Abstract

Bioorthogonal deprotections are readily used to control biological function in a cell-specific manner. To further improve the spatial resolution of these reactions, we here present a lysosome-targeted tetrazine for an organelle-specific deprotection reaction. We show that trans-cyclooctene deprotection with this reagent can be used to control the biological activity of ligands for invariant natural killer T cells in the lysosome to shed light on the processing pathway in antigen presenting cells. We then use the lysosome-targeted tetrazine to show that long peptide antigens used for CD8+ T cell activation do not pass through this organelle, suggesting a role for the earlier endosomal compartments for their processing.
Introduction
Bioorthogonal chemistries are the family of chemical conversions that can be performed in a living system with a high degree of selectivity.1,2 They can be broadly subdivided into bond-forming and bond-breaking reactions.3,4 The latter are often used to control the activation of molecules of interest in cellular systems,3 for example, to locally activate chemotherapeutics and other drugs,5,6 activate enzyme activities in living cells,7−9 and control the activation of T cells.10 Various bond-breaking methods are available for this: from metal-based reactions,11,12 to the inverse electron-demand Diels–Alder (IEDDA) pyridazine elimination (the Click-to-Release reaction). The latter is the reaction between a tetrazine and a trans-cyclooctene (TCO)13 with allylic substitution that results in the formation of a dihydropyridazine intermediate, which tautomerizes and subsequently releases the allylic substituent (colloquially known as the “click-to-release”-approach).3−5,9,10,13−18 It is particularly favorable, as a wide range of chemical functionalities can be protected/deprotected by it (amines,14 alcohols,19,20 and carboxylic acids21).22 It is also very fast and has a strong in vivo pedigree,5,16,23 with a clinical trial using this chemistry having started recently.17,24
A recent development in the Click-to-Release field has been the move toward higher spatial resolution of these reactions. For example, Fox and co-workers have performed an IEDDA ligation reaction using two-photon activation of a tetrazine with submicrometer resolution.25 Dzijak et al.26 and Zheng et al.27 reported a mitochondrion-restricted bioorthogonal deprotection reaction, which they achieved by using both a mitochondrion-targeted deprotecting agent and prodrug to achieve mitochondrion-localized deprotection. We felt these advances could offer an exciting opportunity to unravel the subcellular topology of certain biological processes.
Our interest in this regard lies in the role of the dendritic cell (DC) lysosome during presentation of different classes of antigen.28,29 For example, invariant natural killer T cells (iNKTs) are activated by DCs presenting glycolipid antigens on CD1d receptors,30,31 and CD8+ T cells are activated by peptide antigens presented by these cells.32 Tools to precisely study the subcellular topology of the processes leading to the activation of these cells do not yet exist, but are highly important, as the precise contribution of the lysosome to these processes can be controversial. To study this, an organelle-specific bioorthogonal deprotection chemistry is required, without the requisite that both reagents are pretargeted to the organelle of interest.
iNKT cells are hybrids between T cells and NK cells, expressing semi-invariant T cell receptors (TCRs) as well as NK cell markers (e.g., NK1.1).33,34 They show important dual functions (cell killing and cytokine secretion),35 particularly in the regulation of immune responses against cancer and certain infections.36 These cells are activated by DCs displaying glycolipid antigens on the CD1d receptors at their cell surface. The activation and loading biology of these ligands onto the CD1d receptors is complex. Lipid loading can take place in the endoplasmic reticulum (ER), where endogenous phosphatidylinositols37 and phosphocholines are preferentially loaded.38 The lysosomal population of CD1d on the other hand is mostly loaded by lipids extracted from the lysosomal membrane by Saposin B,39,40 such as lyso-phosphatidylserine and lysophosphocholine,38 which they can attain by taking on a pH-dependent lipid-receptive conformation.41,42 Exogenous glycolipids have not been found loaded on the endo-lysosomal pool of CD1d. This is surprising because the most potent exogenous iNKT-activator is α-galactosyl-ceramide (KRN7000, α-GalCer, 4, Figure 1).43,44 This lipid, which is heavily pursued clinically,36,45−52 and its lower affinity variant α-galactosyl phytosphingosine53 (α-GalPhyto, 5) are both taken up via endocytosis and are therefore most likely loaded onto CD1d54,55 in the endosome or lysosome (Figure 1). However, there is at present no method to determine the relative contribution of lysosomally loaded or endosomally loaded 4 and 5 to the activation of iNKT cells.42,44,56,57
Figure 1.
Bioorthogonal chemistry to study the subcellular routing before antigen presentation of CD1d-glycolipid (top) and MHC-I-peptide (bottom) processing by DCs. With the use of a lysosome-specific tetrazine, the TCO-protecting group will only be removed when the antigens enter the lysosome before presentation at the cell surface. While the glycolipid antigens TCO-α-GalCer (1) and TCO-α-GalPhyto (2) pass the lysosome and will therefore activate the iNKT cells, the mbTCO-OVA18 (6) peptide does not enter the lysosome, resulting in no CD8+ T cell activation.
The activation of CD8+ killer T cells is another area in which the lysosome plays a contentious role.58,59 In this process, polypeptide antigens are taken up by various endocytic mechanisms in the DC, yet end up on the major histocompatibility complex I (MHC-I) that is normally reserved for the presentation of endogenous peptides derived from cytosolic compartments. It has been a long-running debate to what extent cross-presented antigens pass through the lysosome.60 One proposed route suggests that a compartment with active cathepsin S is key in cross presentation,61 whereas other studies have suggested endosomal escape to the cytosol,62 or directly to the ER.63 Whether any of these escape events happen from an early endosome, or whether they occur from the lysosome remains unknown (Figure 1).
To study both these questions, tools for the lysosome-specific activation of these ligands would be essential. We here present such a system, in the form of a lysosome-specific bioorthogonal deprotection approach that allows us to study whether the CD1d ligands α-GalCer (4) and α-GalPhyto (5) as well as a CD8-activating long peptide antigen pass through lysosomes in the process leading to their loading on CD1d and MHC-I (Figure 1), respectively. We achieve this through the development and analysis of a lysosome-targeted tetrazine reagent (3, Figure 1) in combination with TCO-protected derivatives of α-GalCer (TCO α-GalCer, 1, Figure 1), α-GalPhyto (TCO α-GalPhyto, 2, Figure 1), and an 18-mer peptide containing the H-2Kb restricted model epitope SIINFEKL (mbTCO-OVA18, 6, Figure 1). We show with this approach that the glycolipids do pass through the lysosome during the CD1d loading process, but the long peptide antigen does not during MHC-I loading.
