Abstract
Background
Cancer is the second-leading cause of death worldwide. According to a 2018 WHO report, 9.6 million deaths occurred globally due to cancer. Ehrlich carcinoma is characterized by rapid proliferation and a short survival time. Ligustilide is a phthalide derivative and is one of the main compounds in Danggui essential oil and Rhizoma Chuanxiong. It has many protective effects, such as anticancer, anti-inflammatory, antioxidant, and neuroprotective effects.
Aims
We conducted this study to investigate the antitumor activity of ligustilide against Ehrlich solid carcinoma (ESC) in rats by affecting beclin 1, mammalian target of rapamycin (mTOR), B-cell lymphoma 2 (BCL2), and 5' AMP-activated protein kinase (AMPK).
Materials and methods
Twenty rats were intramuscularly implanted in the thigh of the left hind limb with a 200-µL tumor cell suspension in PBS containing 2 × 106 cells. After eight days of inoculation, 10 rats out of the 20 were treated with oral 20 mg/kg ligustilide daily. At the end of the experiment, samples of muscles with ESC were separated. Sections prepared from the muscle samples with ESC were immunohistochemically stained with anti-Ki67 antibodies. Another part of the muscle samples with ESC was used to assess gene expression and protein levels of beclin 1, mTOR, BCL2, and AMPK.
Results
Treatment of carcinoma rats with ligustilide elevated the mean survival time and reduced tumor volume and weight. Moreover, examination of tumor tissue stained with hematoxylin/eosin showed an infiltrative, highly cell-dense mass supported by a small to moderate amount of fibrovascular stroma and intersected with multifocal myofibril necrosis. Treatment with ligustilide ameliorated all these effects in the carcinoma group without affecting the control group. Finally, treatment with ligustilide significantly decreased the expression of beclin 1, mTOR, and AMPK associated with elevated expression of BCL2.
Conclusions
Our study aimed to explore the potential chemotherapeutic activity of ligustilide against ESC. We found that ligustilide effectively reduced tumor size and weight, indicating its antineoplastic activity against ESC. We further investigated that ligustilide inhibits cell proliferation by suppressing Ki67 and mTOR and activates autophagy through beclin 1 activation. Moreover, ligustilide inhibits apoptosis by upregulating BCL2. Finally, ligustilide reduced the expression of AMPK, preventing its ability to promote tumor cell growth.
Keywords: mammalian target of rapamycin (mtor), ki67, b-cell lymphoma 2 (bcl2), beclin 1, 5' adenosine monophosphate-activated protein kinase (ampk)
Introduction
Cancer is a severe disease and the second-leading cause of death worldwide. According to a WHO report in 2018 [1], it is complicated to treat due to many characteristics related to the pathology of the disease and the side effects of the chemotherapy treatment, including loss of hair, fatigue, nausea, and many others [2]. Ehrlich carcinoma is a spontaneous murine mammary adenocarcinoma. Characterized by rapid proliferation and a short survival time, it is usually utilized in many tumor and chemotherapy studies [3]. They are maintained by intraperitoneal passages in an ascitic form (EAC). Ehrlich tumor cells can also develop into solid tumors (ESC) when injected subcutaneously [4].
Beclin 1 is a member of class 3 phosphatidylinositol-3-kinase (PI3KC3). It is a tumor suppressor gene and a primary regulator of autophagy. High values of beclin 1 are associated with a good prognosis in patients with colorectal cancer [5]. It reacts with B-cell lymphoma-2 (BCL-2) to enhance the process of apoptosis through the expression of caspases [6]. In parallel, the mammalian target of rapamycin (mTOR) is an autophagy inhibitor through the enhancement of the signaling pathways of mitogen-activated protein kinase (MAPK) and protein kinase B (AKT) [5].
Ligustilide is a phthalide derivative. It is a leading and most effective compound of Danggui essential oil and Chuanxiong Rhizoma, traditional Chinese herbals. Ligustilide could be a promising compound due to its pharmacological properties; it has antitumor, anti-inflammation, antioxidant neuroprotection, and vasodilatation activities [7]. Ligustilide was also reported to treat hepatocellular carcinoma by regulating cancer cells and the crosstalk between tumor cells and macrophages in the tumor microenvironment [8]. Our objective was to investigate the potential chemotherapeutic impact of ligustilide on EAC in rats by targeting beclin 1, mTOR, B-cell lymphoma 2 (BCL2), and 5' AMP-activated protein kinase (AMPK).
This article was previously presented as a meeting abstract at the 2023 Dubai International Pharmaceuticals and Technologies Conference and Exhibition (DUPHAT) on January 10-12, 2023.
Materials and methods
Animals and treatment protocol
We conducted an experiment on 30 Sprague-Dawley rats weighing 180-200 g, maintaining them under standard temperature conditions with a regular 12-hour light/12-hour dark cycle. The Research Ethics Committee of Horus University, Faculty of Pharmacy, approved our working protocol with the number P2023-003. We divided the rats into three groups, each consisting of ten rats.
The Control Group
The rats in this group were untreated during the entire experiment period.
