Abstract
Background
Guidelines emphasize rapid antibiotic treatment for sepsis, but infection presence is often uncertain at initial presentation. We investigated the incidence and drivers of false-positive presumptive infection diagnosis among emergency department (ED) patients meeting Sepsis-3 criteria.
Methods
For a retrospective cohort of patients hospitalized after meeting Sepsis-3 criteria (acute organ failure and suspected infection including blood cultures drawn and intravenous antimicrobials administered) in 1 of 4 EDs from 2013 to 2017, trained reviewers first identified the ED-diagnosed source of infection and adjudicated the presence and source of infection on final assessment. Reviewers subsequently adjudicated final infection probability for a randomly selected 10% subset of subjects. Risk factors for false-positive infection diagnosis and its association with 30-day mortality were evaluated using multivariable regression.
Results
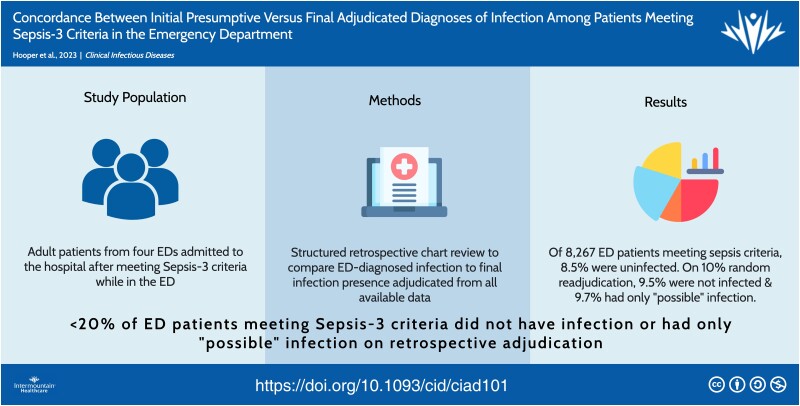
Of 8267 patients meeting Sepsis-3 criteria in the ED, 699 (8.5%) did not have an infection on final adjudication and 1488 (18.0%) patients with confirmed infections had a different source of infection diagnosed in the ED versus final adjudication (ie, initial/final source diagnosis discordance). Among the subset of patients whose final infection probability was adjudicated (n = 812), 79 (9.7%) had only “possible” infection and 77 (9.5%) were not infected. Factors associated with false-positive infection diagnosis included hypothermia, altered mental status, comorbidity burden, and an “unknown infection source” diagnosis in the ED (odds ratio: 6.39; 95% confidence interval: 5.14–7.94). False-positive infection diagnosis was not associated with 30-day mortality.
Conclusions
In this large multihospital study, <20% of ED patients meeting Sepsis-3 criteria had no infection or only possible infection on retrospective adjudication.
Keywords: sepsis, source diagnosis discordance, misdiagnosis, overtreatment, physician practice variation
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/concordance-between-initial-presumptive-versus-final-adjudicated-diagnoses-of-infection-among-patients-meeting-sepsis-3-criteria-in-the-emergency-department
In this large multihospital study, <20% of emergency department (ED) patients meeting Sepsis-3 criteria had no infection or only possible infection on retrospective adjudication and 18% of infected patients had a different source of infection diagnosed in the ED versus final adjudication.
(See the Editorial Commentary by Rhee et al. on pages 2056–8.)
Sepsis is a life-threatening condition caused by a dysregulated immune response to infection [1]. Patients with this syndrome benefit from prompt treatment [2–4], and current international guidelines and regulatory mandates have set rapid timelines for antibiotic delivery and fluid resuscitation when sepsis is suspected [5–7]. Various noninfectious conditions, however, can mimic the signs and symptoms of sepsis [8]. Strict timelines for antibiotic administration in the emergency department (ED) coupled with the complex and multifaceted clinical syndrome of sepsis have raised concerns about false-positive presumptive infection diagnosis (suspected infection initially treated by the ED clinician that is later determined to be absent) and initial/final infection source diagnosis discordance (discrepancy between ED-diagnosed and final sources of infection) and consequent harms from unnecessary administration of broad-spectrum antibiotics and/or delays in treatment for patients’ actual infectious or noninfectious diagnosis [8–18].
Meaningful discussions of tradeoffs involved in accelerating sepsis treatment require accurate figures on the frequency of false-positive diagnosis and source diagnosis discordance. Previous studies have suggested sepsis false-positive diagnosis rates as high as 43%, but some of these studies have been small, included patients meeting legacy definitions of sepsis now reclassified as simple infection, or combined patients with possible infection and absent infection [18–26]. We therefore analyzed a large, multihospital cohort to clarify the incidence of and risk factors for false-positive presumptive infection diagnosis and source diagnosis discordance among patients who met Sepsis-3 criteria in the ED.
