Abstract
Background:
Supervised aerobic exercise training (ET) is recommended for stable outpatients with heart failure with reduced ejection fraction (HFrEF). Frailty, a syndrome characterized by increased vulnerability and decreased physiological reserve, is common in patients with HFrEF and associated with a higher risk of adverse outcomes. The effect modification of baseline frailty on the efficacy of aerobic ET in HFrEF is not known.
Methods:
Stable outpatients with HFrEF randomized to aerobic ET vs. usual care in the HF-ACTION trial were included. Baseline frailty was estimated using Rockwood’s Frailty index (FI), a deficit accumulation-based model of frailty assessment, and participants with FI>0.21 were identified as frail. Multivariable Cox proportional hazard models with multiplicative interaction terms (Frailty*treatment arm) were constructed to evaluate whether frailty modified the treatment effect of aerobic ET on the primary composite endpoint (all-cause hospitalization and mortality), secondary endpoints (composite of cardiovascular death or cardiovascular hospitalization, and cardiovascular death or HF hospitalization), and Kansas City Cardiomyopathy Questionnaire (KCCQ) score. Separate models were constructed for continuous (FI) and categorical (frail vs. not frail) measures of frailty.
Results:
Among 2130 study participants (age: 59±13 y, 28% women), 1266 (59%) were characterized as frail (FI>0.21). Baseline frailty burden significantly modified the treatment effect of aerobic ET (P-interaction: FI*treatment arm=0.02, Frail status [frail vs. non-frail]*treatment arm = 0.04) with a lower risk of primary endpoint in frail (HR[95%CI]: 0.83[0.72, 0.95]) but not non-frail (HR[95%CI]: 1.04 [0.87, 1.25]) participants. The favorable effect of aerobic ET among frail participants was driven by a significant reduction in the risk of all-cause hospitalization (HR[95%CI]: 0.84[0.72, 0.99]). The treatment effect of aerobic ET on all-cause mortality and other secondary endpoints was not different between frail and non-frail patients. (p-interaction>0.1 for each). Aerobic ET was associated with a nominally greater improvement in KCCQ at 3-months among frail vs. non-frail participants without a significant treatment interaction by frailty status (p-interaction>0.2).
Conclusion:
Among patients with chronic stable HFrEF, baseline frailty modified the treatment effect of aerobic ET with a greater reduction in the risk of all-cause hospitalization but not mortality.
Keywords: Frailty, Exercise training, Heart failure with reduced ejection fraction, Hospitalization, Mortality
INTRODUCTION
Aerobic supervised exercise training (ET) and cardiac rehabilitation (CR) are effective non-pharmacological management strategies for patients with HF and reduced ejection fraction (HFrEF).1, 2 The HF-ACTION trial was the largest trial of aerobic ET in outpatients with chronic stable HFrEF. The HF-ACTION trial demonstrated a non-significant reduction in the risk of primary composite endpoint (all-cause mortality or hospitalization) and key secondary endpoints (all-cause mortality, composite of cardiovascular [CV] mortality or CV hospitalization, and composite of CV mortality or HF hospitalization) in the aerobic ET vs. the usual care group. However, the trial demonstrated that aerobic ET is safe and significantly improves exercise capacity and quality of life (QOL). Furthermore, in protocol-specified analysis adjusting for known prognostic markers, aerobic ET was also associated with a significantly lower risk of primary endpoint and key secondary endpoint of CV mortality or HF hospitalization.3, 4 Based on these findings, the Centers for Medicare & Medicaid Services approved aerobic supervised ET and cardiac rehabilitation for outpatients with chronic stable HFrEF.5 However, the utilization of cardiac rehabilitation in patients with HFrEF remains low.6, 7 Furthermore, there is significant heterogeneity in the exercise capacity improvement among individuals who undergo aerobic ET. Up to one-third of aerobic ET participants demonstrate no meaningful improvement in peak exercise oxygen uptake (peak VO2).8, 9 Thus, there is a need for effective strategies to identify patients most likely to benefit from aerobic ET and may be targeted with aerobic ET interventions.
Frailty —a syndrome characterized by a reduced physiologic reserve and impaired homeostatic tolerance to stressors— is common among patients with HFrEF and associated with worse exercise tolerance, poor QOL, and greater risk of adverse CV events.10, 11 Aerobic ET and physical function interventions are efficacious in reducing the frailty burden among older individuals, including patients with HF.12–16 However, it remains unclear whether the effects of aerobic ET on the risk of adverse clinical outcomes, exercise capacity, and QOL are modified by the baseline frailty burden. Accordingly, in this study, we developed a deficit index model of frailty among chronic HFrEF patients who participated in the HF-ACTION trial. We evaluated how the therapeutic effects of the aerobic ET intervention on the risk of adverse clinical outcomes, exercise capacity, and QOL were modified by the baseline frailty burden. Considering the well-established association between frailty and the risk of adverse clinical outcomes and poor functional status and the potential beneficial effects of aerobic ET on frailty burden, we hypothesized that individuals with greater frailty burden at baseline would be more likely to benefit from aerobic ET.
METHODS
Study design and participants
The present study was performed as a secondary analysis of the HF-ACTION trial. De-identified, publicly available data from the HF-ACTION trial was obtained from the National Heart Lung Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). The details of the HF-ACTION study design, rationale, and the primary trial results have been published previously.4, 17 All data used for the present analysis are available at the BioLINCC data repository and can be obtained after approval. The analysis codes used for the present study can be obtained by specific requests to the corresponding author.
In brief, HF-ACTION was a multicenter, randomized control trial that evaluated the clinical efficacy and safety of aerobic ET (vs. usual care) among stable patients with HFrEF (EF≤35%, NYHA class II to IV). All participants underwent detailed cardiopulmonary exercise testing before randomization. The study recruited 2331 participants between 2002 and 2007 who were randomized in a 1:1 ratio to either aerobic ET or usual care. For the present analysis, we used 2130 patients who consented, completed follow-up, and had available data in BioLINCC. The study protocol was approved by the local institutional review board and ethics committee of participating centers, and all participants provided written informed consent for participation in the study.
