Abstract
Background
Pre-existing lower urinary tract symptoms (LUTS), cognitive impairment, and the high prevalence of asymptomatic bacteriuria (ASB) complicate the diagnosis of urinary tract infection (UTI) in older women. The presence of pyuria remains the cornerstone of UTI diagnosis. However, >90% of ASB patients have pyuria, prompting unnecessary treatment. We quantified pyuria by automated microscopy and flowcytometry to determine the diagnostic accuracy for UTI and to derive pyuria thresholds for UTI in older women.
Methods
Women ≥65 years with ≥2 new-onset LUTS and 1 uropathogen ≥104 colony-forming units (CFU)/mL were included in the UTI group. Controls were asymptomatic and classified as ASB (1 uropathogen ≥105 CFU/mL), negative culture, or mixed flora. Patients with an indwelling catheter or antimicrobial pretreatment were excluded. Leukocyte medians were compared and sensitivity–specificity pairs were derived from a receiver operating characteristic curve.
Results
We included 164 participants. UTI patients had higher median urinary leukocytes compared with control patients (microscopy: 900 vs 26 leukocytes/µL; flowcytometry: 1575 vs 23 leukocytes/µL; P < .001). Area under the curve was 0.93 for both methods. At a cutoff of 264 leukocytes/µL, sensitivity and specificity of microscopy were 88% (positive and negative likelihood ratio: 7.2 and 0.1, respectively). The commonly used cutoff of 10 leukocytes/µL had a poor specificity (36%) and a sensitivity of 100%.
Conclusions
The degree of pyuria can help to distinguish UTI in older women from ASB and asymptomatic controls with pyuria. Current pyuria cutoffs are too low and promote inappropriate UTI diagnosis in older women.
Clinical Trials Registration. International Clinical Trials Registry Platform: NL9477 (https://trialsearch.who.int/Trial2.aspx?TrialID=NL9477)
Keywords: urinary tract infection, asymptomatic bacteriuria, pyuria, microscopy, urine flowcytometry
The degree of pyuria can help distinguish urinary tract infection (UTI) in older women from asymptomatic women with and without bacteriuria. We demonstrate that current pyuria cutoffs are too low, promoting inappropriate diagnosis and treatment of UTI.
Urinary tract infection (UTI) incidence increases with age and is higher in women than in men [1]. In older women, diagnosing UTI is complicated for several reasons. First, symptom communication may be affected by cognitive impairment. Second, pre-existing lower urinary tract symptoms (LUTS), such as urinary incontinence and urgency, are common and distinguishing acute from chronic LUTS can be challenging [2]. Finally, 20% of community-dwelling and 50% of institutionalized older women have asymptomatic bacteriuria (ASB), defined as the presence of 1 or more uropathogens with 105 colony-forming units (CFU)/mL or higher in the absence of signs or symptoms attributable to UTI [3–5]. As a result, inappropriate antimicrobial treatment is common, leading to unnecessary side effects, drug interactions, Clostridioides difficile infection, and the selection of antimicrobial-resistant pathogens [6, 7]. Distinguishing ASB from UTI is further complicated by the fact that over 90% of older women with ASB have concomitant pyuria [8, 9]. Consequently, the positive-predictive value of the presence of pyuria for UTI is low in older women. However, it is unclear whether the degree of pyuria differs between older women with UTI and ASB, partly because urine dipstick is the most ordered screening test, providing only semiquantitative results of leukocyte esterase activity. Pyuria can be quantified in different ways. Initially, Mabeck [10] found that a leukocyte excretion rate of 400 000 per hour could distinguish UTI from asymptomatic women. This rate corresponds to a cutoff value of 10 leukocytes/mm3 in non-centrifuged urine [11]. In clinical practice and research, pyuria is most often quantified by direct or automated microscopy of (non)centrifuged urine, usually after initial dipstick screening. Automated microscopy reduces variability in centrifugation and resuspension of urine and is more efficient than direct microscopy [12]. In recent years, an increasing number of laboratories are adopting urine flowcytometry for quantification of pyuria. Although cutoff values for “significant” pyuria vary in the literature and depend on quantification methods, commonly accepted cutoffs include 10 leukocytes/µL and 5–10 leukocytes per high-power field (hpf). These cutoff values are largely derived from studies involving nonpregnant premenopausal women, in whom ASB is uncommon [13]. The objective of this study was to determine sensitivity and specificity of automated microscopy and urine flowcytometry for diagnosing UTI in older women, with the ultimate goal to derive optimal cutoff values for pyuria for UTI in this population, taking ASB into account.
