Abstract
Background
Acute myocardial infarction (AMI) events have been reported among patients with certain viral and bacterial infections. Whether invasive pneumococcal disease (IPD) increases the risk of AMI remains unclear. We examined whether laboratory-confirmed IPD was associated with the risk of AMI.
Methods
We conducted a self-controlled case series analysis among adult Tennessee residents with evidence of an AMI hospitalization (2003–2019). Patient follow-up started 1 year before the earliest AMI and continued through the date of death, 1 year after AMI, or study end (December 2019). Periods for AMI assessment included the 7 to 1 days before IPD specimen collection (pre-IPD detection), day 0 through day 7 after IPD specimen collection (current IPD), day 8 to 28 after IPD specimen collection (post-IPD), and a control period (all other follow-up). We used conditional Poisson regression to calculate incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for each risk period compared with control periods using within-person comparisons.
Results
We studied 324 patients hospitalized for AMI with laboratory-confirmed IPD within 1 year before or after the AMI hospitalization. The incidence of AMI was significantly higher during the pre-IPD detection (IRR, 10.29; 95% CI: 6.33–16.73) and the current IPD (IRR, 92.95; 95% CI: 72.17–119.71) periods but nonsignificantly elevated in the post-IPD risk period (IRR, 1.83; 95% CI: .86–3.91) compared with control periods. The AMI incidence was higher in the post-IPD control period (29 to 365 days after IPD; IRR, 2.95; 95% CI: 2.01–4.32).
Conclusions
Hospitalizations with AMI were strongly associated with laboratory-confirmed IPD.
Keywords: acute myocardial infarction, invasive pneumococcal disease, self-controlled case series
We identified an elevated incidence of hospitalizations with acute myocardial infarction during periods associated with laboratory-confirmed invasive pneumococcal disease (IPD) compared with non-IPD control periods.
Acute myocardial infarction (AMI) is a leading cause of global mortality [1]. Previous studies have reported that acute viral and bacterial respiratory infections can increase the risk of AMI, compounding the burden of those common infections [2]. It has been postulated that acute infections may trigger an AMI through an acute inflammatory process that involves platelet activation and endothelial dysfunction and through increased metabolic demand and stress on the vascular system [2]. Acute bacterial infections have been consistently associated with an intense inflammatory response and, generally, with greater clinical severity than acute viral infections and are thus potentially associated with a higher risk of cardiac events [3–6].
Streptococcus pneumoniae is a leading cause of acute community-acquired infections. Although pneumococcal infections have been linked to the occurrence of cardiac events, previous studies have not clearly distinguished between invasive and noninvasive S. pneumoniae infections [2, 4, 5, 7, 8]. Recent studies have reported that pneumococcus can directly invade and damage the myocardium during bacteremic episodes. However, it remains unclear whether bacteremic or other invasive pneumococcal disease (IPD) is associated with a high risk of AMI that is distinct from the more common respiratory infections with S. pneumoniae [2, 9, 10].
Therefore, we sought to build on prior work in this area by using state-based laboratory- and hospital-based surveillance data to conduct a self-controlled case series (SCCS) study to examine both the short- and longer-term associations between laboratory-confirmed IPD and AMI.
METHODS
Study Population and Data Sources
We identified study patients using data from the Tennessee Active Bacterial Core Surveillance (ABCs) System and linked healthcare encounter data from the Tennessee Hospital Discharge Data System (HDDS; Supplementary Appendix) [11, 12]. Using these data, we identified a retrospective cohort of adult (aged ≥18 years) patients on the date they experienced their first hospitalization for AMI from 1 July 2004 to 31 December 2018 and experienced a laboratory-confirmed IPD-related hospitalization within 365 days before or after the admission date of the hospitalization for AMI. We used this follow-up interval to reduce potential time-varying confounding from the inclusion of longer observation periods. The institutional review boards of Vanderbilt University Medical Center and the Tennessee Department of Health reviewed and approved the study protocol.
