Abstract
Introduction
Falls have major implications for quality of life, independence, and cost of health services. Strength and balance training has been found to be effective in reducing the rate/risk of falls, as long as there is adequate fidelity to the evidence-based programme. The aims of this study were to (1) assess the feasibility of using the “Motivate Me” and “My Activity Programme” interventions to support falls rehabilitation when delivered in practice and (2) assess study design and trial procedures for the evaluation of the intervention.
Methods
A two-arm pragmatic feasibility randomized controlled trial was conducted with five health service providers in the UK. Patients aged 50+ years eligible for a falls rehabilitation exercise programme from community services were recruited and received either (1) standard service with a smartphone for outcome measurement only or (2) standard service plus the “Motivate Me” and “My Activity Programme” apps. The primary outcome was feasibility of the intervention, study design, and procedures (including recruitment rate, adherence, and dropout). Outcome measures include balance, function, falls, strength, fear of falling, health-related quality of life, resource use, and adherence, measured at baseline, three-month, and six-month post-randomization. Blinded assessors collected the outcome measures.
Results
Twenty four patients were randomized to control group and 26 to intervention group, with a mean age of 77.6 (range 62–92) years. We recruited 37.5% of eligible participants across the five clinical sites. 77% in the intervention group completed their full exercise programme (including the use of the app). Response rates for outcome measures at 6 months were 77–80% across outcome measures, but this was affected by the COVID-19 pandemic. There was a mean 2.6 ± 1.9 point difference between groups in change in Berg balance score from baseline to 3 months and mean 4.4 ± 2.7 point difference from baseline to 6 months in favour of the intervention group. Less falls (1.8 ± 2.8 vs. 9.1 ± 32.6) and less injurious falls (0.1 ± 0.5 vs. 0.4 ± 0.6) in the intervention group and higher adherence scores at three (17.7 ± 6.8 vs. 13.1 ± 6.5) and 6 months (15.2 ± 7.8 vs. 14.9 ± 6.1). There were no related adverse events. Health professionals and patients had few technical issues with the apps.
Conclusions
The motivational apps and trial procedures were feasible for health professionals and patients. There are positive indications from outcome measures in the feasibility trial, and key criteria for progression to full trial were met.
Keywords: Rehabilitation, Older, Technology
Introduction
Falls are an important issue for older adults, with implications for independent living and quality of life and with a high cost to health services [1]. Rehabilitation programmes focused on strength and balance exercise are effective in reducing risk and rate of falls if they are progressive, tailored, and carried out regularly by participants [2]. Although we know evidence-based exercise is effective, few interventions are tested as part of routine clinical practice (participants tend to be a healthier population), and there are even fewer evaluations of their effectiveness when implemented in practice rather than clinical trials [3, 4]. We know that adherence to evidence-based strength and balance programmes in clinical settings is poor [5, 6] and older adults do not carry out their exercise programme three times a week as prescribed (dose) to achieve and maintain the benefits [6]. Smartphones could be a way to support falls rehabilitation and ensure adequate exercise dose (adherence) of exercise, by allowing for regular communication and support with the health professional. The proportion of older adults using smartphones is growing rapidly, with 39% of those aged 65–74 and 15% of those aged over 75 using smartphones [7], and 75% of those over 75 now using a mobile phone [8]. Smartphones are portable and can provide support and feedback at any time, improving motivation and compliance; they also do not tend to be switched off in the same way that other portable devices are [9, 10, 11]. The evidence which looks at the role of the smartphone in fall prevention is sparse [12], particularly for interventions focused on rehabilitation. There is evidence that older adults find smartphones more usable than other devices [13, 14] and that they can support healthy ageing [15, 16, 17, 18]. Personalized goal-setting and behavioural feedback have been found to be successful behaviour change techniques with older adults [10, 19, 20].
The Theory of Planned Behaviour [21] is particularly useful for assessing older adults' attitudes in relation to exercise uptake and adherence [22, 23, 24] and has informed the intervention adopted in this feasibility trial [20, 25]. Smartphone technology-based motivational applications underpinned by behaviour change theory and developed with health professionals and older adults could be an effective way of encouraging maintenance of exercise and of successfully supporting adherence to falls rehabilitation exercises. We have carried out usability and acceptability testing of two motivational apps (one for health professionals and one for patients) with positive results before planning this trial [20, 25]. In the current paper, we report a feasibility RCT designed to establish whether it is feasible for smartphone technology to be used to support patients to carry out an evidence-based exercise rehabilitation programme. “Motivate Me” (health professional app) and “My Activity Programme” (patient app) support the setting of short- and long-term goals for rehabilitation exercise, providing personalized feedback, self-reporting of exercise, and monitoring. We assess feasibility of the study design and trial procedures by examining the number of eligible participants approached, number who consent and then willingness to be randomized, success of randomization, follow-up rates, adherence/compliance rates, time needed to collect data, adverse events, standard deviations and effect sizes of outcome measures.
For this paper, we will focus on feasibility of the trial procedures and the intervention. For the secondary aim, which assesses whether technology-based outcome measures (smartphone-based falls alarm, Timed Up and Go (TUG) Test [26] and digital self-report) are reliable when compared to standard methods (e.g., falls calendars), we will report the results elsewhere. In this paper, we focus on reporting gold standard, traditional outcome measures. The qualitative study results collected within the trial further exploring feasibility will also be reported elsewhere.
Materials and Methods
Full details of the trial can be found in the trial protocol [25]; we briefly recap the study here.
Trial Design
This study was a two-arm pragmatic feasibility randomized controlled trial including the collection of economic data (trial registration number: ISRCTN12830220). The trial design framework was exploratory.
Participants
Participants were identified through five community falls rehabilitation services across diverse socio-economic areas in Greater Manchester and South Yorkshire (both urban and suburban) between September 2018 and December 2019. Three services were specific falls services, one was an outpatient rehabilitation unit in the community, and one was delivered as part of a broader community rehabilitation team. Three of the services offered a combination of group and home visits, with two services offering only home-based visits. Thirty patients per arm after attrition (approximately 10%) are normally used for feasibility RCTs [27], and this was our original target for recruitment. The number of sites was increased from two to five during the course of recruiting due to governance delays and slower than expected recruitment (overestimate of proportion of those attending falls services eligible for rehabilitation). Sites joined the study at different points with different length of recruitment time (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000528471).
Eligibility Criteria
Older adults at risk of falls (aged 50+ years) and assessed as requiring a falls rehabilitation exercise programme were eligible to take part. Exclusions included older adults who were unable to follow instructions (unless supported by a family member/carer), unable to understand written English (unless supported by a family member/carer), with severe visual impairment, those in long-term residential or nursing care, and those with terminal illness or expected shortened lifespan, defined as less than 6 months, as determined by the recruiting sites. Participants needed to have good 3G/4G mobile phone reception (able to access webpages) or Wi-Fi in their home assessed by their health professional or by the researcher when taking consent.
