Abstract
Background
Cryptococcal meningitis (CM) is a major cause of morbidity and mortality in persons with human immunodeficiency virus (HIV; PWH). Little is known about CM outcomes and availability of diagnostic and treatment modalities globally.
Methods
In this retrospective cohort study, we investigated CM incidence and all-cause mortality in PWH in the International Epidemiology Databases to Evaluate AIDS cohort from 1996 to 2017. We estimated incidence using quasi-Poisson models adjusted for sex, age, calendar year, CD4 cell count (CD4), and antiretroviral therapy (ART) status. Mortality after CM diagnosis was examined using multivariable Cox models. A site survey from 2017 assessed availability of CM diagnostic and treatment modalities.
Results
Among 518 852 PWH, there were 3857 cases of CM with an estimated incidence of 1.54 per 1000 person-years. Mortality over a median of 2.6 years of post-CM diagnosis follow-up was 31.6%, with 29% lost to follow-up. In total, 2478 (64%) were diagnosed with CM after ART start with a median of 253 days from ART start to CM diagnosis. Older age (hazard [HR], 1.31 for 50 vs 35 years), lower CD4 (HR, 1.15 for 200 vs 350 cells/mm3), and earlier year of CM diagnosis (HR, 0.51 for 2015 vs 2000) were associated with higher mortality. Of 89 sites, 34% reported access to amphotericin B; 12% had access to flucytosine.
Conclusions
Mortality after CM diagnosis was high. A substantial portion of CM cases occurred after ART start, though incidence and mortality may be higher than reported due to ascertainment bias. Many sites lacked access to recommended CM treatment.
Keywords: HIV, AIDS, cryptococcal meningitis, global health
In this global cohort of persons with human immunodeficiency virus spanning across the antiretroviral therapy (ART) era, mortality due to cryptococcal meningitis (CM) was high, and a substantial portion of CM cases occurred after ART start. Many sites lacked access to recommended CM treatment.
Cryptococcal meningitis (CM) is a major cause of morbidity and mortality in persons with human immunodeficiency virus (HIV; PWH), both before and after the advent of combination antiretroviral therapy (ART). Mortality rates from CM in several studies are approximately 40% [1–3], representing a significant burden to the individual patient as well as healthcare systems and resources worldwide. Estimates range from 181 000 to 625 000 annual deaths due to CM [2, 4]. Further, mortality varies significantly according to country income [5]. Although there have been several studies of CM in resource-limited settings, particularly in sub-Saharan Africa, there are gaps in our knowledge regarding the full impact of CM on PWH globally. Few studies have investigated the burden of CM after global availability of ART, recognizing that access to ART varied worldwide. Additionally, although there has been some study of this in sub-Saharan Africa [6], global data of CM incidence in relation to ART start are scarce. Further, lack of access to certain diagnostic and treatment modalities may lead to variance from recommended guidelines and impact patient outcomes, even in today's era.
These knowledge gaps warrant further exploration. The largest study to date of CM outcomes in PWH from Latin America (and 1 clinic in North America) reported an overall mortality of 42% among 340 individuals with CM from 1985 to 2014. In that cohort, the majority developed CM after initiating ART, with a median time to CM diagnosis of 2 years, and 76% of patients had documented virologic failure preceding CM diagnosis [7]. Those with CM after ART start had a 28% higher risk of death compared with those with CM prior to ART start. This study highlighted the need to consider CM as a possible complication even months to years after starting ART, especially in those with virologic failure. It also underscored the high mortality associated with CM, even at the North American site (33%), despite access to state-of-the-art diagnostic and treatment modalities.
We therefore conducted an observational study to examine CM incidence, including before and after ART initiation, and all-cause mortality rates in a large global cohort of PWH. We also sought to describe current practices for treating CM across these global regions.
METHODS
In this retrospective, observational, cohort study, we investigated CM incidence and all-cause mortality after CM diagnosis in the International Epidemiology Databases to Evaluate AIDS (IeDEA) cohort from 1996 to 2017. IeDEA is a research consortium established by the National Institute of Allergy and Infectious Diseases to provide globally diverse HIV/AIDS data (www.iedea.org). This study used data from the North America, Latin America, Asia-Pacific, East Africa, and Southern Africa IeDEA regions. The IeDEA regions of West Africa and Central Africa did not participate as they do not routinely collect data on opportunistic infections. Data were collected from clinical records at local sites; harmonized, cleaned, and merged by each region's data coordinating center; and then merged across regions for analysis. All data collection and analyses were approved by local and regionally centralized institutional review boards, and all study activities adhered to the Declaration of Helsinki standards.
PWH who were aged ≥16 years and enrolled at IeDEA sites between 1 January 1996 and 31 December 2017 were included. Patients were followed from enrollment until the first occurrence of death, loss to follow-up (LTFU), or site-specific database closing date (see Supplementary Material). All-cause mortality was used to define death. LTFU was defined as no clinic or laboratory visit within 12 months prior to the site-specific database closing date; for those lost to follow-up, the date of last visit marked the end of follow-up. Baseline was defined as the date of enrollment in the cohort.
Baseline CD4 cell count (CD4) and plasma HIV-1 RNA level (viral load) were defined as the measurements closest to but no more than 180 days before or 30 days after enrollment. CD4 and viral load at CM diagnosis were defined as the measurements closest to but no more than 90 days before or after date of CM diagnosis. Viral load at enrollment could be undetectable as some patients on ART transferred into care.
