Abstract
Purpose
The purpose of our study was to investigate the profiles of inflammatory cytokines and the macrophage polarization gene in a choroidal neovascularization (CNV) mouse model before and after intravitreal aflibercept treatment.
Methods
The CNV mouse model was conducted by laser photocoagulation. A total of 58 cytokines were measured by the multiplex mouse cytokine antibody array. The macrophage polarization genes were tested by reverse transcription polymerase chain reaction. The relationship between the cytokines and the CNV lesion area was analyzed by correlation.
Results
MIP-1a on day 3 after laser photocoagulation, MCP-5 and Fas-L on day 7, and IL-15 and IL-7 on day 14 were significantly upregulated (p < 0.001, fold change >10.0). After the intravitreal aflibercept treatment, GM-CSF and MCP-1 on day 3 and TIMP-1 on days 7 and 14 were the most significantly upregulated cytokines (p < 0.001, fold change >10.0). MIP-1 on day 3, IL-13 and Fas-L on day 7, and Fas-L on day 14 were the most significantly downregulated cytokines after intravitreal aflibercept treatment (p < 0.001, fold change >5.0). M2 polarization and VEGFA genes were significantly increased in the CNV formation, whereas aflibercept suppressed M2 polarization and VEGFA genes. IL-7 was negatively related to the CNV lesion area on day 14 after intravitreal aflibercept treatment (r = −0.938, p = 0.006).
Conclusion
The inflammatory cytokines and the M1/M2 macrophage genes significantly changed in the CNV mouse model. This result suggests that inflammatory cytokines and macrophages play a critical role in the physiopathology of CNV.
Keywords: Choroidal neovascularization, Inflammatory cytokines, M1/M2 macrophages, Aflibercept treatment
Introduction
Choroidal neovascularization (CNV) is a common pathological feature of several diseases, such as neovascular age-related macular degeneration, pathological myopia, and ocular trauma [1, 2, 3]. CNV stems from the choroid, which invades the subretinal space and causes hemorrhage, exudative photoreceptor cell damage, and fibrous scarring with subsequent visual impairment [3].
Although vascular endothelial growth factor (VEGF) is known as a potent proangiogenic factor [4], the exact pathogenesis of CNV is complex and has not been fully elucidated. Accumulating evidence has demonstrated inflammation to play an important role in the pathogenesis of CNV [5, 6]. Guo et al. [6] reported that serum inflammatory cytokines are significantly elevated and involved in idiopathic CNV patients. Nagineni et al. [7] reported that interferon-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-1 could enhance the secretion of VEGF, given that inflammatory cytokines are associated with CNV formation. After treatment, the levels of inflammatory and angiogenic cytokines decreased [8]. Therefore, knowledge of inflammatory cytokines and their response to treatment is necessary to better understand the pathophysiology of CNV.
The laser-induced CNV animal model can simulate CNV growth in vivo, and it is an important model for CNV research. Thus, in this study, we investigated the inflammatory cytokine profiling and macrophage polarization gene in a CNV mouse model and examined how they changed after an anti-VEGF treatment.
Methods
CNV Model and Animal Treatment
C57BL/6J male mice (6–8 weeks old) were chosen for this research. The operation was performed as in previous reports [9]. In general, laser photocoagulation was performed on 4 laser spots (Visulas 532S; Carl Zeiss Meditec, Dublin, Ireland; power of 120 mW, spot size of 50 µm, and duration of 100 ms) around the optic nerve. Only the laser spots in which a bubble was produced without hemorrhage, indicating perforated Bruch's membranes, were considered effective and included in the study. One microliter of 40 mg/mL aflibercept (Bayer Pharma AG, Berlin, Germany) or 1 μL of PBS solution was intravitreally injected at day 1 after laser photocoagulation. The eyes were enucleated, and RPE-choroid-sclera complexes were extracted on days 3, 7, and 14 after laser treatment. The method is summarized as follows: the mouse eyeball was removed under a surgical microscope. The cornea, lens, and vitreous were removed. The nerve layer of the retina was gently scraped off. RPE-choroid-sclera complexes were retained for detection. Age- and sex-matched mice that did not undergo laser photocoagulation treatment were used as controls.
Cytokine Antibody Array
Fifty-eight selected cytokines were measured using a multiplex quantitative cytokines array (Ray Biotech, Inc., Norcross, GA, USA). The cytokine array was performed according to the manufacturer's guidelines. Samples were detected through use of a GenePix scanner (Axon Instruments, Inc., Foster City, CA, USA) and further analyzed with a GenePix Pro 6.0 software (Axon Instruments, Inc.).
