. “Female” is used in this text to refer those who are assigned female sex at birth. Importantly, however, the term “female” for those who are assigned female at birth can be elusive, as sex can refer to many things, including genitals, hormones, chromosol makeup, among other things. Therefore, we would like to be clear that in this text, we solely use “female” to refer to those who experience menstrual cycles. Furthermore, we use the term “women” to refer to those who identify as such. We do not intent to extend “female” to signify any other biological, social, or identity related factors in this text.
Because Jacobs and D'Esposito (2011) found and reported an interaction between Met and Val homozygous carriers, our main aims only focused on these two groups. However, we also tested a model with all three groups (41 Val/Val, 33 Met/Met, and 65 Val/Met). Our results remained unchanged. There was a significant interaction between COMT × Estradiol (p = .02). The effects revealed that there was a significant effect of estradiol for Val carriers (b = .06, two-tailed p = .01, one-tailed p = .005), trending in the opposite direction for Met carriers (b = −.04, two-tailed p = .15, one-tailed p = .075) and no effect for Val/Met carriers (p = .27).
REFERENCES
- Amunts, J., Camilleri, J. A., Eickhoff, S. B., Heim, S., & Weis, S. (2020). Executive functions predict verbal fluency scores in healthy participants. Scientific Reports, 10, 11141. 10.1038/s41598-020-65525-9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten, A. F. T. (2015). Stress weakens prefrontal networks: Molecular insults to higher cognition. Nature Neuroscience, 18, 1376–1385. 10.1038/nn.4087, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten, A. F., Wang, M., & Paspalas, C. D. (2015). Dopamine's actions in primate prefrontal cortex: Challenges for treating cognitive disorders. Pharmacological Reviews, 67, 681–696. 10.1124/pr.115.010512, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, C., Villringer, A., & Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience, 9, 37. 10.3389/fnins.2015.00037, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D., Mächler, M., Bolker, B., & Walker, S. (2014). Fitting linear mixed-effects models using lme4. arXiv:1406.5823. 10.48550/arXiv.1406.5823 [DOI] [Google Scholar]
- Becker, J. B. (1990). Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neuroscience Letters, 118, 169–171. 10.1016/0304-3940(90)90618-J, [DOI] [PubMed] [Google Scholar]
- Beltz, A. M., & Moser, J. S. (2020). Ovarian hormones: A long overlooked but critical contributor to cognitive brain structures and function. Annals of the New York Academy of Sciences, 1464, 156–180. 10.1111/nyas.14255, [DOI] [PubMed] [Google Scholar]
- Bernal, A., & Paolieri, D. (2022). The influence of estradiol and progesterone on neurocognition during three phases of the menstrual cycle: Modulating factors. Behavioural Brain Research, 417, 113593. 10.1016/j.bbr.2021.113593, [DOI] [PubMed] [Google Scholar]
- Bimonte, H. A., & Denenberg, V. H. (1999). Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology, 24, 161–173. 10.1016/S0306-4530(98)00068-7, [DOI] [PubMed] [Google Scholar]
- Björklund, A., & Dunnett, S. B. (2007). Dopamine neuron systems in the brain: An update. Trends in Neurosciences, 30, 194–202. 10.1016/j.tins.2007.03.006, [DOI] [PubMed] [Google Scholar]
- Brozoski, T. J., Brown, R. M., Rosvold, H. E., & Goldman, P. S. (1979). Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science, 205, 929–932. 10.1126/science.112679, [DOI] [PubMed] [Google Scholar]
- Cohen, E. R., & Taylor, B. N. (1973). The 1973 least-squares adjustment of the fundamental constants. Journal of Physical and Chemical Reference Data, 2, 663–734. 10.1063/1.3253130 [DOI] [Google Scholar]
- Colzato, L. S., & Hommel, B. (2014). Effects of estrogen on higher-order cognitive functions in unstressed human females may depend on individual variation in dopamine baseline levels. Frontiers in Neuroscience, 8, 65. 10.3389/fnins.2014.00065, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools, R., & D'Esposito, M. (2011). Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69, e113–e125. 10.1016/j.biopsych.2011.03.028, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río, J. P., Alliende, M. I., Molina, N., Serrano, F. G., Molina, S., & Vigil, P. (2018). Steroid hormones and their action in women's brains: The importance of hormonal balance. Frontiers in Public Health, 6, 141. 10.3389/fpubh.2018.00141, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, J. A., Makarewicz, J. A., Bunn, J., Nickerson, J., & McGee, E. (2018). Dopamine-dependent cognitive processes after menopause: The relationship between COMT genotype, estradiol, and working memory. Neurobiology of Aging, 72, 53–61. 