Abstract
Background
There is not yet a consensus regarding the in-use effectiveness of ultraviolet irradiation (UV-C) as a supplementary tool for terminal room disinfection.
Aims and Objectives
To summarize and evaluate literature detailing the germicidal effectiveness of UV-C disinfection on high-touch surfaces in the patient environment.
Methods
A literature search was carried out utilizing PRISMA guidelines. Studies were included if intervention included UV-C after standard room disinfection in hospital rooms evaluated microbiologically by surface type.
Findings/Results
Twelve records met our criteria for inclusion. Studies predominantly focused on terminal disinfection of patient rooms, including five reports carried out in isolation rooms and three studies including operating room (OR) surfaces. Bedrails, remote controls, phones, tray tables, assist rails, floors, and toilets were the most commonly reported surfaces. Across study designs, surfaces, and room types, flat surfaces tended to showcase UV-C effectiveness best, particularly isolation room floors. In contrast, handheld surfaces (i.e., bed controls and assist bars) tended to show reduced efficacies (81–93%). In the OR, complex surfaces similarly demonstrated reduced UV-C effectiveness. Bathroom surfaces demonstrated 83% UV-C effectiveness overall, with surface characteristics uniquely impacted depending on the room type. Isolation room studies tended to include effectiveness comparison with standard treatment, reporting UV-C superiority most of the time.
Discussion
This review highlights the enhanced effectiveness of UV-C surface disinfection over standard protocols across various study designs and surfaces. However, surface and room characteristics do appear to play a role in the level of bacterial reduction.
Keywords: Systematic reviews, ultraviolet light, hospital, technology, microbiology, environment
Background
Hospital-associated infections (HAIs) remain the fourth highest risk factor of mortality in the Western world, doubling the mortality risk of the patient and exacerbating the length and cost of hospitalization (Kirkland et al., 1999). Environmental disinfection practices are critical in reducing the likelihood of pathogenic transmission, in some cases reducing bacterial contamination by 75% (Jinadatha et al., 2014) and contamination with pathogenic microorganisms by ∼50% (Bhalla et al., 2004). However, the absence of dedicated guidelines has contributed to much variability in disinfection practice of high-touch surfaces within the patient environment (Manning et al., 2013; Chao Foong et al., 2015). Additionally, the lack of clarity regarding the cleaning responsibilities of housekeeping staff and medical personnel can result in a failure to adequately decontaminate these surfaces (Carling and Bartley, 2010; Carling et al., 2010). Cleaning failures in turn are correlated with increased acquisitions of pathogenic infections (Dancer and Simmons, 2006). For example, patients who are admitted to a room with a prior HAI-positive occupant are at an increased risk of developing an HAI themselves (a 40% increased risk with MRSA, a 135% increased risk with C. difficile, and 280% increased risk with VRE) (Shaughnessy et al., 2011; Huang et al., 2006; Datta et al., 2011; Drees et al., 2008). Due to the lack of direct contact between patients, these effects are likely due to inadequate disinfection of environmental surfaces within the patient room or hand/glove transfer of pathogens by healthcare workers (Carling et al., 2010). Combined with the range of survival (days to months) of many infectious pathogens (Kramer et al., 2006), contamination of the patient environment due to human error can have serious consequences. This is supported by the positive correlation between the frequency of total hygiene failures and the number of ICU-acquired infections (White et al., 2008).
In laboratory settings, VRE, MRSA, Acinetobacter, and C. difficile inoculum have been eradicated by as little as 30 s of ultraviolet (UV-C) irradiation (Nerandzic et al., 2015; Nagaraja et al., 2015; Rutala et al., 2010; Rastogi et al., 2007; Weber et al., 2016). UV-C has also demonstrated real-world effectiveness in reducing HAIs and environmental bacteria across numerous studies (Anderson et al., 2017, 2018; Bernard and Little, 2015; Catalanotti et al., 2016; Donskey, 2013; Haas et al., 2014; Levin et al., 2013; Pegues et al., 2017; McMullen et al., 2016; Miller et al., 2015; Penno et al., 2017; Raggi et al., 2018; Sampathkumar et al., 2016; Vianna et al., 2016). While several reviews have been dedicated to evaluating the effectiveness of enhanced “no-touch” disinfection (including ultraviolet intervention) (Boyce, 2016; Doll et al., 2015; Health Quality, 2018), none to our knowledge have focused on characterizing surface-specific effectiveness of UV-C. Due to the known influence of variables such as frequency of touch, porosity of material, and obstruction of UV light path, it is important to profile potential vectors in the patient environment more precisely, aiming to identify surfaces/areas that may require enhanced treatment.
Methods
Study identification
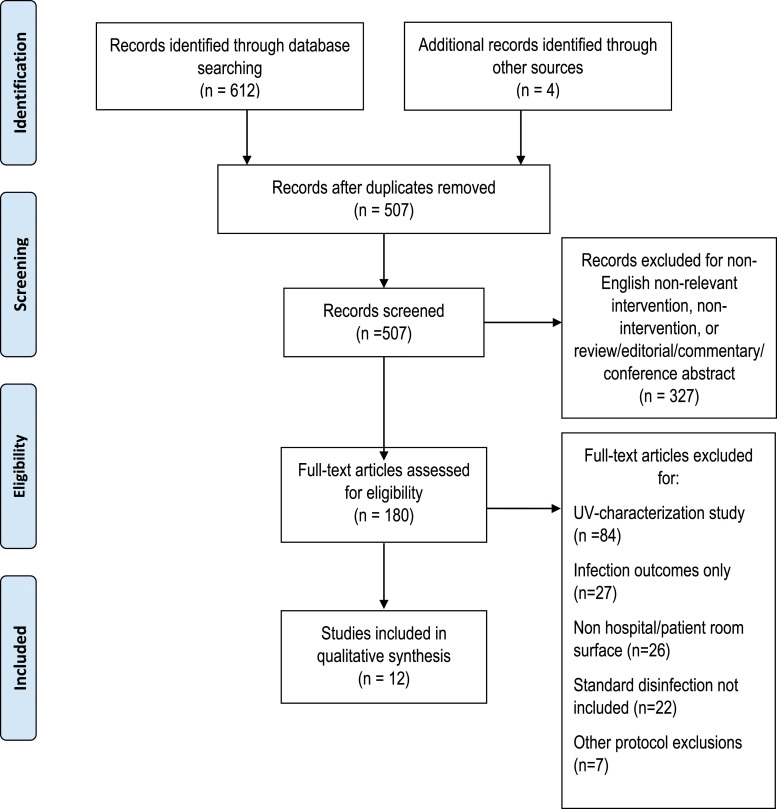
The following databases were used to search for relevant keywords: PubMed, Excerpta Medica database (Embase), Cumulative Index of Nursing and Allied Health Literature (CINAHL), and the Cochrane Central Register of Controlled Trials. The search was performed for studies from inception to July 21, 2020 using the keyword combination “hospital,” “ultraviolet,” and “disinfection.” A PRISMA approach (Figure 1) was used to filter through 507 hits based on relevance and subsequently full-text screening of 180 entries based on the inclusion/exclusion criteria described below. Finally, a methodological quality assessment was performed by two investigators independently using the 11-point GRACE checklist (Dreyer et al., 2016), a validated assessment tool for evaluating the quality of observational cohort studies for decision-making support. This checklist was modified by removing two questions that were not relevant to the review (patient-related factors). The yes/no format of the checklist allowed us to create summary scores. Finally, we included a 10th point to address whether or not studies may have been influenced by commercial UV disinfection device manufacturers (Supplementary Table 1).
