Abstract
Background:
The opioid overdose and opioid use disorder (OUD) epidemics are concomitant with increased metabolic and CVD risk. Although OUD causes adverse pregnancy outcomes, the offspring’s cardiovascular health is understudied. We hypothesized that offspring exposed to morphine (MOR) in utero would show increased CVD risk factors and endogenous opioid system dysregulation.
Methods:
Sprague Dawley dams were treated with saline (VEH, n=10) or escalating doses of MOR (5-20mg/kg/day, s.c, n=10) during gestation. Cardiovascular and metabolic parameters were assessed in adult offspring.
Results:
Litter size and pups’ birth weight were not different in response to MOR exposure. Female and male MOR-exposed offspring showed reduced body length at birth (p<0.05) and body weight from weeks 1-3 of life (p<0.05), followed by a catch-up growth effect. By week 16, female and male MOR-exposed rats showed reduced tibia length (p<0.05) and fat mass. in utero MOR exposure (IUME) increases the mean arterial pressure and the depressor response to mecamylamine (5mg/kg/day, i.p) were abolished by a chronic treatment with an alpha-adrenergic receptor blocker (prazosin, 1mg/kg/day, i.p.). Although circulating levels of angiotensin peptides were similar between groups, IUME exacerbated maximal ex vivo AngII-induced vasoconstriction (p<0.05) and induced endothelial dysfunction in a sex-specific manner (p<0.05). Proenkephalin, an endogenous opioid peptide that lowers blood pressure and sympathetic-mediated vasoconstriction, showed reduced mRNA expression in the heart, aorta, and kidneys from MOR vs. VEH group (p<0.05).
Conclusion:
Among the effects of IUME, neurogenic hypertension, vascular dysfunction, and metabolic dysfunction could be associated with the dysregulation of the endogenous opioid system.
Keywords: opioids, pregnancy, hypertension, offspring cardiovascular health, cardiometabolic risk factors
Graphical Abstract

Introduction
Opioid misuse, OUD, and opioid overdoses are great public health threats in the US1,2. From 2019 to 2020, drug overdose deaths increased by 31%, with 75% of these deaths involving opioids1. In 1999, an initial opioid overdose death wave was caused by prescription opioids, both natural and semisynthetic opioids (e.g., morphine and oxycodone), followed by the second wave of deaths in 2010 caused by heroin (semisynthetic opioid), which is metabolized to MOR 1. However, ‘a third concerning wave caused by synthetic opioids, specifically illicit fentanyl and fentanyl-like analogs, has been on an alarming rise since 2013 1. Nevertheless, an understudied area related to the opioid epidemic is OUD during pregnancy, which results in a 5-fold increase in neonatal opioid withdrawal syndrome (NOWS) incidence2–4, a group of symptoms affecting different body systems (e.g., cardiovascular, gastrointestinal). NOWS severity is influenced by the length of exposure, type of opioid, and other licit and illicit substances that the mother uses. Strikingly, every hour four babies are diagnosed with this syndrome 2,3,5.
A growing number of studies shed light on possible adverse effects on health outcomes associated with long-term opioid use 6–8. The hospitalization trends due to cardiovascular events mirror trends of opioid overdose9. In this sense, OUD is associated with a 2-fold increased risk of hypertension, myocardial infarction, and ischemic heart disease10–12. OUD is associated with an increased risk of a metabolic-like syndrome13, worsening of preexisting diabetes condition by impairing glucose metabolism, promoting insulin resistance, altering lipid profile14–16, atherogenesis, and coronary artery disease11,14,15,17, all major risk factors for CVD. Nevertheless, there are significant gaps in the scarce literature investigating the mechanisms underlying the interaction between opioids and systems modulating cardiovascular function. The current gap in assessing CVD risk in the offspring of women experiencing OUD during pregnancy raises a concern regarding the opportunity to identify a group of individuals at risk and improve the current therapeutic approaches to prevent adverse health outcomes 3,18.
A critical point in the decision-making to treat OUD is understanding the interaction between different types of opioid agonists and antagonists with opioid receptors. The endogenous opioid system (EOS) comprises endogenous opioid peptides (EOPs) and five opioid receptors (ORs): µ -OR, κ-OR, δ-OR, nociception-OR, and z-OR, expressed in most tissues, including the brain, heart, kidneys, liver, and intestinal tract19. ORs are G-protein coupled receptors (GPCRs) that can dimerize and affect the function of other GPCRs involved in cardiovascular regulation20–22. For instance, opioids, angiotensin II, and adrenergic receptors are GPCRs expressed within brain areas responsible for blood pressure control and sympathetic outflow, such as the paraventricular nucleus of the hypothalamus (PVN), the rostroventrolateral medulla (RVLM), and the nucleus of the solitary tract (NTS)23–26. Moreover, the peripheral expression of ORs subtypes in the vasculature have been shown to play a role in endothelial function by impairing or improving vascular relaxation upon the activation of mu-OR or K-OR, respectively 27–29.
While numerous preclinical studies of prenatal opioid exposure have been used to investigate long-term consequences in behavior outcomes, analgesia, reward, learning, and memory processing, only a handful of studies have assessed the detrimental effects of opioid use on cardiovascular function12,29–31. Moreover, the study of potential sex differences secondary to prenatal opioid exposure has been lacking altogether31. Consequently, there is a remarkable gap in the knowledge addressing the link between opioid exposure during developmental life and long-term cardiovascular risk3,18.
This study aimed to develop a rat paradigm of opioid exposure during pregnancy using MOR, the prototypic μ-OR agonist, to assess the long-term cardiovascular effects on the offspring. The ultimate goal of this study is to use this model as a tool to investigate molecular mechanisms, identify targets, and test therapeutic approaches to reduce the effects of opioid exposure increasing cardiovascular risk factors in the offspring. Thus, we investigated litter morphometrics, body composition, blood pressure, and sympathetic activation with and without the effects of a chronic alpha adrenergic receptor blocker, blood and urine biomarkers of cardiovascular and sympathetic function, and the effect of ORs on vascular function in male and female offspring exposed to MOR in utero. In addition, we determined PENK expression in cardiovascular tissues and the locomotor sensitization to MOR exposure in adulthood to assess potential neurobehavioral alterations following in utero MOR exposure (IUME).
Methods
The data supporting the findings of this study are available upon reasonable request from the corresponding author. Detailed protocols can be found in the Supplementary Material section.
Animals.
All experiments followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved and monitored by the Institutional Animal Care and Use Committee at the University of Kentucky. Sprague Dawley female and male rats used for breeding had ad libitum access to food and water and were housed in a pathogen-free environment with constant temperature and humidity, with a 12:12 hour light: dark cycle. Animals were fed a regular chow diet consisting of 24% protein, 58% carbohydrates, and 18% fat with 3.1 kcal/g gross energy (Teklad 8604, Madison, WI).
Morphine exposure during pregnancy.
