Abstract
Background
Outpatient monoclonal antibodies are no longer effective and antiviral treatments for coronavirus disease 2019 (COVID-19) disease remain largely unavailable in many countries worldwide. Although treatment with COVID-19 convalescent plasma (CCP) is promising, clinical trials among outpatients have shown mixed results.
Methods
We conducted an individual participant data meta-analysis from outpatient trials to assess the overall risk reduction for all-cause hospitalizations by day 28 in transfused participants. Relevant trials were identified by searching Medline, Embase, medRxiv, World Health Organization COVID-19 Research Database, Cochrane Library, and Web of Science from January 2020 to September 2022.
Results
Five included studies from 4 countries enrolled and transfused 2620 adult patients. Comorbidities were present in 1795 (69%). The virus neutralizing antibody dilutional titer levels ranged from 8 to 14 580 in diverse assays. One hundred sixty of 1315 (12.2%) control patients were hospitalized, versus 111 of 1305 (8.5%) CCP-treated patients, yielding a 3.7% (95% confidence interval [CI], 1.3%–6.0%; P = .001) absolute risk reduction and 30.1% relative risk reduction for all-cause hospitalization. The hospitalization reduction was greatest in those with both early transfusion and high titer with a 7.6% absolute risk reduction (95% CI, 4.0%–11.1%; P = .0001) accompanied by at 51.4% relative risk reduction. No significant reduction in hospitalization was seen with treatment >5 days after symptom onset or in those receiving CCP with antibody titers below the median titer.
Conclusions
Among outpatients with COVID-19, treatment with CCP reduced the rate of all-cause hospitalization and may be most effective when given within 5 days of symptom onset and when antibody titer is higher.
Keywords: SARS-CoV-2, COVID-19 convalescent plasma, hospitalization, therapy, COVID-19
While this outpatient COVID-19 convalescent plasma meta-analysis indicated heterogeneity in participant risk factors and convalescent plasma titer, the combined efficacy for reducing hospitalization was significant, improving with transfusion within 5 days of symptom onset and high antibody neutralization levels.
(See the Editorial Commentary by Shoham on pages 2087–9.)
The coronavirus disease 2019 (COVID-19) pandemic is responsible for an estimated 18 million excess deaths through 2021 including >1 million in the United States [1]. Despite widespread vaccination in high- and middle-income countries, new variant outbreaks, including the December 2022 outbreak in China, continue to fuel economic disruptions and increased hospitalizations [2]. Novel vaccines and treatments against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been developed, tested, and deployed in record time, yet most arrived too late to benefit the millions of people who died in the pandemic's first year [1]. Three years into the COVID-19 pandemic, it remains unclear how we can respond faster and more effectively to the next pandemic [3, 4].
Antibodies to the SARS-CoV-2 virus, whether induced by vaccination or infused as monoclonal antibodies (mAbs) or polyclonal convalescent plasma, have been shown to reduce the risk of COVID-19–related hospitalization and death, but only convalescent plasma is likely to be both available and affordable for the majority of the world in the early days of the next viral pandemic [5]. COVID-19 convalescent plasma (CCP) was first administered to a hospitalized patient in China in January 2020 [6] and in the United States in March 2020 [7]. Meanwhile, mAbs to prevent hospitalization [8, 9] and vaccines [10, 11] to prevent symptomatic infection, hospitalization, or death were not available until December 2020. By that time, more than 79 million cases of COVID-19 and 1.7 million deaths had been reported worldwide [12]. Effective oral drug therapy for outpatient use was not available until December 2021 [13]. While safe and effective oral agents against SARS-CoV-2 are ideal to prevent COVID-19 hospitalizations, this solution remains unavailable to many patients worldwide due to high costs [14, 15], with effectiveness threatened at any time by new resistant variants.
Escape spike protein mutations leading to acquired resistance during treatment with a single mAb have been repeatedly described in immunocompromised patients [16, 17]. The rapid rise of variants with spike protein mutations has created a dilemma in mAb development, as pharmaceutical companies weigh the high development cost against short-lived utility [18]. Now that all authorized mAbs are no longer effective against recent omicron variants like BQ.1.1 [19–21], CCP, which can be continuously updated from regionally circulating variants, remains an important therapeutic option, especially for severely immunocompromised and other high-risk patients [22, 23].
Most initial randomized controlled trials (RCTs) of CCP were conducted in patients already hospitalized with COVID-19, largely due to the convenience of conducting research in this population. Later in the pandemic, RCTs of CCP targeting outpatients were designed to determine whether early CCP treatment could prevent hospitalization, though few had sufficient power on their own to measure this outcome. Our objective in this study was to conduct an individual patient meta-analysis of all available RCTs of CCP in adult COVID-19 outpatients to determine whether early CCP therapy reduces hospitalization.
METHODS
This study followed the guidelines provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [24].
Objectives
This review aimed to find, assess, and synthesize all RCTs that assessed the efficacy of CCP in preventing all-cause hospitalization among outpatients with confirmed symptomatic SARS-CoV-2 infection.
Eligibility, Search Strategy, RCT Selection, and Data Extraction and Quality
Our PICO (population, intervention, comparator, and outcome) included the following: population = adult (≥18 years) COVID-19 outpatients (not hospitalized at time of transfusion with CCP or placebo) regardless of risk factors; intervention = intravenous CCP transfusion, qualified by antibody titer; comparators = control (nonconvalescent plasma or normal saline); and outcome = all-cause hospitalization within 28 days of transfusion. We used a modified intention-to-treat (mITT) analysis, which included patients for whom transfusion with CCP or placebo was initiated (though not necessarily completed). For 1 study in Argentina, patients meeting prespecified hypoxic respiratory criteria were sometimes admitted to a specific unit within their long-term care facilities, which provided hospital-level care, to avoid overcrowding hospitals. For purposes of trial eligibility, we considered these admissions to be hospitalizations. Only English-language documents were reviewed.