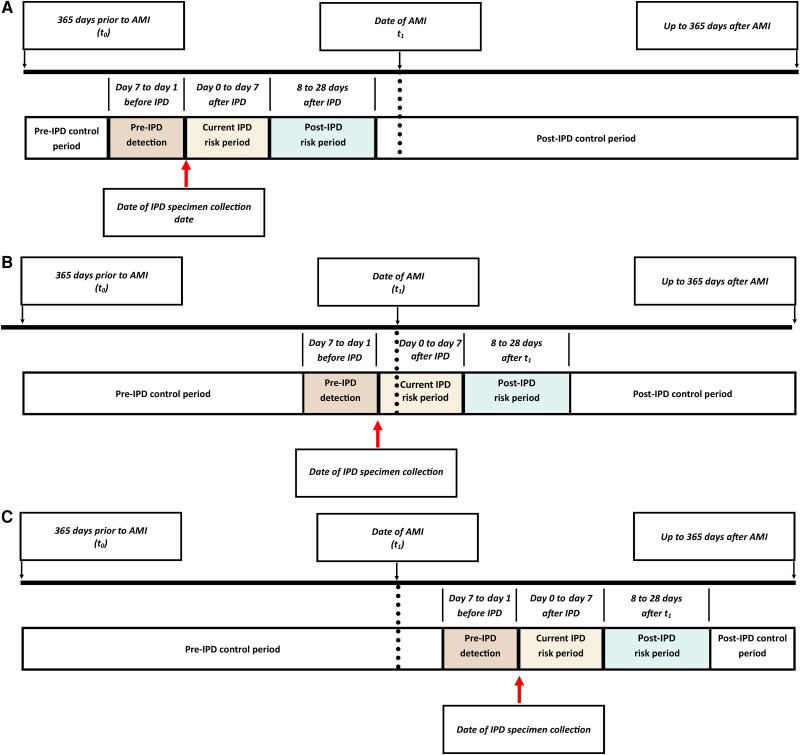
Following the SCCS design, we included only patients with evidence of AMI and IPD identified during follow-up (the date of hospitalization with AMI was classified as t1). The study observation period (t0) started on day 365 before the earliest observed AMI admission date (Figure 1), with follow-up continuing up to t1 and through the earliest of the end of the study (31 December 2019), death, residence in a county not captured by the ABCs surveillance network, or day 365 after t1. We identified patients on the date of their first AMI hospitalization from available data during the study (1998–2019), but only included patients with an AMI hospitalization during the period for which ABCs data were available in the year before and after the AMI hospitalization (ABCs data only available 2003–2019). Therefore, we included a patient's first detected AMI hospitalization with an admission date between 1 July 2004 and 31 December 2018, such that each patient had a look-back period of at least 78 months for prior AMI hospitalizations. We also excluded those with evidence of a non-IPD invasive infection in the 18 months prior to t1.
Figure 1.
Demonstration of baseline, control, and risk periods during follow-up. Patient A represents a patient with an AMI hospitalization identified in the post-IPD control period. Patient B represents a patient with an AMI hospitalization identified during a current IPD risk period (with IPD specimen collection date 1 day prior to date of admission for the AMI hospitalization). Patient C represents a patient with an AMI hospitalization during the pre-IPD control period. Abbreviations: AMI, acute myocardial infarction; IPD, invasive pneumococcal disease.
Outcome
The occurrence of AMI was identified from hospitalizations, 23-hour observational stays, and emergency department visits using diagnosis and procedure codes derived from prior studies that examined infection and AMI and listed in any discharge diagnosis position (Supplementary Table 1) [8, 13, 14]. An AMI hospitalization that occurred within 30 days of a previous AMI (ie, AMI readmission) was considered part of the prior AMI episode. The primary outcome of interest was any evidence of AMI (encompassing ST-elevation myocardial infarction [STEMI], non–ST-elevation myocardial infarction [NSTEMI], or unspecified myocardial infarction). To explore the potential mechanism of the association with AMI, secondary analyses also examined the association between IPD and individual AMI types (STEMI, NSTEMI, unspecified).