METHODS
Study Design and Patient Population
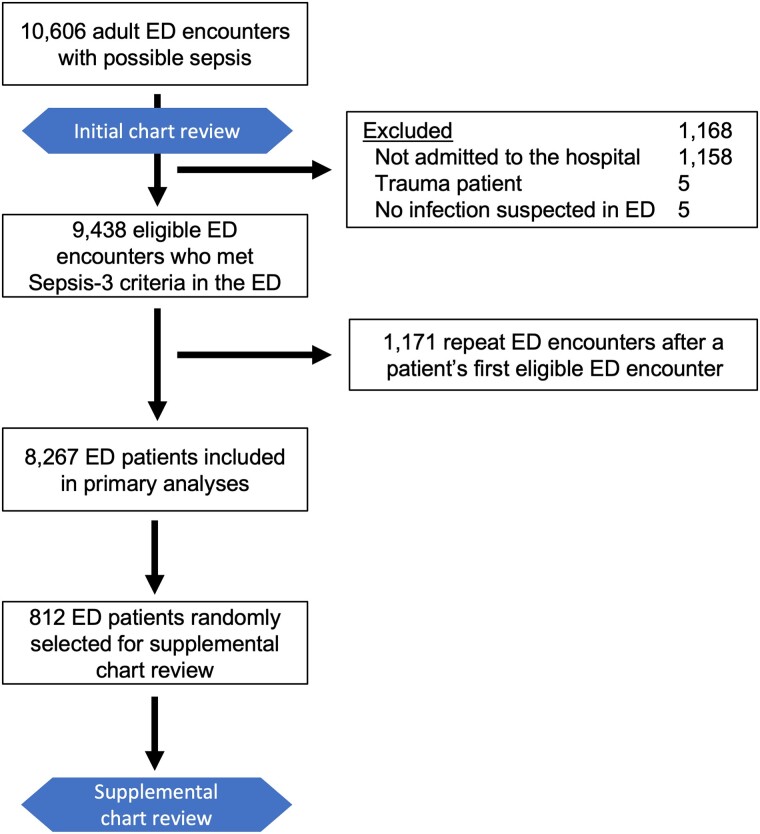
We performed a secondary analysis of a retrospective cohort of ED patients seen at 4 Utah hospitals (see Supplementary Methods) from July 2013 to January 2017 [2, 27, 28]. Patients admitted to a study hospital from the ED were eligible for inclusion if they were 18 years or older and met sepsis consensus criteria (Sepsis-3) [1] prior to ED departure based on a suspected or known infection per ED clinician documentation; blood cultures obtained in the ED; administration in the ED of an intravenous (IV) antimicrobial, oral vancomycin, or oseltamivir; and acute organ failure (≥2-point increase in the Sequential Organ Failure Assessment [SOFA] score [29] from baseline). Exclusion criteria included infection not suspected by the ED clinician (eg, antibiotics given only as prophylaxis), trauma protocol activation, and death or discharge from the ED rather than admission to the hospital. Only a patient's first eligible ED visit was included. This study was approved by the Intermountain Healthcare Institutional Review Board (IRB; #1050172) with waiver of informed consent.
Data Abstraction
Demographic and clinical data were queried from the Intermountain Healthcare Electronic Data Warehouse [30]. Trained personnel (including I. D. P., S. R. M., E. R. M., and C. J. K.) previously verified missing or outlier data, identified the ED diagnosis of infection and source, and adjudicated the final presence/absence and source of infection as described previously and in detail in the Supplementary Methods [2, 27, 28]. In brief, reviewers utilized ED and admitting physician documentation to identify the ED-diagnosed source of infection (interrater agreement kappa score: .96; 95% confidence interval [CI]: .95–.97). Adjudication of the final presence and source of infection utilized all available information including the discharge summary, clinician progress and consultation notes, follow-up hospitalizations and clinic documentation, microbiologic testing, and other radiologic, diagnostic, and laboratory testing. Analyses used 7 infection source categories: pneumonia/pulmonary, urinary, skin/soft tissue, abdominal/gastrointestinal, unknown, other, and not infected.
For the current analysis, a second round of medical record review was conducted, and the final presence and source of infection were independently re-adjudicated for a random 10% sample of all patients (primarily G. A. H., with <4% of re-adjudications performed by I. D. P.). Disagreements were resolved by an experienced critical care physician (I. D. P.). Interrater agreement was “near perfect” (kappa: .83; 95% CI: .80–.86) for the 7-category infection source categorization and “substantial” (kappa: .69; 95% CI: .60–.78) for a binary infection present/not present determination [31]. These reviewers also adjudicated final infection probability as definite, probable, possible, or not infected using criteria adapted from prior reports [21, 32–38]. In general, “definite” infection was defined as a highly consistent infectious syndrome plus detection of a consistent pathogen; “probable” infection was defined as a highly consistent clinical syndrome without microbiologic confirmation that was the most likely cause of the patient's syndrome and/or responded to appropriate treatment; and “possible” infection was defined as a likely consistent clinical syndrome of sufficient clinical concern to merit a full course of antimicrobial therapy (if applicable), although alternative diagnoses were considered as or more likely and/or there was a lack of response to appropriate therapy. See the Supplementary Methods and Supplementary Tables 1–9 for additional details. A random sample of 21% of infection probability determinations by G. A. H. were validated by an experienced critical care clinician (I. D. P.), with “near perfect” interrater agreement (kappa: .86; 95% CI: .79–.92).
Outcomes and Exposures
The primary outcome was false-positive presumptive infection diagnosis, defined as an infection initially suspected and treated in the ED but absent per final adjudication. Secondary outcomes included initial/final source diagnosis discordance (mismatch between ED-diagnosed and final adjudicated infection source); infection probability; total and antibiotic-specific days of IV (or equivalent) antimicrobial exposure through hospital discharge, death, or hospital day 7; the number of unique antimicrobials administered in the ED; the summed values on a validated antibiotic spectrum score [39] for patients’ initial antibiotic regimen (all antibiotics administered in the ED and within 120 minutes of patients’ initial antibiotic); and 30-day mortality. Potential risk factors for false-positive diagnosis and source diagnosis discordance were selected a priori. See the Supplementary Methods for detailed definitions of candidate risk factors and model covariates.
