Abstract
Background
Development of a prediction model using baseline characteristics of tuberculosis (TB) patients at the time of diagnosis will aid us in early identification of the high-risk groups and devise pertinent strategies accordingly. Hence, we did this study to develop a prognostic-scoring model for predicting the death among newly diagnosed drug sensitive pulmonary TB patients in South India.
Methods
We undertook a longitudinal analysis of cohort data under the Regional Prospective Observational Research for Tuberculosis India consortium. Multivariable cox regression using the stepwise backward elimination procedure was used to select variables for the model building and the nomogram-scoring system was developed with the final selected model.
Results
In total, 54 (4.6%) out of the 1181 patients had died during the 1-year follow-up period. The TB mortality rate was 0.20 per 1000 person-days. Eight variables (age, gender, functional limitation, anemia, leukopenia, thrombocytopenia, diabetes, neutrophil–lymphocyte ratio) were selected and a nomogram was built using these variables. The discriminatory power was 0.81 (95% confidence interval: 0.75–0.86) and this model was well-calibrated. Decision curve analysis showed that the model is beneficial at a threshold probability ~15–65%.
Conclusions
This scoring system could help the clinicians and policy makers to devise targeted interventions and in turn reduce the TB mortality in India.
Keywords: India, mortality, nomograms, prognosis, tuberculosis
Introduction
Although significant progress has been achieved in disease control activities, tuberculosis (TB) continues to be a global public health threat.1 According to the Global TB report 2020, 14 million people had access to adequate TB care in 2018 and 2019, a rise from 6.4 million in 2017. There has also been a substantial drop in TB-related deaths in 2018, compared with 2017. Despite the furtherance, the numbers fall far short of what is needed to reach the target of End TB strategy. With over 10 million people falling ill in 2019 for TB, it still stands as one of the top infectious killer diseases worldwide.2
Ever since the establishment of the Revised National Tuberculosis Control Program (RNTCP), now National TB Elimination Program (NTEP), India has ensured better planning, implementation and evaluation of TB prevention, diagnosis and treatment services. Along with advancements in diagnostics (Xpert MTB/RIF), efforts to improve the compliance such as fixed dose combination regimens and newer monitoring mechanisms like 99-DOTS, has considerably reduced the mortality rate among the TB patients.3 However, the program still faces several challenges such as lack of funding, poor access to health resources (infrastructure, testing facilities, drug availability), stigmatization, poverty and lack of compliance.3 Early identification, proper treatment and follow-up can aid us in progressing our steps toward achieving end TB targets by 2035.4–6 Although the NTEP has expanded TB treatment coverage over years, very little progress has been made in improving the success of TB treatment outcomes.7
Previous studies have identified several sociodemographic, behavioral, anthropometric and hematological factors as potential predictors of mortality during TB treatment.8–10 Older age, previous TB treatment, smoking, alcohol use, comorbidities such as HIV, diabetes mellitus (DM), congestive heart failure, liver cirrhosis, hematological abnormalities such as anemia, high neutrophil–lymphocyte ratio (NLR) have been shown to be associated with increased mortality among TB patients during and post-treatment.9–11 The extent to which these predictors impact TB deaths is largely region specific.8,10,11 Several studies exploring models for predicting multidrug resistance, disease transmission among household contacts and treatment failure are available from varied study settings.4,12–14
Nevertheless, work supporting the development of a prediction model for examining the mortality for drug-sensitive TB patients has not previously been attempted in an Indian setting. Furthermore, developing a prediction model using baseline sociodemographic, behavioral, anthropometric and hematological characteristics will aid us in early identification of the high-risk groups and devise pertinent strategies to reduce the risk of death among newly diagnosed TB patients. Hence, we aimed to develop a statistical prediction model to predict the probability of death within 1-year of TB diagnosis among newly diagnosed drug sensitive pulmonary TB patients in Puducherry and Tamil Nadu, South India.
Methods
Study setting and study population
Data was obtained from an ongoing longitudinal study conducted under the Regional Prospective Observational Research for Tuberculosis (RePORT)-International consortium.15 It embodies regional observational cohorts in the following sites: India, China, Brazil, Philippines, South Africa and Indonesia.15 In India, five teams are participating in data and specimen collection process. Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) along with the Boston Medical Center and New Jersey Medical University-Rutgers University is one of these teams that has developed two set of prospective observational cohorts. The first cohort with active TB patients recruited from 2014 to 2018 were included in our analysis.
TB patients were recruited from the tuberculosis units (TU) of the following three districts in South India: (i) Pondicherry—one TU (Puducherry), (ii) Villupuram—two TUs (Tamil Nadu) and (iii) Cuddalore—two TUs (Tamil Nadu). TU acts a central point of data collection, whereas the designated microscopy center and peripheral health institutions are responsible for diagnosing the cases and service delivery. The eligibility criteria for inclusion into the study was newly diagnosed sputum smear-positive TB patients aged ≥6 years. The patients with who had previous history of TB, who were on treatment for 1 week or more and those with multidrug-resistant TB were excluded as per RePORT study protocol. The detailed study protocol has been described previously.16,17
Study procedure
Ethical approval was obtained from the JIPMER Scientific Advisory Committee and Institutional Ethics Committee of JIPMER, and Institutional Review Boards at New Jersey Medical University-Rutgers University and Boston Medical Center. Data collection was initiated after obtaining informed written consent from adult participants (≥18 years) and assent form in addition to parents’ consent from participants < 18 years. Pretested semi-structured questionnaire was used to gather the sociodemographic details of the participants including age (elderly defined as ≥60 years), gender, occupation (employed/unemployed), marital status (never-married/married/separated/widowed/divorced), Bacille Calmette–Guerin (BCG) vaccination history, behavioral characteristics such as self-reported smoking and alcohol use and anthropometric measurements such as height, weight, body mass index (BMI). Karnofsky score was assessed to determine the functional status of the patients at the time of recruitment into the study. We also obtained details related to DM, which was defined as random blood sugar > 200 mg/dl or self-reported DM. Hematological assessment was performed to identify anemia, low white blood cell (WBC) count, low platelet count and high NLR.
