Abstract
Background
Cryptosporidium spp. are responsible for significant diarrheal morbidity and mortality in under-5 children. There is no vaccine; thus, a focus on prevention is paramount. Prior studies suggest that person-to-person spread may be an important pathway for transmission to young children. Here we describe a longitudinal cohort study of 100 families with infants to determine rates of cryptosporidiosis within households during the coronavirus disease 2019 (COVID-19) pandemic.
Methods
Families living in Mirpur, Bangladesh, with 1 infant aged 6–8 months were enrolled and followed with weekly illness survey and stool testing for Cryptosporidium for 8 months.
Results
From December 2020 to August 2021, 100 families were enrolled. Forty-four percent of index children and 35% of siblings had at least 1 Cryptosporidium infection. Shedding of Cryptosporidium occurred for a mean (standard deviation) of 19 (8.3) days in index infants, 16.1 (11.6) days in children 1–5 years, and 16.2 (12.8) days in adults. A longer duration of Cryptosporidium shedding was associated with growth faltering in infants. There was a spike in Cryptosporidium cases in May 2021, which coincided with a spike in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases in the region.
Conclusions
In this intensive, longitudinal study of Cryptosporidium infection in families we found high rates of cryptosporidiosis in infants and children, and prolonged parasite shedding, especially among malnourished children. These data support that transmission within the household is an important route of exposure for young infants and that treatment of nondiarrheal infection to interrupt person-to-person transmission within the home may be essential for preventing cryptosporidiosis in infants.
Keywords: Cryptosporidium, Bangladesh, diarrheal disease
Cryptosporidiosis is a leading cause of morbidity and mortality among children. We followed 100 families with infants living in Bangladesh and studied the incidence of Cryptosporidium infection. We found prolonged Cryptosporidium shedding in stool was common among infants and adults.
Cryptosporidium, an enteric protozoa, has been identified as a leading contributor to moderate-to-severe diarrhea in children younger than 2 years of age worldwide [1]. Cryptosporidium diarrhea and subclinical infection have both been associated with stunted growth and cognitive deficits in children [2–5]. Despite the high morbidity from this infection, there is currently no effective treatment for infants younger than 1 year of age.
Cryptosporidium is transmitted by fecal-oral contamination. Transmission of diarrheal pathogens has been traditionally described as occurring along the “five-F pathways”: fluids, fingers, food, fields, and flies [6]. Cryptosporidium oocysts are readily infectious when excreted in feces, and ingestion of just 10 oocysts can result in infection [7]. Cryptosporidium is also resistant to chlorination [8, 9]. Sporadic waterborne outbreaks have been well documented in industrialized countries, but in endemic areas, like Bangladesh, infection is associated with poor sanitation, poverty, animal rearing, and malnutrition [3, 10, 11].
Efforts to combat Cryptosporidium infection include improving the quality of drinking water and sanitation. However, nonpharmacologic studies to reduce environmental exposure and contamination have not been successful at preventing symptomatic or asymptomatic infections. In a semiurban slum in India, where cryptosporidiosis is endemic, an intervention aimed at providing clean water to households found that children who drank bottled water did not have reduced rates of Cryptosporidium infection compared with those who drank from the municipal water supply [12]. Moreover, a community-based introduction of membrane-filtered drinking water, with pore size small enough to filter Cryptosporidium spp., did produce microbiologically safe drinking water for 1 year; however, this did not result in reduced rates of diarrhea in children under 2 years [13]. The Water quality, Sanitation, and Handwashing (WASH) Benefits study, a cluster-randomized controlled trial, demonstrated success in providing clean water and improved handwashing and sanitation in communities in rural Bangladesh; however, despite these improved measures, there was no improvement in the rate of cryptosporidiosis and there was no significant improvement in child growth [14]. This study highlighted that cryptosporidiosis may not be amenable to traditional WASH interventions because of its low infectious dose.
In a small pilot study, we previously demonstrated that 39% of households with a Cryptosporidium-infected child had a second infected household member. Furthermore, genotyping of Cryptosporidia suggested that transmission in young children primarily occurs via human-to-human transmission rather than zoonosis and emphasized the importance of household transmission in Cryptosporidium infections for children under 2 years of age [15].