Results and Discussion
To perform a selective lysosomal deprotection, we required an uncaging reagent that would accumulate in the lysosome. For this we turned to the (2-aminoethyl)-morpholine group.64 This group is protonated at lysosomal pH-values and therefore accumulates in compartments with pH ≤ 4.5, which effectively leads to its retention in the lysosome. This group has been used to target a wide variety of cargo to the lysosome, including an NO-sensor,64 and the IR780 fluorophore.65 We opted to link this moiety to the recently reported 3-(pyridin-3-yl)-6-aminoethyl-1,2,4,5-tetrazine,66 using a glutaric anhydride ring opening followed by an amide condensation reaction to yield 3 (Figures 1 and S1).
We then characterized the lysosomal targeting properties of 3 by live cell microscopy, as the lysosome retention of the morpholine group is crucially dependent on the pH of the lysosome. We quantified the overlap with the known lysosome-specific fluorophore LysoTracker Deep Red, which is only fluorescent in the lysosome due to a combination of a targeting moiety and a H+-conditional fluorophore. Imaging 3 with a cell-permeable TCO-BODIPY fluorophore (10) resulted in poor signal-to-noise ratios (Figure S2). To reduce this background, we designed a cell-permeable quenched TCO-reagent (9) based on our previously reported fluorogenic bifunctional TCO probe for tetrazine characterization.67 As before, we utilized the bifunctional TCO5 to conjugate a 4-[4-(dimethylamino)phenylazo]benzoyl (DABCYL) quencher to a fluorophore which can undergo elimination, but in this case we used a cell-permeable BODIPY instead of 5-(2-aminoethylamino)naphtha-lene-1-sulfonic acid (EDANS) as the quenched fluorophore (Figures 2A and S1).68 Indeed, the DABCYL quenched the BODIPY fluorescence 10-fold (Figure 2B) without affecting the excitation/emission wavelengths of the dye (Figure S3). When we used DABCYL-TCO-BODIPY (9) to determine whether LysoTz 3 localized to the lysosome (Figure 2C) by confocal microscopy on BMDCs, we found that 3 labeled and colocalized with the commercially available LysoTracker Deep Red (Manders split coefficient M1 = 0.741 ± 0.089 and the Pearson’s correlation coefficient per cell gives r = 0.808 ± 0.052 or per image r = 0.460 ± 0.079). These results suggest good signal overlay between LysoTz 3 and LysoTracker Deep Red, thus confirming the accumulation of 3 in the lysosome. The lack of co-localization for other organelles, such as the nucleus, ER, and mitochondria (Pearson’s scores <0.05) further confirms this finding (Figure S4).
Figure 2.
Evaluation of LysoTz (3). (A) Structure of DABCYL-TCO-BODIPY (9). (B) Turn-on rate of DABCYL-TCO-BODIPY (9, 1 μM) ± LysoTz 3 (10 μM) in H2O measured by increased fluorescent signal (spectral properties can be found in Figure S2). (C) Confocal microscopy images of bone marrow derived DCs (BMDCs) stained with Hoechst 33342 (DNA), CellMask Orange Actin tracking stain and LysoTracker Deep Red as reference. Legend: 1. LysoTz (3) + DABCYL-TCO-BODIPY (9); 2. Negative control for 3; 3. Negative control for 9. All scale bars represent 20 μm.
With lysosome-targeted tetrazine 3 in hand, we next turned to the synthesis of TCO-protected analogues of glycolipids 4 and 5. Previous work by Painter and Hermans has shown that key contact residues for iNKT activation can be masked using a prodrug approach in which an enzymatically cleavable protecting group prevents CD1d/ TCR interaction and N→O acyl migration to form 4.49,53 Furthermore, the Trauner lab modulated the activity of these ligands via azobenzene photoisomerization.69 Inspired by this, we chose to protect the amine functionality of the proform of 4 with an allylic TCO modality to yield a TCO-protected α-GalCer (1, Figures 1 and 3). Upon IEDDA pyridazine elimination, the resulting compound rearranges to form CD1d-ligand 4. A similar approach was used for 5 to obtain TCO-protected α-GalPhyto 2 (Figure 1).
Figure 3.

Synthesis of TCO-protected α-GalCer (1) and α-GalPhyto (2). Reagents/conditions: (a) NIS, TMS-OTf, DCM, −40 °C, 67%; (b) PtO2, H2 (g), THF, rt; (c) TCO-NHS, DIPEA, DMAP, DMF, rt, 89% over two steps; (d) LiOH, THF, H2O, rt, quant.; (e) Et3N·3HF, THF, rt, 84%; (f) hexacosonoic acid, EDC·HCl, DIPEA, DMAP, DCM, rt, 31–34%; (g) Et3N·3HF, THF, rt, 23%.
A key consideration for designing the synthetic route toward 1 and 2 (Figure 3) was the stability of the TCO moiety, which ruled out late-stage (global) deprotection by means of hydrogenolysis or acid, as is often the case for α-GalCer (4) syntheses reported in the literature.70 Formation of 11 was envisaged by combining 4,6-di-tert-butylsilylene (DTBS)-directed α-galactosylation71−73 with an azide protected phytosphingosine acceptor, as reported by Veerapen et al.74 More specifically, we selected 2,3-TBS-4,6-DTBS protected donor 12(75) and 2-azido-3,4-cyclic carbonate acceptor 13(76) as building blocks (full experimental details for 12 and 13 in Figure S5). This approach would enable selective saponification of the cyclic carbonate moiety after glycosylation, in addition to a mild desilylation as the final deprotection step.
For the glycosylation, employing a mixture of NIS and catalytic TMS-OTf at −40°C74 resulted in α-selective glycosylation using donor 12 and 1.5 equivalents of acceptor 13 to obtain 11 in 67% yield (Table S1). Hydrogenation of the α-galactosylated product (11) in the presence of Adam’s catalyst afforded amine 14. Next, axial TCO carbonate 15(10) was employed as a reagent to install the TCO carbamate moiety on 14, in the presence of DIPEA and DMAP, to obtain 16 in 89% over two steps after chromatographic purification. Saponification of the cyclic carbonate functionality was performed with LiOH in a mixture of THF and H2O to obtain 17 as a crude product which could be directly used for subsequent steps. Steglich esterification77 of 17 and hexacosonoic acid in the presence of 1-ethyl-3-(3 -dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), DMAP and DIPEA afforded 18 in 31–34% yield (Table S2).