Identification and Recruitment
Health professionals gave patients the study information sheet and informed them about the intervention on first referral to their service. The health professionals then asked the patient if they were happy to be contacted by the researcher, who demonstrated the technology at the patient's home. Where possible, a former patient who had used the smartphone applications before accompanied the researcher to demonstrate the technology to assist in promoting patient confidence in the use of the technology. Patients consented when the researcher visited and were then randomized into either intervention or control group; the researcher informed participants of their allocation. They then started their rehabilitation programme.
Assessments and Randomization
Baseline and follow-up (3 and 6 months) assessments were carried out by experienced clinicians within each National Health Service Trust (external to the clinical teams participating). They were blinded to which intervention the participants received. As it was an active intervention, it was not possible to blind the health professionals delivering the service or the participants during the intervention. Randomization occurred after the baseline assessment. Study participants were randomized using a computer-generated randomization algorithm at sealedenvelope.com (unknown to the research team), stratified by gender and site, using block randomization (2, 4, 6 blocks) into either intervention or control group. The lead researcher provided technical support to both health professionals and patients and was not blinded.
The Intervention
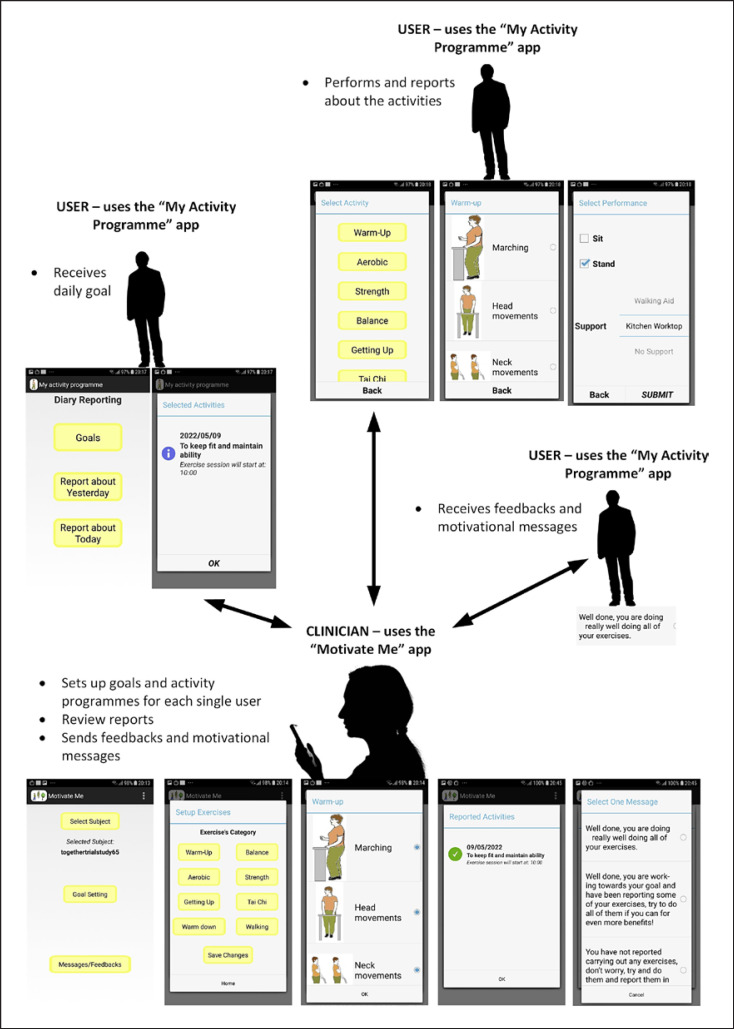
Samsung Galaxy J5 smartphones were provided to all participants (control and intervention) and health professionals. Both the intervention and control groups received standard service. This was variable across the different sites, but all sites delivered a mix of the evidence-based Falls Management Exercise Programme (FaME) [28] and Otago [29] exercises as standard care. They include face-to-face delivery and a home exercise programme (with booklet) (online suppl. Table 1). All sites left participants with a home exercise plan on discharge and, where appropriate, referred on to community-based strength and balance programmes. Those in the intervention group also received support through the “Motivate Me” and “My Activity Programme” apps which included the FaME [28] and Otago [29] exercises (warm up, dynamic endurance, balance, strength, getting up from the floor, warm down, Tai Chi, and walking; floor exercises were not offered as they are not normally used as part of rehabilitation by these services). 12-behaviour change techniques were adopted through the intervention [30] and included goal-setting (behaviour/outcome), action planning (recording plan to exercise in diary on smartphone/reminder text messages when it is time to start the programme), and feedback on behaviour (providing feedback on what they have done/benefits). The main components of the applications are illustrated within our development paper [20], trial protocol [25], and Figure 1 and outlined in a video [31].
Fig. 1.
Components of app.
Motivate Me and My Activity Programme Apps
The “Motivate Me” app is the health professional application. This app was used by the health professional on the first rehabilitation session with the patient to set outcome-based goals (what the patient wanted to achieve/improve through rehabilitation) and then behavioural goals (what exercises they were going to do achieve the outcome goal and when they were going to do them). These goals then transferred from “Motivate Me” to “My Activity Programme,” the patient app. The health professional could then see what exercises the patient reported at home, give them personalized feedback, and check whether they had received messages. They could also update patients' goals through “Motivate Me” (which then transferred to “My Activity Programme”) and were asked to send each patient a personalized feedback message once a week during active rehabilitation. “My Activity Programme” is the patients' application. This app was used by the patient to view their exercises set with the health professional on “Motivate Me,” report the exercises that they had done, and receive messages and prompts to exercise and to confirm whether they liked the messages received. The “Motivate Me” app and “My Activity Programme” app were used to enhance standard service for the intervention group. The health professional only assessed reported exercises, sent personalized messages and made changes to goals whilst the participants were receiving active rehabilitation (time period differed across sites). If the active rehabilitation was shorter than the 6-month intervention period at patient discharge from the service, participants received pre-set messages and accessed the programme set prior to discharge (including pre-set prompts and reminders) until the end of the 6 months (online suppl. Table 1), with no real-time updates or messages from the health professional.
The Control
The control arm also received a study phone with a basic app where they were asked to report their exercises (secondary aim of digital adherence measure and fall detection). Participants were not able to view their exercise programme on the smartphone, receive messages, or receive feedback on the phone but received their exercises in a paper booklet as per standard service. The health professional was not able to view the exercises patients reported through the phone (data collected by basic app is for the research team only).
Outcome Measures
Demographic data (age, gender, socio-economics, health conditions, falls history, previous smartphone/mobile phone use, and Wi-Fi) were recorded at baseline. Health conditions were categorized using the World Health Organization (WHO) International Classification of Diseases codes (ICD-11). We report feasibility and acceptability of design and procedures through:
a. number of eligible patients and number of eligible patients who consented and were willing to be randomized, as well as the success of randomization
b. willingness of clinicians to recruit participants (through focus groups reported elsewhere)
c. whether demonstration by peer of the technology aids recruitment (number of first visits with a peer volunteer and whether they consented)
d. follow-up rates and adherence/compliance rates
e. time needed to collect and analyse data
f. number of adverse events
g. characteristics of the proposed outcome measures including the variability and suggestive effect size to be used for definitive large-scale randomized controlled trial (e.g., balance, falls, and adherence)
We monitored intervention fidelity through the use of a specially developed fidelity checklist with the health professional, observing first sessions and checking on the “Motivate Me” app that weekly messages had been sent to the patient by the health professional. Technical issues with the phones or apps during the trial were recorded in an issue log.