Study intervention: Aerobic ET vs. usual care
The details of the aerobic ET intervention used in the HF-ACTION trial have been reported previously.4, 17 The exercise program included a structured, group-based supervised aerobic ET program with a goal of 3 sessions per week for 36 sessions over 3 months. The supervised ET program included cycle or walk-based exercise regimens according to the patient’s comfort and preference. Supervised aerobic ET was supplemented with home exercise after 18 supervised sessions. After completing the 36 supervised ET sessions, participants were fully transitioned to home-based exercise. Participants in the usual care arm did not receive any formal exercise regimen. Participants of both arms (ET and usual care) received detailed self-management instruction consistent with the ACC/AHA guidelines, including counseling to exercise for at least 30 minutes per day. The participants were followed up at regular intervals with in-person visits and phone calls as determined by the study protocol.
Assessment of frailty burden: Frailty index
The frailty index was constructed using the deficit accumulation approach described by Rockwood et al. as detailed in the Supplemental Methods.18 The frailty index model included 36 items that reflected deficits across multiple domains, including symptoms, signs, disabilities, self-rated health, comorbidities, biomarkers, and functional capacity, as reported in Table S1. The deficits were assessed as binary variables and assigned a 0 (absence of the deficit) or 1 (presence of the deficit). Ordinal variables were coded by converting the number of possible ranks into equally spaced scores ranging from 0 to 1. Variables with < 20% missingness were imputed using random forest imputation (covariate missingness ranged from 0–13%).19 Variables capturing similar information in a specific domain were assessed for the degree of correlation, and only one variable was retained if the correlation was > 0.7. Finally, the frailty index was calculated by dividing the number of deficits present by the number of variables considered. Consistent with prior literature, a frailty index threshold of 0.21 was used to stratify participants into non-frail (frailty index ≤ 0.21) vs. frail (frailty index > 0.21) groups.18, 20, 21
Outcomes of interest
Consistent with the HF-ACTION trial, the primary outcome for our analysis was a composite of all-cause hospitalization or all-cause mortality.4 Secondary endpoints included the composite of CV mortality or CV hospitalization, CV mortality or HF hospitalization, and individual components of the primary endpoint (all-cause mortality and all-cause hospitalization) assessed separately. Outcomes were censored at last known contact with a maximum follow-up of 4 years. The median follow-up for the primary outcome was 2.9 [interquartile range = 2.0 – 3.8] years. QOL was assessed by the 23-item self-administered Kansas City Cardiomyopathy Questionnaire (KCCQ score) every 3 months in the first year of follow-up, followed by annually for the next 3 years.3 Functional endpoints included Peak VO2 and 6-minute walk distance (6-MWD) measurements. Peak VO2 was measured at baseline, 3, 12, and 24 months using cardiopulmonary exercise testing using symptom-limited maximal treadmill test as reported previously.4, 17 6-MWD was measured at baseline, 3 months, and at yearly follow up visits (till year 4) using a standard protocol at all sites.4, 17 Finally, safety outcomes of interest for this analysis were consistent with that reported in the primary study and detailed in Table S2.
Statistical Analysis
Baseline characteristics of the study participants were compared across non-frail vs. frail groups using two-sample t-tests and chi-square tests for continuous and categorical variables, respectively. The unadjusted risk of the primary composite endpoint was compared across the non-frail vs. frail study groups using cumulative incidence curves and log-rank tests. Multivariable Cox proportional hazard models were constructed to evaluate the adjusted association of frailty status and risk of the primary composite endpoint and key secondary endpoints. Separate models were constructed for categorical (frail vs. non-frail [referent]) and continuous measures of frailty (frailty index) for each outcome with adjustment for the following covariates 3, 4: Model 1- age, sex, race, treatment arm; Model 2 - model 1 + left ventricular ejection fraction, HF etiology, Beck’s Depression Inventory score, baseline peak VO2, and atrial fibrillation. The adjustment covariates were selected based on the primary composite outcome models reported in the primary trial analysis and used consistently in prior analyses.4 Sensitivity analysis was also conducted further adjusting for body mass index in Model 2. The interaction between treatment arm and frailty status for the risk of the primary composite endpoint and secondary endpoints was assessed by including a multiplicative interaction term (treatment arm*frailty status) in the most adjusted models (Model 2). Interaction tests were performed for both continuous and categorical measures of frailty. Stratified analysis by frailty status was conducted to evaluate the association between aerobic ET and risk of the primary composite endpoint and secondary endpoints in frail and non-frail participants separately using the model 2 adjustment covariates. Finally, the treatment effect of aerobic ET was also assessed across the continuous distribution of FI using restricted cubic splines.
The association between baseline frailty burden (assessed continuously as FI and categorically as frail vs. non-frail) and measures of QOL, peak VO2, and 6-MWD was evaluated using multivariable-adjusted linear regression models that included age, sex, race, treatment arm, and HF etiology. The longitudinal changes in QOL, peak VO2, and 6-MWD over time in the aerobic ET vs. usual care were also assessed across the baseline frailty strata using separate linear mixed-effect models for repeated measurements of each outcome. Consistent with the approach in the primary trial,3 two slopes were computed with a cut-point at 3 months (baseline to 3 months and slope after 3 months) to account for the non-linearity in the trajectory.3 The models were adjusted for age, sex, race, treatment arm, HF etiology, and interval incidence of the primary composite outcome. Multiplicative interaction terms (treatment arm*frailty status) were also included in the adjusted models using the overall cohort to evaluate the effect modification of frailty status on the treatment effect for the QOL and functional capacity outcomes. Finally, the proportion of participants in usual care and ET arms with safety endpoint incidence was also compared across the frail and non-frail strata. Analyses were performed using R version 3.6.3 (R Foundation) with a P-value < 0.05, indicating significance. P-interaction <0.05 was considered statistically significant.
RESULTS
The study included 2,130 participants (mean age 59±13 years, 28% women, 32% African American), with 1,266 (59.4%) identified as frail (frailty index > 0.21, Figure S1). Frail (vs. non-frail) participants had a higher burden of cardiometabolic co-morbidities, worse symptom burden, worse functional status, and higher burden of HF hospitalization before enrollment (Table 1).