METHODS
This study is an exploratory analysis of an overarching, case-control study registered at the International Clinical Trials Registry Platform (NL9477). The study was conducted across 5 hospitals (4 regional and 1 academic), 4 long-term care facilities (LTCFs), 3 primary care centers, 1 after-hours primary care clinic, and 14 senior housing facilities. This study was approved by the regional medical ethics committee (Medisch-Ethische Toetsingscommissie Leiden Den Haag Delft) and was conducted in accordance with the Declaration of Helsinki [14]. Written informed consent was obtained from all participants.
Study Population
Women aged 65 years and older were eligible for inclusion. Exclusion criteria included inability to express symptoms (eg, due to delirium or cognitive impairment), the presence of an indwelling catheter, immunosuppressive use, antimicrobial use (<48 hours prior to inclusion), current urolithiasis, and a UTI in the previous month. Stringent criteria were applied to both patients with UTI and control patients, as a consensus-based reference standard for UTI is currently missing. To be eligible for the UTI group, patients were required to have at least 2 new-onset LUTS (dysuria, frequency, urgency, or suprapubic pain). Furthermore, patients were required to have pyuria, defined as 10 or more leukocytes/µL or 5 or more leukocytes/hpf or the presence of leukocyte esterase, and a monoculture (ie, 1 uropathogen ≥104 CFU/mL), for the primary analysis. Enterobacterales, enterococci, Pseudomonas aeruginosa, Staphylococcus saprophyticus, and group B streptococci were considered uropathogens. In case of a temperature of 38.0°C or higher, patients were classified as having an upper UTI. Community-dwelling women and LTCF residents who did not have any LUTS or fever were eligible as controls. Patients were eligible regardless of urine culture results and they were subdivided into 3 subgroups: ASB, negative culture, and mixed flora. Asymptomatic bacteriuria was defined as at least 2 consecutive urine cultures (2–4 weeks apart) with the same uropathogen with 105 CFU/mL or greater, and a negative culture was defined as no growth or growth of nonpathogenic micro-organisms with 103 CFU/mL or less. Cases and controls were not matched for age or comorbidities.
Study Procedures and Methods of Measurement
The study team was contacted by the treating physician in case of a potential participant at the emergency department, LTCF, or primary care office. Asymptomatic LTCF residents were asked to participate by their elderly-care physician; community-dwelling older women were recruited through flyers. If eligibility criteria were met, participants were visited by the study team within 1 hour. Baseline data included the following: age, prior medical history (hypertension, chronic kidney disease, diabetes mellitus, and urological history), new-onset LUTS, and fever. All patients underwent a delirium screening and assessment of dependency in activities of daily living (ADLs) through 4AT and Katz questionnaires, respectively, and measurement of vital signs [15, 16].
Urinalysis
Midstream urine was collected in a 100-mL sterile urine container. Urine obtained via single catheterization was accepted; urine collected from a bedpan was not. After collection, the urine was divided into 2 V-monovette 10-mL urine tubes (Sarstedt, Nümbrecht, Germany), 1 for automated microscopy and 1 for urine flowcytometry. Automated microscopy was performed using the Cobas U701 (Roche, Rotkreuz, Switzerland) [17]. After mixing by the analyzer, 170 µL of urine was injected into a polycarbonate cuvette. Next, a monolayer of cells was created by centrifuging the cuvette for 10 seconds at 260 g. Cobas U701 output included quantitative measures of leukocytes in cells/µL with a lower limit of detection (LLD) of 1 cell/µL and an upper limit of detection (ULD) of 900 cells/µL. Urine flowcytometry was carried out with the Sysmex UF-4000 (Sysmex, Kobe, Japan). Within the analyzer, fluorescent dyes were added to 450 µL of urine, after which urine particles were quantified and classified by analysis of scattered light patterns. The LLD was 1 leukocyte/µL and the ULD was 10 000 leukocytes/µL. All urine samples were analyzed at the Leiden University Medical Center, except for urine samples of the participants who were included in regional hospitals. In the latter case, urine was analyzed at the corresponding regional hospital by automated microscopy, as urine flowcytometry was not available. All urine samples were kept at room temperature and analyzed within 4 hours of micturition to ensure stability of all urine components.
Microbiological Assessments
The remaining urine in the sterile container was used for bacteriological culture at the microbiology department. For all included patients with UTI and control patients, 10 µL of noncentrifuged urine was placed on routine culture media and incubated for 1 day. A culture result was deemed positive in case of growth of 104 CFU/mL or more and defined as a monoculture if 90% or more of the cultured colonies were of 1 microorganism.