CM diagnosis was as noted in the clinical record; most IeDEA data are obtained from routine patient care. Information on site-level capacity in treatment and diagnosis of CM was obtained from the 2017 IeDEA Site Assessment Survey (see Supplementary Appendix 2) [8].
We estimated incidence of CM diagnosis and incidence rate ratios (IRRs) using univariate and multivariable quasi-Poisson models with time at risk included as an offset. Univariate models were used to estimate incidence (and 95% confidence intervals [CIs]) of CM diagnosis within regions, across all regions, and as a function of calendar year across all regions. Multivariable models were adjusted for sex, baseline age, calendar year, time-updated CD4 (square-root transformed), time-updated ART status (initiated vs not), and country-level income (gross domestic product [GDP] per capita in 2010). No adherence data were available for analyses. We did not assess HIV exposure category or viral load as risk factors because of high levels of missingness. To relax linearity assumptions, continuous covariates (age, calendar year, and CD4) were included in models using natural splines with 5 knots [9]. Missing data for variables included in multivariable models were imputed separately within regions using multiple imputation by chained equations with 20 replications. Multivariable analyses using multiply imputed data were performed within IeDEA region with standard errors calculated using Rubin's rules. Results were then combined across regions using random-effects meta-analysis techniques.
Mortality after CM diagnosis was examined using Kaplan–Meier curves, cumulative incidence curves that accounted for LTFU as a competing risk, and univariate and multivariable Cox proportional hazards models. The multivariable Cox model included sex and timing of CM diagnosis (before/after ART initiation) and age, calendar year, and CD4 (square-root transformed) at CM diagnosis. The model was stratified (ie, separate “baseline” hazards estimated) by IeDEA region. ART start date (or first ART date in cohort for patients who transferred in) was used to determine timing of CM diagnosis relative to ART initiation. Missing data were multiply imputed with 20 replications, and continuous variables (age, CD4, and calendar year) were expanded using natural splines with 4 knots (1 fewer knot than for incidence analyses because of the fewer number of events).
RESULTS
Incidence per Region
A total of 518 852 PWH, followed for a median of 3.4 years (interquartile range [IQR], 0.9–7.7) from enrollment, were included. Patient characteristics by IeDEA region and across all regions are shown in Table 1. Median year of enrollment was 2009, and 51% of patients were male. At enrollment, median age was 36 years, median CD4 was 261 cells/mm3, and 71% were ART-naive. Of the total follow-up time, 78% was after ART initiation, and the median time from enrollment to ART initiation was 31 days.
Table 1.
Characteristics of Patients by International Epidemiology Databases to Evaluate AIDS Region and Across all Regions
| Characteristic | Asia-Pacific | Latin America | East Africa | North America | South Africa | Overall |
|---|---|---|---|---|---|---|
| (N = 8319) | (N = 20 324) | (N = 289 159) | (N = 157 370) | (N = 43 680) | (N = 518 852) | |
| Age at enrollment, y | ||||||
| Median (IQR) | 35.8 (30.5–42.4) | 33.8 (27.3–41.8) | 34.0 (27.9–41.4) | 42.0 (34.0–49.2) | 33.7 (28.6–40.3) | 36.1 (29.2–44.4) |
| Range | 16.0–83.3 | 16.0–89.0 | 16.0–100.5 | 16.6–99.0 | 16.0–108.8 | 16.0–108.8 |
| Missing (%) | 0 (0.0) | 0 (0.0) | 4 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.0) |
| Year of enrollment | ||||||
| Median (IQR) | 2008 (2005–2011) | 2010 (2005–2014) | 2010 (2007–2013) | 2004 (2000–2010) | 2008 (2006–2011) | 2009 (2005–2012) |
| Range | 1997–2017 | 1996–2017 | 1998–2017 | 1996–2017 | 1996–2017 | 1996–2017 |
| Sex, N (%) | ||||||
| Female | 2606 (31.3) | 4873 (24.0) | 191 161 (66.1) | 25 071 (15.9) | 28 644 (65.6) | 252 355 (48.6) |
