Abstract
Background
The impact of infection-induced immunity on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission has not been well established. Here we estimate the effects of prior infection induced immunity in adults and children on SARS-CoV-2 transmission in households.
Methods
We conducted a household cohort study from March 2020-November 2022 in Managua, Nicaragua; following a housheold SARS-CoV-2 infection, household members are closely monitored for infection. We estimate the association of time period, age, symptoms, and prior infection with secondary attack risk.
Results
Overall, transmission occurred in 70.2% of households, 40.9% of household contacts were infected, and the secondary attack risk ranged from 8.1% to 13.9% depending on the time period. Symptomatic infected individuals were more infectious (rate ratio [RR] 21.2, 95% confidence interval [CI]: 7.4–60.7) and participants with a prior infection were half as likely to be infected compared to naïve individuals (RR 0.52, 95% CI:.38–.70). In models stratified by age, prior infection was associated with decreased infectivity in adults and adolescents (secondary attack risk [SAR] 12.3, 95% CI: 10.3, 14.8 vs 17.5, 95% CI: 14.8, 20.7). However, although young children were less likely to transmit, neither prior infection nor symptom presentation was associated with infectivity. During the Omicron era, infection-induced immunity remained protective against infection.
Conclusions
Infection-induced immunity is associated with decreased infectivity for adults and adolescents. Although young children are less infectious, prior infection and asymptomatic presentation did not reduce their infectivity as was seen in adults. As SARS-CoV-2 transitions to endemicity, children may become more important in transmission dynamics.
Keywords: COVID-19, secondary attack rate, transmission, SARS-CoV-2, cohort study
Infection-induced immunity is associated with decreased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Prior infection is associated with decreased infectivity in adolescents and adults. However, neither prior infection nor asymptomatic presentation are associated with decreased infectivity in young children in a household setting.
Prior studies show that vaccination reduces the likelihood of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission [1, 2], and infection-induced immunity is associated with shorter shedding duration and lower viral load [3]; however, the effect of infection-induced immunity on SARS-CoV-2 transmission has not been well established [4]. Given the high infectivity of SARS-CoV-2 and its emerging variants, most of the population including many children have already been infected worldwide [5–7]. Furthermore, as of November 2022, SARS-CoV-2 vaccine availability and uptake has been limited for children globally [8].
Questions persist about the contribution of children to SARS-CoV-2 transmission. Evidence on the contribution of children to transmission generally shows that children have a lower risk of SARS-CoV-2 transmission when infected compared to adults [9–11], whereas other work, particularly after the emergence of SARS-CoV-2 variants, finds that children have similar or increased risk of transmission [12, 13].
In Nicaragua, as with much of the rest of the world, SARS-CoV-2 transmission picked up in March/April of 2020 with a large wave of the pre-variant virus that ended by August 2020. In our cohort, ∼60% of adults were infected in that initial wave [14] . A second large wave, primarily of Delta and Gamma, occurred in 2021 from April to November [15]. SARS-CoV-2 vaccination did not become widely available until September-November of 2021. Omicron, and its subvariants, was introduced in 2022 quickly becoming the dominant virus [16]. However, by that time a majority of the population had been previously infected and most subsequently vaccinated [15].
We present results from an ongoing, community-based, household transmission study located in Managua, Nicaragua, from March 2020 to November 2022. We evaluate the effect of prior infection-induced immunity on transmission as well as the contribution of children to SARS-CoV-2 household transmission.
METHODS
This study was approved by institutional review boards at the Nicaraguan Ministry of Health and the University of Michigan. Adults and parents/guardians of children provided written informed consent and children 6 years or older provided verbal assent.
Participants included in this analysis are members of the ongoing Household Influenza Cohort Study (HICS) which began in 2017. HICS is a community-based prospective household cohort study located in District II of Managua, Nicaragua. In June 2020, the study was expanded to include a transmission sub-study of SARS-CoV-2. Participants attended the Health Center Sócrates Flores Vivas at the first signs of a fever or respiratory illness. A respiratory sample was collected and tested for influenza and SARS-CoV-2 via reverse-transcription polymerase chain reaction (PCR).