Statistical Analysis
Statistical analysis was performed using SPSS version 27.0 (IBM Corporation, Armonk, NY, USA). Data are presented as percentages, means with standard deviations, or medians with interquartile ranges, as appropriate. A Mann–Whitney U test was performed to compare leukocyte medians between patients with UTI and controls. As a pyuria threshold for UTI in older women is not known, sensitivity–specificity pairs with associated 95% confidence intervals (CIs) were calculated for all possible cutoffs and plotted in a receiver operating characteristic (ROC) curve using GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA). The area under the curve (AUC) was calculated to determine the discriminative ability of the index tests (automated microscopy and urine flowcytometry). Youden's index was used to determine the cutoff value with the optimal trade-off between sensitivity and specificity. In a fraction of UTI cases, automated microscopy results were missing (eg, only semiquantitative results were available [leukocyte esterase or leukocytes/hpf]). The impact of missing automated microscopy results on estimates of accuracy was evaluated by a sensitivity analysis consisting of best- and worst-case scenarios (all missing pyuria results were either considered true positive and negative or false positive and negative, respectively). Twenty-seven patients presenting with LUTSs were not included in the primary analysis because they did not meet the urine culture criteria for the UTI group. Their urine leukocyte counts were evaluated separately, in the secondary analysis.
RESULTS
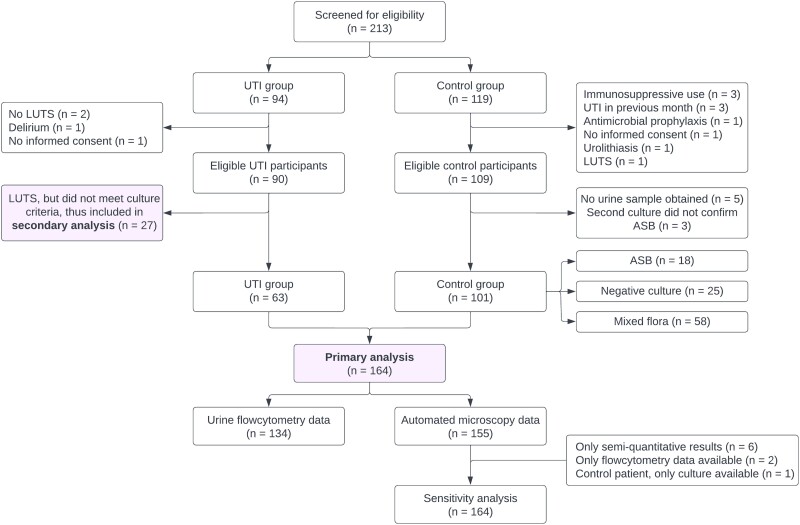
Of the 213 screened participants, 199 were eligible for inclusion, of whom 164 were included in the primary analysis (Figure 1). Baseline characteristics are summarized in Table 1. Urinary tract infection and control groups were comparable in terms of age (overall mean: 78.3 years) and comorbidities. Inclusion sites differed between UTI and control groups—for example, 11% of patients with UTI versus 43% of controls were included in an LTCF. The ADL dependency scores were comparable. Within the UTI group, the most common new-onset symptom was frequency, followed by urgency and dysuria; 13 of 63 patients (21%) had an upper UTI.
Figure 1.
Overview of screening and selection process. All patients in the control group were asymptomatic. Abbreviations: ASB, asymptomatic bacteriuria; LUTS, lower urinary tract symptoms; UTI, urinary tract infection.
Table 1.