Real-Time PCR
The total RNA in RPE-choroid-sclera complexes was extracted using a TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA then underwent reverse transcription using the Prime Script RT Master Mix kit (TaKaRa, Shiga, Japan) and quantitative PCR with the SYBR Green Master Mix (ABI; Applied Biosystems, Foster, CA, USA) on an ABI PRISM 7900HT Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA). Each sample was amplified in triplicate. The data were analyzed using the SDS software. The sequences of the primers for real-time PCR analysis are as follows: iNOS, forward-TGGTCCGCTAGAGAGTGCT, reverse-CCTCATTGGCCAGCTGCTT; TNF-a, forward-CGTAGGCGATTACAGTCACGG, reverse-GACCAGGCTGTCGCTACATCA; Arg-1, forward-GCAGAGGTCCAGAAGAATGG, reverse-AGCATCCACCCAAATGACAC; Ym-1, forward-TCACAGGTCTGGCAATTCTTCTG, reverse-TGCATTCCAGCAAAGGCATA; VEGFA, forward-AGCAGAAGTCCCATGAAGTGA, reverse-ATGTCCACCAGGGTCTCAAT; CD163, forward-TGGACTGTGGCGTGGCAATTAAC, reverse-GCCTGGATGGTGTCTTGTCTGAAG; CD86, forward-ATTTCGCCTGACCAACACCACTG, reverse-GTCCGCCTCTCCACAACACAAG; CD20 6, forward-CTTGCTCTGCTGTCCTGCTTCTC, reverse-GTCCACGGTCAGTGTCCAATGTC; and GAPDH, forward-CACCATCTTCCAGGAGCGAG, reverse-GGGGCCATCCACAGTCTTC.
Choroidal Flatmounts
On days 3, 7, and 14 after laser photocoagulation, RPE-choroid-sclera complexes were surgically isolated, fixed with 4% paraformaldehyde for 1 h, and then incubated with a blocking buffer containing PBS, 0.5% Triton X-100, and 5% bovine serum albumin for 4 h at 4°C. The complexes were then incubated with Alexa Fluor®-594 conjugated isolectin (GS-IB4; Invitrogen, Carlsbad, CA, USA). The CNV lesions were imaged with a confocal microscope (FV 1000; Olympus), and fluorescent signals were quantified (Image-Pro Plus; Media Cybernetics, Inc., Rockville, MD, USA) [9].
H&E Staining
To perform H&E staining examinations, mice were killed and perfused with 0.9% saline followed by cold 10% paraformaldehyde. Eyes were enucleated and postfixed. Alternate sets of serial vertical sections of the eye were cut and mounted. Prepared sections were processed for standard hematoxylin and eosin staining. Serial slices were examined, and specimens representing the thickest and/or widest lesions within the examined specimens were evaluated for each CNV under a light microscope (FV 1000; Olympus, Tokyo, Japan). CNV thickness was measured vertically from the adjacent RPE layer to the top of the CNV, and CNV length was measured as horizontal maximize distance of CNV using IPP 6.0 software.
Results
Inflammatory Cytokine Expression in a CNV Mouse Model
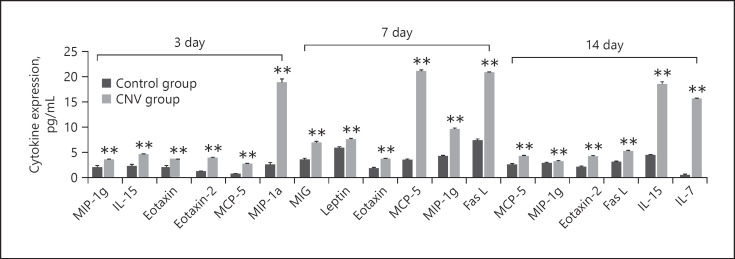
We measured the level of 58 inflammation cytokines in an RPE-choroid-sclera complex on days 3, 7, and 14 in a CNV mouse model and compared them with the normal control group (see online suppl. Table 1; see www.karger.com/doi/10.1159/000513588 for all online suppl. material). Figure 1 shows significantly upregulated cytokines on days 3, 7, and 14 (p < 0.001, fold change >2.0). On day 3, the most significantly upregulated cytokine was macrophage inflammatory protein (MIP)-1a (p < 0.001, fold change >10.0). On day 7, the 2 most significantly upregulated cytokines were monocyte-chemoattractant protein-5 (MCP-5) and Fas ligand (Fas-L) (p < 0.001, fold change >10.0). On day 14, the 2 most significantly upregulated cytokines were IL-15 and IL-7 (p < 0.001, fold change >10.0).
Fig. 1.
