Abstract
Background
With more than 15,000 new cases /year in France and 2,000 deaths, cutaneous melanoma represents approximately 4% of incidental cancers and 1.2% of cancer related deaths. In locally advanced (stage III) or resectable metastatic (stage IV) melanomas, medical adjuvant treatment is proposed and recent advances had shown the benefit of anti-PD1/PDL1 and anti-CTLA4 immunotherapy as well as anti-BRAF and anti-MEK targeted therapy in BRAF V600 mutated tumors. However, the recurence rate at one year is approximately 30% and justify extensive research of predictive biomarkers. If in metastatic disease, the follow-up of circulating tumor DNA (ctDNA) has been demonstrated, its interest in adjuvant setting remains to be precised, especially because of a lower detection rate. Further, the definition of a molecular response could prove useful to personalized treatment.
Methods
PERCIMEL is an open prospective multicentric study executed through collaboration of the Institut de Cancérologie de Lorraine (non-profit comprehensive cancer center) and 6 French university and community hospitals. A total of 165 patients with resected stage III and IV melanoma, eligible to adjuvant imunotherapy or anti-BRAF/MEK kinase inhibitors will be included. The primary endpoint is the presence of ctDNA, 2 to 3 weeks after surgery, defined as mutated ctDNA copy number calculated as the allelic fraction of a clonal mutation relative to total ctDNA. Secondary endpoints are recurrence-free survival, distant metastasis-free survival and specific survival. We will follow ctDNA along treatment, quantitatively through ctDNA mutated copy number variation, qualitatively through the presence of cfDNA and its clonal evolution. Relative and absolute variations of ctDNA during follow-up will be also analyzed.
PERCIMEL study aims at provide scientific evidence that ctDNA quantitative and qualitative variations can be used to predict the recurrence of patients with melanoma treated with adjuvant immunotherapy or kinase inhibitors, thus defining the notion of molecular recurrence.
Keywords: Circulating tumor DNA, Melanoma, Adjuvant therapy, Immunotherapy, Kinase inhibitors
Background
With an estimated 15,500 new cases in 2018 in metropolitan France (7,900 men and 7,600 women), and 1,880 deaths (1,040 men and 840 women), cutaneous melanoma accounts for around 4% of all incident cancers, and 1.2% of all cancer-related deaths among both sexes. It is one of the forms of cancer that has seen its incidence and mortality rise significantly in the last 40 years (data from the National Cancer Institute, www.e-cancer.fr). At locally advanced or metastatic stages, relative survival at 5 years is around 60% in patients with loco-regional cancer, and 15% in those with metastasis. At earlier stages of the disease, relative 5-year survival is above 90%, but although patients can be potentially cured by surgery, 13% will go on to develop loco-regional or metastatic disease within 2 years.
Standard treatment for locally advanced melanoma that is amenable to surgery (stage III disease) is surgical resection, with lymph node dissection in case of macroscopic lymph node involvement, or sentinel node biopsy in the absence of detectable macroscopic lymph node involvement. For metastatic melanoma (stage IV), metastasis amenable to complete surgical removal without residual disease should undergo surgery. In these cases, adjuvant medical therapy is proposed.
Since the advent of the first adjuvant treatments based on interferon α2b, the therapeutic arsenal has increased considerably, with immunotherapies such anti-PD1 [1, 2], and targeted therapies using the tyrosine kinase BRAF and MEK inhibitors for patients harbouring activating mutations of the BRAF gene [3].
Recently published analyses reporting 3 to 5-year survival have confirmed the value of adjuvant treatment for operable stage III and IV melanoma. Accordingly, in the randomized, phase III KEYNOTE-054 study, which investigated the anti-PD1 monoclonal antibody pembrolizumab versus placebo in resected high-risk stage III melanoma, the results showed a significant prolongation of recurrence-free survival (RFS) at 3 years (63.7% vs 44.1%, hazard ratio (HR), 0.56; 95% confidence interval (CI), 0.47 to 0.68) [4]. Similar findings were also reported from the randomized, phase III Checkmate 238 study, which tested the anti-PD1 monoclonal antibody nivolumab versus immunotherapy with the anti-CTLA4 monoclonal antibody ipilimumab in patients with resected stage IIIB–C or stage IV melanoma, and found a sustained RFS benefit in favour of nivolumab at 4 years (51.7% versus 41.2% with ipilimumab (HR, 0.71, 95% CI 0.60–0.86) [5]. In addition, in the randomized, phase III Combi-AD trial, evaluating the association of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib versus placebo in patients with BRAFV600 mutant melanoma, the results showed a significant improvement in RFS at 5 years, with 52% RFS in the dabrafenib plus trametinib arm versus 36% in the control group (HR, 0.51; 95% CI, 0.42 to 0.61) [6].
In a recent published paper, [7] the authors presented the results of a neoadjuvant and adjuvant or adjuvant only Pembroluzumab in locally advances melanoma. They included 313 patients, 154 in the neoadjuvant-adjuvant arm and 159 in the adjuvant only arm. All patients had clinically detectable measurable disease stage IIIB to IIID melanoma or oligometastatic resectable stage IV. Primary objective was EFS (Event Free Survival). With a median follow up of 14,7 months they demonstrated significant benefit for the neoadjuvant – adjuvant arm, with an EFS of 72% at 2 years versus 49% in the adjuvant arm with a p value at 0.004. This new strategy will be considered very soon as a standard of care, and needs to be integrated in our study, an amendment is in preparation.
However, the rate of relapse at one year after the end of treatment is around 30%, underlining the need to find predictive markers of early relapse [6].