Baseline Characteristics of Patients With Urinary Tract Infection and Controls
| Baseline Characteristics | UTI (n = 63) | Controls (n = 101) |
|---|---|---|
| Age, years | 77.1 (8.0) | 79.0 (8.0) |
| Setting | ||
| Hospital | 18 (28.6) | 0 |
| LTCF | 7 (11.1) | 43 (42.6) |
| Primary care office | 38 (60.3) | 0 |
| At home | 0 | 58 (57.4) |
| Urological comorbidity | 8 (12.7) | 8 (7.9) |
| Other comorbidity | ||
| Diabetes mellitus | 14 (22.2) | 14 (13.9) |
| Hypertension | 30 (47.6) | 47 (46.5) |
| History of CKD | 12 (19.0) | 11 (10.9) |
| UTI history | ||
| Ever had UTI | 57 (90.5) | 77 (76.2) |
| Ever hospitalized for UTI | 2 (3.2) | 1 (1.0) |
| Number of UTI in past year | 1 (0–2) | 0 (0–0) |
| Antibiotics in previous month | 16 (25.4) | 20 (19.8) |
| New-onset symptoms | 63 (100) | 0 (0) |
| Dysuria | 49 (77.8) | … |
| Frequency | 57 (90.5) | … |
| Urgency | 53 (84.1) | … |
| Suprapubic pain | 43 (68.3) | … |
| Urethral pain | 33 (52.4) | … |
| Flank pain | 12 (19.0) | … |
| New/worsening urinary incontinence | 31 (49.2) | … |
| Recognition of symptoms | 46 (73.0) | … |
| Fever (≥38.0°C) | 13 (20.6) | … |
| ADL-dependency ≥2 Katz-items | 14 (22.2) | 23 (23.8) |
Age is expressed as mean (SD); number of UTIs in the past year is expressed as median (IQR); and all other variables are expressed as n (%). Urological comorbidity included pelvic organ prolapse, previous procedures for urinary incontinence, and previous malignancies. One patient with UTI had had renal cell carcinoma 12 years prior, and 1 control patient had had non–muscle-invasive bladder cancer 2 years prior. In both patients, there was no evidence of active malignancy. History of CKD was self-reported. Fever was objectified. Thirteen patients had an upper UTI. Abbreviations: ADL, activities of daily living; CKD, chronic kidney disease; IQR, interquartile range; LTCF, long-term care facility; SD, standard deviation; UTI, urinary tract infection.
Nearly all urine samples were midstream samples (162/164; 98.8%). Asymptomatic bacteriuria prevalence in our control group was 18%. Within the UTI group, Escherichia coli was the most common causative pathogen (81%), followed by Klebsiella spp. (4.8%) and Proteus mirabilis (4.8%). Two episodes were caused by extended-spectrum β-lactamase–producing E. coli. In 78% of UTI episodes colony counts were 105 CFU/mL or greater. Asymptomatic bacteriuria was caused by E. coli in 14 cases (78%); other pathogens included Klebsiella spp., Enterococcus faecalis, and streptococci.
Median Urine Leukocyte Values
Median urine leukocyte values in patients with UTI and controls are displayed in Table 2. Patients with UTI had higher median leukocyte levels compared with control patients with both quantification methods (automated microscopy: 900 vs 26 leukocytes/µL [P < .001]; urine flowcytometry: 1575 vs 23 leukocytes/µL [P < .001]). Moreover, median leukocyte values were higher for patients with UTI than for patients with ASB (automated microscopy: 900 vs 296 leukocytes/µL [P = .002]; urine flowcytometry: 1575 vs 197 leukocytes/µL [P = .004]), although interquartile ranges of these groups overlap.
Table 2.
Median Urine Leukocyte Values of Patients With Urinary Tract Infection and Controls (With Subgroups), Measured by Automated Microscopy and Urine Flowcytometry
| Control Group | ||||
|---|---|---|---|---|
| UTI Group | ASB | Negative Culture | Mixed Flora | |
| Automated microscopy | ||||
| n | 56 | 18 | 24 | 57 |
| Automated microscopy in cells/µL, median (IQR) | 900 (430–900) | 296 (49–773) | 4 (1–30) | 18 (5–57) |
| Urine flowcytometry | ||||
| n | 35 | 17 | 24 | 58 |
| Urine flowcytometry in cells/µL, median (IQR) | 1575 (581–4673) | 197 (43–1368) | 6 (1–35) | 20 (4–88) |
All values are expressed as median (IQR) as leukocyte values did not follow a normal distribution. The UTI column contains patients with both lower and upper UTI. Urine flowcytometry data were missing for 28 patients with UTI as they were included in regional hospitals in which urine flowcytometry was not available. For automated microscopy values, 900 cells/µL was the upper limit of detection. Abbreviations: ASB, asymptomatic bacteriuria; IQR, interquartile range; UTI, urinary tract infection.
