Abstract
Organoids are in vitro model systems that mimic the complexity of organs with multicellular structures and functions, which provide great potential for biomedical and tissue engineering. However, their current formation heavily relies on using complex animal-derived extracellular matrices (ECM), such as Matrigel. These matrices are often poorly defined in chemical components and exhibit limited tunability and reproducibility. Recently, the biochemical and biophysical properties of defined hydrogels can be precisely tuned, offering broader opportunities to support the development and maturation of organoids. In this review, the fundamental properties of ECM in vivo and critical strategies to design matrices for organoid culture are summarized. Two typically defined hydrogels derived from natural and synthetic polymers for their applicability to improve organoids formation are presented. The representative applications of incorporating organoids into defined hydrogels are highlighted. Finally, some challenges and future perspectives are also discussed in developing defined hydrogels and advanced technologies toward supporting organoid research.
Keywords: Organoids, Extracellular matrix, Hydrogels, Tunable property
Graphical abstract
Highlights
-
•
Conventional matrices for organoid formation are limited by their poor reproducibility and defined chemical composition.
-
•
Defined hydrogels offer precise tuning of their biochemical and biophysical properties.
-
•
Recent advances for incorporating organoids into defined hydrogels are reviewed.
1. Introduction
The creation of three-dimensional (3D) human organotypic models in vitro represents a major technological breakthrough [1,2]. Organoids have attracted significant attention, as they can recapitulate the key functional and structural features of their in vivo counterparts [[3], [4], [5]]. Over the past decades, consistent efforts to develop strategies for complex biomimetic tissue structures have emerged in growing and maintaining organoids in vitro [[6], [7], [8], [9]]. In vivo, the native matrix provides bioactive factors, 3D support, and morphological guidance to the embedded cellular clusters within all tissues and organs, contributing to the construction of the whole human body [10]. Replicating the key features of the extracellular matrix (ECM) in vitro is necessary to build more physiologically relevant organoids. As the important characteristics of the ECM are better understood, several matrices are used for organoid culture, but some challenges remain. In common protocols, the formation of organoids heavily relies on the basement membrane extract (BME), such as Matrigel, which is derived from tumor origin, exhibiting batch-to-batch variation, high cost, and safety issues [11,12]. Organoids grown in such matrices are greatly limited in translational research, such as regenerative medicine and high-content drug screening [13]. Moreover, some studies have also reported using the whole ECM obtained from decellularized tissues as 3D scaffolds for cell and organoid cultures [[14], [15], [16], [17]]. The use of whole tissue ECM provides the most suitable tissue environment for developing organoids, but the results are still inconstant because of the chemically ambiguous components [18,19]. In addition, the compositional heterogeneity of these matrices is not conducive to the precise tuning of their physical and biochemical properties, which further hinders organoid applications [20].
In this regard, defined hydrogels are hydrophilic, crosslinked polymers that can be designed to mimic the ECM of different tissues [21]. This enables the creation of microenvironments that closely resemble the in vivo niche of the organ of interest, promoting the growth and differentiation of cells within the organoid. Defined hydrogels can be customized by varying their physical, chemical, and mechanical properties, providing researchers with a versatile tool to create organoid models for various organs [20,[22], [23], [24]]. Furthermore, incorporating growth factors, signaling molecules, and other bioactive molecules into the hydrogel matrix further allows for precise control over the organoid microenvironment [25]. In recent years, with the emergence of several natural and synthetic hydrogels with precisely tuned biochemical and biophysical properties, further opportunities have emerged in the field of organoid biomedicine [[26], [27], [28], [29]].
In this review, we aim to provide an overview of using defined hydrogels as cell instructive scaffolds to support organoid research (Fig. 1). Firstly, we provide some basic considerations and strategies of defined hydrogels for mimicking native ECM. Then, we highlight two types of well-defined hydrogels and their remarkable applications of organoids in the fields of organ development, disease modeling, drug testing, and regenerative medicine. Finally, we also discuss the promise of emerging hydrogels with controllable response properties in helping to bridge the gap between in vitro organoid models and their in vivo counterparts.
Fig. 1.
Overview of defined hydrogels to support organoid research. Several basic properties (e.g., degradability, mechanical properties, and cell adhesion) in developing hydrogels to mimic extracellular matrix in vivo should be considered. Two types of hydrogels derived from natural (e.g., animal and plant) and fully synthetic polymers are widely used regulate cell behavior (e.g., differentiation, adhesion, cell-cell interaction, proliferation, and migration). These hydrogels with well-defined properties can be integrateed into organoids facilitating their potential applications in organ development, disease model, drug testing, and regenerative medicine.
2. Basic consideration to defined hydrogel as matrices for organoid culture
The success of organoid culture is mainly dependent on the highly biomimetic dynamic microenvironment required for organ or tissue formation and functional generation in vivo. Mimicking the functional characteristics of native ECM and being compatible with common bioengineering techniques are essential criteria to consider in the development of defined hydrogels for organoid culture [11]. In this section, we describe some basic considerations for constructing defined hydrogels in terms of biological signals, biophysical cues, and crosslinking strategies, which can be conceivable to optimize hydrogel formulations to deliver increased fidelity in replicating the in vivo microenvironment and offering a more credible representation of organ behavior.
2.1. Biochemical signaling
Generally, the native ECM is composed of various proteins, polysaccharides, and growth factors, which possesses bioactive sites to allow cell adhesion, degradation, and activation of the certain signaling pathway (Fig. 2). As such, the defined hydrogels should be designed to contain the key bioactive or functional groups to support the growth and self-assembly of embedded cells for the ultimate organoid formation [30].
Fig. 2.
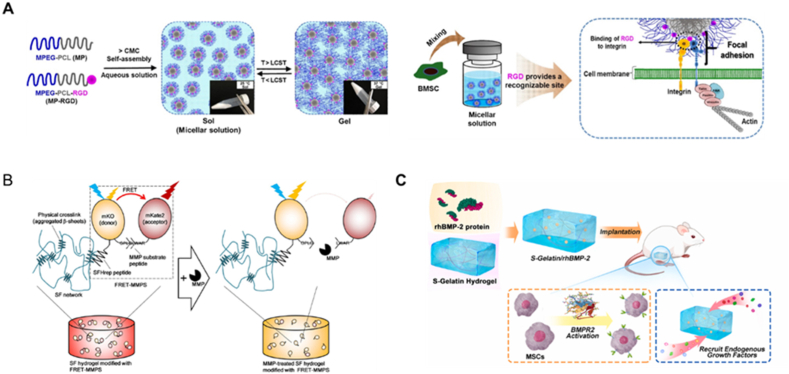
Representative biochemical properties considered in designing hydrogels to mimic ECM. (A). Schematic of the RGD-conjugated hydrogels to improve adhesion-dependent cellular behavior (reprinted with permission from Ref. [161]). (B). Schematic of the MMPs loosening hydrogels to enable immune cells to infiltrate and degrade silk fibroin hydrogel (reprinted with permission from Ref. [162]). (C). Schematic of the bone morphogenetic protein-2 (BMP-2) integrated into hydrogels to accelerate bone regeneration (reprinted with permission from Ref. [163]).
2.1.1. Cell adhesion
Cell adhesion is a crucial process that facilitates the binding of cells to ECM components or other cells, which plays a significant role in various cellular behaviors, such as migration, differentiation, proliferation, and survival [31]. Therefore, defined hydrogels used in organoid culture should contain specific biochemical protein components, such as laminin, fibronectin, and collagen, etc., that can facilitate cell adhesion and further improve the cell spreading and force transmission between cells and the ECM via integrin-mediated mechanotransduction [32,33]. However, this still brings a lot of complexity and uncertainty to organoid culture and is not conducive to the construction of defined hydrogels. Over the past decades, numerous adhesion motifs such as RGD, IKVAV, YIGSR, GFOGER, AG73, and PHSRN, etc., have been identified from some of the above proteins to precisely controlling cell behavior [34,35]. These can be directly modified into defined hydrogels to improve cell adhesion and thus influence cell functions. Among them, RGD is the minimum peptide sequence, i.e., Arginine–Glycine–Aspartic acid required for integrin ligands, commonly used in fabricating a wide range of cell adhesive surfaces [36]. For example, in the process of hMSC condensation, chondrogenic differentiation was greatly promoted by functionalized hyaluronic acid grafted with RGD peptide [37]. Therefore, the inclusion of specific cell adhesion cues in defined hydrogels represents an essential strategy for creating more biomimetic organoid culture systems.
2.1.2. Modulation of signaling pathway
The activation of certain signaling pathways within organoids are essential for cell growth, differentiation, and survival. Defined hydrogels must be designed to facilitate the activation of these signaling pathways by incorporating chemical cues that can activate the corresponding receptors. For example, Wnt signaling pathway plays a crucial role in the formation of intestinal organoids, and therefore, defined hydrogels used for the cultivation of intestinal organoids contain Wnt agonists to facilitate this pathway's activation [38]. Similarly, hydrogels with morphogens, such as bone morphogenetic protein 2 (BMP2) and transforming growth factor-β (TGFβ), hold the potential to enhance their bioactivity and promote cell spreading, viability, and differentiation [[39], [40], [41]].
2.1.3. Degradation and remodeling
As for the matrix's degradation and remodeling, metalloproteinase-sensitive degradation motifs can be modified to the defined hydrogel systems to allow cell spreading and self-assembly. Also, such purpose can be achieved by simply using hydrogels formed via supramolecular interaction, including electrostatic interaction, hydrogen-bond interaction, and hydrophobic interaction, etc. [42,43]. Hydrolytically degradable and photodegradable hydrogels are also promising alternatives for 3D cell culture. Previous studies have demonstrated that introducing labile bonds (e.g., ester bonds) within the crosslinks has become a common strategy for offering hydrolytic degradation to design cell microenvironment, which can simply control the degradation rate of hydrogels by changing the number of ester bonds [44]. As for the photodegradable category, various photolabile groups (such as o-nitrobenzylester, coumarin and disulfides, etc.) were introduced into hydrogel networks, allowing users to more simply and precisely control hydrogel's degradability at positions of interest by external light irradiation [45]. Moreover, altering the molecular weight of defined hydrogels can significantly affect some of the important physical features, like viscosity, stiffness, and stress relaxation, of the artificial matrices, which will be elaborated in the next paragraphs [46,47]. Clearly, fully recapitulating the critical biochemical properties of natural ECM will endow defined hydrogels with the potential to support organoids growth, expansion, morphogenesis, and maintenance of physiological functionalities.
2.2. Biophysical cues
Hydrogels are aqueous polymers with 3D hydrophilic networks, which exhibit swelling and porous properties that allow diffusion of small molecules and hydrophilic biomacromolecules, serving as qualified matrices for cell culture [48,49]. In addition to componential supplementary, defined hydrogels are supposed to provide mechanical clues and geometrical guidance for organoid formation and development (Fig. 3).
Fig. 3.
Representative biophysical properties considered in designing hydrogels to mimic ECM. (A). Schematic of alginate hydrogels with varying stiffening times was used to analyze the influence of dynamic ECM stiffness changes on MSCs' paracrine function (reprinted with permission from Ref. [52]). (B). Schematic of in vitro modeling using PEG-patterned substrates to geometrically confine human pluripotent stem cell colonies and spatially present mechanical stress to simulate such events (reprinted with permission from Ref. [164]). (C). Schematic of a novel droplet microfluidic system for one-step fabrication of hybrid hydrogel capsules enabling 3D culture and growth of hiPSC-derived organoids in a reproducible and high-throughput manner (reprinted with permission from Ref. [165]). (D). Schematic of creating microengineered hydrogel films on the bottom of multiwell plates allows for the simultaneous derivation of thousands of uniform organoids at predefined locations on the same focal plane (reprinted with permission from Ref. [166]).
2.2.1. Mechanics
The key mechanical features, like viscoelasticity, stiffness and stress relaxation, of hydrogels can be significantly changed by altering the molecular weight and structure, and the crosslinking density of prepolymers to build customized or dynamic matrices for certain organoid culture. Stiffness is taken as an example to illustrate the mechanical design criteria of defined hydrogels used in organoid areas [50]. Generally, the stiffness of natural ECM depends on their origins, ranging from 0.1 kPa of brain to 10 MPa of bones in terms of Young's Modulus [51]. And the stiffness of hydrogels can be enhanced by increasing the molecular weight and crosslinking density, as well as the amount of supramolecular interaction, to meet the needs of different type of organoids. In a recent study, the varying ECM stiffness was simulated using alginate hydrogels with different stiffening times. When the matrix stiffness increases, the paracrine function of MSCs can be significantly promoted [52]. Moreover, the stiffness of natural ECM changes significantly during the differentiation of stem cells or pathological state (e.g., cancerization and fibrosis), under which circumstances, dynamic hydrogels with secondary reactive groups or isomeric groups can serve as the optimal matrices for building corresponding organoid models [53,54].
