Abstract
Purpose
There is increasing evidence that a persistent systemic inflammatory response predicts lower survival in patients with malignant disease. The modified Glasgow Prognostic Score (mGPS) is defined by a combination of elevated C-reactive protein (CRP) (>10 mg/L) and hypoalbuminemia (<35 g/L). It is considered as an independent prognostic marker in several organ malignancies. The aim of this study was to investigate the value of mGPS in metastatic penile carcinoma in predicting treatment response and survival.
Methods
One hundred and fifty-six patients with penile carcinoma treated with chemotherapy were included in this retrospective study. The mGPS before chemotherapy was classified into 3 groups (mGPS 0 [CRP <10, any albumin], mGPS 1 [CRP >10 mg/L, albumin >35 g/L], and mGPS 2 [CRP >10 mg/L, albumin <35 g/L]). Overall survival and disease-free survival were calculated by Kaplan-Meier analysis and chemotherapy toxicity by CTC criteria. Univariate Cox proportional hazards models were calculated to estimate the effect of each predictor on OS and DFS.
Results
Survival was significantly different in the 3 mGPS classes, with mGPS 0 patients showing the best treatment response and survival. Univariate analysis showed that mGPS (p < 0.0001), tumor stage (p = 0.004), and venous and lymphatic invasion (p = 0.011) were factors independently associated with prognosis. The response to chemotherapy differed significantly between mGPS groups (mGPS 0, 36/51 [71%]; mGPS 1, 24/70 [34%]; mGPS 2, 9/35 [26%], p = 0.03 and p = 0.37, respectively). mGPS was significantly associated with chemotherapy-associated toxicity, with treatment adaptation (p < 0.01) and toxicity-related deaths (p = 0.028).
Conclusions
Systemic inflammatory response and nutritional status as expressed by the mGPS are independent predictors of treatment response, chemotherapy-associated toxicity, and survival in metastatic penile carcinoma. In addition to other known pathological markers of tumor aggressiveness, the mGPS can be used as a clinical predictor of prognosis.
Keywords: Penile cancer, Response rate, Long-term survival, Chemotherapy, Modified Glasgow Prognostic Score
Introduction
Penile squamous carcinoma is an aggressive disease with rapid invasive growth and early lymphatic metastasis. Its development is associated with either an infection with human papilloma virus or with chronic inflammation [1]. Survival with adequate treatment is high in localized disease (relative 5-year overall survival with pTis/pTa 97%, with pT1 90%). With all stages of regional lymph node metastases (pN1-3), survival is reduced to about 46% [2, 3]. The presence of extranodal extension and lymphovascular invasion is correlated with a significantly higher risk of death and tumor recurrence in patients with penile carcinoma and inguinal lymph node metastasis [4]. However, with adequate treatment in limited lymph node disease, survival can be much better [5] [5, 6, 7, 8].
Systemic chemotherapy is used for the neoadjuvant and/or adjuvant treatment of regional lymph node disease in conjunction with surgical lymphadenectomy as well as for systemic metastatic disease. In the latter case, chemotherapy treatment is mostly palliative. Established regimens are cisplatin and taxane based with the combination of paclitaxel, cisplatin, and 5-fluorouracil mostly used in Europe [9] and that of cisplatin, paclitaxel, and ifosfamide in the USA [10].
There is a systemic tumor response in many patients. Also, patients receiving chemotherapy commonly experience some weight loss and some inflammatory response in the context of the cancer cachexia syndrome [11, 12]. This is characterized by elevated resting energy expenditure, loss of lean tissue mass, and functional decline [12]. C-reactive protein (CRP) is a marker of systemic inflammation and has been related to most of the aforementioned signs of nutritional depletion as well as overall survival [13]. Albumin represents a negative acute-phase protein, and its level decreases as CRP increases [12]. Loss of lean tissue mass is also related to hypoalbuminemia which in turn is related to weight loss, increased morbidity, and adverse outcomes in patients with malignant disease [12, 14].