Differentially expressed cytokines between control samples and CNV groups on days 3, 7, and 14 measured using a multiplex quantitative cytokines array (p < 0.001, fold change >2.0). MIP-1a was the most significantly upregulated cytokine on day 3 after laser induction (p < 0.001, fold change >10.0). MCP-5 and Fas-L were the 2 most significantly upregulated cytokines on day 7 after laser induction (p < 0.001, fold change >10.0). IL-15 and IL-7 were the 2 most significantly upregulated cytokines on day 14 after laser induction (p < 0.001, fold change >10.0). Data are presented as mean + SEM, n = 3 for each time point. **p < 0.001 versus control. CNV, choroidal neovascularization; MIP, macrophage inflammatory protein; MCP, monocyte-chemoattractant protein; Fas-L, Fas ligand; IL, interleukin.
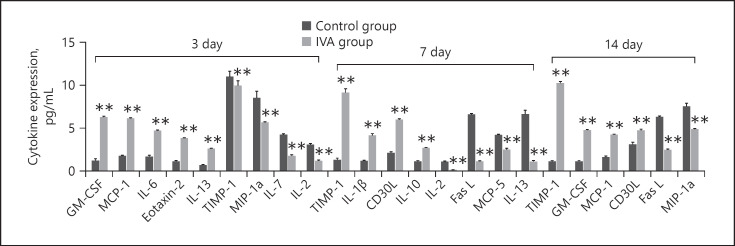
Effect of Aflibercept Treatment on Inflammatory Cytokines
To understand the effect of anti-VEGF treatment on inflammatory factors, aflibercept (IVA group) or the same volume of saline (control group) was intravitreally injected on day 1 after laser photocoagulation. We compared the cytokine levels between the aflibercept treatment group and the control group. Figure 2 shows the significantly changed cytokines on days 3, 7, and 14 (p < 0.001, fold change >2.0). On day 3, the most significantly upregulated cytokines were granulocyte-macrophage colony-stimulating factor (GM-CSF) and MCP-1, and the most significantly downregulated cytokine was MIP-1a (p < 0.001, fold change >5.0). On day 7, the most significantly upregulated cytokine was the tissue inhibitor of metalloproteinases-1 (TIMP-1), and the most significantly downregulated cytokines were IL-13 and Fas-L (p < 0.001, fold change >5.0). On day 14, the top most significantly upregulated cytokine was TIMP-1, and the most significantly downregulated cytokine was Fas-L (p < 0.001, fold change >5.0).
Fig. 2.
Differentially expressed cytokines between CNV groups and aflibercept treatment groups on days 3, 7, and 14 measured using a multiplex quantitative cytokines array (p < 0.001, fold change >2.0). GM-CSF and MCP-1 were the most significantly upregulated cytokines on day 3 after laser induction (p < 0.001, fold change >5.0). MIP-1a was the most significantly downregulated cytokine on day 3 after laser induction (p < 0.001, fold change >5.0). TIMP-1 was the most significantly upregulated cytokine on day 7 after laser induction (p < 0.001, fold change >5.0). IL-13 and Fas-L were the most significantly downregulated cytokines on day 7 after laser induction (p < 0.001, fold change >5.0). TIMP-1 was the most significantly upregulated cytokine on day 14 after laser induction (p < 0.001, fold change >5.0). Fas-L was the most significantly downregulated cytokine on day 14 after laser induction (p < 0.001, fold change >5.0). Data are presented as mean + SEM, n = 3 for each time point. **p < 0.001 versus control. CNV, choroidal neovascularization; GM-CSF, granulocyte-macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; MCP, monocyte-chemoattractant protein; Fas-L, Fas ligand; IL, interleukin; TIMP, tissue inhibitor of metalloproteinase.