| Male | 5713 (68.7) | 15 451 (76.0) | 97 998 (33.9) | 132 297 (84.1) | 15 031 (34.4) | 266 490 (51.4) |
| Missing (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.0) | 5 (0.0) | 7 (0.0) |
| CD4 at enrollment, cells/mm3 | ||||||
| Median (IQR) | 269 (133–427) | 242 (86–436) | 245 (99–443) | 354 (168–562) | 125 (51–217) | 261 (105–468) |
| Range | 0–4022 | 0–2505 | 0–4600 | 0–4868 | 0–3210 | 0–4868 |
| Missing (%) | 889 (10.7) | 4079 (20.1) | 98 334 (34.0) | 41 005 (26.1) | 11 721 (26.8) | 156 028 (30.1) |
| VL at enrollment, copies/mLa | ||||||
| Median (IQR) | 644 (400–75 001) | 49 300 (5118–190 000) | 792 (400–58 538) | 8562 (400–72 680) | 70 350 (4399–308 662) | 10 900 (400–84 439) |
| < 400 | 2288 (48.2) | 2056 (14.9) | 1461 (40.0) | 36 890 (32.5) | 598 (18.5) | 43 293 (31.2) |
| < 200 | 1949 (41.1) | 1864 (13.5) | 1364 (37.4) | 34 489 (30.4) | 586 (18.2) | 40 252 (29.0) |
| Missing (%) | 3572 (42.9) | 6550 (32) | 285 509 (99) | 43 944 (28) | 40 452 (93) | 380 027 (73) |
| ART-naive at enrollment, N (%) | 2866 (34.5) | 16 659 (82.0) | 230 705 (79.8) | 97 834 (62.2) | 19 282 (44.1) | 367 346 (70.8) |
| Follow-up, y | ||||||
| Median (IQR) | 6.8 (3.4–9.6) | 4.6 (1.5–9.5) | 2.4 (0.5–6.4) | 4.7 (1.7–10.0) | 4.6 (1.5–8.1) | 3.4 (0.9–7.7) |
| Range | 0.0–15.4 | 0.0–22.0 | 0.0–19.0 | 0.0–21.8 | 0.0–21.9 | 0.0–22.0 |
| Percent of follow-up time on ART | 94 | 85 | 76 | 76 | 86 | 78 |
| Median, enrollment to ART start, d | 0 | 26 | 47 | 5 | 50 | 31 |
| CM diagnosis, N (%) | 33 (0.4) | 259 (1.3) | 2289 (0.8) | 890 (0.6) | 386 (0.9) | 3857 (0.7) |
| CM diagnosis year | ||||||
| Median (IQR) | 2009 (2006–2011) | 2008 (2004–2012) | 2010 (2007–2012) | 2004 (2000–2009) | 2008 (2005–2011) | 2008 (2005–2012) |
| Range | 2003–2017 | 1996–2017 | 1998–2017 | 1996–2017 | 2000–2017 | 1996–2017 |
| VL at CM diagnosis, copies/mLa | ||||||
| Median (IQR) | 99 660 (400–211 665) |
75 000 (400–397 086) |
5918 (400–112 720) |
29 100 (400–157 449) |
18 600 (400–210 833) |
26 641 (400–186 484) |
| < 400 | 5 (35.7) | 46 (25.6) | 48 (29.6) | 197 (28.9) | 61 (34.4) | 357 (29.4) |
| < 200 | 5 (35.7) | 37 (20.6) | 42 (25.9) | 175 (25.7) | 61 (34.3) | 320 (26.3) |
| Missing (%) | 19 (57.6) | 79 (30.5) | 2127 (92.9) | 209 (23.5) | 208 (53.9) | 26 432 (68.5) |
| ART-naive at CM diagnosis, N (%) | 3 (9.1) | 98 (37.8) | 907 (39.6) | 190 (21.3) | 181 (46.9) | 1379 (35.8) |
Abbreviations: ART, antiretroviral therapy; CM, cryptococcal meningitis; IQR, interquartile range; VL, viral load.
Percentage is among those with nonmissing values.
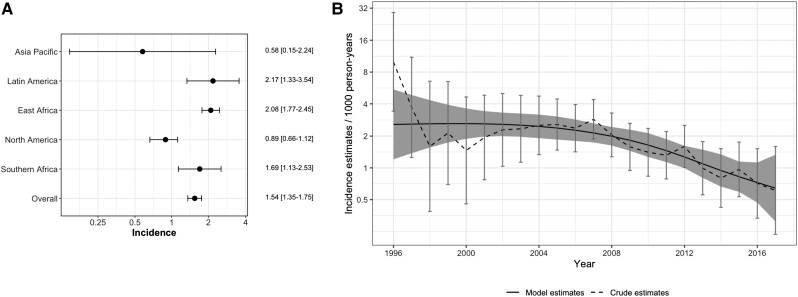
There were 3857 diagnosed cases of CM (0.7%) during follow-up, for an incidence of 1.54 per 1000 person-years (95% CI, 1.35–1.75). Within the regions, Latin America had the highest unadjusted incidence (2.17/1000 person-years) compared with East Africa (2.08/1000 person-years), South Africa (1.69/1000 person-years), North America (0.89/1000 person-years), and Asia-Pacific (0.58/1000 person-years; Figure 1A). Both overall and per region incidence during the first year of follow-up was substantially higher (overall, 5.66/1000 person-years; Supplementary Material). The incidence of CM diagnosis across all sites by calendar year is shown including both crude year-specific estimates and smoothed estimates across years (Figure 1B). In general, the incidence of CM diagnosis decreased over time; for example, the incidence was 2.56/1000 person-years (95% CI, 1.47–4.46) in 2005 compared with 0.97 (95% CI, .54–1.75) in 2015.
Figure 1.
Incidence of cryptococcal meningitis (CM) diagnosis. A, Unadjusted incidence of CM diagnosis per 1000-person years (95% CIs) according to region and overall. B, Incidence (95% CI) of CM diagnosis across all sites according to calendar year; dashed line represents crude estimates for that year, dark line represents model-based estimates smoothing across calendar years. Abbreviation: CI, confidence interval.