Table 1:
Baseline characteristics of study participants stratified by baseline frailty status
| Non-frail (N = 864) | Frail (N = 1,266) | P-value | |
|---|---|---|---|
|
| |||
| Frailty Index | 0.15 (0.04) | 0.31 (0.08) | <0.001 |
|
| |||
| Exercise training intervention arm, % | 420 (48.6) | 640 (50.6) | 0.40 |
|
| |||
| Age, years | 58.1 (13.3) | 58.9 (12.3) | 0.20 |
|
| |||
| Men, % | 589 (68.2) | 942 (74.4) | 0.002 |
|
| |||
| Black race, % | 280 (32.9) | 389 (31.2) | 0.46 |
|
| |||
| Diabetes, % | 170 (19.7) | 508 (40.1) | <0.001 |
|
| |||
| BMI, kg/m2 | 29.9 (6.8) | 31.8 (7.3) | <0.001 |
|
| |||
| Systolic blood pressure, mm Hg | 113.7 (18.1) | 113.8 (18.4) | 0.79 |
|
| |||
| Heart rate, beats per min. | 69.9 (11.5) | 71.5 (11.4) | 0.001 |
|
| |||
| Atrial fibrillation, % | 144 (16.7) | 299 (23.6) | <0.001 |
|
| |||
| Hypertension, % | 415 (48.0) | 855 (67.5) | <0.001 |
|
| |||
| Prior MI, % | 274 (31.7) | 625 (49.4) | <0.001 |
|
| |||
| PVD, % | 19 (2.2) | 127 (10.0) | <0.001 |
|
| |||
| COPD, % | 49 (5.7) | 183 (14.5) | <0.001 |
|
| |||
| Angina, % | 122 (4.1) | 405 (32.0) | <0.001 |
|
| |||
| Stroke, % | 56 (6.5) | 162 (12.8) | <0.001 |
|
| |||
| Depression, % | 76 (8.8) | 376 (29.7) | <0.001 |
|
| |||
| Cancer, % | 30 (3.5) | 54 (4.3) | 0.42 |
|
| |||
| NYHA class, % | |||
| NYHA Class 2 | 699 (80.9) | 652 (51.5) | |
| NYHA Class 3 | 164 (19.0) | 595 (47.0) | <0.001 |
| NYHA Class 4 | 1 (0.1) | 19 (1.5) | |
|
| |||
| Smoking, % | |||
| Never | 408 (47.2) | 377 (29.8) | |
| Former | 106 (12.3) | 256 (20.2) | <0.001 |
| Current | 350 (40.5) | 633 (50.0) | |
|
| |||
| Mobility, % | |||
| No problem | 696 (80.6) | 476 (37.6) | |
| Slight/Moderate | 167 (19.3) | 789 (62.3) | <0.001 |
| Severe | 1 (0.1) | 1 (0.1) | |
|
| |||
| HF hosp. in last 6 months (%), | 162 (18.8) | 402 (31.8) | <0.001 |
|
| |||
| Sodium, mg/dL | 139.4 (3.3) | 138.8 (5.1) | 0.005 |
|
| |||
| BUN, mg/dL | 22.2 (18.2) | 26.8 (30.2) | <0.001 |
|
| |||
| eGFR, mL/min/1.73m2 | 71.4 (22.0) | 65.2 (23.6) | <0.001 |
|
| |||
| Peak VO2 | 16.3 (4.8) | 14.1 (4.3) | <0.001 |
|
| |||
| KCCQ Score | 80.9 (13.3) | 56.4 (18.6) | <0.001 |
|
| |||
| Left Ventricular Ejection Fraction | 25.3 (7.7) | 25.0 (7.4) | 0.31 |
|
| |||
| ACE inhibitor use, % | 667 (77.2) | 924 (73.0) | 0.03 |
|
| |||
| ICD use, % | 298 (34.5) | 567 (44.8) | <0.001 |