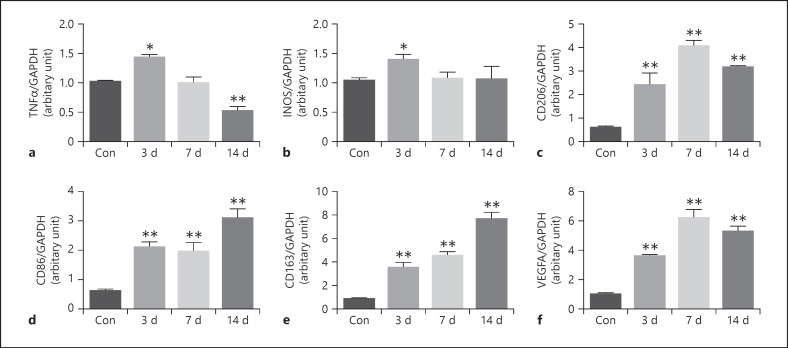
Expression of Macrophage Polarization Markers in the CNV Mouse Model
Our previous study discovered that macrophage polarization occurs during CNV formation. In this study, the mRNA expressions of M1-related markers (iNOS and TNF-α), M2a-related markers (CD206), M2b-related markers (CD86), and M2c-related markers (CD163) were measured before and on days 3, 7, and 14 after the laser induction of the CNV mouse. Figure 3 shows that the mRNAs of TNF-α and iNOS were upregulated on day 3 compared with the control (p < 0.05). TNF-α decreased on days 7 and 14, and iNOS returned to baseline levels on days 7 and 14. CD206 increased on day 3, significantly peaked on day 7, and was sustained on day 14 (p < 0.001). CD86 and CD163 increased on day 3, plateaued on day 7, and peaked on day 14 (p < 0.001). VEGFA increased on day 3, significantly peaked on day 7, and was sustained on day 14 (p < 0.001).
Fig. 3.
RT-PCR analysis of gene expression of TNF-α, iNOS, CD206, CD86, CD163, and VEGFA in the normal RPE-choroid-sclera complex and that of laser-induced CNV mice model at different time points. The level of TNF-α increased on day 3 after laser photocoagulation, while significantly decreased on day 14 after laser photocoagulation (a). The level of iNOS increased on day 3 after laser photocoagulation (b). The level of CD206 increased on days 3, 7, and 14 after laser photocoagulation and peaked on day 7 (c). The level of CD86 (d) and CD163 (e) increased on days 3, 7, and 14 after laser photocoagulation and peaked on day 14. The level of VEGFA increased on days 3, 7, and 14 after laser photocoagulation and peaked on day 7 (f). Data are presented as mean ± SEM, n = 6 for each time point. *p < 0.05, **p < 0.001.
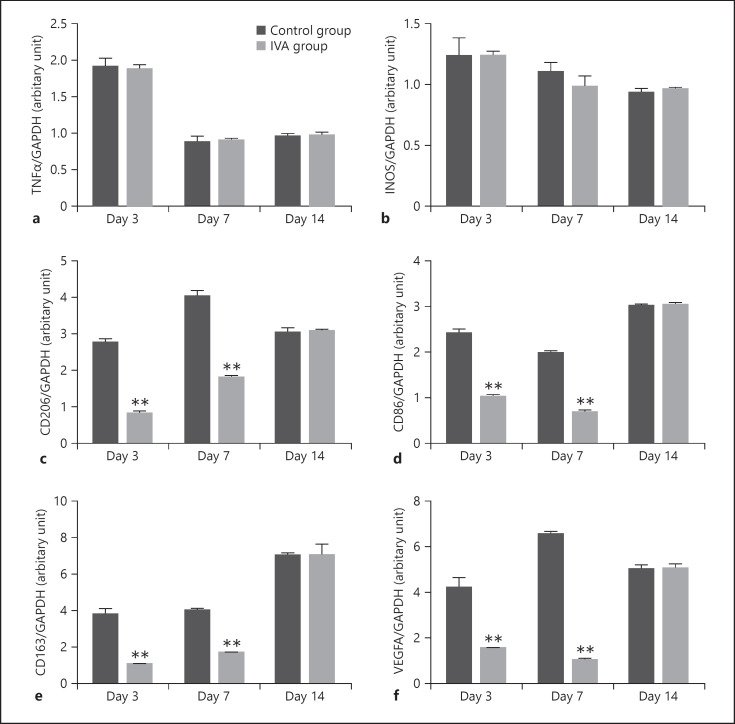
To understand the effect of anti-VEGF treatment on macrophage polarization, aflibercept or the same volume of saline was intravitreally injected on day 1 after laser photocoagulation. Figure 4 shows that the iNOS and TNF-α genes did not change significantly before and after aflibercept treatment on days 3, 7, and 14. CD206, CD86, CD163, and VEGFA decreased on days 3 and 7 (p < 0.001).
Fig. 4.
RT-PCR analysis of gene expression of TNF-α, iNOS, CD206, CD86, CD163, and VEGFA in the RPE-choroid-sclera complex of laser-induced CNV mice groups and aflibercept treatment groups at different time points. The level of TNF-α (a) and iNOS (b) was not significantly different between the 2 groups. The level of CD206 (c), CD86 (d), CD163 (e), and VEGFA (f) significantly decreased on days 3 and 7 in the aflibercept treatment group. Data are presented as mean ± SEM, n = 6 for each time point. *p < 0.05, **p < 0.001.