Sprague-Dawley rats (250–300 g) were bred in-house. A vaginal smear for timed pregnancy was performed between 8:00 and 9:00 AM for two weeks on female breeders. Two days prior to mating, bedding of the male was introduced in the female cage and males were added when the females were in the proestrus phase. Gestational day 0 (G0) was determined by the presence of a sperm plug. Once GD0 was determined, dams were randomly assigned to morphine (MOR, n=10) and vehicle (VEH, n=10) groups. Dams were treated with a daily subcutaneous injection of VEH (sterile 0.9% NaCl) or an escalating dose of morphine sulfate GD1-3 (5mg/kg/day, manufactured by Hospira Laboratory, supplied by Henry Schein using DEA registration number RL0457730). This dose was increased by 5 mg/kg/day every five days from GD4-GD19 to reach the final dose of 20mg/kg/day. At birth, litters were culled to 8-10 pups and balanced for sex. Body weight, composition, and morphometrics data were averaged per litter. For functional tests, one adult offspring per sex per litter was randomly assigned to different experiments.
Experimental design.
Weight gain during pregnancy and lactation was assessed every other day. At birth, body weight and morphometric measurements (head and waist circumference, anogenital distance, tail length, total body length) were taken in all pups during the first 8 hours of life. At weaning, body composition was determined using the Echo Magnetic Resonance Imaging system (Echo-MRI; Echo Medical Systems, Houston, TX). Then, body weight was assessed weekly. At 14 weeks, rats were placed in metabolic cages for 24-hour acclimation. Then, food and water intake and urine output were measured for two consecutive days. A week later, a magnetic resonance imaging (MRI) scan was performed to determine body composition32–34. In a second subset of 16-week old offspring, fasting blood glucose and metabolic function were determined35,36, allowing rats to recover for a few days before the endpoint euthanasia with ketamine/xylazine (100/10mg/kg, IP) for blood collection, tissue harvesting for gene expression37 and vascular function studies38. In a third subset of rats, direct, conscious blood pressure was measured in untreated or treated with an α-adrenergic receptor blocker (prazosin, 1mg/kg, i.p., 2 weeks). In the last subset of rats, a locomotor test was performed39 following repeated exposure to morphine (10mg/kg/day) for 6 consecutive days.
Plasma and serum biomarkers.
Fasting blood glucose was determined by analyzing a drop of blood using a glucometer (Accu-Check, Roche, Germany). ELISA kits were used to measure plasma insulin (Invitrogen by Thermo Fisher Scientific, Waltham, MA) and leptin (Cayman, Ann Harbor, MI). Serum renin-angiotensin system (RAS) fingerprints were determined at the University of Kentucky RAS Analytical Lab Service Center40–42. Serum cholesterol and lipoproteins were determined using fast protein liquid chromatography (FPLC)43,44 and plasma and Urine Norepinephrine (NE) were measured by modifications of HPLC protocols45,46.
Conscious measurement of blood pressure.
Rats were fitted with femoral artery cathethers as previously reported.36 The following morning, rats were awake and freely moving in the housing cage, acclimated for 2 hours, and a baseline blood pressure was measured for 2 hours under continuous monitoring (PowerLab, ADInstruments). Acute angiotensin II (AngII) intraarterial boluses (0.01-1.2 nMol/Kg) were performed randomly during baseline. Thirty minutes after the last AngII dose, a bolus of the ganglion blocker mecamylamine (5mg/kg, i.p.) was performed to determine changes in blood pressure over 1-hour period.
Statistical Analysis.
All data are presented as mean±SEM. Comparison between VEH and MOR was assessed via t-test. When sex was an added factor, a 2-way ANOVA analysis was performed. Data for body composition trajectories were analyzed by 2-Way ANOVA repeated measures followed by Tuckey multiple comparisons post-hoc test for body composition trajectories. Detailed statistic tests are provided in the legend of each figure. Analyses were performed using GraphPad Software version 9.0.0 (La Jolla, CA, www.graphpad.com). Statistical significance was determined by p<0.05.
Results
Prenatal MOR exposure leads to smaller offspring that experience a catch-up growth effect.
Dams treated with escalating doses of MOR showed a similar increase in weight gain during pregnancy compared to VEH-treated dams (26.5±2.5% versus 26±3.8% of BW). The litter size, number of female and male pups (Fig. 1A), and birth weight (Fig. 1B) were similar between groups. Morphometric measurements showed no differences in anogenital distance, head circumference, waist circumference, cephalization index, and tail length between VEH and MOR-exposed offspring. On the contrary, body length (nose to anus) and full length (nose to last caudal vertebra) were significantly reduced in male and female IUME offspring (Fig. 1C–D). Further, IUME offspring showed reduced BW from week 1 to week 3; however, both groups showed similar fat and lean mass composition at weaning (Fig.S1). MOR-exposed females (Fig. 1E) and males (Fig. 1F) experienced catch-up growth by week 4, showing similar BW between VEH and MOR groups until week 16.
Figure 1. Morphine exposure does not affect pregnancy outcomes however results in catch-up growth in the in utero morphine exposed offspring:
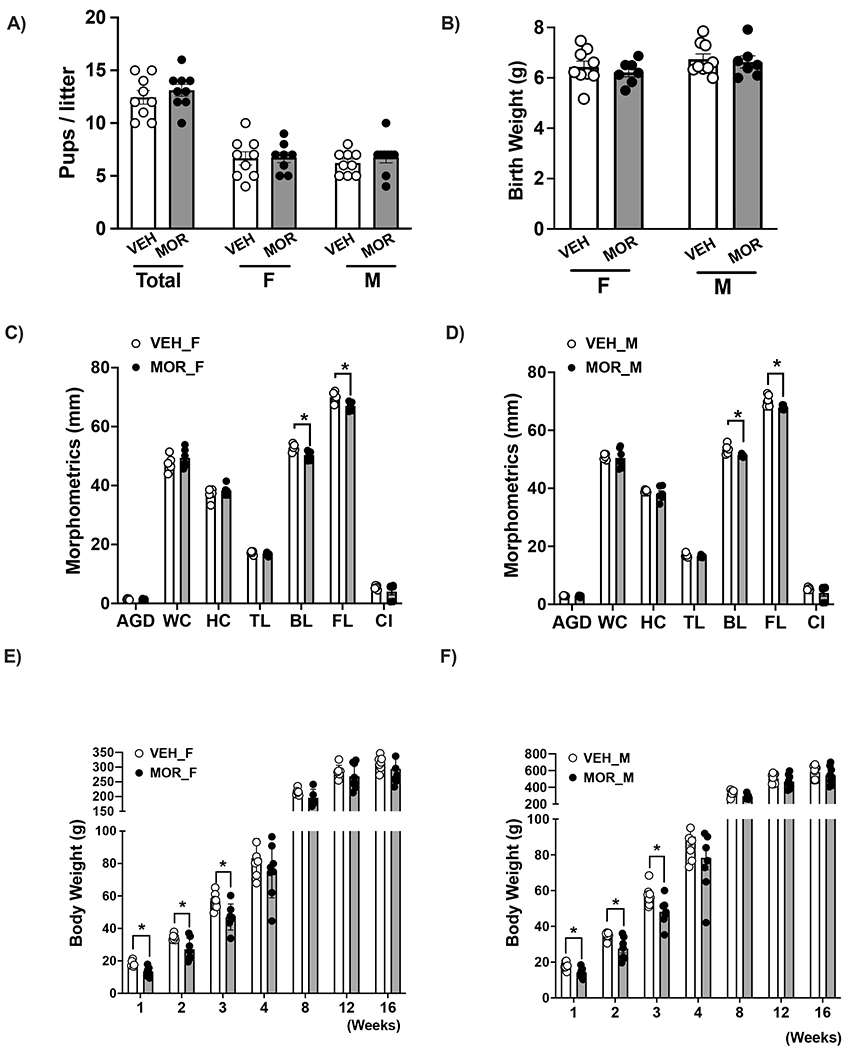
A) total litter size B) female and male neonates birth weight, C) morphometric measurements in female neonates, D) morphometric measurements in male neonates, E) body weight trajectory from weeks 1-16 in female offspring, F) body weight trajectory from weeks 1-16 in male offspring.
