Abstract
Background:
Normal values for regional left ventricular wall motion, although documented in adults, have not been reported in healthy newborns.
Methods:
This study prospectively evaluated global and segmental systolic and diastolic cardiac function by color kinesis in clinically asymptomatic healthy newborns.
Results:
Eighty-eight asymptomatic infants who were less than 48 hours old were studied. Systolic and diastolic parameters of global and regional left ventricular function are reported as means ± SD, medians, 5th and 95th percentiles to establish the normative values for newborns. The reported fractional area changes during systole and diastole are similar to the reported normal values for older subjects. Higher body surface area significantly correlated with an increased peak velocity during systole, and fractional area changes during filling of the lateral wall.
Conclusions:
Our report of left ventricular regional wall-motion characteristics of healthy newborns, as evaluated by color kinesis, may help in the objective evaluation and management of newborns suspected to have global or segmental ventricular dysfunction.
The importance of left ventricular (LV) diastolic function in various myocardial disease states has received increasing attention in infants and children. Doppler echocardiography has been the most widely used method to study diastolic function in neonates because of its ease of use and noninvasive nature.1,2 The blood flow velocities measured with pulsed Doppler echocardiography reflect global LV diastolic function. Recent evidence, however, indicates that there may be differences in regional wall motion as seen by myocardial tissue velocity studies in children.3 A magnetic resonance imaging (MRI) study has demonstrated differences in regional diastolic strain and wall motion.4 The study of regional wall motion may also benefit infants undergoing arterial switch procedure, anomalous origin of the left coronary artery from the pulmonary artery, cardiac transplantation, or newborns with prenatal cocaine exposure.5
Because noninvasive objective evaluation of the regional wall motion in newborns and infants has been difficult, there is a paucity of data in young children regarding normal LV regional wall-motion characteristics. In adults, color kinesis provides a realtime, color-coded display of LV endocardial motion. This enables an objective method for quantifying LV segmental wall motion.6 In a recent multicenter color kinesis study, normal values were published from 187 healthy subjects between the ages of 2 months to 79 years.7 Normal color kinesis values have not been established for the healthy newborns. The purpose of this study was to establish normal values for newborns and to study patterns of LV segmental wall motion with the help of color kinesis.
METHODS
As part of a study on the effects of in utero cocaine exposure on the heart, healthy newborn infants were eligible for the study if they were less than 48 hours old, weighed more than 1500 g, and were between 33 and 42 weeks gestation. Infants were excluded if they had any significant medical problems (for example, receiving any medications including supplemental oxygen, birth asphyxia with 5-minute Apgar < 6); any illicit drug exposure in utero including alcohol, marijuana, cocaine, and nicotine (assessed by maternal drug interview and urine/meconium toxicology studies); and any significant maternal surgical or medical problems (ie, requiring pharmacologic treatment for hypertension, depression, or asthma). Newborns with associated congenital anomalies, including cardiac defects (except patent ductus arteriosus, patent foramen ovale, or physiologic mitral, tricuspid, or pulmonary regurgitation) were also excluded. A convenience sampling strategy was used to enroll these newborns.
Demographic and medical characteristics at the time of infant birth were abstracted from the hospital record.These included maternal age, race, infant Apgar scores, birth weight, length, head circumference, and estimated gestational age.The study was approved by the Institutional Review Board for human investigation at MetroHealth Medical Center at Case Western Reserve University. Informed written consent was obtained from the legal guardians/parents of all participants.