ESC Group
Rats in the ESC group received an intramuscular injection of 0.15 ml of 2 × 106 Ehrlich cells in the thigh of the left hind leg and left for four weeks without any treatment [9].
ESC Treated With Ligustilide Group
After induced ESC in rats, once a solid tumor appeared on day 8, rats were administered 20 mg/kg ligustilide (Sigma Aldrich Chemicals Co., Burlington, MA, USA) by oral gavage and indicated as day 0. The rats were then treated with ligustilide for three more weeks.
Sample collection
The tumor area on the left hind leg's thigh was removed, measured, and weighed. For morphological and immunohistochemistry studies, a portion of the muscle tissue with ESC was preserved in 10% buffered formalin. Another portion was homogenized in an ice-cold sodium-potassium phosphate buffer and then stored at −80 °C.
Immunohistochemistry
Our group previously described the process of performing immunohistochemistry [10-12]. To begin, we cut 5-µm-thick paraffin sections from a block of muscle tissues. Next, we immunostained the cells with monoclonal anti-Ki67 from Santa Cruz Biotechnology, Inc., Dallas, TX, USA, at 4 °C. Afterward, we incubated the sections with a horseradish peroxidase conjugate antibody and counterstained them with hematoxylin. Finally, the cells were examined in a masked manner.
Biochemical investigations using enzyme-linked immunosorbent (ELISA)
The assessment of AMPK (Catalog number MBS1602983), beclin 1 (Catalog number MBS2706719), Ki67 (Catalog number MBS705024), BCL2 (Catalog number MBS2515143), and mTOR (Catalog number MBS744326) was conducted using commercially available ELISA kits from MyBioSource, Inc., San Diego, CA, USA, in accordance with the manufacturer's instructions.
RT-PCR investigations
To analyze gene expression in rat muscle, we followed our group's established protocol [13-16]. We measured the levels of AMPK, beclin 1, BCL2, and mTOR mRNA using specific PCR primers listed in Table 1. We used GAPDH as a housekeeping gene and internal reference control.
Table 1. Primer sets used to detect gene expression in rats.
| Gene symbol | Primer sequence from 5′- 3′ | Accession number |
| GAPDH | F: 5`-CCATCAACGACCCCTTCATT-3` R: 5`-CACGACATACTCAGCACCAGC-3` | NM_017008.4 |
| AMPK | R: 5`CCTTCGGCAAAGTGAAGATTGG-3` R: 5`ATGAAGGAACCCGTTGGAGG-3` | NM_023991.1 |
| BCL2 | F: 5`-AGTTCGGTGGGGTCATGTGTG-3` R: 5`-CCAGGTATGCACCCAGAGTG-3` | NM_016993.2 |
| mTOR | F: 5′-CTGCACTTGTTGTTGCCTCC-3′ R: 5′-ATCTCCCTGGCTGCTCCTTA-3′ | NM_019906.2 |
| Beclin 1 | F: 5`-CTCGTCAAGGCGTCACTTCT-3` R: 5`-CCTCCATTCTTTAGGCCCCG-3` | NM_053739.2 |
| Ki67 | F: 5`-TTCCAGACACCAGACCATGC-3` F: 5`-GGGTTCTAACTGGTCTTCCTGG-3` | NM_001271366.1 |
Statistical analysis
In presenting quantitative variables, we utilized the mean ± standard error. To compare groups, we utilized a one-way analysis of variance (ANOVA) followed by a post hoc Bonferroni correction test. All statistical analyses were performed using SPSS version 20 (Chicago, IL, USA). We defined statistical significance as P < 0.05.
Results
Antitumor activity of ligustilide
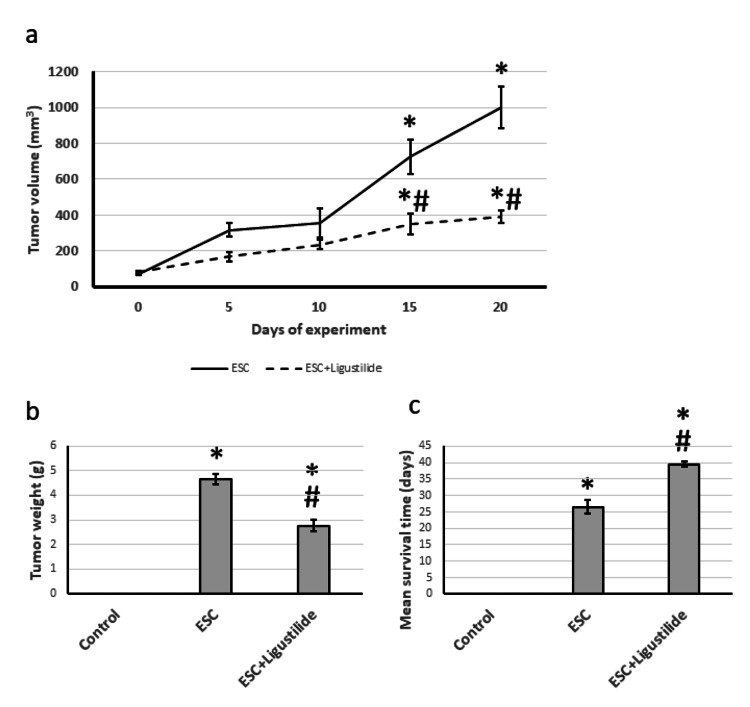
First, we examined the direct effect of ligustilide on the tumor. We found that ligustilide significantly reduced the tumor weight and size by about 60%. In addition, ligustilide significantly increased the survival of rats from 26 days to 41 days (Figure 1).