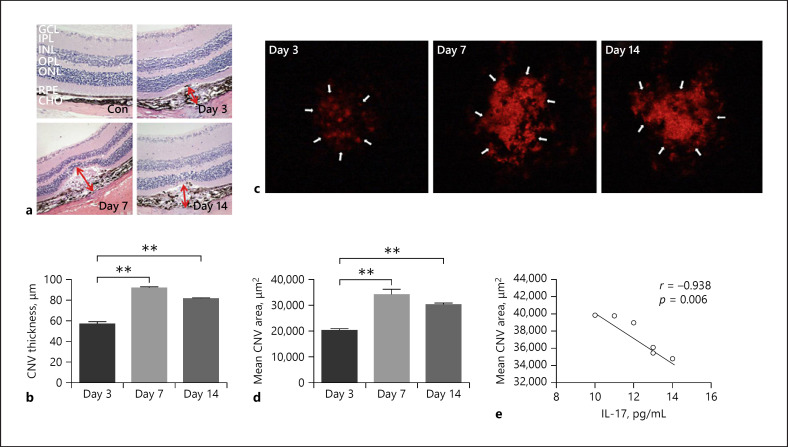
Correlation between Cytokines and CNV Area
To observe CNV, we used the choroidal flatmount method to observe the lesion area on days 3, 7, and 14 after laser induction. As shown in Figure 5, the CNV lesion area was the largest on day 7 and lasted until day 14. In this study, we chose cytokines (p < 0.001, fold change >2.0) on days 3, 7, and 14 and performed the correlation analysis with the CNV lesion area on the corresponding days. The results showed that IL-7 was negatively related to the CNV area on day 14 (r = −0.938, p = 0.006).
Fig. 5.
The hematoxylin-eosin staining results (a, ×200) showed normal retina structure and CNV on days 3, 7, and 14 after laser induction. CNV thickness was measured vertically from the adjacent RPE layer to the top of the CNV, and CNV length was measured as horizontal maximum distance of CNV (red arrows). b CNV thickness significantly increased on days 7 and 14 compared to day 3 after laser induction. c The CNV area measured by choroidal flatmounts. d CNV area significantly increased on days 7 and 14 compared to day 3 after laser induction. The area is the largest on day 7. e IL-7 was negatively related to the CNV area on day 14 (r = −0.938, p = 0.006). Data are presented as mean + SEM, n = 6 for each time point. *p < 0.05, **p < 0.001. GCL, ganglion cells layer; INL, inner nuclear layer; ONL, optic nerve layer; RPE, retina pigment epithelium; CHO, choroid; CNV, choroidal neovascularization.
Discussion
This study is the first to demonstrate the inflammatory cytokine profiles in experimental CNV. We found that the inflammatory cytokines and the M1/M2 macrophage genes significantly changed in the CNV mouse before and after anti-VEGF treatment. The results suggested that inflammatory cytokines and macrophages could play a critical role in the physiopathology of CNV.
In this study, we used aflibercept as an anti-VEGF agent. Aflibercept is composed of the human VEGF receptors; however, it has been considered to react in mouse too. For example, Torimura et al. [10] showed that aflibercept can act on VEGFR-1 and −2 in mice. Amin et al. [11] and Sergeys et al. [12] reported that after intravitreal injection of aflibercept in mice, the leakage of CNV and inflammation were significantly improved [13]. Although the laser-induced CNV mouse model cannot completely simulate the pathophysiological process of CNV in humans, it is a model of acute wound-healing tempo for laser-induced damage. However, as the model is stable and highly accessible, it is widely used to unravel the mechanisms of CNV and to demonstrate the utility of anti-VEGF therapies [14]. In this study, MIP-1a was significantly upregulated on day 3 after laser photocoagulation, MCP-5 and Fas-L were significantly upregulated on day 7, and IL-15 and IL-7 were significantly upregulated on day 14.
MIP-1a is a cytokine belonging to the subgroup of CCL chemokines [15]. It plays a significant role in the chemotactic activity of monocytes and mononuclear phagocytes. Yang et al. [16] found that MIP-1a expression increased in the corneal neovascularization tissue of mice, suggesting that MIP-1a is related to inflammation and neovascularization. Our study showed that MIP-1a was significantly correlated with the CNV lesion area, suggesting that the migration of MIP-1a-mediated macrophages could induce an inflammatory response and affect CNV formation.