|
| |||
| ACE inhibitor/ARB use, % | 827 (95.7) | 1184 93.5) | 0.04 |
|
| |||
| Beta Blockers, % | 820 (94.9) | 1192 (94.2) | 0.52 |
|
| |||
| Loop diuretics, % | 609 (70.5) | 1055 (83.3) | <0.001 |
Baseline characteristics are reported as mean (±standard deviation) or %
Abbreviations: MI, myocardial infarction; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart association; HF, Heart failure; hosp, hospitalization; BMI, Body mass index; GFR, glomerular filtration rate; mg, milligram; mL, milliliter; dL, deciliter; L, liter; m, meter; min, minute; U, unit; BUN, Blood urea nitrogen; KCCQ, Kansas City Cardiomyopathy Questionnaire; ICD, implantable cardioverter defibrillator; ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker
Frailty and effect of aerobic ET on clinical endpoints and safety outcomes
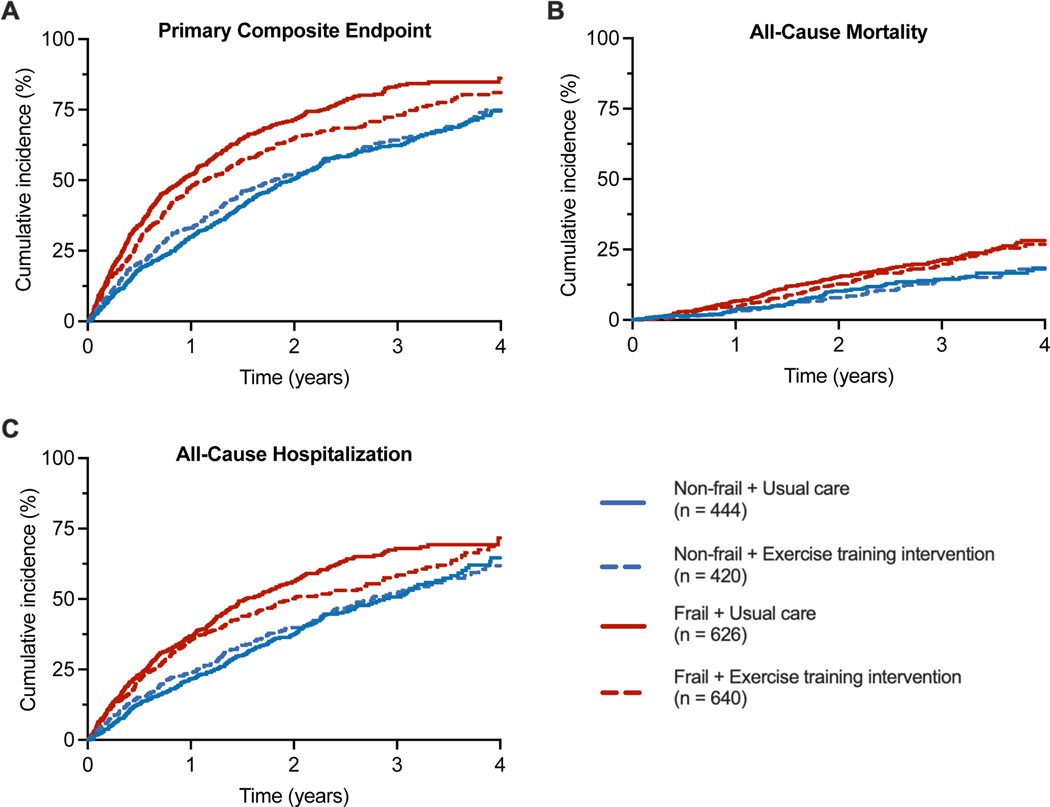
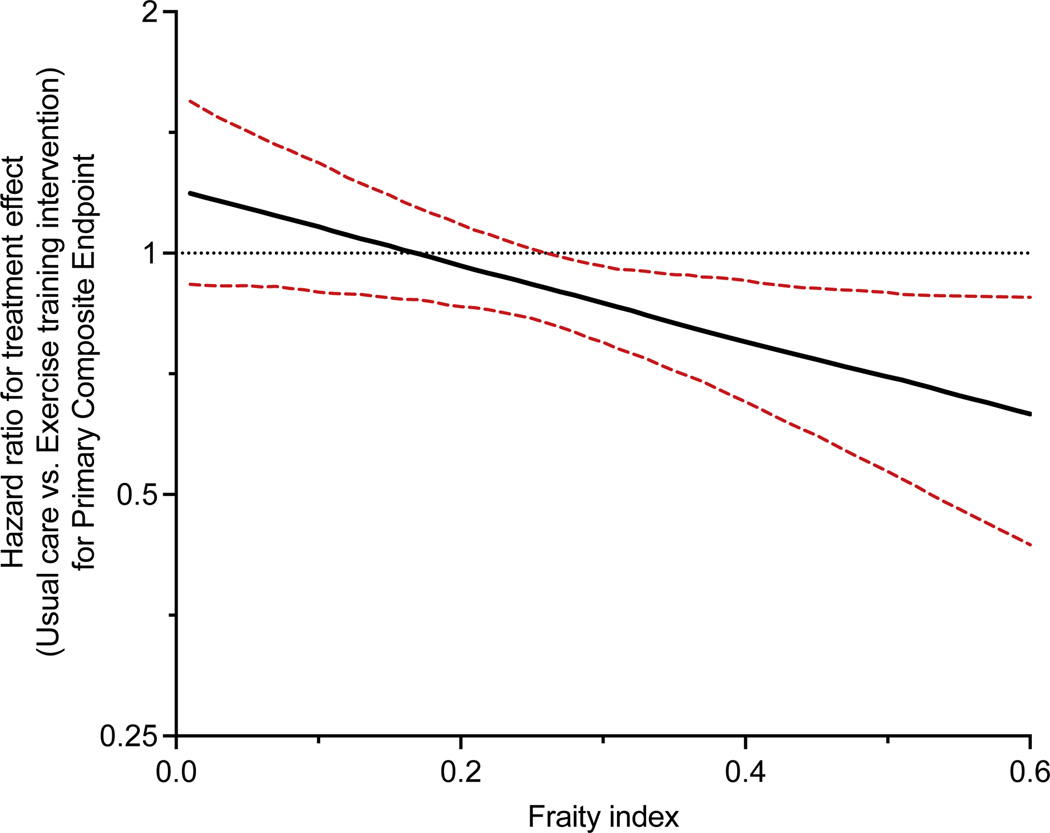
During a median follow-up of 2.9 [interquartile range = 2.0 – 3.8] years, 1426 (67.0%) developed a primary composite endpoint event. Higher frailty burden was significantly associated with a higher risk of the primary composite endpoint and secondary composite endpoints (CV mortality or CV hospitalization and CV mortality or HF hospitalization) in partially and fully adjusted models, including adjustment for BMI (Tables S3,S4). These associations were primarily driven by a higher risk of non-fatal hospitalization outcomes among frail participants. A significant interaction was observed between the frailty status and the treatment arm for the risk of the primary composite endpoint. Aerobic ET was associated with a significantly lower risk of the primary composite endpoint among frail participants but not non-frail participants (HR [95% CI] aerobic ET vs. usual care: Frail participants: 0.83 (0.73 – 0.95); non-frail participants: 1.04 (0.87 – 1.25); P-interaction [frailty status * treatment arm]: 0.04, Table 2, Figure 1). A similar treatment interaction was noted between the continuous measure of frailty index and the treatment arm for the risk of the primary composite outcome (P-interaction: 0.02). In restricted cubic spline analysis, aerobic ET was associated with a significantly lower risk of the primary composite endpoint above the frailty index threshold of 0.26 (Figure 2).