Falls
Falls are expressed as fall rate per person per months of follow-up observation after randomization. We used the internationally agreed Prevention of Falls Network Europe (ProFaNE) falls definition [32] and followed the agreed ProFaNE falls data collection and analysis protocols based on self-report monthly calendars [33].
Balance
The Berg Balance Scale (BERG) was used to assess balance and was seen as one of the primary indicators of potential for the intervention to work. BERG has good validity and sensitivity in this population [34] and is one of the best outcome measures for assessing standing balance [35]. It has also been used for the prediction of falls [36]. The BERG has a score from 0 to 56, with higher scores indicating better balance.
Function
The TUG test was used to assess improvements in mobility and function [26]. Participants were asked to perform the TUG at their self-selected habitual walking speed. A successful intervention would be expected to bring about a reduction in the number of seconds required to complete the task.
Strength
The 30-second chair stand test (30CST) [37], which has good validity and is used throughout health services, was used to assess physical ability, in particular strength. A successful intervention would be expected to bring about an increase in the number of chair rises.
Fear of Falling
The Short Falls Efficacy Scale-International (Short FES-1) was used to measure fear of falling [38]. This is often a measure used by UK falls services as part of standard outcome measures and lower scores indicate less fear of falling.
Adherence
We used the Exercise Adherence Rating Scale (EARS) [39]. This is a validated 16-question tool with a 6-question subscale specifically measuring adherence (remaining questions measure reasons for adherence/non-adherence). We focused on the 6-questions. Higher scores indicate better adherence.
Health Economics
Health-related quality of life was measured using the 5-level EQ-5D instrument (EQ-5D-5L) [40] and an additional instrument used in previous trials related to falls prevention, the ICEpop Capability Measure for Older People (ICECAP-O) [41, 42], at baseline, 3-month, and 6-month follow-up. Healthcare resource use was also captured at the same time points via a resource use questionnaire measuring costs from an NHS and social care perspective, including general practice-related services, hospital-related, community-related services, and medication, as well as a patient perspective (medical devices and travel). The unit cost of health and social care from the Personal Social Services Research Unit (PSSRU) 2019 [43] and other sources were used to estimate costs. The intervention cost was estimated based on receipt of intervention, including resources for staff training, staff time, delivery costs, and equipment costs (phones and sim cards were provided to participants). Costs and quality-adjusted life years (QALYs) over the 6-month trial period were calculated for all participants. Mean differences in costs incurred and QALYs accrued between groups were estimated using a seemingly unrelated regression model (14), adjusted for baseline costs and EQ-5D scores. The incremental cost-effectiveness ratio (ICER) and the incremental net health benefit (NHB) were calculated at the cost-effectiveness threshold range of GBP 20,000 to GBP 30,000 per QALY. Bootstrapping percentile method was used to identify the sampling uncertainty of cost-effectiveness.
Analysis
Quantitative data are analysed using SPSS Release 22.0 and Stata 17.0 for health economics analysis. The main analyses are descriptive, involving the estimation of recruitment rates, attrition rates, non-compliance rates, and app usage rates. Independent sample t-test on continuous variable and χ2 test on categorical variables together with p value were used to compare baseline characteristics between intervention and control group to test the feasibility of the randomization procedure. For outcome measures that would be used in the full trial, we report means and standard deviations, mode (Q1, Q3) of outcomes by group at baseline and end of the trial (supplementary material). For change over time for key outcomes from baseline to 3 months and to 6 months, we report mean (SD) and mode (Q1, Q3) for each group. For two group comparison of change over time, we report mean (SE) and 95% CI. The effect size are reported using Cohen's D, as these could be used to inform the future definitive trial, but no significance level or precision are attached to the effect size as this feasibility trial was not powered to do so. The health economics analysis is focussed on informing relevant measures and means of collection of health related quality of life and resource use for the future definitive study. Only an exploratory cost-effectiveness analysis is conducted; for all measures, we report mean values and sample variability alongside information on missing values. A statistical analysis plan was created before data analysis.
Results
52 participants were recruited to take part in the TOGETHER trial, with 50 participants randomized to either the intervention (N = 26) or control (N = 24) group. Table 1 presents sample characteristics. Our randomization worked as expected with very similar demographics across the two groups (Table 1). Health conditions within the table are categorised under the broad WHO ICD codes. There were only four patients (n = 2 intervention, n = 2 control) who did not have multi-morbidities. Within the nervous system ICD category, two participants had Parkinson's (n = 1 intervention, n = 1 control) and five patients had a diagnosis of Alzheimer's (n = 4 intervention, n = 1 control). All participants had stable medical conditions when they started falls rehabilitation and were undergoing no other rehabilitation for their conditions. Online suppl. Tables 1 and 2 provides more details on delivery characteristics. Twelve health professionals (seven physiotherapists, two occupational therapists, and three rehabilitation assistants) delivered the intervention.
Table 1.
Sample characteristics
| Patient characteristics | Intervention (N = 26) | Control (N = 24) | p value |
|---|---|---|---|
| Age, years | 77.0±8.5 | 78.2±7.4 | 0.61a |
| Female gender | 17 (65.4) | 17 (70.8) | 0.77 |
| Ethnicity | |||
| White | 19 (73.1) | 18 (75.0) | 0.88 |
| White Irish | 2 (7.7) | 1 (4.1) | |
| Black | 3 (11.5) | 3 (12.5) | |
| Asian | 1 (3.8) | 2 (8.3) | |
| Housing | |||
| Own home | 17 (65.4) | 18 (75) | 0.46 |
| Rent | 3 (11.5) | 0 (0) | |
| Housing association | 6 (23.1) | 6 (25) | |
| Education | |||
| Age left school | 15.5±0.26 | 15.9±0.28 | 0.38a |
| Further education | 17 (65.4) | 14 (58.3) | 0.61 |
| Occupation | |||
| Retired | 24 (92.3) | 21 (87.5) | 0.57 |
| Voluntary work | 2 (7.7) | 2 (8.3) | |
| Part time work | 0 (0) | 1 (4.2) | |
| Previous fall (in the last 12 months) | 23 (88.5) | 20 (83.3) | 0.60 |
| Health conditions | |||
| Respiratory disease | 9 (34.6) | 11 (45.8) | 0.56 |
| Endocrine disease | 10 (38.5) | 12 (50) | 0.57 |
| Musculoskeletal disease | 20 (76.9) | 16 (66.7) | 0.42 |
| Circulatory disease | 16 (61.5) | 16 (66.7) | 0.71 |
| Nervous system | 3 (11.5) | 2 (8.3) | 0.71 |
| Mental and behavioural | 6 (23.1) | 7 (29.2) | 0.62 |
| Digestive system | 3 (11.5) | 1 (4.2) | 0.34 |
| Urinary system | 8 (30.8) | 8 (33) | 0.85 |
| Visual system | 7 (26.9) | 5 (20.8) | 0.61 |
| Immune system | 2 (7.7) | 1 (4.2) | 0.60 |
| Mobile phone | 21 (80.8) | 21 (87.5) | 0.52 |
| Smartphone | 12 (46.0) | 11 (45.8) | 0.98 |
| Wi-Fi | 18 (69.2) | 20 (83.3) | 0.24 |
Values represent mean ± SD or n (%) p values from independent sample t test
or χ2 test to illustrate that randomization has worked successfully.