Diagnostic Accuracy
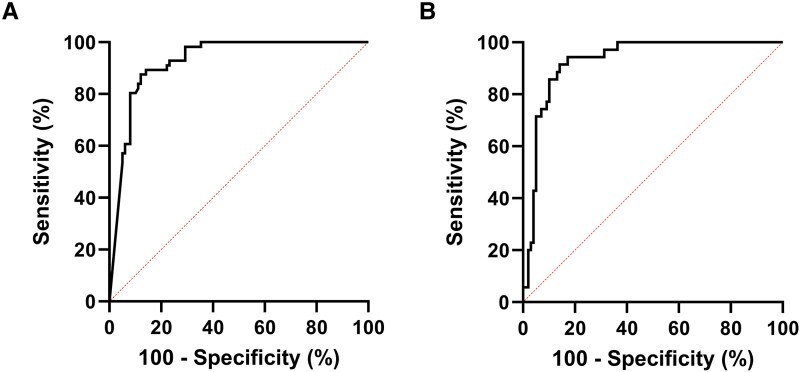
The ROC curves for automated microscopy and urine flowcytometry are displayed in Figure 2 and contingency tables for sensitivity and specificity calculations are shown in Supplementary Tables 1A and 1B. The AUC was 0.93 for both diagnostic methods. At a threshold of 264 leukocytes/µL, the sensitivity of automated microscopy was 88% (95% CI: 77–94%) and specificity was 88% (95% CI: 80–93%), corresponding to a positive likelihood ratio (LR) of 7.2 and a negative LR of 0.1. For urine flowcytometry, sensitivity was 91% (95% CI: 79–98%) and specificity was 86% (95% CI: 78–92%) at a cutoff value of 231 leukocytes/µL, with a positive LR of 6.5 and a negative LR of 0.1. Diagnostic accuracy parameters for several theoretical pyuria thresholds are shown in Table 3. Applying the currently used cutoff of 10 leukocytes/µL resulted in a sensitivity of 100% (95% CI: 94–100%) and specificity of 36% (95% CI: 28–48%). Diagnostic accuracy remained adequate in the sensitivity analysis (Supplementary Material 2). The secondary analysis showed that symptomatic patients with mixed flora or 2 or more uropathogens all had urine leukocyte counts above our “optimal” pyuria threshold (264 leukocytes/µL), and all but 2 patients with negative cultures had counts below this threshold (Supplementary Material 2).
Figure 2.
Receiver operating characteristic curves for automated microscopy (A) and urine flowcytometry (B). For both diagnostic methods, the number of leukocytes (per µl) was used as the test variable and our stringent UTI definition was used for determining disease status. The true positive rate (sensitivity) was plotted against the false positive rate (1-specificity) for different pyuria cutoffs. The area under the curve was 0.93 for both methods. The reference line is represented by the dotted line. Abbreviation: UTI, urinary tract infection.
Table 3.
Sensitivity, Specificity, and Positive and Negative Likelihood Ratios for the Current and Theoretical Pyuria Thresholds for Diagnosing Urinary Tract Infection in Older Women
| 10 cells/µL | 50 cells/µL | 100 cells/µL | 200 cells/µL | 300 cells/µL | 400 cells/µL | |
|---|---|---|---|---|---|---|
| Sensitivity, % (95% CI) | 100 (94–100) | 98 (92–100) | 93 (84–98) | 89 (80–96) | 84 (73–92) | 77 (65–87) |
| Specificity, % (95% CI) | 36 (28–48) | 66 (56–75) | 71 (61–79) | 86 (78–92) | 88 (81–93) | 92 (86–96) |
| LRpos (95% CI) | 1.6 (1.4–1.9) | 2.9 (2.2–3.8) | 3.2 (2.3–4.3) | 6.3 (3.9–10.3) | 6.9 (4.0–11.9) | 9.5 (4.8–18.7) |
| LRneg (95% CI) | 0.0 (0.0–0.1) | 0.03 (0.004–0.2) | 0.1 (0.04–0.3) | 0.1 (0.06–0.3) | 0.2 (0.1–0.3) | 0.3 (0.2–0.4) |
Diagnostic accuracy parameters are based on automated microscopy results. The currently used cutoff value for pyuria is 10 cells/μl. Abbreviations: CI, confidence interval; LRneg, negative likelihood ratio; LRpos, positive likelihood ratio.
DISCUSSION
This explorative study has 2 important findings. First, we show that the degree of pyuria—quantified by automated microscopy or urine flowcytometry—can help distinguish UTI in older women from asymptomatic controls, including those with ASB. Second, we demonstrate that the currently used cutoff for pyuria (10 leukocytes/µL) has a very low specificity for UTI in older women, and therefore should not be applied to this population.