Exposures
For each study participant, each person-day of follow-up was classified into 1 of 5 mutually exclusive periods relative to the first reported IPD specimen collection date (Figure 1), including control period (t0 to day 8 before IPD specimen collection), pre-IPD detection period (7 days to 1 day before IPD specimen collection), current IPD risk period (day 0 through day 7 after IPD specimen collection), post-IPD risk period (day 8 through day 28 after specimen collection), and control period (day 29 after IPD through end of follow-up). The majority of ABCs IPD cases represent specimen collections at the onset of hospitalization for IPD. Thus, we included day 0 in the current IPD risk period for the primary analysis. Additionally, the pre-IPD detection period was used not only as a buffer period in the SCCS design but also as a period a patient may have active infection prior to having a specimen collected or being hospitalized.
Statistical Analyses
We characterized baseline characteristics and distribution of the IPD specimen collection dates relative to the occurrence of AMI. For the primary SCCS analysis, we used conditional Poisson regression to estimate incidence rate ratios (IRRs) with 95% confidence intervals (CIs) comparing the risk of AMI during the 3 periods of interest (pre-IPD detection, current IPD risk, post-IPD risk) relative to both control periods, accounting for patient age, seasonality, and pneumococcal conjugate vaccine (PCV) era at the start of each period (to account for potential secular trends) [8]. In our sample size calculation, we determined that 206 cases were necessary to estimate a relative incidence of 4.0 with 90% power at the 5% significance level for the current IPD risk period (8 days) with a median follow-up of 595 days in our cohort (28 necessary to estimate a relative incidence of 15 for stratified analyses described below).
Secondary analyses stratified estimates by age groups (<65 and ≥ 65 years) and for specific AMI types (STEMI, NSTEMI). To examine the robustness of our findings to the choice of control and risk periods, we conducted 2 additional sensitivity analyses, including an analysis using only the pre-IPD control period as the reference and an analysis classifying an additional period between the pre-IPD detection and current IPD period (day −1 to day 0 of specimen collection).
Since one assumption of the SCCS design is that the length of the observation period is independent of the occurrence of the outcome (AMI), we conducted a sensitivity analysis that excluded patients who died within 28 days of AMI hospitalization and a separate analysis that excluded any patient who died during follow-up. Additionally, as the SCCS design assumes that recurrent events within an individual are independent, we conducted a sensitivity analysis that excluded patients with a subsequent AMI hospitalization detected after the initial AMI hospitalization (t1).
RESULTS
Study Population
Among 5877 patients with evidence of laboratory-confirmed IPD identified from ABCs surveillance data and with evidence of linked hospitalization data in the Tennessee HDDS, 5.5% (n = 324) had a specimen collection within 1 year before or after the patient's earliest admission date for AMI. Among these patients, 14 (4.3%) experienced a subsequent AMI in the year after the earliest AMI (Supplementary Table 2). Approximately 18% of IPDs were identified with a serotype covered by the 7-valent PCV (PCV7) or 13-valent PCV (PCV13), 53% with a nonvaccine serotype, and 29% with an unspecified serotype.
Nearly half of the patients died within 365 days after their earliest AMI, with patients whose earliest AMI did not occur during the pre-IPD detection or IPD risk periods having a longer time to death after AMI (mean, 133 days; median, 110.5 days) compared with those whose earliest AMI did occur during one of these periods (mean, 46.9 days; median, 9 days). The most common AMI type was NSTEMI (74.3%) followed by unspecified AMI type (16.3%) and STEMI (8.9%).