A literature search was performed independently by 2 authors (Y. F., D. J. S.). The Medline, Embase, medRxiv, Cochrane Library, World Health Organization COVID-19 Research Database, and Web of Science were searched for all RCTs as of 30 September 2022. Search strategies were designed with terms related to CCP and COVID-19 (Supplementary Figure 1). All RCTs were included that met the eligibility criteria above. We contacted the corresponding authors for each of the included trials and asked them to contribute data and serve as coauthors for the prepared manuscript.
The investigators for each RCT provided the following data elements: trial design characteristics, descriptions of the intervention and control groups, baseline characteristics of the patients (including underlying comorbidities and days after symptom onset), CCP characteristics (eg, antibody titers), hospitalizations, enrollment period, target enrollment, number of enrollments, number of transfusions, and trial locations. Data not provided in the published reports were collected from the authors.
A risk of bias assessment was independently performed by COVID-19 Network Meta-Analysis [25, 26].
Statistical Analysis
Primary and secondary analyses were done in the mITT population including all randomized participants who received the intervention (CCP or control). The primary outcome used for analysis was all-cause hospitalization within 28 days of transfusion, and the secondary outcome was all-cause hospitalization among those patients admitted to hospitals >24 hours after transfusion. Two subgroup analyses were performed: (1) the reduction in hospital admission for patients with ≤5 versus >5 days of symptoms at the time of intervention; and (2) the reduction in hospitalizations for patients receiving CCP with antibody titers above the median SARS-CoV-2 antibody titer value for each individual RCT versus those receiving CCP not above the median.
Descriptive analysis included the country in which the study was conducted, patient demographics, days since symptom onset, plasma donor antibody levels, and high-risk comorbidities. Box plots were used for visualization and comparison of viral neutralization among studies. Treatment effect was determined using the absolute risk reduction (ARR), relative risk reduction (RRR), and number needed to treat (NNT). Odds ratio (OR), 95% confidence interval (CI), weight of each study (inverse of the variances), heterogeneity (I2), between-study variance (τ2), and significance levels were estimated using mixed random-effects models and displayed in forest plots. A funnel plot was used to estimate the risk of publication bias. The significance level for analyses was set at .05. All of the data manipulation and analyses were performed using Excel software and R software (version 4.2.0, R Foundation, Vienna, Austria) and its statistical packages “meta” (version 6.0-0) and “metafor” (version 3.8-1).
RESULTS
Trial Population
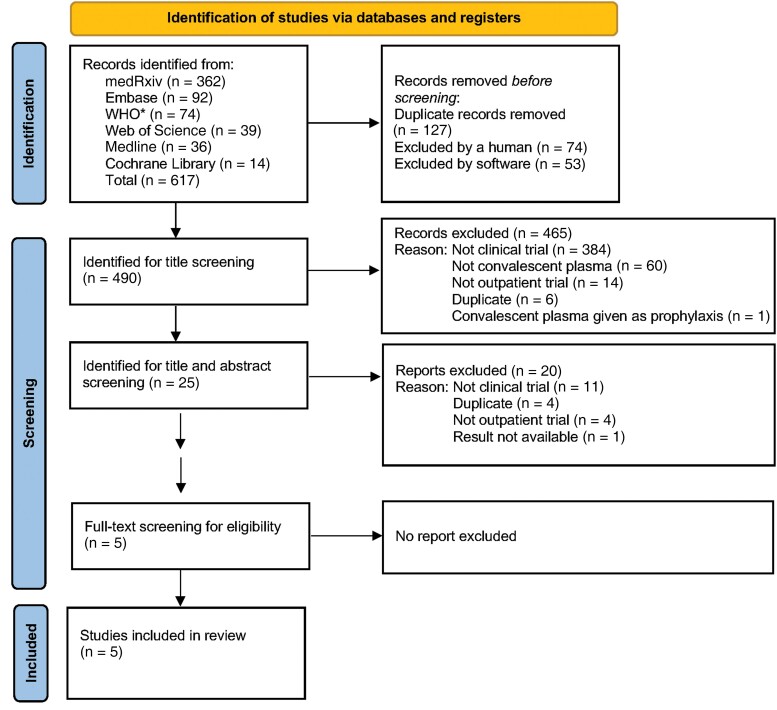
A total of 617 studies were identified by our primary search strategy. After screening and exclusion of ineligible studies, 5 RCTs were included (Figure 1). Two were conducted in the United States [27, 28], 2 in Europe [29, 30], and 1 in Argentina [31]. All of the trials were stopped early: 1 due to slow recruitment as COVID-19 cases in the trial region decreased considerably [31], 3 due to rapid uptake of vaccination resulting in substantial reduction in hospital admission rates [27, 29, 30], and 1 due to a finding of futility to detect the planned difference after the second planned interim analysis of the primary outcome analysis [28].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. The Medline, Embase, medRxiv, Cochrane Library, World Health Organization (WHO) COVID-19 Research Database, and Web of Science were searched for all randomized controlled trials as of 30 September 2022. Abbreviation: COVID-19, coronavirus disease 2019. *WHO COVID-19 global literature on coronavirus disease.