Figure 1.
Systematic review identification and screening of eligible studies.
Inclusion/Exclusion criteria
Studies were included if samples were collected from disinfected patient rooms in hospitals where enhanced ultraviolet disinfection followed standard terminal disinfection. Ultraviolet disinfection could include continuous or pulsed ultraviolet irradiation. Ultraviolet devices had to meet dose requirements for the inactivation of vegetative and sporicidal bacteria (typically 12,000–24,000 µWs/cm2). Any high-touch surface was accepted as a sampling point if it was located within the treated room. All observational cohort studies that included at least two groups (after standard decontamination and after ultraviolet irradiation) were considered. Included studies were required to present microbiological quantification of precisely named high-touch surfaces within the patient room. Studies which did not perform comparative analysis of standard terminal disinfection and ultraviolet disinfection or which presented data only as log reduction or percent positive growth were excluded. Finally, studies where the comparator did not include standard terminal disinfection a priori were excluded.
Data synthesis and analysis
A data extraction form was developed which included study setting characteristics, intervention details, and all reported microbiological study outcome measures. The most common microbiological data were reported as total colony forming units (CFUs), mean CFU, and median CFU. Reports of log reduction, percent reduction, and percent positive cultures were excluded from data analysis. Total CFU, mean CFU, and median CFU were extracted per each high-touch surface as reported. Descriptive averages reported in the results herein reflect author-generated summaries based on study design and surface categorization only.
Study selection
The search among all databases included in this systematic review yielded 612 entries, with an additional 4 studies identified through other sources. Five hundred and seven entries remained after eliminating duplicate entries. These entries underwent expedited screening which included mining summaries and abstracts for compatibility with inclusion/exclusion criteria. After expedited screening, 180 studies were reviewed in full. In 84 studies, ultraviolet characterization occurred in the absence of a controlled comparator such as standard manual terminal disinfection. Twenty seven studies reported infection outcomes only. Twenty six studies reported surface samples outside of patient rooms. Finally, 22 studies did not include an evaluation for standard decontamination. Seven studies were further excluded for protocols which did not meet the inclusion criteria. In sum, 12 observational studies met the aforementioned inclusion criteria for systematic review (Figure 1).
Methodological quality
Methodological quality based on the GRACE checklist (Dreyer et al., 2016) revealed an average score of 7.5/10. Five studies provided important covariates such as MRSA colony counts or detection of other drug-resistant organisms aside from heterotrophic colony counts. Six studies tested the efficacy assumptions using biological indicators and four studies included HAI outcomes. All studies provided adequate details of the treatment exposure and adequate study outcomes. Important covariates and confounders were addressed in 8 of the 11 studies. In 10 studies, comparator evaluation was performed concurrently with the intervention group (two studies were two-arm, crossover-design). Finally, six studies were either financed or authored by the manufacturers or distributors of the UV-C devices, three studies deployed devices on loan from the manufacturer (one of which also received study support services from the manufacturer), and three studies were determined to be free from commercial influence.
Study settings and treatment
Five studies were carried out in US hospitals, including two academic hospitals, two military treatment facilities, and one acute care private hospital. The geographic location included one Canadian hospital, one Ecuadorian hospital, one Japanese hospital, and three British hospitals, including a large teaching hospital and a facility serving elderly patients predominantly. Finally, one additional US study was performed across 23 different hospitals, accumulating results from a convenience sample of mostly short-term acute care facilities agreeing to perform product validation as part of their product selection process. Assessment was carried out after terminal room disinfection (standard or UV-C enhanced) in all studies. Five studies focused specifically on isolation rooms housing patients on contact precautions. Six studies sampled inpatient rooms where contact precaution patients were either not housed or not specified. Finally, three studies included independent OR sampling.
Most studies used UV-C devices delivering between 12 and 22,000 µWs/cm2 dose; however in one study, the UV-C dose was auto-prescribed and thus not specified. The average cycle time of the eight studies that reported this measure (8/12) was 16.25 min. Eight of twelve studies deployed PX-UV devices (Xenex) delivering pulsed xenon UV. One study utilized the Intelligent Automated Syndicate UV-C system (Skytron LLC, Grand Rapids, MI), one study performed comparisons between Tru-D Disinfector (Tru-D; Lumalier Corp, Memphis, TN) and R-D Rapid Disinfector (Steriliz, Rochester, NY), and one study used a combination of the Surfacide Helios (Surfacide) and the Ultra V Disinfection Robot (Hygiene Solutions). Boyce et al., used the Tru-D system as a stand-alone. Comparators in all studies included standard hospital protocols for terminal room disinfection, in some cases containing hydrogen peroxide, troclosene sodium, chlorine (0.1%), bleach, and peracetic acid. All studies were published between August 2011 and February 2020.
Surface sampling
Due to the variation in study type (comparative effectiveness, two-arm crossover, etc.,) it was not possible to quantitatively summarize all bacterial burden before and after enhanced UV disinfection uniformly. Thus, quantitative summary was carried out by study design type. Incidentally, design type mostly corresponded with variations in room type, where pre/post studies (2 time points) were mostly performed in non-isolation rooms. Pre/post designs were also carried out in all operating room (OR) surface studies, so we extracted and summarized all OR sampling separately. Finally, isolation room studies were mostly comparative effectiveness studies, where the percent reduction could be calculated for each intervention (standard and UV-C) independently. In all but one isolation room study, percent reduction for the UV-C intervention was calculated from a post-standard decontamination time point. In Zeber et al. (2018) and Jinadatha et al. (2014), UV-C effectiveness was calculated by pre-intervention independent sampling which represented post-standard decontamination but was not concurrent with control intervention sampling due to the two-arm, crossover nature of the study. These two studies are labeled appropriately within the results tables. The most common high-touch surfaces within the surveyed rooms (across all study types) were bed rails (8 studies), call buttons and/or bedside controls (9 studies), and bed/tray tables (8 studies). Less commonly included surfaces included door handles, drawer/cabinet handles, and light switches. Within the bathroom, handrails and assist bars were most commonly sampled (5 studies), followed by toilet/toilet seats (8 studies). OR surfaces commonly included OR tables, instrument tables, and anesthesia machines (Table 1).