Description of Healthcare Encounters With Admission or Discharge Proximal to IPD Specimen Collection
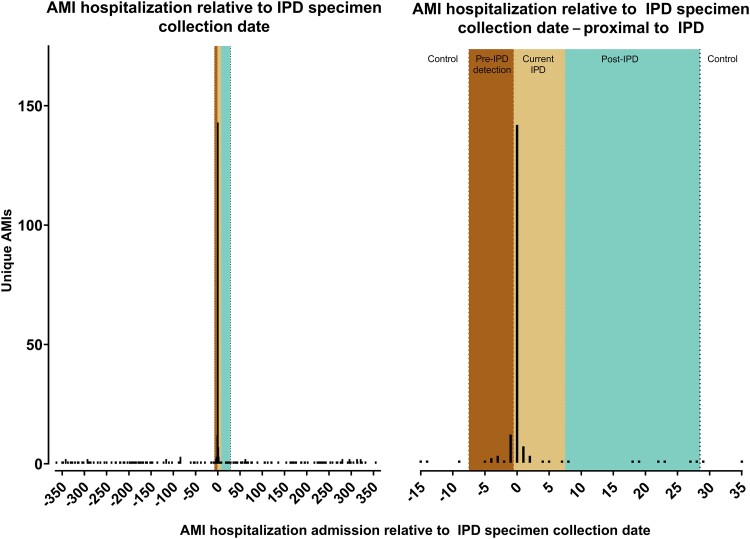
We identified a spike in the frequency of hospitalizations for AMI with an admission date 1 day before or on the same day as IPD specimen collection (Figure 2), with an even distribution of AMIs in the control periods (Figure 2). Of the 338 hospitalizations for AMI in the study period, 56.5% (n = 191) involved an admission during the pre-IPD detection (5.6%), current (48.8%), or post-IPD (2.1%) periods (Table 1). For AMIs in the current IPD period (n = 165), 86.7% had a positive IPD specimen collection on the date of admission for the AMI hospitalization.
Figure 2.
Distribution of dates for IPD specimen collection relative to date of AMI admission, 2004–2019. Day 7 to 1 before IPD specimen collection represents the pre-IPD detection period. Day 0 to Day 7 after IPD specimen collection represents the current IPD risk period. Day 8 to 28 after IPD specimen collection represents the post-IPD risk period. Abbreviations: AMI, acute myocardial infarction; IPD, invasive pneumococcal disease.
Table 1.
Incidence Rate Ratios for Acute Myocardial Infarction (AMI) Episodes by Period Type Among Patients With AMI, Included in Self-Controlled Case Series Analysis, 2004–2019
| IPD Risk and Control Periods | Acute Myocardial Infarction | Cumulative p-y | Incidence Rate per 100 py | 95% CI | Crude IRR | 95% CI | Adjusteda IRR | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Pre- and post-IPD control | 147 | 498.20 | 29.51 | (24.93–34.68) | 1.00 | (reference) | 1.00 | (reference) |
| Pre-IPD detection period (day 1 to day 7 before IPD) | 19 | 6.21 | 305.99 | (184.22–477.83) | 9.78 | (6.07–15.76) | 10.29 | (6.33–16.73) |
| Current IPD risk (day 0 to day 7 after IPD) | 165 | 6.48 | 2547.18 | (2173.34–2966.86) | 88.03 | (69.92–110.83) | 92.95 | (72.17–119.71) |
| Post-IPD risk (day 8 to day 28 after IPD) | 7 | 14.48 | 48.34 | (19.44–99.60) | 1.76 | (.83–3.75) | 1.83 | (.86–3.91) |
| Total | 338 | 525.36 | 64.34 | (57.66–71.57) | … | … | … | … |
Abbreviations: CI, confidence interval; IPD, invasive pneumococcal disease; IRR, incidence rate ratio; py, person-years.
Adjusted for pneumococcal conjugate vaccine era, seasonality, and patient age at start of period.
Self-Controlled Case Series Analysis
The incidence rate of AMI was substantially higher in the pre-IPD detection period (7 to 1 day before IPD specimen collection) and current IPD period (day 0 to day 7 after IPD specimen collection; 306.0 and 2547.2 AMI per 100 person-years [py], respectively) compared with the pre-IPD control (22.9 per 100 py), post-IPD secondary risk (48.3 per 100 py), and post-IPD control (41.8 per 100 py) periods.