Simultaneous deprotection of the cyclic DTBS protecting group and two TBS groups on the galactose moiety was evaluated for both 17 and 18 to obtain 2 and 1, respectively (Table S3). Initial attempts investigated the deprotection of 17 with HF·pyridine and tetra-n-butylammoniumfluoride (TBAF), as individual reports on α-GalCer derivatives have shown both of these reagents to be effective for 4,6-DTBS deprotection.74,78,79 While these conditions resulted in a complex mixture of products or a lack of conversion, (prolonged) exposure to Et3N·3HF in THF afforded 2 in up to 84% yield. Et3N·3HF mediated deprotection conditions also enabled conversion of 18 to 1 in 23% yield without observing hydrolysis of the ester bond. NMR analysis for both 18 and 1 indicated the presence of a regioisomeric byproduct. As migration of the ester moiety was not observed during the deprotection of 18 to 1, we suspect that the ester bond was installed without complete regioselectivity. Additionally, liquid chromatography–mass spectrometry (LC-MS) experiments with a nonreleasing tetrazine (Figure S6A–D) confirmed the trans configuration of the double bond for 2 and 1. Taken together, while further optimization for the esterification and deprotection steps is warranted for 1 specifically, the results described confirm the compatibility of the deprotection conditions toward the envisioned synthetic strategy.
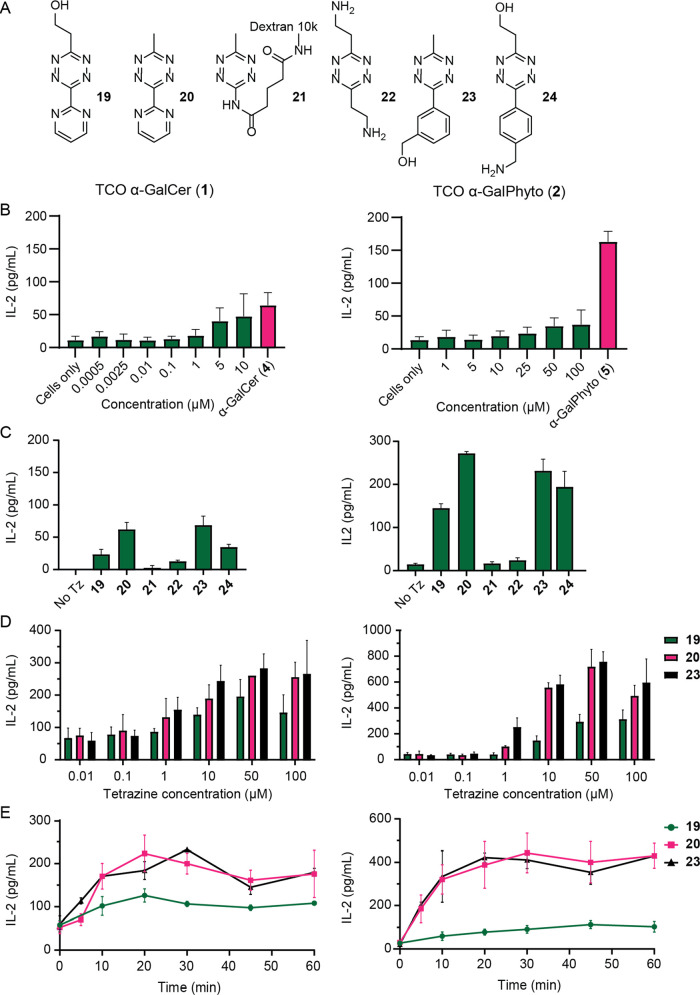
We next studied the biology of TCOα-GalCer (1) and TCOα-GalPhyto (2) in order to gain knowledge about the efficiency of the reaction in our cell-system. We first assessed whether TCO-caging could block iNKT activation (Figure 4). For this we used the DN32.D3 iNKT cell line the activation of which can be measured by IL-2 secretion. For 1, no increase in IL-2 was observed at concentrations <5 μM, whereas for 2 this was <25 μM (Figure 4B).
Figure 4.
Characterization of iNKT cell activation with TCOα-galactosylceramide (α-GalCer, 1) and TCOα-galactosyl phytosphingosine (α-GalPhyto, 2) uncaged with tetrazines; the y-axis shows the IL-2 levels measured with ELISA as readout for DN32.D3 iNKT cell activation. (A) Structures of the six different tetrazines used for characterization. (B) iNKT cell activation measured by IL-2 levels upon addition of TCO α-GalCer (1, 0.3 μM) and TCO α-GalPhyto (2, 15 μM) at different concentrations. α-GalCer (4, 10 nM) and α-GalPhyto (5, 10 μM) were used as positive control. (C) iNKT cell activation measured upon uncaging of TCO with different tetrazines. (D) Concentration optimization of Tz-concentration for both 1 (left) and 2 (right)-based reagents; (E) IL-2 production with increasing Tz-incubation time showing near maximal uncaging after a 20-minutes Tz incubation. All experiments were performed in triplicate and with BMDCs from three different mice.
This led us to choose a maximum concentration of 0.3 μM (for 1) and 15 μM (for 2) for all further experiments. Afterward, we assessed whether the addition of tetrazine to the ligand-loaded BMDCs led to the recovery of iNKT activation capacity. For this, we preincubated the BMDCs with the caged ligands followed by different tetrazines that we and others had previously shown to be capable of uncaging TCO on BMDCs (19–24).5,7,10,66,80 It was found that addition of tetrazines 19, 20, 23, and 24 (Figure 4A) resulted in increased levels of IL-2 compared to the caged controls (Figure 4C). The improved recovery of the iNKT cell activation in living cells of 20 compared to 19 is in consensus with earlier work of Peng Chen et al.7 Tetrazine-functionalized dextran (21) displayed limited uncaging despite having been reported as an exclusively extracellular tetrazine.5,81 This is most likely the result of its hydrophilic and bulky character, which is in discord with the ligand located in the hydrophobic CD1d pocket.82 The toxicity of the tetrazines against BMDCs was also tested, and all were found to be nontoxic at 10 μM concentration (Figure S7).