The 5 RCTs enrolled 2693 patients from June 2020 to October 2021 [27–31], and transfusion was initiated in 2620 patients (Table 1). Seventy-three patients were either hospitalized or withdrew from the study after randomization but before transfusion with CCP or placebo could be initiated. These 5 trials varied in terms of their demographic and clinical profiles, including median age, sex distribution, and the prevalence of major risk factors for COVID-19–related hospitalization (Table 1). The target study populations were all COVID-19 outpatient participants regardless of comorbidities (diabetes, cardiovascular, or lung disease) without contraindication to plasma transfusion. Studies also varied somewhat in the timing of the intervention, although 1562 patients (60%) were transfused within 5 days of symptom onset. Overall, only 159 (6%) of all patients were fully vaccinated (defined as 2 messenger RNA doses or 1 adenovirus-vectored dose). We found that the risk of bias was low for the 5 RCTs (Supplementary Table 1). Funnel plot analysis did not suggest a risk of publication bias (Supplementary Figure 2).
Table 1.
Trial Characteristics
| Characteristic | CSSC-004 | CCP-Argentina | CONV-ERT | C3PO | CoV-Early | Total |
|---|---|---|---|---|---|---|
| Control arm | Plasma | Saline | Saline | Saline/MVC | Plasma | … |
| Enrollment period | June 2020 to Oct 2021 |
June 2020 to Oct 2020 |
Nov 2020 to July 2021 |
Aug 2020 to Feb 2021 | Nov 2020 to July 2021 |
… |
| Trial duration, mo | 16 | 5 | 9 | 7 | 9 | 46 |
| Variants | 614G, Alpha, Beta, Delta | WA-1, D614G | D614G, Alpha | D614G | D614G, Alpha | … |
| Geography | US | Argentina | Spain | US | Netherlands | … |
| Target enrollment, No. | 1280 | 210 | 474 | 900 | 690 | 3554 |
| Enrolled, No. | 1225 | 160 | 376 | 511 | 421 | 2693 |
| mITT (% of target enrollment) | 1181 (92) | 154 (73) | 369 (78) | 500 (55) | 416 (60) | 2620 (74) |
| Age, y, median (range) | 43 (18–85) | 77 (65 to ≥90) | 56 (IQR, 52–62) | 54 (18–93) | 60 (IQR, 55–65) | … |
| ≥1 medical high-risk condition for COVID-19 progression | 470 (40) | 131 (82) | 278 (74) | 511 (100) | 416 (100) | 1806 (68.6) |
| Enrollment symptom duration for inclusion, d | 0–8 | 0–3 | 0–7 | 0–7 | 0–7 | 0–8 |
| Symptoms ≤5 d | 517 (44) | 154 (100) | 283 (77) | 389 (78) | 226 (54) | 1569 (60) |
| Symptoms ≤3 d | 168 (14) | 154 (100) | 101 (27) | 240 (48) | 52 (13) | 715 (27) |
| Median/mean duration of symptoms, median (mean) | 6 | 3 | (4.4) | 4 | 5 | … |
| Total female | 675 (57) | 98 (64) | 169 (46) | 265 (53) | 93 (22) | 1300 (50) |
| Age >50 y | 411 (35) | 154 (100) | 368 (100) | 310 (61) | 414 (100) | 1657 (63) |
| Age >65 y | 80 (7) | 154 (100) | 73 (20) | 95 (19) | 113 (27) | 515 (20) |
| Diabetes | 99 (8) | 35 (23) | 39 (10) | 142 (28) | 29 (7) | 344 (13) |
| Hypertension | 276 (23) | 110 (71) | 244 (66) | 216 (42) | Not reported | 846 (38)a |
| Obesity (BMI >30 kg/m2) | 444 (38) | 11 (7) | 95 (26) | 302 (60) | 126 (30) | 978 (37) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; C3PO, clinical-trial of COVID-19 convalescent plasma in outpatients; CCP-Argentina, COVID-19 convalescent plasma-Argentina; CONV-ERT, convalescent methylene blue treated (MBT) plasma for early treatment; CoV-Early, early convalescent plasma therapy; CSSC-004, COVID-19 serologic studies consortium; COVID-19, coronavirus disease 2019; IQR, interquartile range; mITT, modified intention-to-treat (those transfused); MVC, multivitamin concentrate; US, United States.
Only included 4 reported studies.
Convalescent Plasma
The included studies used diverse assays to qualify and characterize the CCP transfused in study subjects (Supplementary Table 2). There was insufficient residual donor plasma samples available to compare neutralization titers across the different studies using the same assay. Two studies qualified units with 50% viral neutralization dilutional plasma titers >1:160. Two studies qualified with dilutional antibody binding greater than 1000 or 320, while the last measured Euroimmun immunoglobulin G was >6.0 AU. Separate viral neutralization indices, depicted in Supplementary Figure 3, show that COVID-19 serologic studies consortium (CSSC-004) and COVID-19 convalescent plasma-Argentina (CCP-Argentina) had slightly lower viral neutralization metrics, albeit a different viral neutralization assay than clinical-trial of COVID-19 convalescent plasma in outpatients (C3PO), early convalescent plasma therapy (CoV-Early), and convalescent methylene blue treated (MBT) plasma for early treatment (CONV-ERT).