Eligible Patients and Willingness to Be Randomized
136 patients were identified as eligible to participate across the sites during the recruitment period, with 84 patients refusing to participate and 2 withdrawing before randomization (after consent). We recruited 37.5% of eligible participants across the 5 clinical sites. The two who withdrew before randomization following reflection felt that their lives were too challenging at that time to participate in a study (shown in Fig. 2).
Fig. 2.
Consort diagram.
Demonstration by Peer
Not all patients received a demonstration of the technology from a peer as the feasibility of attending with a peer was dependent on location of the patient, other visits scheduled on the same day and availability of the peer. Fourteen participants were asked whether it would be helpful for a peer to attend, with six participants happy for a peer to attend with the researcher to demonstrate the technology. All six participants found it useful for the peer to explain the apps to them and demonstrate how it worked, reporting that the peer explanations were less technical and the demonstration gave them confidence. The remaining participants stated that they would prefer the researcher to attend alone or felt they had sufficient knowledge of smartphones.
Follow-Up Rates and Compliance Rates
20 (77%) of participants in the intervention group completed their full exercise programme (including the use of the app). Twenty participants in both the intervention and control group (N = 40) received the full allocated intervention. One participant from each arm completely withdrew from their rehabilitation programme, and eight participants were involuntarily withdrawn from rehabilitation due to unstable medical conditions. Only one participant (control group) fully withdrew totally from the study and refused to take part in any further data collection. We assessed patients' engagement with the app by looking at how many times they had interacted with it (reported their exercises or liked messages). Patients in the intervention arm interacted with the app a mean of 52.9 ± 43.9 (range 1–152) times over 6 months. In the intervention group, 15 participants were fully compliant in reporting their exercises (reported on a weekly basis throughout the follow-up period), and five participants were mostly compliant in reporting their exercises (reported mostly on a weekly basis but occasionally interrupted by ill-health). Five participants were quite intermittent at reporting their exercise and this was related to reported lack of family support (language and cognition problems so needed additional support to comply or due to an episode of ill-health early on in the intervention period) and one person stopped using the app after 2 months. All other loss of data was caused by ill-health and at 6 months also due to COVID-19 shielding (shown in Fig. 2; Table 2). There were 18 episodes of acute illness and 13 hospital admissions during the study period.
Table 2.
Adverse events
| Type | Intervention (n = 26) | Control (n = 24) |
|---|---|---|
| Expected adverse effects | ||
| Clicky hip | 0 | 1 |
| Dizziness | 2 | 1 |
| Hypertension | 3 | 0 |
| Urinary tract infection | 2 | 6 |
| Delayed muscle soreness | 0 | 1 |
| Postural drop | 0 | 0 |
| Expected serious adverse effects | ||
| Hospitalization for observation or monitoring | 0 | 2 |
| Hospitalization for falls or fracture | 2 | 8 |
| Attendance at A&E for falls or fracture | 0 | 1 |
| Serious unexpected adverse effects (not relateda) | ||
| TIA | 1 | 0 |
| Heart attack (leading to hospitalization) | 1 | 0 |
Deemed by the clinical team as not related to the intervention.
Fidelity to the Intervention
All of the health professionals provided weekly messages to the patients throughout the trial period across all services. There was good fidelity to the delivery of the intervention by health professional, observed through the checklist.
Time Needed to Collect Data
It took approximately an hour and 15 min at baseline and then an hour at three and 6 months for both physical assessments and questionnaire to be completed; the assessments were not seen as a barrier to involvement by participants.
Technical Issues
There were actually very few technical issues with “My Activity Programme” and “Motivate Me” during the trial. Most of the issues related to connectivity at the health professionals' workplaces due to intermittent Wi-Fi. Patients' issues related to missing the messages sent by the health professional because of the touchscreen and the deletion of the app (online suppl. Table 3).
Number of Adverse Events
No incidents occurred whilst participants were actively taking part in their rehabilitation programme. There were two unexpected serious adverse events but neither of these were deemed as related to the intervention (Table 2).
Falls
Descriptive data show that the control group had a higher number of falls, higher falls rate per month (0.39 falls per month compared to 0.29), higher numbers of injurious falls, and a higher proportion of participants falling compared to the intervention group during the follow-up period (Table 3). There were also more multiple fallers within the control group.
Table 3.
Falls
| Intervention (n = 25) | Control (n = 23) | |
|---|---|---|
| Total falls | 1.76±2.8 | 9.09±32.6ab |
| 1 (0, 2) | 2 (0, 4) | |
| Falls rate per person month | 0.29a | 0.39b |
| Injurious falls leading to hospital attendance | 0.08±0.53 | 0.35±0.57 |
| Number of participants falling | 15 (60.0) | 15 (68.1) |
| Multiple fallers | 7 (28) | 12 (52) |
Values represent mean ± SD, median (Q1, Q3), n (%) or a falls rate.
Outlier has been removed from control group with n = 158 falls so n = 22.
Balance
The BERG score increased for both the intervention and control group between baseline and 3 months with the intervention group change achieving a clinically meaningful difference [44] of 4.9 points and a mean difference in change over time of 2.6 ± 1.9 (95% CI −1.3 to 6.5) between groups. The mean difference between groups increased further when looking at change between baseline and 6 months 4.4 ± 2.7 (95% CI −1.1 to 9.8) in favour again of the intervention group (Table 4 and online suppl. Table 4).
Table 4.