Figure 1. The influence of ESC induced in rats and treatment with 20 mg/kg ligustilide on tumor volume (a), tumor weight (b), and mean survival time (c).
*Represented a significant difference when compared to the control group at a significance level of p<0.05. Similarly, #represented a significant difference when compared to the ESC group at a significance level of p<0.05. ESC: Ehrlich solid carcinoma.
Effect of ligustilide on tumor-induced elevation in cell proliferation
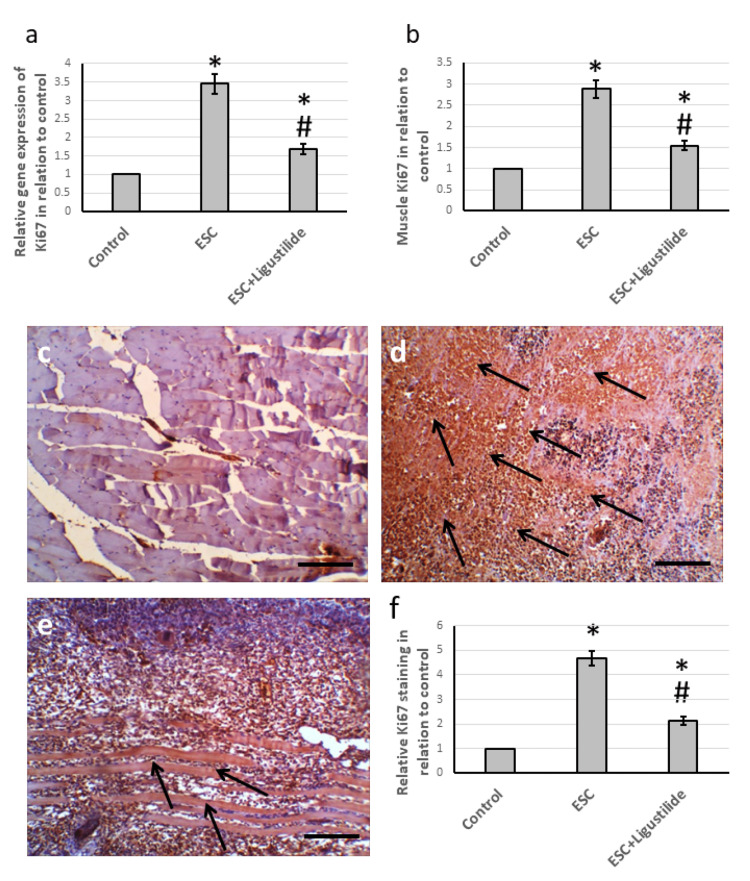
Ki67 is a proliferation marker strongly associated with tumor cell proliferation [17]. ESC resulted in an elevation in the gene expression of Ki67 and its muscle protein level as compared with the control group. In addition, examination of muscle sections immuno-stained with anti-Ki67 revealed an increase in immuno-staining in muscle sections from ESC, which was reduced in sections from rats treated with ligustilide. In addition, ligustilide decreased the analysis of the anti-Ki67 stained area (Figure 2).
Figure 2. The influence of ESC induced in rats and treatment with 20 mg/kg ligustilide on the Ki67 gene expression (a) and protein levels (b). Muscle sections stained with anti-Ki67 antibodies in the control group (c), ESC group (d), and ESC group treated with ligustilide (e). Immunohistochemistry score of positive staining (f).
The immunohistochemistry score was calculated by dividing the total area stained with anti-Ki67 by the total tissue area in the field, measured in 10 different fields of each animal section.
*Represented a significant difference when compared to the control group at a significance level of p<0.05. Similarly, #represented a significant difference when compared to the ESC group at a significance level of p<0.05.
Scale bar 100 μm. ESC: Ehrlich solid carcinoma.
Effect of ligustilide on beclin 1
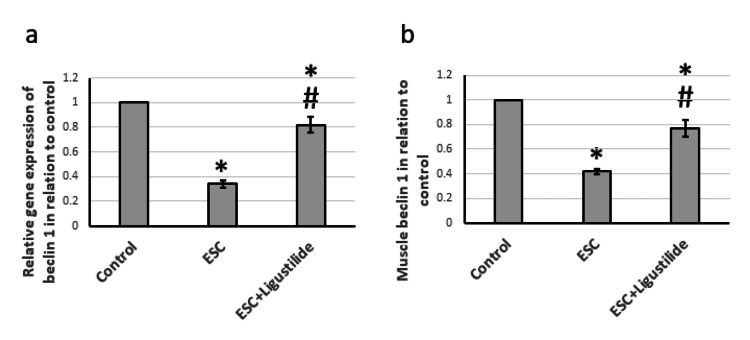
After finishing the examination of the antitumor activity of ligustilide, we looked inside the molecular mechanism of action to investigate the pathological effects on autophagy, a complex catabolic program for lysosomal degradation of proteins and other subcellular constituents that is activated in response to nutrient deprivation, leading to the recycling of organelles and other cytoplasmic substances to provide metabolic precursors. Beclin 1 is the first autophagy core-machinery protein identified as a caspase substrate [18]. ESC reduced gene expression and muscle protein levels of beclin 1 compared to the control rats. Treatment of ESC rats with ligustilide significantly increased the expression of beclin 1, but still less than the control level. Therefore, ESC reduced autophagy, which was restored by treatment with ligustilide (Figure 3).