Statistical Analysis
Between-group comparisons used chi-square or Fisher's exact test for categorical variables and t tests or analysis of variance (ANOVA) for continuous variables. Potential patient-level risk factors for false-positive infection diagnosis and source diagnosis discordance were evaluated using multivariable logistic regression with a fixed effect for study site. Physician-level variation in source diagnosis discordance was evaluated among physicians who treated at least 50 patients with confirmed infection. In the physician variation analysis, patients without confirmed infection and patients not treated by a physician who saw at least 49 other eligible patients were excluded. We tested for significant physician-level variation using a generalized linear mixed model with a random effect for physician and fixed effects for a prespecified list of potential patient- and system-level confounders. Evaluation of the association of false-positive diagnosis and source diagnosis discordance with 30-day mortality used multivariable logistic regression adjusted for prespecified covariates. See the Supplementary Methods for additional details of multivariable modeling for the physician variation and mortality analyses. A 2-tailed P value less than .05 was considered statistically significant. Statistical analysis was conducted using Stata, version 16.1 (StataCorp LLC).
RESULTS
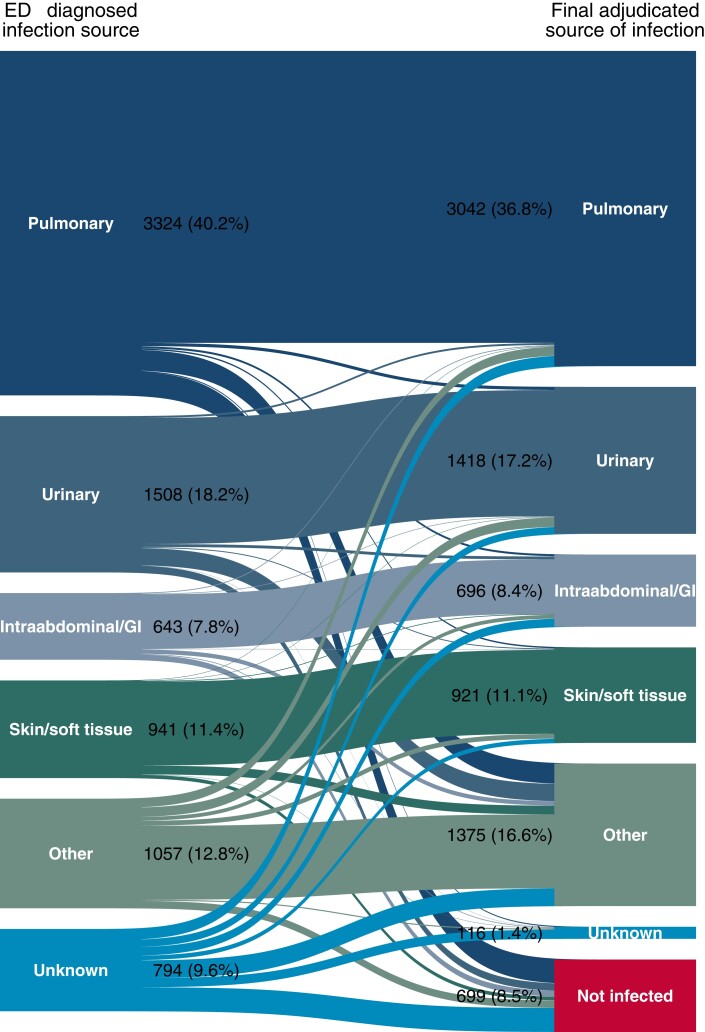
Of 8267 patients admitted to the hospital after meeting Sepsis-3 criteria in the ED (Figure 1), 699 (8.5%) ultimately had a false-positive presumptive infection diagnosis (Table 1). An additional 1488 (18.0%) patients with infection confirmed on final assessment had initial/final infection source diagnosis discordance (Supplementary Table 12). Pneumonia was the most common infection source on final assessment (n = 3042; 36.8%) (Figure 2). Agreement between the ED-diagnosed and final adjudicated source of infection was “substantial” (kappa: .67; 95% CI: .66–.69).
Figure 1.
Patient inclusion flow diagram. Initial medical record review was conducted as part of the parent study and included (1) adjudication of whether the ED clinician suspected infection and the ED-diagnosed source(s) of infection, (2) validation of ED source of infection data by independent adjudication for 30% of patients, and (3) adjudication of the final presence and source of infection. Supplemental medical record review was conducted specifically for the present analysis on a random 10% sample of eligible patients and included (1) validation of the final presence and source of infection adjudication and (2) adjudication of the probability of infection. In addition, a random 21% sample of patients included in supplemental medical record reviews underwent validation of infection probability by independent adjudication. Abbreviation: ED, emergency department.
Table 1.