Optimal tetrazine concentrations were investigated for three of these tetrazines (19, 20, and 23, Figure 4D). Although increased IL-2 levels were already observed at a concentration of 1 μM for 20 and 23, 19 was less efficient at decaging 1 and 2. We determined that 10 μM of 20 and 23 afforded an optimal uncaging yield (Figure 4D). Finally, the optimal in vitro uncaging time was assessed for both ligands and found to plateau after 20 min of incubation with the respective tetrazine (Figure 4E).
Having confirmed the suitability of all reagents, we turned to studying lysosomal routing of ligands 1 and 2. First, we tested whether LysoTz (3) was able to uncage the ligands extracellularly, after presentation by BMDCs (Figure 5A,B). Here, we observed activation of the iNKT cells for both compounds. Second, the lysosomal routing was tested by preincubating the DCs with LysoTz (3, 10 μM), before adding the caged ligands (1, 0.3 μM; 2, 15 μM) and finally adding the iNKT cells, with washes in between all steps (Figure 5C,D). iNKT activation was observed for both ligands, confirming the successful release of the TCO protecting group in the lysosome. Interestingly, the uncaging of TCOα-GalPhyto (2) in the lysosome was more efficient compared to extracellular uncaging, possibly due to the shielding by CD1d when presented at the cell surface. Furthermore, applying our previously reported fluorogenic TCO-reporter-quencher assay67 on 3 at pH 7.2 and 4.5 revealed a high pH dependency of uncaging by 3, both increasing the plateau from <10% elimination yield at pH 7.2 to ≥30% at pH 4.5 with a concomitant increase in the observed reaction rate (Figure S8). While it is generally accepted that a lower pH results in faster and more efficient decaging,22,66,83 this effect is perhaps more drastic for LysoTz 3 due to increased protonation of the morpholine moiety. Results for the other tetrazines investigated again emphasize the discrepancy between uncaging in a mere aqueous buffer and cellular systems80 (Figures 4 and S8). Altogether, we show that ligands 1 and 2 do pass the lysosomes before being presented by DCs.
Figure 5.

TCOα-galactosylceramide (α-GalCer) (1) and TCOα-galactosyl phytosphingosine (α-GalPhyto) (2) are both present in the lysosome before presentation by BMDCs; the y axis shows the IL-2 levels measured with ELISA as readout for DN32.D3 iNKT cell activation. (A) Experimental set-up for glycolipid uncaging with LysoTz (3) in BMDCs; (B) iNKT cell activation measured by IL-2 levels upon addition of TCOα-GalCer (1) and TCOα-GalPhyto (2) with thereafter addition of LysoTz (3); (C) experimental set-up for lysosomal-specific uncaging in BMDCs; (D) iNKT cell activation measured by IL-2 levels after preincubation with LysoTz (3) and after a wash addition of TCOα-GalCer (1) or TCOα-GalPhyto (2). All experiments were performed in triplicate and with BMDCs from three different mice.
We next studied whether peptides also pass through the lysosome during antigen cross-presentation, as the contribution of this compartment in this process is still contentious. We have previously shown that protection of a minimal 8-mer epitope of the model MHC-I restricted epitope SIINFEKL with the bifunctional mbTCO-group allowed control over its recognition by a cognate T cell.10 The peptides that were used in this work could be loaded on the surface of the cell, therefore not requiring any intracellular antigen processing and routing before presentation. In order to study whether peptide antigens pass through the lysosome during antigen cross-presentation, we made a variant peptide containing this same epitope that cannot be loaded onto MHC-I on the surface. This is achieved by extending the peptide at the N-terminus so that it requires proteolysis before the epitope fits in the MHC-I.84 We synthesized a TCO-protected 18-mer peptide 6, containing the MHC-I restricted epitope with TCO protection on the ε-amino group of Lys-7, using the previously established N-terminal methylsulfonylethyloxycarbonyl (MSc)85 protection strategy to selectively install the TCO moiety (Figure S9).10
We first determined whether 3 was capable of deprotecting the caged epitope. The cells were first incubated with the mbTCO-modified minimal SIINFEKL epitope (8, 100 nM) which could be loaded directly in MHC-I on the surface of the DC. After this 1 h loading period, the cells were washed and 3 was added for 1 h at 10 μM. A SIINFEKL-specific T cell hybridoma B3Z86 and its activation (measured by quantifying the NFAT-induced expression of the reporter protein β-galactosidase) served as a proxy for surface MHC-I-restricted SIINFEKL concentration. This assay showed that 3 could uncage approximately 60% of the mbTCO group, which was in a similar range to previously reported tetrazines (Figure 6A).10 Preloading the DCs with 3 followed by incubation with mbTCO-SIINFEKL did not result in uncaging, suggesting no exocytosis of the reagent into the medium over the timescales of the experiment (Figure 6A).
Figure 6.

SIINFEKL peptides do not enter the lysosome before presentation by DCs. B3Z T cell activation assay was used to assess the amount of “uncaged” antigen presented by D1 cells;86 the y axis shows the normalized absorbance at 570 nm. (A) T cell activation upon addition of 100 nM mbTCO-SIINFEKL (8) ± LysoTz (3). As control, LysoTz (3) is also added after peptide presentation and the samples are normalized between the negative cells only and the positive 100 nM SIINFEKL control. (B) T cell activation upon addition of 50 μM mbTCO-OVA18 (6) ± LysoTz (3). LysoTz (3) is also added as control after peptide presentation and the samples are normalized between the negative cells only and the positive 10 nM SIINFEKL (7) controls. All experiments were performed three times and in triplicate.
We then determined whether LysoTz (3) could also uncage the long peptide 6 during antigen processing. We assessed whether preloading the lysosome with 3 (10 μM) followed by the addition of 6 (20 μM) yielded any deprotected MHC-I-loaded SIINFEKL (Figure 6B). In the experiments analogous to those performed for CD1d ligands 1 and 2, no T cell activation was found, suggesting no role for the lysosomal compartments during cross-presentation of this particular peptide.