Figure 3. The influence of ESC induced in rats and treatment with 20 mg/kg ligustilide on beclin 1 gene expression (a) and protein levels (b).
*Represented a significant difference when compared to the control group at a significance level of p<0.05. Similarly, #represented a significant difference when compared to the ESC group at a significance level of p<0.05.
ESC: Ehrlich solid carcinoma.
Effect of ligustilide on BCL2
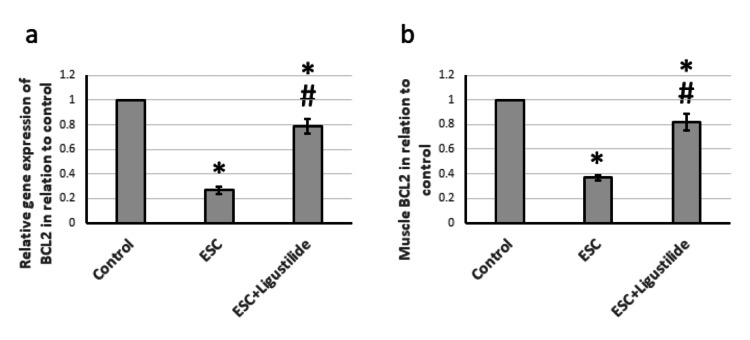
BCL2 is the founding member of the BCL family of regulator proteins that produce anti-apoptotic effects [19]. ESC resulted in reduced gene expression and protein levels of BLC2 compared to the control rats. Treatment with ligustilide significantly increased the expression of BCL2, but still less than the control level (Figure 4). Therefore, ESC reduced cell protection against apoptosis, which was restored by treatment with ligustilide.
Figure 4. The influence of ESC induced in rats and treatment with 20 mg/kg ligustilide on BCL2 gene expression (a), and protein levels (b).
*Represented a significant difference when compared to the control group at a significance level of p<0.05. Similarly, #represented a significant difference when compared to the ESC group at a significance level of p<0.05.
ESC: Ehrlich solid carcinoma, BCL2: B-cell lymphoma 2.
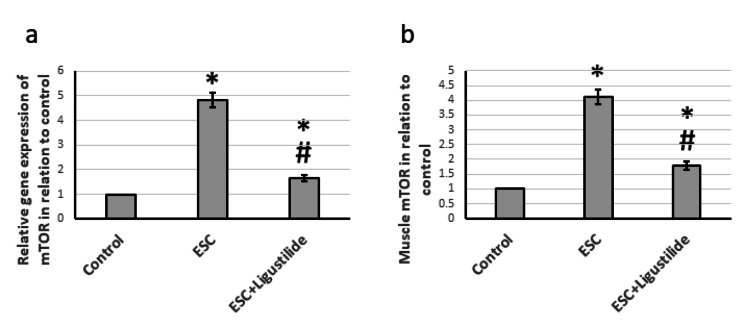
Effect of ligustilide on mTOR
mTOR functions as a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, autophagy, and transcription [20]. In ESC, mTOR had elevated gene expression and protein levels compared to the control group. Treatment with ligustilide significantly reduced the expression of mTOR but was still higher than the control level (Figure 5).
Figure 5. The influence of ESC induced in rats and treatment with 20 mg/kg ligustilide on mTOR gene expression (a), and protein levels (b).
*Represented a significant difference when compared to the control group at a significance level of p<0.05. Similarly, #represented a significant difference when compared to the ESC group at a significance level of p<0.05.
ESC: Ehrlich solid carcinoma, mTOR: mammalian target of rapamycin.
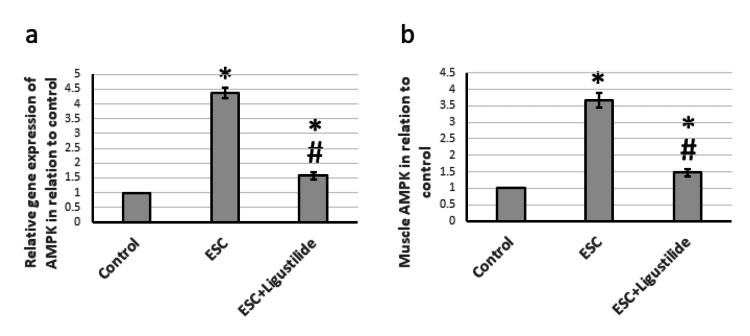
Effect of ligustilide on AMPK
Next, we investigated the effect on AMPK, a highly conserved sensor of low intracellular ATP levels that is rapidly activated after nearly all mitochondrial stresses, even those that do not disrupt the mitochondrial membrane potential [21]. Figure 6 revealed that ESC resulted in elevated gene expression and protein levels of AMPK. Treatment with ligustilide significantly reduced the expression of AMPK, but it was still higher than the control level. Therefore, ESC activated tissue stress, which was reversed by treatment with ligustilide.