After the baseline assessment, the patients were followed-up at the end of intensive phase (2 months after treatment initiation) and continuation phase (6 months after treatment initiation) to check for unfavorable treatment outcomes such as treatment failure (bacteriological and clinical), treatment not complete, lost to follow-up and death. Then, the patients were further followed-up monthly till the end of 1-year follow-up period after TB diagnosis to assess the adverse treatment outcomes including death. In total, 1274 patients were recruited into the cohort during the study period. Out of these, data from 1185 patients were used in this model, as the rest of the patients had missing information among the included variables and outcomes.
Operational definitions
Underweight
BMI was used to classify the study participants as underweight (≤18.50 kg/m2) based on Asia-Pacific guidelines.18
Karnofsky score
A score ≥ 80 was considered as normal, whereas score <80 were considered to have some form of functional impairment.19
Anemia
Participants with hemoglobin count <12 g/dl among females and <13 g/dl among males were considered as having anemia.20
Leukopenia
Participants with WBC count <4000 cells/μml were considered as having leukopenia.21
Thrombocytopenia
Participants with platelet count <150 000 cells/μml were considered as having thrombocytopenia.22
High NLR
Participants with NLR ≥5 were considered as having high NLR.23
One-year mortality
Participants who have died of any cause from the date of diagnosis (T0) till the end of 1-year follow-up period (T365).
Statistical analysis
All analyses were performed using STATA software version 14.2 (College Station, TX: StataCorp LP). Descriptive analysis was performed by summarizing the continuous variables as mean and standard deviation (SD) and categorical variables as proportions. Bivariate analysis was performed using chi-square test for all the baseline characteristics with the 1-year mortality outcome. The entire dataset was randomly split into training and validation set at 8:2 ratio. We developed the prediction model for identifying the patients with risk of 1-year mortality among the newly diagnosed cases using training set. The model was built using the following 14 variables: age, gender, occupation, marital status, BCG vaccination, Karnofsky score, BMI status, smoking, alcohol use, DM, anemia, leukopenia, thrombocytopenia, high NLR, as these variables were presumed to be ones that would be easily available to the NTEP.
Since we had a time-to-event outcome, we performed survival analysis to develop our prediction model. Measurement of survival time was in days between the date of TB diagnosis (T0) and the date of end of the follow-up (T365) or the event of interest (death). Participants who were lost to follow-up before the end of 1-year were ‘censored’ till their last seen time. First, we declared the dataset as survival data using ‘stset’ command package. Then, multivariable cox regression analysis was performed using the stepwise backward elimination procedure to select the variables for the model building with an entry P-value of 0.20 and stay P-value of 0.10. We have set a higher stay P-value threshold to give priority for clinical reasoning in the selection of variables along with the statistical significance.24 In the final selected model, proportional hazards assumption was checked using the statistical test and graphical plot diagnostics based on the scaled Schoenfeld residuals. P-value >0.05 in Schoenfeld test (global and individual) and random pattern of the residuals over time in the plot is indicative of satisfaction of proportional hazards assumption.25 Prediction of mortality by each variable in the final model were interpreted as adjusted hazard ratio (aHR) with 95% confidence interval (CI).
We constructed a nomogram-scoring system to present the final selected model.26 Receiver Operator Characteristic (ROC) analysis was performed to estimate the area under the curve or c-statistic and determine the optimal cut-off of our scoring system. Log rank test and Kaplan–Meier plot was performed to determine the difference in survival probability between the patients having higher or lower scores based on the obtained optimal cut-off. ROC analysis was also used to explain the discriminatory capacity of the prediction model (how well the model can differentiate between the TB patients who died and those who survived at the end of 1 year). Calibration of the model was performed using ‘stcoxcal’ command package. Calibration plot was made to check whether the model calibrates well and no significant deviation from 450 line of perfect fit. The slope, intercept and joint slope and intercept was tested against null hypothesis (i.e. intercept equals 0, slope equals 1), providing evidence for the linear calibration. P value >0.05 is indicative of adequate calibration.27 Both discrimination and calibration analysis were re-run with validation set. Decision curve analysis was performed to quantify the net benefits at various threshold probabilities in our study cohort, which in turn determines the clinical usefulness of the final predictor model. The net benefit was estimated by subtracting percentage of participants with the false-positive results with the percentage of participants with the true-positive results and by weighing hazard ratio of the prediction model compared with the adverse effects of an unnecessary application of the prediction model.28
Population attributable fraction (PAF) was calculated for the modifiable risk factors in the selected model such as anemia, leukopenia, thrombocytopenia and functional limitation. Two kinds of PAF were calculated. First, PAF+1 indicates PAF for each of the risk factors, assuming that every participant in the study changes only one of the above-mentioned modifiable risk factors from ‘baseline scenario’ (observed exposure rate) to ‘fantasy scenario’ (all the participants do not have exposure to either anemia or leukopenia or thrombocytopenia or functional limitation). Second, PAFALL indicates the total modifiable PAF, assuming that every participant in the study changes all the modifiable risk factors from baseline to fantasy scenario. The analysis of PAF was performed using the ‘punafcc’ postestimation command package following the final cox regression model.29
Results
Mean (SD) age of the study population was 43.9 (14.6) years (Table 1). Out of the 1181 patients, 54 (4.6%; 95%CI: 3.4–5.9%) had died during the 1-year follow-up period. Total time at risk was 270 425 days. The rate of TB mortality was 0.20 deaths per 1000 person days (or) 72.8 deaths per 1000 person-years.