To further explore Cryptosporidium transmission, we enrolled a cohort of 100 families with an index child 6–8 months of age and followed them prospectively for 8 months. The extensive longitudinal follow-up provides an opportunity to define the incidence of Cryptosporidium infection within families with infants and to identify factors involved in transmission of the disease. Enrollment began during the early years of the coronavirus disease 2019 (COVID-19) pandemic, allowing a unique perspective on the effect of social-distancing measures on transmission of an enteric protozoal infection.
METHODS
Study Design
Families with infants 6 to 8 months old living in Mirpur, Bangladesh, were identified and approached for enrollment into the study. If families agreed to participate, all household members, defined as any individual sleeping under the same roof or eating from the same cooking pot, were consented for enrollment. Once enrolled, a demographic enrollment form was completed for each participant. Baseline characteristics collected included age, weight, height, and familial relationships from all participants. At the household level, socioeconomic data including household income, parental occupation, maternal education, and crowding were collected. Additionally, data on water, sanitation, and household environment were collected, including source of drinking water, method of treatment of drinking water, toilet type, and presence of domesticated animals in the home.
Subsequently, all index infants and family members were surveyed weekly for any illness, including diarrhea and respiratory illness. A stool specimen was collected from each participant weekly, over an 8-month follow-up period. If a participant was experiencing diarrhea, a sample was collected, and this was counted as that week's stool collection. Stool was not collected more frequently than once a week. A serum specimen was collected from participants at baseline and at the end of the 8-month follow-up.
Laboratory Testing
Stool and serum samples were collected in Mirpur and transported via cold chain to the Parasitology Laboratory at the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,b). Testing for Cryptosporidium was performed using the Cryptosporidium II enzyme-linked immunosorbent assay (ELISA) kit (TechLab, Blacksburg, VA) on all weekly and diarrheal stool samples. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin G (IgG) testing was performed using the TechLab SARS-CoV-2 ELISA (investigational use only) on baseline and 8-month serum samples collected from each participant.
Case Definitions and Statistical Analysis
Symptoms were analyzed based on the weekly clinical survey of each participant. A Cryptosporidium case was defined as a participant having at least 1 stool sample positive for Cryptosporidium during the 8-month follow-up period. A diarrheal episode was defined as 3 or more loose stools per day, recurring daily within a 14-day period. A diarrhea-associated positive Cryptosporidium episode was defined as at least 1 positive diarrhea sample during the episode of infection.
The duration of Cryptosporidium shedding was calculated for each episode of Cryptosporidium infection. An episode was defined as stool samples testing positive within a 14-day period. The duration of the episode was calculated by the difference in days between the first positive stool sample in the episode and the first negative stool sample after the episode. If a positive sample was collected in the last week of study follow-up, this was assigned a minimum 7-day duration of positivity.
The height-for-age adjusted z scores (HAZ) and weight-for-age adjusted z scores (WAZ) were calculated based on World Health Organization standards [14]. Lengths/heights and weights of the participants were measured monthly after baseline enrollment. The ages of children were rounded to the nearest month at the time of each measurement.
Socioeconomic Definitions
“Overcrowding” in the home was classified as more than 3 people per room per household [16]. Drinking water was categorized as water piped into the home, water piped into the yard, and tube well [17]. “Unimproved” toilets were categorized as having no facility or traditional pit latrine without slab. “Improved” toilet included ventilated improved pit toilet with water seal and flush toilet to a piped sewer system, septic tank, or pit latrine [17].
Statistical Analysis
Linear regression was used to estimate the difference in duration of shedding between index children compared with other children compared with adults, adjusting for sex and baseline COVID-19 IgG result. Linear regression was also applied to identify risk factors associated with longer duration of shedding. Both models are presented, and models with the lowest Akaike information criterion (AIC) based on stepwise model selection. Logistic regression was used to identify risk factors for Cryptosporidium-positive cases at the household level. All tests were based on a 2-sided P < .05. Fisher's exact test was used for comparing group differences of categorical variables and Wilcoxon-Mann-Whitney test was used for continuous variables in exploratory analysis because of their relaxed assumptions on large sample sizes. Chi-square tests were used to compare difference in symptoms reported among weeks with Cryptosporidium infection and negative for infection among participants. Analysis was performed using R, version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Ethics Approval
The study was approved by the Research and Ethics Review Committees at the ICDDR,b and by the Institutional Review Board at the Johns Hopkins University Bloomberg School of Public Health. Written consent or assent was obtained from all participants over 11 years old, and parental consent was obtained for all children 11 years old and younger.