2.2.2. Geometrical guidance
Geometrical guidance of defined hydrogels is another important biophysical issue when designing and constructing organoids in vitro due to the complex and hierarchical structures of in vivo tissues and organs [55]. The geometrical guidance can be broadly categorized into uniform arrays and biomimetic architectures. The former are usually superficial patterns, microwells, and microcarriers, which are used for high-throughput generation of uniform organoids to enhance the producibility of organoid systems [[56], [57], [58]]. For example, Liu et al. provided an all-in-water droplet microfluidic system to fabricate hybrid hydrogel capsules composed of alginate and chitosan, allowing for massive formation of functional and uniform islet organoids derived from human iPSCs [57]. The produced organoids exhibit high expression of pancreatic hormone specific genes and proteins, as well as glucose stimulated insulin secretion function, demonstrating the efficiency of these microcarriers for building robust organoid models. The latter are various biomimetic architectures, such as lumens, crypts, and any structures similar to the targeting organs fabricated by 3D printing or other molding technologies [[59], [60], [61]], which are usually utilized for guiding the heterogeneous morphogenesis and functionalities (e.g., vascularization) of organoids. For instance, Nikolaev et al. developed a crypt- and villus-like organoid model using microchannels with lateral branches within a mixture of collagen and Matrigel fabricated by nanosecond laser in a microfluidic chip [61]. With the biomimetic architectures, the proposed organoids exhibit rare, specialized cell types that are seldom found in conventional organoids, indicating the crucial role of morphological guidance during organoid development.
2.3. Crosslinking strategies
The formation of stable polymeric networks is indispensable to combine with some emerging engineering techniques such as microfluidic, bioprinting, and electrospinning, etc. with the incorporation of organoids into defined hydrogels [62]. Hydrogels' mechanical and biochemical properties are closely related to the crosslinking strategies. Hydrogels with different crosslinking structures can exhibit distinct functions, even for the same compositions [34]. Currently, the crosslinking mechanisms of constructing hydrogels into 3D assemblies for cell culture can be divided into two basic types: physical interactions and covalent interactions. The following subsections introduced some common reactions used to crosslink hydrogels based on such interactions. More details regarding these interactions can be found in other excellent reviews [[63], [64], [65]].
2.3.1. Physical interactions
Hydrogels constructed through physical interactions are non-covalent intermolecular associations, which avoid potential cytotoxicity with residue initiators or monomers in some gel reactions [63]. Meanwhile, this crosslinked hydrogel can undergo a reversible phase transition when environmental conditions, such as ion concentration, pH, salt, temperature, and electric field, change to a certain extent, exhibiting self-healing, biocompatibility, and stress relaxation [66].
Ionic/electrostatic interaction represents a typical physical interaction to construct dynamic hydrogels for various biomedical applications, which refers to the crosslinking mechanism of hydrogel via two molecules of opposite electric charges. Multivalent oligomers or polymers can create solid and tough hydrogels by exploiting ionic/electrostatic interactions [64]. The stiffness of these hydrogels is determined by factors such as the polymer molecular weight, composition, and ion crosslinking density, which enables independent control of both stiffness and adhesion ligand concentration. Alginate is a well-known example of hydrogel that undergoes gelation in the presence of divalent cations, particularly Ca2+, due to interactions between the carboxylic groups of one of the sugar constituents and divalent cations, and is commonly used in various biomedical applications [67]. Another type of physical interaction, hydrophobic interaction, can also be exploited for crosslinking water-soluble polymers containing hydrophobic end groups, side chains, or monomers to create hydrogels [68]. This interaction is not affected by the pH or ionic strength of the aqueous solution and can be used to engineer the mechanical microenvironment of organoids. Significantly, the mechanical properties of hydrogels, including their viscoelasticity, can be modified by adjusting the strength of hydrophobic interactions, which is essential to mimic the microstructural dynamics of native ECM. In addition, physical interactions such as hydrogen bonding, supramolecular interactions, and metal-ligand coordination can also be utilized [64]. The combination of multiple physical interactions for developing defined hydrogels enables the investigation of diverse organoids.
2.3.2. Covalent interactions
The hydrogels gelled with covalent interactions exhibit relatively higher mechanical strength, which usually includes free radical polymerization, condensation reactions, and orthogonal coupling reactions such as thiol–ene/yne reactions or alkyne–azide reactions, etc [65]. However, these hydrogels are generally permanent solidified and difficult to be remodeled unless their covalent bonds are cleaved, which may negatively affect cell viability or function in organoid culture. It is highly desirable to design a biomimetic scaffold that possesses stable mechanical properties yet can reasonably simulate the dynamics of the native ECM.
Recently, with the emergence of dynamic covalent chemistry, the seemingly contradictory mechanical properties, i.e., stability and kinetics, have been successfully combined in some stimuli-responsive hydrogels [65,69]. Such dynamic covalent interactions can undergo reversible fragmentation, recombination, and exchange when triggered by specific stimuli and play a crucial role in capturing the viscoelastic properties of soft tissues. Generally, they can be divided into two groups according to the different dependence of their equilibrium constants on temperature and concentration, i.e., reversible addition reactions (e.g., Diels-Alder, Schiff base, boronate, hydrazone, oxime) and reversible exchange reactions (e.g., disulfide, ally sulfide, thioester) [70,71]. Specifically, addition reactions involve the breaking and reformation of crosslinks, which leads to the equilibrium constants being significantly affected by both of the temperature and concentrations of functional groups. Conversely, exchange reactions result in bond rearrangement without crosslink cleavage, making the equilibrium dependent only on the exchange reaction's temperature and kinetics. In fact, these two different dynamic covalent interactions provide abundant possibilities for designing defined hydrogels with unique properties for organoid culture, enabling one to decide the appropriate crosslinking strategy for specific applications [72].
As above, with the comprehensive integration of diverse biochemical signals, biophysical cues and crosslinking strategies, numerous highly promising defined hydrogels that meet such criteria have led to the emergence of construction of the higher-order organoids with dynamic and physiologically relevant properties.
3. Types of defined hydrogels used in organoid culture
Recently, the increased understanding of important properties of native ECM has driven the utilization of chemically and mechanically well-defined hydrogels for organoid culture [73]. Due to the special 3D network structure and easily modifiable properties, they has been considered one of the most promising candidates for the challenge of organoid culture [74]. In this section, we will review relevant defined hydrogels, including the unique properties of natural and synthetic hydrogels and their potential advantages for organoid culture [75]. The major advantages and disadvantages of these hydrogels and the types of organoids generated them are summarized in Table 1, Table 2.
Table 1.
Summary of natural hydrogels suited for organoid culture.
| Hydrogels | Origin | Advantages | Disadvantages | Gelation | Organoid types | Refs. |
|---|---|---|---|---|---|---|
| Collagen | Connective tissue of animals |
|
|
|
|
[22,23,73,169] |
| Fibrin | Formed from fibrinogen isolated from the blood system |
|
|
|
|
[20,87,170] |
| Gelatin | Derived through hydrolytic processes of collagen |
|
|
|
|
[92,93,154] |
| Silk fibroin | Derived from the degumming of silk |
|
|
|
|
[101,102,171,172] |
| Alginate | A byproduct of iodine and mannitol extracted from the brown algae kelp or Sargassum |
|
|
• Physical gelation: Ionic cross-linking, electro-static interaction • Chemical gelation: Glutaraldehyde, EDC/NHS, photopolymerization (methacrylate, thiol deriva-tives |
|
[106,109,[173], [174], [175], [176]] |
| Chitosan | The product of chemical deacetylation of chitin |
|
|
|
|
|
| Hyaluronic acid | Distributed in connective tissue, joint fluid, ocular vitreous, umbilical cord and other tissue |
|
|
|
|
[[119], [120], [121],177] |
Table 2.
Summary of synthetic hydrogels suited for organoid culture.
| Hydrogels | Origin | Advantages | Disadvantages | Gelation | Organoid types | Refs. |
|---|---|---|---|---|---|---|
| PEG | Artificially synthesized |
|
|
|
|
[[129], [130], [131], [132],134,135] |
| PIC | Artificially synthesized |
|
|
|
|
[136,137,178] |
| PAAM | Artificially synthesized |
|
• Relatively chemically inert and lacks cell adhesion sites |
|
|
[140,179] |
| PVA | Artificially synthesized |
|
• Lacks of cell adhesion sites |
|
|
[28,180] |
3.1. Natural hydrogels
In fact, the ECM is extremely complex in composition and biochemical characteristics, dynamically changed as the development of tissues and organs. It is impractical to establish a culture matrix that is completely consistent with ECM in vivo [76]. Some single-component polymers are suitable for selective organoid culture to ensure that the organoid quality is sufficient for certain research needs. These polymers, usually derived from natural proteins or polysaccharides, have been classified as determinable hydrogels in numerous studies due to their single and determinable composition. In order to reduce the complexity and improve the reproducibility of experiments in organoid research, numerous studies have been conducted on biopolymers derived from single components (such as collagen I, gelatin, silk fibroin, hyaluronic acid, alginate, etc.) or their mixtures extracted from natural hydrogels to culture various organoids [[77], [78], [79]]. Each type of hydrogels has different properties, and their physical and biochemical properties can be further controlled by chemical modification or physical methods, which makes them suitable for different scenarios [75].
3.1.1. Collagen
To date, more than 20 different types of collagen have been found in the human body. Collagen accounts for approximately 20–30% of all proteins in mammals by weight and is also the most abundant protein in the ECM [80,81]. In addition, they have low immunogenicity, proper biodegradability and great biocompatibility [82]. Compared with Matrigel, the composition of collagen is simpler, and its physicochemical properties can be easily controlled, making it more reproducible in experiments [83]. Taking advantage of these features of collagen-based hydrogels, many researchers tried to replace the entire ECM with collagen for organoid culture [84]. For instance, Sachs et al. reported a modality for the generation of macroscopic intestinal tubes by embedding small cystic organoids into contracting floating collagen hydrogel with tunable physical properties, which confirmed the potential of collagen to reproduce macroscopic tissue morphogenesis in vitro [22]. However, native collagen has poor thermal stability, mechanical strength, and enzymatic resistance, which limit its application in the biomedical fields. The combination of collagen with other biocompatible materials is expected to solve this problem. In a recent case, Sandilya et al. prepared a more stable collagen-based scaffold by cross-linking collagen with oxidized gum arabic, which supported the culture of iPSC-derived pancreatic organoids for up to 30 days. In addition, the obtained islet clusters showing the presence of differentiated cells were able to release insulin upon glucose stimulation [23].
3.1.2. Fibrin
Fibrin is an essential natural protein component and present in thrombi, serving as a temporary ECM during wound healing. In contrast to collagen hydrogels found in mature tissues, fibrin gels are suitable for directing associated cells to secrete restorative growth factors and ECM components to stimulate tissue repair [85]. Fibrin is approved by the U.S. Food and Drug Administration (FDA) for biomedical use. In vitro, fibrin hydrogels are often used as model ECM and are widely used in the culture of stem cells, macrophages, and cancer cell lines, as well as in the study of angiogenesis [86]. In recent years, fibrin hydrogels have also shown great potential in organoid formation and culture. Broguiere et al. designed a fibrin-based defined hydrogel that can support the long-term culture and expansion of mouse and human epithelial organoids, which opened up new possibilities for the study of complex tissues in 3D in vitro [20]. Meanwhile, Factor XIII-mediated fibrin covalent anchoring sites can be readily coupled to fluorescent nanoparticles, specific peptides, or biomolecules, thus providing an opportunity to study cellular mechanisms by means of mechanical testing, such as 3D traction microscopy. For example, the authors found that increasing the stiffness or blocking the RGD domains in fibrous gel scaffolds inhibited organoid growth, implying that organoid expansion could be controlled by altering the mechanical and biochemical properties of the fibrin matrix. In another study, our group utilized droplet microfluidics to synthesize fibrin-based hydrogel capsules to support the generation of liver organoids from hiPSCs. The hepatocytes and cholangiocyte-like cells encapsulated in the microcapsules possessed favorable cell viability and proliferation activity, and further formed liver organoids of uniform size. Moreover, the organoids in the above microcapsules can maintain key features and specific functions of the human liver such as urea synthesis and albumin secretion [87]. These data demonstrate that organoids derived from different tissues can be generated using fibrin hydrogels. Furthermore, compared to thermosensitive BME, fibrin hydrogels can be easily produced in situ by proteolytic cleavage at room temperature, which provides better convenience for the manipulation process of cultured organoids [88].
3.1.3. Gelatin
Another well-defined hydrogel based on natural protein system has been used clinically for decades represented by gelatin, which is derived from collagen hydrolysis. Compared with collagen, gelatin retains the biological activity signal of its natural progenitor cells while also has low immunogenicity, even if it is derived from animals, it does not affect its translation in biomedicine [89,90]. It is worth noting that the structure of gelatin contains both matrix metalloproteinase (MMP) target sequences and RGD sequences, which makes it very beneficial for cell remodeling and adhesion when used in organoid culture [91]. Recently, to create functional tissue analogs in vitro, Klotz et al. developed a gelPEG hydrogel platform by enzymatic reaction with coagulation factor XIII to form covalent cross-links between gelatin and PEG. This defined hydrogel platform is simple, customizable, and reproducible while replacing basement membrane biological functions. When used in a liver organoid tissue engineering model, cells were effectively stimulated to differentiate in the hydrogel, and the experimental results were better than those of Matrigel [92]. In addition, Bernal et al. successfully developed gelatin-based bio-resins for volumetric additive manufacturing. In this study, the authors demonstrate the potential of using gelatin-based hydrogels for liver tissue modelling and leverage the technological advantages of light-driven volume bioprinting to establish multiscale bio-factories that can guide tissue-specific functions [93]. Indeed, gelatin derivatives represented by GelMA have photosensitive properties, which brings more options for the combination of organoids and bioprinting [[94], [95], [96]].