Table 1.
Sociodemographic, behavioral, anthropometric, hematological characteristics and treatment outcomes of the study participants (N = 1181)
| Sl no. | Characteristics | Frequency | Death in each subgroup |
|---|---|---|---|
| n (%) a | n (%) b | ||
| 1 | Age categories, in years | ||
| <60 | 998 (84.5) | 37 (3.7) | |
| ≥60 | 183 (15.5) | 17 (9.3) | |
| 2 | Gender | ||
| Female | 261 (22.1) | 3 (1.1) | |
| Male | 920 (77.9) | 51 (5.5) | |
| 3 | BMI category c | ||
| Underweight (<18.50) | 720 (61.0) | 44 (6.1) | |
| Normal (18.50–22.99) | 350 (29.6) | 10 (2.9) | |
| Overweight (23.00–24.99) | 63 (5.3) | 0 (0.0) | |
| Obesity (≥25.00) | 48 (4.1) | 0 (0.0) | |
| 4 | Marital status | ||
| Currently married | 855 (72.4) | 39 (4.6) | |
| Never married | 213 (18.0) | 6 (2.8) | |
| Widowed/Divorced/Separated | 113 (9.6) | 9 (8.0) | |
| 5 | Employment status | ||
| Unemployed/Student/Housewife | 296 (25.1) | 13 (4.4) | |
| Employed | 885 (74.9) | 41 (4.6) | |
| 6 | Smoking status d | ||
| Nonsmoker | 887 (75.1) | 33 (3.7) | |
| Smoker | 294 (24.9) | 21 (7.1) | |
| 7 | Alcohol use d | ||
| Alcohol user | 691 (58.5) | 42 (6.1) | |
| Nondrinker | 490 (41.5) | 12 (2.4) | |
| 8 | Karnofsky d | ||
| Able to carry normal activity and work | 339 (28.7) | 3 (0.9) | |
| Unable to work, able to live at home | 842 (71.3) | 51 (6.1) | |
| 9 | DM status | ||
| Present | 367 (31.1) | 8 (2.2) | |
| Absent | 814 (68.9) | 46 (5.6) | |
| 10 | BCG vaccination history | ||
| Present | 1054 (89.3) | 51 (4.8) | |
| Absent | 127 (10.7) | 3 (2.4) | |
| 11 | Anemia e | ||
| Present | 830 (70.3) | 48 (5.8) | |
| Absent | 351 (29.7) | 6 (1.7) | |
| 12 | Leukopenia (WBC < 4000 cells/μml) | ||
| Present | 30 (2.5) | 4 (13.3) | |
| Absent | 1,151 (97.5) | 50 (4.3) | |
| 13 | Thrombocytopenia (platelet < 150 000 cells/μml) | ||
| Present | 18 (1.5) | 3 (16.7) | |
| Absent | 1,163 (98.5) | 51 (4.4) | |
| 14 | NLR (≥5) | ||
| Present | 523 (44.3) | 41 (7.8) | |
| Absent | 658 (55.7) | 13 (2.0) | |
| 15 | Died within the 1-year follow-up period (total) | ||
| Yes | 54 (4.6) | ||
| No | 1,127 (95.4) | ||
Column percentage;
Row percentage;
Asia-Pacific guidelines for obesity;
Self-reported by the participants;
Hemoglobin <12 g/dl (females) and <13 g/dl (males)
Development of predictor model
Stepwise backward cox-regression model in the training set has selected eight variables collected during the baseline assessment (age group, gender, Karnofsky score, DM status, anemia, leukopenia, thrombocytopenia and high NLR) and the final model was run with these variables (Table 2).
Table 2.
Multivariable Cox regression developed from prediction model using the stepwise backward technique among the study participants
| Characteristic | aHR | SE | 95% CI | P-value | |
|---|---|---|---|---|---|
| 1 | Age category | ||||
| <60 | (Ref.) | ||||
| ≥60 | 2.19 | 0.66 | 1.22–3.95 | 0.009 | |
| 2 | Gender | ||||
| Female | (Ref.) | ||||
| Male | 4.36 | 2.61 | 1.34–14.11 | 0.01 | |
| 3 | Karnofsky | ||||
| Able to carry normal activity and work | (Ref.) | ||||
| Unable to work, able to live at home | 4.48 | 2.69 | 1.38–14.52 | 0.01 | |
| 4 | DM status | ||||
| Absent | (Ref.) | ||||
| Present | 0.42 | 0.16 | 0.20–0.90 | 0.02 | |
| 5 | Anemia | ||||
| Present | 2.30 | 1.01 | 0.97–5.44 | 0.06 | |
| Absent | (Ref.) | ||||
| 6 | Leukopenia | ||||
| Present | 3.54 | 1.87 | 1.25–9.99 | 0.02 | |
| Absent | (Ref.) | ||||
| 7 | Thrombocytopenia | ||||
| Present | 3.32 | 1.99 | 1.02–10.80 | 0.04 | |
| Absent | (Ref.) | ||||
| 8 | High NLR | ||||
| Present | 3.18 | 1.04 | 1.68–6.04 | <0.001 | |
| Absent | (Ref.) |
Effect measurements—aHR with its standard error (SE), 95% CI and corresponding P-value—from predictive model. Bold values indicate P-value is statistically significant.