RESULTS
From 29 September 2020 to 22 December 2020, field workers conducted a census in Mirpur wards 2, 3, and 5 and identified 1841 households. Of these, 284 families were identified as being eligible and were screened for enrollment. Between December 2020 and August 2021, 100 families were enrolled into the study, with a mean duration of follow-up of 212.7 days (standard deviation [SD]: 28.8). Of the 100 families enrolled, 4 families were lost to follow-up and had a mean duration of follow-up of 98.6 days (SD: 54.2).
Families were composed of 100 index children between the ages of 6 and 8 months and 242 family members (Table 1) (total n = 342), including 100 mothers, 49 fathers, and 60 siblings. Sociodemographic characteristics of this cohort were similar to prior studies in this area [3, 15, 18]. Seventeen percent of primary caregivers had no formal education. Overcrowding was found in 48% of households. Sixteen percent of households owned animals, and these were chickens or ducks. Nearly one-third of households had an unimproved toilet, which includes no toilet or a pit latrine. Treatment of drinking water was common and included boiling water prior to drinking (64%) or using a filter (6%).
Table 1.
Characteristics of Index Infants and Household Members at Enrollment
| Index infants and household members, n | 342 |
| Index infants | 100 |
| Mothers | 100 |
| Fathers | 49 |
| Siblings | 60 |
| Grandmothers | 15 |
| Aunts | 10 |
| Uncles | 2 |
| Cousins | 6 |
| Age of index infants at enrollment, mean (SD), d | 226.8 (26.7) |
| Female index infants, % | 46 |
| Index infants’ height-for-age adjusted z score at enrollment, mean (SD) | −1.15 (1.16) |
| Index infants” weight-for-age adjusted z score at enrollment, mean (SD) | −0.74 (1.03) |
| No. of individuals per household, median (IQR) | 5 (4, 6) |
| Household income in BDT,a median (IQR) | 15 000 (10 000, 20 000) |
| Educational level of the mother, % | |
| No formal education | 17 |
| Some primary school | 36 |
| Some high school | 36 |
| Secondary school diploma | 15 |
| Households with overcrowding, % | 48 |
| Households with domesticated animals, % | 16a |
| Water source, % | |
| Piped into home | 85 |
| Piped into yard | 2 |
| Deep tube well | 15 |
| Method of water treatment, % | |
| None | 30 |
| Boiling | 64 |
| Filter | 6 |
| Toilet type, % | |
| Improved | |
| Ventilated improved pit water seal | 72 |
| Flush toilet | 1 |
| Unimproved | |
| Traditional pit toilet | 27 |
| Households sharing toilet, % | 98 |
| If sharing, number of other households sharing toilet, mean (SD) | 2.2 (1.56) |
Abbreviations: BDT, Bangladeshi Taka; IQR, interquartile range; SD, standard deviation.
Chicken or ducks were kept at 100% of the households that had any animals.
In total, 9774 surveillance stool samples were collected, with a 1.5% incidence of Cryptosporidium spp. positivity, and in the 384 diarrheal stool samples, Cryptosporidium positivity was 6.8%. Among index infants, Cryptosporidium positivity was 3.9% in surveillance stools and 7.6% in diarrheal stools (Table 2). Among the 100 index infants, there were 239 episodes of diarrhea. The mean duration of diarrhea was 7.6 days (SD: 9.6) (Table 3). Index infants had more episodes of diarrhea and longer duration of diarrhea than all other age groups.
Table 2.
Cryptosporidium-Positive Diarrheal and Surveillance Stools Collected During 8 Months of Follow-up, Listed by Age Group
| Diarrheal Stools, Cryptosporidium Positive, n (%) | Surveillance Stools, Cryptosporidium Positive, n (%) | Total Stools, Cryptosporidium Positive, n (%) | |
|---|---|---|---|
| Index children | 23 (7.6%) | 106 (3.9%) | 129 (4.2%) |
| Children, 1 to 5 y | 2 (20%) | 20 (2.8%) | 22 (3.0%) |
| Children, 5–10 y | 0 (0%) | 5 (0.5%) | 5 (0.5%) |
| Children, 10–17 y | 0 (0%) | 3 (0.8%) | 3 (0.8%) |
| Adults | 1 (2%) | 16 (0.3%) | 17 (0.3%) |
| All | 26 (6.8%) | 150 (1.5%) | 176 (1.7%) |
Table 3.