Table 1.
Study settings and room characteristics.
| Study | Clinical setting | Location | Room type | Intervention | Comparator |
|---|---|---|---|---|---|
| Penno et al. 2017 | 699-bed tertiary care academic medical center | Cincinnati, OH, USA | 11 patient rooms that were about to undergo terminal disinfection | Intelligent Automated Syndicate UV-C system programmed 22,000 µWs/cm2; 15 min treatment per patient room; 1 device in room and 1 device in bathroom | Unmonitored (phase 1) terminal disinfection by environmental services staff |
| Wong et al., 2016 | 728-bed tertiary care academic teaching hospital | British Colombia, Canada | Isolation rooms of recently discharged patients known to have MRSA, VRE, or CD | Tru-D SmartUVC (12,000–22,000 µWs/cm2) or R-D Rapid Disinfector system (46,000 µWs/cm2); both devices automatically calculate duration based on room size; R-D required repositioning of the device after a pre-defined dose | Discharge isolation cleaning with accelerated hydrogen peroxide for surfaces and a neutral detergent for floors removing all mobile equipment, personal items, linens, and curtains |
| Simmons et al., 2018 | 23 facilities with 136 ORs. Including 22 short-term acute facilities and 1 ambulatory surgical center. The short-term acute care hospitals range from 106 to 844 licensed beds (median = 336) and 5 to 30 ORs (median = 12) | NR | 136 ORs | PX-UV xenon flash lamp; two 5–10 min cycles depending on room size; device is placed on either side of the bed each time | Routine terminal manual cleaning at the end of the day using standard disinfectants and following current protocols at each of the study hospitals |
| Villacis et al., 2019 | 329-bed second-level public hospital | Quito, Ecuador | 12 hospital rooms and 4 ORs | PX-UV deployed for one 5 min cycle in bathroom, two 5 min cycles in patient room; two 10 min cycles in OR | Terminal manual cleaning and disinfection with 2500 ppm (0.25%) chlorine disinfectant for 20 min |
| Hoesin et al., 2016 | 700-bed hospital serving a population with a significantly elderly proportion with many comorbidities | North London, the United Kingdom | 40 isolation rooms | In each hospital room, the PX-UV device was deployed for 3 cycles: Two 5 min cycles in the living room (1 cycle on each side of the patient bed) and one 5 min cycle in the bathroom | Standard terminal cleaning using 1000 ppm chlorine disinfectant (0.1%) with detergent (troclosene sodium) |
| Beal et al., 2016 | Single occupancy, isolation, en suite rooms in clinical hematology wards in a large teaching hospital | United Kingdom | 10 patient rooms for environmental sampling excluded VRE-positive discharges | Within each room, PX-UV device was deployed at 3 locations, each for a 5 min disinfection cycle. On average, 25 min were required to perform the room disinfection | Manual clean performed by staff using a general purpose detergent in warm water per national standards |
| Green et al., 2017 | 16-bed ICU burn care center, patients predominantly admitted for thermal injury and occasional trauma and specialized wound care | JBSA Ft. Sam Houston, Texas, USA | 9 inpatient rooms and 2 ORs | PPX-UVD for 5 min, with 4 positions per patient room/anteroom/bathroom combination and two for shower rooms/ancillary areas. Cycle lengths were 10 min for ORs with two positions per room. PPX-UVD was used in patient rooms when vacated for a procedure and after discharge, and in ORs/shower rooms/ancillary areas daily | At discharge/transfer, room is cleaned with hospital-approved disinfectant, including bleach product if patient had CDI |
| Ali et al., 2017 | Unspecified teaching hospital | London, the United Kingdom | 12 single-patient isolation rooms (6 per UV device) | Surfacide Helios (triple emitter system) with laser mapping system to auto-prescribe dose; Ultra V (single-emitter system) with pre-programmed minimum required dose (not-specified) | Manual cleaning with ∼1000 ppm peracetic acid solution. Terminal cleaning was monitored by domestic supervisors using ATP bioluminescence |
| Kitagawa et al., 2020 | 740-bed tertiary care hospital | Hiroshima, Japan | 11 isolation rooms occupied for at least 48 h by a patient with MRSA | PX-UV for 5 min with 2 positions (1 additional cycle for separate bathroom) | Standard manual cleaning including surface cleaning with disposable 0.5% benzalkonium chloride wipes |
| Zeber et al. (2018) | 4 Veterans Affairs facilities | USA | 23 PX-UV rooms sampled, 16 manual rooms sampled; single bed with unshared bathroom, included contact and non-contact patient rooms | PX-UV device placed in the bathroom to complete a 5 min cycle at roughly 450 flashes a cycle; followed by two 5 min cycles in the central room | Terminally disinfected after every patient discharge or transfer. Manual cleaning was conducted according to the local protocol and inspected by research staff using standardized checklist to monitor manual cleaning efforts (CDC) |
| Jinadatha et al., 2014 | 120-bed acute care hospital | Temple, TX, USA | 20 rooms (10 per treatment arm) previously occupied by MRSA-positive patients | PX-UV device emitting ∼450 flashes/cycle was deployed 3 times (5 min each time) in three different positions (2 in main room and 1 in bathroom) | Cleaning visible dirt then soak and wipe cleaning with dispatch disinfection solution (1 min contact time, two applications) on all areas and surfaces in patient rooms regardless of soiling including walls in bathroom and living room up to head height, curtains are replaced if present |
| Boyce et al., 2011 | 500-bed university-affiliated community teaching hospital | New Haven, CT, USA | 20 patient rooms | Tru-D device placed in bathroom for one cycle followed by one cycle in the patient room (UV light dose was pre-set to 22,000 µWs/cm2) | Terminal cleaning performed by hospital housekeeping |
NR: not reported; UV-C: ultraviolet; PX-UV: pulsed xenon UV; OR: operating room; OT: operating theatre; CD (I): Clostridium difficile infection; MRSA: methicillin-resistant Staphylococcus aureus; VRE: vancomycin-resistant Enterococcus.
