Abstract

Shikimic acid (SA) is a compound extracted from the plant anise and has anti-inflammatory effects. However, any impact on intestinal inflammation or mechanisms involved has not been investigated. The present study used a dextran sulfate sodium (DSS)-induced mouse colitis model to investigate the effects of SA on intestinal inflammation. Intragastric administration of SA slowed DSS-induced weight loss, reduced disease activity index (DAI) score, enhanced the intestinal barrier, reduced the destruction of the colonic structure, inhibited the phosphorylation of key proteins in MAPK and NF-κB signaling pathways, inhibited the expression of inflammatory factors TNF-α, IL-1β, and MPO (P < 0.05), decreased IFN-γ expression (P < 0.05), and increased immunoglobulin IgG content (P < 0.05). After 50 mg/kg SA treatment, the content of Bacteroidetes increased and Proteobacteria decreased in the cecal feces of mice with colitis (P < 0.05) and the richness of gut species increased. In conclusion, SA could improve intestinal inflammation and enhance intestinal immunity, indicating its suitability as a therapeutic candidate.
Keywords: shikimic acid, ulcerative colitis, intestinal barrier function, tight junction protein, intestinal flora
1. Introduction
Inflammation is the body’s protective response to eliminate harmful stimuli and repair damaged tissue.1 Inflammation has five typical symptoms of redness, fever, pain, tissue damage, and loss of function and occurs through mechanisms such as increased permeability of the vascular endothelium, leakage of plasma components, and exosmosis of immune cells. These physiological responses are designed to alert the host to possible infection, tissue damage, and cell death in order to initiate complex repair processes by cellular and molecular mechanisms, but if inflammation persists, acute and chronic diseases may result.2 Ulcerative colitis (UC) is a chronic, nonspecific inflammation of the colon, manifested as pathological injury to the intestinal ulcers, colonic constriction, diarrhea, and bloody stool.3 UC is caused by a complex interplay of genetic and environmental factors, dysbiosis of the gut microbiota, immune dysregulation, oxidative stress, and other factors.4 Untreated UC can result in severe complications, including peritonitis and colorectal cancer.5
Shikimic acid (SA) is a compound with a chiral carbon structure. It acts as a precursor to various substances such as cinnamic acid, anthocyanins, flavonols, and tannins. It also serves as an aromatic mediator in the synthesis of essential amino acids such as l-phenylalanine, l-tyrosine, and l-tryptophan, as well as lignans and plant and microbial alkaloids such as mangiferic acid.6 SA is the basic raw material for oseltamivir production, obtained through biosynthesis and microbial metabolism.6,7 SA also has many biological properties, such as antidiabetic, antibacterial, anti-inflammatory, analgesic, antioxidant, and antithrombotic effects.8−11 SA is generally considered pharmacologically safe, and its biological activity has led to its use to treat stomach pain and skin inflammation. SA showed anti-inflammatory activity by inhibiting the reduction of RAW 264.7 cell viability, nitrite accumulation, and production of proinflammatory cytokines induced by lipopolysaccharide (LPS).12 LPS-induced production of cytoproinflammatory cytokines and mechanical hyperalgesia were also alleviated in SA-treated mice.13 SA inhibited osteoclastogenesis via an effect on the NF-κB and MAPK pathways.14 However, activities relevant to intestinal inflammation remain unclear.
Anti-inflammatory and immunosuppressive agents such as 5-aminosalicylic acid (5-ASA), corticosteroids, and immunomodulators have been employed to manage UC.5 However, such drugs are expensive and have many side effects, raising interest in alternative therapies, especially bioactive plant extracts. This study aimed to explore the impact of SA on DSS-induced colitis in mice, focusing on its effects on intestinal ecology, barrier function, and NF-κB and MAPK signaling pathways.
2. Materials and Methods
2.1. Materials and Reagents
SA (99.3%) was purchased from Fujian Fukang Pharmaceutical Co, LTD. (batch no.: FKSA2110007, Fujian, China); 5-aminosalicylic acid was obtained from Maclean’s; DSS (36–50 kDa) was obtained from MP Biomedicals (Cellagen, Aurora, OH, USA).
2.2. Animals
C57BL/6J mice (male, 8–10 weeks old, 20 ± 2 g) were purchased from Guangdong Scajingda Biotechnology Co. (license no. SCXK (Cantonese) 2020-0052) and housed at an ambient temperature of 23–25 °C, a relative humidity of 43% with adequate water and food. Animal experiments were conducted following the guidelines of the South China Agricultural University Animal Ethics Committee and the Guide for the Care and Use of Laboratory Animals (SYXK-2022-0136).