Characteristics of Diagnosed CM Cases
Characteristics of patients with and without a diagnosis of CM are shown (Table 2). Of the 3857 CM cases, the median year of diagnosis was 2008, 56.8% were male. Median age at enrollment and CM diagnosis were 36.8 and 38.5 years, respectively. Median CD4 at CM diagnosis was 64 cells/mm3 (IQR, 20–168), and median viral load at CM diagnosis was 26 641 copies/mL, though 68.5% were missing viral load at CM diagnosis. Seventy-two percent of patients with CM diagnosis were ART-naive at enrollment, but only 36% were ART-naive at CM diagnosis. Of those diagnosed with CM after ART start, the median time to CM diagnosis was 253 days (IQR, 56–1064; Table 3). Median year of enrollment for those without vs with a CM diagnosis was 2009 (IQR, 2005–2012) vs 2007 (IQR, 2004–2010), respectively. Median CD4 at enrollment for those with a CM diagnosis was 59 cells/mm3 (IQR, 20–163 cells/mm3) vs 262 cells/mm3 (IQR, 106–469 cells/mm3) for those without a CM diagnosis. The median CD4 for those diagnosed with CM before starting ART was 47 cells/mm3 (IQR, 16–118) vs 77 cells/mm3 (IQR, 24–195) for those diagnosed after starting ART.
Table 2.
Characteristics of Patients Stratified by Cryptococcal Meningitis Diagnosis
| Characteristic | No CM | CM | Overall |
|---|---|---|---|
| (N = 514 995) | (N = 3857) | (N = 518 848) | |
| Age at enrollment, y | |||
| Median (IQR) | 36.1 (29.2–44.5) | 36.8 (30.8–43.6) | 36.0 (29.1–44.4) |
| Range | 16.0–108.8 | 16.0–88.0 | 16.0–108.8 |
| Missing (%) | 4 (0.0) | 0 (0.0) | 4 (0.0) |
| Age at CM diagnosis, y | |||
| Median (IQR) | … | 38.5 (32.3–45.6) | … |
| Range | … | 16.3–90.2 | … |
| Missing (%) | … | 0 (0.0) | … |
| Year of enrollment | |||
| Median (IQR) | 2009 (2005–2012) | 2007 (2004–2010) | 2009 (2005–2012) |
| Range | 1996–2017 | 1996–2017 | 1996–2017 |
| Year of CM diagnosis | |||
| Median (IQR) | … | 2008 (2005–2012) | … |
| Range | … | 1996–2017 | … |
| Sex | |||
| Female | 250 688 (48.7) | 1667 (43.2) | 252 355 (48.6) |
| Male | 264 300 (51.3) | 2190 (56.8) | 266 490 (51.4) |
| Missing (%) | 7 (0.0) | 0 (0.0) | 7 (0.0) |
| CD4 at enrollment, cells/mm3 | |||
| Median (IQR) | 262 (106–469) | 59 (20–163) | 261 (105–468) |
| Range | 0–4868 | 0–1963 | 0–4868 |
| Missing (%) | 154 530 (30.0) | 1498 (38.8) | 156 028 (30.1) |
| CD4 at CM diagnosis, cells/mm3 | |||
| Median (IQR) | … | 64 (20–168) | … |
| Range | … | 0–3695 | … |
| Missing (%) | … | 1066 (27.6) | … |
| VL at enrollment, copies/mLa | |||
| Median (IQR) | 10 712 (400–83 500) | 87 387 (12 381–312 096) | 10 900 (400–84 439) |
| < 400 | 43 199 (31.3) | 94 (13.1) | 43 293 (8.2) |
| < 200 | 40 165 (29.1) | 87 (12.2) | 40 252 (7.7) |
| Missing (%) | 376 885 (73.2) | 3142 (81.5) | 380 027 (3.2) |
| VL at CM diagnosis, copies/mLa | |||
| Median (IQR) | … | 26 641 (<400–186 484) | … |
| < 400 | … | 357 (29.4) | … |
| < 200 | … | 320 (26.3) | … |
| Missing (%) | … | 2642 (68.5) | … |
| ART-naive at enrollment | 364 572 (70.8) | 2774 (71.9) | 367 346 (70.8) |
| ART-naive at CM diagnosis | … | 1379 (35.8) | … |
Abbreviations: ART, antiretroviral therapy; CM, cryptococcal meningitis; IQR, interquartile range; VL, viral load.
Percentage is among those with nonmissing values.
Table 3.