Global and local Schoenfeld test showed nonsignificant P values (>0.05) and the plot showed a random pattern of residuals for all the variables indicating the satisfaction of proportional hazard (PH) assumption (Supplementary Fig. S1).
Nomogram-scoring system
The prediction model was presented as a nomogram (Fig. 1), which could be used conveniently to predict the 1-year mortality.
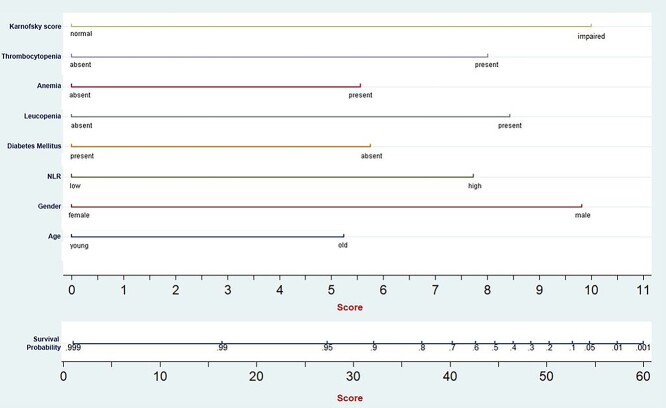
Fig. 1.
Nomogram for predicting survival probability among TB patients.
Each of the eight variables included in predictive models were arranged one-by-one on a horizontal plane with its scoring system, ranging from 0 to 10, at the bottom. Gender, Karnofsky score, leukopenia and thrombocytopenia had the widest range of individual scores. The overall score can then be obtained by the summation of these individual scores, can then be used against the total score axis. Total score ranged from 0 to 60 and each of the components had following scores (in descending order):
Functional impairment (Karnofsky score)–10 points,
Male gender–9.7 points,
Leukopenia–8.4 points,
Thrombocytopenia–8 points.
High NLR–7.6 points,
Absence of DM–5.6 points.
Anemia–5.5 points,
Elderly age group–5.2 points,
The optimal cut-off for this scoring system was found to be 32 points. The rate of TB mortality was 0.51 deaths per 1000 person days (or) 186.1 deaths per 1000 person-years among patients with score ≥ 32 points, whereas the patients with score < 32 points had TB mortality rate of 0.06 deaths per 1000 person days (or) 21.9 deaths per 1000 person years (Supplementary Fig. S2) and this difference was statistically significant. This indicates that the TB patients having score ≥ 32 points at the start of treatment have 7.6-times higher rate of having 1-year mortality compared with patients with score <32 points.
Discrimination and calibration
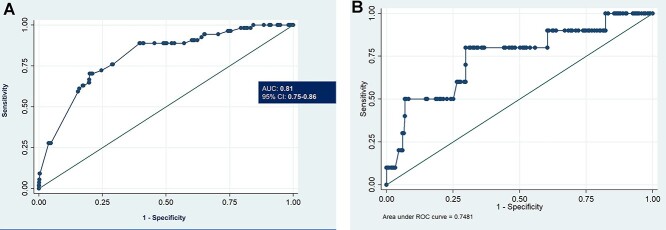
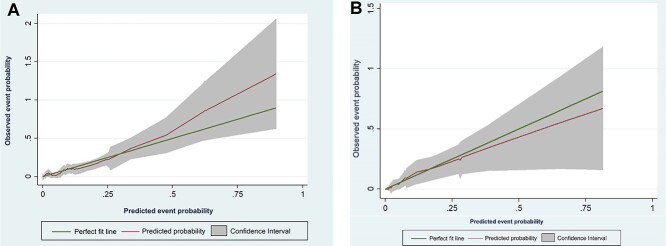
The discriminatory power (c-index) of the predictor model for unfavorable outcomes was 0.81 (95%CI: 0.75–0.86) (Fig. 2A). The validation set had c-index of 0.75 indicating good discriminatory capacity of the model (Fig. 2B). Calibration plot for the proposed model in training and validation set was depicted in the Figure 3A and B. The test for slope, intercept and joint slope and intercept had P-value >0.05 in the final model and validation set indicating that there was no significant departure from a perfect fit between the observed and predicted probability of events.
Fig. 2.
ROC curve for predicting survival using the model developed from the study cohort. (A) Final model. (B) Validation set.
Fig. 3.
Calibration of the predicted model for survival among TB patients. (A) Final model (B) Validation set.
Decision curve analysis
Figure 4 shows decision curve analysis for the model. It showed that the model is beneficial at a threshold probability ~15–65% than either treat-none or treat-all strategies. All the variables in the prediction model are already obtained during the baseline assessment of TB patients. There is no additional cost or risk involved in obtaining any of the information in the model. Hence, the model can be beneficial for application in clinical setting for better decision making.
