Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for the coronavirus disease 2019 pandemic, is capable of infecting a variety of wildlife species. Wildlife living in close contact with humans are at an increased risk of SARS-CoV-2 exposure and, if infected, have the potential to become a reservoir for the pathogen, making control and management more difficult. The objective of this study is to conduct SARS-CoV-2 surveillance in urban wildlife from Ontario and Québec, increasing our knowledge of the epidemiology of the virus and our chances of detecting spillover from humans into wildlife.
Methods
Using a One Health approach, we leveraged activities of existing research, surveillance and rehabilitation programs among multiple agencies to collect samples from 776 animals from 17 different wildlife species between June 2020 and May 2021. Samples from all animals were tested for the presence of SARS-CoV-2 viral ribonucleic acid, and a subset of samples from 219 animals across three species (raccoons, Procyon lotor; striped skunks, Mephitis mephitis; and mink, Neovison vison) were also tested for the presence of neutralizing antibodies.
Results
No evidence of SARS-CoV-2 viral ribonucleic acid or neutralizing antibodies was detected in any of the tested samples.
Conclusion
Although we were unable to identify positive SARS-CoV-2 cases in wildlife, continued research and surveillance activities are critical to better understand the rapidly changing landscape of susceptible animal species. Collaboration between academic, public and animal health sectors should include experts from relevant fields to build coordinated surveillance and response capacity.
Keywords: SARS-CoV-2, wildlife, surveillance, Ontario, Québec
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the global coronavirus disease 2019 (COVID-19) pandemic and has been maintained through human-to-human transmission. However, humans are not the only species susceptible to infection. Over the course of the current pandemic, a range of domestic and wild animal species have been reported to either be naturally infected with SARS-CoV-2 or susceptible to the virus in experimental infections ((1–4)). As of April 30, 2022, 36 countries have reported positive SARS-CoV-2 cases in 23 different animal species to the World Organisation for Animal Health ((5)). Other species have been identified as potential hosts based on sequence analysis of the host cell receptor of SARS-CoV-2, angiotensin 1 converting enzyme 2 and predicted binding affinity ((6,7)).
Many wild animal species, such as raccoons, skunks and bats, thrive in the ecological overlap with humans and are thus at an increased risk of being exposed to SARS-CoV-2 ((8)). Several peri-domestic species have been experimentally shown to become infected with and shed SARS-CoV-2 ((9,10)). SARS-CoV-2 infection has also been reported in wild or free-ranging animals that have been naturally exposed, including American mink (Neovison vison) in Spain ((11)) and, more recently, white-tailed deer (Odocoileus virginianus) in multiple locations across North America ((12–16)). In Ontario, this includes identification of a probable case of deer-to-human viral transmission ((16)). Infection in animals can result in mild to severe symptoms of respiratory disease up to and including death via interstitial pneumonia (e.g. mink) ((17,18)). Other species do not show clinical signs of infection (e.g. skunks) ((9,10)) or show only mild and transient symptoms in some individuals, such as elevated temperature (e.g. white-tailed deer) ((19)).
The concept of One Health recognizes that human and animal health are interdependent ((20)). The spillover of virus from humans or domestic animals into wildlife is concerning not only due to the possible deleterious effects on wildlife, but because these wild populations have the potential to act as reservoirs for SARS-CoV-2. Pathogens that have an animal reservoir are inherently more difficult to control and the spread of SARS-CoV-2 through animal populations could further contribute to the development of variants of concern (VoCs), potentially undermining the efficacy of countermeasures such as antivirals and vaccines ((21,22)). As such, there have been calls for increased surveillance at the human-wildlife interface ((23)). Urban areas around the world have been a particular area of concern and focus ((24–26)). The higher density of both human and some peri-urban wildlife species populations in urban centres can lead to more frequent human-animal contact and increased potential for disease transmission. Additionally, people who have close contact with wildlife, such as biologists, rehabilitators, and hunters and trappers, may be at higher risk of being exposed to the virus and of facilitating its spread among wildlife. The impact of SARS-CoV-2 infection on wildlife health is not fully understood. Early detection of any spillover is therefore critical to preventing and addressing these concerns.
Given the risk of reverse-zoonotic SARS-CoV-2 transmission and our lack of knowledge of the virus in local wildlife, there was an urgent need to elucidate the epidemiology of the virus at the human-wildlife interface to help wildlife management and public health officials better communicate risk and plan management strategies. We therefore conducted SARS-CoV-2 surveillance in wildlife across Ontario and Québec, with a major focus on the southern regions of both provinces. These areas have high human population densities and include major urban centres such as Toronto and Montréal. Between spring 2020 and spring 2021, incidences of COVID-19 peaked in Montréal and the surrounding regions in early January 2021, with rates exceeding 400 cases per 100,000 population in Montréal and Laval ((27)). Incidences between spring 2020 and spring 2021 in the Greater Toronto Area peaked in April 2021, with case rates in the City of Toronto and Peel also exceeding 400 per 100,000 population ((27)).
Methods
Many experts have recommended a One Health approach for animal SARS-CoV-2 testing, which balances concerns for both human and animal health and is based on knowledge of experts in both fields ((28,29)). As such, our work was conducted through consultation and cooperation among a wide variety of agencies: the Public Health Agency of Canada; the Ontario Ministry of Northern Development, Mines, Natural Resources and Forestry (NDMNRF); le Ministère des Forêts, de la Faune et des Parcs du Québec; the Canadian Wildlife Health Cooperative (CWHC); the Ontario Ministry of Agriculture, Food, and Rural Affairs; the Canadian Food Inspection Agency; the Western College of Veterinary Medicine; the Granby Zoo; the National Microbiology Laboratory (NML) of the Public Health Agency of Canada; and Sunnybrook Research Institute. All samples for testing were collected between June 2020 and May 2021 through pre-existing partnerships or over the course of other research, surveillance or rehabilitation work (Table 1).