Results
Evaluation of inpatient rooms
Six studies sampled high-touch surfaces within non-isolation patient rooms after standard decontamination and after enhanced UV-C disinfection. Four studies reported outcomes as median CFU, three reported total CFU, and three reported mean CFU. Percent bacterial reduction was calculated from total CFU or median CFU if total CFU were not reported. For studies where only baseline, standard decontamination, and post-UV-C sampling were performed, post-UV-C enhanced disinfection was subtracted from post-standard decontamination and the total was divided by post-standard decontamination counts, representing enhanced reduction after the standard protocol. In the two-arm study, percent reduction was calculated for each treatment (standard decontamination and UV-C disinfection) based on two independent baseline sampling (post-discharge baseline or post-standard decontamination baseline). Bedrails, one of the most commonly reported surfaces, yielded an average enhanced reduction of 76% based on five studies (Table 2). This was largely driven by a 0% reduction observed in Green et al., (2017), which was an outlier of the five studies and was possibly due to a low baseline sample of just three bacterial colonies. For comparison, percent reduction after UV-C on bedrails in a two-arm study was 92%. Handheld surfaces like TV remotes, telephones, and call buttons demonstrated an average UV-C reduction of 86%. Percent reduction after UV-C in a two-arm study was 74% by comparison. Flat surfaces like tray tables and bedside monitors demonstrated a 99% and 96% post-UV-C reduction, respectively. Patient chairs reported in two studies showed a 100% or 73% post-UV-C reduction, respectively. Finally, toilet surfaces showed a 95% post-UV-C reduction compared to the 94% reduction reported in a two-arm study. In contrast, bathroom hand rails showed only a 70% post-UV-C reduction compared to a 93% reduction in a two-arm study.
Table 2.
Bacterial reduction after enhanced disinfection with UV-C in patient room surfaces.
| Author | High-touch surface | Sample size | % Reduction after standard | % Reduction after UV |
|---|---|---|---|---|
| Villacis et al., 2019 | Handrail stretcher | 12 | 86% | |
| Penno et al. 2017 | Bed rail | 11 | 100% | |
| Boyce et al., 2011 | Bedrail | 20 | 100% | |
| Green et al., 2017 | Bedrail | 9 | 0% | |
| Zeber et al., 2019* | Bedrail | 19 | 62% | 92% |
| Villacis et al., 2019 | Side control bed | 11 | 85% | |
| Penno et al. 2017 | Call button | 11 | 100% | |
| Boyce et al., 2011 | TV remote | 20 | 100% | |
| Penno et al. 2017 | Phone | 11 | 98% | |
| Zeber et al., 2019* | Call button and telephone | 19 | 84% | 74% |
| Beal et al., 2016 | Bed controls | 10 | 50% | |
| Beal et al., 2016 | Telephone on the top of locker | 10 | 92% | |
| Penno et al. 2017 | Overbed table | 11 | 100% | |
| Boyce et al., 2011 | Table | 20 | 100% | |
| Zeber et al., 2019* | Tray table | 19 | 78% | 94% |
| Beal et al., 2016 | Top of patient table | 10 | 100% | |
| Villacis et al., 2019 | Monitor | 15 | 93% | |
| Green et al., 2017 | Bedside monitor | 9 | 99% | |
| Penno et al. 2017 | Patient chair | 11 | 100% | |
| Beal et al., 2016 | Chair arm (left) | 10 | 67% | |
| Beal et al., 2016 | Chair arm (right) | 10 | 80% | |
| Penno et al. 2017 | Toilet lever | 11 | 100% | |
| Penno et al. 2017 | Toilet seat | 11 | 100% | |
| Boyce et al., 2011 | Toilet | 20 | 84% | |
| Zeber et al., 2019* | Toilet seat | 19 | 78% | 94% |
| Beal et al., 2016 | Toilet bin lid | 10 | 97% | |
| Boyce et al., 2011 | Hand rail | 20 | 85% | |
| Zeber et al., 2019* | Toilet handrail | 18 | 0% | 93% |
| Beal et al., 2016 | Top of service rail | 10 | 33% |
Percent reductions were based on reported reductions in either median or total CFUs reported after standard disinfection (before UV-C) and after UV-C treatment in patient room surfaces where contact precaution patients were either not assigned or not specified. High-touch surfaces are grouped into clusters based on similarity between studies. CFUs: colony forming units.
*Studies reported reductions in bacterial burden based on two-arm intervention, where the baseline for UV-C effectiveness was an independent sampling of uncleaned patient rooms after discharge rather than after standard room disinfection, reflecting the effectiveness of UV-C compared to null rather than standard operating procedures. % Reduction after standard disinfection is provided for comparison.
Evaluation of operating rooms
Operating room surfaces were independently sampled in two of the inpatient room studies and exclusively in Simmons et al. (Table 3). Due to the ancillary nature of OR surfaces in two of the three studies, sample sizes were very small in two studies and significantly larger in the dedicated study. Nonetheless, we summarized the three studies by surface type and found that anesthesia machines experienced a 77% post-UV-C reduction as an average of two studies (Green et al., achieved 0 CFU with standard decontamination so a UV-C decrease was not detectable). Flat surfaces like OR tables and instrument tables observed a 95–97% reduction after UV-C irradiation. Document stations were assessed in two studies where UV-C decreased CFUs by 64% or in one study but a slight increase in the other (1 CFU to 2 CFUs). It should be noted that sampling error is increased at very low CFU counts, likely not accurately representing a meaningful difference. Finally, cabinet surfaces observed a similar trend with the Green study reporting an increase in CFUs after UV-C and the Simons study reporting an 85% reduction.
Table 3.
Bacterial reduction after enhanced disinfection with UV-C in operating room surfaces.
| Author | High-touch surface | Sample size | % Reduction after UV |
|---|---|---|---|
| Villacis et al., 2019 | Anesthesia machine | 4 | 67% |
| Simmons et al., 2018 | Anesthesia machine | 147 | 87% |
| Green et al., 2017 | Anesthesia machine | 2 | 0% |
| Villacis et al., 2019 | Instrumentation table | 4 | 94% |
| Green et al., 2017 | Back table | 2 | 100% |
| Simmons et al., 2018 | Back table | 136 | 91% |
| Villacis et al., 2019 | Table | 4 | 95% |
| Green et al., 2017 | Table | 2 | 100% |
| Simmons et al., 2018 | OR table | 123 | 96% |
| Green et al., 2017 | Documentation station | 2 | −100% |
| Simmons et al., 2018 | Nurse’s document station | 140 | 64% |
| Green et al., 2017 | Cabinet | 2 | −150% |
| Simmons et al., 2018 | Supply cabinet doors | 111 | 85% |
Percent reductions reported were based on reported reductions in total CFUs reported after terminal disinfection (before UV-C) and after UV-C treatment in surgical room surfaces. Negative percentages indicate increases in total CFUs after UV-C treatment. High-touch surfaces are grouped into clusters based on similarity between studies. Villacis et al., and Green et al., included OR surfaces as part of a larger hospital study. CFU: colony forming units; OR: operating rooms.