In the SCCS analysis accounting for age, PCV era, and seasonality comparing incidence rates by period type, we observed that the incidence of AMI was nearly 93 times higher in the current IPD period compared with the pre- and post-IPD control periods (95% CI: 72.2–119.7). Furthermore, we observed an elevated incidence of AMI in the 1 to 7 days before IPD detection and a nonsignificant but elevated incidence in the 8 to 28 days after IPD (Table 1).
The secondary analysis stratified by age (<65 and ≥65 years) produced results similar to those from the primary analysis. In the analysis stratified by AMI type, we observed a consistent increase in the incidence of AMI in the current IPD risk period compared with the control period for both STEMI and NSTEMI AMI, although the association was strongest for NSTEMI AMI (Table 2).
Table 2.
Secondary and Sensitivity Analyses for Acute Myocardial Infarction Episodes by Period Type, 2004–2019
| Sensitivity Analysis | No. of Patients | No. of AMI Episodes | Current IPD Risk Period vs Control Period | Pre-IPD Detection Period vs Control Period | ||
|---|---|---|---|---|---|---|
| Adjusted IRR | 95% CI | Adjusted IRR | 95% CI | |||
| Primary analysis | 324 | 338 | 92.95 | (72.17–119.71) | 10.29 | (6.33–16.73) |
| Age-stratified analysisa | ||||||
| <65 y | 141 | 149 | 94.90 | (66.72–134.97) | 10.55 | (5.08–21.90) |
| ≥65 y | 181 | 184 | 91.97 | (63.59–133.01) | 10.53 | (5.46–20.29) |
| AMI-stratified analysis | ||||||
| ST-elevation myocardial infarction | 30 | 30 | 49.51 | (19.42–126.22) | 13.10 | (3.67–46.80) |
| Non–ST-elevation myocardial infarction | 242 | 251 | 91.74 | (68.35–123.12) | 7.32 | (3.77–14.21) |
| Sensitivity analyses | ||||||
| Excluded if death ≤28 day after IPD specimen collection | 235 | 249 | 80.07 | (60.86–105.34) | 11.34 | (6.49–19.83) |
| Excluded if death any time during follow-up | 168 | 175 | 99.57 | (72.73–136.32) | 16.30 | (8.85–30.01) |
| Excluded if >1 AMI episode during follow-up | 310 | 310 | 97.14 | (73.61–128.19) | 10.83 | (6.52–17.99) |
| Including only pre-IPD detection period as reference | 324 | 338 | 151.47 | (109.16–210.18) | 16.13 | (9.63–27.03) |
| Including additional buffer period for day −1 and day 0 of specimen collection | 324 | 338 | 9.49b | (5.55–16.23) | 3.90c,d | (1.82–8.36) |
Abbreviations: AMI, acute myocardial infarction; CI, confidence interval; IPD, invasive pneumococcal disease; IRR, incidence rate ratio.
Includes only patients who were in age group throughout follow-up.
The current IPD risk period in this analysis included only day 1 to day 7 after IPD specimen collection date.
The pre-IPD detection period in this analysis included only day −7 to day −2 before IPD specimen collection date.
The adjusted IRR for the updated concurrent IPD buffered period (day −1 and 0 of specimen collection date) was 316.84 (95% CI: 248.85–403.40).
Results were consistent in our planned sensitivity analyses (Table 2), including the analysis using only the pre-IPD control period as the reference. In this analysis, the risk of AMI was significantly higher in the post-IPD control period (29 to 365 days after IPD specimen collection) compared with the pre-IPD control period (IRR, 2.95; 95% CI: 2.01–4.32). The results from the analyses excluding any patient who died within 28 days after IPD specimen collection or at any time during follow-up or those with more than 1 AMI were consistent with results from the primary analysis (Table 2). We observed a significantly higher incidence, albeit substantially closer to the null, in the current IPD (IRR, 9.49; 95% CI: 5.55–16.23) and pre-IPD detection period (IRR, 3.90; 95% CI: 1.82–8.36) compared with the control period when the period encompassing day −1 and day 0 prior to specimen collection was classified as a separate period.