2.3. Animal Experiments
C57BL6/J mice were randomly divided into six groups: (i) pure water + gavage of 200 μL saline group (control); (ii) DSS + gavage of 200 μL saline group (DSS); (iii) DSS + gavage of 10 mg/kg SA group (SA10); (iv) DSS + gavage of 30 mg/kg SA group (SA30); (v) DSS + gavage of 50 mg/kg SA group (SA50); (vi) DSS + gavage of 40 mg/kg 5-aminosalicylic acid group (ASA). Mice were fed adaptively for 7 days with free drinking and eating. SA and 5-aminosalicylic acid were given by intragastric administration every morning during the experimental period. After intragastric administration on the fourth day, drinking water was replaced with 3% DSS with free drinking. Referring to previous research methods,15 the body weight, mental state, and blood excretion of mice in each group were recorded every day. After drinking 3% DSS for 5 days (on the eighth day of gavage), the body weight of mice in the DSS group decreased by 20%, and then 3% DSS was replaced with drinking water for 2 days. Colon tissues were collected from mice after euthanasia.
2.4. Evaluation of the DAI
The disease activity index (DAI) was scored as described above.16
2.5. Histological Analysis
H&E staining was performed according to a previously established protocol.5 A portion of the proximal colon was preserved in a 10% neutral buffered formalin solution (pH 7.4) by immersion. The colon tissue was sectioned, embedded in paraffin, and stained with H&E for microscopic examination. The 3 mm sections were subsequently observed using a light microscope equipped with an image analysis system (no. DM4000, Leica, Germany).
2.6. Myeloperoxidase Assay
A 100 mg sample of colon tissue was homogenized in 1 mL of PBS, followed by centrifugation at 3000 rpm for 5 min. The resulting supernatant was then frozen. Protein concentration was measured with a bicinchoninic acid (BCA) protein assay kit (PC0020, Solarbio, China), and MPO levels were measured using kits following the manufacturer’s instructions (MEIMIAN, Jiangsu, China).
2.7. Assay of Inflammatory Cytokines, Immune Factors, and Tight Junction Proteins
Colon tissue was homogenized, and the levels of TNF-α, IL-1β, IFN-γ, IgG, ZO-1, claudin-1, and occludin were measured using ELISA kits (MEIMIAN, Jiangsu, China) following the manufacturer’s instructions.
2.8. Western Blot Analysis
Protein concentration in the colon tissue supernatant was determined using the BCA assay, as previously described. The protein samples were then separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, USA). Membranes were incubated with primary antibodies raised against ERK (CST #9102, USA, 1:1000), p-ERK(CST# 4370, USA, 1:500), p38 (CST#54470, USA, 1:1000), p-p38 (CST# 4511, USA, 1:500), JNK (CST# 9252, USA, 1:1000), p-JNK (CST#9255, USA, 1:500), NF-κB P65 (CST# 8242, USA, 1:1000), p-NF-κB P65 (CST#3033, USA, 1:500), and GAPDH (superior antibody # UM4002, 1:2000) overnight at 4 °C, washed three times with PBST, incubated with goat-labeled secondary antibodies, and visualized using an ECL detection kit. An automatic chemiluminescence image analysis system was used for analysis (Tanon 5200, Shanghai, China).
2.9. 16S rDNA Sequencing of Cecal Fecal Microbial Flora
Microbial genomic DNA was extracted using the DNeasy PowerSoil kit (QIAGEN, Inc., The Netherlands) according to the manufacturer’s protocol and stored at −20 °C. DNA quantity and quality were evaluated using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis. 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) primers were used for PCR amplification of the V3–V4 region of 16S rDNA. PCR was conducted at 98 °C for 5 min, followed by 25 cycles of denaturation at 98 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 45 s. A final extension was done at 72 °C for 5 min. The PCR products were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China) and quantified using the Quant-iT PicoGreen dsDNA assay kit (Invitrogen, Carlsbad, CA, USA). The amplicons were pooled in equal amounts and sequenced using pair-end 2250 bp sequencing on the Illumina NovaSeq platform with the NovaSeq 6000 SP Reagent Kit (500 cycles) at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China).
2.10. Statistical Analysis
In this study, all data are shown as mean ± standard deviation (SD) from six independent measurements (n = 6). Statistical analysis was performed using SPSS software, version 26.0. Multiple comparisons were evaluated using one-way ANOVA followed by Tukey’s multiple-comparison test. P < 0.05 was regarded as statistically significant.