In this context, analysis of circulating tumor DNA (ctDNA) is of relevance. The presence of nucleic acid in the blood was first described in 1948, but it has only recently been discovered that tumours are capable of actively and passively releasing their DNA into body fluids (for review, see [8]). Technological progress, notably in molecular biology, now makes it possible to detect ctDNA and to envisage clinical applications using ctDNA detection in the field of medical oncology. The detection of ctDNA, i.e. genetic material released by the tumour during necrosis, apoptosis or other active cell phenomena, could be used for the early detection of cancer, for theranostic applications, for follow-up of patients, to evaluate the quality of surgery or for the early detection of relapse [8]. Indeed, it has been shown that the concentration of ctDNA is correlated to tumour burden and consequently, is higher in patients with metastasis.
In the setting of melanoma, the majority of studies have been performed in metastatic, inoperable disease [9–17]), where ctDNA was more easily detectable, and frequently observed (70–90%).
These studies were performed in patients with non-resectable, stage III or metastatic (stage IV) melanoma, treated with BRAF inhibitors, with or without MEK inhibitors. The concentration of ctDNA before, and 4 weeks after treatment was shown to be a prognostic factor for survival [16]. Prior to the advent of adjuvant therapies, studies also showed the utility of ctDNA in operable advanced melanoma: ctDNA at 12 weeks after surgery seemed to be of use for predicting survival in patients with operable stage II or III melanoma [18], and also in patients with stage III disease, independently of the substage (a/b/c/d), when ctDNA was measured pre-operatively [19, 20]) or post-operatively [20].
A study of non-resectable advanced melanoma treated by immunotherapy or targeted therapy, with or without chemotherapy, showed that variations in ctDNA during follow-up were an indicator of treatment efficacy, suggesting that ctDNA could be a better reflection of tumoral heterogeneity than tissue biopsy [21].
In patients who undergo surgery, the situation is different, and notably, ctDNA can only be detected in a much smaller percentage of cases (20–40%). Few studies were performed before the advent of adjuvant treatment with immunotherapy or BRAF and MEK inhibitors. For example, the study by Tan et al. [20] in patients with operable, stage III melanoma found that the detection of ctDNA at baseline and after treatment was associated with shorter RFS and inferior distant metastasis-free survival (DMFS). In the same way, other studies [18, 19] performed respectively in 161 stage II/III high-risk melanoma patients and 174 patients with stage III melanoma undergoing complete lymph node dissection, found that the detection of ctDNA at baseline was associated with a higher rate of LN involvement, high lactate dehydrogenase (LDH) levels and worse melanoma-specific survival [19], and the presence of ctDNA post-operatively was associated with shorter metastasis-free and overall survival.
These studies also highlight the value of ctDNA for the prediction of survival in patients with operable stage II and III melanoma [18], and in stage III, regardless of substage (a/b/c/d) when ctDNA is measured at baseline [19, 20] or post-operatively [20]. More recently, the study by Gouda et al. [22] in 80 patients with BRAFV600E mutated melanoma of stage ≤ III, detection of ctDNA after surgery was associated with a higher likelihood of melanoma recurrence and shorter disease-free and overall survival. Furthermore, analyses of ctDNA from patients included in the Checkmate 915 study found that the presence of ctDNA predicted early relapse and inferior progression-free and metastasis-free survival, in patients with resected stage IIIB-D/IV melanoma receiving adjuvant nivolumab with or without ipilimumab [23]).
In these studies, which were almost all performed before the introduction onto the market of kinase inhibitors and immunotherapy in the adjuvant setting, none investigated normalized follow-up of ctDNA during treatment, or the link between the course of ctDNA and therapeutic response.
In the current era of adjuvant therapy, one of the aims of the PERCIMEL study is to determine whether the monitoring of ctDNA makes it possible to predict relapse, thereby defining the concept of molecular relapse.
Objectives
The primary objective of PERCIMEL study is to evaluate the predictive value of the presence of ctDNA post-surgery on recurrence-free survival at 24 months in patients undergoing surgery for cutaneous melanoma and treated with immunotherapy or targeted therapy with BRAF and MEK inhibitors, in an adjuvant setting.
The secondary objectives are:
To evaluate the predictive value of baseline (prior to surgery) ctDNA on survival (recurrence-free survival, distant metastasis-free survival and specific survival).
To analyse the quantitative and clonal course of ctDNA during 24 months of follow-up, or at relapse, to predict survival
To describe the clinical characteristics and the quantitative course of ctDNA during follow-up in patients with no detectable ctDNA at pré and/or post-surgery
To determine the predictive value of ctDNA course in subgroups of patients according to clinical and tumour characteristics at inclusion.
Method/design
The PERCIMEL study is an Interventional multicenter cohort study with minimal risks and constraints.
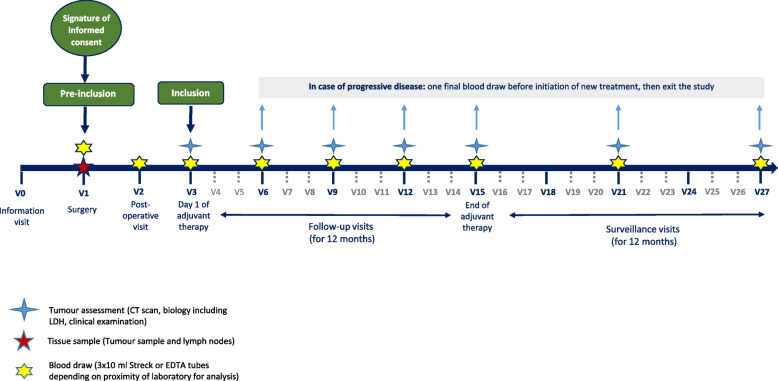
Figure 1 summarizes the study scheduling. The study is registered on ClinicalTrials.gov (NCT04866680), and French Competent Authorithy (ID-RCB: 022-A01904-39).
Fig. 1.
Percimel protocol scheduling
Study population
The PERCIMEL study is proposed to patients diagnosed with histologically-proven cutaneous melanoma, locally advanced, resectable, with complete lymph node dissection upfront in patients presenting with macroscopic lymph node involvement, or with positive sentinel lymph node biopsy (microscopic lesions) or surgery of distant metastasis, followed by adjuvant therapy with immunotherapy or targeted therapy with BRAF and MEK inhibitors, according to currently approved indications.