Fig. 4.
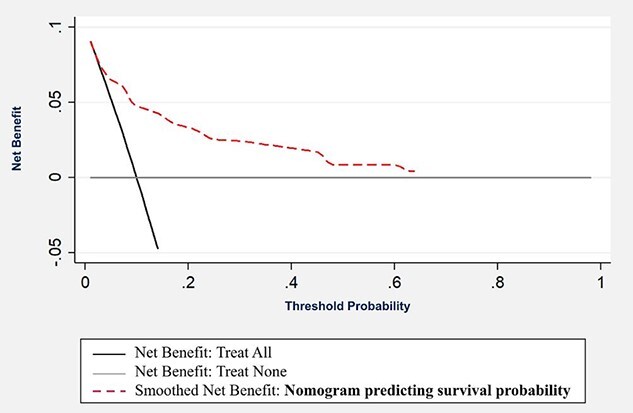
Decision curve analysis for the predicted model.
PAF
PAF+1 estimate was highest for functional limitation (73.3%) indicating that 73.3% of the observed mortality was attributable to the functional limitations among the newly diagnosed TB patients in our study followed by anemia (50.2%), leukopenia (5.3%) and thrombocytopenia (3.9%). PAFALL for total modifiable risk factors was 84.5% indicating that more than four in five of the observed 1-year mortality was attributable to the four modifiable risk factors in our study population.
Discussion
Main findings of this study
We conducted this study as a public health and clinical contribution to the low–middle-income countries with an easy-to-apply and inexpensive prediction tool using baseline characteristics, to identify the TB patients with higher likelihood of dying within 1-year of diagnosis. Hence, our study explored the potential socioeconomic, behavioral, anthropometric, hematological characteristics as predictors of 1-year mortality among TB patients in Puducherry and Tamil Nadu. Of the 1181 patients, 4.6% had died during the 1-year follow-up period, and the TB mortality rate was 0.20 deaths per 1000 person-days. In the present study, we found that elderly, male gender, functional impairment, anemia, leukopenia, thrombocytopenia, absence of DM and high NLR were predictors of 1-year mortality.
What is already known
Our study had a lower mortality rate compared with previous studies in South India,30 but it is far higher than the World Health Organization or the NTEP targets. The National Strategic Plan (NSP) 2017–25 has put forth aggressive targets toward TB elimination in India that emphasizes a four-pronged approach (Detect-Treat-Prevent-Build).31 This calls for a need to understand the predictors for mortality among the pulmonary TB cohort, so that focused interventions can be delivered. A successful TB treatment outcome and survival in any country is a factor of patient characteristics, effectiveness and coverage of treatment programs.
The factors identified in our study were supported by several studies in varied study settings.23,32,33 Mortality among TB patients are influenced by several sociodemographic, behavioral, clinical and genetic characteristics. The effect of sociodemographic and behavioral influence on the treatment is a complex phenomenon.34 These factors, by interacting with each other, finally affect the outcome directly or indirectly.35,36 We also had a contradictory finding that the presence of DM is protective from TB mortality. However, similar protective finding or no association between DM and mortality among TB patients was found in previous studies.37–39 Hence, there is a need for further investigation exploring this possible protective effect of DM.
We tried to explore the mechanism behind biological plausibility of the role of hematological abnormalities in the prediction of TB mortality based on previous literature. First, anemia was identified as a predictor for TB mortality, which was consistent with the previous study findings.40–43 Possible mechanisms could be the consequence of iron redistribution that are known to occur during the anemia of inflammation. The loading and sequestration of the iron in the macrophages where the TB resides and replicates may both facilitate the acquisition of iron required for the growth and inhibit the cellular defense systems.44–46 This promotes a shift from the Th1 to Th2 cytokine responses, reducing cytotoxic activity of the macrophages, preventing the interferon gamma-mediated defense mechanism and ultimately blocking the nitric oxide-dependent bactericidal activity.47,48 Next, leukopenia and high NLR was identified as a predictor for TB mortality, which was also reported by previous evidences.49 In TB context, WBC count is mainly reflective of the neutrophil count as the marker of persistent inflammation or the failure to clear bacteria.50 Sustained inflammation has been expressed as impairment of TB-specific immune responses and marker for active disease.51 Previous studies have also highlighted the role of higher neutrophils, lower lymphocytes, in the severity of TB condition.52,53 Therefore, the hypothesis in our cohort that the patients with lower baseline WBC and higher NLR have higher risk of mortality holds. Finally, thrombocytopenia was identified as a predictor, which was also reported by previous evidences.54 The possible mechanism behind this factor could be the accumulation of platelets in the pulmonary lesions, thereby inhibiting the T-cell responses and replication of TB in macrophages.55 Hence, lower levels of platelets can cause adverse clinical outcomes in TB patients.
What this study adds
The model, through the nomogram, will help the policy makers in addressing challenges with regard to the modifiable predictors identified like, functional limitation (highest attributable fraction) and low blood counts. Removal of these modifiable risk factors has shown to reduce the 1-year mortality by more than three-fourth among our study population. Functional rehabilitation to limit the extent of functional impairment and various nutrition improvement strategies to improve the blood counts can be provided to these target groups. Hence, steps to tackle the burden of these issues (preferably, community-based approach) has to work hand-in-hand with the clinical follow-up of high probability mortality risk TB patients.