Data were averaged by litter, analyzed by t-test and reported as mean ± SEM. Data were analyzed by 2-Way ANOVA repeated measures followed by Tuckey multiple comparisons post-hoc test for body composition trajectories; n=9-10/group; * p<0.05 vs. control. VEH=vehicle, MOR=morphine, F=females, M=males, PD=postnatal day, AGD=anogenital distance, WC=waist circumference, HC=Head circumference, TL=tail length FL=full length, CI=cephalization index
Organs and tissues weights were not influenced by MOR exposure in female rats (Fig. 2A), while male rats showed a significant reduction in subcutaneous and retroperitoneal white adipose tissue fat pads (Fig. 2B). Tibia length was reduced in IUME female and male adult rats (Fig. 2A–B insets).
Figure 2. In utero morphine exposure reduces fat mass in adult offspring:

A) tissue and organ weights and tibia length (inset graph) in adult female rats, B) tissue and organs weights and tibia length (inset graph) in adult male rats, C) representative MRI scan of adult female rats, D) representative MRI scan of adult male rats, E) quantification of the subcutaneous and visceral fat in female rats, F) quantification of the subcutaneous and visceral fat in male rats.
Data were averaged by litter, analyzed by t-test and reported as mean ± SEM; n=6-8/group for tissue, organs weight and tibia length and MRI analysis; * p<0.05 vs. control. VEH=vehicle, MOR=Morphine, F=females, M=males, gWAT=perigonadal white adipose tissue, scWAT= subcutaneous white adipose tissue, periWAT=perirenal white adipose tissue, SC=subcutaneous, VISC=visceral, MESE=mesenteric white adipose tissue, BAT=brown adipose tissue.
MRI full body scans from female rats (Fig. 2C) showed a similar amount of adipose tissue, as the quantification of visceral and subcutaneous fat proportional to BW was similar between groups (Fig. 2E). However, IUME male rats displayed reduced subcutaneous and visceral fat depots proportional to BW (Fig.2D and 2F).
Prenatal MOR exposure is associated with increased circulating cardiovascular risk biomarkers.
IUME adult rats showed increased fasting blood glucose (FBG) (Fig. 3A), reduced plasma insulin (Fig. 3B) and leptin (Fig. 3C). IUME did not change water and food intake and urinary excretion in female or male rats (Table S2). Lipid profile showed IUME increased low-density lipoproteins (LDL) in female and male rats (Fig. 3D). Despite IUME increased albumin to creatinine ratio similarly in both sexes (Figure 3E), only IUME females showed a reduced creatinine clearance compared with vehicle (Fig.S2). Plasma and urine NE were increased in male rats exposed to prenatal MOR (Fig. S3 and 3F, respectively).
Figure 3. In utero morphine exposure increases plasma and urine biomarkers of cardiometabolic disease risk factors:

A) FBG, B) insulin, C) leptin, D) FPLC analysis of plasma male and female rats, E) ACR and F) urinary NE.
Data were analyzed by 2-Way ANOVA followed by Tuckey multiple comparisons post-hoc test and reported as mean ± SEM; n=8-10/group, Fig 3A–F,F, n=6-8/group, Fig. 3G–H;H; * p<0.05 vs. control. VEH=vehicle, MOR=morphine, F=females, M=males, FBG=fasting blood glucose, ACR= albumin creatinine ratio, NE= norepinephrine.
Prenatal MOR exposure increases blood pressure and sympathetic tone.
IUME induced increases in MAP were abolished by the prazosin treatment (Fig. 4A). Furthermore, mecamylamine induced a significant reduction in MAP in IUME female rats compared to their VEH counterparts. Prazosin abrogated this reduction (Fig. 4B). IUME male rats showed the same increase in MAP and significant reduction in response to mecamylamine compared with VEH. The chronic treatment with prazosin reduced blood pressure at baseline and reduced mecamylamine depressor response in IUME male rats (Fig. 4C and 4D). When sex was analyzed as a factor, IUME did not worsen baseline blood pressure or degree of sympathetic tone (not shown). Overall, the expression of adrenergic receptors were downregulated in most of the tissues analyzed. Specifically, MOR-exposed females showed reduced mRNA expression of αA1-AR in heart, αB1 and αB2-AR in renal outer medulla (OM), and β2-AR in renal cortex and OM. Also αB2-AR was increased in heart compared to VEH female rats (Fig. 4E). In males, MOR-exposed offspring showed reduced mRNA expression of αA1-AR in aorta, heart, and renal OM, αB1-AR in aorta and heart, αB2-AR in renal OM and β2-AR in aorta and OM (Fig. 4F).Circulating levels of RAS components were similar between groups of both sexes (Fig. S3A and B). However, the delta dose response to acute doses of AngII was significantly attenuated in MOR-exposed female rats only (Fig. S3C) while showing a subtle effect on the male rat (Fig. S3D).
Figure 4. In utero morphine exposure increases blood pressure and sympathetic activation:

A) Mean arterial pressure in female rats untreated and treated with prazosin, B) acute blood pressure response to mecamylamine in female rats untreated and treated with prazosin, C) mean arterial pressure in male rats untreated and treated with prazosin D) acute blood pressure response to mecamylamine in female rats untreated and treated with prazosin E) cardiovascular tissue adrenergic receptor expression in female rats, and F) cardiovascular tissue adrenergic receptor expression in male rats. Data were analyzed by 2-Way ANOVA followed by Tuckey multiple comparisons post-hoc test for blood pressure in A and B. For AngII-induced pressor response, data were analyzed using a 2-way ANOVA repeated measures, while RAS components were analyzed by t-test. Data were reported as mean ± SEM; n=7-9/group * p<0.05 vs. control. VEH=vehicle, MOR=Morphine, F=females, M=males, MAP=mean arterial pressure, Meca=mecamylamine, PRAZ=prazosin.