Primary Outcome: Hospitalization
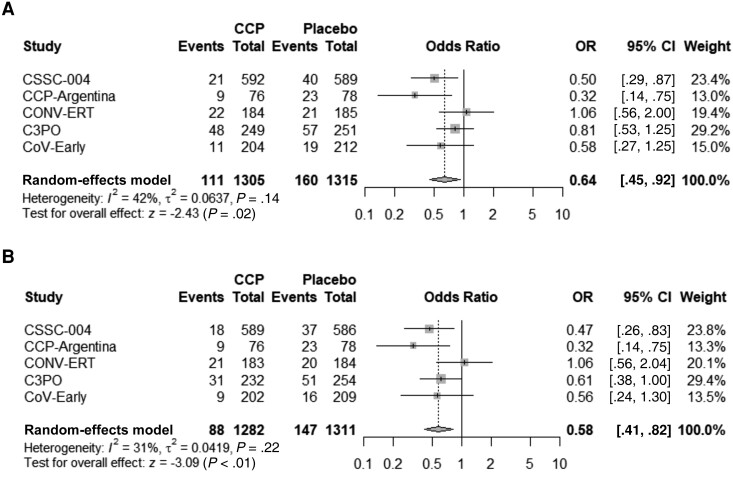
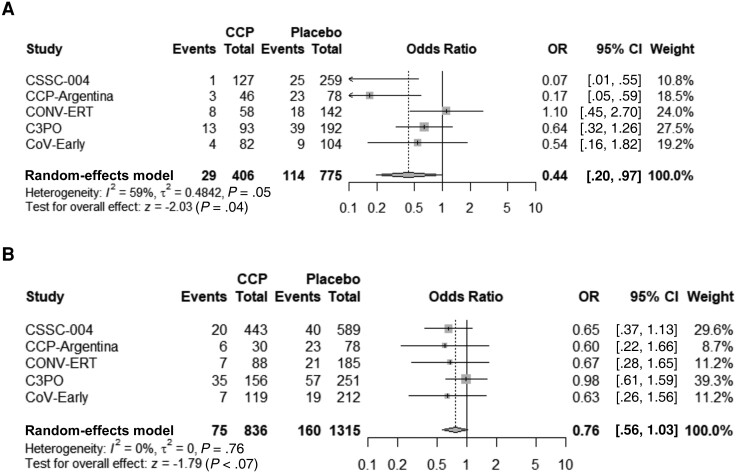
Modified intention-to-treat analysis (Table 2) was performed on patients who received either CCP or control. CSSC-004 added 7 all-cause hospitalizations (4 CCP and 3 control plasma) above reported COVID-19–related hospitalizations and C3PO added 2 participants hospitalized after day 15 but before day 28. Overall, 160 (12.2%) subjects in the control group were hospitalized, compared to 111 (8.5%) in the CCP treatment group, yielding an ARR of 3.7% (95% CI, 1.3%–6.0%), NNT of 27, and RRR of 30.1% (95% CI, 12.0%–44.4%) for all-cause hospitalization (Table 2). The OR for hospitalization was 0.64 (95% CI, .45–.92) in the pooled meta-analysis, and trial heterogeneity was moderate, with an I2 of 42% (Figure 2). A secondary analysis was conducted excluding those patients admitted to the hospital within 24 hours of CCP (25 patients) or control (13 patients) transfusion, yielding an ARR of 4.4% (95% CI, 2.2%–6.6%), NNT of 23, and RRR of 39.2% (95% CI, 21.7%–52.8%). The OR for hospitalization was 0.58 (95% CI, .41–.82), and trial heterogeneity was low in this secondary analysis, with an I2 of 31% (Figure 2).
Table 2.
Overall Numbers and Percentage of Pooled Numbers for Hospitalization and Totals
| Study | Total CCP Outcomes |
Total CCP | Total Control Outcomes | Total Control | Totals | CCP, % | Control, % | ARR, % (95% CI) | RRR % (95% CI) | Significance Level, P Value | NNT Benefit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mITT (all-cause hospitalizations) | 111 | 1305 | 160 | 1315 | 2620 | 8.5 | 12.2 | 3.7 (1.3–6.0) | 30.1 (12.0–44.4) | .0011 | 27 |
| mITT (hospitalizations after 24 h from transfusion) | 88 | 1282 | 147 | 1302 | 2584 | 6.9 | 11.3 | 4.4 (2.2–6.6) | 39.2 (21.7–52.8) | .0001 | 23 |
| Onset ≤5 d | 70 | 787 | 114 | 775 | 1562 | 8.9 | 14.7 | 5.8 (2.6–9.0) | 39.5 (19.9–54.3) | .0002 | 17 |
| Onset ≥6 d | 41 | 518 | 46 | 540 | 1058 | 7.9 | 8.5 | .6 (−2.7 to 3.9) | 7.1 (−39.1 to 37.9) | .3605 | 166 |
| Donor titer ≥ median | 49 | 687 | 157 | 1315 | 2002 | 7.1 | 11.9 | 4.8 (2.2–7.4) | 40.3 (18.8–56.1) | .0004 | 21 |
| Donor titer below median | 62 | 593 | 157 | 1315 | 1908 | 10.5 | 11.9 | 1.5 (−1.5 to 4.5) | 12.4 (−15.6 to 33.7) | .1735 | 67 |
| High titer AND onset ≤5 d | 29 | 406 | 114 | 775 | 1181 | 7.1 | 14.7 | 7.6 (4.0–11.1) | 51.4 (28.3–67.1) | .0001 | 13 |
| Low titer and onset ≤5 d, high titer and onset >5 d, low titer and onset >5 d | 75 | 836 | 160 | 1315 | 2151 | 9.0 | 12.2 | 3.2 (.6–5.8) | 26.3 (4.4–43.2) | .0105 | 31 |
Data are presented as No. unless otherwise indicated.
Abbreviations: ARR, absolute risk reduction; CCP, coronavirus disease 2019 convalescent plasma; CI, confidence interval; mITT, modified intention-to-treat; NNT, number needed to treat; RRR, relative risk reduction.
Figure 2.
Forest plot of modified intention-to-treat (mITT) analysis (A) and of mITT analysis excluding same-day hospital admissions on transfusion day (B). Abbreviations: C3PO, clinical-trial of COVID-19 convalescent plasma in outpatients; CCP, coronavirus disease 2019 convalescent plasma; CCP-Argentina, COVID-19 convalescent plasma-Argentina; CI, confidence interval; CONV-ERT, convalescent methylene blue treated (MBT) plasma for early treatment; CoV-Early, early convalescent plasma therapy; CSSC-004, COVID-19 serologic studies consortium; OR, odds ratio.