Almost all the predictors identified in our study are routinely collected during the NTEP except the assessment of functional impairment. The nomogram created in our study will, thus, help the clinicians in identifying the probability of survival from the baseline characteristics of the patient at the diagnostic stage of the TB care cascade itself. Presence of certain vital sociodemographic, behavioral and hematological predictors at the start treatment might alarm the clinicians to have a tailored follow-up, thereby decreasing such adverse outcome later. Therefore, similar accurate prognosis assessment using such nomograms can help doctors identify those patients who might have higher chances of death in the later part of treatment follow-up. A checklist can be created under the NTEP using the nomogram of our study. This checklist can be applied to the patient at the point of diagnosis itself, and patients scoring high on this checklist should undergo active monitoring and close follow-up. The developed nomogram can be tested and used in the TB clinics around the country for further fine tuning of the scoring system and the predicted probabilities. The same can be used in raising awareness among the general population. External validation of the model in a different cohort is necessary for the approval of this model application in the clinical practice.
In addition, future research should focus on what needs to be done for TB patients scoring high in any severity scoring systems (including our nomogram). One intervention that has been broadly used for patients suspected to develop severe condition or at risk of mortality is the corticosteroids.56 The use of broad-acting corticosteroids such as prednisone or dexamethasone has been found to bring about a balance between insufficient inflammation and immunopathology (major factor determining the TB severity and deaths).57 Previous meta-analysis conducted using 41 trials have also shown that use of steroids has reduced the TB mortality by 17%.58 Several other host-directed therapies are also under development, and such research needs to be intensified as the identification of high-risk patients alone will not reduce the mortality rate without having an effective intervention for these target group patients.56
Strengths and limitations of the study
Our study is one among the very few studies that has devised a prediction model to foresee mortality risk in newly diagnosed TB patients from India. The strength of our model is that the many of these factors are already being collected routinely by the program as compared with other microbiological, pharmacological or genetic approaches to predict such adverse treatment outcomes. Thus, this prediction model is a major advantage to the resource-constrained settings as it is cheap and the data is widely available.
Our study has certain limitations. First, prediction of mortality risk in TB needs to account for regional variations in TB epidemiology for generalizability. Hence, determining if this model is applicable to other regions requires further evaluation. Secondly, we included only certain sociodemographic, behavioral and hematological characteristics into the model. Some clinical variables including radiological findings, drug resistance, delay in initiation of treatment and comorbidities were not included in the analysis due to limitation in the availability of data during the study period. In addition, our study sample was dominated by the employed, middle-aged, males, with a heavy burden of comorbidities like DM and anemia. Patients with Karnofsky score <10 (i.e. moribund patients) were excluded from the study sample. These factors might have influenced the final nomogram model. Third, although the predictive performance was internally validated within the cohort, external validation is not done due to limitation in the data availability. However, since this is an ongoing research program, we will try to apply this model in a later cohort and externally validate the model. Finally, we have excluded nearly 90 participants from the total sample as they had missing information among predictor variables and outcome. However, there was no significant difference in terms of the baseline characteristics among these excluded sample.
Authors’ contributions
Conceived and designed the study: YK, KE and SM; data management and extraction: KE; analyzed the data and wrote the paper: YK, KE, SM, SR, MGM; provided comments and inputs to revise the manuscript: SPB, SS, SL, NMJ, GS, CH, NH, EJ, SK, SRP, PS, GR, JE.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the work done by the field and office staff of the Indo-US TB project under the Department of Preventive & Social Medicine, JIPMER. The authors would like to extend heartfelt gratitude to each of the participant from the study sites.
Yuvaraj Krishnamoorthy, Senior Resident
Komala Ezhumalai, Project Scientist I
Sharan Murali, Senior Resident
Sathish Rajaa, Senior Resident
Marie Gilbert Majella, Senior Resident
Sonali Sarkar, Additional Professor & Head
Subitha Lakshminarayanan, Associate Professor
Noyal Mariya Joseph, Associate Professor
Govindarajan Soundappan, State Tuberculosis Officer
Senbagavalli Prakash Babu, Program Manager
Charles Robert Horsburgh, Professor
Natasha Hochberg, Associate Professor
W. Evan Johnson, Associate Professor
Selby Knudsen, Research Coordinator
Sri Ram Pentakota, Research Associate
Padmini Salgame, Professor
Gautam Roy, Professor
Jerrold Ellner, Director and Professor
Contributor Information
Yuvaraj Krishnamoorthy, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Komala Ezhumalai, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Sharan Murali, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Sathish Rajaa, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Marie Gilbert Majella, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Sonali Sarkar, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Subitha Lakshminarayanan, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Noyal Mariya Joseph, Department of Microbiology, JIPMER, Puducherry 605 006, India.
Govindarajan Soundappan, State TB Cell, Directorate of Health Services, Puducherry 605001, India.
Senbagavalli Prakash Babu, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Charles Horsburgh, Department of Epidemiology, Boston University School of Public Health, Boston, MA 02118, USA.
Natasha Hochberg, Department of Medicine, Section of Infectious Diseases, Boston University School of Medicine, Boston, MA 02118, USA.
W Evan Johnson, Department of Medicine and Biostatistics, Boston University School of Medicine, Boston, MA 02118, USA.
Selby Knudsen, Department of Medicine, Section of Infectious Diseases, Boston University School of Medicine, Boston, MA 02118, USA.
Sri Ram Pentakota, Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey 07103, USA.