Duration of Diarrhea by Age Group
| Age Group | No. of Episodes of Diarrhea | Mean (SD) Duration in Days |
|---|---|---|
| <1 y | 239 | 7.6 (9.6) |
| 1–5 y | 11 | 3.4 (2.2) |
| 6–10 y | 13 | 5 (5.0) |
| 11–17 y | 8 | 3 (1.2) |
| ≥17 y | 54 | 2.9 (4.4) |
Abbreviation: SD, standard deviation.
When we considered Cryptosporidium infections per participant, children under age 5 carried the greatest burden of Cryptosporidium infection. The infected individuals included 44% of index infants, 35% of 2–5-year-olds, 15% of 6–10-year-olds, and 20% of 11–17-year-olds with at least 1 Cryptosporidium infection during the 8-month follow-up period. Among adult caregivers, 6% of mothers and 2% of fathers tested positive for Cryptosporidium. In total, 8 out of 170 (5%) adults tested positive for Cryptosporidium at least once during the study.
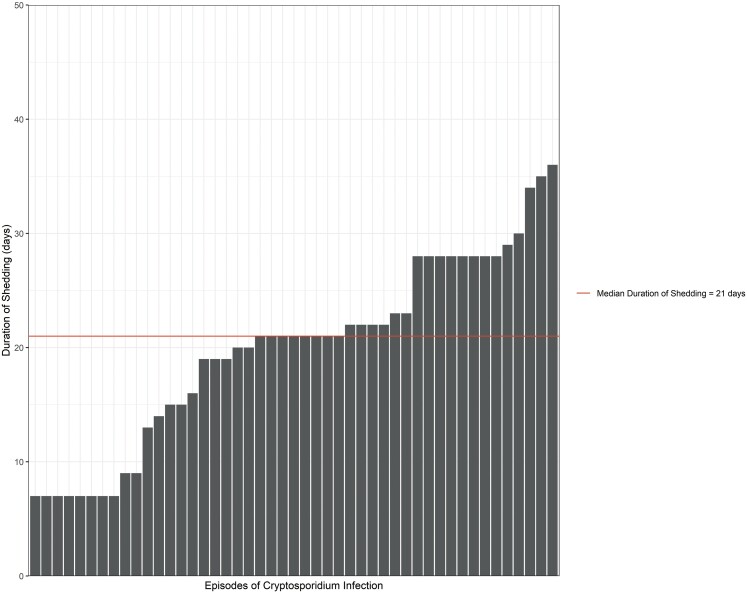
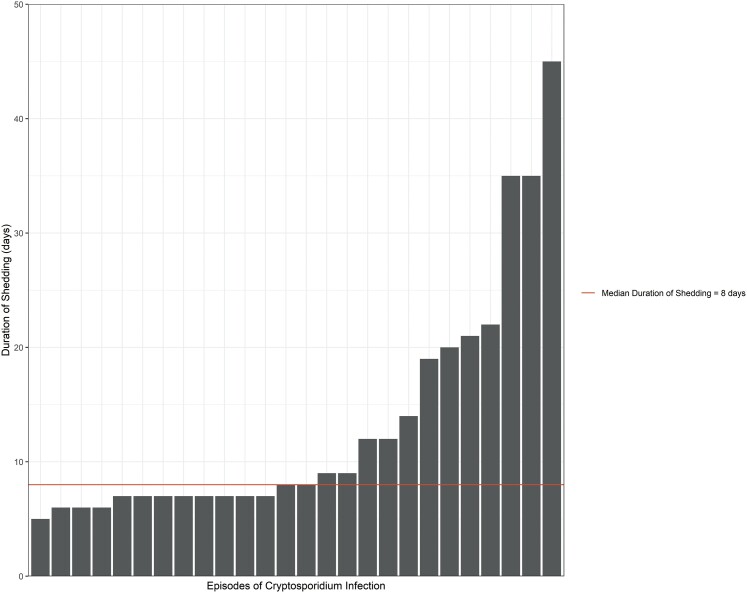
Shedding of Cryptosporidium occurred, on average, for 19.9 days (SD: 8.3) among index infants (Table 4). Index infants had more prolonged shedding than other age groups (Figures 1 and 2). In the linear regression model, older children had a shorter duration of shedding compared with index infants (beta: −7.6; 95% confidence interval [CI]: −13, −2.4) (Supplementary Table 1). As expected, among index infants, we found that growth faltering (HAZ ≤ −2) was significantly associated with a longer duration of shedding (beta: 8.0; 95% CI: .41, 16) (Supplementary Table 2). The presence of animals in the home and treatment of drinking water were negatively associated with the duration of shedding in index children.