Color Kinesis
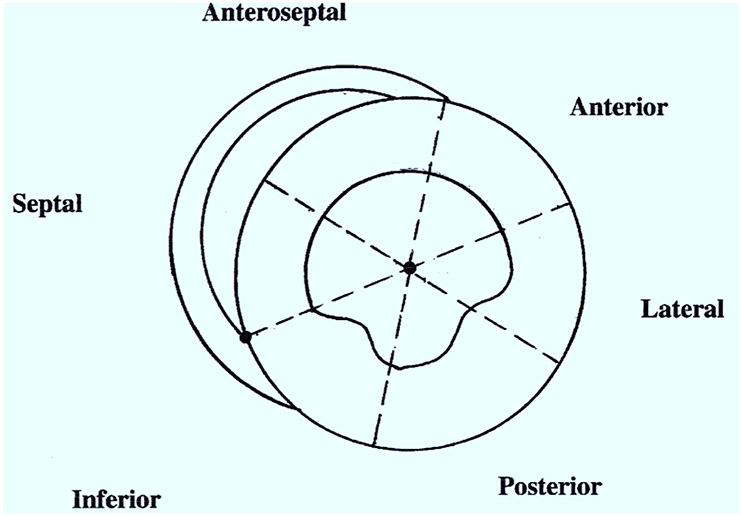
All infants underwent echocardiography and Doppler studies, including color kinesis with a 5 MHz transducer (SONOS 5500 Ultrasonograph, Hewlett-Packard Company, Andover, Mass) by an experienced pediatric-trained sonographer. The parasternal short-axis view, at the level of papillary muscles, was used for the color kinesis studies. Color kinesis images were analyzed off line by a cardiologist (J.P.S.) trained in color kinesis. The analysis was performed with automated software (Quick Color Kinesis, EchoSoft Co, Wilmington, Del). The LV end-systolic and diastolic cavity was segmented into six 60-degree, wedge-shaped segments (clockwise: anterior, lateral, posterior, inferior, septal, and anteroseptal segments). The centroid and a manually determined anatomic landmark at the junction of the right ventricular posterior wall endocardium and the interventricular septum defined the zero line for the segments (Figure 1). Incremental area change was normalized from the end-diastolic area of the corresponding segment resulting in regional fractional area change (%) during systole and diastole. We analyzed global and regional, systolic and diastolic parameters. The following diastolic parameters were also measured: ratio of peak velocity of early (E wave) and late (A wave) diastolic mitral flow (E/A), end-diastolic area (EDA), global fractional area change during filling (GFAC), and index of asynchrony.The index of asynchrony (IA) for LV filling was calculated as the standard deviation of the mean percentage of filling of all segments at 50% filling time.8 The global fractional area change was calculated by dividing the incremental area change by the end-diastolic area.The segmental fractional area change (%) for each segment was calculated by dividing the incremental area change of that segment by its end-diastolic area. Similar variables were measured for global and regional systolic function. A complete echocardiographic study (including M-mode, 2-dimensional, and Doppler) was performed at the same time with careful attention to the gain and filter settings to obtain clear images from endocardial and epicardial surfaces.
Figure 1.
Schematic diagram outlining left ventricular segments in parasternal short axis.
Statistical Analysis
All analyses were performed with SAS v8.1 software (SAS Institute, Cary, NC). Data on demographic characteristics are presented as mean ± SD. Linear regression models were used to evaluate body surface area, age in hours, and heart rate in relation to color kinesis measurements in healthy neonates.The results of the regression models are presented as intercept, slope of the regression line, and standard error of the slope. Scatter plots with a predicted line and corresponding 5th and 95th percentile regression lines are presented only for color kinesis parameters that were significantly correlated with body surface area at the .05 level. The significance level was set a priori at .05 (2-tail). P values should be interpreted with some caution, however, as they need to be compared with an adjusted value because of multiple testing among related variables.To adjust the P value, we used the formula α* = 1 – (1 – α)^(1/k), where α = .05 (significance level) and k is the number of tests in a group of related variables.9,10 For example, there are 6 diastolic regional filling area change variables. Thus, the adjusted significance level is α* = .0085. Therefore, if any individual P value for a diastolic regional filling area change variable is less than or equal to α*, then evidence exists that the regression parameter (slope) is statistically different from zero. However, we report all variables with a P value < .05 for completeness.
RESULTS
Eighty-eight asymptomatic infants who were less than 48 hours old were studied.The patient characteristics for these newborns and their maternal characteristics are reported in Table 1. Fifty-two percent of the infants were male and had a mean gestational age of 39.5 weeks.The color kinesis data are reported as mean ± SD, medians, 5th and 95th percentiles for systolic (Table 2) and diastolic parameters (Table 3). Because of the rapid heart rate and technical difficulties, differentiation between early and late mitral valve filling was not reliable in many newborns; therefore, those cases were eliminated from the analysis of late filling time, rate and peak velocity (Table 3). Hence, our E/A ratio is based on data from 57 patients.The mean values of regional mean time of ejection, systolic fractional area change, and mean time of filling are similar among all 6 segments with similar variability. The mean regional filling area change for the anterior wall is slightly lower than the rest of the 5 LV segments, which have similar means and standard deviations. The lateral wall has the highest mean of regional mean filling time and filling area change; such a change is not apparent during systole (Tables 3 and 4).