Inclusion criteria
Patient aged 18 years or older
WHO performance status 0–2
Patient with histologically-proven cutaneous melanoma, locally advanced (stage III) or metastatic and amenable to complete resection (stage IV)
Naïve of all treatments (except for initial biopsy for diagnostic purposes)
Patient with an indication for adjuvant therapy with BRAF and MEK inhibitors or immunotherapy with anti-PD1 monoclonal antibodies, according to currently approved indications
Biological parameters compatible with the proposed treatment
Patient affiliated to a social security regimen, or beneficiary thereof
Signature of informed consent form
Non-inclusion criteria
Patients with mucous melanoma or choroidal (uveal) melanoma
Patient presenting with a synchronous tumour, or having been treated in the 3 years prior to pre-inclusion (except for cervical carcinoma in situ, or resected cutaneous carcinoma)
Patients with a contra-indication to blood draw of 30 ml
Patients with a contra-indication to surgery
Patients with a contra-indication to the proposed adjuvant therapy
Pregnant or breastfeeding women
Patients under any form of judicial or legal protection
Sample size calculation
Our hypothesis is that 25% of patients will have detectable ctDNA post-operatively, i.e. within 2 to 3 weeks after surgery [20].
Recurrence-free survival at 24 months in this population, based on key studies of adjuvant therapies, can be estimated at 70% (upper limit, to ensure a good statistical power) [1, 2, 24].
Based on an inclusion period of 24 months, an alpha risk of 5% and statistical power of 90%, a total of 124 patients would be required to achieve a hazard ratio of 2.5 [18, 20]. By considering 10% of patients lost to follow-up at 24 months or not analysable, then a total of 136 patients are necessary.
It is expected that almost all patients with stage IIIc/IIId/IV melanoma who are pre-included will subsequently be definitively included. Conversely, we estimate that around 70% of patients with stage IIIa-IIIb melanoma who are pre-included will have negative sentinel lymph nodes and therefore, will not be eligible for definitive inclusion. In view of the active pool of patients, we expect that stage IIIa-IIIb patients will account for 25% of all pre-included patients. Thus, to achieve definitive inclusion of 136 patients, we estimate that 165 patients will need to be pre-included.
Statistical analysis
The main objective is to evaluate the predictive value of the presence of ctDNA post-operatively on RFS. The RFS will be described by the Kaplan–Meier method, and compared according to the presence or absence of ctDNA post-surgery using the log rank test. A multivariate Cox regression model will be used to calculate the hazard ratio and 95% confidence interval (CI). As this is a cohort study, the results will be adjusted for baseline patient characteristics and the type of adjuvant therapy. To this end, bivariate analyses will be performed to identify variables related to the RFS. Variables yielding a p-value < 0.10, and the presence of ctDNA post-surgery will be included in the multivariate model. Results will be presented as hazard ratios with 95% CIs.
The same analysis will be performed for ctDNA presence pre-surgery, for the number of copies of mutant DNA per ml of plasma pre- and post-surgery, and for absolute and relative variations of the quantity of mutated DNA between pre- and post-surgery.
The impact of ctDNA evolution during follow-up on RFS will be evaluated using time-dependent survival models, by considering mutated DNA copy number as a time-dependent variable in the Cox model. This analysis will be adjusted for baseline patient characteristics and for the type of adjuvant therapy to assess whether the course of ctDNA during follow-up is an independent prognostic factor for survival.
To determine whether the predictive value of the course of ctDNA varies according to clinical and tumour characteristics at baseline, interaction tests will be performed.
Distant metastasis-free survival will be analysed using the same approach as for RFS. Melanoma-specific survival will also be analysed using the same approach, but considering death unrelated to the disease as a competing risk. Consequently, specific survival will be described as cumulative incidence according to the Kalbfleisch-Prentice method, and compared using Gray’s test. Bi- and multivariate analysis will be performed using the Fine and Gray subdistribution model.
Baseline characteristics of the study population will be compared between patients with and patients without detectable ctDNA pre- and/or post-operatively. Qualitative variables will be compared using the chi square or Fisher’s exact test, and quantitative variables using the Student t or Mann–Whitney U test, according to whether the distribution of the variable is normal or not. Normality will be tested using the Shapiro–Wilk test. A multivariate logistic regression model will be fit to determine the characteristics that best distinguish the two populations. The discriminant power of the model will be determined using the area under the receiving operating characteristic (ROC) curve. In patients with no detectable ctDNA either pre- or post-operatively, the course of quantification of ctDNA will be described by repeated measure ANOVA using the mixed linear model, to take account of the correlation between measures in a same patient. Time will be considered as a fixed effect, and the patient as a random effect. The analysis will be performed in patients who have progressive disease, and those who do not.
The cut-off timepoint for this analysis will be the date of progression or end of follow-up. The values analysed will be number of copies of mutant DNA, taking account of all values obtained since inclusion.
All statistical analyses will be performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value < 0.05 will be considered statistically significant.
Study procedures
Practical implementation of the study
All study visits will be scheduled to coincide with the standard follow-up appointments. Each patient will have a maximum of 9 blood draws.
Study information and pre-inclusion visit
All patients meeting the following criteria will be eligible for pre-inclusion in the PERCIMEL study before scheduled surgery, i.e.:
Patient presenting with histologically confirmed cutaneous melanoma
- Stage III, and staging has confirmed the absence of distant metastasis,
- with no detectable macroscopic lymph node involvement, and for whom there is an indication for completion lymph node biopsy and investigation of sentinel lymph node (Breslow > 0.8 mm, or regardless of Breslow index in the presence of ulceration)
- with macroscopically detectable lymph node involvement (either synchronously at the time of discovery of the melanoma, or metachronously) and with an indication for complete lymph node dissection
Stage IV, and staging has confirmed the presence of distant metastasis, stage IV, amenable to complete resection, R0.