Household activation occurred when a cohort participant tested positive for SARS-CoV-2 and they and their household members agreed to be monitored intensively for SARS-CoV-2 transmission. Study staff visited the home up to 6 times to collect respiratory samples (days 0, 3, 7, 14, 21, and 30) and conducted a final follow-up visit at day 45–60. Daily symptom data were collected by staff during each visit [14]. The primary case was identified as the household member with earliest symptom onset date.
Each year, blood samples were collected twice from March to April and again from October to December. Serum samples collected from 2019 to 2022 were paired (current vs baseline) and were tested for SARS-CoV-2 immunoglobulin G (IgG) antibodies to the spike receptor binding domain (RBD) via an enzyme-linked immunosorbent assay (ELISA) following a protocol adapted from the Krammer laboratory at Mt Sinai [17]. Blood samples from participants that were previously vaccinated against SARS-CoV-2 were tested for SARS-CoV-2 IgG antibodies to the nucleocapsid (N) via ELISA.
Prior SARS-CoV-2 infection-induced immunity included both PCR and serologically confirmed infections (RBD + before SARS-CoV-2 vaccination or N + after). We categorized SARS-CoV-2 infections into 3 periods: March 2020 to February 2021 (pre-variant era), March 2021 to December 2021 (pre-Omicron variants, predominantly Gamma and Delta), and January 2022 to November 2022 (Omicron variant) [15, 16]. To determine the date of prior infection for serologically confirmed infections, we estimated the infection date as a randomly selected day during the epidemic wave prior to the blood sample collection [3].
SARS-CoV-2 vaccinations in the cohort began in January 2021. Most vaccinated participants received their first vaccine beginning in September of 2021. A variety of vaccines have been used, with AstraZeneca (2 dose, second dose between days 56 and 128), Abdala (3 dose, second dose on day 14 and third on day 28), and the Soberana 02 (2 dose, second dose on day 28) being the 3 most common vaccines administered. Participants are considered fully vaccinated 14 days after the final dose.
We compared age at enrollment, sex, SARS CoV-2 vaccination, and presence of SARS-CoV-2 antibodies before 1 January 2022, between participants who did and did not participate in intensive monitoring using a χ2 and Fisher exact tests. Using these tests, we also compared time period, sex, age, bedroom and bed sharing, prior infections, vaccination, and primary case symptoms between households that did and did not have transmission (an observed SAR-CoV-2 infection among household members) and (except for symptoms) between PCR− and PCR + household contacts.
To estimate the household secondary attack risk (SAR) and rate ratios (RR), we used pairwise survival models. Pairwise survival models are statistical models of disease transmission that overcome weaknesses of binomial models in estimating the household SAR by accounting for multiple generations of transmission. These models can use the entire household observation period to estimate the SAR, not just the infectious period of the primary case, even when who-infects-who is not observed [18, 19]. Additionally, these models account simultaneously for within-household transmission and the risk of infection from outside the household [20]. The SAR from these models can be interpreted as the probability of transmission from one infected household member to one susceptible during the infectious period [18, 19].
We assumed an incubation period of 6 days, a latency period of 3, and a 10-day duration of infectiousness [21–23]; therefore, participants were considered infectious three days before to seven days following symptom onset or their first PCR + test, whichever occurred first. All primary cases were symptomatic and PCR + household members were considered symptomatic during their infectious period if symptoms were reported (loss of taste or smell, fever, cough, rhinorrhea, nasal congestion, headache, sore or itchy throat, joint or muscle pain, diarrhea, vomiting, fatigue, rash, conjunctivitis, loss of appetite, difficulty breathing, rapid breathing, shortness of breath, and chest pain) within 7 days following the infection date.