Several studies support that the combination of CRP and serum albumin termed the modified Glasgow Prognostic Score (mGPS) predicts survival independently of tumor stage and other scoring systems [15, 16]. The original Glasgow Prognostic Score (GPS) is composed of a combination of increased CRP levels in the serum (>10 mg/L) and/or the presence of hypoalbuminemia (<35 g/L). In 2007, the GPS (mGPS) was modified, since hypoalbuminemia alone had no significant prognostic significance in patients with colorectal cancer [17]. We therefore used the current mGPS. In our study, we examined this question for metastatic penile cancer.
Materials and Methods
This is a retrospective study of all patients who were treated with chemotherapy for metastatic penile cancer in the Department of Urology of the Rostock University Medical Center between 2005 and 2019. Included patients had pathologically confirmed metastatic disease with regional lymphatic spread and/or systemic metastases. Patients with additional chronic inflammatory disease and/or active infections were excluded. The included patients have given their written informed consent.
Demographic, pathological, and laboratory parameters before the start of chemotherapy were retrospectively collected from the patients' records. All patients received standard cisplatin- and taxane-based chemotherapy, the majority with cisplatin, paclitaxel, and 5-fluorouracil. Tumor stages are given according to the TNM classification of the Union for International Cancer Control (UICC, eighth edition). Patients operated before 2017 were reclassified with respect to the eighth edition of the UICC after consultation of a pathologist.
All patients were regularly followed up with physical examination and CT or 18-FDG-CT/PET every 3 months for the first 2 years and every 6 months until 5 years after chemotherapy. CRP and serum albumin were measured 1 day before the beginning of chemotherapy.
The mGPS was determined as described previously by McMillan et al. [18]. Patients were stratified into 3 mGPS groups: mGPS 0 (CRP <10, any albumin), mGPS 1 (CRP >10 mg/L, albumin >35 g/L), and mGPS 2 (CRP >10 mg/L, albumin <35 g/L). To illustrate the effects of pathological albumin and CRP on overall survival and disease-free survival (DFS), the score groups were dichotomized (mGPS 0 vs. mGPS 1 + 2).
Overall survival was defined as the time between the beginning of chemotherapy and cancer-related death. Death from causes other than penile cancer or survival until the end of follow-up after 5 years was classed as censored observations. The follow-up period was limited to 5 years, so censorship was set at that time. These patients were considered cured. Longer follow-up data were available for some patients. The other still living patients were no longer actively followed up after 5 years.
DFS was defined from the beginning of chemotherapy until the first evidence of progression. Median follow-up was estimated by the reverse Kaplan-Meier method. Kaplan-Meier curves were plotted to investigate differences in OS and DFS between the 3 mGPS groups. Univariate Cox proportional hazards models were calculated to estimate the effect of each predictor on OS and DFS. All tests were 2-sided, and p values <0.05 were considered significant. All statistical analyses were performed with the statistical software IBM SPSS Statistics 25 (Version 2017).
Results
One hundred and fifty-six patients were included. The mean age was 65.4 years (±13.8 SD). In 99 patients (63%), the primary tumor grade was G3 and in 107 (69%) the primary tumor stage T2. All patients had nodal metastasis, and 44 patients (28%) in addition had evidence of distant metastases (Table 1; Fig. 1a, b). Patients who underwent surgery after 2013 and had distant metastases (n = 22) received neoadjuvant chemotherapy prior to inguinal lymphadenectomy. The investigations regarding the mGPS were performed before the start of the first cycle of adjuvant chemotherapy. The median follow-up was 67.8 months (1–89.5 months) for OS and 68.1 months (1–89 months) for DFS. Fifty-one patients (32.7%) fell into group 0 mGPS, 70 (44.9%) into group 1, and 35 (22.4%) into group 2 (Table 2).
Table 1.