Conclusions
With the use of the bioorthogonal bond-breaking IEDDA reaction,3,4 we created a method for organelle-specific targeting. Here, we synthesized a tetrazine which only accumulates in lysosomes, resulting in lysosome-specific cleavage of the blocking TCO group. With this method, we studied the routing of caged iNKT glycolipids TCO α-GalCer (1) and TCO α-GalPhyto (2) through the late endosomal pathway. We showed that the TCO moiety rendered the CD1d ligands inactive, as iNKT cells incubated with the caged lipids would not activate in the absence of a tetrazine trigger. Only after preincubation with LysoTz (3), followed by the administration of the caged lipids 1 or 2, could the iNKT cells be activated. This in combination with the already confirmed presence of CD1d41,42 in the lysosome suggests that loading of these glycolipids occurs in the late endosome. This lysosome-specific deprotection strategy cannot only be used for the processing pathway of glycolipids but can be well translated to different antigens, such as peptide antigens for CD8+ and CD4+ T cell activation. In this study, we show that mbTCO-OVA18 (6) is not present in the lysosome during cross-presentation, indicating that the processing of this synthetic long peptide (SLP) is confined to early endosomal compartments or cytosolic proteases.
Acknowledgments
We kindly thank Janneke N. Samsom for the DN32.D3 cell line and Tagworks Pharmaceuticals for their kind gift of dextran–tetrazine 21.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c02139.
Experimental procedures and methods, characterization data, and excitation and emission spectra, confocal microscopy images, and LC-MS analysis results (PDF)
Author Present Address
† Department of Chemical Biology II, Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP), Robert-Rössle-Str. 10, 13125 Berlin, Germany
Author Contributions
# N.A.M.L. and M.A.R.d.G. contributed equally. All authors have given approval to the final version of the manuscript.
This work was funded by an ERC Consolidator Grant (kinetic; Grant number 865175) to S.I.v.K.
The authors declare no competing financial interest.
Supplementary Material
References
- Bertozzi C. R. A decade of bioorthogonal chemistry. Acc. Chem. Res. 2011, 44, 651. 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten E. M.; Bertozzi C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem., Int. Ed. 2009, 48, 6974. 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Chen P. R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 2016, 12, 129. 10.1038/nchembio.2024. [DOI] [PubMed] [Google Scholar]
- Tu J.; Xu M.; Franzini R. M. Dissociative Bioorthogonal Reactions. ChemBioChem 2019, 20, 1615. 10.1002/cbic.201800810. [DOI] [PubMed] [Google Scholar]
- Rossin R.; van Duijnhoven S. M.; Ten Hoeve W.; Janssen H. M.; Kleijn L. H.; Hoeben F. J.; Versteegen R. M.; Robillard M. S. Triggered Drug Release from an Antibody-Drug Conjugate Using Fast ″Click-to-Release″ Chemistry in Mice. Bioconjugate Chem. 2016, 27, 1697. 10.1021/acs.bioconjchem.6b00231. [DOI] [PubMed] [Google Scholar]
- Mejia Oneto J. M.; Khan I.; Seebald L.; Royzen M. In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma. ACS Cent. Sci. 2016, 2, 476. 10.1021/acscentsci.6b00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X.; Ge Y.; Lin F.; Yang Y.; Zhang G.; Ngai W. S. C.; Lin Z.; Zheng S.; Wang J.; Zhao J.; et al. Optimized Tetrazine Derivatives for Rapid Bioorthogonal Decaging in Living Cells. Angew. Chem., Int. Ed. 2016, 55, 14046. 10.1002/anie.201608009. [DOI] [PubMed] [Google Scholar]
- Liu L.; Liu Y.; Zhang G.; Ge Y.; Fan X.; Lin F.; Wang J.; Zheng H.; Xie X.; Zeng X.; et al. Genetically Encoded Chemical Decaging in Living Bacteria. Biochemistry 2018, 57, 446. 10.1021/acs.biochem.7b01017. [DOI] [PubMed] [Google Scholar]
- Li J.; Jia S.; Chen P. R. Diels-Alder reaction-triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 2014, 10, 1003. 10.1038/nchembio.1656. [DOI] [PubMed] [Google Scholar]
- van der Gracht A. M. F.; de Geus M. A. R.; Camps M. G. M.; Ruckwardt T. J.; Sarris A. J. C.; Bremmers J.; Maurits E.; Pawlak J. B.; Posthoorn M. M.; Bonger K. M.; et al. Chemical Control over T-Cell Activation in Vivo Using Deprotection of trans-Cyclooctene-Modified Epitopes. ACS Chem. Biol. 2018, 13, 1569. 10.1021/acschembio.8b00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasmal P. K.; Carregal-Romero S.; Han A. A.; Streu C. N.; Lin Z.; Namikawa K.; Elliott S. L.; Köster R. W.; Parak W. J.; Meggers E. Catalytic Azide Reduction in Biological Environments. ChemBioChem 2012, 13, 1116. 10.1002/cbic.201100719. [DOI] [PubMed] [Google Scholar]
- Yusop R. M.; Unciti-Broceta A.; Johansson E. M. V.; Sánchez-Martín R. M.; Bradley M. Palladium-mediated intracellular chemistry. Nat. Chem. 2011, 3, 239. 10.1038/nchem.981. [DOI] [PubMed] [Google Scholar]
- Blackman M. L.; Royzen M.; Fox J. M. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels–Alder Reactivity. J. Am. Chem. Soc. 2008, 130, 13518. 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegen R. M.; Rossin R.; ten Hoeve W.; Janssen H. M.; Robillard M. S. Click to release: instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem., Int. Ed. 2013, 52, 14112. 10.1002/anie.201305969. [DOI] [PubMed] [Google Scholar]
- Neumann K.; Gambardella A.; Bradley M. The Emerging Role of Tetrazines in Drug-Activation Chemistries. ChemBioChem 2019, 20, 872. 10.1002/cbic.201800590. [DOI] [PubMed] [Google Scholar]