3. Results
3.1. Effect of SA on Symptomatic Improvement of DSS-Induced Colitis in Mice
As shown in Figure 1, the DSS-treated mice continued to lose body weight and increased DAI scores. SA inhibited weight loss and decreased the DAI score in a dose-dependent manner (Figure S1).
Figure 1.
Effect of SA on symptomatic improvement of DSS-induced colitis in mice. (A) Body weight, (B) DAI score, control: normal group, DSS: model group, SA10: 10 mg/kg shikimic acid, SA30: 30 mg/kg shikimic acid, SA50: 50 mg/kg shikimic acid, and ASA: 40 mg/kg 5-aminosalicylic acid. Values given are mean ± SD (n = 6).
3.2. Effect of SA on the Histopathological Improvement of DSS-Induced Colitis in Mice
Histological analysis revealed the histopathological status of the colon, as shown in Figure 2. In the DSS group, the colon structure was severely damaged, with a number of inflammatory cells, and the recess was dilated near the ulcer focus. Control and SA groups had normal colonic morphology, clear tissue structure, visible mucosal layer, and crypts.
Figure 2.
Effect of SA on the histopathological improvement of DSS-induced colitis in mice. Typical sections from each group are shown.
3.3. Effect of SA on Regulation of MPO
As shown in Figure 3, compared with the control group, MPO activity in the colon of mice in the DSS group was significantly increased (P < 0.05), and the activity of colon MPO was significantly decreased after treatment with 10–50 mg/kg SA (P < 0.05) in a dose-dependent manner.
Figure 3.

Effect of SA on regulation of MPO. Values given are mean ± SD (n = 6). a,b,c,d,e Values with different letters were significantly different (P < 0.05).
3.4. Effect of SA on Regulation of Inflammatory Cytokines and Immunoglobulin
As shown in Figure 4A, DSS treatment significantly increased the expression of TNF-α and IL-1β (P < 0.05), while SA and 5-aminosalicylic acid treatment significantly inhibited the abnormal increase of TNF-α and IL-1β (P < 0.05).
Figure 4.

Effects of SA on inflammatory factors and immune factors. (A) Colonic expression levels of proinflammatory cytokines, TNF-α and IL-1β; (B) colonic expression levels of IFN-γ and IgG. Values given are mean ± SD (n = 6). a,b,c,d,e Values with different letters were significantly different (P < 0.05).
As shown in Figure 4B, the colon IFN-γ content of mice in the DSS group significantly increased (P < 0.05) and IgG content significantly decreased (P < 0.05). After 10–50 mg/kg SA treatment, the IFN-γ content significantly decreased (P < 0.05) and IgG content increased (P < 0.05).
3.5. Effect of SA on Regulation of Tight Junction Proteins
As shown in Figure 5, compared with the control group, the expression of ZO-1, claudin-1, and occludin decreased significantly after DSS treatment (P < 0.05). SA treatment significantly increased the expression of ZO-1, claudin-1, and occludin (P < 0.05).
Figure 5.
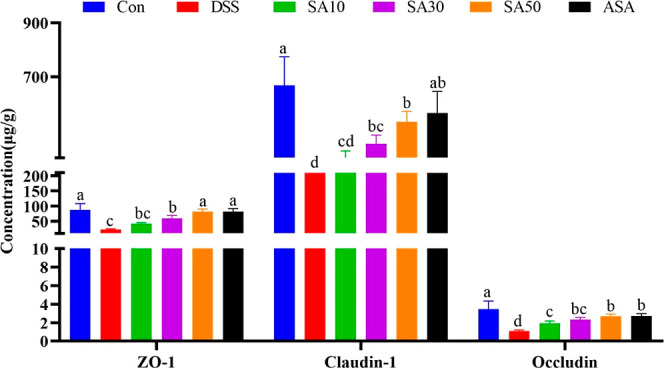
Effect of SA on regulation of tight junction proteins. Values given are mean ± SD (n = 6). a,b,c,d,e Values with different letters were significantly different (P < 0.05).
3.6. Effect of SA on Regulation of NF-κB and MAPK Signaling
As shown in Figure 6A,B, in western blotting experiments, DSS significantly promoted the expression of phosphorylated NF-κB P65 (P < 0.05). SA treatment significantly inhibited the phosphorylation of NF-κB P65 (P < 0.05) with 30 mg/kg SA and 50 mg/kg SA having similar effects, both better than 10 mg/kg SA (P < 0.05).
Figure 6.