Baseline characteristics of included patients
| Characteristics | Included patients (n = 156) |
|---|---|
| Mean age (SD), years | 65.4 (13.8) |
| T-stage, n (%) | |
| pT1 | 0 |
| pT2 | 107 (69) |
| pT3 | 43 (28) |
| pT4 | 6 (4) |
| N-stage, n (%) | |
| pN1 | 57 (37) |
| pN2 | 75 (48) |
| pN3 | 24 (15) |
| Distant metastasis, n (%) | |
| cM0 | 112 (72) |
| cM+ | 44 (28) |
| Grading, n (%) | |
| G1 | 0 |
| G2 | 57 (37) |
| G3 | 99 (63) |
| Neoadjuvant chemotherapy, n (%) | |
| Yes | 22 (7) |
| No | 134 (83) |
| Inguinal lymphadenectomy, n (%) | |
| Yes | 156 (100) |
| No | 0 |
| Adjuvant chemotherapy, n (%) | |
| Yes | 156 (100) |
| No | 0 |
| Follow-up (range), months | |
| OS | 67.8 (1–89.5) |
| DFS | 68.1 (1–89) |
DFS, disease-free survival; OS, overall survival.
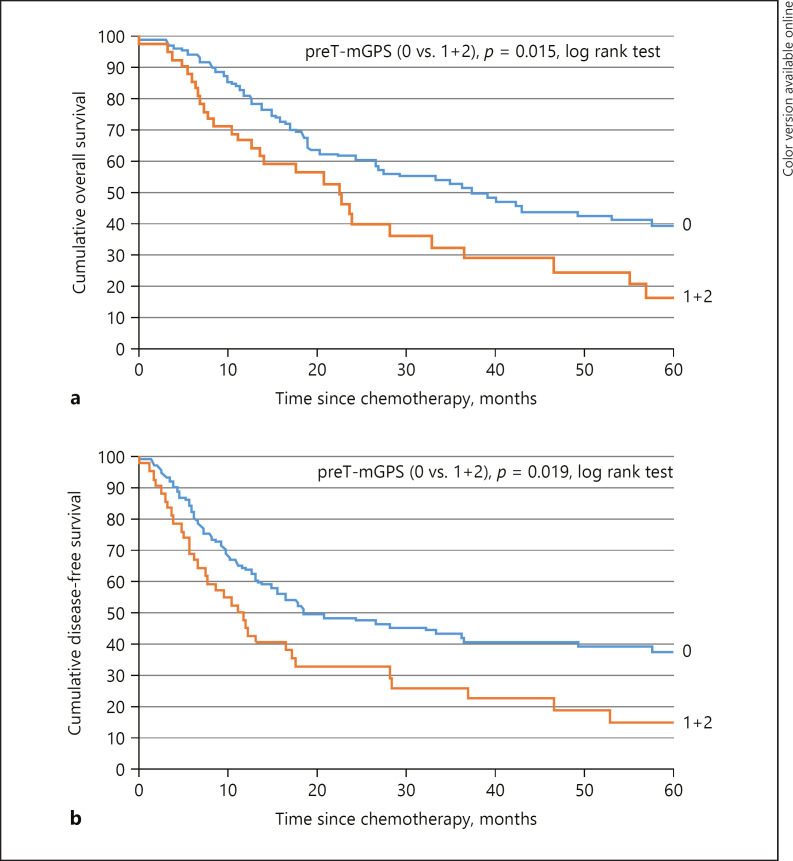
Fig. 1.
a Correlation between mGPS and overall survival. b Correlation between mGPS and disease-free survival. mGPS, modified Glasgow Prognostic Score.
Table 2.
Serum albumin, CRP, and GPS before adjuvant chemotherapy
| Characteristics | Included patients (n = 156) |
|---|---|
| preT-albumin | Mean 41.3 (±SD 6.6) |
| <35.0 g/L, n (%) | 66 (42) |
| ≥35.0 g/L, n (%) | 90 (58) |
| preT-CRP | Mean 5.6 (±SD. 4.1) |
| <10 mg/L, n (%) | 82 (53) |
| ≥10 mg/L, n (%) | 74 (47) |
| preT-mGPS, n (%) | |
| 0 | 51 (33) |
| 1+2 | 105 (67) |
mGPS, modified Glasgow Prognostic Score.