Table 1.
Demographics data
| Mean ± SD | Median | |
|---|---|---|
| Infant characteristics | ||
| Male (%) | 52% | |
| Gestational age (wk) | 39.45 ± 1.22 | 40 |
| Birth weight (g) | 3265.63 ± 431.78 | 3238 |
| Birth length (cm) | 49.95 ± 2.28 | 49.50 |
| Head circumference (cm) | 34.18 ± 1.34 | 34.00 |
| Body surface area (mol/L2) | 0.21 ± 0.02 | 0.21 |
| APGAR 1 min | 9.00 | |
| APGAR 5 min | 9.00 | |
| Age (h) | 29.64 ± 17.73 | 27.83 |
| Maternal characteristics | ||
| Age (y) | 21.61 ± 3.59 | 21.00 |
| White (%) | 20% |
N = 88.
Apgar scores are reported in medians only (at 1 min, 5th and 95th percentile scores were 7 and 9; at 5 min, 5th and 95th percentile scores were 9 and 9, respectively).
Table 2.
Systolic parameters
| Percentiles | |||||
|---|---|---|---|---|---|
| N | Mean ± SD | Median | 5th | 95th | |
| Heart rate (bpm) | 88 | 125.3 ± 12.4 | 125 | 104.2 | 146.3 |
| Time to peak ejection rate (ms) | 88 | 75.4 ± 20.6 | 66 | 66 | 99 |
| Systolic duration or ejection time (ms) | 88 | 247.1 ± 29.6 | 231 | 231 | 297 |
| Peak ejection rate (EDA/s) | 87 | 5.9 ± 0.9 | 5.9 | 4.4 | 7.4 |
| Mean time of ejection (ms) | 86 | 107.1 ± 10.4 | 107 | 92 | 125 |
| Mean time to peak ejection rate (ms) | 87 | 76.6 ± 19.6 | 66 | 49.5 | 116 |
| Regional mean time of ejection (ms) | |||||
| • Anterior | 88 | 106.2 ± 15.9 | 106.7 | 79.5 | 135 |
| • Anteroseptal | 88 | 101.8 ± 16.5 | 102 | 76 | 130 |
| • Septal | 88 | 106.7 ± 16.1 | 107.5 | 80 | 135 |
| • Inferior | 88 | 105.1 ± 13.2 | 104 | 86 | 128 |
| • Posterior | 88 | 109.6 ± 16.2 | 109 | 86 | 137 |
| • Lateral | 88 | 111.9 ± 19.1 | 111 | 85 | 145 |
| Regional systolic fractional area change (%) | |||||
| • Anterior | 86 | 72.6 ± 9.8 | 72.9 | 55.5 | 86.6 |
| • Anteroseptal | 88 | 76.3 ± 11.5 | 77.3 | 57.8 | 94.1 |
| • Septal | 88 | 76.6 ± 9.9 | 78.1 | 56 | 91.7 |
| • Inferior | 87 | 77.3 ± 9.3 | 77 | 62.2 | 93.2 |
| • Posterior | 88 | 78.7 ± 9.7 | 79.5 | 61.9 | 92.2 |
| • Lateral | 87 | 78.4 ± 8.4 | 78.2 | 65.9 | 91.8 |
| Global fractional area change—systole (% EDA) | 87 | 76.5 ± 8.1 | 76.5 | 62 | 89 |
| Index of asynchrony–systole | 88 | 9.6 ± 3.8 | 9.5 | 3.7 | 16.4 |
bpm, Beats per minute; EDA, left ventricular end-diastolic area.
Table 3.