Table 4.
Duration of Cryptosporidium Shedding by Age Group
| Age Group | No. of Episodes of Cryptosporidium Infection | Mean (SD) Duration in Days |
|---|---|---|
| <1 y | 47 | 19.9 (8.3) |
| 1–5 y | 10 | 16.1 (11.6) |
| 6–10 y | 5 | 7.2 (1.1) |
| 11–17 y | 3 | 7.0 (0) |
| ≥17 y | 8 | 16.2 (12.8) |
Abbreviation: SD, standard deviation.
Figure 1.
Duration of Cryptosporidium shedding among index children.
Figure 2.
Duration of Cryptosporidium shedding among household members.
Repeat infections only occurred in young children. Three index infants, plus 1 unrelated sister, experienced repeat infections, and all were aged 3 years and younger (Table 5). Interestingly, 3 of the 4 children had a longer duration of parasite shedding during their second infection compared with their first infection.
Table 5.
Duration of Cryptosporidium Shedding in Individuals With Repeat Infections
| Participant | Age, Y | Sex | Duration of Shedding in First Episode, D | Duration of Shedding in Second Episode, D |
|---|---|---|---|---|
| Index child | <1 | Female | 7 | 7 |
| Index child | <1 | Male | 7 | 29 |
| Index child | <1 | Female | 21 | 28 |
| Sister | 3 | Female | 7 | 20 |
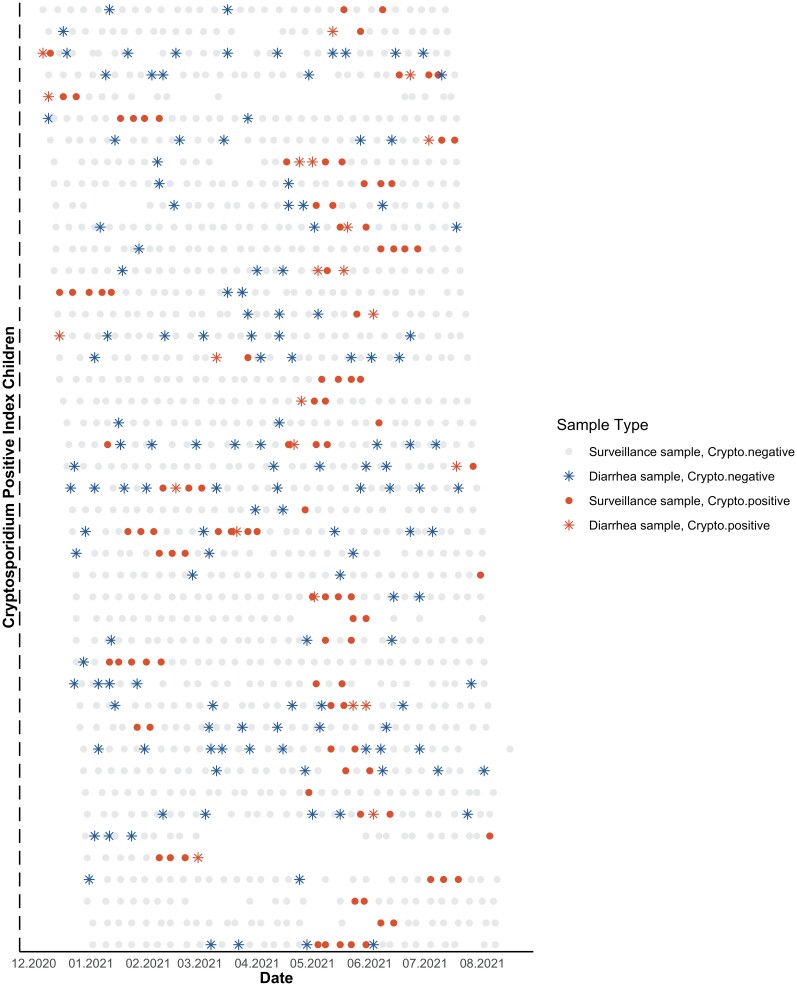
Figure 3 shows weekly stool results among all 44 children who tested positive for Cryptosporidium in at least 1 weekly sample over the 8-month follow-up period. We observed that not all diarrheal episodes were positive for Cryptosporidium, and in some cases, a child may have had multiple diarrheal episodes and only a single weekly positive Cryptosporidium infection. And yet others had prolonged shedding spanning several weeks but no or limited diarrhea.