Information about the PERCIMEL study (and the associated biological collection) will be given to eligible patients by the clinicians in the participating centre during a medical consultation including:
Complete clinical examination.
Assessment of general state (WHO score) and body weight
Collection of personal history of melanoma
Collection of the results of staging examinations (radiological data): echography, abdominal-thoracic-pelvic CT scan, PET scan or brain CT scan
Collection of pathology results with histological confirmation of cutaneous melanoma (from initial biopsy for diagnostic purposes)
Collection of concomitant treatment (any treatment usually taken by the patient at the time of pre-inclusion)
Check for eligibility criteria.
After sufficient time for reflection, and after obtaining the patient’s written informed consent, the patient will be pre-included.
Surgery
Surgery will be performed according to standard practices in line with the recommendations for management of melanoma, and in general, within 28 days after the pre-inclusion visit.
For all pre-included patients:
A blood draw of 30 ml for ctDNA analysis will be performed on the evening before, or on the day of surgery, before the start of surgery.
Tissue samples (operative tumour samples and lymph nodes) will be retrieved and handled according to standard practices.
Post-operative visit
For all pre-included patients, the post-operative visit will be performed within 2 to 3 weeks after surgery, and will consist in:
Recording peri-operative complications (infected lymphocele, lymphoedema, scar disunion…) of grade > 2 or of any grade (according to the CTCAE classification version 5) if the impact on further management is significant
A blood draw of 30 ml on the day of the post-operative visit for the purposes of the study.
Inclusion visit
Patients will be definitively included if histology confirms:
Positive lymph nodes for patients with stage III melanoma
The presence of metastasis for patients with stage IV melanoma
For all patients included, adjuvant therapy must be initiated within 12 weeks after surgery. Adjuvant therapy by immunotherapy or targeted therapy with BRAF and MEK inhibitors will be planned for 12 months, as per standard recommendations.
The inclusion visit will consist in:
A clinical examination of the skin, scalp and lymph node basins, to note any signs and symptoms
Assessment of general state (WHO score) and body weight
Biology, including complete blood count, hemostasis, blood gases, plasma creatinine, liver function tests, and any other biological parameter required for the implementation of adjuvant medical therapy.
Pathology results of the operative sample and/or sentinel lymph nodes
Recording of any adverse events grade > 2 or adverse events of any grade (according to the CTCAE classification version 5) if the impact on medical therapy or further management is significant
Recording of concomitant treatments (if there are any changes compared to those recorded at the pre-inclusion visit)
A blood draw of 30 ml, performed on the day of the consultation where adjuvant therapy is initiated, and before the administration of adjuvant therapy.
Patients who consented to pre-inclusion but who are not definitively included (negative sentinel lymph node, or metastasis not confirmed by lymph node dissection) will exit the study at this point and will be managed as per standard recommendations.
Follow-up visits during adjuvant therapy (up to 12 months)
All follow-up visits will be scheduled to coincide with the standard follow-up appointments, i.e. every 3 to 4 months ± 2 weeks, in order to avoid the patient having to attend visits solely for the purposes of the study.
Each follow-up visit will include:
Complete clinical examination
Assessment of general state (WHO score) and body weight
Biology, including complete blood count, hemostasis, blood gases, plasma creatinine, liver function tests, and any other biological parameter required for the implementation of adjuvant medical therapy.
Recording of any adverse events grade > 2 or adverse events of any grade (according to the CTCAE classification version 5) if the impact on medical therapy or further management is significant
Recording of concomitant treatments (if there are any changes compared to those recorded at the pre-inclusion visit)
Tumour assessment
A blood draw of 30 ml for ctDNA analysis, performed on the day of each follow-up visit
In case of relapse during the 12 months of follow-up, the patient will have one final 30 ml blood draw and will then exit the study.
Follow-up visits (from 12 to 24 months)
All follow-up visits will be scheduled to coincide with the standard follow-up appointments, in order to avoid the patient having to attend visits solely for the purposes of the study. Follow-up visits will be planned according to current guidelines. For the purposes of the study, a follow-up visit will be planned every 6 months ± 2 weeks.
Appropriate, systematic radiological examination (CT or PET scan) is recommended every 6 months during the 12 months of follow-up.
Each follow-up visit will consist in:
Complete clinical examination
Assessment of general state (WHO score) and body weight
Recording of any adverse events grade > 2 or adverse events of any grade (according to the CTCAE classification version 5) if the impact on medical therapy or further management is significant
Recording of concomitant treatments (if there are any changes compared to those recorded at the pre-inclusion visit)
Tumour assessment
A blood draw of 30 ml for ctDNA analysis, performed on the day of each follow-up visit
After the 12 months of follow-up, the patient will have one final blood draw and will then exit the study.
In case of relapse during the 12 months of surveillance, the patient will have one final blood draw and will then exit the study.
Tumour assessment
Tumour assessment will include:
Clinical examination
CT or PET scan or brain CT scan
Biology, including measurement of LDH
Tumour assessment will be scheduled every 3 to 4 months ± 2 weeks during adjuvant therapy and every 6 months ± 2 weeks during follow-up, according to local practices in each centre.
Progressive disease is defined as the appearance of a new clinical or radiological lesion.
In case of progressive disease, the visit will consist in:
A clinical examination of the skin, scalp and lymph node basins, to note any signs and symptoms
Assessment of general state (WHO score) and body weight
Biology, including complete blood count, hemostasis, blood gases, plasma creatinine, liver function tests
Recording of any adverse events grade > 2 or adverse events of any grade (according to the CTCAE classification version 5) if the impact on medical therapy or further management is significant
Recording of concomitant treatments (if there are any changes compared to those recorded at the pre-inclusion visit)
Recording of the tumour assessment performed prior to the follow-up or surveillance visit
A final blood draw of 30 ml
Study stopping rules
The study may be suspended or discontinued by the sponsor, in consultation with the coordinator, or at the request of the competent authorities and/or the Ethics Committee (Comité de Protection des Personnes) if patient accrual is insufficient.