MCP-5 (also known as CCL12) is a member of the MCP family. MCP-5 binds exclusively to CC-chemokine receptor 2 [17], which is a potent chemoattractant for circulating monocytes to inflammation sites, where they become cytokine-secreting macrophages. Previous studies have confirmed that MCP-5 is related to macrophage-related inflammation. For example, Das Sarma et al. [18] found that MCP-5 was related to IL-17A. In ARPE-19 cells, IL-17A triggered a pro-inflammatory state. The intervention of IL-17 could be a strategy for the treatment of CNV [19]. Inflammation is speculated to be involved in the formation of CNV, and MCP-5 is a key factor that promotes inflammation. However, more experiments are needed to verify these assumptions.
It is known that Fas-L increased in proangiogenic M2 macrophages [20]. In our previous study, M2 macrophages promoted CNV in a mouse model [9]. In the present study, the correlation analysis found that Fas-L was positively correlated with the CNV lesion area. We speculate that the elevated Fas-L may be derived from M2 macrophages, which are an important factor in promoting the formation of CNV. Fas-L may be a viable target for therapeutic intervention in CNV. However, more experiments are needed to verify this.
IL-15 can activate macrophages that enhance phagocytosis and local inflammation [21]. Yin and Shchuko [22] showed that IL-15 was elevated in the aqueous humor of CNV patients, suggesting IL-15-related inflammatory processes in CNV formation [23]. In consideration of this study, we speculate that IL-15 may promote inflammation and CNV through macrophage activity.
IL-7 is produced by macrophages and dendritic cells, which play an important role in maintaining the stability of the immune system [24, 25]. When the body is damaged, such as in the oxidative damage of age-related macular degeneration, the expression level of IL-7 is affected [26]. In this study, IL-7 formed a negative correlation with CNV on day 14. The increase in IL-7 is speculated to be related to the fiber repair response and not to the CNV form. Thus, IL-7 is expected to be a viable target for anti-CNV.
Our previous research found that macrophages migrated and occurred in the M1/M2 transformation during experimental CNV. Thus, in the present study, we wanted to understand the macrophage subtype transformation at different time points of CNV formation. The results showed that the M1 marker genes (TNF-α and iNOS) were upregulated on day 3, indicating that the inflammatory response was dominant in the early stage of CNV. The M2a marker gene (CD206) reached its peak on day 7 of the CNV formation, and the M2b marker gene (CD86) and M2c marker gene (CD163) reached their peak on day 14 of CNV. The results suggested that different subtypes of macrophages could play different roles in the different stages of CNV formation. As the CNV area was the largest on day 7 of laser induction, fibrosis increased on day 14 [27]. Thus, we considered that M1a could be related to neovascularization and that M2b and M2c could be associated with fibrosis.
To understand the effect of anti-VEGF on CNV, intravitreal injection of aflibercept was implemented. Our data showed that MIP-1a, which was the most significantly upregulated cytokine, was significantly downregulated on day 3. IL-13 and Fas-L were significantly downregulated, and Fas-L was the most significantly upregulated cytokine on day 7. Fas-L was significantly downregulated on day 14. The results indicated that aflibercept could effectively inhibit the inflammatory factors related to CNV formation.
After the intravitreal injection of aflibercept, GM-CSF and MCP-1 were upregulated significantly on day 3. GM-CSF drives the proliferation of many myeloid cell types, such as granulocytes, macrophages, and monocytes [28]. Previous reports have shown that GM-CSF is significantly elevated in the aqueous humor of CNV patients [6]. Immune/inflammation reaction occurs at this stage of CNV formation. MCP-1, a member of the MCP family, is highly expressed in activated macrophages [29], suggesting a correlation with macrophage-related inflammation. Therefore, MCP-1 may promote inflammation and CNV through macrophage activity.
On days 7 and 14 after the aflibercept injection, the most significantly upregulated cytokine was TIMP-1. TIMP-1 is mainly secreted by monocytes or endothelial cells [30]. TIMP-1 is produced by forming a reversible complex with MMP-9, achieving the purpose of inhibiting the activity of MMP-9 protease [31]. Previous experiments have confirmed that TIMP-1 can inhibit the growth of CNV [32]. Therefore, we speculated that after the aflibercept injection, monocytes or endothelial cells secrete TIMP-1 to inhibit the growth of CNV. Further experiments discovered that M2 macrophage significantly decreased after aflibercept treatment, suggesting that CNV can be suppressed by phenotypic transformation of macrophages.
In conclusion, this study showed the characteristic inflammatory cytokine profiles of the CNV mouse model. Moreover, intravitreal aflibercept treatment was found to change the expression of cytokines and macrophage polarization. However, the results of the CNV mouse model are different from those of clinical samples. Therefore, if possible, clinical samples of the corresponding factors should be obtained, and further investigations, including animal experiments and clinical trials, should be conducted to explore the mutual mechanisms of these cytokines.