Key full trial outcome measures, change over time between group comparisons, and effect sizes
| Changes between baseline and 3 months within group difference mean ± SD; mode (Q1, Q3)a |
Between group difference, mean ± SE (95% CI) | Effect size (Cohen's D) | Changes between baseline and 6 months (within group differences), mean ± SD; mode (Q1, Q3)a |
Between group difference, mean ± SE (95% CI) | Effect size (Cohen's D) | |||
|---|---|---|---|---|---|---|---|---|
| intervention (N = 23) |
control (N = 21) | intervention (N = 20) | control (N = 19) | |||||
| BERG scoreb | 4.9±6.6 | 2.2±6.2 | 2.6±1.9 (−1.3 to 6.5) | 0.41 | 3.9±5.7 | −0.4±10.5 | 4.4±2.7 (−1.1 to 9.8) | 0.52 |
| 4.0 (1.0, 8.0) | 2.0 (−1.5, 5.5) | 3.0 (−0.8, 7.8) | 0 (−4.0, 6.0) | |||||
| TUG, sc | −1.4±6.7 | 5.6±37.7d | 7.0±8.0 (−9.1 to 23.2) | 0.27 | 0.53±24.25e | 4.8±36.8 | 4.3±9.8 (−15.5 to 24.0) | 0.14 |
| 1.5 (−4.9, 0) | 0.3 (−6.5, 4.6) | −1.6 (−5.8, −0.7) | −1.3 (−8.6, | |||||
| 4.9) | ||||||||
| 30-second chair standf | 1.6±3.2 | 1.8±3.9 | 0.2±1.1 (−2.0 to 2.3) | 0.04 | (0.8±2.5) | 0.8±4.4g | 0.1±1.1 (−2.3 to 2.2) | 0.02 |
| 1.0 (0, 4) | 1.0 (−1.0, 4) | 0 (0, 3.0)e | 0 (−0.4, 3) | |||||
| Fallsh Efficacy Scale- | 3.4±9.9 | 0.3±9.4 | 3.1±2.8 (−8.8 to 2.6) | 0.32 | −0.5±8.4i | 2.4±12.5)j | 2.9±3.1 (−3.2 to 9.1) | 0.28 |
| International | 2 (−5.0, 11) | 0 (−7.0, 11.0) | 0 (−7.0, 3.5) | 5 (−8.0, 8.0) | ||||
Mode (Q1 and Q3) are reported due to skewed data.
BERG score is between zero and 56 with higher scores indicating better balance; 5 point change is clinically meaningful.
Timed up and go score in seconds, where the lower the time indicates improved function, so a decrease over time is seen as an improvement.
n = 20.
n = 21.
30 s Chair Stand, where an increase in the number of stands indicates improved strength.
n = 19.
Falls Effiacy Scale-International, where a reduction in score means a reduction in fear of falling.
n = 25.
n = 23.
Function
For the TUG score between baseline and 3 months, the intervention group reduced the time it took to do the test, and show better function with a TUG mean change difference of 7.0 ± 8.0 (95% CI −9.1 to 23.2). The improvement was not maintained at 6 months. Participants in the control group increased the time it took to do the TUG both at three and 6 months (Table 4).
Strength
The number of sit to stands patients can do in 30 s (30CST) increased for both groups between baseline and 3 months, with a slightly better increase for the control group, but very little difference in change over time of 0.2 ± SE 1.1 (95% CI −2.0 to 2.3). Between baseline and 6 months, the intervention group had a slightly better mean change score, but again, the difference was very small of 0.1 ± SE 1.1 (95% CI −2.3 to 2.2).
Fear of Falling
Between baseline and 3 months, both the intervention and control groups saw an increase in fear of falling with the intervention seeing a larger increase and a mean change over time difference of 3.1 ± SE 2.8 (95% CI −8.8 to 2.6). However, between baseline and 6 months, there was a slight decrease in fear of falling from baseline for the intervention but an increase for the control group with a mean difference in change over time of 2.9 ± 3.1 (95% CI −3.2 to 9.1) between groups (Table 4).
Adherence
The Exercise Adherence Rating Scale (EARS) [39], a six-question adherence measure, showed a difference between groups of −4.6 ± 1.9 (95% CI −8.5 to −0.6), with the intervention group having higher adherence levels at 3 months. Participants in the intervention group continued to have better adherence at 6 months when compared with the control group, but both groups' adherence levels had dropped (Table 5).
Table 5.
Adherence
| 3-month scores mean ± SDa mode (Q1, Q3) |
Between group difference (95% CI) | Cohen's D | 6-month scores, mean ± SDa mode (Q1, Q3) |
Between group difference (95% CI) | Cohen's D | |||
|---|---|---|---|---|---|---|---|---|
| intervention (N = 24) | control (N = 22) | intervention (N = 24) | control (N = 23) | |||||
| Exerciseb | 17.7±6.7 | 13.1±6.5 | −4.6±1.9 (−8.5 to −0.6) | 0.69 | 15.2±7.8 | 14.9±6.1 | −0.4±2.0 (−4.5 to 3.7) | 0.05 |
| Adherence | 19.5 (13.0, 24.0) | 12.0 (10.2, 16.5) | 17 (9.8, 23.3) | 12.0 (10.0, 20.0) | ||||
| Rating | ||||||||
| Scale | ||||||||
Mean (SD) and mode (Q1 and Q3 quartiles) are reported due to skewed data.
Exercise Adherence Rating Scale, where the higher the score the better adherence (0–24 points).
Health Economics
Resource Use, Costs, Outcomes, and Cost-Effectiveness
Details of healthcare resource used over the 6-month follow-up period are shown in online suppl. Table 5. The total costs of resource use are shown in Table 6. We found that the ICECAP-O showed similar changes to the EQ-5D, although at 3 months, the ICECAP-O showed no drop in well-being for the intervention group, unlike the EQ-5D (Table 6). There was no significant difference in mean 6-month costs per participant between the intervention and control groups. The mean QALYs over the 6 months were similar (control: 0.3518, intervention: 0.3406) with no significant difference. At the cost-effectiveness thresholds considered, the Net Health Benefit (NHBs) were positive (0.112 and 0.069), and the probability of being cost-effective was 92.3% and 89.6% (Table 6). However, given that there were no statistically different costs and QALYs, the cost-effectiveness of the intervention was not clear.
Table 6.
Total costs of resource use, EQ-5D summary scores and QALYs, and cost-effectiveness results for all participants over the 6-month period
| Control group (n = 23) | Intervention group (n = 24) | p value | |
|---|---|---|---|
| GP-related | 229.5±247.1 | 244.5±282.5 | 0.847 |
| Hospital-related | 2133.4±3028.9 | 812.8±1215.1 | 0.054 |
| Community-related | 2219.8±3833.0 | 1428.4±3233.1 | 0.448 |
| Others (travel, medical devices) | 107.8±315.0 | 156.5±578.1 | 0.723 |
| Medication | 171.8±126.5 | 1249.0±4890.3 | 0.297 |
| Intervention | − | 113.04 | |
| Total costs | 4862.3±6294.0 | 4024.3±6007.5 | 0.643 |
| Incremental costs (95% CI)a | − | −2592.3 | (−2215.3–330.8) |
| Baseline EQ-5D | 0.690±0.161 | 0.723±0.174 | 0.499 |
| 3-month EQ-5D | 0.714±0.174 | 0.638±0.248 | 0.231 |
| 6-month EQ-5D | 0.696±0.145 | 0.726±0.246 | 0.617 |
| Baseline ICECAP-O | 0.769±0.167 | 0.791±0.151 | |
| 3-month ICECAP-O | 0.706±0.201 | 0.788±0.126 | |
| 6-month ICECAP-O | 0.671±0.215 | 0.776±0.143 | |
| QALYs | 0.3518±0.0638 | 0.3406±0.0993 | 0.651 |
| Incremental QALYs (95% CI)a | − | −0.017 | (−0.015–0.017) |
| ICER | N.A. | ||
| Incremental NHB in QALYs at | |||
| GBP 20,000 per QALY | 0.112 | ||
| GBP 30,000 per QALY | 0.069 | ||
| Probability of cost-effectiveness at | |||
| GBP 20,000 per QALY | 92.3% | ||
| GBP 30,000 per QALY | 89.6% |
Adjusted for baseline costs/EQ-5D, age, ethnicity, education level, living condition, and working status. Mean ± SD are reported.