10.1016/j.neurobiolaging.2018.08.009, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan, M. F., Goldberg, T. E., Kolachana, B. S., Callicott, J. H., Mazzanti, C. M., Straub, R. E., et al. (2001). Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences, U.S.A., 98, 6917–6922. 10.1073/pnas.111134598, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton, A., Smith, C. T., Parrish, M. H., & Boettiger, C. A. (2017). COMT Val158Met polymorphism exerts sex-dependent effects on fMRI measures of brain function. Frontiers in Human Neuroscience, 11, 578. 10.3389/fnhum.2017.00578, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. R., Chabris, C. F., & Braver, T. S. (2003). Neural mechanisms of general fluid intelligence. Nature Neuroscience, 6, 316–322. 10.1038/nn1014, [DOI] [PubMed] [Google Scholar]
- Green, A. E., Kraemer, D. J. M., DeYoung, C. G., Fossella, J. A., & Gray, J. R. (2013). A gene–brain–cognition pathway: Prefrontal activity mediates the effect of COMT on cognitive control and IQ. Cerebral Cortex, 23, 552–559. 10.1093/cercor/bhs035, [DOI] [PubMed] [Google Scholar]
- Greendale, G. A., Huang, M.-H., Wight, R. G., Seeman, T., Luetters, C., Avis, N. E., et al. (2009). Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology, 72, 1850–1857. 10.1212/WNL.0b013e3181a71193, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, E. (2018). Estrogens, aging, and working memory. Current Psychiatry Reports, 20, 109. 10.1007/s11920-018-0972-1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson, E., & Morley, E. E. (2013). Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology, 38, 2897–2904. 10.1016/j.psyneuen.2013.07.020, [DOI] [PubMed] [Google Scholar]
- Hara, Y., Yuk, F., Puri, R., Janssen, W. G. M., Rapp, P. R., & Morrison, J. H. (2014). Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proceedings of the National Academy of Sciences, U.S.A., 111, 486–491. 10.1073/pnas.1311310110, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, Y., Yuk, F., Puri, R., Janssen, W. G. M., Rapp, P. R., & Morrison, J. H. (2016). Estrogen restores multisynaptic boutons in the dorsolateral prefrontal cortex while promoting working memory in aged rhesus monkeys. Journal of Neuroscience, 36, 901–910. 10.1523/JNEUROSCI.3480-13.2016, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Lopez, E., & Pletzer, B. (2017). Interactive effects of dopamine baseline levels and cycle phase on executive functions: The role of progesterone. Frontiers in Neuroscience, 11, 403. 10.3389/fnins.2017.00403, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, H. Z., Gilger, J. W., & Brink, T. M. (1986). Effects of menstrual cycle on spatial information-processes. Perceptual and Motor Skills, 63, 743–751. 10.2466/pms.1986.63.2.743, [DOI] [PubMed] [Google Scholar]
- Hoffman, L., & Stawski, R. S. (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6, 97–120. 10.1080/15427600902911189 [DOI] [Google Scholar]
- Jacobs, E., & D'Esposito, M. (2011). Estrogen shapes dopamine-dependent cognitive processes: Implications for women's health. Journal of Neuroscience, 31, 5286–5293. 10.1523/JNEUROSCI.6394-10.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, E. G., & Goldstein, J. M. (2018). The middle-aged brain: Biological sex and sex hormones shape memory circuitry. Current Opinion in Behavioral Sciences, 23, 84–91. 10.1016/j.cobeha.2018.03.009, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, H., Xie, T., Ramsden, D. B., & Ho, S. L. (2003). Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology, 45, 1011–1018. 10.1016/S0028-3908(03)00286-7, [DOI] [PubMed] [Google Scholar]
- Kirchner, W. K. (1958). Age differences in short-term retention of rapidly changing information. Journal of Experimental Psychology, 55, 352–358. 10.1037/h0043688, [DOI] [PubMed] [Google Scholar]
- Kromrey, S. A., Czoty, P. W., & Nader, M. A. (2015). Relationship between estradiol and progesterone concentrations and cognitive performance in normally cycling female cynomolgus monkeys. Hormones and Behavior, 72, 12–19. 10.1016/j.yhbeh.2015.04.017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, C. C., D'Esposito, M., & Moser, J. S. (2021). Investigating interactive effects of worry and the catechol-o-methyltransferase gene (COMT) on working memory performance. Cognitive, Affective, & Behavioral Neuroscience, 21, 1153–1163. 10.3758/s13415-021-00922-9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine, V. N., Richards, S. T., Wu, V. Y., & Beck, K. D. (1998). Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior, 34, 149–162. 10.1006/hbeh.1998.1473, [DOI] [PubMed] [Google Scholar]
- Männistö, P. T., & Kaakkola, S. (1999). Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacological Reviews, 51, 593–628. [PubMed] [Google Scholar]