Subgroup Analyses
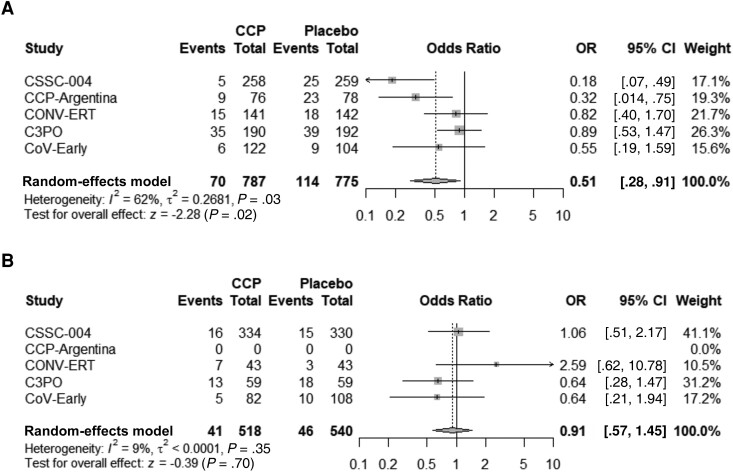
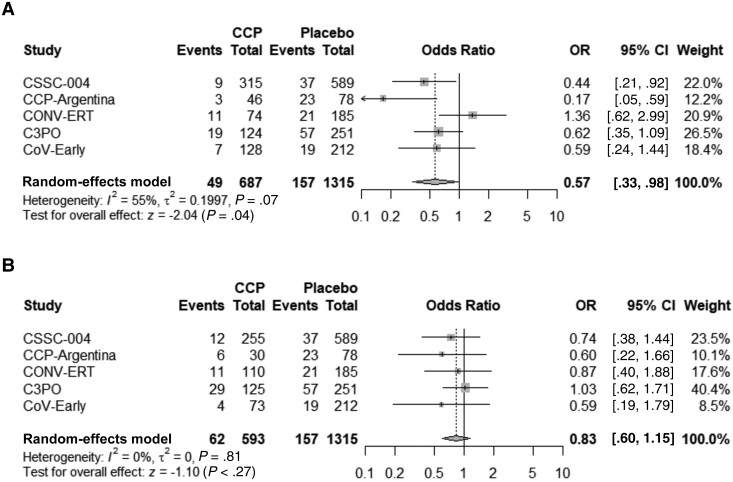
Subgroup analyses were performed based upon the timing of CCP transfusion and the SARS-CoV-2 antibody titer level in transfused CCP units. For subjects transfused within 5 days of symptom onset, pooled analysis among all 5 studies indicated an ARR of 5.8% (95% CI, 2.6%–9.0%), NNT of 17, and RRR of 39.5% (95% CI, 19.9%–54.3%) in hospitalizations when compared to control (Table 2 and Figure 3). Study subjects transfused with high antibody titer CCP (defined as equal to or greater than the median neutralization titer for each individual study) had an ARR of 4.8% (95% CI, 2.2%–7.4%), NNT of 21, and RRR of 40.3% (95% CI, 18.8%–56.1%) in hospitalization compared with subjects given the control (Table 2 and Figure 4). Subjects transfused after 6 days of symptoms or with low antibody titer CCP did not show a significant decrease in hospitalization when compared with control (Table 2). The risk reduction in patients receiving high antibody titer CCP and within 5 days of symptom onset was higher for the combined studies at an ARR of 7.6% (95% CI, 4.0%–11.1%), NNT of 13, and RRR of 51.7% (95% CI, 28.3%–67.1%) (Table 2 and Figure 5).
Figure 3.
Forest plots of transfusion within 5 days (A) or >5 days (B). Abbreviations: C3PO, clinical-trial of COVID-19 convalescent plasma in outpatients; CCP, coronavirus disease 2019 convalescent plasma; CCP-Argentina, COVID-19 convalescent plasma-Argentina; CI, confidence interval; CONV-ERT, convalescent methylene blue treated (MBT) plasma for early treatment; CoV-Early, early convalescent plasma therapy; CSSC-004, COVID-19 serologic studies consortium; OR, odds ratio.
Figure 4.
Forest plots of plasma donor antibody levels at or above median titer (A) or less than median titer (B). Abbreviations: C3PO, clinical-trial of COVID-19 convalescent plasma in outpatients; CCP, coronavirus disease 2019 convalescent plasma; CCP-Argentina, COVID-19 convalescent plasma-Argentina; CI, confidence interval; CONV-ERT, convalescent methylene blue treated (MBT) plasma for early treatment; CoV-Early, early convalescent plasma therapy; CSSC-004, COVID-19 serologic studies consortium; OR, odds ratio.
Figure 5.
Forest plots of plasma donor antibody levels and early treatment at or above median titer AND transfusion within 5 days (A) or total of low titer and onset ≤5 days, high titer and onset over 5 days, low titer and onset over 5 days (B). Abbreviations: C3PO, clinical-trial of COVID-19 convalescent plasma in outpatients; CCP, coronavirus disease 2019 convalescent plasma; CCP-Argentina, COVID-19 convalescent plasma-Argentina; CI, confidence interval; CONV-ERT, convalescent methylene blue treated (MBT) plasma for early treatment; CoV-Early, early convalescent plasma therapy; CSSC-004, COVID-19 serologic studies consortium; OR, odds ratio.