SAS version 9.4 (SAS Institute Inc) and R version 4.1.1 with the transtat package were used to conduct the analysis [18, 24]. The models included time period, characteristics of the susceptible household member (sex, age, prior infection, and vaccination) and characteristics of the infected household member (sex, age, presence of symptoms, cough, rhinorrhea, prior infection, vaccination, number of household members, and bed and bedroom sharing). We also ran separate models that included age and an interaction term for age with infector characteristics (symptoms, cough, rhinorrhea, and prior infection) and for prior infection status of the susceptible household member.
To evaluate if the household SARs were different when considering only households infected with the Omicron variant, we reran the univariate models for household activation for 2020/2021 and 2022 separately. For sensitivity analyses, we adjusted the incubation (4–7 days), latency (2–4 days), and infectious periods (8–15 days). We also reran the univariate models including only households where all household members consented to participate in the household activation and serial swabbing. Finally, we ran a univariate model with time since last infection instead of prior infection (yes/no). To assess the impact of our assumption about the estimated infection date for serologically confirmed infections, we adjusted that date; we shifted all estimates to a random day within the first 15 days of the wave and the last 15 days of the wave prior to the blood sample collection.
RESULTS
From March 2020 to November 2022, there were 2399 active participants in the cohort with 87 new/re-enrollees, 394 withdrawn, and 27 deaths (Supplementary Figure 1). Within the SARS-CoV-2 transmission sub-study, a total of 228 households (51.9% of all cohort houses) were activated (some multiple times) with 349 total activations. Of the 1661 individuals in those households, 1353 (81.5%) household contacts consented to intensive monitoring and 308 (18.5%) declined participation/were not present. Participants in activated households that did not participate in intensive monitoring were generally working-age adults and male (Table 1). They also had lower cohort participation, were more likely to have missed cohort blood collections since the start of the pandemic and were less likely to have reported vaccination or have documented SARS-CoV-2 antibodies. In addition to the 349 primary cases, 553 household contacts (40.8%) were infected.
Table 1.
Demographics of Participants Eligible for SARS-CoV-2 Intensive Monitoring in Managua Nicaragua, March 2020 to November 2022
| Participants (n = 975) | Declined/Not Present for Activation Enrollment (n = 308) | P Value* | |
|---|---|---|---|
| Age at enrollment (%) | … | … | .0001 |
| 0–4 | 233 (23.9) | 44 (14.3) | |
| 5–10 | 197 (20.2) | 46 (14.9) | |
| 11–19 | 136 (13.9) | 71 (23.1) | |
| 20–64 | 379 (38.9) | 142 (46.1) | |
| ≥65 | 30 (3.1) | 5 (1.6) | |
| Female (%) | 614 (63.0) | 161 (52.3) | .0008 |
| SARS-CoV-2 vaccination (%)† | … | … | .0039 |
| Full | 299 (30.1) | 77 (25.0) | |
| Partial | 369 (37.9) | 105 (34.1) | |
| Unvaccinated | 50 (5.1) | 12 (3.9) | |
| No reported vaccination | 257 (26.4) | 114 (37.0) | |
| SARS-CoV-2 antibodies (%)† | … | … | <.0001 |
| Yes | 882 (90.5) | 244 (79.2) | |
| No | 88 (9.0) | 59 (19.2) | |
| Missing | 5 (0.5) | 5 (1.6) | |
| Blood samples collected | … | … | <.0001 |
| 0 | 5 (0.5) | 5 (1.6) | |
| 1 | 15 (1.5) | 10 (3.3) | |
| 2 | 21 (2.2) | 38 (12.3) | |
| 3 | 130 (13.3) | 87 (28.3) | |
| 4 | 804 (82.5) | 168 (54.6) |
A χ2 test was used to compare demographics between those who participated in and declined/were not present for household activation.
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
From χ2 or Fisher exact test.
†Before 1 January 2022.