Leukocyte Counts in Urinary Tract Infection
Thus far, the degree of pyuria in UTI and ASB has not been assessed specifically in women aged 65 and over, while a discriminative biomarker is arguably most needed in this population due to the high prevalence of ASB. In our study, older women with symptomatic UTI had high median urine leukocyte counts (900 and 1575 leukocytes/µL with automated microscopy and urine flowcytometry, respectively). Both Pieretti et al [18] and Kim et al [19] quantified pyuria with urine flowcytometry in men and women of all ages, although no separate leukocyte values were given for older patients. Among patients with positive urine cultures, they found median urine leukocyte values of 117 leukocytes/µL and 189 leukocytes/µL, respectively. However, neither of these studies collected clinical data, so misclassification is likely. The discrepancy between urine leukocyte values between these studies and our cohort is likely explained by the fact that we only included cases that met our strict UTI criteria.
Leukocyte Counts in Asymptomatic Bacteriuria
In our study, women with ASB had median counts of 296 leukocytes/µL. Cai et al [20] included premenopausal women with ASB and a history of recurrent UTI and quantified pyuria with direct microscopy. At baseline, these patients had median urine leukocyte values of 19 per hpf, which corresponds to approximately 100 leukocytes/µL [21, 22]. This study suggests that higher degrees of pyuria, well above 10 leukocytes/µL, do not necessarily mean that a patient has a UTI, even in premenopausal women. Moreover, urine leukocyte values increased to 54 per hpf (∼250 leukocytes/µL) if women developed any LUTS during the study and had a positive urine culture. This is in line with our findings that the degree of pyuria is higher in symptomatic patients with positive urine cultures.
Diagnostic Accuracy of Microscopy and Flowcytometry
The majority of UTI studies investigating the discriminative ability of automated microscopy and urine flowcytometry are limited by the absence of a reference standard for UTI. As a consequence, these studies choose a positive urine culture as the reference test, while this does not discriminate between UTI and ASB. Instead, Foudraine et al [23] defined UTI with an expert panel, taking symptoms and urine culture results into account. They found that automated microscopy had a sensitivity of 86% and a specificity of 82% at a cutoff value of 74 leukocytes/µL. As their study population was younger and antibiotic pretreatment was common, possibly explaining lower pyuria levels, results may not be directly comparable to our study. Diagnostic accuracy parameters are influenced by the studied population—more specifically, how cases and controls are defined. Our control group did not only consist of asymptomatic women with negative urine cultures but rather represents the distribution of urine culture results in asymptomatic older women. For example, the prevalence of ASB in our control group (18%) is very similar to the prevalence of ASB in community-dwelling older women [4].
Leukocyte Counts in Symptomatic Patients With Mixed Flora
Our case group only consisted of patients with clear-cut UTI fulfilling our stringent criteria. However, urine leukocyte levels were also determined in the patients with “suspected UTI” who had new-onset LUTS but were excluded from the primary analysis because they did not meet our culture criteria. Intriguingly, all excluded patients with either mixed flora or 2 uropathogens had leukocyte levels above our “optimal” pyuria threshold and all but 2 patients with negative urine cultures had levels below that threshold. The finding that all symptomatic patients with mixed flora had high degrees of pyuria suggests that these patients might have had a true UTI. This is supported by a study showing that over 90% of symptomatic women with E. coli as part of mixed flora in their midstream urine cultures actually had E. coli bladder bacteriuria as demonstrated by single catheterization [9].
Clinical Implications
In asymptomatic controls, median urine leukocyte values were higher than the most commonly used cutoff value of 10 leukocytes/µL. Therefore, applying the current pyuria threshold to older women leads to misclassification of many of these women, both with and without ASB. This has several consequences. First, the true cause of the symptoms (eg, vaginal atrophy, Candida vulvovaginitis, and overactive bladder) remains unidentified and thus untreated if symptoms are wrongfully attributed to UTI. Second, it leads to overprescription of antimicrobials, contributing to gut dysbiosis, side effects, and selection of resistant pathogens. Gupta et al [24] show that 25% of asymptomatic patients with pyuria on routine preoperative urinalysis (without urine cultures) were treated with antimicrobials, and that the degree of pyuria predicted prescribing of antimicrobials. These findings, combined with our own data, imply that separate, higher reference values are needed for older women with regard to pyuria. For instance, a threshold of 300 leukocytes/µL would be a considerable improvement, increasing specificity to avoid overtreatment while still maintaining a fair sensitivity. As in any diagnostic test, pyuria levels should be interpreted within the clinical context of individual patients and should not be the only deciding factor when diagnosing UTI. Since both older women with UTI and asymptomatic older women have a high pre-test probability of pyuria, and leukocyte esterase activity is a very rough estimate of the absolute number of leukocytes in the urine [21], the role of urine dipsticks in older patients should, at best, be limited to ruling out UTI. In addition to clinical implications, there are also implications for research, as misclassification influences the validity of UTI studies.