Evaluation of isolation rooms
In isolation rooms’ studies, bacteria on bedrails was decreased by an average of 87% after UV-C based on two of three studies (and unchanged from 0 CFU achieved after standard decontamination in the third study). In comparison, the three studies together reported an average 81% reduction after standard decontamination. Across handheld surfaces like call buttons and bed control panels, there was an average 99% reduction after UV-C. It should be noted however, that in Ali et al., they observed an eight-fold increase in bacteria after UV-C based on an increase from 0 to 8 CFUs after Surfacide Helios UV-C treatment but a 100% reduction after Ultra-V treatment. Compared with UV-C enhanced disinfection protocols, standard decontamination observed an average 85% reduction across all handheld surfaces (Table 4). Flat surfaces like bedside tables and floors in isolation rooms observed an 80% and 99% reduction by UV-C, respectively. Interestingly, standard reductions on table surfaces were higher than UV-C at 88% based on four studies. On the other hand, standard decontamination of isolation room floors showed an increase in bacterial presence based on two studies. While floors do not represent the typical clinical touchpoint, it was included in this study on the basis of its potential role in the chain of transmission and its inclusion in at least two of the eligible studies. Finally, toilet surfaces in isolation rooms demonstrated an average 68% reduction in bacteria after UV-C treatment compared to an 80% reduction after standard decontamination. Toilet hand rails were completely cleaned by standard decontamination protocols in Ali et al.; thus, no reduction could be calculated after UV-C. However in the two remaining studies, UV-C reduced toilet hand rail bacteria by 99% compared to 72% average after standard decontamination.
Table 4.
Bacterial reduction after enhanced disinfection with UV-C in contact precaution patient room surfaces.
| Author | High-touch surface | Sample size | % Reduction after standard | % Reduction after UV |
|---|---|---|---|---|
| Kitagawa et al., 2020 | Bed rail | 11 | 47% | 74% |
| Hoesin et al., 2016 | Bedrail | 28 | 100% | 0% |
| Jinadatha et al., 2014 1 | Bedrail | 10 | 96% | 100% |
| Kitagawa et al., 2020 | Bed control panel | 11 | 53% | 95% |
| Ali et al., 2017 (S) | Bed control panel | 6 | 100% | −800% |
| Ali et al., 2017 (U) | Bed control panel | 6 | 83% | 100% |
| Jinadatha et al., 2014 1 | Call button | 10 | 87% | 95% |
| Ali et al., 2017 (S) | Nurse call button (front) | 6 | 78% | 100% |
| Ali et al., 2017 (U) | Nurse call button (front) | 6 | 99% | 100% |
| Ali et al., 2017 (S) | Nurse call button (back) | 6 | 79% | 100% |
| Ali et al., 2017 (U) | Nurse call button (back) | 6 | 98% | 100% |
| Kitagawa et al., 2020 | Over table | 11 | 96% | 92% |
| Kitagawa et al., 2020 | Bedside table | 11 | 72% | 10% |
| Ali et al., 2017 (S) | Bedside table | 6 | 76% | 100% |
| Ali et al., 2017 (U) | Bedside table | 6 | 91% | 100% |
| Hoesin et al., 2016 | Tray table | 39 | 100% | 0% |
| Jinadatha et al., 2014 1 | Tray table | 10 | 93% | 99% |
| Ali et al., 2017 (S) | Floor corner | 6 | −572% | 100% |
| Ali et al., 2017 (U) | Floor corner | 6 | −215% | 98% |
| Wong et al., 2016 2 | Floors | 61 | −145% | 99% |
| Kitagawa et al., 2020 | Toilet seat | 9 | 77% | 11% |
| Ali et al., 2017 (S) | Toilet flush | 6 | 100% | 0% |
| Ali et al., 2017 (U) | Toilet flush | 6 | 90% | 100% |
| Ali et al., 2017 (S) | Toilet seat | 6 | 97% | 0% |
| Ali et al., 2017 (U) | Toilet seat | 6 | 87% | 100% |
| Hoesin et al., 2016 | Toilet seat | 39 | 79% | 100% |
| Jinadatha et al., 2014 1 | Toilet seat | 10 | 31% | 99% |
| Ali et al., 2017 (S) | Toilet handrail | 6 | 100% | 0% |
| Ali et al., 2017 (U) | Toilet handrail | 6 | 100% | 0% |
| Hoesin et al., 2016 | Bathroom handrail | 39 | 67% | 100% |
| Jinadatha et al., 2014 1 | Bathroom handrail | 10 | 77% | 98% |
Percent reductions reported were based on reported reductions in either median or total CFUs reported before standard disinfection, after standard disinfection, and after UV-C treatment in patient room surfaces where contact precaution patients were assigned for a minimum of 48 h before discharge. High-touch surfaces are grouped into clusters based on similarity between studies.
1Studies reported reductions in bacterial burden based on two-arm intervention, where the baseline for UV-C effectiveness was an independent sampling of uncleaned patient rooms after discharge rather than after standard room disinfection, reflecting the effectiveness of UV-C compared to null rather than standard operating procedures.
2Wong et al., reported only mean CFUs. (S) Surfacide intervention and (U) Ultra-V intervention were both UV-C treatments carried out within the same population/study.
Discussion
UV-C in the hospital setting has been a commercial response to the high rates of hospital cleaning failures that were increasingly associated with HAIs (Dancer and Simmons, 2006; Datta et al., 2011; Drees et al., 2008; Huang et al., 2006; Shaughnessy et al., 2011). UV-C has been proposed to solve personnel-related variability in cleaning adequacy and protocol and disinfectant-based variability which can severely impact effectiveness (Boyce, 2016). Rapid cycle time and ease of delivery have also been major selling points of manufacturers. To-date, the majority of the literature has focused on the effectiveness of UV-C to drive down HAIs. Many fundamental aspects of UV-C in the clinical setting remain to be considered, such as the propensity of specific surfaces for contamination and the effectiveness of UV-C under real-world conditions (particularly as reported by surface type). These factors, among others, may help bridge the gap between some of inconsistencies within the body of existing controlled trials.
After a broad search of top academic and clinical literature repositories, 12 records met our strict criteria for inclusion. Hospital settings were diverse yet utilized similar sampling techniques and fairly consistent surface sampling, particularly bedrails, bedside controls, bathroom rails, bed side tables, and toilets. Of 12 international studies, 5 intentionally reported outcomes in rooms previously occupied by patients on contact precautions, including MRSA, C. difficile, and VRE-infected patients. Hand-held surfaces like bedrails and bedside controls in isolation rooms demonstrated a greater overall bacterial reduction than similar surfaces in non-isolation rooms (89% versus 96%, after adjusting for outliers). In both room types, UV-C reductions were greater than standard decontamination protocols, though in non-isolation rooms standard effectiveness comparisons were based on only one study.