Discussion
This is the first trial that we are aware of that explores the potential use of motivational smartphone apps for the support of an evidence-based falls exercise programme. We found that it was feasible for fall prevention services to deliver the intervention with good fidelity to the protocol and few technical issues. We used five very different NHS sites, reflecting the reality of day-to-day practice (three specialist falls services and two more general rehabilitation services) to explore the delivery of the intervention. We met our recruitment target in terms of percentage of those eligible in the timescale we planned, and only one participant in the intervention group decided they no longer wanted to use the application during the follow-up period. 20 (77%) participants in the intervention group completed their full exercise programme (including the use of the app), which is slightly less than the 80% set in our criteria. However, most of the reasons for not using the app for the full intervention period were related to unstable medical conditions and discharge from physiotherapy exercises (n = 5), rather than not wanting to use the application. A large number of our participants have co-morbidities, with only four participants without co-morbidities. The majority of participants had musculoskeletal diseases (n = 36, 72%) and circulatory diseases (n = 32, 64%), leading to a larger risk of ill health. We wanted to ensure we represented the patients who attend our fall services to ensure our findings in future can be transferable to practice. We therefore acknowledge that in doing this, there will be a requirement to recruit more participants to ensure a future trial is adequately powered. Ill-health and co-morbidities is a common issue when recruiting older adults to trials and was to be expected if we were to ensure we were representative of the population, and there were a large number of hospital admissions within our sample [45].
We had very few technical issues during the trial. Most of the issues were related to where the health professional had intermittent Wi-Fi at their sites (as the phone was set up to work through the Wi-Fi, when the Wi-Fi connection was poor, it did not default to the 3/4G through the sim card). Poor Wi-Fi at the clinical site meant that there were a few occasions when the health professional app did not synchronize with the patient app (so exercises and messages did not transfer to the patients' phones). The patients did not really have any technical issues with the “My Activity Programme” app, apart from a few patients deleting the app by accident which was quickly rectified. Usability and technical issues can be a major factor in older adults' engagement with technology [46] but was not reported to be an issue for “My Activity Programme” and “Motivate Me.” We believe this is because we developed the apps with the intended end users [20] and also ensured they would work adequately on 3/4G based on previous experience with patient access to Wi-Fi [20, 47]. The proposed future trial outcome measures were willingly completed by participants (unless they were unwell) which helped us understand the levels of adherence, balance, and number of falls expected within this population, and potential effect sizes for the intervention. The sample size of this feasibility study was not intended to provide an efficacy analysis but to assess the feasibility of a future definitive trial. Therefore, a formal sample size calculation was not undertaken. The sample size of 50 is able to estimate a completion rate (or response rate) of 80% to within a 95% confidence interval of +/−11.09%. Although the improvements across most outcome measures in favour of the intervention cannot be used in any way to demonstrate effectiveness, they do give a positive indication of the potential of the applications and support a move to a full randomized controlled trial to test effectiveness. We observed a much larger effect size of the intervention on adherence at 3 months when compared to 6 months and we speculate whether this is because some patients continued to complete the adherence questionnaire at follow-up but were discharged from physiotherapy because they became medically unstable (Fig. 1). Also, this could be because one of the sites (who recruited half of the patients) discharged most patients after 10 weeks and therefore stopped sending personalized messages at this point (and stopped checking patient reports). This may have diluted the impact of the intervention for this site, as we know that the health professional has an important role in motivation and adherence [48, 49, 50]. We would therefore like to explore how the applications could be used in follow-up community-based strength and balance interventions after falls rehabilitation to support longer term motivation and continuity of care, especially where the service delivers only a short rehabilitation programme. There were no serious unexpected adverse events related to the intervention and very few adverse events that were not expected within this population. This is very similar to other rehabilitation and strength and balance trials [51].
Limitations
As part of the feasibility of the trial, we wanted to explore for whom the apps were suitable, and we did not automatically exclude patients with dementia, Parkinsons, or other degenerative diseases, cognitive issues or where English was not their first language. We recruited five patients with a diagnosis of dementia and two with Parkinsons (both of whom also had dementia). Although it was important for us to be as fully representative of patients attending the services as possible, these conditions could be confounders when exploring effectiveness in a future full trial and will need to be taken into consideration. We recruited three patients for whom English was not their first language where they had the support of someone who lived with them or were committed to supporting them. They reported no technical issues with the phone and needed no more support than the other patients. There were varying levels of support from family members.
Recruitment was slower than expected as we based our estimates on the number of patients coming through the two originally recruited services without fully accounting for the number of patients who would not be suitable for rehabilitation. This meant we did not recruit as many participants as originally planned, although we did recruit enough to demonstrate feasibility [52]. Some 84 patients approached to participate in the study declined. Thus, we were unable to capture whether “My Activity Programme” and “Motivate Me” would work for all patients and this may have led to some self-selection bias. The refusal rate was actually lower than other digital rehabilitation studies [53]. The reasons given for declining to participate included the following: reported fear of technology, not wanting to undertake trial procedures, or, for some attending group rehabilitation, an unwillingness to do any home exercise at all. However, we attempted to recruit as many patients as possible with few exclusion criteria so as to be inclusive and representative. Almost all of our participants once recruited to the study, completed study procedures and assessments at all follow-up assessments where they could. We did have some loss of physical outcome data at both three and 6 months due to ill health and the COVID-19 pandemic (we were unable to send our assessors into patients' homes between the end of March 2020 and June 2020 when the main study data collection ended). We could have used a technology acceptance questionnaire. However, we chose to use qualitative methods, informed by the Technology Acceptance Model [54], to explore participants' and health professionals' experiences of the technology. These results will be reported elsewhere.
Conclusion
This feasibility trial has provided evidence that “Motivate Me” and “My Activity Programme” are feasible when used as part of falls rehabilitation and could have the potential to support adherence to evidence-based programmes and therefore better outcomes for patients. They are the only apps of this type available for falls rehabilitation which have been utilized within the UK health services. Descriptive outcome data has provided some preliminary support for the potential effectiveness of the intervention in supporting older adults' adherence to falls rehabilitation, improving balance, strength and reducing falls, and the potential for cost-effectiveness. A future pragmatic trial engaging with a range of services delivery falls rehabilitation will provide definitive evidence about the effectiveness and cost-effectiveness of this promising intervention.
Statement of Ethics
This study was reviewed and approved by the North West Greater Manchester East Research Ethics Committee (Rec ref: 18/NW/0457, 9/07/2018) and regional and site-specific approvals were obtained.
Conflicts of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This paper presents independent research arising from a National Institute for Health and Care Research (NIHR) Postdoctoral Fellowship Award to Dr Helen Hawley-Hague, (PDF-2015-08-012). The study was further supported by the National Institute for Health and Care Research Applied Research Collaboration-Greater Manchester. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health and Care Research or the Department of Health.