Prenatal MOR exposure induces a sex-specific impairment of vascular function.
The AngII-induced response in isolated rings tended to increase in MOR-exposed female offspring while constricted similarly with and without naloxone pre-incubation (Fig. 5A). Conversely, rings from MOR-exposed male rats displayed increased AngII-induced vasoconstriction that was abrogated by naloxone treatment (Fig. 5B). The mRNA expression for AT1 and ACE was significantly reduced in aortas from MOR-exposed offspring compared to VEH (Fig. S5).
Figure 5. In utero morphine exposure promotes sex-specific impairments of the vascular function:

AngII-induced constriction with and without naloxone in rings from A) female and B) male rats, C) PE-induced constriction in male and female rats, D) KCl-induced constriction in male and female rats, Ach and SNP-induced relaxation in rings from E) female and F) male rats.
Data were reported as mean ± SEM; n=7-9/group * p<0.05 vs. control. VEH=vehicle, MOR=Morphine, F=females, M=males, MAP=mean arterial pressure, AngII=angiotensin II, PE=phenylephrine, Ach=acetylcholine, SNP=sodium nitroprusside.
PE-induced constriction (Fig. 5C) and the maximal response to KCl in isolated aortas (Fig. 5D) were similar in MOR and VEH-exposed groups in both sexes. Moreover, the endothelial function was impaired in IUME females only, as evidenced by the reduced Ach-induced relaxation (Fig. 5E–F), whereas the receptor-independent SNP-induced relaxation was similar between groups in both sexes.
Prenatal MOR exposure reduces the expression of an endogenous opioid peptide in vascular tissues.
PENK mRNA levels were reduced in the aorta and kidney (cortex and OM) of IUME females (Fig. 6A) and male rats (Fig. 6B). IUME induced a reduction in PENK only in the heart of male rats. Conversely, PENK expression was significantly increased in the liver of IUME adult offspring.
Figure 6. In utero morphine (MOR) exposure reduces proenkephalin (PENK) expression in the heart, aorta, and kidney and delays developing tolerance to morphine-induced locomotor hypoactivity:

A) PENK mRNA expression in female rats, B) PENK mRNA expression in male rats, C) distance traveled by female rats after 1 and 6 days of MOR injections, D) distance traveled by male rats after 1 and 6 days of MOR injections.
Data were analyzed by t-test for gene expression and paired t-test for locomotor activity and reported as mean ± SEM; n=6-8/group for gene expression and n=8 per group for locomotor activity test; * p<0.05 vs. control. VEH=vehicle, MOR=morphine, F=females, M=males, PENK=proenkephalin.
Prenatal MOR exposure sensitizes the locomotor response to morphine.
In adult rats, a single dose of morphine induced a depressant effect in locomotor activity that was similar between prenatal exposure to VEH and MOR (Fig. 6C). However, the development of tolerance after six injections of morphine was delayed in MOR-exposed compared to VEH-exposed counterparts (Fig. 6D). Data is presented pooling rats from both sexes since the disaggregated effect of MOR was similar in IUME offspring (Fig. S5).
Discussion
Our study is among the first to develop a rodent model to investigate the effects of prenatal morphine exposure on the offspring’s cardiovascular health outcomes from neonatal life to adulthood, shedding light on the potential link between prenatal MOR exposure and high blood pressure, a risk factor for the development of CVD. Although litter size and birth weight were similar between groups, IUME neonates were shorter and weighed less until weaning, showing a catch-up growth effect by the first month of life. As adults, IUME rats displayed increased sympathetic activation, blood pressure and blood and urine biomarkers associated with increased CVD risk. In addition, we identified endothelial dysfunction in MOR-exposed females as a potential sex-specific contributing risk factor.
OUD during pregnancy has been linked to low birth weight (LBW), preterm birth, birth defects, and NOWS 2–5,18.Several factors associated with pregnancy outcomes have been shown to increase the risk of developing cardiovascular and metabolic diseases 47–49. LBW and catch-up growth are established predictor of CVD risk, catch-up growth, a compensatory weight gain leading to rapid growth in infants or young children who are smaller for their age or adjusted age 50. Causal factors include premature birth, perinatal malnourishment, or placental deficiency resulting in unhealthy growth patterns51. In addition, catch-up growth increases the risk of obesity, insulin resistance, aging, and non-communicable diseases 52.
Our study shows that IUME litters displayed a significantly reduced BW observed during lactation. As a limitation, this outcome does not allow determining whether the reduction in BW is secondary to prenatal programming or a result of a potential effect of opioids on milk production/composition and/or nursing-feeding behavior during lactation53; consequently, it was not feasible to define the origin of the reduced BW during postnatal life. Thus, the design of studies with cross-fostering litters and milk composition assessment could provide insights into how IUME decreases BW and induces catch-up growth, in addition to studies designed to discern the effect of weigh loss during lactation from the opioids exposure on the cardiovascular phenotype. Also, IUME reduced tibia length through adulthood, suggesting the effect on body length is permanent; however, only IUME male rats showed reduced subcutaneous and visceral fat mass, indicating that the effect of IUME can impact the adipose tissue homeostasis mechanisms in a sex-specific manner. A potential explanation for the reduced fat mass could be given by in vitro studies in human and rodent adipocytes showing that EOPs promote lipolysis54. However, the effect of prenatal opioids exposure on the metabolic programming of adipogenesis, adipocyte differentiation process and/or the sympathetic control of adipose tissue lipolytic function lipogenesis has not been systematically investigated. Therefore, one of the limitations of this study is the unfeasibility to define the origin of the reduced BW during postnatal life. Therefore, future studies will be needed to discern the effect of weight loss during lactation from opioid exposure on the cardiovascular phenotype.
OUD has been associated with the development of metabolic-like syndrome13. Tuduri et al. have shown that μ-OR stimulation increased insulin resistance and glucose intolerance, while a whole body μ-OR knockout improved glucose tolerance, insulin secretion, fatty acid oxidation, and insulin sensitivity55, blocking α-ADR reduced sympathetic activation and metabolic dysfunction mediated by μ-OR55. It also has been shown that acute and chronic opioid exposure are associated with alterations in lipid profile (increasing LDL, VLDL, aortic cholesterol content, and decreasing HDL), and islet cell damage in the pancreas11,15. Accordingly, our data show that MOR-exposed offspring display a diabetic-like phenotype compatible with prolonged insulin resistance, evidenced by elevated FBG and reduced insulin levels. The contribution of a potential interaction between ORs and ADRs in liver, pancreas and/or adipose tissue from IUME offspring remains under investigation.