Premature withdrawal from the study
The following reasons may justify premature withdrawal of a patient from the study:
Failure to perform post-operative blood tests
Patient not eligible for treatment with immunotherapy using an anti-PD1 monoclonal antibody or targeted therapy using BRAF and MEK inhibitors in the adjuvant setting
Progressive disease
Death of the patient
Withdrawal of consent
Specific case of patients who:
Present major toxicity requiring definitive discontinuation of adjuvant therapy
Present toxicity requiring postponement of treatment or reduced treatment dose
Refuse to pursue adjuvant therapy
These patients will be maintained in the study, and blood draws will continue to be performed up to 24 months after initiation of adjuvant therapy, with the patient’s consent.
Participants in the study may withdraw their consent at any time without justification, for whatever reason, and this will in no way affect their right to continue to be treated by their physician.
Blood draws, sample preparation and transfer
Blood sampling will be sheduled for ctDNA analysis as follows:
Before surgery (the day before or on the day of surgery before the start of surgery)
At the post-operative visit (within 2 to 3 weeks after surgery)
On the day of the consultation when adjuvant therapy is initiated (before the administration of adjuvant therapy)
At each follow-up for tumour assessment (every 3 to 4 months ± 2 weeks) for the 12 months of adjuvant therapy
At each follow-up for tumour assessment (every 6 months ± 2 weeks) for the 12 months of surveillance
At the time of relapse
Blood samples will be drawn in Cell Free DNA collection tubes (Streck or equivalent). The 3 tubes of 10 ml of blood will be centrifuged on site in each centre for 10 min at 1,600 g at room temperature. The supernatants will be transferred to a 15 ml conical tube and centrifuged for 10 min at 6,000 g at room temperature. Around 10 to 15 ml of plasma will be retrieved in 5 ml cryotubes. ctDNA extracted from 5 ml plasma will be analysed on-site after by droplet digital PCR (ddPCR) (Váraljai R et al., 2019).
The tubes of plasma will be frozen at -80 °C for later analysis, and biobanking. The samples will be collected and transported, as per the rate of accrual in each participating centre, or at least once per year. The tubes will be transported to the laboratory for analysis on dry ice by a dedicated transporter and in compliance with current regulations.
Tissue samples (operative tumour samples and lymph nodes)
Tissue samples (tumour samples and lymph nodes) retrieved during surgery will be analysed according to standard procedures, then transferred, as per the rate of accrual in each participating centre, or at least once per year, by a dedicated transporter and in compliance with current regulations, to the laboratory.
DNA sequencing and digital droplet-PCR
To determine the genetic characteristics of the tumour, high-throughput sequencing will be performed on the initial tissue sample: After extraction of DNA (Qiagen, AllPrep FFPE), analysis of a 517-gene panel of interest in oncology will be performed (Agilent, SureSelect XT) with a depth of at least 1,000x (illumina, NextSeq 550). The results of tumour sequencing will enable exhaustive profiling of the initial lesions and mutations will be classified as clonal and subclonal. A clonal mutation is defined as a mutation that is common to all tumour cells, and a subclonal mutation is defined as a mutation that is present in only a subset of cells in the lesion. The selection of 3 clonal and subclonal mutations per patient will constitute a personalized profile for follow-up for each patient, specific to their tumour. For each mutation, specific, bespoken probes will be synthesized for analysis by droplet digital PCR (ddPCR, BioRad QX200).
The blood samples collected will be centrifuged as per current standards for liquid biopsy (double centrifugation at high then very high speed) and DNA will be extracted from the plasma (Qiagen, free nucleic acids kit). Extracted nucleic acids will be quantified by ddPCR (ID Solutions, quantification kit), and the fragmentation profiles will be analysed (Agilent, Fragment Analyzer). The qualification of the extracted nucleic acids will ensure the absence of any contamination by genomic DNA derived from white blood cell lysis.
All DNA samples extracted from plasma collected in the study will be analysed by ddPCR using the bespoken probes synthesized for each patient. ddPCR uses a preliminary step to denature the DNA strands, enabling detection of variants with low allele frequency (as low as 0.005%). This sensitivity will enable the detection of residual disease by the monitoring of plasma DNA concentration, and also by the follow-up of tumor clones and subclones specific to each patient (Váraljai R et al., 2019).
Biobank collection
Any leftover or unused plasma extracted from the blood samples performed in the course of this study, or leftover or unused DNA extracted from the tissue samples, will be conserved to constitute a biobank, to enable future translational research in oncology.
The collection will be stored at the sponsor’s biobanking center for future research purposes on the same topic, namely to search for gene mutations implicated in tumour processes.
The samples and associated data may be shared with national or international research groups within the European Union.
An information leaflet and consent form will be given to each patient at the start of the study to obtain their consent for participation in the biobank, including leftover and/or unused volumes from plasma and DNA extracts from the blood draws and tissue sampling performed in the course of this study.
Primary endpoint
The primary endpoint is the presence of ctDNA detected with 2 to 3 weeks after surgery, defined as a number of copies of mutant DNA ≥ 1 per ml of plasma, calculated on the basis of allele frequency of a detectable clonal mutation, as a fraction of the total circulating free DNA.ctDNA will be evaluated during follow-up, and is defined as follows:
Quantitatively, by the number of mutant copies of DNA per ml of plasma.
Qualitatively, by the presence or absence of ctDNA and clonal evolution.
The presence of ctDNA is defined as the number of mutant copies of DNA per ml of plasma. Clonal evolution will consist in the monitoring of 3 clonal and subclonal mutations identified by analysis of the initial tumor sample. Absolute and relative variations in ctDNA during follow-up will also be analysed.
Secondary endpoints
Recurrence-free survival (RFS) is defined as the time from inclusion to loco-regional relapse, distant metastasis or all-cause death, whichever occurs first.
Distant metastasis-free survival is defined as the time from inclusion to distant metastasis or all-cause death, whichever occurs first.