3.1.4. Silk fibroin
Silk fibroin (SF) is a natural protein polymer derived from the degumming process of silk, which is widely used in clinics, especially surgical sutures, after FDA approval [97]. The chemical structure of SF is a copolymer composed of hydrophilic and hydrophobic blocks, which brings good elasticity and toughness to SF [98,99]. In recent years, SF has been studied for cell culture in tissue engineering, including exploring its potential in organoid culture due to its excellent mechanical properties, good biocompatibility and slow degradation [100]. For instance, Gupta et al. explored the possibility of using silk as a scaffold for the growth and differentiation of primary cells and hiPSCs into kidney tissue. In this study, cells proliferated well on this material and differentiated into epithelial cells akin to the developing kidney. In addition, these representative structures can be also maintained after transplantation under adult renal capsule [101]. Similarly, this material shows potential for the establishment of human hair follicle dermal papilla in vitro organoid models. In a study, Gupta et al. encapsulated stem cells and hair follicle keratinocytes in a silk-gelatin microenvironment to establish an organoid model involving dermal papilla spheroids. This organoid model system provides an opportunity to elucidate distinct molecular signals associated with different stages of the hair cycle, which can provide important insights into the mechanisms underlying hair follicle morphogenesis [102]. Indeed, these cases provide a promising paradigm that silk can be used as a stem cell scaffold for organoid research, and also provide more references for the construction of various cell culture scaffolds based on protein polymers. In the future, the development of SF-based materials in medical applications can be accelerated by genetic engineering or chemical modification to make them more abundant and controllable in physicochemical properties [98].
3.1.5. Alginate
Polysaccharides are an important class of components that exist in the cellular microenvironment, and are often chemically attached to core proteins to form complexes such as proteoglycans [103]. Various polysaccharides and glycan-based materials have been widely used in 3D cell culture in the past [104]. Among them, alginate has a wide range of sources, easy gelation, good biocompatibility, and a certain degree of tenability [105]. It belongs to FDA-approved medical materials and has been successfully used alone in the culture of intestinal organoids [106]. It is worth noting that alginate hydrogels have specific biological stability and chemical inertness, and are often used for in vitro immobilization of cells to extend the culture period, as well as for spatiotemporal imaging and analysis of cells under specific conditions [107]. However, this intrinsic property also creates an additional barrier to the diffusion of substances in the culture of organoids embedded in 3D gel networks, which is not conducive to the delivery of nutrients during traditional static culture. To address this issue, Patel et al. designed a micro-physiological system capable of continuous dynamic culture and in situ multiparameter detection. In this system, human and rodent islet organoids can maintain functional activity in alginate hydrogels for more than 10 days [108]. In addition, in other studies, alginate were covalently modified with norbornene to obtain hydrogels cross-linked by thiol-ene chemistry. This chemical modification can tune the mechanical properties of hydrogels without Ca2+, is less susceptible to ionic components in the culture medium, and increases the ability to incorporate bioactive components. Further, Geuens et al. used the modified sodium alginate to support kidney organoid growth. In this research, alginate modulated collagen expression to prolong kidney organoid culture in vitro, which can decrease the abnormal collagen expression to advance the clinical translation of kidney organoids [109].
3.1.6. Chitosan
Chitosan, a product of natural polysaccharide chitin, has many physiological functions such as biodegradability, biocompatibility and immune enhancement [110,111]. Structurally, the amino group in chitosan is more reactive than the acetyl group in chitin molecule, which makes chitosan a kind of functional biomaterial with greater application potential. Similar to glycosaminoglycans, chitosan can also be a component of ECM and play a key role in cell attachment, differentiation and morphogenesis [112]. Back to 1998, Murat et al. prepared a chitosan membrane that could be used for hepatocyte culture, which was mixed with collagen, gelatin and other components to improve the surface roughness of the membrane. The fetal pig hepatocytes could survive for more than 14 days after inoculation with chitosan film, showing that chitosan has the potential to be used as the active component of artificial ECM, which provided the basis for subsequent organoid culture studies based on chitosan [113]. Recently, we developed a composite hydrogel system based on chitosan, fibrin and alginate to achieve 3D culture of liver organoids. This cultured liver organoid had specific functions of human liver organs such as urea synthesis and protein secretion [87]. In addition to liver organoids, chitosan was combined with sodium alginate by microfluidic technology to form hydrogel capsules capable of being used for islet organoid culture. The hydrogel capsule could be loaded with hiPSC derived pancreatic endocrine cells to achieve large-scale formation and culture of human islet organoids [114]. Combined with droplet microfluidic technology and stem cell biology, this defined hydrogel system is simple and easy to operate, and can be used to study and cultivate various stem cell organoids, which accelerates the research and transformation of organoids.
3.1.7. Hyaluronic acid
Hyaluronic acid (HA) is the only glycosaminoglycan that can be produced not only in animal tissues but also in bacteria, which has physiological functions such as regulating blood vessel permeability and protein function, playing an important physiological role in cell attachment and proliferation [[115], [116], [117], [118]]. In 2007, Khademhosseini et al. prepared a patterned HA matrix based on microfluidic technology that could be used for 3D cardiomyocyte culture. The methods could be used to rapidly construct a simple model of heart tissue in vitro, providing a research basis for organoid culture based on HA matrix [119]. Recently, Loebel et al. achieved the proliferation, differentiation and self-assembly process of iPSCs-derived alveolar cells to alveolar spheres in the absence of Matrigel by using laminin-modified defined HA hydrogel, which provides a Matrigel-independent method for the culture of lung and other epithelial organoids [120]. Although HA can be used as an active component of ECM, pure HA cannot be formed into defined hydrogels by 3D bioprinting. Therefore, the HA used in 3D printing needs to be chemically modified. Hyaluronic acid methacrylate (HAMA) is an ideal material for the synthesis of defined hydrogels because it is both biocompatible and printable and can rapidly form stable hydrogels. Wang et al. used HAMA and ECM as bioink to realize 3D bioprinting of islet organoids that have the physiological function of islets and can rapidly generate insulin under blood glucose stimulation [121]. Benefit from the regulatory effect of HA on blood vessels, the composite hydrogel obtained by 3D printing can promote angiogenesis, which further optimizes the physiological function of islet organoids and improves the feasibility and safety of islet transplantation.
3.2. Synthetic hydrogels
Given the limitations in customizing the mechanical and degradation properties of natural polymers according to organ specificity, fully synthetic matrices have emerged [122,123]. Synthetic hydrogels are better in this respect, which allow greater control over physical properties, chemical composition and overall structure [124,125]. In the past few years, synthetic hydrogels, represented by polyethylene glycol (PEG) and polyisocyanide (PIC) derivatives, etc., have flourished for applications in organoid culture [126]. These hydrogels themselves can avoid inflammatory and immune responses during cell culture by preventing nonspecific protein adhesion. In addition, they can highly mimic natural ECM characteristics to precisely tailor various topological and mechanical properties over a wide range for different application scenarios. In this section, we reviewed some synthetic hydrogels that have been successfully used in organoid research. In addition, we also selected some other synthetic hydrogel materials that have shown good biochemical properties in tissue engineering and other fields, which have great potential for organoid culture in the future.
3.2.1. Polyethylene glycol
PEG is a typical polymer made of ethylene oxide and water or ethylene glycol by stepwise addition polymerization. Compared with most chemically synthesized polymers, PEG has good water solubility, biocompatibility, physiological inertia and biodegradability, which is suitable for co-culture with biological samples [127,128]. In 1995, Drumheller et al. synthesized a PEG-based cross-linking polymer by photopolymerization, realized the culture of human fibroblasts in chemical matrix, and realized the regulation of the number of adherent cells by changing the molecular weight of PEG [129]. With the development of cell culture research, PEG hydrogel has been proved to be an excellent alternative to ECM due to its low price and resistance to non-specific protein adsorption. Vallmajo-Martin et al. synthesized a hybrid hydrogel based on PEG and HA, and used this defined hydrogel as an ECM to achieve bone marrow organoid culture. More importantly, this defined hydrogel is a more promising because of its superior performance in bone marrow organoid formation and xenotransplantation compared to currently used natural biomaterials [130]. Chemically synthesized PEG hydrogels have a wide range of size distribution, adjustable arm length and controllable molecular weight, which can regulate the physical and chemical properties of the materials, making them excellent biomaterials. Hayashi et al. prepared a hydrogel microsphere by cross-linking PEG with amylopectin, and adjusted and optimized the diameter of the microsphere by changing the composition ratio of the components. The microspheres could be used as cell scaffolds for the cultivation of bone marrow mesenchymal stem cells, laying the foundation for large-scale organoid production [131]. In addition to the microsphere material, Ng et al. developed an inverted colloidal crystal based on PEG hydrogel to achieve the formation and culture of iPSC-derived liver organoids. The liver organoids formed on the basis of such crystals are very close to adult tissues in appearance and physiological characteristics in all aspects, and can also form more complete liver tissues through further vascularization [132].
In addition, PEG is easy to be modified on the surface and can change matrix properties by various functionalization (such as RGD), co-modification of integrins and proteins, etc., which is especially suitable for organoid culture [133]. Hernandez-Gordillo et al. for the first time constructed a Matrigel-independent fully synthetic ECM by covalently modifying commercially available 8-arm vinyl sulfone activated PEG macromolecule monomer with integrin-binding peptide and ECM-binding peptide, combined with peptide cross-linking agent containing matrix metalloproteinase-sensitive degradation sites [134]. Later, they confirmed that this artificial ECM could replace Matrigel in the culture of epithelial organoids such as intestinal and endometrial organoids, providing technical support for organoid culture based on artificial ECM. Recently, Wilson et al. successfully realized the culture of monolayer organoids on PEG hydrogels, and reproduced part of the physiological function of the human intestinal epithelium. This PEG hydrogel can be modified on the surface to change the charge through amino acid functionalization, and then adsorb ECM protein through electrostatic interaction to form artificial ECM, which mediates the formation of monolayer intestinal epithelial organoids. This method is different from the traditional method of covalently modifying cell adhesion peptide and ECM protein on the surface of hydrogel [135]. Electrostatic adsorption can lead to the formation of monolayer tissue, which is more suitable for the construction of epithelial organoids. However, PEG still has the disadvantages of easy hyperexpansion and insufficient cell scale, which still needs further research and optimization.
3.2.2. Polyisocyanide
The fully synthetic polyisocyanide (PIC) hydrogels have an inherent property of reverse thermo-responsive due to the hydrophobic interactions between the oligoglycol substituent on their skeleton. In particular, they gel when heated above around 18 °C, forming low-viscosity aqueous solutions at low temperatures, which can facilitate cell and organoid extraction. A recent study demonstrated that PIC hydrogels modified with the cell adhesion peptide RGD sequence could be used alone to support cell growth of dissected mouse mammary gland fragments and further to generate cystic mammary organoids (MGOs). In addition, the advantage of this hydrogel is that its mechanical properties can be easily achieved by adjusting the polymer molecular weight or concentration, which is very beneficial for studying the effect of matrix stiffness on cell behavior. Taking advantage of this property, the authors prepared four PIC hydrogels with different stiffnesses and RGD ligand densities to culture mammary fragments and further investigated the growth state of basal and luminal cell populations during MGO formation. The results showed that the major cell types and ratios in MGO were closely related to the physicochemical properties of the hydrogel, such as gel stiffness affecting cell colony formation efficiency, while high RGD concentration could improve basal cell population proliferation [136]. In another study, PIC have also been used as advanced platforms for human liver organoid culture. Ye et al. developed a new hydrogel based on PIC combined with laminin-111. Organoid cultures using this hydrogel allow cells to effectively differentiate into hepatocyte-like phenotype with key liver functions. Meanwhile, the organoid reproduction and differentiation can be maintained for more than 14 generations exhibiting the potential for long-term culture based this hydrogel. Similarly, in this study, the authors also adjusted PIC mechanical properties by modulation of molecular weights and concentrations. By comparing the proliferation trend of liver organoids under different conditions, they found that the matrix with lower stiffness is more favorable for organoids [137]. In addition, the thermoreversible properties of PIC hydrogels allow the cells embedded in the gel to be easily released, which will significantly improve the portability of organoids for subsequent functional characterization at the gene and protein levels. Moreover, PIC can be well compatible with engineering technologies such as bioprinting and in vivo cell therapy, helping to realize the great potential of organoids in vivo.