Demographic and Clinical Characteristics of Subjects by Final Infection Presence
| Variable | Confirmed Infection (n = 7568) |
False-Positive Presumptive Infection Diagnosis (n = 699) |
P |
|---|---|---|---|
| Age, y | 61.6 (±18.4) | 61.1 (±18.7) | .50 |
| Female sex | 3860 (51.0%) | 348 (49.8%) | .54 |
| Race | .52 | ||
| Hispanic/Latino | 676 (8.9) | 67 (9.6) | |
| Non-Hispanic/Latino, White | 6357 (84.0) | 576 (82.4) | |
| Non-Hispanic/Latino, other race | 535 (7.1) | 56 (8.0) | |
| Arrival to ED from long-term care facility | 515 (6.8) | 46 (6.6) | .82 |
| Arrival to ED by EMS | 2320 (30.7) | 264 (37.8) | <.001 |
| Charlson Comorbidity Index | 3 (1–7) | 4 (2–7) | .001 |
| SOFA score | 4.8 (±2.8) | 4.8 (±2.5) | .86 |
| Initial vital signs and laboratory values | |||
| Heart rate, beats/minute | 105 (±22.4) | 102 (±24.2) | .001 |
| White blood cell count, 1000/µL | 13.6 (±14.7) | 12.0 (±6.7) | <.001 |
| Temperature | <.001 | ||
| <36˚C | 620 (8.2) | 122 (17.5) | |
| 36–38˚C | 3825 (50.5) | 413 (59.1) | |
| >38˚C | 3123 (41.3) | 164 (23.5) | |
| Glasgow Coma Scale ≤14 | 571 (7.5) | 84 (12.0) | <.001 |
| Hypotension in the ED | 2350 (31.1) | 219 (31.3) | .88 |
| Lactate checked and >2 mg/dL | 3005 (39.7) | 293 (41.9) | .25 |
| ED occupancy rate | 0.66 (±0.29) | 0.67 (±0.28) | .36 |
| ED-diagnosed source of infection | <.001 | ||
| Pulmonary | 3088 (40.8) | 236 (33.8) | |
| Urinary | 1437 (19.0) | 71 (10.2) | |
| Intra-abdominal/gastrointestinal | 588 (7.8) | 55 (7.9) | |
| Skin and soft tissue | 907 (12.0) | 34 (4.9) | |
| Other | 985 (13.0) | 72 (10.3) | |
| Unknown | 563 (7.4) | 231 (33.1) | |
| Days of IV antibiotics to hospital day 7 | 3 (2–5) | 1 (1–3) | <.001 |
| Unique antibiotics administered in ED | 1.7 (±0.7) | 1.6 (±0.6) | .005 |
| Initial antibiotic regimen total spectrum score | 5.4 (±2.6) | 5.4 (±2.6) | .97 |
Data are presented as mean (±SD), median (IQR), or number of patients (%).
Abbreviations: ED, emergency department; EMS, emergency medical services; IQR, interquartile range; IV, intravenous; SD, standard deviation; SOFA, Sequential Organ Failure Assessment.
Figure 2.
Alluvial diagram illustrating the relationship between the ED-diagnosed source of infection and the presence and source of infection on final adjudication. The “other” category includes less common sources of infection (CNS/meningitis, bloodstream/endocarditis, osteoarticular, etc) and cases with multiple infectious sources. Abbreviations: CNS, central nervous system; ED, emergency department; GI, gastrointestinal.
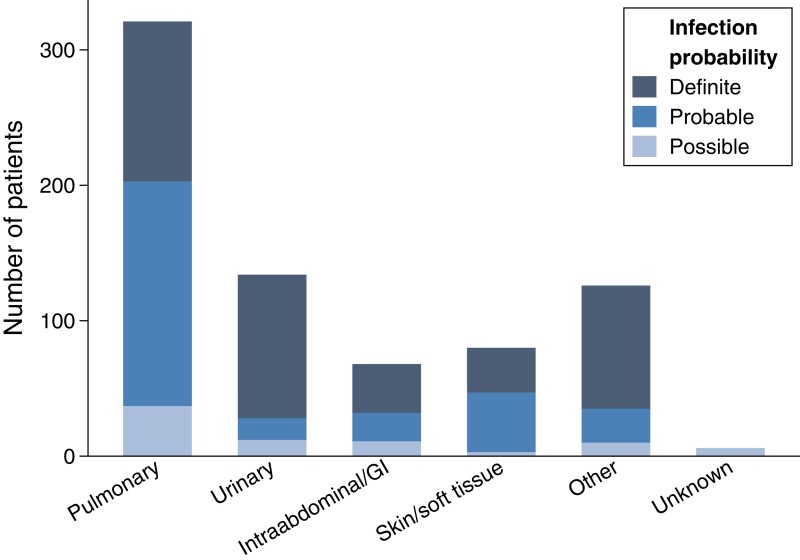
In the subset of 812 patients for whom probability of infection was adjudicated, 77 (9.5%) patients were determined to not be infected, 79 (9.7%) had a possible infection, 272 (33.5%) had a probable infection, and 384 (47.3%) had a definite infection. Aside from temperature at triage, patient characteristics and illness severity did not correlate with infection probability (see Supplementary Table 10). Patients with pneumonia were more likely to have a “probable” infection likelihood, whereas patients with a urinary source were more likely to have a “definite” infection likelihood (Figure 3).
Figure 3.
Adjudicated probability of infection according to final source of infection diagnosis (total N = 812). “Unknown” final source of infection was classified as a possible infection. Abbreviation: GI, gastrointestinal.
False-Positive Infection Diagnosis Risk Factors
In unadjusted analyses, false-positive presumptive infection diagnosis was associated with Charlson Comorbidity Index, arrival by emergency medical services (EMS), heart rate, temperature, white blood cell (WBC) counts, decreased Glasgow Coma Scale score, and the initial source of infection (Table 1). Multivariable modeling identified a lower likelihood of false-positive infection diagnosis with increasing SOFA score (odds ratio [OR]: .92; 95% CI: .89–.95; P < .001), WBC level (OR: .96; 95% CI: .95–.97; P < .001), and fever (OR: .41; 95% CI: .33–.50; P < .001) (Table 2). In contrast, a higher Charlson Comorbidity Index (OR: 1.03; 95% CI: 1.01–1.06; P = .01), hypothermia (OR: 1.65; 95% CI: 1.30–2.10; P < .001), and altered mental status (OR: 1.78; 95% CI: 1.33–2.38; P < .001) were all associated with an increased likelihood of false-positive infection diagnosis. Compared with an ED diagnosis of pneumonia as the source of infection, urinary (OR: .68; 95% CI: .51–.90; P = .007) and skin and soft tissue (OR: .50; 95% CI: .34–.73; P < .001) infections were associated with a lower likelihood of false-positive diagnosis, whereas the risk was substantially higher if the ED physician diagnosed infection with an unknown source (OR: 6.39; 95% CI: 5.14–7.94; P < .001).