Univariable Cox proportional hazard regression showed that hypoalbuminemia was associated with lower OS and DFS (p = 0.05 and p = 0.03). Significant differences for OS and DFS were seen between mGPS group 0 versus group 1 and group 2 (p = 0.015 for OS and p = 0.019 for DFS). Based on univariable Cox regression analysis, hypoalbuminemia, tumor differentiation, and mGPS were identified as significant prognostic factors for OS and hypoalbuminemia, mGPS, and T-stage for DFS, respectively (Table 3).
Table 3.
Multivariate Cox regression analysis to estimate the influence of GPS and clinicopathological parameters on the OS and DFS
| preT | p value | HR | 95% CI |
|---|---|---|---|
| OS | |||
| preT-albumin (≥35.0 g/L vs. <35.0 g/L) | 0.05 | 0.53 | 0.31–0.85 |
| Grading (G2 vs. G3) | 0.01** | 0.48 | 0.33–0.77 |
| mGPS (1+2 vs. 0) | 0.015 | 1.76 | 1.10–2.87 |
| DFS | |||
| preT-albumin (≥35.0 g/L vs. <35.0 g/L) | 0.03 | 0.51 | 0.33–0.80 |
| T-stages (pT2 vs. pT3) | 0.05 | 0.57 | 0.35–0.87 |
| mGPS (0 vs. 1+2) | 0.019 | 1.66 | 1.11–2.53 |
DFS, disease-free survival; OS, overall survival; mGPS, modified Glasgow Prognostic Score.
Indicates statistical significance.
The response to chemotherapy differed between mGPS groups (36/51 [71%] in group 0, 24/70 [34%] in group 1, and 9/35 [26%] in group 2). These differences were statistically significant (p < 0.01) (Table 4).
Table 4.
Correlation between mGPS and response rate
| mGPS | Patients (n = 156) | Response rate (CR + PR), n (%) | p value | Clinical benefit (CR + PR + SD), n (%) | p value |
|---|---|---|---|---|---|
| mGPS 0 | 51 | 36 (71) | 0.05 | 39 (76) | 0.03 |
| mGPS 1 | 70 | 24 (34) | 0.25 | 35 (50) | 0.37 |
| mGPS 2 | 35 | 9 (26) | 12 (34) |
mGPS, modified Glasgow Prognostic Score; CR, complete remission; PR, partial remission; SD, stable disease.
mGPS was also significantly associated with chemotherapy-associated toxicity, with resultant treatment modifications and with treatment-related deaths (Tables 5, 6). A higher mGPS correlated with higher toxicity as measured by Common Toxicity Criteria (CTC) and consequently with the adaptation of further treatment. Thus, the mGPS class was significantly associated with the need for dose reductions, treatment discontinuations (p < 0.01), and also toxicity-related deaths (p = 0.028).
Table 5.
Relationship between mGPS and therapy-related toxicities
| Toxicities (CTC) ≥ grade 2 | mGPS 0 (n = 51), n (%) | mGPS 1 (n = 70), n (%) | mGPS 2 (n = 35), n (%) | p value |
|---|---|---|---|---|
| Anemia | 5 (9) | 24 (34) | 21 (60) | <0.01** |
| Neutropenia | 6 (12) | 32 (46) | 30 (86) | <0.01** |
| Nausea/emesis | 8 (16) | 25 (36) | 23 (74) | <0.01** |
| Anorexia | 8 (16) | 26 (37) | 21 (60) | <0.01** |
| Mucositis | 3 (6) | 7 (10) | 12 (34) | <0.05 |
| Fatigue | 9 (18) | 21 (30) | 17 (49) | <0.01** |
| Neurotoxicity | 4 (8) | 10 (14) | 15 (43) | <0.01** |
mGPS, modified Glasgow Prognostic Score.
Indicates statistical significance.
Table 6.
Association of mGPS and toxicity-related therapy modifications
| Toxicity variables | mGPS 0 (n = 51) | mGPS 1 (n = 70) | mGPS 2 (n = 35) | p value |
|---|---|---|---|---|
| Toxicity-related therapy modifications, n (%) | 5 (10) | 15 (21) | 17 (49) | <0.01** |
| Dose delay | 2 | 6 | 5 | 0.028 |
| Dose reduction | 2 | 5 | 5 | |
| Therapy demolition | 1 | 4 | 7 | |
| Toxicity-related death, n (%) | 0 | 1 (1) | 5 (14) |
mGPS, modified Glasgow Prognostic Score.