Padmini Salgame, Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey 07103, USA.
Gautam Roy, Department of Preventive & Social Medicine, JIPMER, Puducherry 605 006, India.
Jerrold Ellner, Department of Medicine, Rutgers New Jersey Medical School, Newark, New Jersey 07103, USA.
Funding
This work was supported by in whole or in part with Federal funds from the Government of India’s (GOI) Department of Biotechnology (DBT), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR) and distributed in part by CRDF Global. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the NIH or CRDF Global. Any mention of trade names, commercial projects or organizations does not imply endorsement by any of the sponsoring organizations. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Written consent or assent in addition to parents’ consent (in case of participant <18 years) was obtained from all participants enrolled in the study. The study protocol was approved by the Institute Ethics Committee and Scientific Advisory Committee of JIPMER, and the Institutional Review Boards at Boston University Medical Campus and Rutgers-New Jersey Medical School.
Consent for publication
The authors declare that they have no consent for publication.
Availability of data and materials
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. World Health Organization [WHO] . Global Tuberculosis Report 2017. Geneva, 2017, 26 October 2020, date last accessed. [Google Scholar]
- 2. World Health Organization [WHO] . Global Tuberculosis Report 2020. Geneva, 2020, 26 October 2020, date last accessed. [Google Scholar]
- 3. Pai M, Bhaumik S, Bhuyan SS. India’s plan to eliminate tuberculosis by 2025: converting rhetoric into reality. BMJ Glob Health 2017;2:e000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauer CM, Sasson D, Paik KE et al. Feature selection and prediction of treatment failure in tuberculosis. PLoS One 2018;13(11):e0207491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization [WHO] . Treatment of tuberculosis: guidelines. In: Who/HTM/TB/2009. Switzerland: Geneva, 2010, 426 26 October 2020, date last accessed. [Google Scholar]
- 6. Central TB Division . India TB Report 2020 National Tuberculosis Elimination Programme Annual Report. New Delhi, 2020, 26 October 2020, date last accessed. [Google Scholar]
- 7. Gebrezgabiher G, Romha G, Ejeta E et al. Treatment outcome of tuberculosis patients under directly observed treatment short course and factors affecting outcome in southern Ethiopia: a five-year retrospective study. PLoS One 2016;11(2):e0150560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaves Torres NM, Quijano Rodrıguez JJ, Porras Andrade PS et al. Factors predictive of the success of tuberculosis treatment: a systematic review with meta-analysis. PLoS One 2019;14:e0226507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moosazadeh M, Bahrampour A, Nasehi M, Khanjani N. Survival and predictors of death after successful treatment among smear positive tuberculosis: a cohort study. Int J Prev Med 2014;5:1005. [PMC free article] [PubMed] [Google Scholar]
- 10. Millet J-P, Orcau A, Rius C et al. Predictors of death among patients who completed tuberculosis treatment: a population-based cohort study. PLoS One 2011;6:e25315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bastos HN, Osório NS, Castro AG et al. A prediction rule to stratify mortality risk of patients with pulmonary tuberculosis. PLoS One 2016;11:e0162797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang S. Development of a predictive model of tuberculosis transmission among household contacts. Can J Infect Dis Medical Microbiol 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu L, Chang W, Song Y, Wang L. Predicting treatment failure risk in a Chinese Drug-Resistant Tuberculosis with surgery therapy: development and assessment of a new predictive nomogram. Int J Infect Dis 2020;96:88–93. [DOI] [PubMed] [Google Scholar]
- 14. Madan C, Chopra KK, Satyanarayana S et al. Developing a model to predict unfavourable treatment outcomes in patients with tuberculosis and human immunodeficiency virus co-infection in Delhi, India. PLoS One 2018;13:e0204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamilton CD, Swaminathan S, Christopher DJ et al. Report international: advancing tuberculosis biomarker research through global collaboration. Clin Infect Dis 2015;61:S155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hochberg NS, Sarkar S, Horsburgh CR et al. Comorbidities in pulmonary tuberculosis cases in Puducherry and Tamil Nadu, India: opportunities for intervention. PLoS One 2017;12:e0183195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Ness SE, Chandra A, Sarkar S et al. Predictors of delayed care seeking for tuberculosis in southern India: an observational study. BMC Infect Dis 2017;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 19. Karnofsky Performance Status . [Internet]. Available: https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=44156 (26 October 2020, date last accessed).
- 20. World Health Organization . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1) http://www.who.int/vmnis/indicators/haemoglobin.pdf, (accessed 26 October 2020). [Google Scholar]
- 21. Kabat GC, Kim MY, Manson JE et al. White blood cell count and total and cause-specific mortality in the women's health initiative. Am J Epidemiol 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gauer RL, Braun MM. Thrombocytopenia. Am Fam Physician 2012;85:612–22. [PubMed] [Google Scholar]
- 23. Han Y, Kim SJ, Lee SH et al. High blood neutrophil-lymphocyte ratio associated with poor outcomes in miliary tuberculosis. J Thorac Dis 2018;10:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chowdhury MZI, Turin TC. Variable selection strategies and its importance in clinical prediction modelling. Fam Med Com Health 2020;8:e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grambsch PTT. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- 26. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015;162:55–63. [DOI] [PubMed] [Google Scholar]
- 27. Sundström J, Byberg L, Gedeborg R et al. Useful tests of usefulness of new risk factors: tools for assessing reclassification and discrimination. Scand J Public Health 2011;39:439–41. [DOI] [PubMed] [Google Scholar]
- 28. Van Calster B, Wynants L, Verbeek JFM et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol 2018;74:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013;13:672–98. [Google Scholar]
- 30. Kolappan C, Subramani R, Kumaraswami V et al. Excess mortality and risk factors for mortality among a cohort of TB patients from rural south India. Int J Tuberc Lung Dis 2008;12:81–6. [PubMed] [Google Scholar]
- 31. RNTCP National Strategic Plan 2017 - 2025 [Internet] . Central TB Division, Di- rectorate General of Health Services, Ministry of Health with Family Wel- fare, Nirman Bhavan, New Delhi–110108; 2018. [cited 24 November 2018]: https://www.tbfacts.org/wpcontent/uploads/2018/06/NSP- Draft-2017-2025.pdf (26 October 2020, date last accessed).