Author Contributions
Dr. Helen Hawley-Hague led the research project and its design, managed the trial overall, and has led the writing of the protocol, analysis, and manuscript. Dr. Carlo Tacconi and Dr. Sabato Mellone gave technical support for the study and advised on outcomes and the manuscript. Professor Jorunn Helbostad and Professor Lorenzo Chiari provided scientific advice around the design of the study and commented on the manuscript. Professor Chris Todd provided scientific advice around the design of the study and commented on the manuscript. Dr. Ting-Li Su has given advice on statistics and Dr. Fan Yang has assisted with the health economic part of design, including carrying out some of the analysis, and has commented on the manuscript. Ellen Martinez has given advice on the operationalization of the study and commented on the manuscript.
Data Availability Statement
The data that support the findings of this study are not publicly available due to containing information that could compromise the privacy of research participants and formal consent not being given for it to be shared. Anonymized data requests or further enquiries may be directed to Dr. Helen Hawley-Hague (corrosponding author).
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
We thank Professor Dawn Skelton and Later Life Training for allowing us to use their images and the name “Motivate Me” for the health professional app. We also thank Helen Skelton for changes made to some of the images for us based on previous feedback. We thank Caburn Telecom for their assistance with connectivity. We thank Mr Oliver Storey for his help with the medication costs for the health economics section. We thank all the participants in this study, both patients and healthcare professionals. We thank two PPI representatives who sat on our advisory group and three peer volunteers who have supported recruitment to the trial. We dedicate the paper in particular memory of Mr. George Street, who died recently aged 97 and Mr. Kevin Duffy.
Funding Statement
This paper presents independent research arising from a National Institute for Health and Care Research (NIHR) Postdoctoral Fellowship Award to Dr Helen Hawley-Hague, (PDF-2015-08-012). The study was further supported by the National Institute for Health and Care Research Applied Research Collaboration-Greater Manchester. The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health and Care Research or the Department of Health.
References
- 1.Public Health England Muscle and bone strengthening and balance activities for general health benefits in adults and older adults. Summary of a rapid evidence review for the UK Chief Medical Officers' update of the physical activity guidelines. 2018. Available from https://www.//assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/721874/MBSBA_evidence_review.pdf.
- 2.Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healthy Active Ageing Group Raising the bar on strength and balance report. 2019. Available from https://www.//ageing-better.org.uk/publications/raising-bar-strength-balance.
- 4.Hawley-Hague H, Roden A, Abbott J. The evaluation of a strength and balance exercise program for falls prevention in community primary care. Physiother Theor Pract. 2017;33((8)):611–621. doi: 10.1080/09593985.2017.1328721. [DOI] [PubMed] [Google Scholar]
- 5.Nyman, Victor CR. Older people's participation in and engagement with falls prevention interventions in community settings an augment to the Cochrane systematic review. Age Ageing. 2012;41((1)):16–23. doi: 10.1093/ageing/afr103. [DOI] [PubMed] [Google Scholar]
- 6.Royal College of Physicians Older people's experiences of therapeutic exercise as part of a falls prevention service-patient and public involvement. London, RCP. 2012.
- 7.Ofcom Rise of the social seniors revealed. Available from https://www.ofcom.org.uk/about-ofcom/latest/media/media-releases/2017/rise-social-seniors.
- 8.Ofcom Adults media use and attitudes. 2020. Available from https://www.ofcom.org.uk/__data/assets/pdf_file/0031/196375/adults-media-use-and-attitudes-2020-report.pdf.
- 9.Mellone S, Tacconi C, Schwickert L, Klenk J, Becker C, Chiari L. Smartphone-based solutions for fall detection and prevention the FARSEEING approach. Z Gerontol Geriatr. 2012;45((8)):722–727. doi: 10.1007/s00391-012-0404-5. [DOI] [PubMed] [Google Scholar]
- 10.King AC, Hekler EB, Grieco LA, Winter SJ, Sheats JL, Buman MP, et al. Harnessing different motivational frames via mobile phones to promote daily physical activity and reduce sedentary behavior in aging adults. PLoS One. 2013;8((4)):e62613. doi: 10.1371/journal.pone.0062613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Rosario MB, Redmond SJ, Lovell NH. Tracking the evolution of smartphone sensing for monitoring human movement. Sensors. 2015;15((8)):18901–933. doi: 10.3390/s150818901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGarrigle L, Boulton E, Todd C. Map the apps a rapid review of digital approaches to support the engagement of older adults in strength and balance exercises. BMC Geriatr. 2020;20((1)):483. doi: 10.1186/s12877-020-01880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaMonica HM, Davenport TA, Roberts AE, Hickie IB. Understanding technology preferences and requirements for health information technologies designed to improve and maintain the mental health and well-being of older adults participatory design study. JMIR Aging. 2021;4((1)):e21461. doi: 10.2196/21461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker SJ, Jessel S, Richardson JE, Reid MC. Older adults are mobile too! Identifying the barriers and facilitators to older adults' use of mHealth for pain management. BMC Geriatr. 2013;13:43. doi: 10.1186/1471-2318-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuoka Y, Kamitani E, Bonnet K, Lindgren T. Real-time social support through a mobile virtual community to improve healthy behavior in overweight and sedentary adults a focus group analysis. J Med Internet Res. 2011;13((3)):e49. doi: 10.2196/jmir.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vankipuram M, McMahon S, Fleury J. ReadySteady app for accelerometer-based activity monitoring and wellness-motivation feedback system for older adults. AMIA Annu Symp Proc. 2012;2012:931–939. [PMC free article] [PubMed] [Google Scholar]
- 17.Worringham C, Rojek A, Stewart I. Development and feasibility of a smartphone. ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PloS one. 2011;6((2)):e14669. doi: 10.1371/journal.pone.0014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin C, Bock B. Desired features of smartphone applications promoting physical activity. Telemed J E Health. 2011;17((10)):801–803. doi: 10.1089/tmj.2011.0055. [DOI] [PubMed] [Google Scholar]
- 19.Epton T, Currie S, Armitage CJ. Unique effects of setting goals on behavior change systematic review and meta-analysis. J Consult Clin Psychol. 2017;85((12)):1182–1198. doi: 10.1037/ccp0000260. 2017, 85. [DOI] [PubMed] [Google Scholar]
- 20.Hawley-Hague H, Martinez E, Tacconi C, Mellone S, Chiari L, Helbostad J, et al. Smartphone applications to support falls rehabilitation exercise application development, usability and acceptability study. J Med Internet Res mHealth uHealth. 2020;8:9. doi: 10.2196/15460. https://mhealth.jmir.org/2020/9/e15460/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajzen I. Maidenhead, UK: Open University Press; 1998. Attitudes personality and behaviour. [Google Scholar]