Our study showed that hypertension in IUME offspring is most likely from a neurogenic origin supported by reduced blood pressure in response to prazosin treatment and the greater depressor response to mecamylamine, along with the increased NE levels in plasma and urine. Nevertheless, IUME female rats displayed similar levels of NE compared with VEH. This lack in NE increase could be due to multiple factors. Females have reduced adreno-medullary derived synthesis of NE early in life, while after sexual maturity, estradiol can modulate the release, reuptake, and degradation. Therefore, although we found the expected reduced NE levels in females compared to males, IUME females may display mechanisms to enable a tight regulation of the NE metabolism despite increased sympathetic outflow. Studies in adult rats have shown that C1 adrenergic neurons in the rostroventrolateral medulla contain μ-ORs play a key role in blood pressure regulation during withdrawal. These neurons maintain tonic and reflex control of arterial pressure and sympathetic vasomotor tone.59 In rat models, chronic administration of morphine is associated with μ-OR redistribution in C1 neurons,58 while precipitated withdrawal induced by a μ-OR antagonist has been shown to reduce the availability of μ-OR in C1 neurons, increasing neuronal activation, which may facilitate the profound sympathetic hyperactivity observed in this model.
The EOS has been shown to interact centrally and peripherally with other GPCRs involved in the modulation of cardiovascular and sympathetic systems 28,61,62. It has been shown that adrenergic and angiotensin receptors dimerize with μ-ORs in brain areas contributing to the pathogenesis of neurogenic hypertension by increasing the sympathetic tone. Further, EOPs can stimulate the release of catecholamines, increasing blood pressure, while α-ADR stimulation can increase the levels of β-endorphins63,64. Spontaneously hypertensive rats (SHR) and other hypertensive animal models overexpress Gi proteins, the G proteins activated by ORs and α-ADRs, and are considered a contributing factor to the pathogenesis of hypertension65,66. Furthermore, knocking out α-ADR eliminated the sympathoexitatory response to cocaine and morphine in mice67, whereas blocking the μ-OR reduces plasma catecholamines and muscle sympathetic activation in humans68. Further studies assessing tissue-specific α-ADRs and ORs expression and/or density, as well as their interaction, will clarify the possible mechanism by which IUME increases sympathetic activation and, consequently, blood pressure in our model. Interactions between ORs and ADRs can happen via ligand stimulation and/or dimerization, initiating a cascade of signaling increasing cardiovascular reactivity.
The heterodimerization of μ-OR can also occur with angiotensin type 1 receptor (AT1R), another GPCR implicated in blood pressure control18,61,69. Numerous studies have investigated the interactions between the RAS and the sympathetic system28,62,70,71. Centrally, EOPs, and morphine infusion inhibit AngII-induced pressor response, an effect that was reversed by naloxone administration. Peripherally, EOPs could indirectly mediate Ang II actions by increasing circulating levels of renin and angiotensin-converting enzyme (ACE). Additionally, ACE plays a role in endogenous opioid signaling by cleaving and degrading EOPs72. Furthermore, naloxone administration attenuated the development of hypertension in the SHR model known to show sympathetic and RAS activation20,28,29. In the current study, the AngII-induced ex-vivo constriction was increased in IUME rats while the expression of AT1R and ACE were downregulated in the aorta,heart, and kidney (data not shown). This finding suggests the increased AngII-induced constriction is most likely facilitated by the ORs, potentially increasing the affinity to AT1R and amplifying the signaling cascade, as we showed that blocking ORs with naloxone can revert this effectherefore, IUME could induce chronic activation of the AT1R potentially enhanced by the heterodimerization with μ-OR, resulting in reduced AT1R expression. Notably, reduced levels of ACE in the vasculature could be linked to impaired degradation of enkephalins, leading to the co-stimulation of dimerized mu and AT1 receptors resulting in a potentiated constrictive effect. Despite these increases in AngII reactivity ex-vivo, IUME significantly attenuated the whole animal dose-response to acute doses of AngII only in female rats. While the isolated aorta is a conductance vessel, the acute pressor response primarily involves the contribution of several vascular beds of resistance vessels combined with the central effects of AngII combined with tissue-specific status of the EOS. Our study identified endothelial dysfunction in MOR-exposed female rats but not in males. Similarly, Cheon et al. reported that female mice showed a greater risk of morphine-induced vascular dysfunction, increased arterial stiffness, and resistance via the cofilin-ERK signaling pathway, suggesting that impaired endothelial function may contribute to the high blood pressure in female rats along with sympathetic activation73. Taken together, the results from our study provide evidence that prenatal exposure to exogenous opioids may alter the programming and sensitivity of the vasculature to AngII in a sex-specific manner. Although the circulating levels of RAS components were similar between VEH and MOR-exposed rats, because of the discrepancy between in-vivo and ex-vivo responses, differences in RAS peptides at the central nervous system level cannot be ruled out.
Pre-clinical reports have shown opiates as important modulators of cardiovascular function in both normotensive and hypertensive states and studies have reported that EOPs are elevated in models of hypertension of various etiologies, including genetic and renovascular hypertension29,74. The levels of EOPs fluctuate during the lifespan. Physiologically, β-endorphin plasma levels increase during pregnancy from week 6 (20 fmol/mL) to delivery (>120 fmol/mL) 75. By PD5, EOPs levels in neonates and mothers decrease to physiological levels 75. However, β-endorphin and met-enkephalin levels remain increased in opioid-exposed infants 40 days after birth75, and continue to increase about 100-fold than young age-matched controls75,76. Interestingly, pharmacologic treatments to reduce NOWS did not affect EOPs levels, and there was no relationship between NOWS symptom severity and EOPs plasma levels77. ORs are expressed in the placenta and the brain of neonates in gestational weeks 12-13, where EOPs modulate brain development 78,79. Therefore, prolonged exogenous stimulation of opioids could contribute to decreasing the energy level required for initiating an active cascade of GPCR signaling76,80,81. These data support that exogenous prenatal opioid exposure could cause alterations in the EOS that have lifelong effects on cardiovascular and metabolic function. Therefore, fetal programming of the EOS could contribute to the phenotype depicted in our study and induce cardiovascular dysfunction and affect exogenous opioids response later in life, noting that specific periods of life and lengths of exposure affect the EOS status in a different manner.
In order to investigate the long-term behavioral consequences of IUME, we conducted a locomotor activity test in adult rats. Similar to a previous finding82, there was no difference in baseline locomotor activity between VEH and MOR prenatal exposed groups. In addition, a morphine dose (10mg/kg) known to produce acute hypoactivity 82 showed no difference in response between in-utero VEH and MOR groups. However, in response to repeated MOR exposure, healthy VEH rats became tolerant to the hypoactivity effect of MOR, whereas tolerance development was delayed in IUME rats. Thus, the neurobehavioral mechanism underlying tolerance to the behavioral effect of morphine appears to be impaired by IUME. While the mechanism underlying tolerance is not well understood, perhaps long-lasting alterations in opioid receptor densities in dopaminergic brain regions involved in locomotion may play a role83.