Table 2.
Patient and System Characteristics Associated With False-Positive Presumptive Infection Diagnosis
| Variable | Adjusted Odds Ratio (95% CI) | P |
|---|---|---|
| Age (per 10-year increase) | .95 (.91–1.01) | .082 |
| Female sex | .96 (.81–1.14) | .63 |
| Race | ||
| Non-Hispanic/Latino, White | Reference | |
| Hispanic/Latino | 1.11 (.84–1.47) | .48 |
| Non-Hispanic/Latino, other race | 1.20 (.88–1.64) | .24 |
| Arrival to ED from long-term care facility | .89 (.63–1.26) | .50 |
| Charlson Comorbidity Index | 1.03 (1.01–1.06) | .011 |
| SOFA score | .92 (.89–.95) | <.001 |
| Initial vital signs and laboratory values | ||
| Heart rate, beats/minute | 1.00 (.99–1.00) | .18 |
| White blood cell count, per 1000/µL increase | .96 (.95–.97) | <.001 |
| Temperature | ||
| 36–38˚C | Reference | |
| <36˚C | 1.65 (1.30–2.10) | <.001 |
| >38˚C | .41 (.33–.50) | <.001 |
| Glasgow Coma Scale ≤14 | 1.78 (1.33–2.38) | <.001 |
| Hypotension in the ED | .85 (.70–1.04) | .12 |
| ED occupancy rate | 1.06 (.78–1.46) | .70 |
| ED-diagnosed source of infection | ||
| Pulmonary | Reference | |
| Urinary | .68 (.51–.90) | .007 |
| Intra-abdominal/gastrointestinal | 1.36 (.99–1.85) | .055 |
| Skin and soft tissue | .50 (.34–.73) | <.001 |
| Other | .96 (.73–1.28) | .80 |
| Unknown | 6.39 (5.14–7.94) | <.001 |
Multivariable logistic regression comparing patients adjudicated to be infected versus not infected.
Abbreviations: CI, confidence interval; ED, emergency department; SOFA, Sequential Organ Failure Assessment.
Antibiotic Utilization
Patients with false-positive presumptive infection diagnoses received fewer days of antibiotics (median: 1 day; interquartile range [IQR]: 1–3 days) compared with patients with confirmed infection (3 days; IQR: 2–5 days; P < .001). These patients also received slightly fewer unique antibiotics in the ED (mean: 1.6 [standard deviation (SD): 0.6] vs 1.7 [SD: 0.7], respectively; P = .005) and fewer days of therapy for each of the 10 most prescribed antibiotics (see Supplementary Table 11).
Source Diagnosis Discordance Risk Factors
The 1488 patients (18.0%) with initial/final source diagnosis discordance were more often admitted from a long-term care facility and transported by EMS and had more chronic illnesses and higher illness severity (Supplementary Table 12). In contrast to false-positive diagnosis, factors associated with source diagnosis discordance in multivariable models (Table 3) included female sex (OR: 1.17; 95% CI: 1.02–1.35; P = .027), arrival from a long-term care facility (OR: 1.48; 95% CI: 1.14–1.92; P = .003), increasing SOFA score (OR: 1.09; 95% CI: 1.06–1.11; P < .001), and fever (OR: 1.23; 95% CI: 1.07–1.42; P = .004). As with false-positive diagnosis, increasing Charlson Comorbidity Index (OR: 1.04; 95% CI: 1.02–1.06; P < .001) was associated with source diagnosis discordance. Compared with an ED diagnosis of pneumonia, ED diagnoses of urinary (OR: 1.81; 95% CI: 1.49–2.20; P < .001), skin/soft tissue (OR: 1.43; 95% CI: 1.12–1.83; P = .004), other (OR: 6.07; 95% CI: 5.05–7.30; P < .001), and unknown (OR: 54.57; 95% CI: 41.97–70.95; P < .001) infectious sources were all associated with a higher risk of source diagnosis discordance.
Table 3.
Patient and System Characteristics Associated With Initial/Final Infection Source Diagnosis Discordance
| Variable | Odds Ratio (95% CI) | P |
|---|---|---|
| Age (per 10-year increase) | 1.04 (.99–1.08) | .12 |
| Female sex | 1.17 (1.02–1.35) | .027 |
| Race | ||
| Non-Hispanic/Latino, White | Reference | |
| Hispanic/Latino | 1.06 (.84–1.35) | .60 |
| Non-Hispanic/Latino, other race | .98 (.74–1.30) | .90 |
| Arrival to ED from long-term care facility | 1.48 (1.14–1.92) | .003 |
| Charlson Comorbidity Index | 1.04 (1.02–1.06) | <.001 |
| SOFA score | 1.09 (1.06–1.11) | <.001 |
| Initial vital signs and laboratory values | ||
| Heart rate, beats/minute | 1.00 (1.00–1.01) | .083 |
| White blood cell count, per 1000/µL increase | 1.00 (1.00–1.00) | .99 |
| Temperature | ||
| 36–38˚C | Reference | |
| <36˚C | 1.21 (.94–1.56) | .14 |
| >38˚C | 1.23 (1.07–1.42) | .004 |
| Glasgow Coma Scale ≤14 | 1.01 (.77–1.31) | .96 |
| Hypotension in the ED | .91 (.78–1.06) | .24 |
| ED occupancy rate | 1.19 (.92–1.53) | .19 |
| ED-diagnosed source of infection | ||
| Pulmonary | Reference | |
| Urinary | 1.81 (1.49–2.20) | <.001 |
| Intra-abdominal/gastrointestinal | 1.20 (.90–1.62) | .22 |
| Skin and soft tissue | 1.43 (1.12–1.83) | .004 |
| Other | 6.07 (5.05–7.30) | <.001 |
| Unknown | 54.57 (41.97–70.95) | <.001 |
Multivariable logistic regression compared patients who had the same source of infection diagnosed in the ED as on final adjudication with patients who had different ED-diagnosed and final adjudicated sources of infection (ie, source diagnosis discordance). Patients adjudicated as not infected on final assessment were excluded from this analysis.