Statement of Ethics
The study has been approved by the Animal Ethics Committee of Wannan Medical College (Approval No. 2019-028) and follows ARRIVE guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the National Science Foundation of Anhui Province, China (Grant No. 1808085MH253), National Natural Science Foundation of China (Grant No. 81700867), and the Talent Cultivation and International Academic Visiting Project for College Scholar of Anhui Province, China (Grant No. gxgwfx2019034).
Author Contributions
C.W., R.Z., Q.Z., and H.J. designed and conducted the study. H.J., C.W., and L.M. analyzed data. C.W. and L.M. wrote the manuscript. Y.L. and P.Z. revised the manuscript. All authors have read and approved the final manuscript.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request (zhangpengfei1023@126.com).
Supplementary Material
Supplementary data
Funding Statement
This work was supported by the National Science Foundation of Anhui Province, China (Grant No. 1808085MH253), National Natural Science Foundation of China (Grant No. 81700867), and the Talent Cultivation and International Academic Visiting Project for College Scholar of Anhui Province, China (Grant No. gxgwfx2019034).
References
- 1.Kulkarni AD, Kuppermann BD. Wet age-related macular degeneration. Adv Drug Deliv Rev. 2005;57((14)):1994–2009. doi: 10.1016/j.addr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.De Benedetto U, Battaglia Parodi M, Knutsson KA, Librando A, Bandello F, Lanzetta P, et al. Intravitreal bevacizumab for extrafoveal choroidal neovascularization after ocular trauma. J Ocul Pharmacol Ther. 2012;28((5)):550–552. doi: 10.1089/jop.2012.0022. [DOI] [PubMed] [Google Scholar]
- 3.Shao J, Choudhary MM, Schachat AP. Neovascular age-related macular degeneration. Dev Ophthalmol. 2016;55:125–136. doi: 10.1159/000438969. [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirasawa M, Takubo K, Osada H, Miyake S, Toda E, Endo M, et al. Angiopoietin-like protein 2 is a multistep regulator of inflammatory neovascularization in a murine model of age-related macular degeneration. J Biol Chem. 2016;291((14)):7373–7385. doi: 10.1074/jbc.M115.710186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo S, Yin H, Zheng M, Tang Y, Lu B, Chen X, et al. Cytokine profiling reveals increased serum inflammatory cytokines in idiopathic choroidal neovascularization. BMC Ophthalmol. 2019;19((1)):94. doi: 10.1186/s12886-019-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagineni CN, Kommineni VK, Ganjbaksh N, Nagineni KK, Hooks JJ, Detrick B. Inflammatory cytokines induce expression of chemokines by human retinal cells: role in chemokine receptor mediated age-related macular degeneration. Aging Dis. 2015;6((6)):444–455. doi: 10.14336/AD.2015.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezar-Dreindl S, Sacu S, Eibenberger K, Pollreisz A, Bühl W, Georgopoulos M, et al. The intraocular cytokine profile and therapeutic response in persistent neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016;57((10)):4144–4150. doi: 10.1167/iovs.16-19772. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Wang H, Luo X, Liu H, Lu B, Li T, et al. MicroRNA-155 inhibits polarization of macrophages to M2-type and suppresses choroidal neovascularization. Inflammation. 2018;41((1)):143–153. doi: 10.1007/s10753-017-0672-8. [DOI] [PubMed] [Google Scholar]
- 10.Torimura T, Iwamoto H, Nakamura T, Abe M, Ikezono Y, Wada F, et al. Antiangiogenic and antitumor activities of aflibercept, a soluble VEGF receptor-1 and −2, in a mouse model of hepatocellular carcinoma. Neoplasia. 2016 Jul;18((7)):413–424. doi: 10.1016/j.neo.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin SM, Gonzalez A, Guevara J, Bolch C, Andersen L, Smith WC, et al. Efficacy of aflibercept treatment and its effect on the retinal perfusion in the oxygen-induced retinopathy mouse model of retinopathy of prematurity. Ophthalmic Res. 2021;64((1)):91–98. doi: 10.1159/000509380. [DOI] [PubMed] [Google Scholar]
- 12.Sergeys J, Etienne I, Van Hove I, Lefevere E, Stalmans I, Feyen JHM, et al. Longitudinal in vivo characterization of the streptozotocin-induced diabetic mouse model: focus on early inner retinal responses. Invest Ophthalmol Vis Sci. 2019 Feb 1;60((2)):807–822. doi: 10.1167/iovs.18-25372. [DOI] [PubMed] [Google Scholar]
- 13.Qiu B, Tan A, Veluchamy AB, Li Y, Murray H, Cheng W, et al. Apratoxin S4 inspired by a marine natural product, a new treatment option for ocular angiogenic diseases. Invest Ophthalmol Vis Sci. 2019 Jul 1;60((8)):3254–3263. doi: 10.1167/iovs.19-26936. [DOI] [PubMed] [Google Scholar]