Figure 6. The influence of ESC induced in rats and treatment with 20 mg/kg ligustilide on AMPK gene expression (a) and protein levels (b).
*Represented a significant difference when compared to the control group at a significance level of p<0.05. Similarly, #represented a significant difference when compared to the ESC group at a significance level of p<0.05.
ESC: Ehrlich solid carcinoma, AMPK: 5'-adenosine monophosphate-activated protein kinase.
Discussion
According to a 2018 report by the World Health Organization, cancer is the second leading cause of death globally, responsible for over 9.6 million deaths. It is projected to increase to 21.4 million deaths worldwide by 2030 [22]. Cancer treatment often results in severe symptoms, emotional distress, and physical limitations, which can lead to a decreased quality of life and depression. Additionally, chemotherapy can be extremely expensive and cause serious side effects. The aim of our current research is to investigate the potential chemotherapeutic effects of ligustilide. For our study, we utilized the ESC tumor model, which is characterized by rapid growth [23]. We implanted Ehrlich cancer cells subcutaneously in the thigh of the left hind leg, resulting in the appearance of a lump that increased in size over several weeks due to tumor growth. The diagnosis was confirmed through the separation of the tumor after animal sacrifice and inspection of micro-images stained with anti-Ki67 antibodies.
In an effort to find a chemotherapeutic agent, the effectiveness of ligustilide was investigated. Previous studies had shown that ligustilide had anti-inflammation, antioxidant, and antitumor properties [7]. When tested on ESC rats, ligustilide significantly reduced tumor volume and weight while increasing the mean survival time from 26 to 41 days. Additionally, examining the micro-images of the rats treated with ligustilide revealed a decrease in immunostaining with Ki67 antibodies. Previous studies had shown that ligustilide had chemotherapeutic properties against bladder cancer [24], oral cancer cells [25], breast cancer [26], and prostate cancer [27] due to its antioxidant and antiapoptotic properties. However, this is the first study to show that ligustilide is effective in reducing ESC.
We examined muscle sections stained with Ki67, which is a proliferative marker. The Ki67 protein has long been used as a prognostic marker for cancer treatment [17]. It has also been studied in many experimental models of cancer [28,29]. Ki67 is overexpressed throughout all the active phases of the cell cycle. It was previously commonly used as a marker of Ehrlich tumor proliferation. We discovered elevated gene expression and protein levels of Ki67 associated with increased immune staining of Ki67 in ESC rats. Treating rats with ligustilide significantly reduced the expression of Ki67. Ligustilide was previously reported to alleviate insulin resistance and lipid accumulation in experimentally induced diabetes mellitus in rats through activation of AMPK [30]. However, this is the first research to report the ability of ligustilide to reduce the expression of Ki67 in ESC.
Beclin-1 is a critical protein that controls the activity of lipid kinase, leading to the activation of autophagy. It interacts with the sodium-potassium ATPase pump, regulating the survival and death cycles of cells [31]. Our data suggest that ligustilide enhances autophagy and attenuates tissue stress. Activation of beclin 1 can inhibit tumorigenesis by eliminating damaged organelles and other cellular components. In addition, beclin 1 is included in the formation of class III PI3K complexes, which are essential for autophagy initiation [32]. Ligustilide was previously reported to activate autophagy in A7r5 fibroblast-like cell cells [33], PC12 brain cells [34], and breast cancer cells [26].
We investigated the apoptotic pathway as a possible mechanism for the pathogenicity of ESC. This process is regulated by a balance of proapoptotic proteins (BAX, BAK, and p38) and antiapoptotic proteins (BCL2, BCLX, and MCl-1) within cells. Our findings revealed an overexpression of BAX and a downregulation of BCL2, causing an imbalance that can affect apoptosis and trigger caspase activation. BCL2 is a well-known anti-apoptotic compound that protects cells from apoptosis by regulating antioxidant activities. On the other hand, BAX promotes apoptosis by activating cytochrome C and caspase-3 [19]. However, treatment with ligustilide in rats effectively upregulated BCL2.
Our investigation examined the impact of ligustilide on cell function by analyzing the role of mTOR, which is a serine/threonine kinase. It is a key factor in autophagy induction and plays a vital role in cell growth, proliferation, autophagy, and survival. Promising results have been seen from testing mTOR inhibitors against tumor cells [20]. We found that ligustilide significantly reduced the ESC-induced elevation in the expression of mTOR. Ligustilide was reported previously to inhibit mTOR signaling in angiotensin II-induced A7r5 cell autophagy [33] and protect PC12 cells from oxygen-glucose deprivation/reoxygenation-induced apoptosis [34]. However, no previous report illustrated the ability of ligustilide to inhibit mTOR in ESC.