DISCUSSION
We demonstrated a higher incidence of AMI-related hospitalizations during IPD episodes compared with control periods. More than 85% of IPD identified cases had a specimen collected on the admission date for the hospitalization during which an AMI was identified. A significantly elevated incidence of AMI-related hospitalizations was observed in the 1 to 7 days prior to the IPD specimen collection date and in the 8 to 28 days after IPD specimen collection, indicating an elevated risk of AMI diagnosis prior to laboratory confirmation of active IPD infection and an ongoing elevated risk of subsequent AMI in the intermediate and long-term follow-up period after IPD.
The literature supports a causal relationship between pneumonia and short-term cardiac risks involving several postulated mechanisms, including direct invasion of the myocardium during infection, formation of microscopic lesions due to infection of heart tissue, and destabilization of atherosclerotic plaques due to the inflammatory response or scar formation after antibiotic treatment among patients at high risk for cardiac events [2, 15]. These mechanisms are also plausible in IPD. Previous studies have also suggested that pneumolysin may play a role in myocardial infarction specific to S. pneumoniae infection [9, 16–19]. There is also evidence to suggest that myocardial injury can occur earlier than myocardial inflammation in acute experimental myocarditis, suggesting that injury can occur during infection without distinct clinical findings of myocarditis [20].
The biological plausibility of AMI induced by IPD is further supported by prior studies among patients with influenza and hospitalized with community-acquired pneumonia (CAP). One Ontario-based SCCS study examined the role of influenza infection on the risk of AMI and found that compared with control periods before and after influenza infection, the risk of AMI was 6.3 times higher in the first 1–3 days after infection and 5.8 times higher from day 4 to day 7 after infection, though no difference was observed after day 7 post-infection [21]. A meta-analysis of 24 studies examining the association between CAP and cardiac complications reported that 17.7% of patients hospitalized with CAP experienced cardiovascular complications, including heart failure, acute coronary syndromes, and cardiac arrhythmias [7]. In a study among 4988 hospitalized patients with CAP but without heart failure (2000–2002) who were matched to 23 060 adult patients without pneumonia or heart failure, the hazard of incident heart failure or death was 61% higher among patients with CAP compared with patients without (95% CI, 1.44–1.81) and 86% higher within 1 year [22]. Among 2 cohorts of hospitalized patients with pneumonia through 2010, the largest risk of AMI, stroke, or fatal coronary heart disease was observed in the first 30 days after pneumonia hospitalization (4.07 and 2.38 in each respective cohort), with elevated risks through 1 year after hospitalization [4]. While those studies have described the association between pneumonia and cardiovascular events, the etiology of pneumonia in those studies remains unclear, and whether specific pneumonia etiologies may directly increase the risk of subsequent cardiovascular events needs further study.
Prior studies specifically examining the association between IPD and AMI have reported rates of AMI during hospital admission among patients hospitalized with pneumococcal pneumonia (7.1%) and IPD (1.7%), with higher rates observed when considering all major adverse cardiovascular events [23–25]. In an SCCS study among patients aged ≥40 years with laboratory-confirmed respiratory pneumococcal or viral infections (not limited to hospitalizations) experiencing an AMI or stroke identified from the national health registry in Scotland (2002–2014; n = 1227 with AMI; 762 with stroke), the age- and seasonality-adjusted IRR for pneumococcal infections and AMI was 5.98 (95% CI, 2.47–14.4) in day 1–3 after infection and remained elevated through day 7 [8]. Yet, pneumococcal infections identified in these studies included sputum samples. In contrast, all IPD identified in our study was based on demonstration of bacterial invasion defined as isolation of pneumococci from sterile sites. Our findings were also consistent, though effect estimates were higher, with a similar study examining AMI and infections with S. pneumoniae (including but not limited to laboratory-confirmed IPD) in a nationally representative population from Denmark [26]. In our study, IPD cases were commonly identified in patients who required hospitalization, thus representing a population with more severe disease and requiring the use of different risk periods than the prior 2 studies. We do note that the effect estimates in this study were substantially closer to the null and more comparable to estimates from both prior studies when the day before and day of specimen collection were excluded from the current IPD risk interval. Furthermore, the current study included hospitalizations with an AMI diagnosis code listed in both the primary and secondary positions. Thus, although our findings are qualitatively consistent, the focus on only IPD as an outcome and choice of risk periods likely explains most of the difference in observed effect estimate magnitude.