Table 1. Metadata for 776 animals from Ontario and Québec screened for severe acute respiratory syndrome coronavirus 2.
| Species | Sampling agency | Sample source | Sample location(s) | Dates of collection | Number of individuals sampled | Types of samples tested | Test performeda |
|---|---|---|---|---|---|---|---|
| Raccoon (Procyon lotor) | CWHC | Rabies surveillance (Québec samples), post-mortem exam | Southern Ontario, Southern Québec | Aug 2020–Feb 2021 | 11 | Respiratory tissue | PCR |
| Southern Québec | Nov–Dec 2020 | 68 | Respiratory tissue, rectal swab | ||||
| Southern Ontario, Southern Québec | Oct 2020–June 2021 | 15 | Respiratory and intestinal tissue | ||||
| Southwestern Québec | Jan 2021 | 3 | Nasal swab | ||||
| Southern Québec | Jan–June 2021 | 54 | Nasal and rectal swabs | ||||
| NDMNRF and CWHC | Rabies surveillance, post-mortem exam | Hamilton, Ontario | Dec 2020 | 1 | Oral and rectal swabs, respiratory and intestinal tissue | ||
| NDMNRF | Rabies surveillance | Southwestern Ontario | June 2020–Jan 2021 | 100 | Oral and rectal swabs | ||
| Rabies seroprevalence study | Oakville, Ontario | Sept–Oct 2020 | 141 | Oral and rectal swabs | |||
| Sera | Antibody | ||||||
| Total raccoons sampled | 393 | - | |||||
| Striped skunk (Mephitis mephitis) | CWHC | Rabies surveillance (Québec samples), post-mortem exam | Southern Québec | Jan–June 2021 | 66 | Nasal swab | PCR |
| Southern Ontario, Southern Québec | July–Dec 2020 | 55 | Respiratory tissue | ||||
| Southern Ontario, Southwestern Québec, Saint-Félicien, Québec | Oct 2020–Apr 2021 | 9 | Respiratory and intestinal tissue | ||||
| NDMNRF | Rabies surveillance, rabies seroprevalence study | Southwestern Ontario | Sept 2020–May 2021 | 104 | Oral and rectal swabs | ||
| Rabies seroprevalence study | Oakville, Ontario | Sept–Oct 2020 | 36 | Oral and rectal swabs | |||
| Sera | Antibody | ||||||
| Total skunks sampled | 270 | - | |||||
| American mink (Neovision vison) | CWHC | Post-mortem exam | Thornhill, Ontario | July 2020 | 1 | Respiratory tissue | PCR |
| NDMNRF | Registered fur harvesters, roadkill, rabies surveillance | Southern Ontario | Fall 2020–Spring 2021 | 42b | Oral and rectal swabs, lung and intestinal tissue | ||
| Cardiac blood or Nobuto strips | Antibody | ||||||
| Total mink sampled | 43 | - | |||||
| Big brown bat (Eptesicus fuscus) | Granby Zoo | Rehabilitation program | Southwestern Québec | Nov 2020–Mar 2021 | 15 | Oral swabs | PCR |
| 2 | Guano | ||||||
| 15 | Oral swabs and guano | ||||||
| Total big brown bats sampled | 32 | - | |||||
| Hoary bat (Lasiurus cinerus) | CWHC | Post-mortem exam | Etobicoke, Ontario | Dec 2020 | 1 | Respiratory and intestinal tissue | PCR |
| American marten (Martes americana) | CWHC | Post-mortem exam | Sainte-Anne-de-Bellevue, Québec | Nov 2020 | 1 | Respiratory and intestinal tissue | PCR |
| Fisher (Pekania pennanti) | CWHC | Post-mortem exam | Western Québec | May 2021 | 2 | Respiratory and intestinal tissue | PCR |
| American black bear (Ursus americanus) | CWHC | Post-mortem exam | Northern Ontario | Sept 2020 | 2 | Respiratory tissue | PCR |
| Killaloe, Ontario | Oct 2020 | 1 | Respiratory and intestinal tissue | ||||
| Total black bears sampled | 3 | - | |||||
| Atlantic white-sided dolphin (Lagenorhynchus actus) | CWHC | Post-mortem exam | Carleton-sur-Mer, Québec | June 2021 | 1 | Intestinal tissue | PCR |
| Sept-Îles, Québec | March 2021 | 1 | Respiratory and intestinal tissue | ||||
| Total Atlantic white-sided dolphins sampled | 2 | - | |||||
| Harbour porpoise (Phocoena phocoena) | CWHC | Post-mortem exam | La Montée, Québec | Dec 2020 | 1 | Respiratory and intestinal tissue | PCR |
| Harbour seal (Phoca vitulina) | CWHC | Post-mortem exam | Matane, Québec | Dec 2020 | 1 | Respiratory and intestinal tissue | PCR |
| Coyote (Canis latrans) | CWHC | Post-mortem exam | Saint-Alexandre-d'Iberville, Québec | April 2021 | 1 | Respiratory and intestinal tissue | PCR |
| Eastern wolf (Canus lupus lycaon) | CWHC | Post-mortem exam | Algonquin Provincial Park, Ontario | Oct 2020 | 1 | Respiratory tissue | PCR |
| Southern and central Ontario | 4 | Respiratory and intestinal tissue | |||||
| Total eastern wolves sampled | 5 | - | |||||