Melanoma specific survival is defined as the time from inclusion to cancer-related death.
Discussion
Recent results have been reported [23], showing that liquid biopsy proved suitable for follow-up of patients with stgae III B-D, IV melanoma, receiving adjuvant immunotherapy. In this translational study, baseline ctDNA positivity in resected stage III and IV melanoma was reported to be not highly prevalent (16%) and predictive of a poorer recurrence-free survival (HR 1.87) and distant-metastasis-free survival (HR 2.86). Further, baseline ctDNA combined with other biomarkers (tumor molecular burden, IFNγ) were more predictive of recurrence than any other biomarker alone.
In the landscape of studies investigating the predictive value of ctDNA for treatment response or prognosis in melanoma [25], the present PERCIMEL study has several original features:
Standardized follow-up at key timepoints during management with blood tests performed, at baseline, prior to surgery; at the first post-operative visit; at initiation of adjuvant therapy; at each follow-up visit up during the 12 months of adjuvant therapy; at each follow-up visit during the 12 months of surveillance; at the time of occurrence of relapse
Personalized follow-up: to determine the genetic characteristics of the tumour, high-throughput sequencing of 517 genes known to be of interest in cancer will be performed on tissue samples from the initial lesion of each patient. The selection of 3 clonal and subclonal mutations per patient will make it possible to obtain a personalized follow-up marker for each patient, specific to each tumour. For each mutation, the use of tailor-made detection probes will enable personalized analysis by droplet digital PCR (ddPCR) during follow-up.
Optimized technique: by partitioning samples into thousands of nanoliter-sized droplets, ddPCR enables the detection of variants with low allele frequency (0.005%), making it the most sensitive technique available today. This high sensitivity technique also enables the detection of any potential residual disease, both via monitoring of the DNA concentration in the plasma, and via the monitoring of tumoral clones and subclones specific to each patient.
PERCIMEL study is designed to provide scientific evidence that ctDNA can be used to predict the recurrence of patients with melanoma treated with adjuvant immunotherapy or kinase inhibitors, and to define the notion of molecular recurrence in resected stage III/IV melanomas.
Acknowledgements
Not applicable.
Abbreviations
- PD1/PDL1
Programmed cell death-1 / programmed cell death ligand-1
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
- BRAF
B-Raf proto-oncogene
- MEK
Mitogen-activated protein kinase kinase
- RFS
Recurrence-Free Survival
- HR
Hazard Ratio
- ctDNA
Circulating Tumor DNA
- LDH
Lactate Dehydrogenase
- EudraCT
European Union Drug Regulating Authorities Clinical Trials
- WHO
World Health Organization
- ROC
Receiving Operating Characteristic
- ANOVA
Analysis Of Variance
- CTCAE
Common Toxicity Criteria For Adverse Events
- CT
Computed Tomography
- PET
Positon Emission Tomography
- PCR
Polymerase Chain Reaction
- ddPCR
Droplet Digital PCR
- IFNγ
Interferon Gamma
- GIRCI
Groupement Interrégional de Recherche clinique et d'Innovation
Authors’ contribution
LG, AHa, NS and JLM are responsible for the study design, NS for methodolgy and statistics, LG, AH and JLM for drafting the manuscript and revision of the manuscript, CHS and AS for editing and registrating the protocol. FGB, AHe, LM, GJ, CM, and CN gave additional comments on the manuscript. All authors read and approved the final manuscript.
Funding
This study received funding from the French Ministry of Health (grant number PHRCI-2021–86) after full external peer review by the funding body as part of the peer review process of the GIRCI Est scientific committee for clinical research.
Availability of data and materials
The datasets obtained during the current study, data management procedures or the full protocol will be available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Regulatory requirements
The study will be performed in compliance with:
• The ethical principles as outlined in the latest version of the Declaration of Helsinki;
• Good Clinical Practice, as defined in the version dated 24 November 2005 from the International Conference on Harmonization (ICH-E6);
• French National legislation on privacy of personal data (“Informatique et Libertés” law number 78–17 dated 6 January 1978, modified by law number 2004–801 dated 6 August 2004) relating to the protection of privacy in the management of personal data;
• French Bioethics law number 2004–800 dated 6 August 2004;
• French Decree number 2016–1537 dated 16 November 2016 relating to research involving human subjects;
• French decree dated 7 May 2017 listing the types of research mentioned in paragraph 2 of article L. 1121–1 of the code of public health;
• French decree dated 9 May 2017 modifying certain regulatory dispositions relating to research involving human subjects.
Ethics committee, competent authority, CNIL (national data privacy commission)
Prior to initiating any research involving human subjects, the sponsor is required to submit the project for approval to an Ethics Committee with competence for the location in which the lead investigator practices, and for information to the French health products safety agency (ANSM). The sponsor will submit a request for approval to the competent authority for the conduct of an interventional research study with minimal risks and constraints.
Requests for substantial modifications to the initial protocol will also be sent by the sponsor to the Ethics Committee for approval, and to the competent authority for information.
This study is performed in compliance with the reference methodology MR-001 of the CNIL (national data privacy commission). The promoter, Institut de Cancerologie de Lorraine, has declared to be in conformity with the MR-001 methodology for the treatment of patients’ personal data for the purposes of biomedical research (declaration number 2203858 dated 24 July 2018).
Patient information and informed consent from the patient must be handled in accordance with the French regulation. Prior to the participation of a patient in the trial, this patient will be informed both verbally and in writing about the objectives of the trial, its methods, anticipated benefits and potential risks and the discomfort to which they may be exposed. The informed consent form for study and ancillaries studies, must be personally dated and signed by the patient and investigator.
The present protocol was approved by the Ethics Committee (Comité de Protection des Personnes Sud Est V) on 13/12/2022 under the reference number SI RIPH 2G: 22.04019.000105, and is registered on ClinicalTrials.gov with the number NCT04866680, and in ID-RCB with the number 2022-A01904-39.