Safety
Due to small numbers, we did not combine severe adverse events related to transfusion in a meta-analysis; however, they were collected for each trial. In CSSC-004, 1 subject experienced a transfusion reaction that required cessation of the transfusion [27]. The CCP-Argentina trial did not report any instances of volume overload, allergic reactions, or vasovagal syndromes, but did report 1 case of thrombophlebitis in the control arm. The C3PO authors noted 3 serious transfusion reactions in the CCP arm resulting in steroid or epinephrine administration or hospitalization [28]. The CONV-ERT team communicated no severe adverse events related to transfusion, but 3 vasovagal reactions and mild allergic reactions in 12 of 188 (6.4%) subjects transfused with CCP [30]. A participant with pulmonary embolism was reported 7 days after transfusion. The CoV-Early investigators reported 3 severe adverse events possibly related to plasma transfusion (all with nonconvalescent plasma). Two developed an anaphylactic reaction shortly after receiving plasma for which no hospital admission was required, and 1 patient developed generalized urticaria requiring hospitalization.
DISCUSSION
This meta-analysis of all available RCTs found that early outpatient therapy with CCP in adult patients with COVID-19 was associated with a 30% all-cause hospitalization RRR (NNT, 27) and a 39% RRR (NNT, 23) when excluding patients admitted on the same day as treatment (Table 2). Early treatment with high antibody titer CCP demonstrated a 51% hospitalization RRR (NNT, 13) in all-cause hospitalization among adult patients with COVID-19. Despite differences in the demographics and clinical characteristics of the 5 study populations, overall study heterogeneity was low to moderate, suggesting the appropriateness of combining these studies in a single meta-analysis and broadly generalizing these results. While the effectiveness of early CCP treatment in reducing all-cause hospitalization was less than that of many mAb treatments [32, 33] and antiviral therapies [13, 34], this should be balanced against its increased availability and potential for activity against variant strains of SARS-CoV-2.
Two of the 5 RCTs included in this meta-analysis (CONV-ERT and C3PO) failed to demonstrate a reduction in all-cause hospitalization with CCP, while the other 3 trials (CCP-Argentina, CSSC-004, CoV-Early) all showed approximately 50% reductions in hospitalizations. One potential explanation for the lack of effectiveness for CCP in the CONV-ERT trial is that methylene blue photoinactivation was used for pathogen reduction in transfused units. This might have affected the constant regions of antibody function without interfering with the viral neutralization assay [35]. The C3PO trial, unlike the other RCTs, enrolled only patients presenting to the emergency department (ED) with COVID-19, which likely included a more severely ill patient population further along in the inflammatory phase of disease. Indeed, there are often less tangible factors signifying more severe illness that lead a patient to present to the ED rather than to their primary care doctor. This is evidenced by the much larger number of subjects in the C3PO trial (23% of all hospitalizations) who were admitted directly to the hospital from the ED on the same visit in which they were transfused. Eliminating these same-day admissions (as in our secondary analysis) bring the C3PO results in line with those from the other studies and greatly reduces heterogeneity among the 5 studies.
Antibody levels for the transfused CCP used across these 5 trials varied substantially, despite the fact that donors had been selected based upon a minimum antibody level cutoff in each trial. However, different cutoffs were used as well as different antibody tests. Our observation that the reduction in hospital admission was limited to patients receiving CCP with titers above the median concentration level in each of the trials suggests that the CCP selection process was suboptimal. It is likely that more stringent antibody titer criteria for CCP units may further improve the effectiveness of this intervention [36].
Plasma transfusion, unlike the use of antiviral and mAb agents, presents a risk of transfusion reactions, which may vary from easily treatable conditions (eg, urticaria) to life-threatening reactions such as anaphylaxis. Rates of severe adverse reactions, however, appeared to be low in these outpatient trials.
This study does have several important limitations. While CSSC-004 enrolled both COVID-19–vaccinated and unvaccinated individuals, the other RCTs primarily included unvaccinated patients, which limits our ability to analyze the effectiveness of CCP for reducing COVID-19 hospitalization in a primarily vaccinated population. The NNT with CCP may be much higher in a primarily vaccinated population, although this difference may be mitigated by the rise of mutant variants that undermine the effectiveness of vaccines and mAbs. All 5 included studies also ended before meeting their transfusion goals, reducing their individual power to detect a difference in hospitalizations between treatment and control groups, and therefore increasing the need for this meta-analysis.
Our meta-analysis chose to use an mITT analysis, excluding 73 patients who were randomized to a given treatment but did not receive it due to hospitalization or withdrawal prior to transfusion, which could introduce bias. However, this represents <3% of enrolled patients and would be unlikely to significantly affect our results. More importantly, patients and providers did not know of their randomization assignment, so the risk of bias due to our analysis methodology is low. In the CCP-Argentina study, some patients not actually admitted to a hospital were considered to meet the primary outcome, but these patients did meet standard hospital admission criteria (ie, hypoxia/respiratory distress) and were instead provided with hospital-level care within their long-term care unit. As described above, the actual donor antibody titer levels varied across the 5 RCTs, and the studies used varying assays to measure antibody titer, making it difficult to compare absolute antibody titers across studies. Consequently, we chose to look at median antibody titers within the individual studies as a means of comparing the CCP used in the various RCTs.
Although there are several implementation considerations that could affect the real-world efficacy and sustainability of CCP transfusion programs [37], our pooled meta-analysis including 5 large, rigorously conducted RCTs suggests that high-titer CCP administered early to adult outpatients with COVID-19 significantly reduces the risk of all-cause hospitalizations across a diverse range of demographic and clinical profiles, geographic locations, and transfusion settings. We believe that CCP should be considered as an outpatient treatment option (especially for patients at high-risk for poor outcomes) in settings where mAbs or antivirals are not currently accessible, or when new variants arise that undermine the effectiveness of these interventions. Pandemic preparedness should also incorporate flexible antibody neutralization assay systems for model organisms. Future research should focus on defining the optimal antibody titer and dosage for CCP and evaluating its effectiveness among immunocompromised vaccinated patients. Despite its limitations, CCP has the potential to be an effective, readily available, and highly adaptable intervention for use in both this and future pandemics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Adam C Levine, Department of Emergency Medicine, Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Yuriko Fukuta, Infectious Disease, Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Moises A Huaman, Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA.