Diastolic parameters
| Percentiles | |||||
|---|---|---|---|---|---|
| N | Mean ± SD | Median | 5th | 95th | |
| Heart rate (bpm) | 88 | 125.3 ± 12.4 | 125 | 104.2 | 146.3 |
| Peak filling rate (EDA/s) | 82 | 6.8 ± 1.6 | 6.9 | 4.3 | 9 |
| Time to peak filling rate (ms) | 82 | 69.4 ± 36.7 | 66 | 33 | 132 |
| Mean time of filling (ms) | 81 | 108.1 ± 24.5 | 106 | 70 | 150 |
| Mitral valve | |||||
| • Time to early peak filling rate (EDA/s) | 82 | 63.9 ± 27.1 | 66 | 33 | 99 |
| • Peak velocity during early filling (E) | 82 | 6.4 ± 1.5 | 6.5 | 4.1 | 8.6 |
| • Early filling time (ms) | 81 | 171.5 ± 53.1 | 165 | 99 | 264 |
| • Time to late peak filling rate (EDA/s) | 58 | 200.8 ± 50.3 | 198 | 132 | 297 |
| • Peak velocity during late filling (A) | 57 | 3.0 ± 1.1 | 2.9 | 1.5 | 5.3 |
| • Late filling time (ms) | 58 | 270.2 ± 59.7 | 264 | 165 | 396 |
| • E/A | 57 | 2.4 ± 1.1 | 2.2 | 0.9 | 4.7 |
| Regional mean time of filling (ms) | |||||
| • Anterior | 82 | 102.7 ± 21.7 | 105 | 69 | 139 |
| • Anteroseptal | 82 | 104.9 ± 29.8 | 98.5 | 63 | 161.5 |
| • Septal | 81 | 106.0 ± 28.3 | 104 | 67 | 164 |
| • Inferior | 82 | 108.6 ± 28.3 | 106 | 68 | 157 |
| • Posterior | 82 | 107.0 ± 28.8 | 103 | 68.7 | 160 |
| • Lateral | 82 | 119.9 ± 33.0 | 119 | 70 | 182 |
| Regional filling area change (%EDA) | |||||
| • Anterior | 80 | 65.4 ± 13.1 | 65.8 | 45.6 | 88.9 |
| • Anteroseptal | 78 | 73.1 ± 11.6 | 73 | 51.9 | 93.9 |
| • Septal | 80 | 71.9 ± 11.8 | 73.2 | 51.1 | 92.7 |
| • Inferior | 80 | 73.3 ± 11.1 | 73 | 53.4 | 92.6 |
| • Posterior | 80 | 72.0 ± 11.4 | 72.1 | 48.8 | 90.6 |
| • Lateral | 80 | 74.3 ± 11.9 | 76.2 | 52.7 | 92 |
| Global fractional area change—diastole (% EDA) | 81 | 72.0 ± 9.1 | 72.3 | 57 | 87 |
| Index of asynchrony–diastole | 82 | 11.6 ± 4.2 | 11.4 | 5.4 | 19.3 |
bpm, Beats per minute; EDA, left ventricular end-diastolic area.
Table 4.
Regression coefficients for color kinesis data controlling for body surface area
| Intercept | Slope | Correlation (r) | |
|---|---|---|---|
| Index of asynchrony–systole | 10.2 | −2.8 (23.5) | −0.01 |
| Index of asynchrony–diastole | 14.7 | −14.5 (26.4) | −0.06 |
| Global fractional area change during diastole (%) | 59.1 | 60.8 (57.1) | 0.12 |
| Global fractional area change during systole (%) | 58.9 | 83.0 (50.1) | 0.18 |
| Peak velocity during systole* | 3.1 | 12.0 (5.9) | 0.21 |
| E/A | 2.7 | −1.6 (8.1) | 0.03 |
| Regional filling area change | |||
| • Anterior | 35.8 | 139.7 (80.9) | 0.19 |
| • Anteroseptal | 56.9 | 76.6 (73.3) | 0.12 |
| • Septal† | 40.0 | 150.9 (72.3) | 0.23 |
| • Inferior | 54.8 | 87.3 (68.9) | 0.14 |
| • Posterior | 56.3 | 74.1 (71.4) | 0.12 |
| • Lateral‡ | 29.4 | 211.6 (70.9) | 0.32 |
All data were acquired in parasternal short-axis view.
P – .04.
P – .04 unadjusted (P > .0085 adjusted for test-wise error).
P – .004 unadjusted (P < .0085 adjusted for test-wise error).