Flat surfaces like tables and floors in isolation rooms demonstrated an 87% reduction after UV-C treatment, cumulatively. However, on closer inspection, isolation room floors demonstrated a 99% reduction compared to 80% reduction on overbed/tray tables. By comparison, flat surfaces like tables and bedside monitor display panels were reduced by 98% in non-isolation rooms, cumulatively, with little deviation by surface type. Interestingly, while standard decontamination (based on Zeber et al., 2018) in non-isolation rooms was 78% for patient tables, the standard reduction in isolation rooms was actually higher than with UV-C (88%). On the other hand, standard decontamination procedures on floor surfaces demonstrated a negative percent bacterial reduction based on two studies, indicating that manual decontamination was worse than no treatment at all. Flat, table surfaces in the OR demonstrated similar UV-C reductions to those in non-isolation rooms, between 95 and 97%. By comparison, complex surfaces like anesthesia machines and document stations ranged from 64 to 87%, with one additional study reporting a two-fold increase in colonies after UV-C treatment. Overall, there was a tendency for simplistic surfaces in non-isolation rooms to benefit the most from UV-C enhanced treatment. Interestingly, this was not the case in isolation rooms, where tables demonstrated a diminished effectiveness compared to standard decontamination. On the other hand, floor surfaces, which were only characterized in isolation rooms, demonstrated the most dramatic bacterial reduction compared to standard treatment.
Bathroom surface characterization was separated from other en suite surfaces as bathrooms usually receive an independent cycle/dose of UV-irradiation. Bathroom surfaces were categorized according to the two most commonly reported, bathroom handrails or commode surfaces (toilet, toilet lever, toilet lid, etc.). In isolation room bathrooms, while commode surfaces averaged a reduction of 68% after UV-C (compared to 80% after standard decontamination), hand rails demonstrated a 99% reduction (compared to 86% after standard decontamination). Interestingly, in non-isolation rooms, the trend was reversed, with hand rails exhibiting a 70% bacterial reduction based on three studies and commode surfaces demonstrating a 95% reduction after UV-C.
Characterizing UV-C effectiveness by surface type paints a more complex picture than that typically depicted in “overall” findings reported in the literature. For example, of the six non-isolation room studies, four provided their own overall assessment of UV-C effectiveness, ranging from 44 to 90% reduction from standard decontamination to UV-C treatment (based on total or median CFUs provided for “combined” or “overall” surfaces). As discussed above, a detailed assessment of surface complexity reveals that flat items like tables and bedside monitors achieve a higher than 95% reduction in bacteria versus 76–86% reductions on handheld surfaces. Similarly, on bathroom surfaces, where an independent UV-C cycle is typically deployed, commode surfaces displayed much higher UV-C effectiveness than bathroom hand rails. This may be due to the curvature of bathroom hand rails, where shadowing is more inherent by design and penetrance of UV-C therefore variable. In non-isolation rooms, it is logical that flat surfaces like tables and flat monitors may benefit increasingly from UV-C treatment, as the surfaces are more easily exposed directly to UV-C irradiation waves than more complex surfaces with undersides and folds (which create shadowing that restricts direct UV-C exposure/dose). Additional nuances impacting the dynamics of UV-C irradiation may be at play among different surfaces, most notably the relative position/angle of surface to the irradiating device. The majority of protocols include at least 10 min of UV-C irradiation spread across two positions (often on either side of the patient bed) and an additional 5 min cycle in bathrooms. It is therefore plausible that surfaces closest to the patient bed would receive a slightly higher dose of UV-C than surfaces further away. Based on surfaces summarized in Table 2, items further from the patient bed (chairs) did observe the lowest UV-C enhanced bacterial reduction (avg 82%) after bedrails were adjusted for an outlier. However, a greater amount of flat/complex surfaces could be evaluated across studies than proximal/distal surfaces, limiting confidence.
Four studies also reported overall efficacies in isolation rooms, where standard terminal cleaning protocols include a higher degree of manual decontamination, whether by increased cleaning or increased concentration/potency of cleaning agent. In these four studies, combined or overall reductions (based on total, median, or mean CFUs provided) ranged between 88 and 100% bacterial reduction. Additionally, we know from surface-type characterization that surfaces within isolation rooms and bathrooms varied greatly after UV-C treatment, such as percent reductions as low as 68% on commode surfaces and 80% on overbed tables. In this case, surface simplicity does not explain the discrepancy. Interestingly, comparative effectiveness between manual protocols and enhanced UV-C treatment was only performed in isolation room studies, where UV-C demonstrated superior reduction of bacterial colonies except in two surface categories (overbed tables and commode surfaces). In most cases the reduction of UV-C was only 6–17% greater, though it should be noted that this summary is based on only a handful of studies at this time. UV-C was particularly effective on floor surfaces in comparison to manual decontamination (which actually exhibited an increase in floor bacteria).
A recent review by Boyce and Donskey emphasizes the notion that the delivery of UV irradiation can vary depending on the surface type due to factors like distance, orientation, and shadowing. However, the authors found that surface material (i.e., vinyl, plastic, metal, and laminate) was not a predictor of germicidal effectiveness. Rather, the only notable differences in surface type were found in bathroom versus non-bathroom surfaces. This was true during UV treatment arms as well as standard decontamination (Boyce and Donskey, 2019) and may reflect the germicidal resistance of C. difficile spores which may be more prevalent in bathroom surfaces. Additionally, this may be due to the distance-dependent drop off of UV dose that occurs in regions further from the source lamps. Not enough distal surfaces were consistent across studies in this review to adequately assess whether distance from the UV-C lamp was a factor of effective bacterial reduction. This is an important question for future study nonetheless. While Boyce and Donskey highlight important factors impacting the penetrance and effectiveness of UV disinfection in patient accessible surfaces, the majority of the work described therein focuses on experimental inoculation of various pathogenic strains rather than an exploration of UV effectiveness in the heterogeneous real-world context of the included studies herein.
The findings of this study do suggest a need for more careful characterization of hospital room UV-C effectiveness by surface topography, distance from the UV-C source, and angle of incidence (height relative to the UV-C source) among other variables. Additionally, since effectiveness in this review was profiled by percent bacterial reduction, it was inherently dependent on the performance of standard decontamination protocols, which can vary by institution. For example, some studies report detailed accounts of their standard decontamination protocols (chemicals, concentrations, specific medical equipment targeted, etc.,) while others do not. In either case, there is some degree of human variability introduced in this phase. Despite UV-C irradiation being touted as an “automated” procedure, in a majority of cases, the UV-C protocol is operated by a human tasked with programming the cycle and often repositioning the device in the room for various cycles. This step includes maximizing exposure of surfaces by opening drawers, cabinets, letting down curtains, etc., all tasks of a human operator. Moreover, while most UV-C devices in this review work based on preset dose and duration, three devices represented in two studies operate based on variable duration determined by the sensors in the device themselves, limiting the generalizability of their performance. Another limitation of this study was the low number of included studies which was a result of the stringent inclusion criteria that was required in order to ensure as much methodological similarity as possible. This in addition to the inherent variability in study designs of the included studies precluded us from a more robust, statistically evaluated quantitative analysis. As such, this review presents only a preliminary qualitative summary based on best efforts to pool in-use effectiveness between surface types and on transformation of common outcome measures. Percent reduction provided a glimpse of the comparative performance of the two treatments (standard vs UV-C).