| Grey fox (Urocyon cinereoargenteus) | CWHC | Post-mortem exam | Châteauguay, Québec | Dec 2020 | 1 | Respiratory and intestinal tissue | PCR |
| Red fox (Vulpes vulpes) | CWHC | Post-mortem exam | Mercier, Québec | Jan 2021 | 1 | Nasal and rectal swabs | PCR |
| Southwestern Québec | Nov–Dec 2020 | 4 | Respiratory tissue, rectal swabs | ||||
| Southern, Ontario | July–Oct 2020 | 5 | Respiratory tissue | ||||
| Dunham, Québec | Dec 2020 | 1 | Respiratory and intestinal tissue | ||||
| Total red foxes sampled | 11 | - | |||||
| Virginia opossum (Didelphis virginiana) | CWHC | Post-mortem exam | Bolton-Est, Québec | June 2021 | 1 | Nasal and rectal swabs | PCR |
| Southern Ontario | July–Oct 2020 | 2 | Respiratory tissue | ||||
| Southwestern Ontario, Saint-Jean-sur-Richelieu, Québec | Oct 2020, March 2021 | 3 | Respiratory and intestinal tissue | ||||
| Total Virginia opossums sampled | 6 | - | |||||
| White-tailed deer (Odocoileus virginianus) | CWHC | Post-mortem exam | London, Ontario, Southwestern Québec | Oct–Dec 2020 | 3 | Respiratory and intestinal tissue | PCR |
Abbreviations: CWHC, Canadian Wildlife Health Cooperative; NDMNRF, Northern Development, Mines, Natural Resources and Forestry; PCR, polymerase chain reaction; -, not applicable
a All PCR testing was performed at Sunnybrook Research Institute and all antibody testing was performed at the Public Health Agency of Canada’s National Microbiology Laboratory
b Due to the condition of the carcasses, we were unable to collect lung tissue or cardiac blood from one individual, cardiac blood from a further two individuals and rectal swabs from two individuals. In cases where we could not collect cardiac blood, we instead submitted a Nobuto strip soaked in fluid from the chest cavity for antibody testing
Raccoons and skunks
Raccoons (Procyon lotor) and striped skunks (Mephitis mephitis) are peri-domestic species that are good candidates for reverse-zoonotic disease surveillance due to their high density in urban areas and their frequent close contact with people, pets and refuse. They are also subject to ongoing rabies surveillance operations in both Ontario and Québec, making them easy to sample. In Ontario, wildlife rabies surveillance and testing are conducted by the NDMNRF on roadkill, animals found dead for other reasons, and wildlife that were sick or acting strangely. Submissions are received mainly from southwestern Ontario, and most animals received by the program and subsequently sampled and tested for SARS-CoV-2 came from urban centres within this region or had a case history of close contact with people (Figure 1). In Québec, a similar wildlife rabies surveillance program is coordinated by le Ministère des Forêts, de la Faune et des Parcs du Québec and testing and other post-mortem examinations are performed by the Québec CWHC. As was the case in Ontario, animals sampled by the Québec CWHC for SARS-CoV-2 testing came mainly from urban areas (Figure 1). The Ontario CWHC laboratory also contributed a small number of raccoon and skunk samples from animals submitted to them for post-mortem examination. Carcasses were sampled using a combination of oral, nasal, and rectal swabs, respiratory tissue and intestinal tissue (Table 1). Swabs were stored in individual 2 mL tubes with ~1 mL of universal transport medium (UTM; Sunnybrook Research Institute) and 30–60 mg tissue samples were stored dry in tubes.
Figure 1.
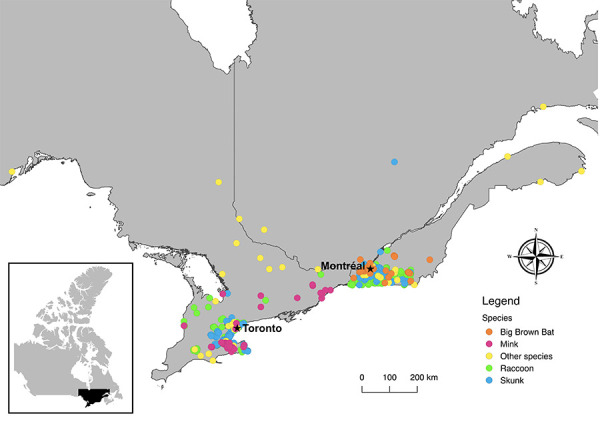
Original locations of animals submitted for severe acute respiratory syndrome coronavirus 2 testing June 2020–May 2021 (N=776)
Additionally, samples were collected from live raccoons and skunks during an annual seroprevalence study conducted by the NDMNRF in Oakville, Ontario to assess the effectiveness of rabies vaccine baiting (NDMNRF Wildlife Animal Care Committee Protocol #358). Animals were captured in live traps and transported to a central processing station where they were anaesthetized. Oral and rectal swabs were collected for polymerase chain reaction (PCR) testing. Blood was drawn from the brachiocephalic vein and 0.2–1.0 mL of sera was collected for antibody testing. Following reversal and successful recovery, animals were returned to their point of capture and released.