Of note, this animal model does not rely on self-administration, an important component of OUD in humans; our study approach involved experimenter-delivered MOR. We used escalating doses to mimic the development of tolerance, one of the key features of OUD in humans, and the pain management regimens used in the clinical setting. Our primary goal was to model the effects of IUME on the offspring’s health outcomes, providing a valuable tool for testing potential interventions and therapeutic approaches, providing critical insight into the potential underlying mechanisms in the programming of hypertension in response to opioid exposure.
In conclusion, our study shows that in-utero opioid exposure affects blood pressure regulation and vascular function in adult female and male rats, increasing their future CVD risk. We showed that prenatal MOR exposure induces the dysregulation of EOPs expression in the heart, aorta, kidney, and liver and the sensitization to secondary MOR exposures and increased CVD biochemical risk factors. Understanding the molecular mechanisms by which morphine, a well-characterized opioid, impacts cardiovascular function will provide valuable fundamental knowledge to prevent and treat OUD-related cardiovascular comorbidities. In addition, this model will allow the investigation of mechanisms in opioids such as fentanyl, currently at the top of overdose death cases and challenging the conventional therapies to treat OUD and withdrawal syndrome in mothers and newborns.
Perspectives
This is the first study modeling the effect of in-utero opioid exposure on cardiovascular disease risk in female and male adult rats. Morphine exposure could induce changes in the EOS that may impact the regulation of cardiovascular function. This model will provide important insights for the mechanistic study of the effects of opioid exposure during developmental stages of life and pregnancy outcomes while establishing a valuable tool for testing interventions and potential therapeutic approaches for mothers and neonates.
Supplementary Material
What Is New?
We established a pre-clinical model to investigate the long-term health effects of in-utero opioid exposure, wallowing us for the testing of potential interventions aiming to improve the pregnancy outcomes and the offspring’s health in addition to testing current therapies
This study pinpoints dysregulation of the endogenous opioid peptides as a potential mechanism by which in-utero opioid exposure increases the cardiovascular disease risk in the adult offspring
The identification of potential sex-specific risk to develop endothelial dysfunction and vascular reactivity that warrants the investigation of personalized approaches to reduce cardiovascular risk secondary to in-utero opioid exposure
What Is Relevant?
It is challenging to predict the long-term impact on the cardiovascular health of children from women with opioid misuse, OUD, and opioid overdoses due to the lack of follow-ups later in life. This study provides insights regarding how opioids could affect the programming of the mechanisms regulating cardiovascular function.
Investigating further the interaction of EOS with the mechanisms implicated in blood pressure regulation are required to understand potential targets for interventions.
Understanding the effect of early-life opioid exposure on the endogenous opioid system programming may help identify potential biomarkers of risk factors for CVD. This study also highlights the importance of screening for in utero opioid exposure as a factor for increasing CVD risk.
Pathophysiological Implications?
Prenatal morphine exposure affects fetal programming of the EOS and systems implicated in the regulation of cardiovascular and metabolic function
Individuals with a history of prenatal opioid exposure can present comorbidities with sex-specific etiologies
Along with the effect of morphine on the overall cardiovascular function, we identified a sex-specific dysregulation of the vascular function that involves mechanisms relevant to potential personalized medicine approach.
Summary
Opioids use during pregnancy profoundly affects the adult offspring’s cardiovascular function, activating the sympathetic system, increasing blood pressure, and impairing vascular function. The endogenous opioid system dysregulation secondary to the in-utero exposure to morphine could be mediating the overactivation of the principal central and peripheral systems in blood pressure regulation.
Acknowledgments
We thank Dr. Michelle Lofwall for providing expertise in the clinical aspects of substance misuse research.
Sources of Funding:
This study was supported by the Department of Pharmacology and Nutritional Sciences and pilot projects from Alliance for Diabetes and Obesity Research (ADORE), Substance Use Research Priority Area (SUPRA), and the Center of Research in Obesity and Cardiovascular Disease COBRE (P20 GM103527).
Nonstandard abbreviations:
- MOR
Morphine
- NOWS
Neonatal Opioid Withdrawal Syndrome
- IUME
In Utero Morphine Exposure
- OUD
Opioid Use Disorder
- EOPs
Endogenous Opioid Peptides
- EOS
Endogenous Opioid System
- RAS
Renin Angiotensin System
- BW
Body Weight
Footnotes
Disclosures: None
References
- 1.Centers for Disease Control and Prevention NCfIPaC. Drug Overdose. 2022; https://www.cdc.gov/drugoverdose/deaths/index.html. Accessed October, 2022.
- 2.Prevention CfDCa. Pregnancy. 2021; https://www.cdc.gov/pregnancy/opioids/data.html. Accessed October 2022.
- 3.Hirai AH, Ko JY, Owens PL, Stocks C, Patrick SW. Neonatal Abstinence Syndrome and Maternal Opioid-Related Diagnoses in the US, 2010-2017. JAMA. 2021;325(2):146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetzl L, Thompson-Felix T, Darbinian N, et al. Novel biomarkers to assess in utero effects of maternal opioid use: First steps toward understanding short- and long-term neurodevelopmental sequelae. Genes Brain Behav. 2019;18(6):e12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weller AE, Crist RC, Reiner BC, Doyle GA, Berrettini WH. Neonatal Opioid Withdrawal Syndrome (NOWS): A Transgenerational Echo of the Opioid Crisis. Cold Spring Harb Perspect Med. 2021;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran EL, Kim SY, England LJ, et al. The MATernaL and Infant NetworK to Understand Outcomes Associated with Treatment of Opioid Use Disorder During Pregnancy (MAT-LINK): Surveillance Opportunity. J Womens Health (Larchmt). 2020;29(12):1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow SL, Sasson C, Benjamin IJ, et al. Opioid Use and Its Relationship to Cardiovascular Disease and Brain Health: A Presidential Advisory From the American Heart Association. Circulation. 2021;144(13):e218–e232. [DOI] [PubMed] [Google Scholar]
- 8.Stevens S, Mohan S. Opioid withdrawal behavior in spiny mice: A novel preclinical model of neonatal opioid withdrawal syndrome (NOWS). Heliyon. 2021;7(4):e06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi R, Majmundar M, Kansara T, et al. Frequency of Cardiovascular Events and In-hospital Mortality With Opioid Overdose Hospitalizations. Am J Cardiol. 2019;124(10):1528–1533. [DOI] [PubMed] [Google Scholar]