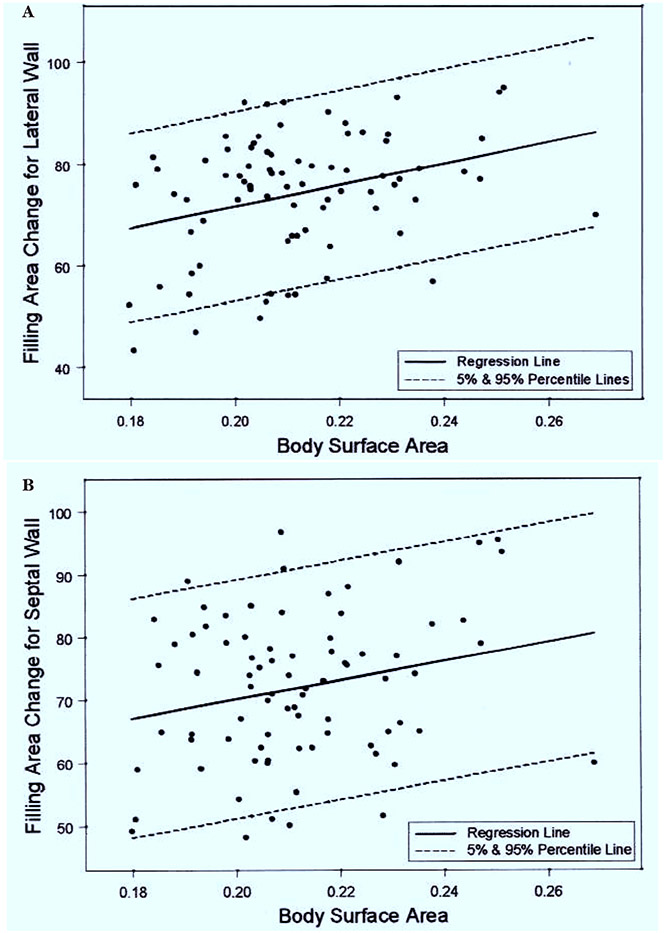
Among the various color kinesis parameters measured, lateral (r = 0.32: unadjusted for possible test-wise error P = .004; adjusted P < .0085) and septal wall (r = 0.23: unadjusted for possible test-wise error P = .04; adjusted P > .0085) filling area change increases with increasing body surface area (Table 4). An increase of 0.01 m2 in body surface area increases the lateral and septal wall filling area change by 2.12% (SE = 0.71) and 1.51% (SE = 0.72), respectively (Figure 2, A and B). A similar relationship was noted for peak velocity during systole and body surface area (P = .04) (Table 4).The body surface area does not significantly influence other systolic and diastolic parameters such as global fractional area changes and IA. In addition, regional and global area changes for systole and diastole were unrelated to heart rate.
Figure 2.
A, Regression plots of body surface area (m2) and filling area changes for lateral wall (P = .004) of left ventricle. B, Regression plots of body surface area and filling area changes for septal wall (P = .04) of left ventricle.
DISCUSSION
With the help of color kinesis, we have established normal regional systolic and diastolic parameters that will permit an objective evaluation of regional wall-motion abnormalities in newborns.We have also observed that higher body surface area significantly increases lateral and septal wall fractional area changes during filling in healthy newborns during the first 48 hours of postnatal period.
In adults, the role of color kinesis in the study of qualitative and quantitative evaluation of global and regional wall motion has been well documented.11 It permits fast, objective, and automated evaluation of regional wall motion with high sensitivity. Wall-motion abnormalities detected by color kinesis have been confirmed in subjects with dobutamine-in-duced regional wall-motion abnormalities.12 Color kinesis also provides an objective quantitative assessment of decreased global LV function.13 Quantitative analysis of color kinesis images was also used to identify diastolic dysfunction in 25 patients with cardiac hypertrophy whose systolic function was preserved. In addition to decreased filling seen during the first half of diastole, these subjects had increased IA with wide intersegmental variability in the regional LV filling times.8
It is of interest to note that means and SD of regional systolic fractional area changes noted in our newborns are similar to the normal data reported in 187 subjects aged 2 months to 79 years by Mor-Avi et al.7 Except for the slightly lower means for the anterior wall, diastolic filling fractions were also similar, indicating that these parameters are independent of the heart rate and age.These finding are similar to other color kinesis studies reported in adults.8,11-13 The lower regional mean time of ejection and filling observed in the current study are probably due to the higher heart rate in newborns. In contrast to adults, the healthy newborn LV myocardium has immature calcium transport and is subject to wide variations in right ventricular hemodynamics.This is reflected in an elevated IA compared with the data reported in older children and healthy adults.7
Our reported normal values of newborns would be helpful in obtaining objective information about regional wall-motion abnormalities in several clinical conditions. Baseline regional wall-motion abnormalities were noted in 80% of patients during the first year of cardiac transplantation.14 Infants who have undergone arterial switch procedure may have transmural perfusion defects15 that may lead to segmental abnormalities. Although global myocardial function of newborns has been studied in clinical conditions such as infants of diabetic mothers (including gestational diabetes), and neonatal asphyxia, it is unknown whether these conditions have selective segmental involvement or affinity for certain segments of the left ventricle.