- Rossin R.; Versteegen R. M.; Wu J.; Khasanov A.; Wessels H. J.; Steenbergen E. J.; Ten Hoeve W.; Janssen H. M.; van Onzen A.; Hudson P. J.; et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 2018, 9, 1484. 10.1038/s41467-018-03880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Yee N. A.; Srinivasan S.; Mahmoodi A.; Zakharian M.; Mejia Oneto J. M.; Royzen M. Click activated protodrugs against cancer increase the therapeutic potential of chemotherapy through local capture and activation. Chem. Sci. 2021, 12, 1259. 10.1039/D0SC06099B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Graaff M. J.; Oosenbrug T.; Marqvorsen M. H. S.; Nascimento C. R.; de Geus M. A. R.; Manoury B.; Ressing M. E.; van Kasteren S. I. Conditionally Controlling Human TLR2 Activity via Trans-Cyclooctene Caged Ligands. Bioconjugate Chem. 2020, 31, 1685. 10.1021/acs.bioconjchem.0c00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.; Oliveira B. L.; Bernardes G. J. L. Development of a self-immolative linker for tetrazine-triggered release of alcohols in cells. Org. Biomol. Chem. 2019, 17, 5725. 10.1039/C9OB01167F. [DOI] [PubMed] [Google Scholar]
- De Geus M. A. R.; Groenewold G. J. M.; Maurits E.; Araman C.; Van Kasteren S. I. Synthetic methodology towards allylic trans-cyclooctene-ethers enables modification of carbohydrates: bioorthogonal manipulation of the lac repressor. Chem. Sci. 2020, 11, 10175. 10.1039/D0SC03216F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S.; Qiao L.; Oliveira B. L.; Navo C. D.; Jiménez-Osés G.; Bernardes G. J. L. Tetrazine-Triggered Release of Carboxylic-Acid-Containing Molecules for Activation of an Anti-inflammatory Drug. ChemBioChem 2019, 20, 1541. 10.1002/cbic.201900098. [DOI] [PubMed] [Google Scholar]
- Versteegen R. M.; ten Hoeve W.; Rossin R.; de Geus M. A. R.; Janssen H. M.; Robillard M. S. Click-to-Release from trans-Cyclooctenes: Mechanistic Insights and Expansion of Scope from Established Carbamate to Remarkable Ether Cleavage. Angew. Chem., Int. Ed. 2018, 57, 10494. 10.1002/anie.201800402. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Li J.; Xie R.; Fan X.; Liu Y.; Zheng S.; Ge Y.; Chen P. R. Bioorthogonal Chemical Activation of Kinases in Living Systems. ACS Cent. Sci. 2016, 2, 325. 10.1021/acscentsci.6b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.Phase 1/2a Study of SQ3370 in Patients With Advances Solid Tumors. ClinicalTrials.gov identifier: NCT04106492. Updated February 9, 2023. Accessed 2023–05 -23. https://clinicaltrials.gov/ct2/show/record/NCT04106492.
- Jemas A.; Xie Y.; Pigga J. E.; Caplan J. L.; Am Ende C. W.; Fox J. M. Catalytic Activation of Bioorthogonal Chemistry with Light (CABL) Enables Rapid, Spatiotemporally Controlled Labeling and No-Wash, Subcellular 3D-Patterning in Live Cells Using Long Wavelength Light. J. Am. Chem. Soc. 2022, 144, 1647. 10.1021/jacs.1c10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzijak R.; Galeta J.; Vázquez A.; Kozák J.; Matoušová M.; Fulka H.; Dračínský M.; Vrabel M. Structurally Redesigned Bioorthogonal Reagents for Mitochondria-Specific Prodrug Activation. J. Am. Chem. Soc. 2021, 1, 23. 10.1021/jacsau.0c00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Ji X.; Yu B.; Ji K.; Gallo D.; Csizmadia E.; Zhu M.; Choudhury M. R.; De La Cruz L. K. C.; Chittavong V.; et al. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat. Chem. 2018, 10, 787. 10.1038/s41557-018-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M.; Silk J. D.; Yvonne Jones E.; Cerundolo V. Biology of CD1- and MR1-Restricted T Cells. Annu. Rev. Immunol. 2014, 32, 323. 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- Guermonprez P.; Valladeau J.; Zitvogel L.; Théry C.; Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621. 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- Lantz O.; Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994, 180, 1097. 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman E. M.; Porcelli S. A.; Morita C. T.; Behar S. M.; Furlong S. T.; Brenner M. B. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 1994, 372, 691. 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Jung S.; Unutmaz D.; Wong P.; Sano G.-I.; De los Santos K.; Sparwasser T.; Wu S.; Vuthoori S.; Ko K.; Zavala F.; et al. In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity 2002, 17, 211. 10.1016/S1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupin E.; Kinjo Y.; Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 2007, 5, 405. 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- Song L.; Asgharzadeh S.; Salo J.; Engell K.; Wu H.-W.; Sposto R.; Ara T.; Silverman A. M.; DeClerck Y. A.; Seeger R. C.; et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J. Clin. Invest. 2009, 119, 1524. 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D. I.; MacDonald H. R.; Kronenberg M.; Smyth M. J.; Kaer L. V. NKT cells: what’s in a name?. Nat. Rev. Immunol. 2004, 4, 231. 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- Waldowska M.; Bojarska-Junak A.; Roliński J. A brief review of clinical trials involving manipulation of invariant NKT cells as a promising approach in future cancer therapies. Cent. Eur. J. Immunol. 2017, 42, 181. 10.5114/ceji.2017.69361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. J.; Kang S. J.; De Silva A. D.; Stanic A. K.; Casorati G.; Hachey D. L.; Cresswell P.; Joyce S. Lipid-protein interactions: biosynthetic assembly of CD1 with lipids in the endoplasmic reticulum is evolutionarily conserved. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1022. 10.1073/pnas.0307847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W.; Kang S. J.; Evans J. E.; Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J. Immun. 2009, 182, 4784. 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter T.; Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005, 21, 81. 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- Yuan W.; Qi X.; Tsang P.; Kang S.-J.; Illarionov P. A.; Besra G. S.; Gumperz J.; Cresswell P. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 5551. 10.1073/pnas.0700617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y.-H.; Park S.-H.; Benlagha K.; Forestier C.; Jayawardena-Wolf J.; Savage P. B.; Teyton L.; Bendelac A. Multiple defects in antigen presentation and T cell development by mice expressing cytoplasmic tail–truncated CD1d. Nat. Immunol. 2002, 3, 55. 10.1038/ni740. [DOI] [PubMed] [Google Scholar]