- 22.Hawley-Hague H, Horne M, Campbell M, Demack S, Skelton DA, Todd C. Multiple levels of influence on older adults' attendance and adherence to community exercise classes. Gerontologist. 2014;54((4)):599–610. doi: 10.1093/geront/gnt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yardley L, Donovan-Hall M, Francis K, Todd C. Attitudes and beliefs that predict older people's intention to undertake strength and balance training. J Gerontol B Psychol Sci Soc Sci. 2007;62:P119–P125. doi: 10.1093/geronb/62.2.p119. [DOI] [PubMed] [Google Scholar]
- 24.Lucidi F, Grano C, Barbaranelli C, Violani C. Social-cognitive determinants of physical activity attendance in older adults. J Aging Phys Act. 2006;14((3)):344–359. doi: 10.1123/japa.14.3.344. [DOI] [PubMed] [Google Scholar]
- 25.Hawley-Hague H, Tacconi C, Mellone S, Martinez E, Easdon A, Yang FB, et al. Can smartphone TechnolOGy be used to support an EffecTive Home ExeRcise intervention to prevent falls amongst community dwelling older people? The TOGETHER feasibility RCT. BMJ Open. 2019;9((9)):e028100. doi: 10.1136/bmjopen-2018-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podsiadlo D, Richardson S. The timed “Up & Go” a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39((2)):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies recommendations for good practice. J Eval Clin Pract. 2004;10((2)):307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 28.Skelton D, Dinan S, Campbell M, Rutherford O. Tailored group exercise (Falls Management Exercise — FaME) reduces falls in community-dwelling older frequent fallers (an RCT) Age Ageing. 2005;34((6)):636–639. doi: 10.1093/ageing/afi174. [DOI] [PubMed] [Google Scholar]
- 29.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ. 1997;315((7115)):1065–1069. doi: 10.1136/bmj.315.7115.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46((1)):81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 31.Hawley-Hague H. Together using smartphone technology in home exercise interventions. Available from: https://www.youtube.com/watch?v=JsIROlvCVvE. [Google Scholar]
- 32.Lamb SE, Jorstad-Stein EC, Hauer K, Becker C, Prevention of., Falls Network Europe and Outcomes Consensus Group. Development of a common outcome data set for fall injury prevention trials the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53((9)):1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 33.Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C, PROFANE-Group Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing. 2006;35((1)):5–10. doi: 10.1093/ageing/afi218. [DOI] [PubMed] [Google Scholar]
- 34.Berg KO, Wood-Dauphinée SL, Williams JI, Maki B. Measuring balance in the elderly validation of an instrument. Can J Public Health. 1992;83((Suppl 2)):S7–S11. [PubMed] [Google Scholar]
- 35.Sibley KM, Howe T, Lamb SE, Lord, Maki BE, Rose DJ, et al. Recommendations for a core outcome set for measuring standing balance in adult populations a consensus-based approach. PLoS One. 2015;10((3)):e0120568. doi: 10.1371/journal.pone.0120568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lajoie Y, Gallagher SP. Predicting falls within the elderly community comparison of postural sway, reaction time, the Berg Balance Scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr. 2004;38((1)):11–26. doi: 10.1016/s0167-4943(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 37.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70((2)):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 38.Kempen GIJM, Yardley L, Van Haastregt JCM, Zijlstra GAR, Beyer N, Hauer K. The Short FES-I a shortened version of the Falls Efficacy Scale-International to assess fear of falling. Age Ageing. 2008;37((1)):45–50. doi: 10.1093/ageing/afm157. [DOI] [PubMed] [Google Scholar]
- 39.Newman-Beinart NA, Norton S, Dowling D, Gavriloff D, Vari C, Weinman JA. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise the Exercise Adherence Rating Scale (EARS) Physiotherapy. 2017;103((2)):180–185. doi: 10.1016/j.physio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups a multi-country study. Qual Life Res. 2013;22((7)):1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people preferences or capabilities? Soc Sci Med. 2006;62((8)):1891–1901. doi: 10.1016/j.socscimed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Davis JC, Liu-Ambrose T, Richardson CG, Bryan S. A comparison of the ICECAP-O with EQ-5D in falls prevention clinical setting are they complements or substitutes? Qual Life Res. 2013;22((5)):969–977. doi: 10.1007/s11136-012-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CurtisBurns LA, Unit costs of health and social care 2019 Personal Social Services Research Unit. University of Kent. Available from https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2019/ [Google Scholar]
- 44.Donoghue D; Physiotherapy Research and Older People PROP group, Stokes EK. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J Rehabil Med. 2009;41((5)):343–346. doi: 10.2340/16501977-0337. [DOI] [PubMed] [Google Scholar]
- 45.Forsat ND, Palmowski A, Palmowski Y, Boers M, Buttgereit F. Recruitment and retention of older people in clinical research a systematic literature review. J Am Geriatr Soc. 2020;68((12)):2955–2963. doi: 10.1111/jgs.16875. [DOI] [PubMed] [Google Scholar]
- 46.Hawley-Hague H, Boulton E, Hall A, Pfeiffer K, Todd C. Older adults' perceptions of technologies aimed at falls prevention detection or monitoring a systematic review. Int J Med Inform. 2014;83((6)):416–426. doi: 10.1016/j.ijmedinf.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Hawley-HagueMartinez HE, Tacconi C, Mellone S, Chiari L, Helbostad J, et al. The acceptability and usability of virtual one to one and group based teleconferencing for falls rehabilitation. J Med Internet Res Rehabil Assistive Tech. 2021 doi: 10.2196/19690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawley-Hague H, Roden A, Abbott J. The evaluation of a strength and balance exercise program for falls prevention in community primary care. Physiother Theor Pract. 2017;33((8)):611–621. doi: 10.1080/09593985.2017.1328721. [DOI] [PubMed] [Google Scholar]
- 49.MacNeill V, Sanders C, Fitzpatrick R, Hendy J, Barlow J, Knapp M, et al. Experiences of front-line health professionals in the delivery of telehealth a qualitative study. Br J Gen Pract. 2014;64((624)):e401–e407. doi: 10.3399/bjgp14X680485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright BJ, Galtieri NJ, Fell M. Non-adherence to prescribed home rehabilitation exercises for musculoskeletal injuries the role of the patient-practitioner relationship. J Rehabil Med. 2014;46((2)):153–158. doi: 10.2340/16501977-1241. [DOI] [PubMed] [Google Scholar]
- 51.Delbaere K, Valenzuela T, Lord, Clemson L, Zijlstra GAR, Close JCT, et al. E-health StandingTall balance exercise for fall prevention in older people results of a two year randomised controlled trial. BMJ. 2021;373:n740. doi: 10.1136/bmj.n740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaplin E, Hewitt S, Apps L, Bankart J, Pulikottil-Jacob R, Boyce S, et al. Interactive webbased pulmonary rehabilitation programme a randomised controlled feasibility trial. BMJ Open. 2017;7((3)):e013682. doi: 10.1136/bmjopen-2016-013682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis FD. Perceived usefulness perceived ease of use and user acceptance of information technology. MIS Q. 1989 Sep;13((3)):319. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
The data that support the findings of this study are not publicly available due to containing information that could compromise the privacy of research participants and formal consent not being given for it to be shared. Anonymized data requests or further enquiries may be directed to Dr. Helen Hawley-Hague (corrosponding author).