Figure 3.
Weekly stools of Cryptosporidium–positive index children aged over 8 months. Abbreviation: Crypto, Cryptosporidium.
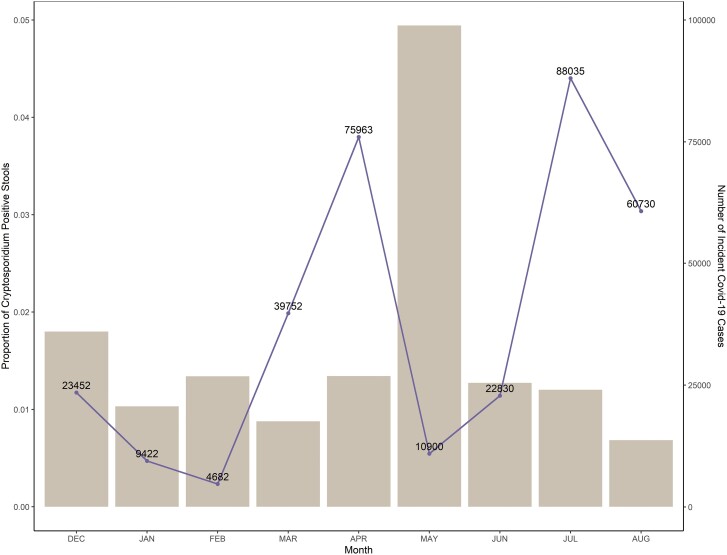
The monthly rate of Cryptosporidium positivity among stool samples was consistent throughout the study; however, there was a large increase in infections during May 2021 (Figure 4) [19]. There was a spike in COVID-19 cases in April 2020, after which the Bangladesh government mandated a strict lockdown. These restrictions were lifted in May 2020, which is when the corresponding spike in Cryptosporidium cases was observed [20]. Of all the enrolled participants (n = 342), 30.4% were SARS-CoV-2 IgG positive at baseline and this percentage increased to 45.8% at the end of the study. Eighty-nine participants went from a negative to positive SARS-CoV-2 serology during the 8-month follow-up period. SARS-CoV-2 IgG positivity in children was not associated with markers of malnutrition, and there were no observed differences in positivity by HAZ or height-for-weight adjusted z scores (t test, P > .05).
Figure 4.
Proportion of positive stool samples by month from December 2020 to August 2021, with the number of monthly incident COVID-19 cases in Dhaka District. Abbreviation: COVID-19/Covid-19, coronavirus disease 2019.
DISCUSSION
In this prospective, longitudinal study of infants and families living in an urban area in Bangladesh, there was a high rate of Cryptosporidium infection and shedding in young children with or without diarrhea. We also observed that household family members had higher rates of infection, including 35% percent of children aged 2–5 years and 6% of mothers.
Shedding of Cryptosporidium lasted from 5 to 36 days among index infants. Although infection rates were lower in older ages, prolonged shedding was observed across the age spectrum. Notably, even in adult participants, we found Cryptosporidium shedding to range from 5 to 35 days. We observed Cryptosporidium shedding in both diarrheal and nondiarrheal infections. Interestingly, growth faltering was associated with prolonged duration of shedding. The presence of domestic fowl in the home and treatment of drinking water were marginally inversely associated with duration of shedding. Treatment of drinking water may be directly related to decreased shedding by reducing the burden of parasite exposure through participants’ gastrointestinal tracts. Alternatively, treatment of drinking water may be a proxy for other conditions that predispose to reduced parasite shedding.