- 32. Nguyen DT, Jenkins HE, Graviss EA. Prognostic score to predict mortality during TB treatment in TB/HIV co-infected patients. PLoS One 2018;13:e0196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen DT, Graviss EA. Development and validation of a risk score to predict mortality during TB treatment in patients with TB-diabetes comorbidity. BMC Infect Dis 2019;19:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munro SA, Lewin HJSSA, Engel AFME, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med 2007;4:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Llongo I. Tuberculosis health belief gaps of tuberculosis and suspected tuberculosis cases in New York City. Int J Clin Heal Psychol 2004;4:69–90. [Google Scholar]
- 36. Barnhoon NF, Adriaanse H. Search of factors responsible for non-compliance among tuberculosis patients in Wardha district, India. Soc Sci Med 1992;34:291–306. [DOI] [PubMed] [Google Scholar]
- 37. Dos Santos Feltrin AF, Vendramini SH, Neto FC et al. Death in patients with tuberculosis and diabetes: associated factors. Diabetes Res Clin Pract 2016;120:111–6. [DOI] [PubMed] [Google Scholar]
- 38. Ajagbe OB, Kabair Z, O’Connor T. Survival analysis of adult tuberculosis disease. PLoS One 2014;10:e0118013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39. Magee MJ, Foote M, Maggio DM et al. Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009-2012. Ann Epidemiol 2014;24:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Isanaka S, Mugusi F, Urassa W et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr 2012;142(2):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kourbatova EV, Borodulin BE, Borodulina EA, del Rio C, Blumberg HM, Leonard MK., Jr. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia Int J Tuberc Lung Dis 2006; 10:1224–30 [PubMed] [Google Scholar]
- 42. Mehta JB, Fields CL, Byrd RP Jr, Roy TM. Nutritional status and mortality in respiratory failure caused by tuberculosis. Tenn Med 1996;89:369–71. [PubMed] [Google Scholar]
- 43. Sacks LV, Pendle S. Factors related to in-hospital deaths in patients with tuberculosis. Arch Intern Med 1998;158:1916–22. [DOI] [PubMed] [Google Scholar]
- 44. Cronjé L, Edmondson N, Eisenach KD, Bornman L. Iron and iron chelating agents modulate Mycobacterium tuberculosis growth and monocyte-macrophage viability and effector functions. FEMS Immunol Med Microbiol 2005;45:103–12. [DOI] [PubMed] [Google Scholar]
- 45. Raghu B, Sarma GR, Venkatesan P. Effect of iron on the growth and siderophore production of mycobacteria. Biochem Mol Biol Int 1993;31:341–8. [PubMed] [Google Scholar]
- 46. Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol 1973;60:1–3. [DOI] [PubMed] [Google Scholar]
- 47. Serafin-López J, Chacon-Salinas R, Munoz-Cruz S et al. The effect of iron on the expression of cytokines in macrophages infected with Mycobacterium tuberculosis. Scand J Immunol 2004;60:329–37. [DOI] [PubMed] [Google Scholar]
- 48. Weiss G, Meusburger E, Radacher G et al. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int 2003;64:572–8. [DOI] [PubMed] [Google Scholar]
- 49. Chedid C, Kokhreidze E, Tukvadze N et al. Association of baseline white blood cell counts with tuberculosis treatment outcome: a prospective multicentered cohort study. Int J Infect Dis 2020;100:199–206. [DOI] [PubMed] [Google Scholar]
- 50. Srivastava S, Ernst JD, L. Desvignes beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev 2014;262(1):179–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sia JK, Rengarajan J. Immunology of Mycobacterium tuberculosis Infections. Microbiol Spectr 2019;7(4):3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naghavi M, Abajobir AA, Abbafati C et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Panteleev AV, Nikitina IY, Burmistrova IA et al. Severe tuberculosis in humans correlates best with neutrophil abundance and lymphocyte deficiency and does not correlate with antigen-C specific CD4 T-Cell response. Front Immunol 2017;8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goto H, Horita N, Tashiro K et al. The platelet count can predict in-hospital death in HIV-negative smear-positive pulmonary tuberculosis inpatients. Intern Med 2018;57(10):1391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. La Manna MP, Orlando V, Badami GD et al. Platelets accumulate in lung lesions of tuberculosis patients and inhibit T-cell responses and Mycobacterium tuberculosis replication in macrophages. Euro J Immunol 2022;52:784–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young C, Walzl G, Du Plessis N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol 2020;13:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 2003;1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:223–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and analyzed during the current study are available from the corresponding author on reasonable request.