- Jayawardena-Wolf J.; Benlagha K.; Chiu Y.-H.; Mehr R.; Bendelac A. CD1d Endosomal Trafficking Is Independently Regulated by an Intrinsic CD1d-Encoded Tyrosine Motif and by the Invariant Chain. Immunity 2001, 15, 897. 10.1016/S1074-7613(01)00240-0. [DOI] [PubMed] [Google Scholar]
- Kinjo Y.; Wu D.; Kim G.; Xing G.-W.; Poles M. A.; Ho D. D.; Tsuji M.; Kawahara K.; Wong C.-H.; Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 2005, 434, 520. 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Kawano T.; Cui J.; Koezuka Y.; Toura I.; Kaneko Y.; Motoki K.; Ueno H.; Nakagawa R.; Sato H.; Kondo E.; et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997, 278, 1626. 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Nicol A. J.; Tazbirkova A.; Nieda M. Comparison of Clinical and Immunological Effects of Intravenous and Intradermal Administration of α-GalactosylCeramide (KRN7000)-Pulsed Dendritic Cells. Clin. Cancer Res. 2011, 17, 5140. 10.1158/1078-0432.CCR-10-3105. [DOI] [PubMed] [Google Scholar]
- Nieda M.; Okai M.; Tazbirkova A.; Lin H.; Yamaura A.; Ide K.; Abraham R.; Juji T.; Macfarlane D. J.; Nicol A. J. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 2004, 103, 383. 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- Giaccone G.; Punt C. J.; Ando Y.; Ruijter R.; Nishi N.; Peters M.; von Blomberg B. M.; Scheper R. J.; van der Vliet H. J.; van den Eertwegh A. J.; Roelvink M.; Beijnen J.; Zwierzina H.; Pinedo H. M. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002, 8, 3702. [PubMed] [Google Scholar]
- Anderson R. J.; Compton B. J.; Tang C.-W.; Authier-Hall A.; Hayman C. M.; Swinerd G. W.; Kowalczyk R.; Harris P.; Brimble M. A.; Larsen D. S.; et al. NKT cell-dependent glycolipid–peptide vaccines with potent anti-tumour activity. Chem. Sci. 2015, 6, 5120. 10.1039/C4SC03599B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. J.; Tang C.-W.; Daniels N. J.; Compton B. J.; Hayman C. M.; Johnston K. A.; Knight D. A.; Gasser O.; Poyntz H. C.; Ferguson P. M.; et al. A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat. Chem. Biol. 2014, 10, 943. 10.1038/nchembio.1640. [DOI] [PubMed] [Google Scholar]
- Fujii S.-I.; Shimizu K.; Kronenberg M.; Steinman R. M. Prolonged IFN-γ–producing NKT response induced with α-galactosylceramide–loaded DCs. Nat. Immunol. 2002, 3, 867. 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- Kunii N.; Horiguchi S.; Motohashi S.; Yamamoto H.; Ueno N.; Yamamoto S.; Sakurai D.; Taniguchi M.; Nakayama T.; Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009, 100, 1092. 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi S.; Ishikawa A.; Ishikawa E.; Otsuji M.; Iizasa T.; Hanaoka H.; Shimizu N.; Horiguchi S.; Okamoto Y.; Fujii S.; Taniguchi M.; Fujisawa T.; Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer. Res. 2006, 12, 6079. 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- Compton B. J.; Farrand K. J.; Tang C.-W.; Osmond T. L.; Speir M.; Authier-Hall A.; Wang J.; Ferguson P. M.; Chan S. T. S.; Anderson R. J.; et al. Enhancing T cell responses and tumour immunity by vaccination with peptides conjugated to a weak NKT cell agonist. Org. Biomol. Chem. 2019, 17, 1225. 10.1039/C8OB02982B. [DOI] [PubMed] [Google Scholar]
- Natori T.; Morita M.; Akimoto K.; Koezuka Y. Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge Agelas mauritianus. Tetrahedron 1994, 50, 2771. 10.1016/S0040-4020(01)86991-X. [DOI] [Google Scholar]
- Morita M.; Motoki K.; Akimoto K.; Natori T.; Sakai T.; Sawa E.; Yamaji K.; Koezuka Y.; Kobayashi E.; Fukushima H. Structure-Activity Relationship of .alpha.-Galactosylceramides against B16-Bearing Mice. J. Med. Chem. 1995, 38, 2176. 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- Kang S. J.; Cresswell P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 2002, 21, 1650. 10.1093/emboj/21.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody D. B.; Porcelli S. A. Intracellular pathways of CD1 antigen presentation. Nat. Rev. Immunol. 2003, 3, 11. 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- van Montfoort N.; Camps M. G.; Khan S.; Filippov D. V.; Weterings J. J.; Griffith J. M.; Geuze H. J.; van Hall T.; Verbeek J. S.; Melief C. J.; et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 6730. 10.1073/pnas.0900969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre O. P.; Segura E.; Savina A.; Amigorena S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557. 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Basha G.; Lizée G.; Reinicke A. T.; Seipp R. P.; Omilusik K. D.; Jefferies W. A. MHC class I endosomal and lysosomal trafficking coincides with exogenous antigen loading in dendritic cells. PLoS One 2008, 3, e3247 10.1371/journal.pone.0003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.; Sigal L. J.; Boes M.; Rock K. L. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 2004, 21, 155. 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M.; Rock K. L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 1995, 267, 243. 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Cebrian I.; Visentin G.; Blanchard N.; Jouve M.; Bobard A.; Moita C.; Enninga J.; Moita L. F.; Amigorena S.; Savina A. Sec22b Regulates Phagosomal Maturation and Antigen Crosspresentation by Dendritic Cells. Cell 2011, 147, 1355. 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Yu H.; Xiao Y.; Jin L. A Lysosome-Targetable and Two-Photon Fluorescent Probe for Monitoring Endogenous and Exogenous Nitric Oxide in Living Cells. J. Am. Chem. Soc. 2012, 134, 17486. 10.1021/ja308967u. [DOI] [PubMed] [Google Scholar]
- Mai Y.; Qu X.; Ding S.; Lv J.; Li X.; Gao P.; Liu Y.; Yuan Z. Improved IR780 derivatives bearing morpholine group as tumor-targeted therapeutic agent for near-infrared fluorescence imaging and photodynamic therapy. Dyes Pigm. 2020, 177, 107979 10.1016/j.dyepig.2019.107979. [DOI] [Google Scholar]