Our work adds to the body of literature by demonstrating an association between AMI-related hospitalizations and laboratory-confirmed IPD. The timing of AMI was strongly centered around the time of invasive infection. Future work will be necessary to better understand the association between IPD and specific AMI types, as well as to identify the subgroups of patients with IPD and pneumococcal pneumonia at highest risk for cardiac events. Additional work should examine whether pneumococcal vaccination is protective against specific cardiac outcomes and whether different treatment strategies for infection might be associated with different risks of subsequent cardiac outcomes [15].
One important strength of our study was the identification of laboratory-confirmed IPD from the ABCs active surveillance system that limits concern of potential exposure misclassification. However, this highly specific outcome limited our ability to characterize the risk of AMI among patients with noninvasive or less severe S. pneumoniae infections or other infections that may not have been detected (eg, if cultures were not obtained or if prior antibiotic use precluded bacterial isolation). Although we identified each patient's earliest hospitalization for AMI during the study period and used coding algorithms used in prior work examining hospitalizations for AMI from observational data, we cannot rule out the possibility of ascertainment bias from hospitalizations for AMI representing comorbid conditions rather than incident AMI due to the inclusion of AMI diagnoses in secondary coding positions for the outcome definition. We do not suspect ascertainment bias related to increased detection of IPD related to the AMI hospitalization though, as the majority of IPD cases reported to the ABCs Surveillance System represent specimen collections at the onset of hospitalization for IPD. Additionally, we recognize the potential for reverse causation in our study (ie, AMI hospitalization increases the risk of hospital-acquired IPD) but expect it to be unlikely to explain the observed association as nearly all IPD cases reported to the ABCs surveillance system in the current IPD risk period had a positive specimen identified on the same day of admission or day 1 after admission for the AMI-related hospitalization. Furthermore, we identified confirmed IPD cases and AMI hospitalizations among a large, representative population in the state of Tennessee not limited to patients from a single insurance payor or those receiving care within a single hospital healthcare system, thus improving the generalizability of our findings. However, the specific relative IRRs may differ in other settings with different underlying burdens of comorbidities and cardiac risk. The use of the SCCS design allowed us to account for person-specific measurable and unmeasurable factors, though we were unable to examine stratified analyses based on IPD treatment type and pneumococcal vaccination status, as these data were not available.
CONCLUSIONS
Laboratory-confirmed IPD is associated with a greater incidence of hospitalizations with diagnosed AMI. Since IPD is vaccine-preventable, effective vaccination programs that prevent IPD may also reduce the risk of major acute cardiovascular events.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Andrew D Wiese, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Ed Mitchel, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Danielle Ndi, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Tiffanie M Markus, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
H Keipp Talbot, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
William Schaffner, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Carlos G Grijalva, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Veteran Affairs TN Valley Health Care System, Nashville, TN, USA.
Notes
Acknowledgments. We are indebted to the Tennessee Department of Health for providing data for the study.
Disclaimer. A. D. W. had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Financial support. The study was supported by the National Institute on Drug Abuse (A. D. W., K01DA051683), the National Institutes of Health (NIH)—National Institute on Aging (C. G. G., R01AG043471), National Institute for Allergy and Infectious Diseases (C. G. G., K24AI148459), and the Centers for Disease Control and Prevention (CDC; H. K. T. and W. S., 1U50CK000491—Emerging Infections Program).