The only other study that has reported on Cryptosporidium shedding with the same intensity as the present study was in Scotland in 1988, which looked at immunocompetent children and adults ages 9 months to 88 years. The Scottish study also reported a range of Cryptosporidium shedding from 2 to 35 days [21] using daily household follow-up of all Cryptosporidium-positive patients from their region. This differs from modeling-based projections, which have reported longer shedding times but may represent a lack of precision in those models [22].
We did not observe any reduced duration in shedding in second versus primary infections, suggesting that prior infection does not protect or prime for repeat episodes of shedding. A potential explanation for this could be that the primary and secondary infection involved distinct Cryptosporidium species. In gnotobiotic pigs, infection with Cryptosporidium hominis did not protect against infection from Cryptosporidium parvum, suggesting a lack of cross-species immunity [23]. The next phase of our study will further explore this question by sequencing all collected Cryptosporidium isolates.
The duration of oocyst shedding is important for transmission, as it represents the period of time an individual is infectious and can spread the infection to close contacts. The prolonged shedding observed in all age groups provides multiple opportunities for transmission within a household. In addition, we report shedding not only in the diarrheal episodes but also in surveillance samples. Efforts to treat or disrupt Cryptosporidium infections that solely target diarrheal infections will not be adequate to control transmission.
Our data, combined with the Scottish data, demonstrate that, in a healthy population or in a malnourished population, Cryptosporidium shedding still occurs for a prolonged period, especially in children. Interventions to prevent Cryptosporidium infection in vulnerable populations must address the fact that shedding is occurring even when participants are not experiencing diarrhea. With this prolonged shedding in the household environment, and the failure of WASH in other studies, pharmacologic treatment of nondiarrheal infection in children and perhaps entire households may be needed, as this may be the only way to eliminate Cryptosporidium from the household environment.
One limitation of our study is that we used a commercially available Cryptosporidium ELISA, which may be slightly less sensitive than polymerase chain reaction (PCR) or direct immunofluorescence; thus, there could be the potential for misclassification [24]. Additionally, stool specimens were collected once per week; thus, our granularity for measuring the duration of infection could be improved by biweekly or daily stool collection.
This study is unique in that it took place during the SARS-CoV-2 pandemic. Nearly one-third of participants had a positive SARS-CoV-2 IgG response. After enacting a series of nationwide lockdowns in 2020, all restrictions on movement of individuals were lifted in September 2020. Seven months later, Bangladesh experienced a record number of COVID-19 cases in April 2021, and the government imposed a strict lockdown once again. After the spike in COVID-19 cases in April 2021, we observed a corresponding spike in Cryptosporidium cases in May 2021 [19]. We propose the corresponding spike in Cryptosporidium cases could be attributed to the nationwide lockdown, as all family members were required to remain within their homes. Therefore, while social distancing was enacted to protect against the spread of SARS-CoV-2, it may have inadvertently reduced social distancing in the home, allowing for increased Cryptosporidium transmission among household contacts. One limitation is that the SARS-CoV-2 data are at a country-wide level. If we had accurate rates of SARS-CoV-2 infection in the Mirpur region, it would have been possible to link social-distancing measures for SARS-CoV-2 to Cryptosporidium cases at a community level.
This is the largest study to follow entire households over 8 months to describe the incidence of cryptosporidiosis in an endemic region. Notably, we found that prolonged shedding of cryptosporidiosis occurs among infants and adults, increasing the risk for exposure. Determining methods for interrupting transmission within the household should be prioritized to protect infants from cryptosporidiosis, especially given the lack of vaccine and approved pharmacologic treatments in this vulnerable population.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Poonum Korpe, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Zhanmo Ni, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Mamun Kabir, Emerging Infections and Parasitology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Masud Alam, Emerging Infections and Parasitology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Tahsin Ferdous, Emerging Infections and Parasitology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Rifat Ara, Emerging Infections and Parasitology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Rebecca M Munday, Department of Genetic Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Rashidul Haque, Emerging Infections and Parasitology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh.
Priya Duggal, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Notes
Acknowledgments. The authors thank the families of Mirpur for participating in this study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number 5R01AI146123 to P. K.).