- 14.Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, et al. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 2013;8((11)):2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad R, Akhter N, Al-Roub A, Kochumon S, Wilson A, Thomas R, et al. MIP-1α induction by palmitate in the human monocytic cells implicates TLR4 signaling mechanism. Cell Physiol Biochem. 2019;52((2)):212–224. doi: 10.33594/000000015. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Zhang Y, Liu X, Wang N, Song Z, Wu K. A comparison of the effects of benzalkonium chloride on ocular surfaces between C57BL/6 and BALB/c mice. Int J Mol Sci. 2017;18((3)):509. doi: 10.3390/ijms18030509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117((4)):902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J, et al. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2009;6:14. doi: 10.1186/1742-2094-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coughlin B, Schnabolk G, Joseph K, Raikwar H, Kunchithapautham K, Johnson K, et al. Connecting the innate and adaptive immune responses in mouse choroidal neovascularization via the anaphylatoxin C5a and γδT-cells. Sci Rep. 2016;6:23794. doi: 10.1038/srep23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Roychoudhury J, Doggett TA, Apte RS, Ferguson TA. Age-dependent changes in FasL (CD95L) modulate macrophage function in a model of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54((8)):5321–5331. doi: 10.1167/iovs.13-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim EW, Teles RMB, Haile S, Liu PT, Modlin RL. Vitamin D status contributes to the antimicrobial activity of macrophages against Mycobacterium leprae. PLoS Negl Trop Dis. 2018;12((7)):e0006608. doi: 10.1371/journal.pntd.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin H, Fang X, Ma J, Chen M, Yang Y, Guo S, et al. Idiopathic choroidal neovascularization: intraocular inflammatory cytokines and the effect of intravitreal ranibizumab treatment. Sci Rep. 2016;6:31880. doi: 10.1038/srep31880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shchuko AG, Zaitseva NV, Yurieva TN, Chernykh ER, Mikhalevich IM, Shevela EY, et al. Intraocular cytokines and their correlations with clinical parameters in patients with myopic choroidal neovascularization. Ophthalmologica. 2017;237((2)):96–104. doi: 10.1159/000455271. [DOI] [PubMed] [Google Scholar]
- 24.Kubin N, Richter M, Sen-Hild B, Akintürk H, Schönburg M, Kubin T, et al. Macrophages represent the major pool of IL-7Rα expressing cells in patients with myocarditis. Cytokine. 2020;130:155053. doi: 10.1016/j.cyto.2020.155053. [DOI] [PubMed] [Google Scholar]
- 25.Kim SJ, Chang HJ, Volin MV, Umar S, Van Raemdonck K, Chevalier A, et al. Macrophages are the primary effector cells in IL-7-induced arthritis. Cell Mol Immunol. 2020 Jul;17((7)):728–740. doi: 10.1038/s41423-019-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-Guilloty F, Saeed AM, Duffort S, Cano M, Ebrahimi KB, Ballmick A, et al. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS One. 2014;9((2)):e88201. doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa K, Kannan R, Hinton DR. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp Eye Res. 2016;142:19–25. doi: 10.1016/j.exer.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trus E, Basta S, Gee K. Who's in charge here? Macrophage colony stimulating factor and granulocyte macrophage colony stimulating factor: competing factors in macrophage polarization. Cytokine. 2020;127:154939. doi: 10.1016/j.cyto.2019.154939. [DOI] [PubMed] [Google Scholar]
- 29.Kotwal GJ, Chien S. Macrophage differentiation in normal and accelerated wound healing. Results Probl Cell Differ. 2017;62:353–364. doi: 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grünwald B, Schoeps B, Krüger A. Recognizing the molecular multifunctionality and interactome of TIMP-1. Trends Cell Biol. 2019;29((1)):6–19. doi: 10.1016/j.tcb.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Lu Y, Zhao Z, Wang J, Li J, Wang W, et al. Relationships of MMP-9 and TIMP-1 proteins with chronic obstructive pulmonary disease risk: a systematic review and meta-analysis. J Res Med Sci. 2016;21:12. doi: 10.4103/1735-1995.178737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert V, Wielockx B, Munaut C, Galopin C, Jost M, Itoh T, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17((15)):2290–2. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request (zhangpengfei1023@126.com).