Jiangda Ou, Division of Brain Injury Outcomes, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Barry R Meisenberg, Department of Hematology–Oncology, Anne Arundel Medical Center, Annapolis, Maryland, USA.
Bela Patel, Division of Critical Care Medicine, McGovern Medical School, University of Texas Health Science Center, Houston, Texas, USA.
James H Paxton, Department of Emergency Medicine, Wayne State University School of Medicine, Detroit, Michigan, USA.
Daniel F Hanley, Division of Brain Injury Outcomes, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Bart J A Rijnders, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, University Medical Center, Rotterdam, The Netherlands.
Arvind Gharbharan, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, University Medical Center, Rotterdam, The Netherlands.
Casper Rokx, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Center, University Medical Center, Rotterdam, The Netherlands.
Jaap Jan Zwaginga, Department of Haematology, Leiden University Medical Centre, Leiden, The Netherlands; Center for Clinical Transfusion Research, Sanquin Blood Supply, Amsterdam, The Netherlands.
Andrea Alemany, Fight Infectious Diseases Foundation, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Infectious Diseases Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Oriol Mitjà, Fight Infectious Diseases Foundation, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Infectious Diseases Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Lihir Medical Centre, International SOS, Lihir Island, Papua New Guinea.
Dan Ouchi, Fight Infectious Diseases Foundation, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Infectious Diseases Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Pere Millat-Martinez, ISGlobal, Department of Infectious Diseases, Hospital Clínic, Universitat de Barcelona, Barcelona, Spain.
Valerie Durkalski-Mauldin, Department of Public Health Sciences, Medical University of South Carolina, Charleston, South Carolina, USA.
Frederick K Korley, Department of Emergency Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Larry J Dumont, Vitalant Research Institute, Research Department, Denver, Colorado, USA; Department of Pathology, University of Colorado School of Medicine, Aurora, Colorado, USA.
Clifton W Callaway, Department of Emergency Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Romina Libster, Fundación INFANT, Buenos Aires, Argentina; Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Gonzalo Perez Marc, Fundación INFANT, Buenos Aires, Argentina.
Diego Wappner, Fundación INFANT, Buenos Aires, Argentina.
Ignacio Esteban, Fundación INFANT, Buenos Aires, Argentina.
Fernando Polack, Fundación INFANT, Buenos Aires, Argentina; Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
David J Sullivan, Department of Molecular Microbiology and Immunology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Notes
Author Contributions. A. C. L. and D. J. S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: D. J. S., D. F. H., A. C. L. Acquisition, analysis, or interpretation of data: A. C. L., Y. F., M. A. H., J. O., B. R. M., B. P., J. H. P., D. F. H., B. J. A. R., A. G., C. R., J. J. Z., A. A., O. M., V. D.-M., L. J. D., F. K. K., C. W. C., R. L., D. J. S. Drafting of the manuscript: D. J. S., A. C. L., Y. F., M. A. H., J. O., B. R. M., B. P., J. H. P. Critical revision of the manuscript for important intellectual content: A. C. L., Y. F., M. A. H., J. O., B. R. M., B. P., J. H. P., D. F. H., B. J. A. R., A. G., A. A., O. M., V. D.-M., F. K. K., C. W. C., R. L., D. J. S., C. R., J. J. P., P. M.-M. Statistical analysis: J. O., D. J. S., A. C. L. Obtained funding: D. J. S., B. J. A. R., O. M., C. W. C., F. P. Administrative, technical, or material support: C. R., J. J. Z., P. M.-M. Supervision: D. J. S., A. G., A. A., C. W. C., R. L.
Acknowledgments. The authors thank the patients who participated, all of the plasma donors, and the study nurses involved at the study sites. The authors also thank the 5 separate consortiums that performed the trials.
Data sharing. Data are available from individual authors upon request.
Disclaimer. The funders had no role in the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Financial support. This work was supported principally by the US Department of Defense (DOD) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense, in collaboration with the Defense Health Agency (contract number W911QY2090012), with additional support from Bloomberg Philanthropies, the State of Maryland, the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID; grant number 3R01AI152078-01S1), NIH National Center for Advancing Translational Sciences (cooperative agreement number U24TR001609), Division of Intramural Research of the NIAID/NIH, Mental Wellness Foundation, Moriah Fund, Octapharma, HealthNetwork Foundation, and the Shear Family Foundation. CoV-Early was supported by ZonMw, the Netherlands (grant number 10430062010001). Sanquin Blood Supply provided convalescent plasma free of charge for study sites in the Netherlands. CONV-ERT was sponsored by the Fight AIDS and Infectious Diseases Foundation (Badalona, Spain) with funding from the pharmaceutical company Grifols Worldwide Operations (Dublin, Ireland) and the crowdfunding campaign YoMeCorono. The study received support from the Hospital Universitari Germans Trias i Pujol and Banc de Sang i Teixits de Catalunya. CCP-Argentina was supported by the Bill & Melinda Gates Foundation and by the Fundación INFANT Pandemic Fund, which received contributions from Laboratorio Roemmers, Bodega Vistalba, Swiss Medical Group, Laboratorios Bago, Laboratorio Raffo, Laboratorios Monserrat y Eclair, Tuteur Sacifia, TASA Logistica, Fundación Inversiones y Representaciones, Puerto Asís Investments, and Fundación Hematológica Sarmiento and individual contributions from Alec Oxenford, Carlos Kulish and family, Renato Montefiore and family, Irene Gorodisch, Alejandro Gorodisch, the Braun family, Agustín Otero-Monsegur, and Luis R. Otero. C3PO was supported by awards (1OT2HL156812-01, U24NS100659, and U24NS100655) from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Neurological Disorders and Stroke of the NIH and by a contract (number 75A50120C00094) with the Biomedical Advanced Research and Development Authority (BARDA) through the Department of Health and Human Services and the Operation Warp Speed interagency program. Support included funding and material support in the form of plasma and testing supplies.