3.2.3. Polyacrylamide
Polyacrylamide (PAAm) is a biocompatible synthetic polymer with good hydrophilic characteristics and swelling properties [138]. Due to its easily regulated biochemical and mechanical properties, the hydrogel shows significant advantages in studying the effects of matrix stiffness and chemical signals on cell behavior [139]. PAAm has long been used for cell culture in tissue engineering. Recently Shkumatov et al. prepared PAAm hydrogels with elastic moduli ranging from 0.2 kPa to 40 kPa for control of embryoid body (EB) differentiation. In this work, cardiovascular organoids were successfully obtained with altered matrix stiffness, which provides a new strategy for strengthening stem cells during development [140]. However, PAAm does not have cell adhesion sites, so blending with other hydrogels to form interpenetrating polymer networks to achieve tailoring for different organ tissue cultures has become an effective solution.
3.2.4. Polyvinyl alcohol
Another type of defined hydrogel matrix is based on polyvinyl alcohol (PVA), which has a large number of hydroxyl groups on the polymer chain, which makes it hydrophilic, accompanied by a certain degree of biodegradability and good biocompatibility, has been applied in various biomedical fields [141]. To mimic native ECM, some studies have coupled RGD sequences on PVA to ensure cell interaction with synthetic polymers and provide cells with an incentive to bind. Furthermore, to meet the matrix tunability of PVA for organoid culture and clinical translation, the authors selected two linkers, PEG-link and HyLink. The stability and degradability of these two linkers are pretty different. By changing their respective concentrations, the physical properties of hydrogel matrix can be adjusted in a wide range, which is expected to provide stable support for the formation of 3D structure while supporting the growth and development of organoids [28].
3.2.5. Others
Apart from the above discussed ones, some other synthetic hydrogels also have the potential for sustaining organoids growth. In previous studies, polymers with ester groups in their backbones generally exhibit biodegradability and facilitate binding to various ECM proteins and cell growth factors. Such conditions are common in polyglycolic acid (PGA), polylactic acid derivatives (such as PLA, PLLA, PDLLA, PDLA) and poly(caprolactone) (PCL), etc, which have been widely used in the encapsulation and culture of various cells. In one study, the authors doped NaCl particles as a porogen into PLG to obtain a microporous scaffold with excellent permeability. The scaffold can be used for the culture and regulation of islet organoids by loading different functional factors (such as promoting hESC pancreatic progenitor cell maturation through exendin-4). In addition, these PLG scaffolds can adsorb collagen IV to help diabetic mice quickly restore normal blood glucose levels after loading islet cells. In addition, 3D printing technology can also be used to prepare PCL scaffolds containing macroporous structures, which have good oxygen diffusion effect and recovery performance, and can deliver islets into epididymal fat pad in the long-term [142].
Due to the highly complex and dynamic processes of the ECM matrix of different tissues or organs in vivo, it is difficult for a single type of hydrogel to be used to culture various organoids. Based on chemical copolymerization or modification and physical blending, a new type of hybrid hydrogel with a broader range of adjustable mechanical and biochemical properties can be prepared, which provides an excellent solution to meet the needs of different organoid cultures. Specifically, there are two types of hybrid hydrogels based on synthetic polymers, namely hybrid synthetic-synthetic hydrogels and hybrid synthetic-natural hydrogels. These hybrid hydrogels consisting of two or more polymers can be tailored for biodegradability, reactivity, and stiffness for different organoids. Under normal circumstances, fully synthetic hydrogels are stable and easy to customize, and naturally derived hydrogels have excellent biochemical properties when used in cell culture. Using hybrid synthetic-natural hydrogels to integrate their respective advantages for organoid culture may be a powerful option. For instance, a novel hybrid hydrogel obtained by enzymatic reaction incorporating laminin onto a chemically fully synthesized PEG polymer can be used for pancreatic progenitor cell culture with comparable efficacy to commercial Matrigel [114].
4. Applications of defined hydrogels in organoid research
The incorporation of defined hydrogels into organoids has brought about significant advancements in the field of tissue engineering and disease modeling. By offering precise control over tissue architecture and cellular signaling, defined hydrogels allow researchers to more accurately mimic the complex microenvironments of various organs in vitro. Compared to traditional animal models and monolayer cultures, organoids grown in defined hydrogels offer attractive organotypic models in biomedical research. Additionally, the use of well-defined hydrogels can help to control the high variability in organoid architecture, phenotype, and cellular composition, further expanding their applications to broader fields of organ development, disease studies, drug testing, and regenerative medicine (Fig. 4).
Fig. 4.
The representative applications of incorporating organoids into defined hydrogels. (A). PEG hydrogels were implanted with mouse intestinal stem cells to assess whether the hydrogels could support intestinal stem cell expansion and organoid formation (reprinted with permission from Ref. [144]). (B). Hyaluronan was used to emulate the matrix components and mechanical transduction pathways of cholangiocyte self-assembly involved to establish a model for exploring hepatobiliary diseases (reprinted with permission from Ref. [167]). (C). Biomimetic hydrogel composed of hyaluronan and matrix metalloproteinase-cleavable were used to mimic extracellular matrix of breast cancer organoidsfor drug testing (reprinted with permission from Ref. [27]). (D). Fully defined synthetic hydrogel based on a four-armed, maleimide-terminated poly (ethylene glycol) macromer was developed for robust and highly repeatable in vitro human intestinal organoids production and enhancement of colonic wound repair (reprinted with permission from Ref. [168]).
4.1. Organ formation and development
During organogenesis, engineered matrices influence tissue development, maturation and maintenance by regulating stem cell niches, with great potential to help uncover developmental processes. Biophysical factors in the extracellular environment can cause organoid self-organization and morphogenetic rearrangement.
With the development in the molecularly defined hydrogels, multiple biophysical factors that regulate and influence cell fate, pluripotency, and epigenetic inheritance have been further identified [143]. A classic study reported for the first time a fully defined ECM-based organoid culture system expanded from human intestinal stem cells (ISC) [144]. In particular, Lutolf et al. developed RGD-functionalized PEG gels to create the smallest chemically defined environment [144,145]. The results showed that enteric stem cells cultured in PEG-RGD could maintain cell morphology and Lgr5 expression after four passages, indicating that enteric stem cells could be successfully expanded in this artificial ECMs and re-formed into organoids if re-embedded in Matrigel. In addition, intestinal stem cells and the cultured microenvironment can communicate signals through mechanical factors to regulate cell fate and development. In human embryonic development, mechanical factors are also involved in the formation of organoid patterns and organogenesis. In vitro simulations, the stem cell niche enables precise control of the physicochemical properties of the cellular microenvironment and the study of the influence of mechanical factors, such as matrix hardness or other applied forces on stem cell differentiation and function [146]. YAP is a key effector of Hippo pathway in intestinal organoid formation and tissue regeneration in vitro. The defined hydrogels can more conveniently control the physicochemical and mechanical properties of the niche to study the regulatory effect of mechanical properties on stem cell growth and development. Indeed, the PEG hydrogel just described can also be also used to study the separation morphology outcomes in artificial ECMs [144,145]. The results showed that the activation of YAP in soft matrix was significantly higher than that in high stiffness matrix during stem cell differentiation. These studies indicate that composition-defined hydrogels possess tunable mechanical properties and can be used to explore the effects of 3D microenvironment on organoid formation processes, such as stem cell proliferation and self-organizing assembly.
4.2. Disease studies
Since organoids are more complex than single cell cultures and have morphology and function closer to the maternal organ, organoid technology has great potential for application in disease modeling. Current research has developed organ models for multiple diseases to reproduce chronic diseases, bacterial infections, or cancer, and has demonstrated that organoids can reproduce typical pathological features of human diseases [147]. For example, a 3D inner ear organ derived from stem cells was used to study TMPRSS3-related degeneration of defective hair cells (HCs), shedding light on the development of HCs and the cellular role of TMPRSS3 [148].
Hydrogel materials can provide similar microenvironments related to disease states and serve as artificial ECMs for 3D multicellular tissue culture in vitro. In recent years, the cellular microenvironment has attracted more and more attention due to its tunability, which has promoted the study of hydrogel-based disease models in vitro. For example, hypoxia is a microenvironmental feature of many diseases, especially cancer. Some pathological tissues, such as rheumatoid arthritis, inflammatory diseases, diabetes, and cancer, suffer from hypoxia to varying degrees compared with normal tissues and organs. To date, researchers have developed defined hydrogel systems that mimic the tumor hypoxic microenvironment to study tumor growth and enable anti-cancer drug screening and mechanistic studies. When malignant tumors grow, tumor cells rapidly consume oxygen and other nutrients due to infinite proliferation. Shen et al. developed a 3D culture system based on acrylic hyaluronic acid (AHA) hydrogel material to study the effects of matrix mechanical strength and oxygen tension on tumor cell growth. This culture system promotes the generation of functional vascular networks and stimulates endothelial cell (EC) proliferation and angiogenesis in tumors [149]. Indeed, the development of compositional hydrogels has facilitated the conversion of organoid models to clinical applications more than natural substrates. Recently, Rizwan et al. designed a series of compositive determined viscoelastic HA hydrogels for culture of primary bile duct cells. By simulating the stress relaxation rate of liver tissue, the development of cholangiocytes organoids was promoted and the expression of YAP target genes was significantly increased, paving the way for clinical application of defined hydrogels in disease modeling [150]. In fact, organoid disease modeling still has the limitation that it is difficult to control organoid function after assembly, which limits the further utilization of organoids. Compositive biomaterials can further regulate organoid growth and development by modulating the mechanical properties of the substrate to mimic the in vivo environment, thus allowing organoids to be cultured in a controlled manner [151]. In view of the booming development of organoid technology, organoids will provide more convenience for disease mechanism modeling and clinical treatment in the future.
4.3. Drug testing
Defined hydrogel materials will allow organ-specific compartmentalization and spatiotemporal definition of niche factors, and these complex microphysiological systems that reconstruct the in vivo cross-talk between different organs will provide powerful models of human physiology for drug screening [152]. Rajan et al. constructed a microfluidic device with biocompatible thermoplastic polymers to achieve the synthesis of 3D cell-loaded hydrogels and construct an organoid system. They tested functional drug responses with capecitabine and isophosphoramide, respectively, using 20 different cell types (primary and IPS-derived) that mimic basic circulatory and physiological functions in the complex human body. These cells remained highly active in a simulated cycle for 21 days, after which they studied the liver metabolism of the prodrug capecitabine and observed physiological toxicity to lung and heart organoids. By the drug test, they demonstrated that multiple organoid systems produce more complex responses, with changes in one organoid affecting the physiological functions of several others [153]. This organoid system based on defined hydrogels provides a platform for exploring complex drug and toxin interactions between tissue types in vitro.
Drug screening based on tumor organoids is to establish tumor organoid model and tumor organoid library to evaluate the anti-tumor activity and toxicity of drugs and to screen drug targets and drug sensitivities. Tumor microenvironment is an important factor affecting cell proliferation, differentiation, metastasis, and other biological characteristics. In addition to tumor cells themselves, there are ECM and cytokines in the tumor microenvironment. Therefore, ECM components are indispensable to the construction of tumor microenvironment in vitro. Compositional defined hydrogels offer a broader opportunity to optimize and reconstruct tissue-specific microenvironments. Some chemically synthesized composition-determined hydrogel materials enable efficient and consistent network formation and uniform cell distribution, providing customized advantages over biochemical, mechanical, and degradation-related features. Ng et al. used enzyme cross-linked hydrogels synthesized from gelatin-phenol and hyaluronic acid-phenol to identify the mechanical and biochemical properties of organoids, and found that gelatin-based ECM hydrogels could maintain the activity of transplanted organoids derived from colorectal cancer patients. They tested the sensitivity of tumor organoids grown on the defined hydrogel, and the results were comparable to clinical trials [154]. With the further development of organoid technology, organoids can be modified at the gene level in the future, which will provide better conditions for the research of cancer occurrence and development. The screening of anticancer drugs using organoids as preclinical cancer models has the characteristics of precision, convenience, and high throughput, which makes up for the defects of existing major cancer models to the greatest extent.
4.4. Regenerative medicine
Currently commercially available ECMs use animal-derived substrates, which have limitations in clinical application, as it contains some residual ECM-derived DNA, endotoxins and inflammatory proteins, which may interfere with organoid growth and tissue repair effects [11,19,155].
Polymer-based synthetic hydrogels are less likely to elicit immunogenic or pathogenic responses than animal-derived matrices. op ‘t Veld et al. synthesized a thermosensitive hydrogel with properties similar to collagen from polyisocyanate peptides and functionalized it with RGD peptide to improve biocompatibility. Based on experiments in mice, they found that the number of granulocytes in the wound was significantly reduced in the PIC gel treatment group, and other treatment effects were consistent with the results of PIC-RGD. Therefore, this PIC hydrogel can be used as a wound dressing without RGD functionalization, which proves that the defined hydrogel is chemically more stable and has good biocompatibility [156]. In another typical study, Cruz-Acuña et al. synthesized four-armed, maleimide-terminated poly (ethylene glycol) hydrogel and used it as an ECM to generate 3D intestinal organoid in vitro from human ESC and iPSCs. They used hydrogel as an injection carrier to deliver intestinal organs to intestinal wounds through colonoscopy, thus achieving the implantation of intestinal organs and improving the repair of colon wounds. This 3D organoid culture based on defined hydrogels is expected to be extended to the in vitro generation and in vivo repair of other organoids in regenerative medicine [157].