- 10.Singleton JH, Abner EL, Akpunonu PD, Kucharska-Newton AM. Association of Nonacute Opioid Use and Cardiovascular Diseases: A Scoping Review of the Literature. Journal of the American Heart Association. 2021;10(13):e021260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawal H, Patel BM. Opioids in Cardiovascular Disease: Therapeutic Options. Journal of Cardiovascular Pharmacology and Therapeutics. 2018;23(4):279–291. [DOI] [PubMed] [Google Scholar]
- 12.Weerakoon SM, Chen B, Harrell MB, Vidot DC, Messiah SE. Effect of in-utero polysubstance exposure on adolescent cardiovascular disease risk: Results from the Maternal Lifestyle Study. Progress in Pediatric Cardiology. 2022;66:101528. [Google Scholar]
- 13.Kazemi M, Bazyar M, Naghizadeh MM, et al. Lipid profile dysregulation in opium users based on Fasa PERSIAN cohort study results. Scientific Reports. 2021;11(1):12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mysels DJ, Sullivan MA. The relationship between opioid and sugar intake: review of evidence and clinical applications. J Opioid Manag. 2010;6(6):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafipour H, Beik A. The Impact of Opium Consumption on Blood Glucose, Serum Lipids and Blood Pressure, and Related Mechanisms. Frontiers in Physiology. 2016;7(436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Balhara Y. Opioid use and diabetes: An overview. Journal of Social Health and Diabetes. 2016;4:6. [Google Scholar]
- 17.Fagerholm V, Haaparanta M, Scheinin M. α2-Adrenoceptor Regulation of Blood Glucose Homeostasis. Basic & Clinical Pharmacology & Toxicology. 2011;108(6):365–370. [DOI] [PubMed] [Google Scholar]
- 18.Asdjodi S, Rubarth RB, Hardy J, Lee H. The Effects of Opioids During Pregnancy: A Literature Review. Georgetown Medical Review. 2020;4(1):16759. [Google Scholar]
- 19.Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: A common substrate in drug addiction. Drug and Alcohol Dependence. 2010;108(3):183–194. [DOI] [PubMed] [Google Scholar]
- 20.Levin ER, Mills S, Weber MA. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides. 1986;7(6):977–981. [DOI] [PubMed] [Google Scholar]
- 21.Root-Bernstein R, Turke M, Subhramanyam UKT, Churchill B, Labahn J. Adrenergic Agonists Bind to Adrenergic-Receptor-Like Regions of the Mu Opioid Receptor, Enhancing Morphine and Methionine-Enkephalin Binding: A New Approach to “Biased Opioids”? Int J Mol Sci. 2018;19(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Zhang J-T, Hang L, Liu T. Mu opioid receptor heterodimers emerge as novel therapeutic targets: recent progress and future perspective. Frontiers in Pharmacology. 2020:1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont AG, Légat L. GABA is a mediator of brain AT1 and AT2 receptor-mediated blood pressure responses. Hypertension Research. 2020;43(10):995–1005. [DOI] [PubMed] [Google Scholar]
- 24.Mendonça MM, Santana JS, da Cruz KR, et al. Involvement of GABAergic and Adrenergic Neurotransmissions on Paraventricular Nucleus of Hypothalamus in the Control of Cardiac Function. Frontiers in physiology. 2018;9:670–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4(2):126–131. [DOI] [PubMed] [Google Scholar]
- 26.Kiritsy-Roy JA, Appel NM, Bobbitt FG, Van Loon GR. Effects of mu-opioid receptor stimulation in the hypothalamic paraventricular nucleus on basal and stress-induced catecholamine secretion and cardiovascular responses. J Pharmacol Exp Ther. 1986;239(3):814–822. [PubMed] [Google Scholar]
- 27.Tian F, Zheng XY, Li J, et al. κ-Opioid Receptor Stimulation Improves Endothelial Function via Akt-stimulated NO Production in Hyperlipidemic Rats. Sci Rep. 2016;6:26807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bali A, Randhawa PK, Jaggi AS. Interplay between RAS and opioids: opening the Pandora of complexities. Neuropeptides. 2014;48(4):249–256. [DOI] [PubMed] [Google Scholar]
- 29.Skiba DS, Szczepaniak P, Siedliński M, et al. Hypertensive Effect of Downregulation of the Opioid System in Mouse Model of Different Activity of the Endogenous Opioid System. Int J Mol Sci. 2021;22(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alipio JB, Brockett AT, Fox ME, et al. Enduring consequences of perinatal fentanyl exposure in mice. Addict Biol. 2021;26(2):e12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrnes EM, Vassoler FM. Modeling prenatal opioid exposure in animals: Current findings and future directions. Front Neuroendocrinol. 2018;51:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–186. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- 34.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. [DOI] [PubMed] [Google Scholar]
- 35.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loria AS, Osborn JL. Maternal separation diminishes α-adrenergic receptor density and function in renal vasculature from male Wistar-Kyoto rats. Am J Physiol Renal Physiol. 2017;313(1):F47–f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leachman JR, Cincinelli C, Ahmed N, et al. Early life stress exacerbates obesity in adult female mice via mineralocorticoid receptor-dependent increases in adipocyte triglyceride and glycerol content. Life Sci. 2022;304:120718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension. 2011;58(4):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez-Sanroman DB, Arlington Wilson G, Bardo MT. Effects of Social Isolation on Perineuronal Nets in the Amygdala Following a Reward Omission Task in Female Rats. Mol Neurobiol. 2021;58(1):348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Basu R, Poglitsch M, Bakal JA, Oudit GY. Elevated Angiotensin 1-7/Angiotensin II Ratio Predicts Favorable Outcomes in Patients With Heart Failure. Circ Heart Fail. 2020;13(7):e006939. [DOI] [PubMed] [Google Scholar]
- 41.Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J Am Coll Cardiol. 2017;69(7):805–819. [DOI] [PubMed] [Google Scholar]
- 42.Haschke M, Schuster M, Poglitsch M, et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet. 2013;52(9):783–792. [DOI] [PubMed] [Google Scholar]
- 43.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. Journal of proteome research. 2010;9(10):5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon SM, Chung JH, Playford MP, et al. High density lipoprotein proteome is associated with cardiovascular risk factors and atherosclerosis burden as evaluated by coronary CT angiography. Atherosclerosis. 2018;278:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood AT, Hall MR. Reversed-phase high-performance liquid chromatography of catecholamines and indoleamines using a simple gradient solvent system and native fluorescence detection. J Chromatogr B Biomed Sci Appl. 2000;744(1):221–225. [DOI] [PubMed] [Google Scholar]
- 46.Mercolini L, Gerra G, Consorti M, Somaini L, Raggi MA. Fast analysis of catecholamine metabolites MHPG and VMA in human plasma by HPLC with fluorescence detection and a novel SPE procedure. Talanta. 2009;78(1):150–155. [DOI] [PubMed] [Google Scholar]