Effect of SA on regulation of NF-κB and MAPK signaling. (A) Strip plot of NF-κB P65 western blot in the mouse colon, (B) relative quantitative plot of NF-κB P65 phosphorylated protein in the mouse colon, (C) strip plot of P38/JNK/ERK western blot in the mouse colon, and (D) relative quantitative plot of P38/JNK/ERK phosphorylated protein in the mouse colon. Values given are mean ± SD (n = 3). a,b,c,d,e,f Values with different letters were significantly different (P < 0.05).
As shown in Figure 6C,D, the phosphorylation levels of p38, JNK, and ERK were significantly increased in the DSS group (P < 0.05). 10 mg/kg SA could significantly inhibit the phosphorylation of P38 and ERK (P < 0.05), while 30 mg/kg SA could significantly inhibit the phosphorylation of JNK (P < 0.05). Moreover, the inhibitory effect of 50 mg/kg SA on phosphorylation of the three proteins was significantly better than that of 10 mg/kg SA and 30 mg/kg SA (P < 0.05).
3.7. Effect of SA on Regulation of Cecal Microflora
3.7.1. Rarefaction Curves and OTU Cluster Analysis
Specific primers were used for sequencing analysis of V3–V4 regions of cecal contents 16S rDNA. The dilution curve (Figure 7A) shows that the number of operational taxonomic units (OTUs) detected in samples with the increase of detection sequences was not further increased after reaching a plateau, indicating that the sequencing depth meets the requirements of experimental analysis and the microorganisms in the samples have been detected. Cluster analysis was performed on OTUs with a sequence similarity greater than 97%, and the results were drawn as petals (Figure 7B). The operable taxa of blank, model, SA low-dose, SA medium-dose, SA high-dose, and 5-aminosalicylic acid groups were 4700, 1528, 1133, 1917, 2744, and 2370, respectively. There were 338 OTUS in the six groups. These results indicated that the intestinal flora of the high-dose SA group was closer to that of the controls than that of the model group.
Figure 7.
Sparse curve and OTU petal diagram. (A) Sparse curve of mouse blind feces and (B) OTU petal diagram of mouse blind feces. Values given are mean ± SD (n = 6).
3.7.2. α-Diversity Analysis
The α-diversity index (Chao1, Observed_species, Shannon, and Simpson) of different samples under the 97% similarity threshold was analyzed (Figure 8). Compared with the control group, the Chao 1 index, Observed_species index, Shannon index, and Simpson index in the DSS group were significantly reduced (P < 0.05) but significantly higher for the Chao and Shannon indices in the 50 mg/kg SA group than in the DSS group (P < 0.05). There was no effect on the Observed_species index and Simpson index (P > 0.05).
Figure 8.

Effect of SA on α-diversity in mice cecal feces. (A) Chao1 and observed species and (B) Shannon and Simpson. Values given are mean ± SD (n = 6).
3.7.3. Beta-Diversity Analysis
Based on the unweighted binary Jaccard algorithm, principal coordinate analysis (PCoA) was performed for the cecum microorganisms to determine the beta diversity of the microbial community. As shown in Figure 9, the cecal microbial community of the 50 mg/kg SA-treated group appeared to be different from that of the DSS group.
Figure 9.

PCoA diagram. Values given are mean ± SD (n = 6).
3.7.4. Species Relative Abundance Analysis
To reveal the detailed composition of the gut microbiome, differences at different levels of phyla and genera were assessed. As shown in Figure 10A,B, at the phylum level, 10 main bacterial phyla were identified, including Firmicutes (46.96%), Bacteroidetes (34.42%), Proteobacteria (3.4%), and Bacteroidetes (34.42%). Campilobacterota (6.03%) and Spirochaetota (6.45%) are the top five phyla with a total relative abundance of more than 97%. The abundance of Firmicutes decreased significantly after DSS treatment (P < 0.05), and Proteobacteria abundance increased significantly (P < 0.05). In contrast, 50 mg/kg SA treatment significantly increased the abundance of Bacteroidetes (P < 0.05). In addition, the abundance of Proteobacteria significantly decreased after 30 mg/kg SA and 50 mg/kg SA treatment (P < 0.05). At the genus level (Figure 10C,D), Akkermansia, Lactobacillus, Allobaculum, Oscillospira, and Desulfovibrio were dominant bacteria in the colon feces of controls. Shigella was the dominant genus after DSS treatment, but the proportion of Shigella decreased significantly after 50 mg/kg SA treatment (P < 0.01).
Figure 10.