Characteristics of Patients Diagnosed With Cryptococcal Meningitis by Timing of Antiretroviral Therapy
| Characteristic | Diagnosis Before ART | Diagnosis After ART | Overall |
|---|---|---|---|
| (N = 1379) | (N = 2478) | (N = 3857) | |
| Region | |||
| Asia-Pacific | 3 (0.2) | 30 (1.2) | 33 (0.9) |
| Latin America | 98 (7.1) | 161 (6.5) | 259 (6.7) |
| East Africa | 907 (65.8) | 1382 (55.8) | 2289 (59.3) |
| North America | 190 (13.8) | 700 (28.2) | 890 (23.1) |
| South Africa | 181 (13.1) | 205 (8.3) | 386 (10.0) |
| Age at enrollment, y | |||
| Median (IQR) | 36.5 (30.9–43.2) | 36.8 (30.8–43.8) | 36.8 (30.8–43.6) |
| Range | 16.8–88.0 | 16.0–73.2 | 16.0–88.0 |
| Age at CM diagnosis, y | |||
| Median (IQR) | 37.0 (31.4–43.6) | 39.3 (32.9–46.3) | 38.5 (32.3–45.6) |
| Range | 16.8–90.2 | 16.3–77.5 | 16.3–90.2 |
| Year of enrollment | |||
| Median (IQR) | 2008 (2005–2011) | 2006 (2003–2010) | 2007 (2004–2010) |
| Range | 1996–2017 | 1996–2017 | 1996–2017 |
| CM diagnosis year | |||
| Median (IQR) | 2008 (2005–2012) | 2009 (2005–2012) | 2008 (2005–2012) |
| Range | 1996–2017 | 1996–2017 | 1996–2017 |
| Male sex | 745 (54.0) | 1445 (58.3) | 2190 (56.8) |
| CD4 at enrollment, cells/mm3 | |||
| Median (IQR) | 52 (18–143) | 66 (21–170) | 59 (20–163) |
| Range | 1–1175 | 0–1963 | 0–1963 |
| Missing (%) | 621 (45.0) | 877 (35.4) | 1498 (38.8) |
| CD4 at CM diagnosis, cells/mm3 | |||
| Median (IQR) | 47 (16–118) | 77 (24–195) | 64 (20–168) |
| Range | 1–1308 | 0–3695 | 0–3695 |
| Missing (%) | 464 (33.6) | 602 (24.3) | 1066 (27.6) |
| VL at enrollment, copies/mLa | |||
| Median (IQR) | 147 865 (38 562–475 134) | 70 850 (6356–235 777) | 87 387 (12 381–312 096) |
| < 400 | 14 (1.0) | 80 (3.2) | 94 (2.4) |
| < 200 | 13 (0.9) | 74 (3.0) | 87 (2.3) |
| Missing (%) | 1176 (85) | 1966 (79) | 3142 (81) |
| VL at CM diagnosis, copies/mLa | |||
| Median (IQR) | 145 740 (39 091–438 590) | 7076 (400–106 123) | 26 641 (400–186 484) |
| < 400 | 20 (1.5) | 337 (13.6) | 357 (9.3) |
| < 200 | 18 (1.3) | 302 (12.2) | 320 (8.3) |
| Missing (%) | 1088 (79) | 1554 (63) | 2642 (68) |
| ART-naive at enrollment | 1379 (100.0) | 2063 (83.3) | 3442 (89.2) |
| Time to CM from ART, d | |||
| Median (IQR) | – | 253 (56–1064) | – |
| Range | – | 1–6604 | – |
| Follow-up after CM diagnosis, y | |||
| Median (IQR) | 1.3 (0.2–6.2) | 3.2 (0.6–7.4) | 2.6 (0.3–7.0) |
| Range | 0.0–21.5 | 0.0–19.5 | 0.0–21.5 |
| Loss to follow-up | 530 (38.4) | 591 (23.8) | 1121 (29.1) |
| Mortality status: dead | 424 (30.7) | 793 (32.0) | 1217 (31.6) |
| Time to death, y | |||
| Median (IQR) | 0.3 (0.1–1.6) | 0.7 (0.1–2.6) | 0.5 (0.1–2.3) |
| Range | 0.0–17.6 | 0.0–18.8 | 0.0–18.8 |
Abbreviations: ART, antiretroviral therapy; CM, cryptococcal meningitis; IQR, interquartile range; VL, viral load.
Percentage is among those with nonmissing values.
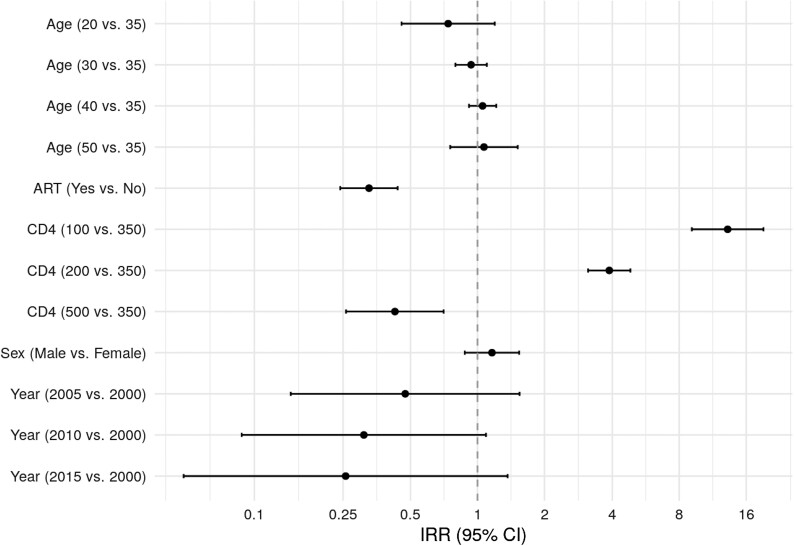
Figure 2 shows risk factors associated with CM diagnosis, combining estimates across all regions. Current CD4 was highly predictive of CM. In adjusted analyses, the incidence rate for a patient with CD4 = 200 cells/mm3 was 3.89 times higher than for a patient with CD4 = 350 cells/mm3 (95% CI, 3.13–4.84). Being on ART was also highly protective of CM, with an estimated IRR of 0.33 (95% CI, .24–.44). The risk of CM tended to be lower in more recent years (IRR, 0.23; 95% CI, .04–1.28, comparing 2015 vs 2000), although this was quite variable across regions. Age and sex were not statistically associated with CM diagnosis in adjusted models.