Table 2:
Treatment effects of aerobic exercise training on clinical outcomes among non-frail and frail participants
| Outcomes of interest | 2Treatment effect of Aerobic ET across Frailty Strata | 1P-int (Treatment*frailty) | |||
|---|---|---|---|---|---|
| Non-frail Participants | Frail Participants | ||||
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | ||
| Primary composite endpoint | 1.04 (0.87, 1.25) | 0.65 | 0.83 (0.72, 0.95) | 0.007 | 0.04 |
| All-cause mortality | 0.98 (0.66, 1.47) | 0.94 | 0.93 (0.71, 1.22) | 0.59 | 0.75 |
| All-cause hospitalization | 1.05 (0.85, 1.29) | 0.67 | 0.84 (0.72, 0.99) | 0.04 | 0.09 |
| HF hospitalization | 0.80 (0.57, 1.12) | 0.20 | 0.91 (0.73, 1.13) | 0.39 | 0.56 |
| CV death or HF hospitalization | 0.81 (0.57, 1.15) | 0.23 | 0.93 (0.75, 1.17) | 0.56 | 0.55 |
| CV death or CV hospitalization | 0.90 (0.71,1.13) | 0.36 | 0.94 (0.79, 1.13) | 0.51 | 0.77 |
Abbreviations: CI, confidence interval; HF, heart failure; CV, Cardio-vascular
The interaction between treatment arm and frailty status for the risk of the primary composite endpoint and secondary endpoints was assessed by including a multiplicative interaction term (treatment arm*frailty status) in the most adjusted model evaluating the association of treatment with outcomes in the overall cohort with following covariates: age, sex, race, treatment arm, left ventricular ejection fraction, HF etiology, Beck’s Depression Inventory score, baseline peak VO2, and atrial fibrillation. Separate model was constructed for each outcome.
Stratified Cox models were constructed frail and non-frail participants separately and for each outcome with adjustment for the same covariates as above (except for the stratifying variable)
Figure 1.
Cumulative incidence of the primary composite endpoints and its components (all-cause mortality and all-cause hospitalization) stratified by frailty status and treatment arm. A frailty index threshold of 0.21 was used to stratify participants into non-frail (frailty index ≤ 0.21) vs. frail (frailty index > 0.21) groups.
Figure 2:
Continuous association between frailty index and risk of primary composite end point in patients with aerobic exercise training intervention vs. usual care. (P-interaction = 0.03). The graph shows continuous measure of frailty index on x-axis and treatment effect of aerobic exercise on the primary composite endpoint on y-axis. Dotted line represents the 95% confidence interval around the point estimate for the treatment effect (black line).
Among components of the primary composite endpoint, aerobic ET was significantly associated with lower risk of all-cause hospitalization among frail but not non-frail participants (HR [95% CI] frail participants: 0.84 [0.72 – 0.99]; non-frail participants: 1.05 [0.85 – 1.29], p-interaction [frailty status * treatment arm]: 0.09 Table 2, Figure 1). There was no significant reduction in risk of all-cause mortality with aerobic ET among frail (HR [95% CI]: 0.93 [0.71 – 1.22]) and non-frail participants (HR [95% CI]: 0.98 [0.66 – 1.47], p-interaction [frailty status * treatment arm]: 0.75, Table 2, Figure 1). Among key secondary endpoints, aerobic ET was associated with non-significant reductions in the risk of composite endpoint of CV mortality or CV hospitalization and CV mortality or HF hospitalizations in frail and non-frail groups with no significant treatment interaction by frailty status (Table 2).
Among safety outcomes, aerobic ET was not associated with the risk of any adverse safety events (worsening HF, myocardial infarction, angina, arrhythmia, stroke, transient ischemic attack, implantable cardioverter defibrillator firing, or hospitalization immediately after exercise) in frail as well as non-frail participants (Table S2).
Frailty and effect of aerobic ET on quality of life and functional capacity
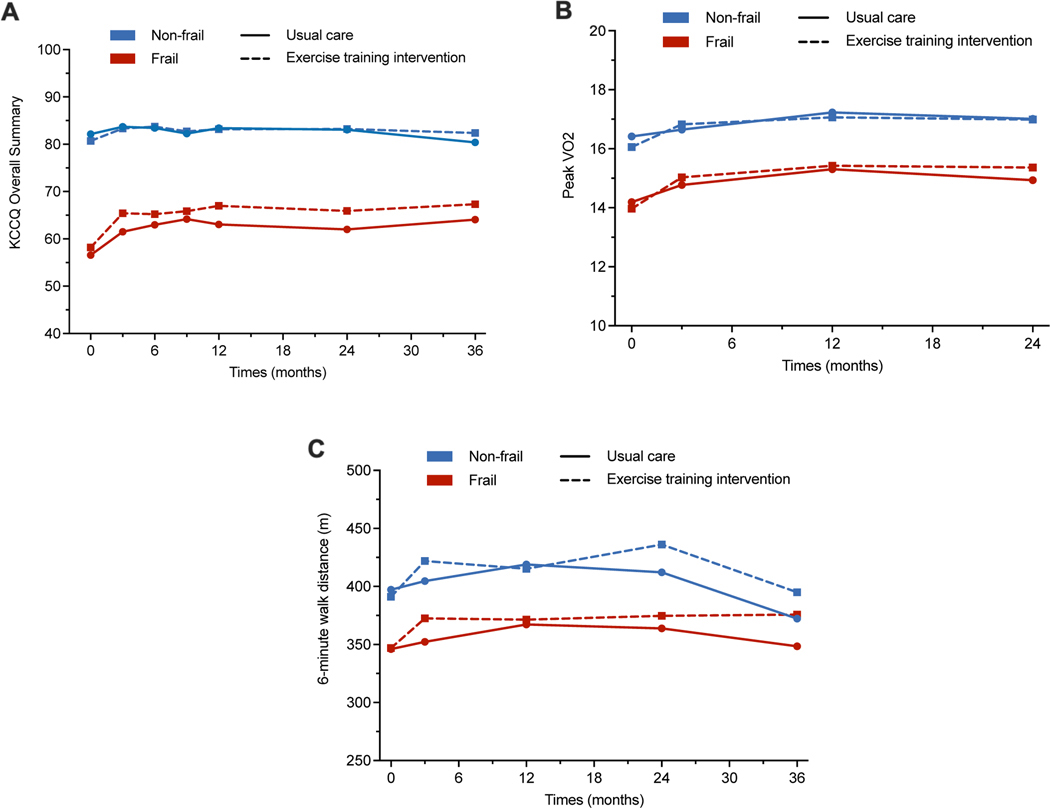
A higher burden of frailty was significantly associated with lower baseline KCCQ score, indicating worse QOL, lower peak VO2, and lower 6-MWD (Table S5). From baseline to 3-month follow-up, the improvement in QOL with aerobic ET, assessed by the KCCQ score, was comparable among frail vs. non-frail participants (p-interaction = 0.48). However, the magnitude of improvement in KCCQ was nominally greater among frail vs. non-frail participants (Figure 3, Table 3). On longer-term follow-up, there was no further improvement in KCCQ score with exercise in frail and non-frail participants (Figure 3, Table 3). Among measures of exercise capacity, aerobic ET was associated with a significant improvement in peak VO2 and 6-MWD in both frail and non-frail patients in the short-term (up to 3 months) with no further improvement in longer-term follow-up (Figure 3, Table 3).