Our finding of increased septal fractional area change during filling may be related to altered right ventricular pressure because of a drop in pulmonary vascular resistance during the postnatal period. Earlier experimental studies indicate that diastolic properties of the left ventricle can be modified by changes in right ventricular pressure and compliance even without a change in right ventricular volume.16 Furthermore, diastolic ventricular interaction has been associated with septal shift and deformation.17 At birth, normal newborns have pulmonary hypertension that is known to cause flattening of the interventricular septum.18 A rapid drop in the pulmonary vascular resistance and pulmonary artery pressure during the postnatal period has been well documented in humans and in various animal studies.The pulmonary arterial pressure remains elevated if the ductus arteriosus is still widely patent (PDA).19 A drop in pulmonary artery pressure would permit increased septal motion. It is likely that this change is accentuated in larger newborns (with higher body surface area) by earlier closure of the PDA. It is unclear whether increased septal fractional area changes during filling are due to changes in the right ventricular pressures or LV diastolic compliance with increasing body surface area.
The lateral wall of the left ventricle appears to be most affected by strain. The possible mechanism may be that lateral wall is farthest away from the right ventricle, and therefore is less restricted and less influenced by the right ventricular myocardium and its mechanics.4 A recent study of the regional differences in ventricular shape and load in patients with hypertrophic cardiomyopathy and right ventricular pressure overload, as demonstrated by 3-dimensional tagged MRI, demonstrated significantly higher lateral wall motion than other segments when compared with normal hearts.20 Non-uniformity in temporal and regional distribution of load and inactivation is one of the major factors influencing LV relaxation.21 Because multiple inter-connected factors may influence LV myocardium, particularly during the postnatal period, the cause of our finding of increased lateral wall fractional area change during filling remains speculative. It could be the result of postnatal hemodynamic changes and or maturational changes in the myocardium as reflected by the body surface area.
In contrast to adults, in whom delineation of the endocardium may be difficult, we had optimal delineation of the endocardial surface of the left ventricle in all infants. In our pilot study, the parasternal short-axis view in infants had more consistent landmarks than the apical 4-chamber view. In addition, the parasternal short-axis view in infants is easier to acquire with higher reproducibility. In adults, there is also greater intrasubject variability in the 4-chamber view in contrast to the short-axis view.22 In addition, MRI studies have shown less rotational error in the parasternal short-axis view than in the 4-chamber view.4 Therefore, we elected to study and report the color kinesis data only in the parasternal short-axis view. In older healthy individuals, compared with the other 3 views (parasternal long-axis, apical 4- and 2-chamber views), parasternal short-axis view yielded the maximum fractional area changes during systole and diastole. Furthermore, mean times of ejection and filling showed minor differences between the 4 standard views.7
It should be noted that fractional area changes do not necessarily reflect volume changes or the cardiac output. These area changes, measured only in the short-axis view, may or may not be reflective of changes in the long-axis view or in other LV dimensions. The possible contribution of LV rotational changes to our measurements of global or regional fractional area changes during the relaxation period cannot be estimated by the current noninvasive methods. Therefore, the above parameters for the evaluation of possible segmental wall-motion abnormalities should be compared with data obtained only in the parasternal short-axis view.We have concerns regarding temporal resolution, which may not be high enough for accurate measurements of the endocardial velocity rates at the high heart rates seen in our subjects.This needs further study before our data of mitral inflow peak velocity rate and velocity rate at E wave can be compared with other clinical states.