Figure 2.
Estimated IRR (95% CI) of risk factors for cryptococcal meningitis combined across all regions, in adjusted analyses. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; IRR, incidence rate ratio.
Timing of CM Diagnosis and All-Cause Mortality
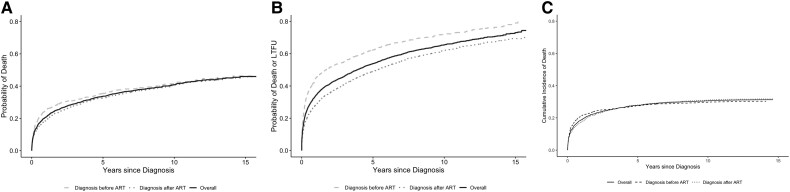
Overall mortality for those diagnosed with CM was 31.6% (95% CI, 30.1–33.0) over a median follow-up of 2.6 years after CM diagnosis (IQR, 0.3–7.0); 1121 (29%) were lost to follow-up (Table 3). Mortality differed by region; Asia-Pacific had a mortality of 24.2% (8 of 33) among those with CM diagnosis, followed by East Africa at 26.5% (607 of 2289), South Africa at 28.2% (109 of 386), Latin America at 42.5% (110 of 259), and North America at 43% (383 of 890). However, the proportions lost to follow-up were high and inversely correlated with mortality rates by region: Asia-Pacific 36.4%, East Africa 32.3%, South Africa 37.6%, Latin America 19.3%, and North America 19.6%. LTFU was higher for those who were diagnosed with CM before ART initiation: 38.4% vs 23.8%. Figure 3 shows over time after CM diagnosis the estimated probability of death (Figure 3A), the estimated probability of death or LTFU (Figure 3B), and the cumulative incidence of death treating LTFU as a competing event (Figure 3C), both overall and stratified by whether the diagnosis was before or after ART initiation. At 5 years after CM diagnosis, for those diagnosed before ART initiation and after ART initiation, respectively, the estimated probabilities of death were 0.35 (95% CI, .32–.38) and 0.33 (95% CI, .35–.31); the estimated probabilities of death or LTFU were 0.62 (95% CI, .60–.65) and 0.49 (95% CI, .47–.51); and the estimated cumulative incidences of death treating LTFU as a competing event were 0.27 (95% CI, .25–.30) and 0.28 (95% CI, .26–.30).
Figure 3.
All-cause mortality after diagnosis of cryptococcal meningitis, overall and stratified by whether diagnosis was before or after ART initiation. A, Estimated probabilities based on Kaplan–Meier curves. B, Estimated probability of death or loss to follow-up based on Kaplan–Meier curves. C, Estimated cumulative incidence of death treating loss to follow-up as a competing event. Abbreviation: ART, antiretroviral therapy.
Risk Factors for Mortality
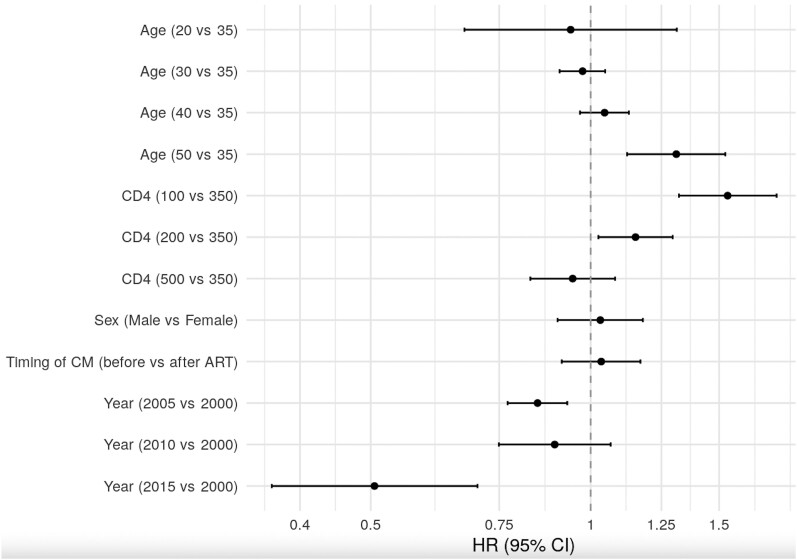
In unadjusted analyses, the cause-specific hazard of death was higher for those diagnosed with CM before ART initiation (hazard ratio [HR], 1.16; 95% CI, 1.03–1.30), but this association was not significant (HR, 1.03; 95% CI, .91–1.17) adjusting for CD4 at CM diagnosis, sex, age, and year of CM diagnosis and stratifying by IeDEA region. Figure 4 shows the association between these other variables and all-cause mortality. Older age (HR, 1.31 for 50 vs 35 years; 95% CI, 1.12–1.53), lower CD4 (HR, 1.15 for 200 vs 350 cells/mm3; 95% CI, 1.03–1.30), and earlier year of CM diagnosis (HR, 0.51 for 2015 vs 2000; 95% CI, .37–.70) were associated with higher risks of mortality (Figure 4).
Figure 4.
Estimated HRs (95% CIs) of risk factors for all-cause mortality after diagnosis of CM combined across regions, in adjusted analyses. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CM, cryptococcal meningitis; HR, hazard ratio.