- 47.Dong M, Zheng Q, Ford SP, Nathanielsz PW, Ren J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. Journal of Molecular and Cellular Cardiology. 2013;55:111–116. [DOI] [PubMed] [Google Scholar]
- 48.Palinski W Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation. 2014;129(20):2066–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drake AJ, Reynolds RM. Focus on obesity: Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140:387–398. [DOI] [PubMed] [Google Scholar]
- 50.Verduin ML. Update on the Treatment of Opioid Use Disorders in Pregnancy. Focus (Am Psychiatr Publ). 2017;15(4):10s–11s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life sciences. 2011;89(13–14):417–421. [DOI] [PubMed] [Google Scholar]
- 52.Arisaka O, Ichikawa G, Koyama S, Sairenchi T. Childhood obesity: rapid weight gain in early childhood and subsequent cardiometabolic risk. Clin Pediatr Endocrinol. 2020;29(4):135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito S Opioids in Breast Milk: Pharmacokinetic Principles and Clinical Implications. J Clin Pharmacol. 2018;58 Suppl 10:S151–s163. [DOI] [PubMed] [Google Scholar]
- 54.Vettor R, Pagano C, Fabris R, Lombardi AM, Macor C, Federspil G. Lipolytic effect of beta-endorphin in human fat cells. Life Sci. 1993;52(7):657–661. [DOI] [PubMed] [Google Scholar]
- 55.Tudurí E, Nogueiras R. Mu opioid receptor: from pain to glucose metabolism. Oncotarget. 2017;8(4):5643–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Adrenaline synthesizing neurons in the rostral ventrolateral medulla: a possible role in tonic vasomotor control. Brain Res. 1983;273(2):356–361. [DOI] [PubMed] [Google Scholar]
- 57.Milner TA, Hernandez FJ, Herrick SP, Pierce JP, Iadecola C, Drake CT. Cellular and subcellular localization of androgen receptor immunoreactivity relative to C1 adrenergic neurons in the rostral ventrolateral medulla of male and female rats. Synapse. 2007;61(5):268–278. [DOI] [PubMed] [Google Scholar]
- 58.Drake CT, Aicher SA, Montalmant FL, Milner TA. Redistribution of mu-opioid receptors in C1 adrenergic neurons following chronic administration of morphine. Exp Neurol. 2005;196(2):365–372. [DOI] [PubMed] [Google Scholar]
- 59.Guyenet PG, Stornetta RL, Schreihofer AM, et al. Opioid signalling in the rat rostral ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29(3):238–242. [DOI] [PubMed] [Google Scholar]
- 60.Baraban SC, Stornetta RL, Guyenet PG. Respiratory control of sympathetic nerve activity during naloxone-precipitated morphine withdrawal in rats. J Pharmacol Exp Ther. 1993;265(1):89–95. [PubMed] [Google Scholar]
- 61.Goldfarb SS, Stanwood GD, Flynn HA, Graham DL. Developmental opioid exposures: Neurobiological underpinnings, behavioral impacts, and policy implications. Exp Biol Med (Maywood). 2020;245(2):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones JE, Jurgens JA, Evans SA, Ennis RC, Villar VAM, Jose PA. Mechanisms of Fetal Programming in Hypertension. International Journal of Pediatrics. 2012;2012:584831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bozkurt B Enkephalins and the Opioid System of the Heart. Circulation: Heart Failure. 2019;12(5):e005851. [DOI] [PubMed] [Google Scholar]
- 64.Schaz K, Stock G, Simon W, et al. Enkephalin effects on blood pressure, heart rate, and baroreceptor reflex. Hypertension. 1980;2(4):395–407. [DOI] [PubMed] [Google Scholar]
- 65.Anand-Srivastava MB. Modulation of Gi Proteins in Hypertension: Role of Angiotensin II and Oxidative Stress. Curr Cardiol Rev. 2010;6(4):298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dehe L, Shaqura M, Nordine M, et al. Chronic Naltrexone Therapy Is Associated with Improved Cardiac Function in Volume Overloaded Rats. Cardiovasc Drugs Ther. 2021;35(4):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004;20(11):3073–3084. [DOI] [PubMed] [Google Scholar]
- 68.Kienbaum P, Heuter T, Michel Martin C, Scherbaum N, Gastpar M, Peters J. Sympathetic Neural Activation Evoked by μ-Receptor Blockade in Patients Addicted to Opioids Is Abolished by Intravenous Clonidine. Anesthesiology. 2002;96(2):346–351. [DOI] [PubMed] [Google Scholar]
- 69.Mao C, Shi L, Xu F, Zhang L, Xu Z. Development of fetal brain renin-angiotensin system and hypertension programmed in fetal origins. Prog Neurobiol. 2009;87(4):252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kakall ZM, Nedoboy PE, Farnham MMJ, Pilowsky PM. Activation of μ-opioid receptors in the rostral ventrolateral medulla blocks the sympathetic counterregulatory response to glucoprivation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2018;315(6):R1115–R1122. [DOI] [PubMed] [Google Scholar]
- 71.Arttamangkul S, Plazek A, Platt EJ, et al. Visualizing endogenous opioid receptors in living neurons using ligand-directed chemistry. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trieu BH, Remmers BC, Toddes C, et al. Angiotensin-converting enzyme gates brain circuit–specific plasticity via an endogenous opioid. Science. 2022;375(6585):1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheon S, Tomcho JC, Edwards JM, et al. Opioids Cause Sex-Specific Vascular Changes via Cofilin-Extracellular Signal-Regulated Kinase Signaling: Female Mice Present Higher Risk of Developing Morphine-Induced Vascular Dysfunction than Male Mice. J Vasc Res. 2021;58(6):392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szilagyi JE. Endogenous opiates and the pathogenesis of hypertension. Clin Exp Hypertens A. 1989;11(1):1–24. [DOI] [PubMed] [Google Scholar]
- 75.PANERAI AE, MARTINI A, GIULIO AMD, et al. Plasma β-endorphin, β-lipotropin, and met-enkephalin concentrations during pregnancy in normal and drug-addicted women and their newborn. The Journal of Clinical Endocrinology & Metabolism. 1983;57(3):537–543. [DOI] [PubMed] [Google Scholar]
- 76.Harder HJ, Murphy AZ. Early life opioid exposure and potential long-term effects. Neurobiol Stress. 2019;10:100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Distler W Human Plasma β-Endorphin Levels in Pregnant Women and in Newborns. Paper presented at: Endorphins in Reproduction and Stress; 1990//, 1990; Berlin, Heidelberg. [Google Scholar]
- 78.Rosenfeld CS. The placenta as a target of opioid drugs†. Biology of Reproduction. 2022;106(4):676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ray SB, Wadhwa S. Mu opioid receptors in developing human spinal cord. The Journal of Anatomy. 1999;195(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev. 2008;84(1):29–35. [DOI] [PubMed] [Google Scholar]
- 81.Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen H-H, Chiang Y-C, Yuan ZF, et al. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatric disease and treatment. 2015;11:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vathy I, Šlamberová R, Rimanóczy Á, Riley MA, Bar N. Autoradiographic evidence that prenatal morphine exposure sex-dependently alters μ-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Progress in neuro-psychopharmacology & biological psychiatry. 2003;27(3):381–393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