Effects of SA on species composition. (A) Effect of SA on the fecal taxonomic composition of the phylum, (B) relative abundance of Firmicutes, Bacteroidetes, and Proteobacteria, (C) effect of SA on the fecal taxonomic composition of the genus, and (D) relative abundance of Shigella (n = 6). a,b,c,d,e,f Values with different letters were significantly different (P < 0.05).
3.7.5. Species Difference and Marker Species Analysis
Given that the above analysis did not distinguish the dominant groups, LEf Se was used to generate a clad map to identify the specific bacteria associated with DSS (Figure 11). In the colon feces of DSS mice, the major opportunistic bacteria Gammaproteobacteria were overabundant [LDA score (log 10) > 5]. After 50 mg/kg SA treatment, Bacteroidaceae were the richest flora [LDA score (log 10) > 4].
Figure 11.
Cladistic map of the phylogenetic distribution of related microbiota; values given are mean ± SD (n = 6).
4. Discussion
SA has exhibited anti-inflammatory properties,12,13 but the effects of SA on intestinal inflammatory signaling pathways and intestinal epithelial barrier function remain unclear. The current study shows that SA interferes with NF-κB and MAPK signaling pathways, protects against intestinal epithelial barrier dysfunction induced by inflammatory cytokines TNF-α, IL-1β, and IFN-γ, and enhances intestinal barrier function.
UC neutrophils cause barrier loss, epithelial cell apoptosis, and tissue damage.17 MPO, present in neutrophils, is a marker of acute inflammation that reflects the degree of neutrophil infiltration due to its enzymatic activity.17,18 Previous reports have shown that isopropylshikimic acid (a derivative with a similar molecular structure to SA) alleviates colitis by reducing MPO activity,19 consistent with the current findings. MPO activity was found to be higher after DSS treatment and reduced by SA treatment in a dose-dependent manner.
In the gut, there is a balance between proinflammatory and anti-inflammatory factors. However, in UC, the activation of mast cells and T cells leads to the production of an abundance of inflammatory factors.20 SA inhibited the production of IFN-γ, TNF-α, and IL-1β induced by DSS and increased IgG levels in the present work. Previous studies have shown that SA alleviates hyperalgesia by inhibiting TNF-α and IL-1β expression.13 In this study, we found that SA has anti-inflammatory properties as it increased colonic IgG content, and inhibited the production of IFN-γ, TNF-α, and IL-6 in mice with DSS-induced colitis.
NF-κB is a transcription factor affecting inflammatory mediators involved in innate and acquired immune responses in inflammatory cascades.17,21 Elevated levels of TNF-α and IL-1β have been linked to the translocation of NF-κB from the cytoplasm to the nucleus, resulting in the expression of several proinflammatory target genes.22 NF-κB binds to the p65/p50 subunit and the inhibitory protein IκB as a heterodimer in the cytoplasm. Normal intestinal epithelial cells also show a low level of NF-κB expression to regulate normal physiological function.23 However, inflammation causes NF-κB P65 overactivation, contributing to the progression of inflammation.17 In this study, we observed elevated expression of NF-κB in the colonic mucosa of mice with colitis, which was reduced upon SA treatment. Previous research has suggested that SA may inhibit the NF-κB and MAPK pathways and osteoclast formation in vitro by blocking the RANK-TRAF6 link.14 These results indicated that shikimic acid inhibited the phosphorylation of NF-κB P65 to produce anti-inflammatory activity.
The MAPK pathway is a key extracellular signal transduction pathway activated by inflammatory mediators, which helps regulate the production of inflammatory cytokines.24 MAPK signaling regulates cellular activities such as growth, differentiation, survival, and death. In mammals, there are three major groups of MAPKs: ERK, JNK, and p38, which phosphorylate downstream substrates, including signal response transcription factors.25,26 ERK regulates cell proliferation and differentiation. JNK responds to various stresses in cells and participates in cellular responses to radiation, osmotic pressure, and temperature changes. p38 plays a role in inflammation and apoptosis and is targeted in the development of anti-inflammatory drugs.27 Thus, we investigated the modulation of macrophage polarization in DSS-induced colitis by analyzing alterations in p38, ERK, and JNK signaling pathways through the integration of network pharmacology and existing literature. DSS induced phosphorylation of p38, ERK, and JNK in this study’s animal model, and SA at 50 mg/kg inhibited this phosphorylation. These results are consistent with previous findings that SA suppresses inflammation by inhibiting ERK, p38, and JNK phosphorylation.13,14
Overexpression of TNF-α, IL-1β, and IFN-γ also leads to dysfunction of the intestinal TJ barrier.28,29 Intestinal epithelial cells and tight junction proteins are key constituents of the intestinal mucosal barrier.30 Tight junction proteins are composed of adhesion molecules such as occludin, claudin, and transmembrane proteins and intracellular proteins such as ZOs and actin. Tight junction proteins play a critical role in preserving the integrity of the intestinal mucosal mechanical barrier by preventing the entry of intestinal pathogens, antigens, and other substances into the intestinal mucosa. This, in turn, helps to prevent immune cell activation and abnormal immune responses of the intestinal mucosa, ultimately maintaining the stability of mucosal barrier function and intestinal permeability.17,31 SA treatment increased the expression of ZO-1, occludin, and claudin-1 tight junction proteins in UC mice. This suggests that the beneficial effect of SA on DSS-induced colitis may be due to its protective effect on the intestinal mucosal barrier by enhancing the expression of tight junction proteins.