Close to half of household activations (n = 164, 47.0%) occurred from March 2021 to December 2021, a period when multiple variants circulated, and Delta predominated. Additionally, there were 29 (8.3%) participating households from March 2020 to February 2021 and 156 (44.7%) households from January 2022 to November 2022. In total, 79.9% of household activations began within 6 days of primary case symptom onset. Overall, transmission occurred in 70.2% of households.
Primary Cases and Household Members
Next, we looked for differences in primary cases in households where transmission did and did not occur as well as differences in PCR + and PCR− household members. There were a greater proportion of primary cases aged 20–64 years old in households that had transmission compared to those where no transmission occurred (49.0% vs 34.6%) and the overall age group distribution was significantly different (P value: .0134) (Supplementary Table 1). PCR− household members overall had a greater number of prior SARS-CoV-2 infections (P-value: .0029) (Supplementary Table 2). Around half of all young children (aged 0–4), children (aged 5–10), and adults and adolescents (aged ≥11) had been previously infected at the start of intensive monitoring (Table 2).
Table 2.
Prior Infection by Case Status and Age
| Prior Infection | Overall (%) | Age (%*) | ||
|---|---|---|---|---|
| 0–4 | 5–10 | ≥11 | ||
| All | 1702 | 142 | 348 | 1212 |
| Yes | 1017 (59.8) | 70 (49.3) | 184 (52.9) | 648 (53.5) |
| Primary cases | 349 | 28 | 66 | 255 |
| Yes | 177 (32.0) | 12 (28.6) | 32 (27.1) | 133 (33.8) |
| PCR + household members | 553 | 42 | 118 | 393 |
| Yes | 311 (56.2) | 21 (50.0) | 54 (45.8) | 236 (60.1) |
| PCR− household members | 800 | 72 | 164 | 564 |
| Yes | 529 (66.1) | 37 (51.4) | 102 (62.2) | 390 (69.1) |
Data are grouped by primary cases, PCR + household members, and PCR− household members.
Abbreviation: PCR, polymerase chain reaction.
%s are of the corresponding age within each case status group.
SAR and Susceptibility
Next, we evaluated the household SAR and variables associated with susceptibility. The overall estimated household SAR was 12.5% and ranged from 8.1% to 13.9% depending on the study period (Figure 1). Compared to those with no prior SARS-CoV-2 infection, participants with a prior infection had half the risk of infection within the household (RR 0.52, 95% CI: .38, .70).
Figure 1.
Estimated secondary attack risk and rate ratios. The models are univariate and only include the intercepts, and log-shape parameters in addition to the single variable of interest. Variables are grouped by susceptible variables (characteristics of the susceptible individual in the paired data) and infector variables (characteristics of the infectious individual in the paired data). Abbreviations: CI, confidence interval; PCR, polymerase chain reaction; RR, rate ratio; SAR, secondary attack risk.
SAR and Infectivity
We also evaluated factors associated with infectivity. The household SAR was smaller for larger households (8.0% compared to 16.4% for households with 10 + and 2–5 members, respectively). Children, adults, and adolescents were much more likely to infect others compared to young children (RR 3.6, 95% CI: 1.4, 9.4, and RR 6.1, 95% CI: 2.5, 15.0, respectively). In absolute terms, the difference in the secondary attack risk between young children, and adults and adolescents was 11.2% (SAR 3.4% vs 14.6%). Symptomatic infected individuals were 21.2 times (95% CI: 7.4, 60.7) more likely to transmit the virus compared to asymptomatic individuals, with an absolute difference in the probability of transmission of 14.6% (SAR 15.9% vs 1.3%). Overall, prior infection was not associated with decreased infectivity.