Mink
Instances of SARS-CoV-2 infection in mink have already been identified in multiple countries, including Canada, and infected farmed mink have proven capable of passing the virus to naive conspecifics, humans and companion animals ((17,30–33)). At the time of writing no mink farm outbreaks have been reported in Ontario or Québec, but mink farms in Ontario have previously been shown to act as points of infection for other viruses (e.g. Aleutian mink disease), which can spread to wild mink populations ((34)).
The majority of mink carcasses we sampled for SARS-CoV-2 testing were submitted to the NDMNRF by licensed fur harvesters through a collaboration with the Ontario Fur Managers Federation. The NDMNRF staff collected oral and rectal swabs, lung tissue and intestinal tissue from the carcasses, as well as cardiac blood samples via cardiac puncture for antibody testing. If blood could not be obtained from the heart, fluid was collected from the chest cavity on a Nobuto filter strip (Advantec MFS, Inc, Dublin, California, United States [US]). Nobuto strips were allowed to air dry, then placed in individual coin envelopes.
Big brown bats
Bats are known carriers of coronaviruses ((35–37)). As such, concerns have been raised over the possible susceptibility of North American bats to SARS-CoV-2 ((38)). Species such as the big brown bat (Eptesicus fuscus) frequently roost in buildings, which brings them into close contact with people and increases the likelihood of SARS-CoV-2 exposure. Big brown bat oral swabs and guano samples for SARS-CoV-2 PCR testing were collected by staff at the Granby Zoo, which runs a rehabilitation program over the winter to care for bats that have been disturbed during their hibernation. Guano samples were stored dry in 2 mL tubes.
Other species
Other samples for SARS-CoV-2 PCR testing were obtained opportunistically through the Ontario and Québec regional CWHC laboratories, which receive a wide variety of wildlife species for post-mortem examination (Table 1). Animals were selected for sampling based on potential for SARS-CoV-2 infection. This could be due to urban habitat, human contact or to predicted species susceptibility based on prior research. The number and type of samples collected varied by carcass and depended on carcass condition (Table 1).
Ribonucleic acid extraction
Ribonucleic acid (RNA) extraction and PCR testing were performed at the Sunnybrook Research Institute in Toronto, Ontario. All swab, tissue and guano samples were stored at -80°C prior to testing. For oral, rectal or nasal swab samples, RNA extractions were performed using 140 µL of sample via the QIAmp viral RNA mini kit (Qiagen, Mississauga, Ontario) or the Nuclisens EasyMag using Generic Protocol 2.0.1 (bioMérieux Canada Inc., St-Laurent, Québec) according to manufacturer’s instructions. The RNA from guano samples (80 mg) were extracted via the QIAmp viral RNA mini kit and eluted in 40 µL in containment level 3 at the University of Toronto. Tissue samples were thawed, weighed, minced with a scalpel, and homogenized in 600 µL of lysis buffer using the Next Advance Bullet Blender (Next Advance, Troy, New York, US) and a 5 mm stainless steel bead at 5 m/s for 3 minutes. The RNA from 30 mg tissue samples was extracted via the RNeasy Plus Mini kit (Qiagen, Mississauga, Ontario) or the Nuclisens EasyMag using Specific Protocol B 2.0.1; RNA was eluted in 50 µL. All extractions were performed with a positive and negative control. Extraction efficiency between kits was assessed through comparison of positive extraction controls.
Severe acute respiratory syndrome coronavirus 2 polymerase chain reaction analysis
Real-time polymerase chain reaction (RT-PCR) was performed using the Luna Universal Probe One-Step RT-qPCR kit (NEB). Two gene targets were used for SARS-CoV-2 RNA detection: the 5’ untranslated region (UTR) and the envelope (E) gene ((39)). This assay was adapted from the Shared Hospital Labs from The Research Institute of St. Joseph Hamilton for use in animals. The cycling conditions were as follows: one cycle of denaturation at 60°C for 10 minutes then 95°C for 2 minutes followed by 44 amplification cycles of 95°C for 10 seconds and 60°C for 15 seconds. Quantstudio 3 software (Thermo Fisher Scientific Inc., Waltham, Massachusetts, US) was used to determine cycle thresholds (Ct). All samples were run in duplicate and samples with Cts less than 40 for both gene targets in at least one replicate were considered positive.
Antibody testing
Antibody testing was performed on cardiac blood, chest cavity fluid and serum samples at the NML in Winnipeg, Manitoba. All samples were stored at -20°C prior to testing. Cardiac blood samples were collected onto Nobuto filter strips by saturating the length of the strip with 100 µl of blood. To obtain the 1:9 dilution required for testing, saturated Nobuto strips were cut into 4–5 pieces and placed into a 2 mL tube containing 360 µl phosphate buffered saline pH 7.4 containing 0.05% Tween 20 and eluted overnight at 4°C. Nobuto strips collected from chest cavity fluid were processed in the same way, whereas serum samples were diluted 1:9 with Sample Dilution Buffer. Samples were mixed by vortexing and tested using the GenScript cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript US, Inc. Piscataway, New Jersey, US) according to the manufacturer’s protocol.
Briefly, 60 µl of a sample was added to 60 µl HRP-conjugated RBD solution and incubated at 37°C for 30 minutes. A 100 µl aliquot of the mixture was transferred to the ELISA microwell test plate and incubated at 37°C for 15 minutes. Microwells were washed four times with 260 µl wash buffer then 100 µl TMB substrate was added to each well. Following a 20-minutes incubation in the dark at room temperature, 50 µl of Stop Solution was added to each well. Absorbance was read immediately at 450 nm.