UC is often accompanied by an abnormal increase in pathogenic intestinal bacteria.32 The Chao 1 index reflects species richness and is a qualitative measure of alpha diversity.33 The Chao 1, Shannon, and Simpson indices of the mouse colon after SA treatment were higher than those in the DSS model. It is suggested that SA may regulate intestinal health by altering the microbiome, inhibiting pathogen proliferation, and promoting the proliferation of beneficial bacteria. In addition, PCoA showed a different bacterial community after treatment with 50 mg/kg SA, implying that intestinal flora was regulated.
Four phyla, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, account for about 99% of the human gut microbiota.34Firmicutes and Bacteroidetes are also dominant in the mouse gut. DSS treatment reduced the abundance of Firmicutes and increased Bacteroides.35Glycophilic bacteria, such as Bacteroides, generate LPS to invade the inner mucus layer, degrade glycan, and spread in the mucus. Thinning of the mucus layer allows further bacterial infiltration, causing intestinal inflammation and eventually leading to UC.36 Thus, Bacteroides is an important target for anti-UC therapies. Elevated Proteobacteria have been shown to cause hyphal abnormalities and disease.37 Treatment with 50 mg/kg SA reversed DSS-induced changes in the relative abundance of Bacteroidetes and Proteobacteria in the mouse gut and reduced Proteobacteria. This suggests that 50 mg/kg SA gavage can promote the recovery of intestinal flora imbalance and maintain intestinal health.
In conclusion, under the conditions of this experiment, SA inhibited the production of inflammatory factors by inhibiting the NF-κB and MAPK pathways, improved the abnormal immune response of the intestinal mucosa, regulated intestinal flora, prevented intestinal pathogens, antigens, and other substances from entering the intestinal mucosa, and improved intestinal mucosal barrier function and permeability.
Acknowledgments
This study received funding from the National Key Research and Development Program of China (no. 2022YFD1802105).
Glossary
Abbreviations
- NF-κB
nuclear factor kappa-B
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinase
- ERK
extracellular regulated protein kinases.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.3c00283.
Effect of SA on symptomatic improvement of DSS-induced colitis in mice (PDF)
Author Contributions
X.H., L.L., and X.L. designed the experiments; X.L. performed the experiments and wrote the manuscript; X.L., G.T., J.Z., and J.G. performed the animal experiments; X.L. and K.M. analyzed the data; X.L. revised the manuscript. All authors agree to be accountable for all aspects of the work and ensure the integrity and accuracy of the data.
The authors declare no competing financial interest.