Indicates statistical significance.
Discussion
To the best of our knowledge, this is the first study assessing the value of calculating the mGPS in patients with metastatic penile cancer. There are some markers of systemic inflammatory response, for example, cytokines, differential blood counts, and some serum proteins that have been reported to have prognostic value in some malignant diseases [19, 20, 21, 22, 23]. It seems that tumor-associated inflammation, both locally in the tumor microenvironment and systemically as a host response, does have prognostic significance [24, 25, 26].
In addition, there is a relationship between the systemic inflammatory response and nutritional status, and this also has been reported to be of prognostic significance [27, 28]. Not surprisingly therefore, the combination of a systemic inflammatory response and malnutrition is associated with poor outcome in patients with advanced cancer stages. There is an obvious association between advanced malignancy and malnutrition. The role of hypoalbuminemia in this context may be multifactorial as it is also decreased in response to systemic inflammatory reactions.
Recent studies have reported that inflammation-based prognostic scores, including the mGPS, are useful scoring systems for the prognostication in cancer patients [21, 22, 23, 29, 30, 31, 32, 33]. The mGPS combines albumin and CRP into a prognostic stratification system combining an assessment of inflammatory response and nutritional status and may therefore be particularly useful for predicting clinical outcomes in cancer patients. The neutrophil-to-lymphocyte ratio before inguinal lymph node dissection might be useful for predicting the prognosis of patients with penile squamous cell carcinoma [33]. A study by Djajadiningrat et al. [34] showed no association between BMI of penile cancer patients and their disease stage at the time of treatment.
Our results demonstrate that patients with higher mGPS at baseline had significantly shorter survival compared to patients with lower mGPS. These results are consistent with studies in other malignancies [21, 35, 36]. In the present study, patients with higher mGPS had shorter survival, poorer response to therapy, and a higher rate of chemotherapy toxicity. This suggests that the mGPS is a particularly useful prognostic marker that gives a lot of information about the expected fate of a given patient with metastatic penile carcinoma who is considered a candidate for chemotherapy.
Our study has obvious limitations in that it is a retrospective data collection. The retrospective design implies the risk of unidentified confounding factors, that of missing data, and the potential for miscoding of data. It would therefore be useful to validate our findings in a prospective study.
This study demonstrates that malnutrition and systemic inflammatory response to malignant disease, as determined by the mGPS, are significant independent predictors of survival, chemotherapy response, and chemotherapy-related toxicity in patients with penile cancer. This is an important finding as there are clinical implications. The mGPS may be used to assess individual patients and better counsel them regarding their prognosis if they undergo chemotherapy. Then, the patients' nutritional status can be improved therapeutically, and this might improve treatment response and perhaps reduce toxicity of chemotherapy.
The role of the inflammatory response to malignant disease is complex, and it is not easily modifiable by therapeutic interventions. Thus, at present, only targeting the nutritional status may improve an individual patient's prognosis with chemotherapy in metastatic penile cancer.
Conclusion
mGPS is a useful clinical marker of outcome in patients with metastatic penile cancer who are candidates for chemotherapy. It correlates with tumor differentiation and stage, survival, treatment response, and chemotherapy toxicity.
Clinical Practice Points
Penile squamous carcinoma is an aggressive disease.
Systemic chemotherapy is used for the neoadjuvant and/or adjuvant treatment of regional lymph node disease in conjunction with surgical lymphadenectomy as well as for systemic metastatic disease.
Systemic inflammatory response and nutritional status as expressed by the mGPS are independent predictors of treatment response, chemotherapy-associated toxicity, and survival in metastatic penile carcinoma.
Statement of Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of University Medical Center Rostock. The included patients have given their written informed consent. In the case of the retrospective evaluation of existing, internal patient and examination data, there is only a simple obligation to notify the Ethics Committee of the University of Rostock. Furthermore, it concerns register data from the Penile Cancer Register of the Rostock University Medical Center, which have been anonymized.