Figure 3:
Change in quality of life (Kansas City Cardiomyopathy Questionnaire score), peak exercise capacity, and 6-minute walk distance over time in the usual care vs. aerobic exercise training arms among frail and non-frail participants. A frailty index threshold of 0.21 was used to stratify participants into non-frail (frailty index ≤ 0.21) vs. frail (frailty index > 0.21) groups.
Table 3:
Treatment effects of aerobic ET on quality of life and exercise capacity outcomes among non-frail and frail participants
| Outcomes of interest | Treatment effect of Aerobic ET | P-int (Treatment*frailty) | |||
|---|---|---|---|---|---|
| Non-frail Participants | Frail Participants | ||||
| Quality of life and Functional Outcomes | Parameter Estimate (95% CI) | P-value | Parameter Estimate(95% CI) | P-value | |
| Δ KCCQ 0 to 3 m | 0.45 (−0.10, 0.99) | 0.11 | 0.76 (0.14, 1.38) | 0.02 | 0.48 |
| Δ KCCQ 3 m to 3 y | 0.05 (−0.03, 0.12) | 0.22 | −0.01 (−0.09, 0.07) | 0.90 | 0.37 |
| Δ Peak VO2 0– 3 m | 0.17 (0.05, 0.29) | 0.005 | 0.17 (0.06, 0.27) | 0.002 | 0.95 |
| Δ Peak VO2 3 m to 2 y | 0.02 (−0.03, 0.07) | 0.39 | −0.01 (−0.05, 0.04) | 0.71 | 0.41 |
| Δ 6-MWD 0– 3 m | 7.18 (3.62, 10.74) | <0.001 | 5.54 (2.59, 8.49) | <0.001 | 0.50 |
| Δ 6-MWD 3 m to 3 y | −1.71 (−3.12, −0.3) | 0.02 | −1.23 (−2.51, 0.04) | 0.06 | 0.64 |
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; Peak VO2, peak exercise oxygen uptake; 6-MWD, 6-minute walk distance, m, month; y, year; Δ, change
Parameter estimate for functional, and quality of life outcomes represents the change in outcome variable per 1-month in the treatment arm vs. the usual care arm
DISCUSSION
In this post-hoc analysis of the HF-ACTION trial, we observed that frailty status significantly modified the treatment effects of aerobic ET in patients with chronic stable HFrEF. Aerobic ET was associated with a significant reduction in the risk of primary endpoint, driven by a greater reduction in the risk of all-cause hospitalization among frail but not non-frail participants. Aerobic ET was also associated with a comparable improvement in QOL among frail vs. non-frail participants without a significant treatment interaction. Overall, these findings support that frailty may identify patients with HFrEF who are at a higher risk and are more likely to benefit from aerobic ET.
Frailty is a complex clinical condition related to aging characterized by a decline in physiological reserve across several organ systems and increased susceptibility to stressors.10, 11 The two most common tools to assess frailty burden are Fried’s phenotype model and The Rockwood Frailty index.22 The Fried phenotype model assesses impairments across five physical function domains — weight loss, weakness, poor endurance, slowness, and low physical activity level. Frailty is identified by the presence of impairment is noted in 3 or domains as based on pre-defined cutoffs.11 While frailty identified by Fried phenotype has been associated with worse outcomes in patients with HF, it is resource- and time-intensive, needs prospective evaluations, and primarily represents impairments in physical function.11 The Rockwood frailty index quantifies frailty based on deficits’ cumulative burden across multiple domains. The high burden of frailty among participants of the HF-ACTION trial is consistent with these prior observations.21, 23–25 In the PARADIGM and ATMOSPHERE trials, Dewan et al. reported an even higher burden of frailty (65%) —assessed using a frailty index—among patients with chronic HFrEF.21 The modestly lower burden of frailty in the HF-ACTION may be due to younger age and enrollment criteria of ability to perform cardiorespiratory fitness test. These findings support the high frailty burden among patients with HF, even among relatively young, functional, stable outpatients.
Consistent with findings from prior studies, we observed that frailty burden was associated with a higher risk of adverse clinical outcomes.21, 25–28 High frailty burden may predispose to an increased risk of adverse clinical outcomes through several mechanisms. Frail patients are often sicker with more severe disease, contributing to an increased risk of adverse events. However, findings from our study suggest that the prognostic relevance of frailty in HFrEF extends beyond identifying individuals with more severe disease. Specifically, we observed that frailty was associated with the risk of primary (all-cause mortality or any hospitalization) and secondary composite endpoints (CV mortality or CV hospitalization and CV mortality or HF hospitalization) independent of other prognostic measures and indices of disease severity such as low exercise capacity, NYHA class, and ejection fraction.29–31 The associations were related to a greater risk of non-fatal hospitalization events, which may be driven by greater impairments in functional status and reduced global physiologic reserve. Thus, the prognostic implications of frailty in HF extend beyond disease severity and may be more driven by impairments in functional status and increased vulnerability to physiologic stressors that lead to a greater risk of hospitalization.