Abbreviations: CI, confidence interval; ED, emergency department; SOFA, Sequential Organ Failure Assessment.
Physician-Level Variation
Among the 69 ED physicians who saw at least 50 infected patients (see Supplementary Table 13), the physician-level source diagnosis discordance rate varied from 10.3% to 28.8% (Supplementary Figure 1), with a median diagnosis discordance rate of 19.5% (IQR: 16.9–22.4%). However, after accounting for individual case mix, physician variation was not significantly associated with discordance likelihood (P = .48).
Mortality
Among all patients meeting ED sepsis criteria, 73 (10.4%) patients who had false-positive presumptive infection diagnoses died within 30 days compared with 666 (8.8%) who had infection confirmed (P = .15). Multivariable analysis also showed no association between risk-adjusted 30-day mortality and false-positive diagnosis (adjusted OR [aOR]: 1.04; 95% CI: .77–1.40; P = .78). While unadjusted 30-day mortality was higher among patients with source diagnosis discordance (n = 182; 12.2%) compared with patients with concordance (n = 484; 8.0%) (P < .001), there was no association between 30-day mortality and source diagnosis discordance (aOR: 1.04; 95% CI: .83–1.29; P = .74) after adjustment for patient characteristics and illness severity.
DISCUSSION
In this retrospective analysis of over 8000 patients, we found that 8.5% of patients who met sepsis criteria in the ED had false-positive presumptive infection diagnoses (ie, were suspected to be infected in the ED but were ultimately determined to not be infected) and 18% had initial/final infection source diagnosis discordance (ie, different ED-diagnosed source of infection vs final adjudication). Among patients whose final infection probability was adjudicated, 9.7% had “possible” infection and 9.5% were not infected. Illness severity was associated with lower false-positive diagnosis risk, whereas comorbidity burden, lower WBC count, hypothermia, altered mental status, and “unknown” diagnosis in the ED were all associated with higher false-positive diagnosis risk. False-positive presumptive infection diagnosis was not associated with mortality at 30 days. To our knowledge, this is the largest study to date of false-positive infection diagnosis in patients meeting sepsis criteria and the first study on this topic to enroll patients based on Sepsis-3 criteria.
A major concern regarding efforts incentivizing accelerated sepsis treatment has been the potential to increase the rate of false-positive infection diagnosis in sepsis care. False-positive diagnosis rates in our study were lower than in prior studies that reported rates of 13% [19], 18% [21], and 27% [26]. Importantly, lower false-positive diagnosis rates did not reflect an increase in the proportion of “possible” rather than probable infections: only 19% of our cohort was either uninfected or only possibly infected, which was substantially lower than the 43% uninfected/possibly infected rate reported by Klein Klouwenberg et al [19]. False-positive diagnosis may be less likely among sepsis populations defined by concurrent organ failure compared with these cohorts based on the nonspecific systemic inflammatory response syndrome [40], suggesting that mandates that continue to incorporate this syndrome may pose a higher risk of contributing to false-positive diagnosis. Differences may also reflect our general rather than intensive care unit–only population, as false-positive diagnosis rates of 3–8% in recent cohorts of general ED patients with suspected infection are in line with our findings [18, 20]. Other differences in patient cohorts (eg, exclusion of patients without a specific infection source diagnosed by ED clinicians [20]) and differences in infection adjudication criteria or their application may also contribute to between-study variation and limit study comparisons. Nevertheless, our data suggest that the risk of false-positive diagnosis for patients meeting Sepsis-3 criteria may be less than previously feared.
False-positive diagnosis was associated with factors increasing the complexity of assessment, such as increasing comorbidity burden and, likely due to difficulty obtaining history, altered mental status. Patients treated for suspected infection despite lacking canonical laboratory or vital sign evidence of infection in the form of leukocytosis or fever, respectively, were also more likely to have infection disproven on final assessment. Lack of a clear infectious source in the ED (“unknown” source of infection) was the strongest risk factor for false-positive presumptive infection diagnosis. This finding suggests that clinical decision support or other methods aiding evaluation of patients with an “unknown” infection source diagnosis as well as recent recommendations advocating less urgency for antibiotic initiation when there is uncertainty around the sepsis diagnosis and shock is absent could help reduce false-positive infection diagnoses [5, 41, 42]. Conversely, while both theoretical models [41, 43] and guidelines [5] for decision making in sepsis would assume that increasing organ severity should be associated with a lower threshold for initiating antibiotics with regard to the perceived probability of infection, an increasing SOFA score was actually associated with a lower probability of false-positive infection diagnosis. The mechanisms underlying this unexpected finding require further study. It is possible that clinicians were less likely to discontinue antibiotics for more severely ill patients, decreasing the proportion of patients adjudicated as false-positive infections (although we did not observe a concomitant increase in the frequency of “possible” infections).