Conflict of Interest Statement
The authors declare no potential conflicts of interest.
Funding Sources
The authors have no funding to declare.
Author Contributions
D.D. and O.H. contributed to conceptualization; D.D. and O.W. contributed to methodology; D.D. and T.B. contributed to software; D.D. and O.H. contributed to validation; D.D. contributed to formal analysis; all authors contributed to investigation; O.H. contributed to resources; D.D. contributed to data curation; D.D. contributed to writing − original draft preparation; all authors contributed to writing − review and editing and visualization; O.H. contributed to supervision; D.D. contributed to project administration. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data are available upon request from the corresponding author.
Funding Statement
The authors have no funding to declare.
References
- 1.Brierley JD, Gospodarowicz MK, Wittekind C, Union for International Cancer Control . TNM classification of malignant tumours. Oxford: John Wiley & Sons; 2017. [Google Scholar]
- 2.Hakenberg OW, Dräger DL, Erbersdobler A, Naumann CM, Jünemann KP, Protzel C. The diagnosis and treatment of penile cancer. Dtsch Arztebl Int. 2018;115:646–652. doi: 10.3238/arztebl.2018.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirrander P, Sherif A, Friedrich B, Lambe M, Håkansson U. Swedish National Penile Cancer Register: incidence, tumour characteristics, management and survival. BJU Int. 2016;117:287–292. doi: 10.1111/bju.12993. [DOI] [PubMed] [Google Scholar]
- 4.da Costa WH, Rosa de Oliveira RA, Santana TB, Benigno BS, da Cunha IW, de Cássio Zequi S, et al. Prognostic factors in patients with penile carcinoma and inguinal lymph node metastasis. Int J Urol. 2015;22((7)):669–673. doi: 10.1111/iju.12759. [DOI] [PubMed] [Google Scholar]
- 5.Djajadiningrat RS, Graafland NM, van Werkhoven E, Meinhardt W, Bex A, van der Poel HG, et al. Contemporary management of regional nodes in penile cancer-improvement of survival? J Urol. 2014;191:68–73. doi: 10.1016/j.juro.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 6.Leijte JA, Kirrander P, Antonini N, Windahl T, Horenblas S. Recurrence patterns of squamous cell carcinoma of the penis: recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol. 2008;54:161–168. doi: 10.1016/j.eururo.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Necchi A, Lo Vullo S, Perrone F, Raggi D, Giannatempo P, Calareso G, et al. First-line therapy with dacomitinib, an orally available pan-HER tyrosine kinase inhibitor, for locally advanced or metastatic penile squamous cell carcinoma: results of an open-label, single-arm, single-centre, phase 2 study. BJU Int. 2018;121:348–356. doi: 10.1111/bju.14013. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein RJ, Munsell MF, Pagliaro LC, Pettaway CA. Prognostic factors influencing survival from regionally advanced squamous cell carcinoma of the penis after preoperative chemotherapy. BJU Int. 2016;117:118–125. doi: 10.1111/bju.12946. [DOI] [PubMed] [Google Scholar]
- 9.Pizzocaro G, Piva L. Adjuvant and neoadjuvant vincristine, bleomycin, and methotrexate for inguinal metastases from squamous cell carcinoma of the penis. Acta Oncol. 1988;27:823–824. doi: 10.3109/02841868809094366. [DOI] [PubMed] [Google Scholar]
- 10.Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol. 2010;28:3851–7. doi: 10.1200/JCO.2010.29.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 2011;2011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4:250–255. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 14.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98:1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer. 2006;94:227–230. doi: 10.1038/sj.bjc.6602922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer. 2007;109:205–212. doi: 10.1002/cncr.22400. [DOI] [PubMed] [Google Scholar]