Site Survey Results
Eighty-nine sites across all 5 geographical regions responded to questions in 2017 regarding availability of diagnostic testing and treatment for CM (Supplementary Appendix 2). In total, 53% of sites reported access to serum cryptococcal antigen testing in 2017, and 37% had access to lateral flow assays for detection of cryptococcal antigen. Nearly two-thirds of sites reported access to fluconazole, with 8% responding that they did not have access to fluconazole in 2017 (29% had missing data). Only 34% of sites reported access to amphotericin B, with 37% responding that it was not available. Only 11 of 89 (12%) sites reported they had access to 5-flucytosine (5-FC) in 2017.
DISCUSSION
This study highlights several key findings with implications for clinical care of CM globally. First, the incidence of CM diagnosis decreased over time and varied across regions. Second, diagnosis of CM occurred frequently after ART initiation. Third, mortality after CM diagnosis was high but varied across regions, and LTFU was also high. Finally, CM testing/screening and currently recommended CM treatment regimens that are safe and effective are often out of reach for many in low- to middle-income settings.
This study documents the ongoing high mortality rate associated with CM and raises the question as to why survival has not improved more substantially over time. We report an overall mortality similar to that from past studies [10, 11]. Regional differences in mortality shown in our study may be due to ascertainment but also to variability in the ability to diagnose and treat CM. Lack of access to “gold standard” treatment regimens may be one possible explanation. Even in 2017, only 12% of sites reported access to 5-FC, which has been shown to lower mortality when used in combination treatment of CM compared with amphotericin and fluconazole, or non-flucytosine-containing regimens [1, 12]. At that time, the World Health Organization’s (WHO’s) first-line recommended regimen and 1 of the 2 alternative regimens included flucytosine [13]. In our survey, 38% of sites reported no access to amphotericin and 8% reported no access to fluconazole. These data, and the ongoing high mortality repeatedly demonstrated in global cohorts of PWH diagnosed with CM, highlight an urgent need for health equity in access to CM diagnostic tools and standard-of-care treatments.
It is significant that in the 4 decades since recognition of HIV/AIDS and accompanying opportunistic infections, research on new CM treatments has been scarce. Antifungal agents with improved ease of administration and favorable central nervous system penetration (such as voriconazole) [14] have been little studied against CM in the global south. In the randomized, controlled Advancing Cryptococcal Meningitis Treatment for Africa (ACTA) trial of 721 patients with CM and HIV in Africa, it was found that 1 week of AmB plus 5-FC and 2 weeks of fluconazole plus 5-FC were effective regimens for induction therapy for CM in resource-limited settings [1]. Based on these results, the WHO in 2018 recommended induction therapy with the less toxic and more efficacious 1-week regimen of AmB and 5-FC in resource-limited settings [13]. In the AMBisome Therapyinduction OptimizatioN (AMBITION) trial, an open-label, phase 3, randomized, controlled trial conducted in Africa among 814 PWH with CM, single-dose liposomal AmB combined with 5-FC and fluconazole was noninferior to the WHO-recommended treatment for HIV-associated CM and was associated with fewer adverse events [15]. Based on results from the AMBITION trial, the WHO further updated their guidance in 2022 [16], recommending that a single dose of liposomal AmB with 14 days of flucytosine and fluconazole is the recommended regimen for induction. Alternative induction regimens are listed for situations in which amphotericin or flucytosine is not available [16]. However, these regimens may not be available in most affected regions, as our survey data show. While more randomized, controlled trials for the treatment of CM in PWH are needed, they must be paired with emphatic calls for access to the medications proven safer and more effective.
One of the most surprising findings of this analysis (as reported in our Latin America cohort [7]) was the high rate of CM diagnosis after ART start, representing 64% of all diagnosed CM cases. Here, the median time to CM diagnosis from ART start was 8 months, suggesting that most often, this did not reflect an “unmasking” of CM after ART start or immune reconstitution syndrome. Indeed, in the Latin America cohort, 76% of those with CM after ART start had evidence of virologic failure [7]. Although we were limited by a high level of missingness, the median viral load at CM diagnosis after ART start was 7076 copies/mL. This high rate of CM diagnosis after ART start as well as a detectable viral load around the time of CM diagnosis hint at CM as a complication of virologic failure.
Our study has important limitations. First, because of diagnostic limitations that varied across time and clinical sites, there may have been differential ascertainment of CM. We did not have data on availability of diagnostic tools for CM over time in all regions. Hence, it is possible that in certain regions, the incidence of CM may be higher than our reported estimates. Our study had high rates of LTFU, including after CM diagnosis; those who are lost to follow-up might be expected to have higher mortality than those who remain in care. Additionally, the study is limited by high rates of missing data at the time of CM diagnosis, particularly missing viral load data and measures of adherence. Other limitations include potential measurement errors in other analysis variables, in part, due to harmonizing clinical data collected differently at multiple sites. The use of GDP in 2010 was a crude measure of socioeconomic differences between countries within regions. Furthermore, our study only captured CM diagnoses among individuals engaged in care, so our estimates may not be generalizable to all PWH. There may also be differences in definitions of variables (eg, LTFU) when comparing our results with other studies. Despite these potential shortcomings, an important strength of this study is the pooling of data from clinical cohorts across multiple sites globally, resulting in a rich source of data with diversity of clinical and patient characteristics analyzed using robust methods and including adjustment for important confounders.