SAR Stratified by Age
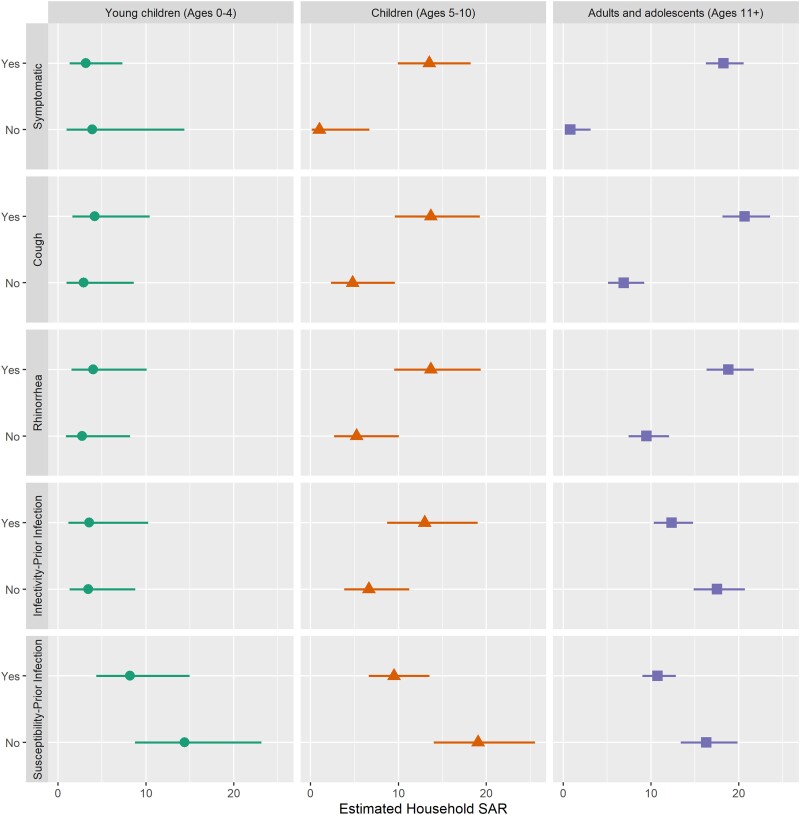
We also compared the age-specific associations between symptom presentation and prior infection with infectivity. The probability of transmission was lower for asymptomatic compared to symptomatic children, and adults and adolescent (Figure 2; 9.8% vs 13.5% and 0.8% vs 18.2%, respectively). For infected young children, we observed no difference by symptom status in the risk of transmitting the virus. Of note, prior infection was associated with decreased infectivity in adults and adolescents (SAR 12.3, 95% CI: 10.3, 14.8, and SAR 17.5, 95% CI: 14.8, 20.7) but not children. When evaluating susceptibility stratified by age, prior infection was associated with decreased SAR in all age groups, but the difference was not significant in young children.
Figure 2.
SAR stratified by age. The presented models include age and an interaction term of age and the infectivity (symptomatic, cough, rhinorrhea, and prior infection) or susceptibility variable (prior infection). The results are stratified by age group: young children (ages 0–4), children (ages 5–10), and adults and adolescents (ages ≥11). Abbreviation: SAR, secondary attack risk.
SAR and Omicron
Next, we evaluated susceptibility and infectivity during the Omicron era. Consistent with the pre-Omicron era results, prior infection was associated with protection against infection. Likewise, susceptibility did not vary by age (Supplementary Figure 2, 3); however, the estimated SAR for infected young children in the Omicron era was almost 3 times that of the pre-Omicron era (2.7% vs 7.5%), although there was little difference seen for adults and adolescents (14.7% vs 15.9%). Infectivity was still associated with symptomatic presentation (RR = 9.0, 95% CI: 2.7, 29.9).
SAR and Time Since Last Infection
Because not only prior infection status, but how recently someone was infected might affect susceptibility and infectivity, we next ran models examining the effects of time since last infection. When examining the association of time since last infection and susceptibility, the results were similar to the association between prior infection (yes/no) and susceptibility (Supplementary Figure 4). At each time point (up to 6 months, 6–12 months, and ≥12 months since), those with a prior infection, were less likely to be infected compared to those who never had a prior infection. Not surprisingly, the SAR was lowest for household members who had a prior infection within 6 months (SAR 9.5, 95% CI: 6.3, 14.1). However, it was still similar to the SAR of those with any prior infection (SAR 10.3, 95% CI: 8.8, 12.0). For an infected household member, there was no association between time since the last infection and infectivity.