Frail patients with HFrEF were more responsive to aerobic ET with a greater reduction in the risk of the primary composite endpoint driven by a significant reduction in all-cause hospitalization. These findings suggest that aerobic ET may modify the frailty-related higher risk of all-cause hospitalization. In the recent REHAB-HF trial, a novel, multidomain physical rehabilitation intervention was associated with greater improvements in physical function among frail (vs. pre-frail) patients with acute decompensated HF.14 Similar findings have also been reported from other observational studies and small randomized controlled trials.31, 32 It is noteworthy that aerobic ET was not associated with a significant reduction in the risk of secondary CV composite endpoints in frail and non-frail patients. Thus, the favorable effects of aerobic ET on frailty-related adverse outcomes may not be all related to improvement in cardiac structure, function, and CV reserve. The improvement in extracardiac (skeletal muscle and peripheral microvascular) abnormalities that underlie the functional impairment among frail HF patients may also contribute to the beneficial effects of aerobic ET. Specifically, aerobic ET has been associated with downregulation of pro-inflammatory pathways and significant improvement in sarcopenia, endothelial function, mitochondrial function, and skeletal muscle oxygen utilization—the key drivers of frailty progression in patients with HF.33–38
Aerobic ET was not associated with improvement in primary or secondary clinical endpoints in non-frail patients with HFrEF, suggesting a potential lack of clinical efficacy in this subgroup of patients. These observations are hypothesis-generating only and would need to be confirmed in future studies and should not be viewed as diminishing the importance of aerobic ET in non-frail patients with HFrEF. Aerobic ET was associated with significant improvement in aerobic exercise capacity of non-frail patients, which was comparable to the improvements noted among frail patients, highlighting its therapeutic utility for all patients with HFrEF. Moreover, aerobic ET may benefit non-frail patients in the long term by lowering the future risk of frailty, improving exercise capacity, and other favorable cardiometabolic effects of exercise. Finally, it is plausible that alternative exercise regimens that include resistance training and/or high-intensity interval training, which were not part of the HF-ACTION exercise intervention, may be more effective in reducing the risk of adverse clinical outcomes among non-frail patients.
Our study findings have important clinical implications. It highlights the potential role of routine frailty assessment in identifying high-risk ambulatory patients with HFrEF who are most likely to benefit from aerobic ET and cardiac rehabilitation. This is particularly relevant since despite the class I recommendation for cardiac rehabilitation in patients with chronic stable HFrEF, its uptake in the contemporary clinical practice remains low.6 The low rates of utilization for cardiac rehabilitation in HFrEF are multifactorial, including poor referral rates, lack of widespread availability of cardiac rehabilitation, logistical challenges related to travel to cardiac rehabilitation sites, and high cost. A targeted approach focused on increasing supervised cardiac rehabilitation utilization among frail patients at the highest risk and most likely to benefit may be an efficient, cost-effective, and high-value care strategy to promote its widespread use. Efficient frailty screening algorithms that use readily available clinical data can be implemented in routine clinical practice to identify frail patients with HF.24, 39 Randomized controlled trials evaluating the efficacy of aerobic ET among frail HFrEF patients are needed to provide a more definitive answer regarding its therapeutic benefits in this high-risk population who may have challenges with adherence to aerobic ET owing to a greater burden of comorbidities, depression, and cognitive function impairment. Future studies are needed to determine if such a risk-based targeted approach to cardiac rehabilitation may improve its uptake and reduce the risk of adverse outcomes in high-risk frail patients with HFrEF.
Several limitations of this study are noteworthy. First, participants of the HF-ACTION trial were relatively young (mean age 60 years) persons with chronic stable HFrEF who had a relatively modest burden of comorbidities and could exercise at baseline. Thus, our study findings may not be generalizable to patients with HFrEF who may not fit the study inclusion/exclusion criteria. Second, adherence to aerobic ET was lower than planned, particularly beyond the first three months of supervised training. Thus, the lack of long-term improvements in quality of life and exercise capacity may be related to lower adherence. However, the adherence was not different among frail vs. non-frail patients. Furthermore, high levels of non-adherence would be expected to bias our study findings related to the primary composite endpoint towards null and potentially underestimate the benefits of aerobic ET in the intervention arm. Third, we did not have measures of grip strength, gait speed, and weight change that limited our ability to quantify physical function-based measures of frailty using the Fried phenotype. Finally, we could not assess the frailty burden on follow-up due to the lack of consistent availability of frailty index components. Thus, we do not have data on changes in frailty status with aerobic ET among the study participants.
In conclusion, among patients with chronic, stable HFrEF who participated in the HF-ACTION trial, the prevalence of frailty was high. Higher frailty burden at baseline identified a high-risk subset of patients who were more responsive to aerobic ET with a greater reduction in the risk of all-cause hospitalization but not mortality.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Baseline frailty modified the treatment effect of supervised aerobic exercise training among patients with chronic, stable heart failure with reduced ejection fraction.
Aerobic exercise training was associated with a significant reduction in the risk of primary endpoint, driven by a greater reduction in the risk of all-cause hospitalization among frail but not non-frail participants.
What are the clinical implications?
Baseline frailty may identify patients with heart failure with reduced ejection fraction who are more likely to benefit from supervised aerobic exercise training.
ACKNOWLEDGEMENTS:
We would like to thank NHLBI BioLINCC for access to data and participants of HF-ACTION.
FUNDING SOURCES:
Dr Pandey is supported by the Gilead Sciences Research Scholar Program, the National Institute of Aging GEMSSTAR Grant (1R03AG067960-01). Dr Kitzman is supported in part by NIH grants R01AG18915, R01AG045551, P30AG021332, and U24AG059624, and by the Kermit G. Phillips Endowed Chair in Cardiovascular Medicine
DISCLOSURES:
Dr. Pandey received grant funding outside the present study from Applied Therapeutics; has received honoraria outside of the present study as an advisor/consultant for Tricog Health Inc and Lilly, USA, Rivus, and Roche Diagnostics, and has received nonfinancial support from Pfizer and Merck. Dr Kitzman has received honoraria outside the present study as a consultant for AbbVie, Bayer, Merck, Medtronic, Relypsa, Merck, Corvia Medical, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Novartis; has received grant funding outside the present study from Novartis, Bayer, Novo Nordisk, and AstraZeneca; and has stock ownership in Gilead Sciences. Dr Whellan received research support and consulting fees from Amgen, CVRx, Cytokinetics, Fibrogen, Novartis, and NovoNordisk. Dr Upadhya received research support from Novartis and Corvia. Dr Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Medtronic, Merck, Novartis, Roche, Sanofi, and Vifor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the United States Department of Health and Human Services.