CONCLUSIONS
Our large study across 5 IeDEA regions straddles the ART era and represents CM outcomes in low-, middle-, and high-income countries. We describe an ongoing high, albeit decreasing, incidence of CM in PWH, with substantial immediate and delayed all-cause mortality in PWH and CM. Moreover, providing data on global availability of testing and treatment capacity for CM highlights inequities in resources that could contribute to poor clinical outcomes. Further studies are needed to identify novel and accessible diagnostic modalities and improved treatment regimens that are readily accessible to resource-rich and resource-poor regions alike.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Anna K Person, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Brenda Crabtree-Ramirez, Departamento de Infectología, Instituto Nacional de Ciencias Médicas y Nutrición, Mexico City, Mexico.
Ahra Kim, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Valdiléa Veloso, Intituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
Fernanda Maruri, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Gilles Wandeler, Department of Infectious Diseases, Bern University Hospital, University of Bern, Bern, Switzerland.
Matthew Fox, Department of Global Health, Boston University, Boston, Massachusetts, USA.
Richard Moore, Center for Global Health, Johns Hopkins University, Baltimore, Maryland, USA.
M John Gill, Department of Medicine, University of Calgary, Calgary, Alberta, Canada.
Darma Imran, Cipto Mangunkusumo Hospital, Jakarta, Indonesia.
Kinh Van Nguyen, National Hospital of Tropical Diseases, Hanoi, Viet Nam.
Elizabeth Nalitya, Infectious Diseases Institute, College of Health Sciences, Makerere University, Kampala, Uganda.
Winnie Muyindike, Department of Internal Medicine, Mbarara University of Science and Technology and Mbarara Regional Referral Hospital, Mbarara, Uganda.
Bryan E Shepherd, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Catherine C McGowan, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Notes
Disclaimer. This work is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions listed here.
Financial support. The International Epidemiology Databases to Evaluate AIDS is supported by the National Institutes of Health's (NIH’s) National Institute of Allergy and Infectious Diseases; the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute of Mental Health; the National Institute on Drug Abuse; the National Heart, Lung, and Blood Institute; the National Institute on Alcohol Abuse and Alcoholism; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Fogarty International Center: Asia-Pacific, U01AI069907; Caribbean, Central and South America network for HIV epidemiology (CCASAnet), U01AI069923 (A. K. P. is listed as an investigator); East Africa, U01AI069911; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), U01AI069918; Southern Africa, U01AI069924. Informatics resources are supported by the Harmonist Project, R24AI24872. REDCap support was provided by UL1 TR000445 from National Center for Advancing Translational Sciences (NCATS)/NIH.
References
- 1. Molloy SF, Kanyama C, Heyderman RS, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 2. Lawrence DS, Boyer-Chammard T, Jarvis JN. Emerging concepts in HIV-associated cryptococcal meningitis. Curr Opin Infect Dis 2019; 32:16–23. [DOI] [PubMed] [Google Scholar]
- 3. Beardsley J, Wolbers M, Kibengo FM, et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajasingham R, Govender NP, Jordan A, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 2022; 22:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rhein J, Hullsiek KH, Evans EE, et al. Detrimental outcomes of unmasking cryptococcal meningitis with recent ART initiation. Open Forum Infect Dis 2018; 5:ofy122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramírez B C, Vega Y C, Shepherd BE, et al. Outcomes of HIV-positive patients with cryptococcal meningitis in the Americas. Int J Infect Dis 2017; 63:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duda SN, Farr AM, Lindegren ML, et al. Characteristics and comprehensiveness of adult HIV care and treatment programmes in Asia-Pacific, sub-Saharan Africa and the Americas: results of a site assessment conducted by the International epidemiologic Databases to Evaluate AIDS (IeDEA) Collaboration. J Int AIDS Soc 2014; 17:19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shepherd BE, Rebeiro PF, Caribbean, Central and South America Network for HIV Epidemiology . Brief report: assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J Acquir Immune Defic Syndr 1999. 2017; 74(3):e60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. AIDS 2007; 21:2119–29. [DOI] [PubMed] [Google Scholar]
- 11. Bamba S, Lortholary O, Sawadogo A, Millogo A, Guiguemdé RT, Bretagne S. Decreasing incidence of cryptococcal meningitis in West Africa in the era of highly active antiretroviral therapy. AIDS 2012; 26:1039–41. [DOI] [PubMed] [Google Scholar]
- 12. Tenforde MW, Shapiro AE, Rouse B, et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev 2018; 7:CD005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children: supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. World Health Organization; 2018. Accessed 27 January 2021. Available at: http://www.ncbi.nlm.nih.gov/books/NBK531449/. [PubMed]
- 14. Yao Y, Zhang JT, Yan B, et al. Voriconazole: a novel treatment option for cryptococcal meningitis. Infect Dis 2015; 47:694–700. [DOI] [PubMed] [Google Scholar]
- 15. Jarvis JN, Lawrence DS, Meya DB, et al. Single-dose liposomal amphotericin B treatment for cryptococcal meningitis. N Engl J Med 2022; 386:1109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. Accessed 29 June 2022. Available at: https://www.who.int/publications-detail-redirect/9789240052178. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.