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC, et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2. Korpe PS, Haque R, Gilchrist C, et al. . Natural history of cryptosporidiosis in a longitudinal study of slum-dwelling Bangladeshi children: association with severe malnutrition. PLoS Negl Trop Dis 2016; 10:e0004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korpe PS, Valencia C, Haque R, et al. . Epidemiology and risk factors for cryptosporidiosis in children from 8 low-income sites: results from the MAL-ED study. Clin Infect Dis 2018; 67:1660–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lima AA, Moore SR, Barboza MS Jr, et al. . Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J Infect Dis 2000; 181:1643–51. [DOI] [PubMed] [Google Scholar]
- 5. Khalil IA, Troeger C, Rao PC, et al. . Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health 2018; 6:e758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Julian TR. Environmental transmission of diarrheal pathogens in low and middle income countries. Environ Sci Process Impacts 2016; 18:944–55. [DOI] [PubMed] [Google Scholar]
- 7. Chalmers RM. Waterborne outbreaks of cryptosporidiosis. Ann Ist Super Sanita 2012; 48:429–46. [DOI] [PubMed] [Google Scholar]
- 8. DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of cryptosporidium parvum in healthy volunteers. N Engl J Med 1995; 332:855–9. [DOI] [PubMed] [Google Scholar]
- 9. Okhuysen PC, Chappell CL, Crabb JH, Sterling CR, DuPont HL. Virulence of three distinct cryptosporidium parvum isolates for healthy adults. J Infect Dis 1999; 180:1275–81. [DOI] [PubMed] [Google Scholar]
- 10. MacKenzie WR, Schell WL, Blair KA, et al. . Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis 1995; 21:57–62. [DOI] [PubMed] [Google Scholar]
- 11. D'Antonio RG, Winn RE, Taylor JP, et al. . A waterborne outbreak of cryptosporidiosis in normal hosts. Ann Intern Med 1985; 103:886–8. [DOI] [PubMed] [Google Scholar]
- 12. Sarkar R, Ajjampur SS, Prabakaran AD, et al. . Cryptosporidiosis among children in an endemic semiurban community in southern India: does a protected drinking water source decrease infection? Clin Infect Dis 2013; 57:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Francis MR, Sarkar R, Roy S, et al. . Effectiveness of membrane filtration to improve drinking water: a quasi-experimental study from rural southern India. Am J Trop Med Hyg 2016; 95:1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin A, Ercumen A, Benjamin-Chung J, et al. . Effects of water, sanitation, handwashing, and nutritional interventions on child enteric protozoan infections in rural Bangladesh: a cluster-randomized controlled trial. Clin Infect Dis 2018; 67:1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korpe PS, Gilchrist C, Burkey C, et al. . Case-control study of Cryptosporidium transmission in Bangladeshi households. Clin Infect Dis 2018; 68:1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Principles and recommendations for population and housing censuses (revision 2). New York: United Nations, 2007.
- 17. World Health Organization/United Nations Children’s Fund Joint Monitoring Programme for Water Supply, Sanitation and Hygiene . Improved and unimproved water sources and sanitation facilities 2015. Available at: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/population-using-improved-sanitation-facilities-(-). Accessed 4 October 2022.
- 18. Mondal D, Minak J, Alam M, et al. . Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clin Infect Dis 2012; 54:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. COVID-19 dynamic dashboard for Bangladesh. Available at: http://dashboard.dghs.gov.bd/webportal/pages/covid19.php. Accessed 4 October 2022.
- 20. COVID-19 timeline in Bangladesh. Available at: https://betterwork.org/portfolio/covid-timeline-in-bangladesh/. Accessed 4 October 2022.
- 21. Shepherd RC, Reed CL, Sinha GP. Shedding of oocysts of cryptosporidium in immunocompetent patients. J Clin Pathol 1988; 41:1104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMurry TL, McQuade ETR, Liu J, et al. . Duration of postdiarrheal enteric pathogen carriage in young children in low-resource settings. Clin Infect Dis 2021; 72:e806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheoran A, Wiffin A, Widmer G, Singh P, Tzipori S. Infection with Cryptosporidium hominis provides incomplete protection of the host against Cryptosporidium parvum. J Infect Dis 2012; 205:1019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hencke JD, Garcia LS, Herbein JF. Detection of Cryptosporidium spp. Antigen in Human Fecal Specimens using the Cryptosporidium II ELISA. Presented at: ASTMH 55th Annual Meeting; Atlanta, GA; November 2006.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.