5. Conclusions and perspectives
The development of organoid technology has brought opportunities to reproduce human tissues and organs in vitro as an advanced tool in basic biology and clinical medical research. Complex components and uncertain properties of existing matrices brought many limitations to the realization of the concept. Establishing a good manufacturing practice (GMP) standard system for organoid expansion is the premise of clinical application. Several defined hydrogels, including natural and synthetic sources, have shown promising results in revolutionize the organoid ranging from basic disease modelling to personalized medicine. Specifically, natural hydrogels based on single component polymers have potential to be an ideal choice for organoid transformation due to their clear composition and adjustable properties. However, the hydrogels still have some limitations, such as poor mechanical properties, rapid degradation and low stability, which also stimulated the exploration of directly using fully synthetic polymers to construct well-defined hydrogels for organoid culture. The above two types of defined hydrogels are not mutually exclusive, and it is common to complement their advantages in many specific research works for constructing organoid culture systems with richer mechanical and biochemical properties [158].
Indeed, the ideal organoid culture matrix should mimic the dynamic characteristics of ECM, including viscoelasticity, erosion rate and degradation sensitivity, which change with cell growth and differentiation. These are becoming a reality with breakthroughs in materials science and processing technology. Integrating some stimuli-responsive (such as temperature, ions, pH, and light) components into those hydrogel 3D networks for organoid culture can better mimic the constantly changing microenvironment in vivo. Among these, Light is emerging as the most promising stimulus, which can provide dose tunability, wavelength orthogonality and high spatiotemporal controllability. Recently, photoisomerization is an attractive method to design the defined hydrogel scaffolds with light response. Azobenzene is a typical photochromic molecule, which is trans isomer under visible light, and can be transformed into cis isomerization under ultraviolet light. This structural transition is reversible, which allows the hydrogel cross-linked by the molecule to reversibly harden or soften in response to light stimulation [159]. Additionally, for constructing 3D bioartificial organs, the size and complexity of organoids needs to be expanded. Addressing some concerns about reproducibility, automation, and standardization when mimicking organ tissue structures becomes critical. The utilizing of some engineering methods such as micromolding, microfluidics and 3D printing in the context of defined hydrogels could help overcome the limit of large scale tissue sizes, precise architectures and automation in organoid production [160]. Moreover, the combination of defined hydrogels with more functional signaling cues, such as those biochemical and biophysical properties, etc., we have mentioned at the beginning, may also be a significant opportunity for tailoring specific organoids to exclusive niches. We envision that one day, organoid cells can be directly transformed into specific tissues or organs via customized “off-the-shelf” defined hydrogels according to user needs without adding various inducing factors or supplementing with more cumbersome operation processes.
To improve cell survival and integration in organoids, the interactions between donor cells, material and host must be further clarified and coordinated, which requires the concerted efforts of researcher from a multidisciplinary field. In the years to come, as the improvement of these defined biomaterials and engineering techniques, organoid platforms will undoubtedly play a critical role in understanding disease mechanisms, revolutionizing personalized medicine, and developing new regenerative therapies.
Ethics approval and consent to participate
The authors declare no studies related to ethics problems.
Declaration of competing interest
The authors declare no conflict of interest.
All authors have read and approved the submission of this manuscript. And we also confirm that this manuscript, or its contents in some other form, has not been published previously by any of the authors and/or is not under consideration for publication in another journal at the time of submission.
Acknowledgement
This research was supported by the National Key R&D Program of China (No. 2022YFA1104700), National Natural Science Foundation of China (No. 31971373, 32101163), the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant (No. XDB29050301), Yunnan Key Research and Development Program (No.202003AD150009), Innovation Program of Science and Research from the DICP, CAS (DICP I202128).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Youhanna S., Kemas A.M., Preiss L., Zhou Y., Shen J.X., Cakal S.D., Paqualini F.S., Goparaju S.K., Shafagh R.Z., Lind J.U., Sellgren C.M., Lauschke V.M. Organotypic and microphysiological human tissue models for drug discovery and development—current state-of-the-art and future perspectives. Pharmacol. Rev. 2022;74:141. doi: 10.1124/pharmrev.120.000238. [DOI] [PubMed] [Google Scholar]
- 2.Corrò C., Novellasdemunt L., Li V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020;319:C151–C165. doi: 10.1152/ajpcell.00120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organoids Nat. Biotechnol. 2021;39:1076. doi: 10.1038/s41587-021-01055-7. 1076. [DOI] [PubMed] [Google Scholar]
- 4.de Souza N. Organoid culture. Nat. Methods. 2017;14:35. doi: 10.1038/nmeth.4122. 35. [DOI] [Google Scholar]
- 5.Kogler S., Kømurcu K.S., Olsen C., Shoji J.-y., Skottvoll F.S., Krauss S., Wilson S.R., Røberg-Larsen H. Organoids, organ-on-a-chip, separation science and mass spectrometry: an update. TrAC, Trends Anal. Chem. 2023;161 doi: 10.1016/j.trac.2023.116996. [DOI] [Google Scholar]
- 6.Takebe T., Wells J.M. Organoids by design. Science. 2019;364:956–959. doi: 10.1126/science.aaw7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kratochvil M.J., Seymour A.J., Li T.L., Paşca S.P., Kuo C.J., Heilshorn S.C. Engineered materials for organoid systems. Nat. Rev. Mater. 2019;4:606–622. doi: 10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magno V., Meinhardt A., Werner C. Polymer hydrogels to guide organotypic and organoid cultures. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202000097. [DOI] [Google Scholar]
- 9.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y., Li R., Han C., Huang L. Extracellular matrix grafts: from preparation to application (Review) Int. J. Mol. Med. 2021;47:463–474. doi: 10.3892/ijmm.2020.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozlowski M.T., Crook C.J., Ku H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021;4:1387. doi: 10.1038/s42003-021-02910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang G., Chen Y.-C., Lu H., Jin D. Advances in spheroids and organoids on a chip, advanced functional materials n/a. 2023. [DOI]
- 13.LeSavage B.L., Suhar R.A., Broguiere N., Lutolf M.P., Heilshorn S.C. Next-generation cancer organoids. Nat. Mater. 2022;21:143–159. doi: 10.1038/s41563-021-01057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ergun C., Parmaksiz M., Vurat M.T., Elcin A.E., Elcin Y.M. Decellularized liver ECM-based 3D scaffolds: compositional, physical, chemical, rheological, thermal, mechanical, and in vitro biological evaluations. Int. J. Biol. Macromol. 2022;200:110–123. doi: 10.1016/j.ijbiomac.2021.12.086. [DOI] [PubMed] [Google Scholar]
- 15.Jiang K., Chaimov D., Patel S.N., Liang J.P., Wiggins S.C., Samojlik M.M., Rubiano A., Simmons C.S., Stabler C.L. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 2019;198:37–48. doi: 10.1016/j.biomaterials.2018.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saldin L.T., Cramer M.C., Velankar S.S., White L.J., Badylak S.F. Extracellular matrix hydrogels from decellularized tissues: structure and function. Acta Biomater. 2017;49:1–15. doi: 10.1016/j.actbio.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willemse J., van Tienderen G., van Hengel E., Schurink I., van der Ven D., Kan Y., de Ruiter P., Rosmark O., Westergren-Thorsson G.G., Schneeberger K., van der Eerden B., Roest H., Spee B., van der Laan L., de Jonge J., Verstegen M. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121473. [DOI] [PubMed] [Google Scholar]
- 18.Kaur S., Kaur I., Rawal P., Tripathi D.M., Vasudevan A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021;504:58–66. doi: 10.1016/j.canlet.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L., Yuhan J., Yu H., Zhang B., Huang K., Zhu L. Decellularized extracellular matrix for remodeling bioengineering organoid's microenvironment, small n/a. 2023. [DOI] [PubMed]
- 20.Broguiere N., Isenmann L., Hirt C., Ringel T., Placzek S., Cavalli E., Ringnalda F., Villiger L., Züllig R., Lehmann R., Rogler G., Heim M.H., Schüler J., Zenobi-Wong M., Schwank G. Growth of epithelial organoids in a defined hydrogel. Adv. Mater. 2018;30 doi: 10.1002/adma.201801621. [DOI] [PubMed] [Google Scholar]
- 21.Naahidi S., Jafari M., Logan M., Wang Y., Yuan Y., Bae H., Dixon B., Chen P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017;35:530–544. doi: 10.1016/j.biotechadv.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Sachs N., Tsukamoto Y., Kujala P., Peters P.J., Clevers H. Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development. 2017;144:1107–1112. doi: 10.1242/dev.143933. [DOI] [PubMed] [Google Scholar]
- 23.Sandilya S., Singh S. Development of islet organoids from human induced pluripotent stem cells in a cross-linked collagen scaffold. Cell Regen. 2021;10:38. doi: 10.1186/s13619-021-00099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillion K., Mahe M.M. Redesigning hydrogel geometry for enhanced organoids. Nat. Methods. 2022;19:1347–1348. doi: 10.1038/s41592-022-01656-3. [DOI] [PubMed] [Google Scholar]
- 25.Unal A.Z., West J.L. Synthetic ECM: bioactive synthetic hydrogels for 3D tissue engineering. Bioconjugate Chem. 2020;31:2253–2271. doi: 10.1021/acs.bioconjchem.0c00270. [DOI] [PubMed] [Google Scholar]
- 26.Chooi W.H., Ng C.Y., Ow V., Harley J., Ng W., Hor J.-H., Low K.E., Malleret B., Xue K., Ng S.-Y. Advanced Healthcare Materials n/a; 2022. Defined Alginate Hydrogels Support Spinal Cord Organoid Derivation, Maturation, and Modeling of Spinal Cord Diseases. [DOI] [PubMed] [Google Scholar]
- 27.Baker A.E.G., Bahlmann L.C., Xue C., Lu Y.H., Chin A.A., Cruickshank J., Cescon D.W., Shoichet M.S. Chemically and mechanically defined hyaluronan hydrogels emulate the extracellular matrix for unbiased in vivo and in vitro organoid formation and drug testing in cancer. Mater. Today. 2022;56:96–113. doi: 10.1016/j.mattod.2022.01.023. [DOI] [Google Scholar]
- 28.Jung N., Moreth T., Stelzer E.H.K., Pampaloni F., Windbergs M. Non-invasive analysis of pancreas organoids in synthetic hydrogels defines material-cell interactions and luminal composition. Biomater. Sci. 2021;9:5415–5426. doi: 10.1039/D1BM00597A. [DOI] [PubMed] [Google Scholar]
- 29.Chrisnandy A., Blondel D., Rezakhani S., Broguiere N., Lutolf M.P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 2022;21:479–487. doi: 10.1038/s41563-021-01136-7. [DOI] [PubMed] [Google Scholar]
- 30.Davidov T., Efraim Y., Hayam R., Oieni J., Baruch L., Machluf M. Extracellular matrix hydrogels originated from different organs mediate tissue-specific properties and function. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung S.Y., Sergeeva Y., Pan G., Mittler S., Ederth T., Dam T., Jönsson P., El-Schich Z., Wingren A.G., Tillo A., Hsiung Mattisson S., Holmqvist B., Stollenwerk M.M., Sellergren B. Reversible self-assembled monolayers with tunable surface dynamics for controlling cell adhesion behavior. ACS Appl. Mater. Interfaces. 2022;14:41790–41799. doi: 10.1021/acsami.2c12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Below C.R., Kelly J., Brown A., Humphries J.D., Hutton C., Xu J., Lee B.Y., Cintas C., Zhang X., Hernandez-Gordillo V., Stockdale L., Goldsworthy M.A., Geraghty J., Foster L., O'Reilly D.A., Schedding B., Askari J., Burns J., Hodson N., Smith D.L., Lally C., Ashton G., Knight D., Mironov A., Banyard A., Eble J.A., Morton J.P., Humphries M.J., Griffith L.G., Jorgensen C. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater. 2022;21:110–119. doi: 10.1038/s41563-021-01085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz M. Mechanotransduction through integrin-mediated adhesions. Faseb. J. 2020;34 doi: 10.1096/fasebj.2020.34.s1.00148. [DOI] [Google Scholar]