Color kinesis is an evolving technology that offers objective evaluation of wall-motion abnormalities. Similar to other technologies, there is a learning curve, but a major challenge is in obtaining the optimum images in a moving newborn infant. It is relatively easy to perform in parasternal short-axis view; however, before considering its routine use to evaluate the cardiac status of all newborn infants, more studies are needed to define its contributions and limitations in the diagnosis of various cardiovascular abnormalities—specifically conditions that are expected to alter myocardial wall motion.
In summary, color kinesis provides a quantitative method for the analysis of segmental wall-motion abnormalities in newborns. By using color kinesis, we observed that maturational changes, as reflected by increasing body surface area, have a significant effect on septal and lateral wall motion. By establishing normal values, our study will aid in the diagnosis and management of numerous pathologic conditions of the newborn myocardium.
Acknowledgments
We are thankful to the wonderful staff of General Clinical Research Center (grant from NIH #MO1RR00080 awarded to Case Western Reserve University) for their constant support and help in providing great care to our patients and their families.We are also grateful to Dr Victor Mor-Avi for his helpful comments and for his help in the color kinesis analysis.The study would not have been possible without the dedication and expertise of Maureen Babjak, Linda Wiersma, Cindy Holliday, and Allen Borowski.
Supported by NIDA (NIH) RO1-DA09049 (S.K.M.).
Footnotes
Presented in part at the 11th Annual Scientific Sessions, American Society of Echocardiography, Chicago, Illinois, June 11-14, 2000.
Contributor Information
Jing Ping Sun, Cleveland Clinic Foundation, Fairview Hospital, Case Western Reserve University; Department of Pediatrics, MetroHealth Medical Center, Case Western Reserve University..
Ann Salvator, Rainbow Babies and Children’s Hospital, Fairview Hospital, Case Western Reserve University; Department of Pediatrics, MetroHealth Medical Center, Case Western Reserve University..
Lynn Singer, Rainbow Babies and Children’s Hospital, Fairview Hospital, Case Western Reserve University; Department of Pediatrics, MetroHealth Medical Center, Case Western Reserve University..
H. Lester Kirchner, Rainbow Babies and Children’s Hospital, Fairview Hospital, Case Western Reserve University; Department of Pediatrics, MetroHealth Medical Center, Case Western Reserve University..
James D. Thomas, Cleveland Clinic Foundation, Fairview Hospital, Case Western Reserve University; Department of Pediatrics, MetroHealth Medical Center, Case Western Reserve University..
Sudhir Ken Mehta, Chairman of Pediatrics, Fairview Hospital, 18101 Lorain Ave, Cleveland, OH.
REFERENCES
- 1.Mehta S, Nuamah I, Kalhan S. Altered diastolic function in infants of mothers with gestational diabetes: no correlation to macrosomia. Pediatr Cardiol 1995;16:24–7. [DOI] [PubMed] [Google Scholar]
- 2.Li JS, Bengur AR, Ungerleider RM, Herlong JR, Sanders SP. Abnormal left ventricular filling after neonatal repair of congenital heart disease: association with increased mortality and morbidity. Am Heart J 1998;136:1075–80. [DOI] [PubMed] [Google Scholar]
- 3.Rychik J, Tian ZY. Quantitative assessment of myocardial tissue velocities in normal children with Doppler tissue imaging. Am J Cardiol 1996;77:1254–7. [DOI] [PubMed] [Google Scholar]
- 4.Fogel MA, Weinberg PM, Hubbard A, Haselgrove J. Diastolic biomechanics in normal infants utilizing MRI tissue tagging. Circulation 2000;102:218–24. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SK, Super DP, Salvator A, Singer L, Connuck D, Fradley LG, et al. Diastolic filling abnormalities by color kinesis in newborns exposed to intrauterine cocaine. J Am Soc Echocardiogr 2000;13:443(Abst P1F). [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Vignon P, Weinert L, Bednarz J, Korcarz C, Sandelski J, et al. Echocardiographic quantification of regional left ventricular wall motion with color kinesis. Circulation 1996;93:1877–85. [DOI] [PubMed] [Google Scholar]