Sensitivity Analyses
To examine the effect of our assumptions on our estimates, we varied the incubation, latency, and infectious parameters (Supplementary Figure 5). Overall, there were minor differences in the estimated SARs; however, our main findings held. To examine the effect of non-participation, we reran models limiting to households where all members participated. The overall SAR was slightly higher, but there were no differences in the direction of the association age, infection-induced immunity, or any other variable (Supplementary Figure 6). The associations between SAR and time since the last infection for both susceptible and infected household members had little variation when we adjusted the estimated infection date for serologically detected infections.
DISCUSSION
We found that prior infection impacted both susceptibility and infectivity of SARS-CoV-2 in a household setting. Overall, and as expected, prior infection reduced susceptibility. However, the decrease in susceptibility was less marked in young children. We found that the effect of prior infection on infectivity was age dependent. Previously infected adults and adolescents were less infectious compared to those who did not have a prior SARS-CoV-2 infection. But in children aged ≤10, we did not observe any reduction in infectivity associated with prior infection.
Our finding of decreased risk of transmission for previously infected adults and adolescents is consistent with decreased shedding duration and viral load among those previously infected individuals aged 10 years and older [3]. Our results also concur with the finding that prior infection was also associated with decreased infectivity during the Omicron wave [4]. Similarly, SARS-CoV-2 vaccination has been associated with decreased infectivity [1, 2, 4]. We note that these results are from a population, like many in the world, where most were infected prior to the availability of SARS-CoV-2 vaccines [15]. However, both infection then vaccination and vaccination then infection produces broad, hybrid immunity to SARS-CoV-2 with no observed differences by sequence [25, 26]. Thus, we expect that as robust immunity develops globally through expanded vaccination efforts and repeat and breakthrough infections occur there will be a decrease in infectivity and lower rates of SARS-CoV-2 infections.
In children, prior infection was not associated with decreased infectivity. Additionally, infectiousness was similar between symptomatic and asymptomatic young children (aged 0–4); the increased likelihood of asymptomatic presentation for pediatric SARS-CoV-2 infections does not account for the differences in infectiousness between adults and children [27]. These results suggest distinct immune responses to natural SARS-CoV-2 infection between younger and older individuals that may impact transmission dynamics [28, 29].
Consistent with recent work, prior infection in the Omicron era was still associated with protection against infection [30]. Although we observed increased infectivity for each age for during the Omicron era, infectivity was proportionally higher for young children compared to adults. Although children are generally less infectious [9–11], the changes in infectivity by age during the Omicron era may suggest changing SARS-CoV-2 dynamics [13, 31].
As expected and consistent with the findings of a recent meta-analysis, more recent prior SARS-CoV-2 infections are associated with lower risk of infection [30]. However, this protection may be attenuated in our study due to the mixing of effects of increased infectivity of variants with time and the antigenic differences between the prior and current infecting strains. Future work should expand on the current research by comparing susceptibility by SARS-CoV-2 infection histories that include information about the specific strains of prior infection in addition to the timing.