Each assay plate included positive and negative controls that met required quality control parameters. Percentage inhibition was calculated for each sample using the following equation:
% inhibition = (1- optical density sample / optical density negative control) x 100%
Samples with greater than or equal to 30% inhibition were considered positive for SARS-CoV-2 neutralizing antibodies.
Results
We tested 776 individual animals from 17 different wildlife species for SARS-CoV-2. These animals were collected primarily from urban areas in southern Ontario and Québec between June 2020 and May 2021 (Table 1). We found no evidence of SARS-CoV-2 viral RNA in any of the tested samples and no evidence of neutralizing antibodies in a subset of 219 individuals (141 raccoons, 36 striped skunks, 42 mink).
Discussion
Our study did not detect any spillover of SARS-CoV-2 to wildlife in Ontario and Québec. Raccoons and skunks were the most commonly tested species. Results from experimental studies have suggested these species may be susceptible to SARS-CoV-2, but the lack of and low quantity of infectious virus shed by raccoons and skunks, respectively, suggest they are an unlikely reservoir for SARS-CoV-2 in the absence of viral adaptations ((9,10)). Similarly, a challenge study with big brown bats found that they are resistant to SARS-CoV-2 infection and do not shed infectious virus ((40)). Conversely, minks are susceptible to SARS-CoV-2 infection, but no evidence of SARS-CoV-2 was detected in any of the mink sampled. While this could be attributed to low effective sample size, to date SARS-CoV-2 has been infrequently detected in wild mink populations globally. It should be noted, however, that these experimental studies on raccoons, skunks and big brown bats ((9,10,40)) were conducted using parental SARS-CoV-2. The susceptibility of these species to VoCs is presently not known and may differ from susceptibility to the parental strain ((41)). Additionally, challenge studies assessing susceptibility tend to be conducted on small numbers of young, healthy individuals, so results may not be reflective of the full range of possible responses to infection in the wild.
As the pandemic progresses, new evidence is emerging on susceptible wildlife that may act as competent reservoirs for the virus. For example, white-tailed deer are now considered a highly relevant species for SARS-CoV-2 surveillance in light of their experimentally determined susceptibility as well as evidence of widespread exposure to the virus via antibody and PCR testing across North America ((12–16,19)). Continued surveillance efforts should be adaptive and include targeted testing of highly relevant species as they are identified. In Ontario and Québec, these would include mink, white-tailed deer and deer mice (Peromyscus maniculatus) ((9,42)). Continuing to include less susceptible species remains important given ongoing viral genomic plasticity and changing host range of VoCs.
Limitations
There are several limitations for this study that need to be acknowledged. First, the majority of our SARS-CoV-2 testing was done by RT-PCR, which is only capable of detecting active infection. Antibody testing, which identifies resolved infection or exposure, is more likely to find evidence of SARS-CoV-2 in surveillance studies since results are less dependent on timing of sample collection. Antibody testing typically requires samples from live animals or fresh carcasses, which limited our ability to use it; however, the testing performed allowed for test validation in raccoons, skunks and mink, which may facilitate more antibody testing in future. Second, we relied on different kits for RNA extraction due to logistical challenges. Based on our extraction controls, the QIAamp RNA mini kit performed slightly better compared to the Nuclisens EasyMag (~2 Cts) for swab samples. Conversely, the Nuclisens EasyMag performed slightly better (~2 Cts) compared to the RNeasy mini plus kit for tissue samples. Third, the type of samples we collected may also have limited our ability to detect SARS-CoV-2 infection. Viral replication can vary among tissue types and therefore some tissues are more optimal for viral RNA detection than others ((1)). In the present work, animals were sampled opportunistically as a part of pre-existing programs, and we were not able to consistently collect the same sample sets. Additionally, the sample types were from live animals and carcasses and not optimized; certain sample types were sometimes unavailable (e.g. tissue samples from live animals) or were not sufficient for collection.
Conclusion
A One Health approach is critical to understanding and managing the risks of an emerging zoonotic pathogen such as SARS-CoV-2. We leveraged activities of existing research, surveillance, and rehabilitation programs and expertise from multiple fields to efficiently collect and test 1,690 individual wildlife samples. The absence of SARS-CoV-2-positive wildlife samples does not exclude spillover from humans to Canadian wildlife, given the limitations cited above. Continued research in this area is both important and pressing, particularly as novel VoCs emerge. Public and animal health sectors should continue to work collaboratively with academic and government partners to help prevent the spread of SARS-CoV-2 from people to wildlife, monitor for spillover, and address any issues should they arise. There is an urgent need for a coordinated wildlife surveillance program for SARS-CoV-2 in Canada. This approach will help protect the health of both Canadians and wildlife, now and in the future.