- 34.Lou J., Mooney D.J. Chemical strategies to engineer hydrogels for cell culture. Nat. Rev. Chem. 2022;6:726–744. doi: 10.1038/s41570-022-00420-7. [DOI] [PubMed] [Google Scholar]
- 35.Yamada Y., Katagiri F., Hozumi K., Kikkawa Y., Nomizu M. Cell behavior on protein matrices containing laminin α1 peptide AG73. Biomaterials. 2011;32:4327–4335. doi: 10.1016/j.biomaterials.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 36.Huettner N., Dargaville T.R., Forget A. Discovering cell-adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. 2018;36:372–383. doi: 10.1016/j.tibtech.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Teng B., Zhang S., Pan J., Zeng Z., Chen Y., Hei Y., Fu X., Li Q., Ma M., Sui Y., Wei S. A chondrogenesis induction system based on a functionalized hyaluronic acid hydrogel sequentially promoting hMSC proliferation, condensation, differentiation, and matrix deposition. Acta Biomater. 2021;122:145–159. doi: 10.1016/j.actbio.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 38.Merenda A., Fenderico N., Maurice M.M. Wnt signaling in 3D: recent advances in the applications of intestinal organoids. Trends Cell Biol. 2020;30:60–73. doi: 10.1016/j.tcb.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Lee S.S., Hsu E.L., Mendoza M., Ghodasra J., Nickoli M.S., Ashtekar A., Polavarapu M., Babu J., Riaz R.M., Nicolas J.D., Nelson D., Hashmi S.Z., Kaltz S.R., Earhart J.S., Merk B.R., McKee J.S., Bairstow S.F., Shah R.N., Hsu W.K., Stupp S.I. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv Healthc Mater. 2015;4:131–141. doi: 10.1002/adhm.201400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis J.A., Freeman R., Carrow J.K., Clemons T.D., Palmer L.C., Stupp S.I. Transforming growth facto beta-1 binding by peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. 2020;6:4551–4560. doi: 10.1021/acsbiomaterials.0c00679. [DOI] [PubMed] [Google Scholar]
- 41.Fan V.H., Tamama K., Au A., Littrell R., Richardson L.B., Wright J.W., Wells A., Griffith L.G. Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cell. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 42.Diba M., Spaans S., Hendrikse S.I.S., Bastings M.M.C., Schotman M.J.G., van Sprang J.F., Wu D.J., Hoeben F.J.M., Janssen H.M., Dankers P.Y.W. Engineering the dynamics of cell adhesion cues in supramolecular hydrogels for facile control over cell encapsulation and behavior. Adv. Mater. 2021;33 doi: 10.1002/adma.202008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chrisnandy A., Blondel D., Rezakhani S., Broguiere N., Lutolf M.P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 2022;21:479. doi: 10.1038/s41563-021-01136-7. [DOI] [PubMed] [Google Scholar]
- 44.Neumann A.J., Quinn T., Bryant S.J. Nondestructive evaluation of a new hydrolytically degradable and photo-clickable PEG hydrogel for cartilage tissue engineering. Acta Biomater. 2016;39:1–11. doi: 10.1016/j.actbio.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenfeld A., Göckler T., Kuzina M., Reischl M., Schepers U., Levkin P.A. Designing inherently photodegradable cell-adhesive hydrogels for 3D cell culture. Adv. Healthcare Materi. 2021;10 doi: 10.1002/adhm.202100632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calitz C., Pavlovic N., Rosenquist J., Zagami C., Samanta A., Heindryckx F. A biomimetic model for liver cancer to study tumor-stroma interactions in a 3D environment with tunable bio-physical properties. J. Vis. Exp. 2020 doi: 10.3791/61606. [DOI] [PubMed] [Google Scholar]
- 47.El-Rashidy A.A., El Moshy S., Radwan I.A., Rady D., Abbass M.M.S., Dorfer C.E., Fawzy El-Sayed K.M. Effect of polymeric matrix stiffness on osteogenic differentiation of mesenchymal stem/progenitor cells: concise review. Polymers. 2021;13 doi: 10.3390/polym13172950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z., Pearce A.K., Dove A.P., O'Reilly R.K. Precise tuning of polymeric fiber dimensions to enhance the mechanical properties of alginate hydrogel matrices. Polymers. 2021;13:2202. doi: 10.3390/polym13132202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rizwan M., Baker A.E.G., Shoichet M.S. Designing hydrogels for 3D cell culture using dynamic covalent crosslinking. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202100234. [DOI] [PubMed] [Google Scholar]
- 50.Ting M.S., Travas-Sejdic J., Malmström J. Modulation of hydrogel stiffness by external stimuli: soft materials for mechanotransduction studies. J. Mater. Chem. B. 2021;9:7578–7596. doi: 10.1039/D1TB01415C. [DOI] [PubMed] [Google Scholar]
- 51.Jung M., Ghamrawi S., Du E.Y., Gooding J.J., Kavallaris M. Advances in 3D bioprinting for cancer biology and precision medicine: from matrix design to application. Adv Healthc Mater. 2022 doi: 10.1002/adhm.202200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin C., Xu K., He Y., Tao B., Yuan Z., Li K., Li X., Xia Z., Cai K. A dynamic matrix potentiates mesenchymal stromal cell paracrine function via an effective mechanical dose. Biomater. Sci. 2020;8:4779–4791. doi: 10.1039/D0BM01012J. [DOI] [PubMed] [Google Scholar]
- 53.Rosales A.M., Vega S.L., DelRio F.W., Burdick J.A., Anseth K.S. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew Chem. Int. Ed. Engl. 2017;56:12132–12136. doi: 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee I.N., Dobre O., Richards D., Ballestrem C., Curran J.M., Hunt J.A., Richardson S.M., Swift J., Wong L.S. Photoresponsive hydrogels with photoswitchable mechanical properties allow time-resolved analysis of cellular responses to matrix stiffening. ACS Appl. Mater. Interfaces. 2018;10:7765–7776. doi: 10.1021/acsami.7b18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T., Nanda S.S., Papaefthymiou G.C., Yi D.K. Mechanophysical cues in extracellular matrix regulation of cell behavior. Chembiochem. 2020;21:1254–1264. doi: 10.1002/cbic.201900686. [DOI] [PubMed] [Google Scholar]
- 56.Ma Z., Wang J., Loskill P., Huebsch N., Koo S., Svedlund F.L., Marks N.C., Hua E.W., Grigoropoulos C.P., Conklin B.R., Healy K.E. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 2015;6:7413. doi: 10.1038/ncomms8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H., Wang Y., Wang H., Zhao M., Tao T., Zhang X., Qin J. A droplet microfluidic system to fabricate hybrid capsules enabling stem cell organoid engineering. Adv. Sci. 2020;7 doi: 10.1002/advs.201903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brandenberg N., Hoehnel S., Kuttler F., Homicsko K., Ceroni C., Ringel T., Gjorevski N., Schwank G., Coukos G., Turcatti G., Lutolf M.P. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020;4:863. doi: 10.1038/s41551-020-0565-2. [DOI] [PubMed] [Google Scholar]
- 59.Tseng T.C., Hsieh F.Y., Theato P., Wei Y., Hsu S.H. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials. 2017;133:20–28. doi: 10.1016/j.biomaterials.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019;6 doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikolaev M., Mitrofanova O., Broguiere N., Geraldo S., Dutta D., Tabata Y., Elci B., Brandenberg N., Kolotuev I., Gjorevski N., Clevers H., Lutolf M.P. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 2020;585:574. doi: 10.1038/s41586-020-2724-8. [DOI] [PubMed] [Google Scholar]
- 62.Liu H., Wang Y., Cui K., Guo Y., Zhang X., Qin J. Advances in hydrogels in organoids and organs-on-a-chip. Adv. Mater. 2019;31 doi: 10.1002/adma.201902042. [DOI] [PubMed] [Google Scholar]
- 63.Ma Y., Han T., Yang Q., Wang J., Feng B., Jia Y., Wei Z., Xu F. Viscoelastic cell microenvironment: hydrogel-based strategy for recapitulating dynamic ECM mechanics. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202100848. [DOI] [Google Scholar]
- 64.Hu W., Wang Z., Xiao Y., Zhang S., Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019;7:843–855. doi: 10.1039/C8BM01246F. [DOI] [PubMed] [Google Scholar]
- 65.Kloxin C.J., Bowman C.N. Covalent adaptable networks: smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013;42:7161–7173. doi: 10.1039/C3CS60046G. [DOI] [PubMed] [Google Scholar]
- 66.Yu L., Ding J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008;37:1473–1481. doi: 10.1039/B713009K. [DOI] [PubMed] [Google Scholar]
- 67.Janarthanan G., Noh I. Recent trends in metal ion based hydrogel biomaterials for tissue engineering and other biomedical applications. J. Mater. Sci. Technol. 2021;63:35–53. doi: 10.1016/j.jmst.2020.02.052. [DOI] [Google Scholar]
- 68.Fredrick R., Podder A., Viswanathan A., Bhuniya S. Synthesis and characterization of polysaccharide hydrogel based on hydrophobic interactions. J. Appl. Polym. Sci. 2019;136 doi: 10.1002/app.47665. [DOI] [Google Scholar]
- 69.Perera M.M., Ayres N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem. 2020;11:1410–1423. doi: 10.1039/C9PY01694E. [DOI] [Google Scholar]
- 70.Tang S., Richardson B.M., Anseth K.S. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater. Sci. 2021;120 doi: 10.1016/j.pmatsci.2020.100738. [DOI] [Google Scholar]
- 71.Ghanian M.H., Mirzadeh H., Baharvand H. In situ forming, cytocompatible, and self-recoverable tough hydrogels based on dual ionic and click cross-linked alginate. Biomacromolecules. 2018;19:1646–1662. doi: 10.1021/acs.biomac.8b00140. [DOI] [PubMed] [Google Scholar]
- 72.Rizwan M., Baker A.E.G., Shoichet M.S. Designing hydrogels for 3D cell culture using dynamic covalent crosslinking. Adv. Healthcare Materi. 2021;10 doi: 10.1002/adhm.202100234. [DOI] [PubMed] [Google Scholar]
- 73.Agarwal T., Celikkin N., Costantini M., Maiti T.K., Makvandi P. Recent advances in chemically defined and tunable hydrogel platforms for organoid culture. Bio-Design. Manuf. 2021;4:641–674. doi: 10.1007/s42242-021-00126-7. [DOI] [Google Scholar]
- 74.Kim B.-S., Park I.-K., Hoshiba T., Jiang H.-L., Choi Y.-J., Akaike T., Cho C.-S. Design of artificial extracellular matrices for tissue engineering. Prog. Polym. Sci. 2011;36:238–268. doi: 10.1016/j.progpolymsci.2010.10.001. [DOI] [Google Scholar]
- 75.Catoira M.C., Fusaro L., Di Francesco D., Ramella M., Boccafoschi F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019;30:115. doi: 10.1007/s10856-019-6318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kort-Mascort J., Bao G., Elkashty O., Flores-Torres S., Munguia-Lopez J.G., Jiang T., Ehrlicher A.J., Mongeau L., Tran S.D., Kinsella J.M. Decellularized extracellular matrix composite hydrogel bioinks for the development of 3D bioprinted head and neck in vitro tumor models. ACS Biomater. Sci. Eng. 2021;7:5288–5300. doi: 10.1021/acsbiomaterials.1c00812. [DOI] [PubMed] [Google Scholar]
- 77.Ahmed A., Joshi I.M., Mansouri M., Ahamed N.N.N., Hsu M.C., Gaborski T.R., Abhyankar V.V. Engineering fiber anisotropy within natural collagen hydrogels. Am. J. Physiol. Cell Physiol. 2021;320:C1112–C1124. doi: 10.1152/ajpcell.00036.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blanco-Fernandez B., Gaspar V.M., Engel E., Mano J.F. Proteinaceous hydrogels for bioengineering advanced 3D tumor models. Adv. Sci. 2021;8 doi: 10.1002/advs.202003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu T., Lo A.C.Y. Collagen-alginate composite hydrogel: application in tissue engineering and biomedical sciences. Polymers. 2021;13 doi: 10.3390/polym13111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salamone M., Rigogliuso S., Nicosia A., Campora S., Bruno C.M., Ghersi G. 3D collagen hydrogel promotes in vitro langerhans islets vascularization through ad-MVFs angiogenic activity. Biomedicines. 2021;9 doi: 10.3390/biomedicines9070739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwab A., Hélary C., Richards R.G., Alini M., Eglin D., D'Este M. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater. Today Bio. 2020;7 doi: 10.1016/j.mtbio.2020.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adali T., Kalkan R., Karimizarandi L. The chondrocyte cell proliferation of a chitosan/silk fibroin/egg shell membrane hydrogels. Int. J. Biol. Macromol. 2019;124:541–547. doi: 10.1016/j.ijbiomac.2018.11.226. [DOI] [PubMed] [Google Scholar]