- Sarris A. J. C.; Hansen T.; de Geus M. A. R.; Maurits E.; Doelman W.; Overkleeft H. S.; Codée J. D. C.; Filippov D. V.; van Kasteren S. I. Fast and pH-Independent Elimination of trans-Cyclooctene by Using Aminoethyl-Functionalized Tetrazines. Chem. – Eur. J. 2018, 24, 18075. 10.1002/chem.201803839. [DOI] [PubMed] [Google Scholar]
- de Geus M. A. R.; Maurits E.; Sarris A. J. C.; Hansen T.; Kloet M. S.; Kamphorst K.; ten Hoeve W.; Robillard M. S.; Pannwitz A.; Bonnet S. A.; Codée J. D. C.; Filippov D. V.; Overkleeft H. S.; Kasteren S. I. Fluorogenic Bifunctional trans-Cyclooctenes as Efficient Tools for Investigating Click-to-Release Kinetics. Chem. – Eur. J. 2020, 26, 9900. 10.1002/chem.201905446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder K. E.; Metcalfe E.; Nanjappan P.; Arunachalam T.; Ramos K.; Skedzielewski T. M.; Marinelli E. R.; Tweedle M. F.; Nunn A. D.; Swenson R. E. Synthesis, in vitro evaluation, and in vivo metabolism of fluor/quencher compounds containing IRDye 800CW and Black Hole Quencher-3 (BHQ-3). Bioconjugate Chem. 2011, 22, 1287. 10.1021/bc100457s. [DOI] [PubMed] [Google Scholar]
- Hartrampf N.; Seki T.; Baumann A.; Watson P.; Vepřek N. A.; Hetzler B. E.; Hoffmann-Röder A.; Tsuji M.; Trauner D. Optical Control of Cytokine Production Using Photoswitchable Galactosylceramides. Chemistry 2020, 26, 4476. 10.1002/chem.201905279. [DOI] [PubMed] [Google Scholar]
- Tashiro T. Structure-Activity Relationship Studies of Novel Glycosphingolipids That Stimulate Natural Killer T-Cells. Biosci., Biotechnol., Biochem. 2012, 76, 1055. 10.1271/bbb.120072. [DOI] [PubMed] [Google Scholar]
- Imamura A.; Ando H.; Korogi S.; Tanabe G.; Muraoka O.; Ishida H.; Kiso M. Di-tert-butylsilylene (DTBS) group-directed α-selective galactosylation unaffected by C-2 participating functionalities. Tetrahedron Lett. 2003, 44, 6725. 10.1016/S0040-4039(03)01647-2. [DOI] [Google Scholar]
- Kimura A.; Imamura A.; Ando H.; Ishida H.; Kiso M. A Novel Synthetic Route to α-Galactosyl Ceramides and iGb3 Using DTBS-Directed α-Selective Galactosylation. Synlett 2006, 2006, 2379. 10.1055/s-2006-949649. [DOI] [Google Scholar]
- Imamura A.; Matsuzawa N.; Sakai S.; Udagawa T.; Nakashima S.; Ando H.; Ishida H.; Kiso M. The Origin of High Stereoselectivity in Di-tert-butylsilylene-Directed α-Galactosylation. J. Org. Chem. 2016, 81, 9086. 10.1021/acs.joc.6b01685. [DOI] [PubMed] [Google Scholar]
- Veerapen N.; Brigl M.; Garg S.; Cerundolo V.; Cox L. R.; Brenner M. B.; Besra G. S. Synthesis and biological activity of alpha-galactosyl ceramide KRN7000 and galactosyl (alpha1-->2) galactosyl ceramide. Bioorg. Med. Chem. Lett. 2009, 19, 4288. 10.1016/j.bmcl.2009.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold H.; Boot R. G.; Aerts J. M. F. G.; Overkleeft H. S.; Codée J. D. C.; Van Der Marel G. A. A Concise Synthesis of Globotriaosylsphingosine. Eur. J. Org. Chem. 2011, 2011, 1652. 10.1002/ejoc.201001690. [DOI] [Google Scholar]
- Panza L.; Compostella F.; Imperio D. A versatile synthesis of αGalCer and its analogues exploiting a cyclic carbonate as phytosphingosine 3,4-diol protecting group. Carbohydr. Res. 2019, 472, 50. 10.1016/j.carres.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Neises B.; Steglich W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem., Int. Ed. 1978, 17, 522. 10.1002/anie.197805221. [DOI] [Google Scholar]
- Lee A.; Farrand K. J.; Dickgreber N.; Hayman C. M.; Jürs S.; Hermans I. F.; Painter G. F. Novel synthesis of α-galactosyl-ceramides and confirmation of their powerful NKT cell agonist activity. Carbohydr. Res. 2006, 341, 2785. 10.1016/j.carres.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Veerapen N.; Leadbetter E. A.; Brenner M. B.; Cox L. R.; Besra G. S. Synthesis of a Novel α-Galactosyl Ceramide Haptenated-Lipid Antigen, a Useful Tool in Demonstrating the Involvement of iNKT Cells in the Production of Antilipid Antibodies. Bioconjugate Chem. 2010, 21, 741. 10.1021/bc9005255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertheussen K.; van de Plassche M.; Bakkum T.; Gagestein B.; Ttofi I.; Sarris A. J. C.; Overkleeft H. S.; van der Stelt M.; van Kasteren S. I. Live-Cell Imaging of Sterculic Acid-a Naturally Occurring 1,2-Cyclopropene Fatty Acid-by Bioorthogonal Reaction with Turn-On Tetrazine-Fluorophore Conjugates. Angew. Chem. Int. Ed. 2022, 61, e202207640 10.1002/anie.202207640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj N. K.; Thurber G. M.; Keliher E. J.; Marinelli B.; Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 4762. 10.1073/pnas.1113466109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z.-H.; Castaño A. R.; Segelke B. W.; Stura E. A.; Peterson P. A.; Wilson I. A. Crystal Structure of Mouse CD1: An MHC-Like Fold with a Large Hydrophobic Binding Groove. Science 1997, 277, 339. 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- Carlson J. C. T.; Mikula H.; Weissleder R. Unraveling Tetrazine-Triggered Bioorthogonal Elimination Enables Chemical Tools for Ultrafast Release and Universal Cleavage. J. Am. Chem. Soc. 2018, 140, 3603. 10.1021/jacs.7b11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hos B. J.; Tondini E.; van Kasteren S. I.; Ossendorp F. Approaches to Improve Chemically Defined Synthetic Peptide Vaccines. Front. Immunol. 2018, 9, 884. 10.3389/fimmu.2018.00884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesser G. I.; Balvert-Geers I. C. The methylsulfonylethyloxycarbonyl group, a New and versatile amino protective function. Int. J. Pept. Protein Res. 1975, 7, 295. 10.1111/j.1399-3011.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Karttunen J.; Sanderson S.; Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 6020. 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.