Acknowledgements
The authors wish to thank B Pickering and J Tataryn for facilitating the inter-agency partnerships that made this work possible, and for their thoughtful review and comments on the manuscript. We also wish to acknowledge B Pickering for helping to arrange antibody testing, and N Toledo for performing the antibody testing. We wish to thank M Anderson of the Ontario Ministry of Agriculture, Food and Rural Affairs and V Misra of the Western College of Veterinary Medicine for their expert advice and participation in discussions regarding this study. We are grateful for the work of D Bulir in developing the PCR testing assay used in this study. We wish to thank L Lazure and the staff of the Granby Zoo, the technicians of the Québec rabies surveillance program, and V Casaubon and J Viau of the Canadian Wildlife Health Cooperative (CWHC) Québec for their help with sample collection. We are also grateful for the sample collection assistance of N Pulham, S Konieczka, J Adams, G McCoy, T McGee, L Pollock, and K Bennett at the Wildlife Research and Monitoring Section of the Northern Development, Mines, Natural Resources and Forestry (NDMNRF) and L Dougherty, L Shirose, and M Alexandrou at the CWHC Ontario. Finally, we wish to thank the licensed fur harvesters who submitted mink for testing.
Competing interests: None.
Funding: This work was supported by the Public Health Agency of Canada, with in-kind contributions provided by all collaborating partners.
References
- 1.Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z, Zhao Y, Liu P, Liang L, Cui P, Wang J, Zhang X, Guan Y, Tan W, Wu G, Chen H, Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 2020;368(6494):1016–20. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobbs EC, Reid TJ. Animals and SARS-CoV-2: species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound Emerg Dis 2021;68(4):1850–67. 10.1111/tbed.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla-Aldana DK, García-Barco A, Jimenez-Diaz SD, Bonilla-Aldana JL, Cardona-Trujillo MC, Muñoz-Lara F, Zambrano LI, Salas-Matta LA, Rodriguez-Morales AJ. SARS-CoV-2 natural infection in animals: a systematic review of studies and case reports and series. Vet Q 2021;41(1):250–67. 10.1080/01652176.2021.1970280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meekins DA, Gaudreault NN, Richt JA. Natural and experimental SARS-CoV-2 infection in domestic and wild animals. Viruses 2021;13(10):1993. 10.3390/v13101993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Organization for Animal Health. SARS-CoV-2 in animals – Situation Report 12. Paris (France); OIE; 2022. https://www.oie.int/app/uploads/2022/05/sars-cov-2-situation-report-12.pdf
- 6.Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, Hiller M, Koepfli KP, Pfenning AR, Zhao H, Genereux DP, Swofford R, Pollard KS, Ryder OA, Nweeia MT, Lindblad-Toh K, Teeling EC, Karlsson EK, Lewin HA. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci USA 2020;117(36):22311–22. 10.1073/pnas.2010146117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander MR, Schoeder CT, Brown JA, Smart CD, Moth C, Wikswo JP, Capra JA, Meiler J, Chen W, Madhur MS. Predicting susceptibility to SARS-CoV-2 infection based on structural differences in ACE2 across species. FASEB J 2020;34(12):15946–60. 10.1096/fj.202001808R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franklin AB, Bevins SN. Spillover of SARS-CoV-2 into novel wild hosts in North America: A conceptual model for perpetuation of the pathogen. Sci Total Environ 2020;733:139358. 10.1016/j.scitotenv.2020.139358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosco-Lauth AM, Root JJ, Porter SM, Walker AE, Guilbert L, Hawvermale D, Pepper A, Maison RM, Hartwig AE, Gordy P, Bielefeldt-Ohmann H, Bowen RA. Peridomestic mammal susceptibility to severe acute respiratory syndrome coronavirus 2 infection. Emerg Infect Dis 2021;27(8):2073–80. 10.3201/eid2708.210180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francisco R, Hernandez SM, Mead DG, Adcock KG, Burke SC, Nemeth NM, Yabsley MJ. Experimental susceptibility of North American raccoons (Procyon lotor) and striped skunks (Mephitis mephitis) to SARS-CoV-2. Front Vet Sci 2022;8:715307. 10.3389/fvets.2021.715307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguiló-Gisbert J, Padilla-Blanco M, Lizana V, Maiques E, Muñoz-Baquero M, Chillida-Martínez E, Cardells J, Rubio-Guerri C. First description of SARS-CoV-2 infection in two feral American mink (Neovison vison) caught in the wild. Animals (Basel) 2021;11(5):1422. 10.3390/ani11051422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler JC, Bevins SN, Ellis JW, Linder TJ, Tell RM, Jenkins-Moore M, Root JJ, Lenoch JB, Robbe-Austerman S, DeLiberto TJ, Gidlewski T, Kim Torchetti M, Shriner SA. SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proc Natl Acad Sci USA 2021;118(47):e2114828118. 10.1073/pnas.2114828118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, Ehrlich M, Grieser J, Winston J, Lombardi D, Gibson S, Saif L, Killian ML, Lantz K, Tell RM, Torchetti M, Robbe-Austerman S, Nelson MI, Faith SA, Bowman AS. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 2022;602(7897):481–6. 10.1038/s41586-021-04353-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuchipudi SV, Surendran-Nair M, Ruden RM, Yon M, Nissly RH, Vandegrift KJ, Nelli RK, Li L, Jayarao BM, Maranas CD, Levine N, Willgert K, Conlan AJ, Olsen RJ, Davis JJ, Musser JM, Hudson PJ, Kapur V. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proc Natl Acad Sci USA 2022;119(6):e2121644119. 10.1073/pnas.2121644119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotwa JD, Massé A, Gagnier M, Aftanas P, Blais-Savoie J, Bowman J, Buchanan T, Chee HY, Dibernardo A, Kruczkiewicz P, Nirmalarajah K, Soos C, Yip L, Lindsay LR, Lung O, Pickering B, Mubareka S. First detection of SARS-CoV-2 infection in Canadian wildlife identified in free-ranging white-tailed deer (Odocoileus virginianus) from southern Québec, Canada. bioRxiv 2022. 10.1101/2022.01.20.476458 [DOI] [Google Scholar]
- 16.Pickering B, Lung O, Maguire F, Kruczkiewicz P, Kotwa JD, Buchanan T, Gagnier M, Guthrie JL, Jardine CM, Marchand-Austin A, Massé A, McClinchey H, Nirmalarajah K, Aftanas P, Blais-Savoie J, Chee H-Y, Chien E, Yim W, Banete A, Griffin BD, Goolia M, Suderman M, Pinette M, Smith G, Sullivan D, Rudar J, Adey E, Nebroski M, Goyett G, Finzi A, Laroche G, Ariana A, Vahkal B, Côté M, McGeer AJ, Nituch L, Mubareka S, Bowman J. Highly divergent white-tailed deer SARS-CoV-2 with potential deer-to-human transmission. bioRxiv 2022;02.22.481551. 10.1101/2022.02.22.481551 [DOI]
- 17.Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, Gerhards N, Tolsma P, Bouwstra R, Sikkema RS, Tacken MG, de Rooij MM, Weesendorp E, Engelsma MY, Bruschke CJ, Smit LA, Koopmans M, van der Poel WH, Stegeman A. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill 2020;25(23):2001005. 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molenaar RJ, Vreman S, Hakze-van der Honing RW, Zwart R, de Rond J, Weesendorp E, Smit LA, Koopmans M, Bouwstra R, Stegeman A, van der Poel WH. Clinical and pathological findings in SARS-CoV-2 disease outbreaks in farmed mink (Neovison vison). Vet Pathol 2020;57(5):653–7. 10.1177/0300985820943535 [DOI] [PubMed] [Google Scholar]
- 19.Palmer MV, Martins M, Falkenberg S, Buckley A, Caserta LC, Mitchell PK, Cassmann ED, Rollins A, Zylich NC, Renshaw RW, Guarino C, Wagner B, Lager K, Diel DG. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol 2021;95(11):e00083–21. 10.1128/JVI.00083-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Organization for Animal Health. One Health. Paris (France): OIE; 2021. https://www.oie.int/en/what-we-do/global-initiatives/one-health/
- 21.Larsen HD, Fonager J, Lomholt FK, Dalby T, Benedetti G, Kristensen B, Urth TR, Rasmussen M, Lassaunière R, Rasmussen TB, Strandbygaard B, Lohse L, Chaine M, Møller KL, Berthelsen AN, Nørgaard SK, Sönksen UW, Boklund AE, Hammer AS, Belsham GJ, Krause TG, Mortensen S, Bøtner A, Fomsgaard A, Mølbak K. Preliminary report of an outbreak of SARS-CoV-2 in mink and mink farmers associated with community spread, Denmark, June to November 2020. Euro Surveill 2021;26(5):2100009. 10.2807/1560-7917.ES.2021.26.5.210009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashor L, Gagne RB, Bosco-Lauth AM, Bowen RA, Stenglein M, VandeWoude S. SARS-CoV-2 evolution in animals suggests mechanisms for rapid variant selection. Proc Natl Acad Sci USA 2021;118(44):e2105253118. 10.1073/pnas.2105253118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montagutelli X, van der Werf S, Rey FA, Simon-Loriere E. SARS-CoV-2 Omicron emergence urges for reinforced One-Health surveillance. EMBO Mol Med 2022;14(3):e15558. 10.15252/emmm.202115558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo VC, Sluydts V, Mariën J, Vanden Broecke B, Van Houtte N, Leirs W, Jacobs L, Iserbyt A, Hubert M, Heyndrickx L, Goris H, Delputte P, De Roeck N, Elst J, Ariën KK, Leirs H, Gryseels S. SARS-CoV-2 surveillance in Norway rats (Rattus norvegicus) from Antwerp sewer system, Belgium. Transbound Emerg Dis 2021;69(5):3016–21. 10.1111/tbed.14219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacchetto L, Chaves BA, Costa ER, de Menezes Medeiros AS, Gordo M, Araújo DB, Oliveira DB, da Silva AP, Negri AF, Durigon EL, Hanley KA, Vasilakis N, de Lacerda MV, Nogueira ML. Lack of evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spillover in free-living neotropical non-human primates, Brazil. Viruses 2021;13(10):1933. 10.3390/v13101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miot EF, Worthington BM, Ng KH, de Lataillade LG, Pierce MP, Liao Y, Ko R, Shum MH, Cheung WY, Holmes EC, Leung KS, Zhu H, Poon LL, Peiris MJ, Guan Y, Leung GM, Wu JT, Lam TT. Surveillance of rodent pests for SARS-CoV-2 and other coronaviruses, Hong Kong. Emerg Infect Dis 2022;28(2):467–70. 10.3201/eid2802.211586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government of Canada. COVID-19 daily epidemiology update: current situation. Ottawa, ON: Government of Canada, (modified 2022). https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html?stat=rate&measure=total_last7&map=hr#a2