- 83.Rico-Llanos G.A., Borrego-González S., Moncayo-Donoso M., Becerra J., Visser R. Polymers; 2021. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jain R., Roy S. Designing a bioactive scaffold from coassembled collagen-laminin short peptide hydrogels for controlling cell behaviour. RSC Adv. 2019;9:38745–38759. doi: 10.1039/c9ra07454f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pathmanapan S., Periyathambi P., Anandasadagopan S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair - in vitro and in vivo study. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102251. [DOI] [PubMed] [Google Scholar]
- 86.Keating M., Lim M., Hu Q., Botvinick E. Selective stiffening of fibrin hydrogels with micron resolution via photocrosslinking. Acta Biomater. 2019;87:88–96. doi: 10.1016/j.actbio.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y., Liu H., Zhang M., Wang H., Chen W., Qin J. One-step synthesis of composite hydrogel capsules to support liver organoid generation from hiPSCs. Biomater. Sci. 2020;8:5476–5488. doi: 10.1039/d0bm01085e. [DOI] [PubMed] [Google Scholar]
- 88.Deller R.C., Richardson T., Richardson R., Bevan L., Zampetakis I., Scarpa F., Perriman A.W. Artificial cell membrane binding thrombin constructs drive in situ fibrin hydrogel formation. Nat. Commun. 2019;10:1887. doi: 10.1038/s41467-019-09763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Contessi Negrini N., Angelova Volponi A., Sharpe P.T., Celiz A.D. Tunable cross-linking and adhesion of gelatin hydrogels via bioorthogonal click chemistry. ACS Biomater. Sci. Eng. 2021;7:4330–4346. doi: 10.1021/acsbiomaterials.1c00136. [DOI] [PubMed] [Google Scholar]
- 90.Asim S., Tabish T.A., Liaqat U., Ozbolat I.T., Rizwan M. Advanced Healthcare Materials n/a; 2023. Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang T., Zhao J., Yu S., Mao Z., Gao C., Zhu Y., Mao C., Zheng L. Untangling the response of bone tumor cells and bone forming cells to matrix stiffness and adhesion ligand density by means of hydrogels. Biomaterials. 2019;188:130–143. doi: 10.1016/j.biomaterials.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klotz B.J., Oosterhoff L.A., Utomo L., Lim K.S., Vallmajo-Martin Q., Clevers H., Woodfield T.B.F., Rosenberg A.J.W.P., Malda J., Ehrbar M., Spee B., Gawlitta D. A versatile biosynthetic hydrogel platform for engineering of tissue analogues. Adv. Healthcare Materi. 2019;8 doi: 10.1002/adhm.201900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bernal P.N., Bouwmeester M., Madrid-Wolff J., Falandt M., Florczak S., Rodriguez N.G., Li Y., Größbacher G., Samsom R.-A., van Wolferen M., van der Laan L.J.W., Delrot P., Loterie D., Malda J., Moser C., Spee B., Levato R. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv. Mater. 2022;34 doi: 10.1002/adma.202110054. [DOI] [PubMed] [Google Scholar]
- 94.O'Grady B.J., Balotin K.M., Bosworth A.M., McClatchey P.M., Weinstein R.M., Gupta M., Poole K.S., Bellan L.M., Lippmann E.S. Development of an N-cadherin biofunctionalized hydrogel to support the formation of synaptically connected neural networks. ACS Biomater. Sci. Eng. 2020;6:5811–5822. doi: 10.1021/acsbiomaterials.0c00885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peter M., Singh A., Mohankumar K., Jeenger R., Joge P.A., Gatne M.M., Tayalia P. Gelatin-based matrices as a tunable platform to study in vitro and in vivo 3D cell invasion. ACS Appl. Bio Mater. 2019;2:916–929. doi: 10.1021/acsabm.8b00767. [DOI] [PubMed] [Google Scholar]
- 96.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kambe Y. Functionalization of silk fibroin-based biomaterials for tissue engineering. Polym. J. 2021;53:1345–1351. doi: 10.1038/s41428-021-00536-5. [DOI] [Google Scholar]
- 98.Wang Y., Kim H.-J., Vunjak-Novakovic G., Kaplan D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Liu W., Sun Y., Dong X., Yin Q., Zhu H., Li S., Zhou J., Wang C. Cell-derived extracellular matrix-coated silk fibroin scaffold for cardiogenesis of brown adipose stem cells through modulation of TGF-beta pathway. Regen Biomater. 2020;7:403–412. doi: 10.1093/rb/rbaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao D., Li M., Wang T., Sun F., Su C., Shi T. Viscoelastic silk fibroin hydrogels with tunable strength. ACS Biomater. Sci. Eng. 2021;7:636–647. doi: 10.1021/acsbiomaterials.0c01348. [DOI] [PubMed] [Google Scholar]
- 101.Gupta A.K., Coburn J.M., Davis-Knowlton J., Kimmerling E., Kaplan D.L., Oxburgh L. Scaffolding kidney organoids on silk. J. Regen. Med. Tissue Eng. 2019;13:812–822. doi: 10.1002/term.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gupta A.C., Chawla S., Hegde A., Singh D., Bandyopadhyay B., Lakshmanan C.C., Kalsi G., Ghosh S. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J. Cell. Physiol. 2018;233:9015–9030. doi: 10.1002/jcp.26853. [DOI] [PubMed] [Google Scholar]
- 103.Neves M.I., Araújo M., Moroni L., da Silva R.M.P., Barrias C.C. Glycosaminoglycan-Inspired biomaterials for the development of bioactive hydrogel networks. Molecules. 2020;25:978. doi: 10.3390/molecules25040978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pahlevanzadeh F., Mokhtari H., Bakhsheshi-Rad H.R., Emadi R., Kharaziha M., Valiani A., Poursamar S.A., Ismail A.F., RamaKrishna S., Berto F. Recent trends in three-dimensional bioinks based on alginate for biomedical applications. Materials. 2020;13 doi: 10.3390/ma13183980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fujita S., Wakuda Y., Matsumura M., Suye S.-i. Geometrically customizable alginate hydrogel nanofibers for cell culture platforms. J. Mater. Chem. B. 2019;7:6556–6563. doi: 10.1039/c9tb01353a. [DOI] [PubMed] [Google Scholar]
- 106.Capeling M.M., Czerwinski M., Huang S., Tsai Y.-H., Wu A., Nagy M.S., Juliar B., Sundaram N., Song Y., Han W.M., Takayama S., Alsberg E., Garcia A.J., Helmrath M., Putnam A.J., Spence J.R. Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Rep. 2019;12:381–394. doi: 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang S.M., Lee J.H., Huh Y.S., Takayama S. Alginate microencapsulation for three-dimensional in vitro cell culture. ACS Biomater. Sci. Eng. 2021;7:2864–2879. doi: 10.1021/acsbiomaterials.0c00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.S.N. Patel, M. Ishahak, D. Chaimov, A. Velraj, D. LaShoto, D.W. Hagan, P. Buchwald, E.A. Phelps, A. Agarwal, C.L. Stabler, Organoid microphysiological system preserves pancreatic islet function within 3D matrix, Sci. Adv. 7 eaba5515. 10.1126/sciadv.aba5515. [DOI] [PMC free article] [PubMed]
- 109.Geuens T., Ruiter F.A.A., Schumacher A., Morgan F.L.C., Rademakers T., Wiersma L.E., van den Berg C.W., Rabelink T.J., Baker M.B., LaPointe V.L.S. Thiol-ene cross-linked alginate hydrogel encapsulation modulates the extracellular matrix of kidney organoids by reducing abnormal type 1a1 collagen deposition. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120976. [DOI] [PubMed] [Google Scholar]
- 110.Shariatinia Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019;263:131–194. doi: 10.1016/j.cis.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 111.Coşkun S., Akbulut S.O., Sarıkaya B., Çakmak S., Gümüşderelioğlu M. Formulation of chitosan and chitosan-nanoHAp bioinks and investigation of printability with optimized bioprinting parameters. Int. J. Biol. Macromol. 2022;222:1453–1464. doi: 10.1016/j.ijbiomac.2022.09.078. [DOI] [PubMed] [Google Scholar]
- 112.Liu C., Jin Z., Ge X., Zhang Y., Xu H. Decellularized annulus fibrosus matrix/chitosan hybrid hydrogels with basic fibroblast growth factor for annulus fibrosus tissue engineering. Tissue Eng. 2019;25:1605–1613. doi: 10.1089/ten.tea.2018.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murat E., Dixit V., Gitnic G. Hepatocyte attachment on biodegradable modified chitosan membranes: in vitro evaluation for the development of liver organoids. Artif. Organs. 1998;22:837–846. doi: 10.1046/j.1525-1594.1998.06182.x. [DOI] [PubMed] [Google Scholar]
- 114.Liu H., Wang Y., Cui K., Guo Y., Zhang X., Qin J. Advances in hydrogels in organoids and organs-on-a-chip. Adv. Mater. 2019;31 doi: 10.1002/adma.201902042. [DOI] [PubMed] [Google Scholar]
- 115.Choi J.H., Kim J.S., Kim W.K., Lee W., Kim N., Song C.U., Jung J.J., Song J.E., Khang G. Evaluation of hyaluronic acid/agarose hydrogel for cartilage tissue engineering biomaterial. Macromol. Res. 2020;28:979–985. doi: 10.1007/s13233-020-8137-6. [DOI] [Google Scholar]
- 116.Hauptstein J., Bock T., Bartolf-Kopp M., Forster L., Stahlhut P., Nadernezhad A., Blahetek G., Zernecke-Madsen A., Detsch R., Jungst T., Groll J., Tessmar J., Blunk T. Hyaluronic acid-based bioink composition enabling 3D bioprinting and improving quality of deposited cartilaginous extracellular matrix. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.202000737. [DOI] [PubMed] [Google Scholar]
- 117.Suo A., Xu W., Wang Y., Sun T., Ji L., Qian J. Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells. Carbohydr. Polym. 2019;211:336–348. doi: 10.1016/j.carbpol.2019.01.115. [DOI] [PubMed] [Google Scholar]
- 118.Yasin A., Ren Y., Li J., Sheng Y., Cao C., Zhang K. Advances in hyaluronic acid for biomedical applications. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.910290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khademhosseini A., Eng G., Yeh J., Kucharczyk P.A., Langer R., Vunjak-Novakovic G., Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed. Microdevices. 2007;9:149–157. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- 120.Loebel C., Weiner A.I., Eiken M.K., Katzen J.B., Morley M.P., Bala V., Cardenas-Diaz F.L., Davidson M.D., Shiraishi K., Basil M.C., Ferguson L.T., Spence J.R., Ochs M., Beers M.F., Morrisey E.E., Vaughan A.E., Burdick J.A. Microstructured hydrogels to guide self-assembly and function of lung alveolospheres. Adv. Mater. 2022;34 doi: 10.1002/adma.202202992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang D., Guo Y., Zhu J., Liu F., Xue Y., Huang Y., Zhu B., Wu D., Pan H., Gong T., Lu Y., Yang Y., Wang Z. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2022 doi: 10.1016/j.actbio.2022.06.036. [DOI] [PubMed] [Google Scholar]
- 122.Brown J.W., Mills J.C. Implantable synthetic organoid matrices for intestinal regeneration. Nat. Cell Biol. 2017;19:1307–1308. doi: 10.1038/ncb3635. [DOI] [PubMed] [Google Scholar]
- 123.Liu K., Veenendaal T., Wiendels M., Ruiz-Zapata A.M., van Laar J., Kyranas R., Enting H., van Cranenbroek B., Koenen H.J.P.M., Mihaila S.M., Oosterwijk E., Kouwer P.H.J. Synthetic extracellular matrices as a toolbox to tune stem cell secretome. ACS Appl. Mater. Interfaces. 2020;12:56723–56730. doi: 10.1021/acsami.0c16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu S.Q., Tay R., Khan M., Rachel Ee P.L., Hedrick J.L., Yang Y.Y. Synthetic hydrogels for controlled stem cell differentiation. Soft Matter. 2010;6:67–81. doi: 10.1039/B916705F. [DOI] [Google Scholar]
- 125.Davidson C.D., Jayco D.K.P., Matera D.L., DePalma S.J., Hiraki H.L., Wang W.Y., Baker B.M. Myofibroblast activation in synthetic fibrous matrices composed of dextran vinyl sulfone. Acta Biomater. 2020;105:78–86. doi: 10.1016/j.actbio.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Balion Z., Sipailaite E., Stasyte G., Vailionyte A., Mazetyte-Godiene A., Seskeviciute I., Bernotiene R., Phopase J., Jekabsone A. Investigation of cancer cell migration and proliferation on synthetic extracellular matrix peptide hydrogels. Front. Bioeng. Biotechnol. 2020;8:773. doi: 10.3389/fbioe.2020.00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao Y., Lee B.H., Irvine S.A., Wong Y.S., Bianco Peled H., Venkatraman S. Inclusion of cross-linked elastin in gelatin/PEG hydrogels favourably influences fibroblast phenotype. Polymers. 2020;12 doi: 10.3390/polym12030670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhan H., Löwik D.W.P.M. A hybrid peptide amphiphile fiber PEG hydrogel matrix for 3D cell culture. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201808505. [DOI] [Google Scholar]
- 129.Drumheller P.D., Hubbell J.A. Densely crosslinked polymer networks of poly(ethylene glycol) in trimethylolpropane triacrylate for cell-adhesion-resistant surfaces. J. Biomed. Mater. Res. 1995;29:207–215. doi: 10.1002/jbm.820290211. [DOI] [PubMed] [Google Scholar]
- 130.Vallmajo-Martin Q., Broguiere N., Millan C., Zenobi-Wong M., Ehrbar M. PEG/HA hybrid hydrogels for biologically and mechanically tailorable bone marrow organoids. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201910282. [DOI] [Google Scholar]
- 131.Hayashi S., Sasaki Y., Kubo H., Sawada S.-i., Kinoshita N., Marukawa E., Harada H., Akiyoshi K. Construction of hybrid cell spheroids using cell-sized cross-linked nanogel microspheres as an artificial extracellular matrix. ACS Appl. Bio Mater. 2021;4:7848–7855. doi: 10.1021/acsabm.1c00796. [DOI] [PubMed] [Google Scholar]