Supplementary Material
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Calder P. C. n-3 polyunsaturated fatty acids, inflammation and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. 10.1093/ajcn/83.6.1505s. [DOI] [PubMed] [Google Scholar]
- Fuentes E.; Guzman-Jofre L.; Moore-Carrasco R.; Palomo I. Role of PPARs in inflammatory processes associated with metabolic syndrome (Review). Mol. Med. Rep. 2013, 8, 1611–1616. 10.3892/mmr.2013.1714. [DOI] [PubMed] [Google Scholar]
- Kim Y. R.; Hwang J.; Koh H. J.; Jang K.; Lee J. D.; Choi J.; Yang C. S. The targeted delivery of the c-Src peptide complexed with schizophyllan to macrophages inhibits polymicrobial sepsis and ulcerative colitis in mice. Biomaterials 2016, 89, 1–13. 10.1016/j.biomaterials.2016.02.035. [DOI] [PubMed] [Google Scholar]
- Jeon Y. D.; Lee J. H.; Lee Y. M.; Kim D. K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. 10.1016/j.biopha.2020.109847. [DOI] [PubMed] [Google Scholar]
- M Estevez A.; J Estevez R. A Short Overview on the Medicinal Chemistry of (-)-Shikimic Acid. Mini-Rev. Med. Chem. 2012, 12, 1443–1454. 10.2174/138955712803832735. [DOI] [PubMed] [Google Scholar]
- Bochkov D. V.; Sysolyatin S. V.; Kalashnikov A. I.; Surmacheva I. A. Shikimic acid: review of its analytical, isolation, and purification techniques from plant and microbial sources. J. Chem. Biol. 2012, 5, 5–17. 10.1007/s12154-011-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Malki A. L. Shikimic acid from Artemisia absinthium inhibits protein glycation in diabetic rats. Int. J. Biol. Macromol. 2019, 122, 1212–1216. 10.1016/j.ijbiomac.2018.09.072. [DOI] [PubMed] [Google Scholar]
- Tripathi P.; Rawat G.; Yadav S.; Saxena R. K. Shikimic acid, a base compound for the formulation of swine/avian flu drug: statistical optimization, fed-batch and scale up studies along with its application as an antibacterial agent. Antonie Van Leeuwenhoek 2015, 107, 419–431. 10.1007/s10482-014-0340-z. [DOI] [PubMed] [Google Scholar]
- Sun J. Y.; You C. Y.; Dong K.; You H. S.; Xing J. F. Anti-inflammatory, analgesic and antioxidant activities of 3,4-oxo-isopropylidene-shikimic acid. Pharm. Biol. 2016, 54, 2282–2287. 10.3109/13880209.2016.1153663. [DOI] [PubMed] [Google Scholar]
- Veach D.; Hosking H.; Thompson K.; Santhakumar A. B. Anti-platelet and anti-thrombogenic effects of shikimic acid in sedentary population. Food Funct. 2016, 7, 3609–3616. 10.1039/c6fo00927a. [DOI] [PubMed] [Google Scholar]
- Kolodziej H.; Radtke O. A.; Kiderlen A. F. Stimulus (polyphenol, IFN-γ, LPS)-dependent nitric oxide production and antileishmanial effects in RAW 264.7 macrophages. Phytochemistry 2008, 69, 3103–3110. 10.1016/j.phytochem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Rabelo T. K.; Guimaraes A. G.; Oliveira M. A.; Gasparotto J.; Serafini M. R.; de Souza Araújo A. A.; Quintans-Junior L. J.; Moreira J.; Gelain D. P. Shikimic acid inhibits LPS-induced cellular pro-inflammatory cytokines and attenuates mechanical hyperalgesia in mice. Int. Immunopharmacol. 2016, 39, 97–105. 10.1016/j.intimp.2016.07.016. [DOI] [PubMed] [Google Scholar]
- Chen X.; Li X.; Zhai X.; Zhi X.; Cao L.; Qin L.; Su J. Shikimic acid inhibits isteoclastogenesis in vivo and in vitro by blocking RANK/TRAF6 association and suppressing NF-κB and MAPK signaling pathways. Cell. Physiol. Biochem. 2018, 51, 2858–2871. 10.1159/000496039. [DOI] [PubMed] [Google Scholar]
- Shen P.; Zhang Z.; He Y.; Gu C.; Zhu K.; Li S.; Li Y.; Lu X.; Liu J.; Zhang N.; Cao Y. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018, 196, 69–76. 10.1016/j.lfs.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Wang S. K.; Chen T. X.; Wang W.; Xu L. L.; Zhang Y. Q.; Jin Z.; Liu Y. B.; Tang Y. Z. Aesculetin exhibited anti-inflammatory activities through inhibiting NF-kB and MAPKs pathway in vitro and in vivo. J. Ethnopharmacol. 2022, 296, 115489. 10.1016/j.jep.2022.115489. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Zou J.; Chen M.; Zhang Z.; Liu C.; Jiang S.; Qian D.; Duan J. A. Protective effects of Lizhong decoction on ulcerative colitis in mice by suppressing inflammation and ameliorating gut barrier. J. Ethnopharmacol. 2020, 259, 112919. 10.1016/j.jep.2020.112919. [DOI] [PubMed] [Google Scholar]