References
- 1. Mechanic O, Gavin M, Grossman S. Acute myocardial infarction. StatPearls, Treasure Island, FL: StatPearls Publishing, 2022. [Google Scholar]
- 2. Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med 2019; 380:171–6. [DOI] [PubMed] [Google Scholar]
- 3. Bello S, Mincholé E, Fandos S, et al. . Inflammatory response in mixed viral-bacterial community-acquired pneumonia. BMC Pulm Med 2014; 14:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. . Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015; 313:264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation 2012; 125:773–81. [DOI] [PubMed] [Google Scholar]
- 6. Corrales-Medina VF, Fatemi O, Serpa J, et al. . The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis 2009; 41(6–7):511–4. [DOI] [PubMed] [Google Scholar]
- 7. Corrales-Medina VF, Suh KN, Rose G, et al. . Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med 2011; 8:e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51:1701794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brissac T, Shenoy AT, Patterson LA, Orihuela CJ. Cell invasion and pyruvate oxidase-derived H(2)O(2) are critical for Streptococcus pneumoniae-mediated cardiomyocyte killing. Infect Immun 2018; 86:e00569–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ciszewski A. Cardioprotective effect of influenza and pneumococcal vaccination in patients with cardiovascular diseases. Vaccine 2018; 36:202–6. [DOI] [PubMed] [Google Scholar]
- 11. Moore MR, Whitney CG. Use of pneumococcal disease epidemiology to set policy and prevent disease during 20 years of the emerging infections program. Emerg Infect Dis 2015; 21:1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langley G, Schaffner W, Farley MM, et al. . Twenty years of active bacterial core surveillance. Emerg Infect Dis 2015; 21:1520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J 2002; 144:290–6. [DOI] [PubMed] [Google Scholar]
- 14. Wei KC, Sy CL, Wang WH, Wu CL, Chang SH, Huang YT. Major acute cardiovascular events after dengue infection—A population-based observational study. PLoS Negl Trop Dis 2022; 16:e0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brack MC, Lienau J, Kuebler WM, Witzenrath M. Cardiovascular sequelae of pneumonia. Curr Opin Pulm Med 2019; 25:257–62. [DOI] [PubMed] [Google Scholar]
- 16. Brown AO, Mann B, Gao G, et al. . Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog 2014; 10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson R, Nel JG, Feldman C. Multifaceted role of pneumolysin in the pathogenesis of myocardial injury in community-acquired pneumonia. Int J Mol Sci 2018; 19:1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shenoy AT, Beno SM, Brissac T, Bell JW, Novak L, Orihuela CJ. Severity and properties of cardiac damage caused by Streptococcus pneumoniae are strain dependent. PLoS One 2018; 13:e0204032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pavasini R, Fabbri G, Marchini F, et al. . Procalcitonin predicts bacterial infection, but not long-term occurrence of adverse events in patients with acute coronary syndrome. J Clin Med 2022; 11:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim BK, Shin JO, Choe SC, et al. . Myocardial injury occurs earlier than myocardial inflammation in acute experimental viral myocarditis. Exp Mol Med 2005; 37:51–7. [DOI] [PubMed] [Google Scholar]
- 21. Kwong JC, Schwartz KL, Campitelli MA, et al. . Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 2018; 378:345–53. [DOI] [PubMed] [Google Scholar]
- 22. Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up. BMJ 2017; 356:j413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Effect of age on the manifestations and outcomes of invasive pneumococcal disease in adults. Am J Med 2018; 131:100.e1–.e7. [DOI] [PubMed] [Google Scholar]
- 24. Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis 2007; 45:158–65. [DOI] [PubMed] [Google Scholar]
- 25. Africano HF, Serrano-Mayorga CC, Ramirez-Valbuena PC, et al. . Major adverse cardiovascular events during invasive pneumococcal disease are serotype dependent. Clin Infect Dis 2021; 72:e711–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohland J, Warren-Gash C, Blackburn R, et al. . Acute myocardial infarctions and stroke triggered by laboratory-confirmed respiratory infections in Denmark, 2010 to 2016. Euro Surveill 2020; 25:1900199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.