The first patient was included on February 6th, 2023.
Consent for publication
Not applicable.
Competing interests
JLM received honoraria for consulting and financial supports for participation to meetings by Novartis, Bristol Myers Squibb, MSD, Pierre Fabre, Roche.
JLM and AHa received financial research grants from Novartis.
AHa received honoraria for consulting and financial supports for participation to meetings by Biocartis, BioRad, BMS, MSD, Novartis, Pierre Fabre, Roche, Sophia Genetics.
GJ received honoria for consulting and financial supports for participation to mmeetings by Novartis, BMS, MSD, Pierre Fabre.
FGB received honoraria for consulting and financial supports for participation to meetings by Novartis, Bristol Myers Squibb, MSD, Pierre Fabre, Sun Pharma.
CM received honoraria for consulting and financial supports for participation to meetings by BMS, Novartis, Pierre Fabre,.
CN received honoraria for consulting and financial supports for participation to meetings by Novartis, Bristol Myers Squibb, MSD, Pierre Fabre.
LM received honoraria for consulting and financial supports for participation to meetings by Novartis, Bristol Myers Squibb, MSD, Merck, Pierre Fabre.
AHe received honoraria for consulting by Novartis, Pierre Fabre, MSD.
All other authors declared that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, Grob JJ, Butler MO, Middleton MR, Maio M, Atkinson V, Queirolo P, Gonzalez R, Kudchadkar RR, Smylie M, Meyer N, Mortier L, Atkins MB, Long GV, Bhatia S, Lebbé C, Rutkowski P, Yokota K, Yamazaki N, Kim TM, de Pril V, Sabater J, Qureshi A, Larkin J, Ascierto PA, CheckMate 238 Collaborators Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 2.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Maio M, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan P, Ibrahim N, Marreaud S, van Akkooi ACJ, Suciu S, Robert C. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Hauschild A, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, Larkin J, Nyakas M, Dutriaux C, Haydon A, Robert C, Mortier L, Schachter J, Schadendorf D, Lesimple T, Plummer R, Ji R, Zhang P, Mookerjee B, Legos J, Kefford R, Dummer R, Kirkwood JM. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, Haydon AM, Meshcheryakov A, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Di Giacomo AM, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan PC, van Akkooi ACJ, Krepler C, Ibrahim N, Marreaud S, Kicinski M, Suciu S, Robert C. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J Clin Oncol. 2020;38(33):3925–3936. doi: 10.1200/JCO.20.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, Cowey CL, Schenker M, Grob JJ, Chiarion-Sileni V, Márquez-Rodas I, Butler MO, Maio M, Middleton MR, de la Cruz-Merino L, Arenberger P, Atkinson V, Hill A, Fecher LA, Millward M, Khushalani NI, Queirolo P, Lobo M, de Pril V, Loffredo J, Larkin J, Weber J. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 6.Dummer R, Brase JC, Garrett J, Campbell CD, Gasal E, Squires M, Gusenleitner D, Santinami M, Atkinson V, Mandalà M, Chiarion-Sileni V, Flaherty K, Larkin J, Robert C, Kefford R, Kirkwood JM, Hauschild A, Schadendorf D, Long GV. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAFV600-mutant, stage III melanoma (COMBI-AD): exploratory biomarker analyses from a randomised, phase 3 trial. Lancet Oncol. 2020;21(3):358–372. doi: 10.1016/S1470-2045(20)30062-0. [DOI] [PubMed] [Google Scholar]
- 7.Patel SP, Othus M, Chen Y, Wright GP, Jr, Yost KJ, Hyngstrom JR, Hu-Lieskovan S, Lao CD, Fecher LA, Truong TG, Eisenstein JL, Chandra S, Sosman JA, Kendra KL, Wu RC, Devoe CE, Deutsch GB, Hegde A, Khalil M, Mangla A, Reese AM, Ross MI, Poklepovic AS, Phan GQ, Onitilo AA, Yasar DG, Powers BC, Doolittle GC, In GK, Kokot N, Gibney GT, Atkins MB, Shaheen M, Warneke JA, Ikeguchi A, Najera JE, Chmielowski B, Crompton JG, Floyd JD, Hsueh E, Margolin KA, Chow WA, Grossmann KF, Dietrich E, Prieto VG, Lowe MC, Buchbinder EI, Kirkwood JM, Korde L, Moon J, Sharon E, Sondak VK, Ribas A. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N Engl J Med. 2023;388(9):813–823. doi: 10.1056/NEJMoa2211437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franczak C, Filhine-Tressarieu P, Broséus J, Gilson P, Merlin JL, Harlé A. Clinical Interest of Circulating Tumor DNA in Oncology. Arch Med Res. 2018;49(5):297–305. doi: 10.1016/j.arcmed.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Xi L, Pham THT, Payabyab EC, Sherry RM, Rosenberg SA, Raffeld M. Circulating tumor DNA as an early indicator of response to T-cell transfer immunotherapy in metastatic melanoma. Clin Cancer Res. 2016;22(22):5480–5486. doi: 10.1158/1078-0432.CCR-16-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forthun RB, Hovland R, Schuster C, Puntervoll H, Brodal HP, Namløs HM, Aasheim LB, Meza-Zepeda LA, Gjertsen BT, Knappskog S, Straume O. ctDNA detected by ddPCR reveals changes in tumour load in metastatic malignant melanoma treated with bevacizumab. Sci Rep. 2019;9(1):17471. doi: 10.1038/s41598-019-53917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with antieprogrammed cell death 1 antibodies. JAMA Oncol. 2018;4(5):717–721. doi: 10.1001/jamaoncol.2017.