AMPK serves as a crucial regulator of energy metabolism, controlling anabolic pathways like fatty acid synthesis and promoting catabolic pathways like fatty acid oxidation to maintain ATP levels in cells [21]. While some studies suggest that it can hinder cancer cell proliferation, recent research indicates that it can also enhance tumor cell growth by maintaining cell energy in certain models. The hyperactive signaling of AMPK plays a dominant role in the biology of cancer and its progression. The roles of AMPK and the mTOR complex are emerging in the molecular pathways that connect autophagy and senescence [35]. Our findings show that ligustilide significantly reduces the expression of AMPK induced by ESC. Ligustilide was previously reported to decrease the expression of AMPK in ischemic stroke [36], cardiomyocyte dysfunction [37], and memory deficit [38]. However, no previous report has demonstrated the ability of ligustilide to inhibit AMPK in ESC.
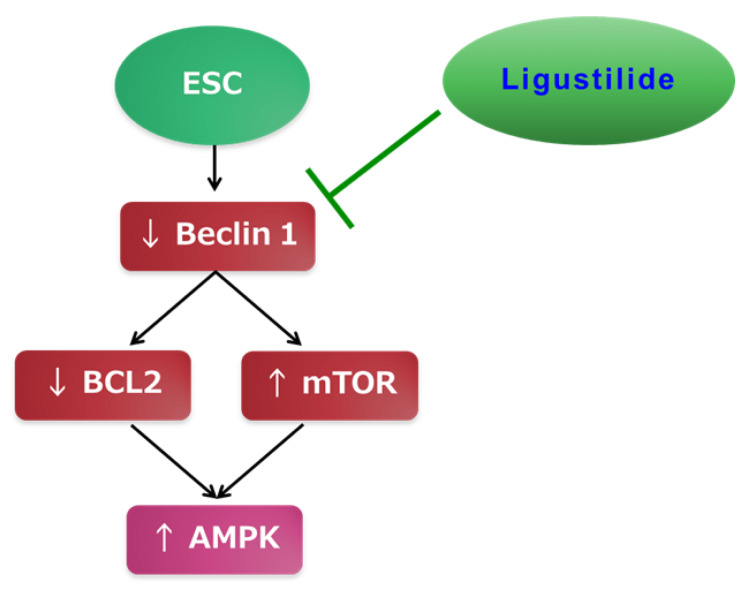
Ligustilide is a promising option for chemotherapy due to its natural occurrence, affordability, and safety. Figure 7 summarizes the mechanism of its chemotherapeutic effects in ESC. However, the current research has certain limitations. For instance, rats have different metabolic pathways than humans, which can lead to varying effects of certain drugs. Additionally, there are multiple animal models for inducing cancer, but we only used one method in our study.
Figure 7. The mechanism of the protective effects of ligustilide in ESC.
ESC: Ehrlich solid carcinoma, AMPK: 5'-adenosine monophosphate-activated protein kinase, BCL2: B-cell lymphoma 2.
Image Credits: The authors of the manuscript.
Conclusions
Our study aimed to explore the potential chemotherapeutic activity of ligustilide against ESC. We found that ligustilide effectively reduced tumor size and weight, indicating its antineoplastic activity against ESC. Upon further investigation, we discovered that ligustilide inhibits cell proliferation by suppressing Ki67 and mTOR and activates autophagy through beclin 1 activation. Moreover, ligustilide inhibits apoptosis by upregulating BCL2. Finally, ligustilide reduced the expression of AMPK, preventing its ability to promote tumor cell growth. AMPK plays a controversial role in tumorigenesis.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
The research ethics committee of Faculty of Pharmacy, Horus University Issued protocol number P2023-003
References
- 1.Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Ferlay J, Colombet M, Soerjomataram I, et al. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Side effects of chemotherapy among cancer patients in a Malaysian General Hospital: experiences, perceptions and informational needs from clinical pharmacists. Chan HK, Ismail S. Asian Pac J Cancer Prev. 2014;15:5305–5309. doi: 10.7314/apjcp.2014.15.13.5305. [DOI] [PubMed] [Google Scholar]
- 3.Ehrlich ascites tumour unbalances splenic cell populations and reduces responsiveness of T cells to Staphylococcus aureus enterotoxin B stimulation. Segura JA, Barbero LG, Marquez J. Immunol Lett. 2000;74:111–115. doi: 10.1016/s0165-2478(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 4.The relationship between (99m)Tc-MIBI uptakes and tumor cell death/proliferation state under irradiation. Zhu X, Wu H, Xia J, Zhao M, Xianyu Z. Cancer Lett. 2002;182:217–222. doi: 10.1016/s0304-3835(02)00079-4. [DOI] [PubMed] [Google Scholar]
- 5.Diagnostic value of beclin-1 and mTOR in ovarian cancer. Lu H, Hu H, Li S. https://pubmed.ncbi.nlm.nih.gov/33564356/ Int J Clin Exp Pathol. 2021;14:238–246. [PMC free article] [PubMed] [Google Scholar]