Our study has several strengths and limitations. Strengths include close monitoring of participants inside of an ongoing cohort, which allows us to know infection histories prior to SARS-CoV-2 entering the household as well as detect mild and asymptomatic infections. Our study is also large and spans both pre-variant and variant eras. Because of our use of a statistical transmission model that also accounts for risk of external infection, each of the SAR estimates in this study can be properly interpreted as the probability of transmission. One limitation of our study is that although PCR testing occurred frequently during monitoring, it is possible that SARS-CoV-2 infections were missed and thus we may underestimate the household SAR. As prior infection was in part determined using serological testing, it is possible that we miscategorized some participants as non-previously infected because they did not seroconvert to their first infection, or their antibodies waned rapidly. In addition, household members that declined or were not available for intensive monitoring were different from those that did participate. The exclusion of these participants likely leads to an underestimation of the household SAR; however, when analyzing only households where the associations between prior infection and infectivity and susceptibility did not change significantly. Finally, sequencing results were not available for all household infections, which limits our ability to evaluate strain-specific infection induced immunity effects.
Our study highlights that infection-induced immunity is associated with decreased infectivity for adults and adolescents. Even with the emergence of the Omicron variant, infection-induced immunity remained associated with protection against infection. However, for young children, neither infection-induced immunity nor symptom presentation was associated with infectivity. At the beginning of the SARS-CoV-2 pandemic, it was established that the contribution of children to SARS-CoV-2 transmission was minor [11]. The absence of decreased infectivity from infection-induced immunity among children and the changing transmission dynamics from emerging SARS-CoV-2 variants suggests that children may already have more meaningful contributions to SARS-CoV-2 transmission; this contribution may further increase as new children are born without immunity to SARS-CoV-2 and increasingly represent the greatest proportion of primary cases [32].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Aaron M Frutos, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Guillermina Kuan, Health Center Sócrates Flores Vivas, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Roger Lopez, Sustainable Sciences Institute, Managua, Nicaragua; Laboratorio Nacional de Virología, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
Sergio Ojeda, Sustainable Sciences Institute, Managua, Nicaragua.
Abigail Shotwell, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Nery Sanchez, Sustainable Sciences Institute, Managua, Nicaragua.
Saira Saborio, Laboratorio Nacional de Virología, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua; Sustainable Sciences Institute, Managua, Nicaragua.
Miguel Plazaola, Sustainable Sciences Institute, Managua, Nicaragua.
Carlos Barilla, Sustainable Sciences Institute, Managua, Nicaragua.
Eben Kenah, Biostatistics Division, College of Public Health, The Ohio State University, Columbus, Ohio, USA.
Angel Balmaseda, Sustainable Sciences Institute, Managua, Nicaragua; Laboratorio Nacional de Virología, Centro Nacional de Diagnóstico y Referencia, Ministry of Health, Managua, Nicaragua.
Aubree Gordon, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Notes
Author Contributions. A. M. F. and A. G. contributed to the conceptualization of the article. G. K., R. L., S. O., N. S., S. S., M. P., C. B., and A. B. contributed to the investigation for the article. A. M. F. conducted the statistical analysis in consultation with E. K. A. S. was responsible for data curation. A. M. F. and A. G. wrote the original draft of the article, and all co-authors contributed to the review and editing of the article. A. F., A. S., G. K., A. B., and A. G. had access to data and verify its authenticity.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health through awards given to A. G. (grant numbers R01 AI120997, HHSN272201400006C, 75N93021C00016, and U01AI144616) and through an award from Open Philanthropy. A. G. is supported by the Biosciences Initiative at the University of Michigan through a Mid-career Biosciences Faculty Achievement Award (MBioFAR).
References
- 1. Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household secondary attack rates of SARS-CoV-2 by variant and vaccination Status: an updated systematic review and meta-analysis. JAMA Netw Open 2022; 5:e229317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jung J, Kim JY, Park H, et al. Transmission and infectious SARS-CoV-2 shedding kinetics in vaccinated and unvaccinated individuals. JAMA Netw Open 2022; 5:e2213606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maier HE, Plazaola M, Lopez R, et al. SARS-CoV-2 infection-induced immunity and the duration of viral shedding: results from a Nicaraguan household cohort study. Influenza Other Respir Viruses 2022; 17:e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan ST, Kwan AT, Rodriguez-Barraquer I, et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat Med 2023; 29:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kubale J, Balmaseda A, Frutos AM, et al. Association of SARS-CoV-2 seropositivity and symptomatic reinfection in children in Nicaragua. JAMA Netw Open 2022; 5:e2218794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Madhi SA, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with Omicron variant in South Africa. N Engl J Med 2022; 386:1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almudarra S, Kamel S, Saleh E, et al. High seroprevalence of SARS-CoV-2 among high-density communities in Saudi Arabia. Infection 2022; 50:643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Interim statement on COVID-19 vaccination for children and adolescents. World Health Organization, 2021.