In closing, there is still a lot of work to be carried out in the resolution of whether or not UV disinfection as a supplementary step in terminal room disinfection can significantly improve bacterial colonization, particularly as the investment in medical-grade UV solutions requires substantial cost, time, and trained labor. Penno et al., suggest that a buddy team approach may be as effective as and potentially more cost effective than UV-C. While our findings did not provide a clear case either for or against UV-C treatment, one study has suggested that sustained improvements in cleaning behaviors are difficult to maintain without ongoing institutional programming (Carling et al., 2008). We describe the need to streamline methodological designs, reporting of outcome measures (i.e., total CFU, mean CFU, and median CFU), and evaluate technology by surface topology, distance from UV-C source, study design similarity, and standard decontamination protocols to name a few. It would also be worthwhile to further examine whether bathroom surfaces are indeed more resistant to terminal disinfection treatments and why. Finally, this review found that a majority of these studies that fit our criteria for inclusion retained some level of commercial interest, whether that be in direct sponsorship/authorship of the study or the provision of microbiological services/financing. Thus, there remains a need for controlled, UV-C hospital effectiveness studies that originate from diverse, unaffiliated institutions.
Supplemental Material
Supplemental Material for A systematic review of the germicidal effectiveness of ultraviolet disinfection across high-touch surfaces in the immediate patient environment by Marisol Resendiz, Dawn Blanchard, and Gordon F West in Journal of Infection Prevention.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by a grant (19S16) awarded to GFW by the TriService Nursing Research Program. The authors have no conflicts of interest to disclose.
Disclaimer: The views expressed in this abstract/manuscript are those of the author(s) and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Gordon F West https://orcid.org/0000-0003-0295-3123
References
- Ali S., Yui S., Muzslay M., Wilson A. P. R. (2017). Comparison of two whole-room ultraviolet irradiation systems for enhanced disinfection of contaminated hospital patient rooms. J Hosp Infect, 97(2), 180-184. 10.1016/j.jhin.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Chen LF, Weber DJ, et al. (2017) Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and clostridium difficile (the benefits of enhanced terminal room disinfection study): a cluster-randomised, multicentre, crossover study. The Lancet 389(10071): 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Moehring RW, Weber DJ, et al. (2018) Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: a secondary analysis of a multicentre cluster randomised controlled trial with crossover design (BETR Disinfection). The LancetInfectious Diseases 18(8): 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal A, Mahida N, Staniforth K, Vaughan N, Clarke M, Boswell T. (2016). First UK trial of Xenex PX-UV, an automated ultraviolet room decontamination device in a clinical haematology and bone marrow transplantation unit. J Hosp Infect, 93(2), 164-168. 10.1016/j.jhin.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Bernard H, Little J. (2015) The impact of ultraviolet (uv) disinfection system coupled with evidence-based interventions on the incidence of hospital onset clostridium difficile (ho-c-diff).42nd annual conference abstracts, APIC 2015, Nashville, TN June 2015. American Journal of Infection Control 43: S27.-S. [Google Scholar]
- Bhalla A, Pultz NJ, Gries DM, et al. (2004) Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infection Control and Hospital Epidemiology 25(2): 164–167. [DOI] [PubMed] [Google Scholar]
- Boyce JM, Donskey CJ. (2019) Understanding ultraviolet light surface decontamination in hospital rooms: A primer. Infection Control and Hospital Epidemiology 40(9): 1030–1035. [DOI] [PubMed] [Google Scholar]
- Boyce JM. (2016) Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrobial Resistance and Infection Control 5: 10–16. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M., Havill N. L., Moore B. A. (2011). Terminal decontamination of patient rooms using an automated mobile UV light unit. Infect Control Hosp Epidemiol, 32(8), 737-742. 10.1086/661222 [DOI] [PubMed] [Google Scholar]
- Carling PC, Bartley JM. (2010) Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. American Journal of Infection Control 38(5 Suppl 1): S41–S50. [DOI] [PubMed] [Google Scholar]
- Carling PC, Parry MM, Rupp ME, et al. (2008) Improving cleaning of the environment surrounding patients in 36 acute care hospitals. Infection Control and Hospital Epidemiology 29(11): 1035–1041. [DOI] [PubMed] [Google Scholar]
- Carling PC, Parry MF, Bruno-Murtha LA, et al. (2010) Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Critical Care Medicine 38(4): 1054–1059. [DOI] [PubMed] [Google Scholar]
- Catalanotti A, Abbe D, Simmons S, et al. (2016) Influence of pulsed-xenon ultraviolet light-based environmental disinfection on surgical site infections. American Journal of Infection Control 44(6): e99–e101. [DOI] [PubMed] [Google Scholar]
- Chao Foong Y, Green M, Zargari A, et al. (2015) Mobile phones as a potential vehicle of infection in a hospital setting. Journal of Occupational and Environmental Hygiene 12(10): D232–D235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer SJ, Simmons NA. (2006) MRSA behind bars? The Journal of Hospital Infection 62(3): 261–263. [DOI] [PubMed] [Google Scholar]
- Datta R, Platt R, Yokoe DS, et al. (2011) Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Archives of Internal Medicine 171(6): 491–494. [DOI] [PubMed] [Google Scholar]
- Doll M, Morgan DJ, Anderson D, et al. (2015) Touchless technologies for decontamination in the hospital: a review of hydrogen peroxide and uv devices. Current Infectious Disease Reports 17(9): 498. [DOI] [PubMed] [Google Scholar]
- Donskey CJ. (2013) Does improving surface cleaning and disinfection reduce health care-associated infections? American Journal of Infection Control 41(5 Suppl): S12–S19. [DOI] [PubMed] [Google Scholar]
- Drees M, Snydman DR, Schmid CH, et al. (2008) Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America 46(5): 678–685. [DOI] [PubMed] [Google Scholar]
- Dreyer NA, Bryant A, Velentgas P. (2016) The GRACE checklist: a validated assessment tool for high quality observational studies of comparative effectiveness. Journal of Managed Care & Specialty Pharmacy 22(10): 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C, Pamplin JC, Chafin KN, et al. (2017) Pulsed-xenon ultraviolet light disinfection in a burn unit: Impact on environmental bioburden, multidrug-resistant organism acquisition and healthcare associated infections. Burns 43(2): 388–396. [DOI] [PubMed] [Google Scholar]
- Haas JP, Menz J, Dusza S, et al. (2014) Implementation and impact of ultraviolet environmental disinfection in an acute care setting. American Journal of Infection Control 42(6): 586–590. [DOI] [PubMed] [Google Scholar]