Strengths and Limitations
Strengths of our study include the use of a stringent UTI definition instead of urine culture as a reference standard, the consistency of results across 2 quantification methods (identical AUCs), inclusion of participants from multiple settings, and the rapid analysis of urine samples, increasing the reliability of results. Our study also has several limitations. Results may not be generalizable to institutionalized older people with high frailty and/or advanced dementia. However, our population was chosen to prove a concept for which a clear definition and reliable assessment of UTI and ASB were deemed necessary. Moreover, our control group contained a higher proportion of LTCF residents than our UTI group. Nonetheless, ADL dependency scores were similar between the UTI and control groups, and median leukocyte values within the LTCF subgroup were comparable to the values of the overall group.
Conclusions
In conclusion, the degree of pyuria should be taken into account when evaluating older women for UTI. Current pyuria cutoffs for UTI are too low and promote inappropriate UTI diagnosis in this population, affecting patient care, antimicrobial stewardship efforts, and research. The impact of higher cutoff values on prescription behavior and UTI-related outcomes in older women deserves further study.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Manu P Bilsen, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Margaretha J Aantjes, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Esther van Andel, Department of Clinical Chemistry and Laboratory Medicine, Leiden University Medical Center, Leiden, The Netherlands.
Janneke E Stalenhoef, Department of Internal Medicine, OVLG, Amsterdam, The Netherlands.
Cees van Nieuwkoop, Department of Internal Medicine, Haga Teaching Hospital, The Hague, The Netherlands; Department of Public Health and Primary Care, Leiden University Medical Center, The Hague, The Netherlands.
Eliane M S Leyten, Department of Internal Medicine, Haaglanden Medisch Centrum, The Hague, The Netherlands.
Nathalie M Delfos, Department of Internal Medicine, Alrijne Hospital, Leiderdorp, The Netherlands.
Martijn Sijbom, Department of Public Health and Primary Care, Leiden University Medical Center, The Hague, The Netherlands.
Mattijs E Numans, Department of Public Health and Primary Care, Leiden University Medical Center, The Hague, The Netherlands.
Wilco P Achterberg, Department of Public Health and Primary Care, Leiden University Medical Center, The Hague, The Netherlands.
Simon P Mooijaart, Department of Gerontology and Geriatrics, Leiden University Medical Center, Leiden, The Netherlands.
Martha T van der Beek, Department of Medical Microbiology, Leiden University Medical Center, Leiden, The Netherlands.
Christa M Cobbaert, Department of Clinical Chemistry and Laboratory Medicine, Leiden University Medical Center, Leiden, The Netherlands.
Simon P Conroy, Medical Research Council Unit for Lifelong Health and Ageing at University College London, University College London, London, United Kingdom.
Leo G Visser, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Merel M C Lambregts, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Notes
Author Contributions. Conceptualization and methodology: M. P. B., J. E. S., C. v. N., M. E. N., W. P. A., M. T. v. d. B., C. M. C., S. P. C., L. G. V., M. M. C. L.; recruitment: M. P. B., M. J. A., M. M. C. L., writing—original draft preparation: M. P. B.; data interpretation: M. P. B., M. M. C. L., L. G. V.; writing—review and editing: M. P. B, M. J. A., E. v. A., J. E. S., C. v. N., E. M. S. L., N. M. D., M. S., M. E. N., W. P. A., S. P. M., M. T. v. d. B., C. M. C., S. P. C., L. G. V., M. M. C. L.; supervision: M. M. C. L. and L. G. V. All authors have read and agreed to the final version of the manuscript.
Acknowledgments. The authors thank Brenda Elzer and Lenneke Vonk for their contribution to participant recruitment. Furthermore, they thank the following inclusion sites: Huisartsenpraktijk de Doelder (Barbara de Doelder), Huisarstenpraktijk Meskers (Angelique Meskers-van Geel), Huisartsenpraktijk Hubertusduin (Maaike Lunstroot, Marja Koppejan), Marente van Wijckerslooth (Johan Verloop, Els van Dijk), WZH Sammersbrug (Jens van Leeuwen), WZH Het Anker (Sander van den Haselkamp), Topaz Overrhyn (Fleur van Zuylen-Jongejan), and Topaz Revitel (Sylvia van der Drift-Verbree).