- 6.Prognostic value of Beclin 1, EGFR and ALK in non-squamous non-small cell lung cancer. Wan Y, Qian Y, Wang Y, Fang F, Wu G. Discov Oncol. 2022;13:127. doi: 10.1007/s12672-022-00586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ligustilide improves cognitive impairment via regulating the SIRT1/ire1α/XBP1s/chop pathway in vascular dementia rats. Peng D, Wang YX, Huang TH, et al. Oxid Med Cell Longev. 2022;2022:6664990. doi: 10.1155/2022/6664990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligustilide counteracts carcinogenesis and hepatocellular carcinoma cell-evoked macrophage M2 polarization by regulating yes-associated protein-mediated interleukin-6 secretion. Yang J, Xing Z. Exp Biol Med (Maywood) 2021;246:1928–1937. doi: 10.1177/15353702211010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antitumor activity of luteolin against Ehrlich solid carcinoma in rats via blocking Wnt/β-catenin/Smad4 pathway. Aljohani H, Khodier AE, Al-Gayyar MM. Cureus. 2023;15:0. doi: 10.7759/cureus.39789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curative effects of crocin in ulcerative colitis via modulating apoptosis and inflammation. Albalawi GA, Albalawi MZ, Alsubaie KT, Albalawi AZ, Elewa MA, Hashem KS, Al-Gayyar MM. Int Immunopharmacol. 2023;118:110138. doi: 10.1016/j.intimp.2023.110138. [DOI] [PubMed] [Google Scholar]
- 11.Therapeutic effects of genistein in experimentally induced ulcerative colitis in rats via affecting mitochondrial biogenesis. Alharbi TS, Alshammari ZS, Alanzi ZN, Althobaiti F, Elewa MA, Hashem KS, Al-Gayyar MM. Mol Cell Biochem. 2023 doi: 10.1007/s11010-023-04746-8. [DOI] [PubMed] [Google Scholar]
- 12.Evaluating the anticancer activity of blocking TNF type 1 receptors in thioacetamide-induced hepatocellular carcinoma in a rat model. Bagalagel A, Diri R, Noor A, Almasri D, Bakhsh H, Kutbi HI, Al-Gayyar MM. Cureus. 2022;14:0. doi: 10.7759/cureus.32519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therapeutic effects of sulforaphane in ulcerative colitis: effect on antioxidant activity, mitochondrial biogenesis and DNA polymerization. Alattar A, Alshaman R, Al-Gayyar MM. Redox Rep. 2022;27:128–138. doi: 10.1080/13510002.2022.2092378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curative effects of fucoidan on acetic acid induced ulcerative colitis in rats via modulating aryl hydrocarbon receptor and phosphodiesterase-4. Bagalagel A, Diri R, Noor A, Almasri D, Bakhsh HT, Kutbi HI, Al-Gayyar MM. BMC Complement Med Ther. 2022;22:196. doi: 10.1186/s12906-022-03680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The therapeutic effects of cycloastragenol in ulcerative colitis by modulating SphK/MIP-1α/miR-143 signalling. Bagalagel A, Diri R, Noor A, Almasri D, Bakhsh HT, Kutbi HI, Al-Gayyar MM. Basic Clin Pharmacol Toxicol. 2022;131:406–419. doi: 10.1111/bcpt.13788. [DOI] [PubMed] [Google Scholar]
- 16.Genistein ameliorated experimentally induced gastric ulcer in rats via inhibiting gastric tissues fibrosis by modulating Wnt/β-catenin/TGF-β/PKB pathway. Hassan HM, Alatawi NM, Bagalagel A, et al. Redox Rep. 2023;28:2218679. doi: 10.1080/13510002.2023.2218679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ki67 is a promising molecular target in the diagnosis of cancer (review) Li LT, Jiang G, Chen Q, Zheng JN. Mol Med Rep. 2015;11:1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 18.The Beclin 1 network regulates autophagy and apoptosis. Kang R, Zeh HJ, Lotze MT, Tang D. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therapeutic effect of Arthrocnemum machrostachyum methanolic extract on Ehrlich solid tumor in mice. Sharawi ZW. BMC Complement Med Ther. 2020;20:153. doi: 10.1186/s12906-020-02947-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Vochysia tucanorum Mart. butanol fraction presents antitumoral activity in vivo and prevents the installation of cachexia in solid Ehrlich tumor model. Morgan HJ, Delgado AQ, Saldanha LL, Camaforte NA, Dokkedal AL, Bosqueiro JR. BMC Complement Med Ther. 2021;21:20. doi: 10.1186/s12906-020-03190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AMPK activation protects against prostate cancer by inducing a catabolic cellular state. Penfold L, Woods A, Pollard AE, et al. Cell Rep. 2023;42:112396. doi: 10.1016/j.celrep.2023.112396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epidemiology of cancer in Saudi Arabia thru 2010-2019: a systematic review with constrained meta-analysis. Alqahtani WS, Almufareh NA, Domiaty DM, et al. AIMS Public Health. 2020;7:679–696. doi: 10.3934/publichealth.2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.