- Choudhary S.; Keshavarzian A.; Yong S.; Wade M.; Bocckino S.; Day B. J.; Banan A. Novel antioxidants zolimid and AEOL11201 ameliorate colitis in rats. Dig. Dis. Sci. 2001, 46, 2222–2230. 10.1023/a:1011975218006. [DOI] [PubMed] [Google Scholar]
- Xing J. F.; Sun J. N.; Sun J. Y.; You C. Y.; Dong K.; Lv J.; Dong Y. L. Protective effects of 3,4-oxo-isopropylidene-shikimic acid on experimental colitis induced by trinitrobenzenesulfonic acid in rats. Dig. Dis. Sci. 2012, 57, 2045–2054. 10.1007/s10620-012-2155-y. [DOI] [PubMed] [Google Scholar]
- Osaka T.; Moriyama E.; Arai S.; Date Y.; Yagi J.; Kikuchi J.; Tsuneda S. Meta-analysis of fecal microbiota and metabolites in experimental colitic mice during the inflammatory and healing phases. Nutrients 2017, 9, 1329. 10.3390/nu9121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. NF-κB and cancer: mechanisms and targets. Mol. Carcinog. 2006, 45, 355–361. 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Matsuhisa K.; Watari A.; Iwamoto K.; Kondoh M.; Yagi K. Lignosulfonic acid attenuates NF-κB activation and intestinal epithelial barrier dysfunction induced by TNF-α/IFN-γ in Caco-2 cells. J. Nat. Med. 2018, 72, 448–455. 10.1007/s11418-017-1167-5. [DOI] [PubMed] [Google Scholar]
- Huo X.; Zhang L.; Gao L.; Guo Y.; Zhang L.; Li L.; Si J.; Cao L. Anti-inflammatory and analgesic aActivities of ethanol extract and isolated compounds from millettia pulchra. Biol. Pharm. Bull. 2015, 38, 1328–1336. 10.1248/bpb.b15-00187. [DOI] [PubMed] [Google Scholar]
- Ma L.; Ni L.; Yang T.; Mao P.; Huang X.; Luo Y.; Jiang Z.; Hu L.; Zhao Y.; Fu Z.; Ni Y. Preventive and therapeutic spermidine treatment attenuates acute colitis in mice. J. Agric. Food Chem. 2021, 69, 1864–1876. 10.1021/acs.jafc.0c07095. [DOI] [PubMed] [Google Scholar]
- Higashiyama S.; Abraham J. A.; Miller J.; Fiddes J. C.; Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991, 251, 936–939. 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Tormos A. M.; Talens-Visconti R.; Nebreda A. R.; Sastre J. p38 MAPK: a dual role in hepatocyte proliferation through reactive oxygen species. Free Radic. Res. 2013, 47, 905–916. 10.3109/10715762.2013.821200. [DOI] [PubMed] [Google Scholar]
- Yan S.; Wei H.; Jia R.; Zhen M.; Bao S.; Wang W.; Liu F.; Li J. Wu-Mei-Wan ameliorates murine ulcerative colitis by regulating macrophage polarization. Front. Pharmacol. 2022, 13, 859167. 10.3389/fphar.2022.859167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S. Y.; Soderholm J. D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- Pastorelli L.; De Salvo C.; Mercado J. R.; Vecchi M.; Pizarro T. T. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front. Immunol. 2013, 4, 280. 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 2005, 62, 2932–2938. 10.1007/s00018-005-5481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. 10.1007/s00018-008-8646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasova D.; Crhanova M.; Babak V.; Jerabek M.; Brzobohaty L.; Matesova Z.; Rychlik I. Development of piglet gut microbiota at the time of weaning influences development of postweaning diarrhea - a field study. Res. Vet. Sci. 2021, 135, 59–65. 10.1016/j.rvsc.2020.12.022. [DOI] [PubMed] [Google Scholar]
- Mo K.; Li J.; Liu F.; Xu Y.; Huang X.; Ni H. Superiority of microencapsulated essential oils compared with common essential oils and antibiotics: effects on the intestinal health and gut microbiota of weaning piglet. Front. Nutr. 2022, 8, 808106. 10.3389/fnut.2021.808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg J. L.; Xu J.; Leip D. D.; Chen C. H.; Westover B. P.; Weatherford J.; Buhler J. D.; Gordon J. I. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 2005, 307, 1955–1959. 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Martini E.; Krug S. M.; Siegmund B.; Neurath M. F.; Becker C. Mend Your Fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Xu Z. Z.; He Y.; Yang Y.; Liu L.; Lin Q.; Nie Y.; Li M.; Zhi F.; Liu S.; Amir A.; Gonzalez A.; Tripathi A.; Chen M.; Wu G. D.; Knight R.; Zhou H.; Chen Y. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. Msystems 2018, 3, e00188 10.1128/msystems.00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.; Deng Q.; Xu J.; Wang X.; Hu C.; Tang H.; Huang F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. 10.1016/j.foodres.2018.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