- 132.Ng S.S., Saeb-Parsy K., Blackford S.J.I., Segal J.M., Serra M.P., Horcas-Lopez M., No D.Y., Mastoridis S., Jassem W., Frank C.W., Cho N.J., Nakauchi H., Glenn J.S., Rashid S.T. Human iPS derived progenitors bioengineered into liver organoids using an inverted colloidal crystal poly (ethylene glycol) scaffold. Biomaterials. 2018;182:299–311. doi: 10.1016/j.biomaterials.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hernandez-Gordillo V., Kassis T., Lampejo A., Choi G., Gamboa M.E., Gnecco J.S., Brown A., Breault D.T., Carrier R., Griffith L.G. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials. 2020;254 doi: 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilson R.L., Swaminathan G., Ettayebi K., Bomidi C., Zeng X.-L., Blutt S.E., Estes M.K., Grande-Allen K.J. Protein-functionalized poly(ethylene glycol) hydrogels as scaffolds for monolayer organoid culture. Tissue Eng. C Methods. 2020;27:12–23. doi: 10.1089/ten.tec.2020.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y., Tang C., Span P.N., Rowan A.E., Aalders T.W., Schalken J.A., Adema G.J., Kouwer P.H.J., Zegers M.M.P., Ansems M. Polyisocyanide hydrogels as a tunable platform for mammary gland organoid formation. Adv. Sci. 2020;7 doi: 10.1002/advs.202001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye S., Boeter J.W.B., Mihajlovic M., van Steenbeek F.G., van Wolferen M.E., Oosterhoff L.A., Marsee A., Caiazzo M., van der Laan L.J.W., Penning L.C., Vermonden T., Spee B., Schneeberger K. A chemically defined hydrogel for human liver organoid culture. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garreta E., Prado P., Tarantino C., Oria R., Fanlo L., Marti E., Zalvidea D., Trepat X., Roca-Cusachs P., Gavalda-Navarro A., Cozzuto L., Campistol J.M., Izpisua Belmonte J.C., Hurtado Del Pozo C., Montserrat N. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019;18:397–405. doi: 10.1038/s41563-019-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jafari A., Hassanajili S., Ghaffari F., Azarpira N. Modulating the physico-mechanical properties of polyacrylamide/gelatin hydrogels for tissue engineering application. Polym. Bull. 2022;79:1821–1842. doi: 10.1007/s00289-021-03592-2. [DOI] [Google Scholar]
- 140.Shkumatov A., Baek K., Kong H. Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kumar A., Han S.S. PVA-based hydrogels for tissue engineering: a review. Int. J. Poly. Mater. Polym. Biomater. 2017;66:159–182. doi: 10.1080/00914037.2016.1190930. [DOI] [Google Scholar]
- 142.Hunckler M.D., García A.J. Engineered biomaterials for enhanced function of insulin-secreting β-cell organoids. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.202000134. [DOI] [Google Scholar]
- 143.Yi S.A., Zhang Y., Rathnam C., Pongkulapa T., Lee K.-B. Bioengineering approaches for the advanced organoid research. Adv. Mater. 2021;33 doi: 10.1002/adma.202007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gjorevski N., Sachs N., Manfrin A., Giger S., Bragina M.E., Ordóñez-Morán P., Clevers H., Lutolf M.P. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 145.Sorrentino G., Rezakhani S., Yildiz E., Nuciforo S., Heim M.H., Lutolf M.P., Schoonjans K. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 2020;11:3416. doi: 10.1038/s41467-020-17161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rossi G., Manfrin A., Lutolf M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018;19:671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 148.Tang P.-C., Alex A.L., Nie J., Lee J., Roth A.A., Booth K.T., Koehler K.R., Hashino E., Nelson R.F. Defective tmprss3-associated hair cell degeneration in inner ear organoids. Stem Cell Rep. 2019;13:147–162. doi: 10.1016/j.stemcr.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen Y.-I., Abaci H.E., Krupski Y., Weng L.-C., Burdick J.A., Gerecht S. Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting. Biomater. Sci. 2014;2:655–665. doi: 10.1039/C3BM60274E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rizwan M., Ling C., Guo C., Liu T., Jiang J.-X., Bear C.E., Ogawa S., Shoichet M.S. Viscoelastic notch signaling hydrogel induces liver bile duct organoid growth and morphogenesis. bioRxiv. 2022:2022. doi: 10.1101/2022.06.09.495476. 06.09.495476. [DOI] [PubMed] [Google Scholar]
- 151.Rauth S., Karmakar S., Batra S.K., Ponnusamy M.P. Recent advances in organoid development and applications in disease modeling. Biochim. Biophys. Acta Rev. Canc. 2021;1875 doi: 10.1016/j.bbcan.2021.188527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Antunes J., Gaspar V.M., Ferreira L., Monteiro M., Henrique R., Jeronimo C., Mano J.F. In-air production of 3D co-culture tumor spheroid hydrogels for expedited drug screening. Acta Biomater. 2019;94:392–409. doi: 10.1016/j.actbio.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 153.Rajan S.A.P., Aleman J., Wan M., Pourhabibi Zarandi N., Nzou G., Murphy S., Bishop C.E., Sadri-Ardekani H., Shupe T., Atala A., Hall A.R., Skardal A. Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater. 2020;106:124–135. doi: 10.1016/j.actbio.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ng S., Tan W.J., Pek M.M.X., Tan M.-H., Kurisawa M. Mechanically and chemically defined hydrogel matrices for patient-derived colorectal tumor organoid culture. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.119400. [DOI] [PubMed] [Google Scholar]
- 155.Aisenbrey E.A., Murphy W.L. Synthetic alternatives to matrigel. Nat. Rev. Mater. 2020;5:539–551. doi: 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Veld R.C. op ‘t, van den Boomen O.I., Lundvig D.M.S., Bronkhorst E.M., Kouwer P.H.J., Jansen J.A., Middelkoop E., Von den Hoff J.W., Rowan A.E., Wagener F.A.D.T.G. Thermosensitive biomimetic polyisocyanopeptide hydrogels may facilitate wound repair. Biomaterials. 2018;181:392–401. doi: 10.1016/j.biomaterials.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 157.Cruz-Acuña R., Quirós M., Farkas A.E., Dedhia P.H., Huang S., Siuda D., García-Hernández V., Miller A.J., Spence J.R., Nusrat A., García A.J. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017;19:1326–1335. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guvendiren M., Burdick J.A. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013;24:841–846. doi: 10.1016/j.copbio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Homma K., Chang A.C., Yamamoto S., Tamate R., Ueki T., Nakanishi J. Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling. Acta Biomater. 2021;132:103–113. doi: 10.1016/j.actbio.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 160.Rawal P., Tripathi D.M., Ramakrishna S., Kaur S. Prospects for 3D bioprinting of organoids. Bio-Design. Manuf. 2021;4:627–640. doi: 10.1007/s42242-020-00124-1. [DOI] [Google Scholar]
- 161.Kim H.J., You S.J., Yang D.H., Chun H.J., Kim M.S. Preparation of novel RGD-conjugated thermosensitive mPEG-PCL composite hydrogels and in vitro investigation of their impacts on adhesion-dependent cellular behavior. J. Ind. Eng. Chem. 2020;84:226–235. doi: 10.1016/j.jiec.2020.01.001. [DOI] [Google Scholar]
- 162.Kambe Y., Yamaoka T. Initial immune response to a FRET-based MMP sensor-immobilized silk fibroin hydrogel in vivo. Acta Biomater. 2021;130:199–210. doi: 10.1016/j.actbio.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Q., Liu Y., Li J., Wang J., Liu C. Recapitulation of growth factor-enriched microenvironment via BMP receptor activating hydrogel. Bioact. Mater. 2023;20:638–650. doi: 10.1016/j.bioactmat.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma Z., Wang J., Loskill P., Huebsch N., Koo S., Svedlund F.L., Marks N.C., Hua E.W., Grigoropoulos C.P., Conklin B.R., Healy K.E. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 2015;6:7413. doi: 10.1038/ncomms8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu H., Wang Y., Wang H., Zhao M., Tao T., Zhang X., Qin J. A droplet microfluidic system to fabricate hybrid capsules enabling stem cell organoid engineering. Adv. Sci. 2020;7 doi: 10.1002/advs.201903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brandenberg N., Hoehnel S., Kuttler F., Homicsko K., Ceroni C., Ringel T., Gjorevski N., Schwank G., Coukos G., Turcatti G., Lutolf M.P. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020;4:863–874. doi: 10.1038/s41551-020-0565-2. [DOI] [PubMed] [Google Scholar]
- 167.Rizwan M., Ling C., Guo C., Liu T., Jiang J.X., Bear C.E., Ogawa S., Shoichet M.S. Viscoelastic notch signaling hydrogel induces liver bile duct organoid growth and morphogenesis. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202200880. [DOI] [PubMed] [Google Scholar]
- 168.Sampaziotis F., Muraro D., Tysoe O.C., Sawiak S., Beach T.E., Godfrey E.M., Upponi S.S., Brevini T., Wesley B.T., Garcia-Bernardo J., Mahbubani K., Canu G., Gieseck R., Berntsen N.L., Mulcahy V.L., Crick K., Fear C., Robinson S., Swift L., Gambardella L., Bargehr J., Ortmann D., Brown S.E., Osnato A., Murphy M.P., Corbett G., Gelson W.T.H., Mells G.F., Humphreys P., Davies S.E., Amin I., Gibbs P., Sinha S., Teichmann S.A., Butler A.J., See T.C., Melum E., Watson C.J.E., Saeb-Parsy K., Vallier L. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–846. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Buchmann B., Engelbrecht L.K., Fernandez P., Hutterer F.P., Raich M.K., Scheel C.H., Bausch A.R. Mechanical plasticity of collagen directs branch elongation in human mammary gland organoids. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-22988-2. ARTN 2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.de Melo B.A.G., Jodat Y.A., Cruz E.M., Benincasa J.C., Shin S.R., Porcionatto M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020;117:60–76. doi: 10.1016/j.actbio.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 171.Zheng H., Zuo B. Functional silk fibroin hydrogels: preparation, properties and applications. J. Mater. Chem. B. 2021;9:1238–1258. doi: 10.1039/D0TB02099K. [DOI] [PubMed] [Google Scholar]
- 172.Thakuri P.S., Liu C., Luker G.D., Tavana H. Biomaterials-based approaches to tumor spheroid and organoid modeling. Adv. Healthcare Materi. 2018;7 doi: 10.1002/adhm.201700980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Devina N., Eriwati Y.K., Santosa A.S. The purity and viscosity of sodium alginate extracted from Sargassum brown seaweed species as a basic ingredient in dental alginate impression material. J. Phys. Conf. 2018;1073 doi: 10.1088/1742-6596/1073/5/052012. [DOI] [Google Scholar]
- 174.Crispim J.F., Ito K. De novo neo-hyaline-cartilage from bovine organoids in viscoelastic hydrogels. Acta Biomater. 2021;128:236–249. doi: 10.1016/j.actbio.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 175.Cassel de Camps C., Aslani S., Stylianesis N., Nami H., Mohamed N.-V., Durcan T.M., Moraes C. Hydrogel mechanics influence the growth and development of embedded brain organoids. ACS Appl. Bio Mater. 2022;5:214–224. doi: 10.1021/acsabm.1c01047. [DOI] [PubMed] [Google Scholar]
- 176.Guo Y., Wang X., Li B., Shen Y., Shen L., Wu J., Yang J. Oxidized sodium alginate crosslinked silk fibroin composite scaffold for skin tissue engineering. J. Biomed. Mater. Res. Part B: Applied Biomaterials n/a. 2022 doi: 10.1002/jbm.b.35119. [DOI] [PubMed] [Google Scholar]
- 177.Bejoy J., Wang Z., Bijonowski B., Yang M., Ma T., Sang Q.-X., Li Y. Differential effects of heparin and hyaluronic acid on neural patterning of human induced pluripotent stem cells. ACS Biomater. Sci. Eng. 2018;4:4354–4366. doi: 10.1021/acsbiomaterials.8b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.op 't Veld R.C., Joosten L., van den Boomen O.I., Boerman O.C., Kouwer P., Middelkoop E., Rowan A.E., Jansen J.A., Walboomers X.F., Wagener F.A.D.T.G. Monitoring 111In-labelled polyisocyanopeptide (PIC) hydrogel wound dressings in full-thickness wounds. Biomater. Sci. 2019;7:3041–3050. doi: 10.1039/C9BM00661C. [DOI] [PubMed] [Google Scholar]
- 179.Norris S.C.P., Soto J., Kasko A.M., Li S. Photodegradable polyacrylamide gels for dynamic control of cell functions. ACS Appl. Mater. Interfaces. 2021;13:5929–5944. doi: 10.1021/acsami.0c19627. [DOI] [PubMed] [Google Scholar]
- 180.Wang M., Bai J., Shao K., Tang W., Zhao X., Lin D., Huang S., Chen C., Ding Z., Ye J. Poly(vinyl alcohol) hydrogels: the old and new functional materials. Int. J. Polymer Sci.. 2021;2021 doi: 10.1155/2021/2225426. [DOI] [Google Scholar]