- 9. Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol 2021; 17:e1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim J, Choe YJ, Lee J, et al. Role of children in household transmission of COVID-19. Arch Dis Child 2021; 106:709–11. [DOI] [PubMed] [Google Scholar]
- 11. Zhu Y, Bloxham CJ, Hulme KD, et al. A meta-analysis on the role of children in severe acute respiratory syndrome coronavirus 2 in household transmission clusters. Clin Infect Dis 2021; 72:e1146–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li F, Li YY, Liu MJ, et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis 2021; 21:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu Y, Xia Y, Pickering J, Bowen AC, Short KR. The role of children in SARS-CoV-2 variant of concerns transmission within households: a meta-analysis. medRxiv 2022: 2022.07.21.22277914. [DOI] [PMC free article] [PubMed]
- 14. Maier HE, Kuan G, Saborio S, et al. Clinical spectrum of SARS-CoV-2 infection and protection from symptomatic re-infection. Clin Infect Dis 2021; 75:e257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maier HE, Balmaseda A, Saborio S, et al. Protection associated with previous SARS-CoV-2 infection in Nicaragua. N Engl J Med 2022; 387:568–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kubale JT, Frutos AM, Balmaseda A, et al. High co-circulation of influenza and severe acute respiratory syndrome coronavirus 2. Open Forum Infect Dis 2022; 9:ofac642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 2020; 57:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharker Y, Kenah E. Estimating and interpreting secondary attack risk: binomial considered biased. PLoS Comput Biol 2021; 17:e1008601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenah E. Handbook of infectious disease data analysis. In: Lee H, Nee H, PDee O, Jee W, eds. Chapman & Hall/CRC handbooks of modern statistical methods. New York, USA: CRC Press, 2020:221–42. [Google Scholar]
- 20. Sharker Y, Kenah E. Pairwise accelerated failure time models for infectious disease transmission with external sources of infection. arXiv 2019.
- 21. Ending isolation and precautions for people with COVID-19: interim guidance. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html. Accessed 5 June 2022.
- 22. Xin H, Li Y, Wu P, et al. Estimating the latent period of coronavirus disease 2019 (COVID-19). Clin Infect Dis 2022; 74:1678–81. [DOI] [PubMed] [Google Scholar]
- 23. Zhao S, Tang B, Musa SS, et al. Estimating the generation interval and inferring the latent period of COVID-19 from the contact tracing data. Epidemics 2021; 36:100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenah E, Yang Y. Transtat: statistical methods for infectious disease transmission. Available at: https://github.com/ekenah/transtat. Accessed 6 May 2022.
- 25. Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021; 595:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med 2022; 386:2201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silverberg SL, Zhang BY, Li SNJ, et al. Child transmission of SARS-CoV-2: a systematic review and meta-analysis. BMC Pediatr 2022; 22:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan T, Rahman M, Ali FA, et al. Distinct antibody repertoires against endemic human coronaviruses in children and adults. JCI Insight 2021; 6:e144499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Chiara C, Cantarutti A, Costenaro P, et al. Long-term immune response to SARS-CoV-2 infection among children and adults after mild infection. JAMA Netw Open 2022; 5:e2221616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen F, Tian Y, Zhang L, Shi Y. The role of children in household transmission of COVID-19: a systematic review and meta-analysis. Int J Infect Dis 2022; 122:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lavine JS, Bjornstad ON, Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 2021; 371:741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.