We found no significant association between 30-day mortality and false-positive presumptive infection diagnosis. Although our study may have been underpowered for this analysis, this could suggest that anchoring related to false-positive infection diagnosis may not have dramatically impaired identification and treatment of noninfectious problems. Among patients with infection, source diagnosis discordance also was not associated with 30-day mortality. These findings are in line with some [20] but not all [18] prior studies. Standardized treatment algorithms in place at study hospitals matching antibiotic treatment to infection source, hospital resistance patterns, and patients’ risk for resistant pathogen infection and recommending broad-spectrum antibiotics for sepsis of unknown source may mitigate the impact of source diagnosis discordance. For both false-positive diagnosis and source diagnosis discordance, we cannot rule out an impact on other patient-relevant outcomes, including patient functional status, costs or length of stay, antibiotic side effects, opportunistic infections, and patient- and community-level antimicrobial resistance.
Strengths of our study include rigorous, validated infection source and probability adjudication and a multicenter patient cohort identified using modern sepsis criteria that was 4–8 times larger than prior studies on this topic. However, our study has several limitations. This study was a retrospective observational study that can measure associations but not determine causality. Risk factor identification and mortality analyses are limited in power and may be subject to residual confounding. Studying a broader population of patients with suspected infection might have yielded different results. We were unable to evaluate the appropriateness of the spectrum of administered antimicrobials in relation to the causative pathogen or how frequently patients with pure viral sepsis were treated with unnecessary antibiotics.
Our detailed protocols for structured evaluation of the medical record and rigorous abstractor training yielded replicable adjudication results, but retrospective determination of infection presence and probability is fundamentally challenging on several fronts. Adjudicators were mostly not clinicians and may have neglected key indicators apparent to bedside clinicians. Adjudication incorporated the clinical interpretation of the bedside clinician and may thus have been vulnerable to incomplete or inaccurate recording of key information in clinical documentation. Apparent treatment responses may actually have resulted from clinical interventions for or spontaneous clinical resolution of a noninfectious problem, such as aspiration pneumonitis, leading to undercounting of false-positive infection diagnoses. Variation in the frequency and yield of microbiologic sampling likely contributed to differences in definite versus probable infection across infection sources. Specific diagnoses documented by ED clinicians may mask varying degrees of diagnostic uncertainty. Other limitations include the study being conducted within a single healthcare system and practice group, which may limit generalizability due to unique antibiotic-prescribing practices, and the fact that primary abstraction of a small number of medical records was performed by the same critical care attending physician reviewer who resolved interrater disagreements.
Conclusions
In a large cohort of patients who met current criteria for sepsis prior to ED discharge, the rate of false-positive presumptive infection diagnosis was less than 10%, as was the proportion of patients whose final infection probability was only possible rather than probable or definite. These findings provide real-world evidence to inform debates on the risks and benefits of efforts to accelerate sepsis care.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Gabriel A Hooper, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Carolyn J Klippel, Department of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA.
Sierra R McLean, University of Utah School of Medicine, Salt Lake City, Utah, USA; Department of Physical Medicine and Rehabilitation, University of North Carolina Health, Chapel Hill, North Carolina, USA.
Edward A Stenehjem, Division of Infectious Diseases and Epidemiology, Department of Medicine, Intermountain Medical Center, Salt Lake City, Utah, USA.
Brandon J Webb, Department of Medicine, University of Wisconsin School of Medicine, Madison, Wisconsin, USA.
Emily R Murnin, University of Utah School of Medicine, Salt Lake City, Utah, USA; Department of Medicine, University of Wisconsin School of Medicine, Madison, Wisconsin, USA.
Catherine L Hough, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Oregon Health and Sciences University, Portland, Oregon, USA.
Joseph R Bledsoe, Department of Emergency Medicine, Intermountain Medical Center, Murray, Utah, USA; Department of Emergency Medicine, Stanford University, Palo Alto, California, USA.
Samuel M Brown, Department of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA; Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Ithan D Peltan, Department of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA; Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Notes
Financial support. This work was supported by grants from the Intermountain Research and Medical Foundation and National Institute of General Medical Sciences (K23GM129661) to I. D. P. and from the National Heart, Lung, and Blood Institute (T35HL007744) to G. A. H. and S. R. M.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peltan ID, Brown SM, Bledsoe JR, et al. ED door-to-antibiotic time and long-term mortality in sepsis. Chest 2019; 155:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017; 196:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 376:2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 2021; 49:e1063–e143. [DOI] [PubMed] [Google Scholar]
- 6.. New York State Department of Health. New York State Report on Sepsis Care Improvement Initiative: Hospital Quality Performance. Available at: https://www.health.ny.gov/press/reports/docs/2015_sepsis_care_improvement_initiative.pdf. Accessed 4 August 2022.
- 7. Centers for Medicare & Medicaid Services . Sepsis bundle algorithm. Available at: file:///C:/Users/2seam/Downloads/SEP_Bundle_Algorithm_2021_Q3_Q4.pdf. Accessed 4 August 2022.