[1,2,4] Triazolo [3,4-a]isoquinoline chalcone derivative exhibits anticancer activity via induction of oxidative stress, DNA damage, and apoptosis in Ehrlich solid carcinoma-bearing mice. WalyEldeen AA, El-Shorbagy HM, Hassaneen HM, Abdelhamid IA, Sabet S, Ibrahim SA. Naunyn Schmiedebergs Arch Pharmacol. 2022;395:1225–1238. doi: 10.1007/s00210-022-02269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ligustilide induces apoptosis and reduces proliferation in human bladder cancer cells by NFκB1 and mitochondria pathway. Yin L, Ying L, Guo R, et al. Chem Biol Drug Des. 2023;101:1252–1261. doi: 10.1111/cbdd.14207. [DOI] [PubMed] [Google Scholar]
- 25.Z-ligustilide induces c-myc-dependent apoptosis via activation of ER-stress signaling in hypoxic oral cancer cells. Hsu RJ, Peng KY, Hsu WL, Chen YT, Liu DW. Front Oncol. 2022;12:824043. doi: 10.3389/fonc.2022.824043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sensitization of tamoxifen-resistant breast cancer cells by Z-ligustilide through inhibiting autophagy and accumulating DNA damages. Qi H, Jiang Z, Wang C, Yang Y, Li L, He H, Yu Z. Oncotarget. 2017;8:29300–29317. doi: 10.18632/oncotarget.16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Su ZY, Khor TO, Shu L, et al. Chem Res Toxicol. 2013;26:477–485. doi: 10.1021/tx300524p. [DOI] [PubMed] [Google Scholar]
- 28.Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. El-Far YM, Khodir AE, Emarah ZA, Ebrahim MA, Al-Gayyar MM. Redox Rep. 2022;27:9–20. doi: 10.1080/13510002.2022.2031515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Therapeutic effects of blocking β-catenin against hepatocellular carcinoma-induced activation of inflammation, fibrosis and tumor invasion. Hassan HM, El-Kannishy SM, Alattar A, Alshaman R, Hamdan AM, Al-Gayyar MM. Biomed Pharmacother. 2021;135:111216. doi: 10.1016/j.biopha.2021.111216. [DOI] [PubMed] [Google Scholar]
- 30.Ligustilide alleviates the insulin resistance, lipid accumulation, and pathological injury with elevated phosphorylated AMPK level in rats with diabetes mellitus. Guo S, Wang G, Yang Z. J Recept Signal Transduct Res. 2021;41:85–92. doi: 10.1080/10799893.2020.1789877. [DOI] [PubMed] [Google Scholar]
- 31.Impact of zinc oxide nanoparticles and thymoquinone in Ehrlich ascites carcinoma induced in mice. Ghanem HB. J Biochem Mol Toxicol. 2021;35:0. doi: 10.1002/jbt.22736. [DOI] [PubMed] [Google Scholar]
- 32.Autophagy-related beclin 1 and head and neck cancers. Hu YJ, Zhong JT, Gong L, Zhang SC, Zhou SH. Onco Targets Ther. 2020;13:6213–6227. doi: 10.2147/OTT.S256072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ligustilide inhibited angiotensin II induced A7r5 cell autophagy via Akt/mTOR signaling pathway. Luo Z, Zeng A, Chen Y, et al. Eur J Pharmacol. 2021;905:174184. doi: 10.1016/j.ejphar.2021.174184. [DOI] [PubMed] [Google Scholar]
- 34.Ligustilide protects PC12 cells from oxygen-glucose deprivation/reoxygenation-induced apoptosis via the LKB1-AMPK-mTOR signaling pathway. Zhao DY, Yu DD, Ren L, Bi GR. Neural Regen Res. 2020;15:473–481. doi: 10.4103/1673-5374.266059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senolytic flavonoids enhance type-I and type-II cell death in human radioresistant colon cancer cells through AMPK/MAPK pathway. Russo M, Moccia S, Luongo D, Russo GL. Cancers (Basel) 2023;15:2660. doi: 10.3390/cancers15092660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ligustilide attenuates ischemic stroke injury by promoting Drp1-mediated mitochondrial fission via activation of AMPK. Wu Q, Liu J, Mao Z, et al. Phytomedicine. 2022;95:153884. doi: 10.1016/j.phymed.2021.153884. [DOI] [PubMed] [Google Scholar]
- 37.Alleviation of glucolipotoxicity-incurred cardiomyocyte dysfunction by Z-ligustilide involves in the suppression of oxidative insult, inflammation and fibrosis. Cao Y, Dong Z, Yang D, Ma X, Wang X. Chem Phys Lipids. 2021;241:105138. doi: 10.1016/j.chemphyslip.2021.105138. [DOI] [PubMed] [Google Scholar]
- 38.Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 mice. Zhu WL, Zheng JY, Cai WW, et al. Aging (Albany NY) 2020;12:3175–3189. doi: 10.18632/aging.102793. [DOI] [PMC free article] [PubMed] [Google Scholar]