- 17.Leitch EF, Chakrabarti M, Crozier JE, McKee RF, Anderson JH, Horgan PGPG, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer. 2007 Nov 5;97((9)):1266–1270. doi: 10.1038/sj.bjc.6604027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMillian DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22((8)):881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 19.Inoue Y, Iwata T, Okugawa Y, Kawamoto A, Hiro J, Toiyama Y, et al. Prognostic significance of a systemic inflammatory response in patients undergoing multimodality therapy for advanced colorectal cancer. Oncology. 2013;84:100–107. doi: 10.1159/000343822. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton TD, Leugner D, Kopciuk K, Dixon E, Sutherland FR, Bathe OF. Identification of prognostic inflammatory factors in colorectal liver metastases. BMC Cancer. 2014;14:542. doi: 10.1186/1471-2407-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jomrich G, Hollenstein M, John M, Baierl A, Paireder M, Kristo I, et al. The modified glasgow prognostic score is an independent prognostic indicator in neoadjuvantly treated adenocarcinoma of the esophagogastric junction. Oncotarget. 2018;9((6)):6968–6976. doi: 10.18632/oncotarget.24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He L, Li H, Cai J, Chen L, Yao J, Zhang Y, et al. Prognostic value of the glasgow prognostic score or modified glasgow prognostic score for patients with colorectal cancer receiving various treatments: a systematic review and meta-analysis. Cell Physiol Biochem. 2018;51((3)):1237–1249. doi: 10.1159/000495500. [DOI] [PubMed] [Google Scholar]
- 23.Soria F, Giordano A, DʼAndrea D, Moschini M, Rouprêt M, Margulis V, et al. Prognostic value of the systemic inflammation modified Glasgow prognostic score in patients with upper tract urothelial carcinoma (UTUC) treated with radical nephroureterectomy: results from a large multicenter international collaboration. Urol Oncol. 2020;38:602–e19. doi: 10.1016/j.urolonc.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg. 2016;263:326–336. doi: 10.1097/SLA.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 25.Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 26.Vayrynen JP, Tuomisto A, Klintrup K, Makela J, Karttunen TJ, Makinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839–1847. doi: 10.1038/bjc.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li QQ, Lu ZH, Yang L, Lu M, Zhang XT, Li J, et al. Neutrophil count and the inflammation-based glasgow prognostic score predict survival in patients with advanced gastric cancer receiving first-line chemotherapy. Asian Pac J Cancer Prev. 2014;15:945–950. doi: 10.7314/apjcp.2014.15.2.945. [DOI] [PubMed] [Google Scholar]
- 28.Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22:803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 30.Lindenmann J, Fink-Neuboeck N, Koesslbacher M, Pichler M, Stojakovic T, Roller RE, et al. The influence of elevated levels of C-reactive protein and hypoalbuminemia on survival in patients with advanced inoperable esophageal cancer undergoing palliative treatment. J Surg Oncol. 2014;110:645–650. doi: 10.1002/jso.23711. [DOI] [PubMed] [Google Scholar]
- 31.Melling N, Grüning A, Tachezy M, Nentwich M, Reeh M, Uzunoglu FG, et al. Glasgow Prognostic Score may be a prognostic index for overall and perioperative survival in gastric cancer without perioperative treatment. Surgery. 2016;159:1548–1556. doi: 10.1016/j.surg.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–5. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Li X, Zhang X, Chen P, Wang B, Chen X, et al. Prognostic significance of common preoperative laboratory variables in penile squamous cell carcinoma. Int J Urol. 2020 Jan;27((1)):76–82. doi: 10.1111/iju.14137. [DOI] [PubMed] [Google Scholar]
- 34.Djajadiningrat RS, van Werkhoven E, Horenblas S. Penile cancer stage, survival and body mass index. Urol Int. 2015;94((2)):220–224. doi: 10.1159/000367927. [DOI] [PubMed] [Google Scholar]
- 35.Gioulbasanis I, Pallis A, Vlachostergios PJ, Xyrafas A, Giannousi Z, Perdikouri IE, et al. The Glasgow Prognostic Score (GPS) predicts toxicity and efficacy in platinum-based treated patients with metastatic lung cancer. Lung Cancer. 2012;77:383–388. doi: 10.1016/j.lungcan.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Zhang P, Xi M, Li QQ, He LR, Liu SL, Zhao L, et al. The modified glasgow prognostic score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer. 2014;5((8)):689–695. doi: 10.7150/jca.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request from the corresponding author.