Financial support. This work was supported by the Netherlands Organisation for Health Research and Development (ZonMw) (grant number 10150511910054). The Sysmex Corporation kindly provided reagents for flowcytometry measurements but was not involved in the study design or any other study-related processes.
References
- 1. Ahmed H, Farewell D, Jones HM, Francis NA, Paranjothy S, Butler CC. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care 2004–2014. PLoS One 2018; 13:e0190521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006; 50:1306–14; discussion 14–5. [DOI] [PubMed] [Google Scholar]
- 3. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68:1611–5. [DOI] [PubMed] [Google Scholar]
- 4. Rodhe N, Löfgren S, Matussek A, et al. Asymptomatic bacteriuria in the elderly: high prevalence and high turnover of strains. Scand J Infect Dis 2008; 40:804–10. [DOI] [PubMed] [Google Scholar]
- 5. Ouslander JG, Schapira M, Fingold S, Schnelle J. Accuracy of rapid urine screening tests among incontinent nursing home residents with asymptomatic bacteriuria. J Am Geriatr Soc 1995; 43:772–5. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med 2014; 174:1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rotjanapan P, Dosa D, Thomas KS. Potentially inappropriate treatment of urinary tract infections in two Rhode Island nursing homes. Arch Intern Med 2011; 171:438–43. [DOI] [PubMed] [Google Scholar]
- 8. Boscia JA, Abrutyn E, Levison ME, Pitsakis PG, Kaye D. Pyuria and asymptomatic bacteriuria in elderly ambulatory women. Ann Intern Med 1989; 110:404–5. [DOI] [PubMed] [Google Scholar]
- 9. Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med 2013; 369:1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mabeck CE. Studies in urinary tract infections. IV. Urinary leucocyte excretion in bacteriuria. Acta Med Scand 1969; 186:193–8. [DOI] [PubMed] [Google Scholar]
- 11. Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med 1983; 75:53–8. [DOI] [PubMed] [Google Scholar]
- 12. Oyaert M, Delanghe J. Progress in automated urinalysis. Ann Lab Med 2019; 39:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hooton TM, Roberts PL, Stapleton AE. Asymptomatic bacteriuria and pyuria in premenopausal women. Clin Infect Dis 2021; 72:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Medical Association General Assembly . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–4. [DOI] [PubMed] [Google Scholar]
- 15. Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014; 43:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 1983; 31:721–7. [DOI] [PubMed] [Google Scholar]
- 17. Cobbaert CM, Arslan F, Caballé Martín I, et al. Automated urinalysis combining physicochemical analysis, on-board centrifugation, and digital imaging in one system: a multicenter performance evaluation of the Cobas 6500 urine work area. Pract Lab Med 2019; 17:e00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pieretti B, Brunati P, Pini B, et al. Diagnosis of bacteriuria and leukocyturia by automated flow cytometry compared with urine culture. J Clin Microbiol 2010; 48:3990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Kim HR, Kim TH, Lee MK. Age-specific cutoffs of the Sysmex UF-1000i automated urine analyzer for rapid screening of urinary tract infections in outpatients. Ann Lab Med 2019; 39:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai T, Lanzafame P, Caciagli P, et al. Role of increasing leukocyturia for detecting the transition from asymptomatic bacteriuria to symptomatic infection in women with recurrent urinary tract infections: a new tool for improving antibiotic stewardship. Int J Urol 2018; 25:800–6. [DOI] [PubMed] [Google Scholar]
- 21. van den Broek D, Keularts IM, Wielders JP, Kraaijenhagen RJ. Benefits of the iQ200 automated urine microscopy analyser in routine urinalysis. Clin Chem Lab Med 2008; 46:1635–40. [DOI] [PubMed] [Google Scholar]
- 22. Ichiyanagi Y. Field volume of urine sediment test—comparison of theoretical volume with practical volume. Sysmex J Int 2014; 24:1–6. [Google Scholar]
- 23. Foudraine DE, Bauer MP, Russcher A, et al. Use of automated urine microscopy analysis in clinical diagnosis of urinary tract infection: defining an optimal diagnostic score in an academic medical center population. J Clin Microbiol 2018; 56:e02030–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta K, O'Brien W, Gallegos-Salazar J, Strymish J, Branch-Elliman W. How testing drives treatment in asymptomatic patients: level of pyuria directly predicts probability of antimicrobial prescribing. Clin Infect Dis 2020; 71:614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.