- Meyer-Lindenberg, A., Nichols, T., Callicott, J. H., Ding, J., Kolachana, B., Buckholtz, J., et al. (2006). Impact of complex genetic variation in COMT on human brain function. Molecular Psychiatry, 11, 867–797. 10.1038/sj.mp.4001860, [DOI] [PubMed] [Google Scholar]
- Mier, D., Kirsch, P., & Meyer-Lindenberg, A. (2010). Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Molecular Psychiatry, 15, 918–927. 10.1038/mp.2009.36, [DOI] [PubMed] [Google Scholar]
- Papaleo, F., Sannino, S., Piras, F., & Spalletta, G. (2015). Sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in both health and schizophrenia. European Neuropsychopharmacology, 25, 2349–2363. 10.1016/j.euroneuro.2015.10.005, [DOI] [PubMed] [Google Scholar]
- Rosenberg, L., & Park, S. (2002). Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology, 27, 835–841. 10.1016/S0306-4530(01)00083-X, [DOI] [PubMed] [Google Scholar]
- Shanmugan, S., & Epperson, C. N. (2014). Estrogen and the prefrontal cortex: Towards a new understanding of estrogen's effects on executive functions in the menopause transition. Human Brain Mapping, 35, 847–865. 10.1002/hbm.22218, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky, R. M., Glavis-Bloom, C., Lerman, D., McRae, P., Benson, C., Miller, K., et al. (2004). Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Molecular Psychiatry, 9, 531–538. 10.1038/sj.mp.4001435, [DOI] [PubMed] [Google Scholar]
- Shansky, R. M., & Lipps, J. (2013). Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Frontiers in Human Neuroscience, 7, 123. 10.3389/fnhum.2013.00123, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein, M., Kolachana, B., Simpson, E. H., Tabares, P., Cheng, B., Duvall, M., et al. (2008). COMT genotype predicts cortical-limbic D1 receptor availability measured with [11C]NNC112 and PET. Molecular Psychiatry, 13, 821–827. 10.1038/mp.2008.19, [DOI] [PubMed] [Google Scholar]
- Smith, C. T., Sierra, Y., Oppler, S. H., & Boettiger, C. A. (2014). Ovarian cycle effects on immediate reward selection bias in humans: A role for estradiol. Journal of Neuroscience, 34, 5468–5476. 10.1523/JNEUROSCI.0014-14.2014, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. T., Swift-Scanlan, T., & Boettiger, C. A. (2014). Genetic polymorphisms regulating dopamine signaling in the frontal cortex interact to affect target detection under high working memory load. Journal of Cognitive Neuroscience, 26, 395–407. 10.1162/jocn_a_00501, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. M., Furman, D. J., Berry, A. S., White, R. L., Jagust, W. J., D'Esposito, M., et al. (2022). Striatal dopamine synthesis and cognitive flexibility differ between hormonal contraceptive users and non-users. bioRxiv. 10.1101/2022.10.20.513082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C. M., Pritschet, L., & Jacobs, E. G. (2021). The scientific body of knowledge—Whose body does it serve? A spotlight on oral contraceptives and women's health factors in neuroimaging. Frontiers in Neuroendocrinology, 60, 100874. 10.1016/j.yfrne.2020.100874, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge, E. M., Harrison, P. J., & Weinberger, D. R. (2006). Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological Psychiatry, 60, 141–151. 10.1016/j.biopsych.2005.10.024, [DOI] [PubMed] [Google Scholar]
- Tunbridge, E. M., Narajos, M., Harrison, C. H., Beresford, C., Cipriani, A., & Harrison, P. J. (2019). Which dopamine polymorphisms are functional? Systematic review and meta-analysis of COMT, DAT, DBH, DDC, DRD1–5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biological Psychiatry, 86, 608–620. 10.1016/j.biopsych.2019.05.014, [DOI] [PubMed] [Google Scholar]
- Williams, G. V., & Castner, S. A. (2006). Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience, 139, 263–276. 10.1016/j.neuroscience.2005.09.028, [DOI] [PubMed] [Google Scholar]
- Wu, C., Ding, Y., Chen, B., Gao, Y., Wang, Q., Wu, Z., et al. (2019). Both Val158Met polymorphism of catechol-O-methyltransferase gene and menstrual cycle affect prepulse inhibition but not attentional modulation of prepulse inhibition in younger-adult females. Neuroscience, 404, 396–406. 10.1016/j.neuroscience.2019.02.001, [DOI] [PubMed] [Google Scholar]
- Xiao, L., & Becker, J. B. (1994). Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: Effects of estrous cycle and gonadectomy. Neuroscience Letters, 180, 155–158. 10.1016/0304-3940(94)90510-X, [DOI] [PubMed] [Google Scholar]
- Yavich, L., Forsberg, M. M., Karayiorgou, M., Gogos, J. A., & Männistö, P. T. (2007). Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. Journal of Neuroscience, 27, 10196–10209. 10.1523/JNEUROSCI.0665-07.2007, [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code can be made available upon request.