- 28.World Organization for Animal Health. Considerations on monitoring SARS-CoV-2 in animals. Paris (France): OIE; 2022. https://www.oie.int/app/uploads/2022/02/en-sars-cov-2-surveillance-.pdf
- 29.Delahay RJ, de la Fuente J, Smith GC, Sharun K, Snary EL, Flores Girón L, Nziza J, Fooks AR, Brookes SM, Lean FZ, Breed AC, Gortazar C. Assessing the risks of SARS-CoV-2 in wildlife. One Health Outlook 2021;3:7. 10.1186/s42522-021-00039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ProMED. Coronavirus disease update (531): animal, Canada (British Columbia) mink, OIE. ProMED; 2020. https://promedmail.org/promed-post/?id=8008864
- 31.Hammer AS, Quaade ML, Rasmussen TB, Fonager J, Rasmussen M, Mundbjerg K, Lohse L, Strandbygaard B, Jørgensen CS, Alfaro-Núñez A, Rosenstierne MW, Boklund A, Halasa T, Fomsgaard A, Belsham GJ, Bøtner A. SARS-CoV-2 transmission between mink (Neovison vison) and humans, Denmark. Emerg Infect Dis 2021;27(2):547–51. 10.3201/eid2702.203794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, van der Spek A, Tolsma P, Rietveld A, Brouwer M, Bouwmeester-Vincken N, Harders F, Hakze-van der Honing R, Wegdam-Blans MC, Bouwstra RJ, GeurtsvanKessel C, van der Eijk AA, Velkers FC, Smit LA, Stegeman A, van der Poel WH, Koopmans MP. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science 2021;371(6525):172–7. 10.1126/science.abe5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Aart AE, Velkers FC, Fischer EA, Broens EM, Egberink H, Zhao S, Engelsma M, Hakze-van der Honing RW, Harders F, de Rooij MM, Radstake C, Meijer PA, Oude Munnink BB, de Rond J, Sikkema RS, van der Spek AN, Spierenburg M, Wolters WJ, Molernaar RJ, Koopmans MP, van der Poel WH, Stegeman A, Smit LA. SARS-CoV-2 infection in cats and dogs in infected mink farms. Transbound Emerg Dis 2022;69(5):3001–7. 10.1111/tbed.14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nituch LA, Bowman J, Beauclerc KB, Schulte-Hostedde AI. Mink farms predict Aleutian disease exposure in wild American mink. PLoS One 2011;6(7):e21693. 10.1371/journal.pone.0021693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez SR, O’Shea TJ, Oko LM, Holmes KV. Detection of group 1 coronaviruses in bats in North America. Emerg Infect Dis 2007. Sep;13(9):1295–300. 10.3201/eid1309.070491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misra V, Dumonceaux T, Dubois J, Willis C, Nadin-Davis S, Severini A, Wandeler A, Lindsay R, Artsob H. Detection of polyoma and corona viruses in bats of Canada. J Gen Virol 2009;90(Pt 8):2015–22. 10.1099/vir.0.010694-0 [DOI] [PubMed] [Google Scholar]
- 37.Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013;503(7477):535–8. 10.1038/nature12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olival KJ, Cryan PM, Amman BR, Baric RS, Blehert DS, Brook CE, Calisher CH, Castle KT, Coleman JT, Daszak P, Epstein JH, Field H, Frick WF, Gilbert AT, Hayman DT, Ip HS, Karesh WB, Johnson CK, Kading RC, Kingston T, Lorch JM, Mendenhall IH, Peel AJ, Phelps KL, Plowright RK, Reeder DM, Reichard JD, Sleeman JM, Streicker DG, Towner JS, Wang LF. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: A case study of bats. PLoS Pathog 2020;16(9):e1008758. 10.1371/journal.ppat.1008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeBlanc JJ, Gubbay JB, Li Y, Needle R, Arneson SR, Marcino D, Charest H, Desnoyers G, Dust K, Fattouh R, Garceau R, German G, Hatchette TF, Kozak RA, Krajden M, Kuschak T, Lang AL, Levett P, Mazzulli T, McDonald R, Mubareka S, Prystajecky N, Rutherford C, Smieja M, Yu Y, Zahariadis G, Zelyas N, Bastien N; COVID-19 Pandemic Diagnostics Investigation Team of the Canadian Public Health Laboratory Network (CPHLN) Respiratory Virus Working Group. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol 2020;128:104433. 10.1016/j.jcv.2020.104433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall JS, Knowles S, Nashold SW, Ip HS, Leon AE, Rocke T, Keller S, Carossino M, Balasuriya U, Hofmeister E. Experimental challenge of a North American bat species, big brown bat (Eptesicus fuscus), with SARS-CoV-2. Transbound Emerg Dis 2021;68(6):3443–52. 10.1111/tbed.13949 [DOI] [PubMed] [Google Scholar]
- 41.Montagutelli X, Prot M, Levillayer L, Salazar EB, Jouvion G, Conquet L, Donati F, Albert M, Gambaro F, Behillil S, Enouf V, Rousset D, Jaubert J, Rey F, van der Werf S, Simon-Loriere E. The B1.351 and P.1 variants extend SARS-CoV-2 host range to mice. bioRxiv 2021;03.18.436013.
- 42.Griffin BD, Chan M, Tailor N, Mendoza EJ, Leung A, Warner BM, Duggan AT, Moffat E, He S, Garnett L, Tran KN, Banadyga L, Albietz A, Tierney K, Audet J, Bello A, Vendramelli R, Boese AS, Fernando L, Lindsay LR, Jardine CM, Wood H, Poliquin G, Strong JE, Drebot M, Safronetz D, Embury-Hyatt C, Kobasa D. SARS-CoV-2 infection and transmission in the North American deer mouse. Nat Commun 2021;12(1):3612. 10.1038/s41467-021-23848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