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray ES, Rizos H, Reid AL, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget. 2015;6(39):42008–42018. doi: 10.18632/oncotarget.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEvoy AC, Warburton L, Al-Ogaili Z, Celliers L, Calapre L, Pereira MR, Khattak MA, Meniawy TM, Millward M, Ziman M, Gray ES. Correlation between circulating tumour DNA and metabolic tumour burden in metastatic melanoma patients. BMC Cancer. 2018;18(1):726. doi: 10.1186/s12885-018-4637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seremet T, Jansen Y, Planken S, Njimi H, Delaunoy M, El Housni H, Awada G, Schwarze JK, Keyaerts M, Everaert H, Lienard D, Del Marmol V, Heimann P, Neyns B. Undetectable circulating tumor DNA (ctDNA) levels correlate with favourable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J Transl Med. 2019;17(1):303. doi: 10.1186/s12967-019-2051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniotti M, Vallacchi V, Rivoltini L, Patuzzo R, Santinami M, Arienti F, Cutolo G, Pierotti MA, Parmiani G, Rodolfo M. Detection of mutated BRAFV600E variant in circulating DNA of stage III-IV melanoma patients. Int J Cancer. 2007;120:2439–2444. doi: 10.1002/ijc.22598. [DOI] [PubMed] [Google Scholar]
- 16.Syeda MM, Wiggins JM, Corless BC, Long GV, Flaherty KT, Schadendorf D, Nathan PD, Robert C, Ribas A, Davies MA, Grob JJ, Gasal E, Squires M, Marker M, Garrett J, Brase JC, Polsky D. Circulating tumour DNA in patients with advanced melanoma treated with dabrafenib or dabrafenib plus trametinib: a clinical validation study. Lancet Oncol. 2021;22(3):370–380. doi: 10.1016/S1470-2045(20)30726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braune J, Keller L, Schiller F, Graf E, Rafei-Shamsabadi D, Wehrle J, Follo M, Philipp U, Hussung S, Pfeifer D, Mix M, Duyster J, Fritsch R, von Bubnoff D, Meiss F, von Bubnoff N. Circulating Tumor DNA Allows Early Treatment Monitoring in BRAF- and NRAS-Mutant Malignant Melanoma. JCO Precis Oncol. 2020;4:20–31. doi: 10.1200/PO.19.00174. [DOI] [PubMed] [Google Scholar]
- 18.Lee RJ, Gremel G, Marshall A, Myers KA, Fisher N, Dunn JA, Dhomen N, Corrie PG, Middleton MR, Lorigan P, Marais R. Circulating tumor DNA predicts survival in patients with resected high-risk stage II/III melanoma. Ann Oncol. 2018;29(2):490–496. doi: 10.1093/annonc/mdx717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JH, Saw RP, Thompson JF, Lo S, Spillane AJ, Shannon KF, Stretch JR, Howle J, Menzies AM, Carlino MS, Kefford RF, Long GV, Scolyer RA, Rizos H. Pre-operative ctDNA predicts survival in high-risk stage III cutaneous melanoma patients. Ann Oncol. 2019;30(5):815–822. doi: 10.1093/annonc/mdz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan L, Sandhu S, Lee RJ, Li J, Callahan J, Ftouni S, Dhomen N, Middlehurst P, Wallace A, Raleigh J, Hatzimihalis A, Henderson MA, Shackleton M, Haydon A, Mar V, Gyorki DE, Oudit D, Dawson MA, Hicks RJ, Lorigan P, McArthur GA, Marais R, Wong SQ, Dawson SJ. Prediction and monitoring of relapse in stage III melanoma using circulating tumor DNA. Ann Oncol. 2019;30(5):804–814. doi: 10.1093/annonc/mdz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Váraljai R, Wistuba-Hamprecht K, Seremet T, Diaz JMS, Nsengimana J, Sucker A, Griewank K, Placke JM, Horn PA, von Neuhoff N, Shannan B, Chauvistré H, Vogel FCE, Horn S, Becker JC, Newton-Bishop J, Stang A, Neyns B, Weide B, Schadendorf D, Roesch A. Application of Circulating Cell-Free Tumor DNA Profiles for Therapeutic Monitoring and Outcome Prediction in Genetically Heterogeneous Metastatic Melanoma. JCO Precis Oncol. 2019;3:PO.18.00229. doi: 10.1200/PO.18.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gouda MA, Polivka J, Huang HJ, Treskova I, Pivovarcikova K, Fikrle T, Woznica V, Dustin DJ, Call SG, Meric-Bernstam F, Pesta M, Janku F. Ultrasensitive detection of BRAF mutations in circulating tumor DNA of non-metastatic melanoma. ESMO Open. 2021;7(1):100357. doi: 10.1016/j.esmoop.2021.100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long GV, Desai K, Tang T, Weber J, Dolfi S, Ritchings C, Huang SP, Bolisetty M, Sausen M, Del Vecchio M, Larkin J, Baden J, Balli D, Chang H, Loffredo J, Zhang N, Wind-Rotolo M, Tenney DJ. Association of pre-treatment ctDNA with disease recurrence and clinical and translational factors in patients with stage IIIB-D/IV melanoma treated with adjuvant immunotherapy (CheckMate 915) Ann Oncol. 2022;33(suppl7):S904. doi: 10.1016/j.annonc.2022.07.914. [DOI] [Google Scholar]
- 24.Maio M, Lewis K, Demidov L, Mandalà M, Bondarenko I, Ascierto PA, Herbert C, Mackiewicz A, Rutkowski P, Guminski A, Goodman GR, Simmons B, Ye C, Yan Y, Schadendorf D, BRIM8 Investigators Adjuvant vemurafenib in resected, BRAFV600 mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018;19(4):510–520. doi: 10.1016/S1470-2045(18)30106-2. [DOI] [PubMed] [Google Scholar]
- 25.Sacco A, Forgione L, Carotenuto M, Luca A, Ascierto PA, Botti G, Normanno N. Circulating Tumor DNA Testing Opens New Perspectives in Melanoma Management. Cancers (Basel) 2020;12(10):2914. doi: 10.3390/cancers12102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets obtained during the current study, data management procedures or the full protocol will be available from the corresponding author upon reasonable request.