References
- 1. COVID-19 Excess Mortality Collaborators . Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet 2022; 399:1513–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paredes MI, Lunn SM, Famulare M, et al. . Associations between SARS-CoV-2 variants and risk of COVID-19 hospitalization among confirmed cases in Washington state: a retrospective cohort study. medRxiv [Preprint]. Posted online 16 February 2022. 10.1101/2021.09.29.21264272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newland M, Durham D, Asher J, et al. . Improving pandemic preparedness through better, faster influenza vaccines. Expert Rev Vaccines 2021; 20:235–42. [DOI] [PubMed] [Google Scholar]
- 4. Kachali H, Haavisto I, Leskela RL, Valja A, Nuutinen M. Are preparedness indices reflective of pandemic preparedness? A COVID-19 reality check. Int J Disaster Risk Reduct 2022; 77:103074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramachandran R, Ross JS, Miller JE. Access to COVID-19 vaccines in high-, middle-, and low-income countries hosting clinical trials. JAMA Netw Open 2021; 4:e2134233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen C, Wang Z, Zhao F, et al. . Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salazar E, Christensen PA, Graviss EA, et al. . Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 2020; 190:2290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen P, Nirula A, Heller B, et al. . SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weinreich DM, Sivapalasingam S, Norton T, et al. . REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polack FP, Thomas SJ, Kitchin N, et al. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson EJ, Rouphael NG, Widge AT, et al. . Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization (WHO) . Weekly epidemiological update—29 December 2020. Geneva, Switzerland: WHO, 2020.
- 13. Hammond J, Leister-Tebbe H, Gardner A, et al. . Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Plata GG. The black market for covid-19 antiviral drugs. BMJ 2022; 377:o1282. [DOI] [PubMed] [Google Scholar]
- 15. Hill A, Ellis L, Wang J, Pepperrell T. Prices versus costs of production for molnupiravir as a COVID-19 treatment. Research Square [Preprint]. Posted online 15 December 2021. 10.21203/rs.3.rs-1169509/v1 [DOI] [Google Scholar]
- 16. Huygens S, Munnink BO, Gharbharan A, Koopmans M, Rijnders B. Sotrovimab resistance and viral persistence after treatment of immunocompromised patients infected with the SARS-CoV-2 Omicron variant. Clin Infect Dis 2023; 76:e507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Birnie E, Biemond JJ, Appelman B, et al. . Development of resistance-associated mutations after sotrovimab administration in high-risk individuals infected with the SARS-CoV-2 Omicron variant. JAMA 2022; 328:1104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernandez AV, Piscoya A, Pasupuleti V, et al. . Beneficial and harmful effects of monoclonal antibodies for the treatment and prophylaxis of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Am J Med 2022; 135:1349–61.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Q, Iketani S, Li Z, et al. . Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023; 186:279–86.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arora P, Kempf A, Nehlmeier I, et al. . Omicron sublineage BQ.1.1 resistance to monoclonal antibodies. Lancet Infect Dis 2023; 23:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Planas D, Bruel T, Staropoli I, et al. . Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies. Nat Commun 2023; 14:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malahe SRK, Hoek RAS, Dalm V, et al. . Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the Omicron variant: a prospective observational study. Clin Infect Dis 2023; 76:e172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhimraj AMR, Shumaker AH, Baden L, et al. . Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 30 September 2022. [DOI] [PMC free article] [PubMed]
- 24. Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boutron I, Chaimani A, Meerpohl JJ, et al. . The COVID-NMA project: building an evidence ecosystem for the COVID-19 pandemic. Ann Intern Med 2020; 173:1015–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nguyen TV, Ferrand G, Cohen-Boulakia S, et al. . RCT studies on preventive measures and treatments for COVID-19. Zenodo [Preprint]. Posted online 1 April 2020. 10.5281/zenodo.4266529 [DOI] [Google Scholar]
- 27. Sullivan DJ, Gebo KA, Shoham S, et al. . Early outpatient treatment for Covid-19 with convalescent plasma. N Engl J Med 2022; 386:1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Korley FK, Durkalski-Mauldin V, Yeatts SD, et al. . Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med 2021; 385:1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gharbharan A, Jordans C, Zwaginga L, et al. . Outpatient convalescent plasma therapy for high-risk patients with early COVID-19. A randomized placebo-controlled trial. Clin Microbiol Infect 2023; 29:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alemany A, Millat-Martinez P, Corbacho-Monne M, et al. . High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med 2022; 10:278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libster R, Perez Marc G, Wappner D, et al. . Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med 2021; 384:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verderese JP, Stepanova M, Lam B, et al. . Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate coronavirus disease 2019 (COVID-19): a real-world experience. Clin Infect Dis 2022; 74:1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jenks JD, Aslam S, Horton LE, et al. . Early monoclonal antibody administration can reduce both hospitalizations and mortality in high-risk outpatients with coronavirus disease 2019 (COVID-19). Clin Infect Dis 2022; 74:752–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. . Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ross V. Photodynamic action of methylene blue on diphtheric antitoxin. J Immunol 1938; 35:371–7. [Google Scholar]
- 36. Rijnders BJA, Huygens S, Mitjà O. Evidence-based dosing of convalescent plasma for COVID-19 in future trials. Clin Microbiol Infect 2022; 28:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bloch EM, Tobian AAR, Shoham S, et al. . How do I implement an outpatient program for the administration of convalescent plasma for COVID-19? Transfusion 2022; 62:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.