- 7.Mor-Avi V, Spencer K, Gorcsan J, DeMaria A, Kimball T, Monaghan M, et al. Normal values of regional left ventricular endocardial motion: multicenter color kinesis study. Am J Physiol Heart Circ Physiol 2000:279:H2464–76. [DOI] [PubMed] [Google Scholar]
- 8.Vignon P, Mor-Avi V, Weinert L, Koch R, Spencer KT, Lang RM. Quantitative evaluation of global and regional left ventricular diastolic function with color kinesis. Circulation 1998;97:1053–61. [DOI] [PubMed] [Google Scholar]
- 9.Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 1967;62:626–33. [Google Scholar]
- 10.Miller RG Jr. Simultaneous statistical inference. New York: Springer-Verlag; 1981. [Google Scholar]
- 11.Vandenberg BF, Oren RM, Lewis J, Aeschilman S, Burns TL, Kerber RE. Evaluation of color kinesis, a new echocardiographic method for analyzing regional wall motion in patients with dilated left ventricle. Am J Cardiol 1997;79:645–50. [DOI] [PubMed] [Google Scholar]
- 12.Koch R, Lang RM, Garcia MJ, Weinert L, Bednarz J, Korcarz C, et al. Objective evaluation of regional left ventricular wall motion during dobutamine stress echocardiographic studies using segmental analysis of color kinesis images. J Am Coll Cardiol 1999;34:409–19. [DOI] [PubMed] [Google Scholar]
- 13.Godoy IE, Mor-Avi V, Weinert L, Vignon P, Korcarz C, Spencer KT, et al. Use of color kinesis for evaluation of left ventricular filling in patients with dilated cardiomyopathy and mitral regurgitation. J Am Coll Cardiol 1998;31:1598–606. [DOI] [PubMed] [Google Scholar]
- 14.Donofrio MT, Kakavand B, Moskowitz WB. Evaluation of regional wall motion and quantitative measures of ventricular function during dobutamine stress echocardiography in pediatric cardiac transplant patients. J Am Soc Echocardiogr 2000;13:932–40. [DOI] [PubMed] [Google Scholar]
- 15.Yates RW, Marsden PK, Badawi RD, Cronin BF, Anderson DR, Tynan MJ, et al. Evaluation of myocardial perfusion using positron emission tomography in infants following a neonatal arterial switch operation. Pediatr Cardiol 2000;21:111–8. [DOI] [PubMed] [Google Scholar]
- 16.Santamore WP, Constantinescu M, Vinten-Johansen J, Johnston WE, Little WC. Alterations in left ventricular compliance due to changes in right ventricular volume, pressure and compliance. Cardiovasc Res 1988;22:768–76. [DOI] [PubMed] [Google Scholar]
- 17.Beyar R, Dong SJ, Smith ER, Belenkie I, Tyberg JV. Ventricular interaction and septal deformation: a model compared with experimental data. Am J Physiol 1993;265(6 Pt 2):H2044–56. [DOI] [PubMed] [Google Scholar]
- 18.Kinsella JP, Neish SR, Abman S, Wolfe RR. Therapy for pulmonary hypertension. In: Garson A Jr, Bricker JT, Fisher DJ, Neish SR, editors. The science and practice of pediatric cardiology. Philadelphia: Williams & Wilkins; 1998. p. 2357. [Google Scholar]
- 19.Rudolph AM. Congenital diseases of the heart. Chicago: Year Book Medical Publishers; 1974. p. 29–48. [Google Scholar]
- 20.Petrank YF, Dong SJ, Tyberg J, Sideman S, Beyar R. Regional differences in shape and load in normal and diseased hearts studied by three dimensional tagged magnetic resonance imaging. Int J Card Imaging 1999;15:309–21. [DOI] [PubMed] [Google Scholar]
- 21.Brutsaert DL, Rademakers FE, Sys SU. Triple control of relaxation: implications in cardiac disease. Circulation 1984;1:190–6. [DOI] [PubMed] [Google Scholar]
- 22.Mor-Avi V, Vignon P, Koch R, Weinert L, Garcia MJ, Spencer KT, et al. Segmental analysis of color kinesis images. Circulation 1997;95:2082–97. [DOI] [PubMed] [Google Scholar]