- Health Quality O. (2018) Portable ultraviolet light surface-disinfecting devices for prevention of hospital-acquired infections: a health technology assessment. Ontario Health Technology Assessment Series 18(1): 1–73. [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Datta R, Platt R. (2006) Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Archives of Internal Medicine 166(18): 1945–1951. [DOI] [PubMed] [Google Scholar]
- Hoesin I., Madeloso R., Nagaratnam W., Villamaria F., Stock E., Jinadatha C. (2016). Evaluation of a pulsed xenon ultraviolet light device for isolation room disinfection in a United Kingdom hospital. Am J Infect Control, 44(9), e157-161. 10.1016/j.ajic.2016.01.044 [DOI] [PubMed] [Google Scholar]
- Jinadatha C, Quezada R, Huber TW, et al. (2014) Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infectious Diseases 14: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland KB, Briggs JP, Trivette SL, et al. (1999) The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection Control and Hospital Epidemiology 20(11): 725–730. [DOI] [PubMed] [Google Scholar]
- Kitagawa H., Mori M., Kashiyama S., Sasabe Y., Ukon K., Shimokawa N., Shime N., Ohge H. (2020). Effect of pulsed xenon ultraviolet disinfection on methicillin-resistant Staphylococcus aureus contamination of high-touch surfaces in a Japanese hospital. Am J Infect Control, 48(2), 139-142. 10.1016/j.ajic.2019.08.033 [DOI] [PubMed] [Google Scholar]
- Kramer A, Schwebke I, Kampf G. (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases 6: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J, Riley LS, Parrish C, et al. (2013) The effect of portable pulsed xenon ultraviolet light after terminal cleaning on hospital-associated Clostridium difficile infection in a community hospital. American Journal of Infection Control 41(8): 746–748. [DOI] [PubMed] [Google Scholar]
- Manning ML, Davis J, Sparnon E, et al. (2013) iPads, droids, and bugs: Infection prevention for mobile handheld devices at the point of care. American Journal of Infection Control 41(11): 1073–1076. [DOI] [PubMed] [Google Scholar]
- McMullen K, Dunn G, Wade R, et al. (2016) Impact of no-touch ultraviolet-c light room disinfection system on hospital acquired infection rates. American Journal of Infection Control 44: S93. [DOI] [PubMed] [Google Scholar]
- Miller R, Simmons S, Dale C, et al. (2015) Utilization and impact of a pulsed-xenon ultraviolet room disinfection system and multidisciplinary care team on Clostridium difficile in a long-term acute care facility. American Journal of Infection Control 43(12): 1350–1353. [DOI] [PubMed] [Google Scholar]
- Nagaraja A, Visintainer P, Haas JP, et al. (2015) Clostridium difficile infections before and during use of ultraviolet disinfection. American Journal of Infection Control 43(9): 940–945. [DOI] [PubMed] [Google Scholar]
- Nerandzic MM, Thota P, Sankar CT, et al. (2015) Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infection Control and Hospital Epidemiology 36(2): 192–197. [DOI] [PubMed] [Google Scholar]
- Pegues DA, Han J, Gilmar C, et al. (2017) Impact of ultraviolet germicidal irradiation for no-touch terminal room disinfection on clostridium difficile infection incidence among hematology-oncology Patients. Infection Control and Hospital Epidemiology 38(1): 39–44. [DOI] [PubMed] [Google Scholar]
- Penno K, Jandarov RA, Sopirala MM. (2017) Effect of automated ultraviolet C-emitting device on decontamination of hospital rooms with and without real-time observation of terminal room disinfection. American Journal of Infection Control 45(11): 1208–1213. [DOI] [PubMed] [Google Scholar]
- Raggi R, Archulet K, Haag CW, et al. (2018) Clinical, operational, and financial impact of an ultraviolet-C terminal disinfection intervention at a community hospital. American Journal of Infection Control 46: 1224–1229. [DOI] [PubMed] [Google Scholar]
- Rastogi VK, Wallace L, Smith LS. (2007) Disinfection of Acinetobacter baumannii-contaminated surfaces relevant to medical treatment facilities with ultraviolet C light. Military Medicine 172(11): 1166–1169. [DOI] [PubMed] [Google Scholar]
- Rutala WA, Gergen MF, Weber DJ. (2010) Room decontamination with UV radiation. Infection Control and Hospital Epidemiology 31(10): 1025–1029. [DOI] [PubMed] [Google Scholar]
- Sampathkumar P, Nation L, Folkert C, et al. (2016) 2-107 - A trial of pulsed xenon ultraviolet disinfection to reduce clostridioides difficile infection. American Journal of Infection Control 44: S32–S33. [DOI] [PubMed] [Google Scholar]
- Shaughnessy MK, Micielli RL, DePestel DD, et al. (2011) Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infection Control and Hospital Epidemiology 32(3): 201–206. [DOI] [PubMed] [Google Scholar]
- Simmons S., Dale C. J., Holt J., Passey D. G., Stibich M. (2018). Environmental effectiveness of pulsed-xenon light in the operating room. Am J Infect Control, 46(9), 1003-1008. 10.1016/j.ajic.2018.02.027 [DOI] [PubMed] [Google Scholar]
- Vianna PG, Dale CR, Jr., Simmons S, et al. (2016) Impact of pulsed xenon ultraviolet light on hospital-acquired infection rates in a community hospital. American Journal of Infection Control 44(3): 299–303. [DOI] [PubMed] [Google Scholar]
- Villacís J. E., Lopez M., Passey D., Santillán M. H., Verdezoto G., Trujillo F., Paredes G., Alarcón C., Horvath R., Stibich M. (2019). Efficacy of pulsed-xenon ultraviolet light for disinfection of high-touch surfaces in an Ecuadorian hospital. BMC Infect Dis, 19(1), 575. 10.1186/s12879-019-4200-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, Kanamori H, Rutala WA. (2016) 'No touch' technologies for environmental decontamination: focus on ultraviolet devices and hydrogen peroxide systems. Current Opinion in Infectious Diseases 29(4): 424–431. [DOI] [PubMed] [Google Scholar]
- White LF, Dancer SJ, Robertson C, et al. (2008) Are hygiene standards useful in assessing infection risk? American Journal of Infection Control 36(5): 381–384. [DOI] [PubMed] [Google Scholar]
- Wong T., Woznow T., Petrie M., Murzello E., Muniak A., Kadora A., Bryce E. (2016). Postdischarge decontamination of MRSA, VRE, and Clostridium difficile isolation rooms using 2 commercially available automated ultraviolet-C-emitting devices. Am J Infect Control, 44(4), 416-420. 10.1016/j.ajic.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Zeber J. E., Pfeiffer C., Baddley J. W., Cadena-Zuluaga J., Stock E. M., Copeland L. A., Hendricks J., Mohammadi J., Restrepo M. I., Jinadatha C. (2018). Effect of pulsed xenon ultraviolet room disinfection devices on microbial counts for methicillin-resistant Staphylococcus aureus and aerobic bacterial colonies. Am J Infect Control, 46(6), 668-673. 10.1016/j.ajic.2018.02.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A systematic review of the germicidal effectiveness of ultraviolet disinfection across high-touch surfaces in the immediate patient environment by Marisol Resendiz, Dawn Blanchard, and Gordon F West in Journal of Infection Prevention.