- 8. Rhee C, Chiotos K, Cosgrove SE, et al. Infectious Diseases Society of America position paper: recommended revisions to the national severe sepsis and septic shock early management bundle (SEP-1) sepsis quality measure. Clin Infect Dis 2021; 72:541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. IDSA Sepsis Task Force . Infectious Diseases Society of America (IDSA) position statement: why IDSA did not endorse the surviving sepsis campaign guidelines. Clin Infect Dis 2018; 66:1631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klompas M, Calandra T, Singer M. Antibiotics for sepsis-finding the equilibrium. JAMA 2018; 320:1433–4. [DOI] [PubMed] [Google Scholar]
- 11. Prescott HC, Seelye S, Wang XQ, et al. Temporal trends in antimicrobial prescribing during hospitalization for potential infection and sepsis. JAMA Intern Med 2022; 182:805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arulkumaran N, Routledge M, Schlebusch S, Lipman J, Conway Morris A. Antimicrobial-associated harm in critical care: a narrative review. Intensive Care Med 2020; 46:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown K, Valenta K, Fisman D, Simor A, Daneman N. Hospital ward antibiotic prescribing and the risks of Clostridium difficile infection. JAMA Intern Med 2015; 175:626–33. [DOI] [PubMed] [Google Scholar]
- 15. Hussain F, Cooper A, Carson-Stevens A, et al. Diagnostic error in the emergency department: learning from national patient safety incident report analysis. BMC Emerg Med 2019; 19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wachter RM, Flanders SA, Fee C, Pronovost PJ. Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure. Ann Intern Med 2008; 149:29–32. [DOI] [PubMed] [Google Scholar]
- 18. Abe T, Tokuda Y, Shiraishi A, et al. In-hospital mortality associated with the misdiagnosis or unidentified site of infection at admission. Crit Care 2019; 23:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein Klouwenberg PM, Cremer OL, van Vught LA, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care 2015; 19:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dregmans E, Kaal AG, Meziyerh S, et al. Analysis of variation between diagnosis at admission versus discharge and clinical outcomes among adults with possible bacteremia. JAMA Netw Open 2022; 5:e2218172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis 2010; 50:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burston J, Adhikari S, Hayen A, et al. A role for antimicrobial stewardship in clinical sepsis pathways: a prospective interventional study. Infect Control Hosp Epidemiol 2017; 38:1032–8. [DOI] [PubMed] [Google Scholar]
- 23. Minderhoud TC, Spruyt C, Huisman S, Oskam E, Schuit SCE, Levin MD. Microbiological outcomes and antibiotic overuse in emergency department patients with suspected sepsis. Neth J Med 2017; 75:196–203. [PubMed] [Google Scholar]
- 24. Tidswell R, Parker T, Brealey D, Singer M. Sepsis—the broken code how accurately is sepsis being diagnosed? J Infect 2020; 81:e31–e2. [DOI] [PubMed] [Google Scholar]
- 25. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31: 1250–6. [DOI] [PubMed] [Google Scholar]
- 26. Taylor SP, Rozario N, Kowalkowski MA, et al. Trends in false-positive code sepsis activations in the emergency department. Ann Am Thorac Soc 2020; 17:520–2. [DOI] [PubMed] [Google Scholar]
- 27. Peltan ID, Bledsoe JR, Oniki TA, et al. Emergency department crowding is associated with delayed antibiotics for sepsis. Ann Emerg Med 2019; 73:345–55. [DOI] [PubMed] [Google Scholar]
- 28. Peltan ID, McLean SR, Murnin E, et al. Prevalence, characteristics, and outcomes of emergency department discharge among patients with sepsis. JAMA Netw Open 2022; 5:e2147882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 30. Clayton PD, Narus SP, Huff SM, et al. Building a comprehensive clinical information system from components. The approach at Intermountain Health Care. Methods Inf Med 2003; 42:1–7. [PubMed] [Google Scholar]
- 31. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 32. Klouwenberg PM K, Ong DS, Bos LD, et al. Interobserver agreement of centers for disease control and prevention criteria for classifying infections in critically ill patients. Crit Care Med 2013; 41:2373–8. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Patient Safety Component Manual. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. Accessed 26 May 2022.
- 34. Calandra T, Cohen J; International Sepsis Forum Definition of Infection in the ICUCC . The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Crit Care Med 2005; 33:1538–48. [DOI] [PubMed] [Google Scholar]
- 35. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 36. Shappell CN, Klompas M, Ochoa A, Rhee C; CDC Prevention Epicenters Program . Likelihood of bacterial infection in patients treated with broad-spectrum IV antibiotics in the emergency department. Crit Care Med 2021; 49:e1144–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valik JK, Ward L, Tanushi H, et al. Validation of automated sepsis surveillance based on the Sepsis-3 clinical criteria against physician record review in a general hospital population: observational study using electronic health records data. BMJ Qual Saf 2020; 29:735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Healthcare Safety Network. CDC/NHSN surveillance definitions for specific types of infections. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf. Accessed 26 May 2022.
- 39. Stenehjem E, Hersh AL, Sheng X, et al. Antibiotic use in small community hospitals. Clin Infect Dis 2016; 63:1273–80. [DOI] [PubMed] [Google Scholar]
- 40. Sprung CL, Sakr Y, Vincent JL, et al. An evaluation of systemic inflammatory response syndrome signs in the Sepsis Occurrence in Acutely Ill Patients (SOAP) study. Intensive Care Med 2006; 32:421–7. [DOI] [PubMed] [Google Scholar]
- 41. Prescott HC, Iwashyna TJ. Improving sepsis treatment by embracing diagnostic uncertainty. Ann Am Thorac Soc 2019; 16:426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Contou D, Roux D, Jochmans S, et al. Septic shock with no diagnosis at 24 hours: a pragmatic multicenter prospective cohort study. Crit Care 2016; 20:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med 1980; 302:1109–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.