ABBRIVIATIONS
- ET
exercise training
- CR
cardiac rehabilitation
- QOL
quality of life
- HFrEF
heart failure with reduced ejection fraction
- peak VO2
peak exercise oxygen uptake
- BioLINCC
Biologic Specimen and Data Repository Information Coordinating Center
- 6-MWD
6-minute walk distance
- KCCQ score
Kansas City Cardiomyopathy Questionnaire
REFERENCES:
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 2.Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Piña IL, Poole DC, Reeves GR, et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardiac Rehabiliataion (CR) Programs – Chronic Heart Failure. Cemters fpr Medicare & Medicaid Services. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=270. Date of Access: 11/11/2021.
- 6.Pandey A, Keshvani N, Zhong L, Mentz RJ, Pina IL, DeVore AD, Yancy C, Kitzman DW, Fonarow GC. Temporal Trends and Factors Associated With Cardiac Rehabilitation Participation Among Medicare Beneficiaries With Heart Failure. JACC Heart Fail. 2021;9:471–481. [DOI] [PubMed] [Google Scholar]
- 7.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, et al. Tracking Cardiac Rehabilitation Participation and Completion Among Medicare Beneficiaries to Inform the Efforts of a National Initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A, Ayers C, Blair SN, Swift DL, Earnest CP, Kitzman DW, Khera A, Church TS, Berry JD. Cardiac determinants of heterogeneity in fitness change in response to moderate intensity aerobic exercise training: the DREW study. J Am Coll Cardiol. 2015;65:1057–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey A, Swift DL, McGuire DK, Ayers CR, Neeland IJ, Blair SN, Johannsen N, Earnest CP, Berry JD, Church TS. Metabolic Effects of Exercise Training Among Fitness-Nonresponsive Patients With Type 2 Diabetes: The HART-D Study. Diabetes Care. 2015;38:1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. JACC Heart Fail. 2019;7:1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courel-Ibanez J, Pallares JG, Garcia-Conesa S, Buendia-Romero A, Martinez-Cava A, Izquierdo M. Supervised Exercise (Vivifrail) Protects Institutionalized Older Adults Against Severe Functional Decline After 14 Weeks of COVID Confinement. J Am Med Dir Assoc. 2021;22:217–219 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 14.Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med. 2021;385:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376:1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. [DOI] [PubMed] [Google Scholar]
- 18.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatrics. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stekhoven DJ, Buhlmann P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. [DOI] [PubMed] [Google Scholar]
- 20.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–740. [DOI] [PubMed] [Google Scholar]
- 21.Dewan P, Jackson A, Jhund PS, Shen L, Ferreira JP, Petrie MC, Abraham WT, Desai AS, Dickstein K, Kober L, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction - an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail. 2020;22:2123–2133. [DOI] [PubMed] [Google Scholar]
- 22.Cesari M, Gambassi G, Abellan van Kan G, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age and Ageing. 2013;43:10–12. [DOI] [PubMed] [Google Scholar]
- 23.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int J Cardiol. 2017;236:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Chen H, Reeves GR. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Heart Fail. 2019;7:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018;20:1570–1577. [DOI] [PubMed] [Google Scholar]
- 26.Vidan MT, Blaya-Novakova V, Sanchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–875. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Lupon J, Vidan MT, Ferguson C, Gastelurrutia P, Newton PJ, Macdonald PS, Bueno H, Bayes-Genis A, Woo J et al. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7:e008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segar MW, Singh S, Goyal P, Hummel SL, Maurer MS, Forman DE, Butler J, Pandey A. Prefrailty, impairment in physical function, and risk of incident heart failure among older adults. J Am Geriatr Soc. 2021;69:2486–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Groote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004;43:1584–1589. [DOI] [PubMed] [Google Scholar]
- 30.Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell TR, Pina IL, Leifer ES, Fleg JL, Blackburn G, Fonarow GC, et al. Variables Measured During Cardiopulmonary Exercise Testing as Predictors of Mortality in Chronic Systolic Heart Failure. J Am Coll Cardiol. 2016;67:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mudge AM, Pelecanos A, Adsett JA. Frailty implications for exercise participation and outcomes in patients with heart failure. J Am Geriatr Soc. 2021;69:2476–2485. [DOI] [PubMed] [Google Scholar]
- 32.Kehler DS, Giacomantonio N, Firth W, Blanchard CM, Rockwood K, Theou O. Association Between Cardiac Rehabilitation and Frailty. Can J Cardiol. 2020;36:482–489. [DOI] [PubMed] [Google Scholar]
- 33.Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, Mobius-Winkler S, Sandri M, Krankel N, Hambrecht R, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3:486–494. [DOI] [PubMed] [Google Scholar]
- 34.Feiereisen P, Vaillant M, Gilson G, Delagardelle C. Effects of different training modalities on circulating anabolic/catabolic markers in chronic heart failure. J Cardiopulm Rehabil Prev. 2013;33:303–308. [DOI] [PubMed] [Google Scholar]
- 35.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guimaraes GV, Ribeiro F, Castro RE, Roque JM, Machado ADT, Antunes-Correa LM, Ferreira SA, Bocchi EA. Effects of the exercise training on skeletal muscle oxygen consumption in heart failure patients with reduced ejection fraction. Int J Cardiol. 2021;343:73–79. [DOI] [PubMed] [Google Scholar]
- 38.Parrinello G, Torres D, Paterna S, Di Pasquale P, Trapanese C, Licata G. Short-term walking physical training and changes in body hydration status, B-type natriuretic peptide and C-reactive protein levels in compensated congestive heart failure. Int J Cardiol. 2010;144:97–100. [DOI] [PubMed] [Google Scholar]
- 39.Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of Frailty in Chronic Heart Failure. JACC Heart Fail. 2019;7:291–302. [DOI] [PubMed] [Google Scholar]
- 40.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legge A, Kirkland S, Rockwood K, Andreou P, Bae SC, Gordon C, Romero-Diaz J, Sanchez-Guerrero J, Wallace DJ, Bernatsky S, et al. Construction of a frailty index as a novel health measure in systemic lupus erythematosus. J Rheumatol. 2020;47:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. [DOI] [PubMed] [Google Scholar]
- 43.Rogers NT, Steptoe A, Cadar D. Frailty is an independent predictor of incident dementia: Evidence from the English Longitudinal Study of Ageing. Sci Rep. 2017;7:15746–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.