Abstract
Angiogenesis, the sprouting of new blood vessels from existing vessels, is one of six known mechanisms employed by solid tumors to recruit blood vessels necessary for their initiation, growth, and metastatic spread. The vascular network within the tumor facilitates the transport of nutrients, oxygen, and immune cells and is regulated by pro- and anti-angiogenic factors. Nearly four decades ago, vascular endothelial growth factor (VEGF) was identified as a critical factor promoting vascular permeability and angiogenesis, followed by identification of VEGF family ligands and their receptors (VEGFRs). Since then, over a dozen drugs targeting the VEGF/VEGFR pathway have been approved for ~20 solid tumor types, usually in combination with other therapies. Initially designed to starve tumors, these agents transiently “normalize” tumor vessels in preclinical and clinical studies, and in the clinic, increased tumor blood perfusion or oxygenation in response to these agents is associated with improved outcomes. Nevertheless, the survival benefit has been modest in most tumor types, and there are currently no biomarkers in routine clinical use for identifying which patients are most likely to benefit from treatment. However, the ability of these agents to reprogram the immunosuppressive tumor microenvironment into an immunostimulatory milieu has rekindled interest and has led to the FDA-approval of 7 different combinations of VEGF/VEGFR pathway inhibitors with immune checkpoint blockers for many solid tumors in the past 3 years. In this review, we discuss our understanding of the mechanisms of response and resistance to blocking VEGF/VEGFR, and potential strategies to develop more effective therapeutic approaches.
INTRODUCTION/ BACKGROUND
The development of new blood vessels from existing vasculature, termed angiogenesis, is a crucial step in the expansive growth and metastasis of tumors. As tumors increase in diameter beyond several millimeters, they become more hypoxic which limits further growth, but the initiation of angiogenesis can enable small, dormant, avascular tumors to rapidly expand. Angiogenesis was proposed as a therapeutic target for cancer in the 1970s, by Dr. Judah Folkman [1], but it was not until years of preclinical and clinical validation later that this hypothesis was fully accepted as a viable theory. However, the original paradigm of targeting angiogenesis to deprive tumors of nutrients for their growth could not explain why anti-angiogenesis improved the efficacy of co-administered cytotoxic drugs that required the blood supply to reach tumors. A potential explanation for this paradox was provided when one of us (R.K.J.) introduced a counterintuitive hypothesis in 2001 that anti-angiogenic drugs – originally developed to starve tumors – can transiently “normalize” tumor vessels, improve perfusion, decrease hypoxia and improve drug delivery and the outcome of concurrent cytotoxic therapy [2–4].
Vascular endothelial growth factor (VEGF or VEGF-A), initially identified as vascular permeability factor (VPF), is one of the major molecules associated with angiogenesis [5–7]. Since these early discoveries, angiogenesis has been found to be regulated by the balance of numerous pro-angiogenic factors such as VEGF-A, basic fibroblast growth factors (bFGF), platelet-derived growth factor (PDGF), and angiopoietin molecules, as well as endogenous angiogenesis inhibitors such as thrombospondin-1. Moreover, in addition to angiogenesis, tumors can establish a vascular network through the multiple non-angiogenic mechanisms, including the recruitment of bone-marrow derived progenitor cells which then differentiate into endothelial cells; co-opting existing vessels; vessel “mimicry” in which channels comprised of tumor cells act as vasculature; transdifferentiation of cancer cells into endothelial cells; and the splitting of blood vessels, a process known as intussusception – that can confer resistance to antiangiogenic therapy [8].
VEGF/VEGFR family members
VEGF is a 40–45 kDa homodimeric protein secreted by a wide variety of cells in both physiologic and pathologic settings. The VEGF family ligands also include VEGF-B, VEGF-C, VEGF-D, and placenta growth factor (PlGF). VEGF-A is known to be a critical factor modulating endothelial cell sprouting, mitogenesis, cell migration, vasodilation, and vascular permeability. VEGF-B activates embryonic angiogenesis and can increase perivascular cell coverage and mediate metastasis independent of effects via VEGF-A [9]. VEGF-C and VEGF-D regulate lymphangiogenesis, and PlGF is critical for a variety of functions including vasculogenesis, inflammation, wound healing, and survival of certain cancer cells (e.g. medulloblastoma) [10]. VEGF family members exert their biological functions through binding to VEGF receptors (VEGFRs). VEGF-A, VEGF-B, and PIGF binds VEGFR1 primarily localized on blood vascular endothelial cells. VEGFR2 is expressed on both blood vascular and growing lymphatic vessels, and VEGF-A and processed VEGF-C/VEGF-D are ligands for this receptor. VEGFR3 serves as a receptor for VEGF-C and VEGF-D and is expressed on blood vascular and lymphatic endothelial cells. Neuropilins-1 and −2 (NRP-1 and NRP-2) also serve as co-receptors for VEGF ligands [10]. VEGF is comprised of 8 exons and alternative splicing of its mRNA yields six distinct transcripts of 111, 121, 145, 165, 189, and 206 amino acids. VEGF165 is the predominant molecular species produced by normal and cancer cells. There are also multiple anti-angiogenic splice isoforms of VEGF, VEGFxxxb, which can counteract activity of their respective pro-angiogenic spliced isoforms. Tumors may overexpress proangiogenic isoforms but downregulate expression of anti-angiogenic isoforms. For example, VEGF165b is a dominant isoform which can act as a competitive inhibitor to VEGF165 [11]. Moreover, studies have identified specific splicing factors which regulate expression of these isoforms and could serve as therapeutic targets to inhibit tumor angiogenesis [12, 13].
Regulation of VEGF expression
VEGF-A is upregulated in tumors by both hypoxia-dependent and -independent mechanisms. In response to hypoxic stress, hypoxia-inducible factor (HIF) is stabilized and directly promotes VEGF-A transcription [14–16]. The hypoxia-dependent accumulation of HIF-1α protein is mediated by a mechanism through which in low oxygen conditions HIF-1α becomes de-hydroxylated and in turn is no longer targeted for degradation by the von Hippel-Lindau (VHL) protein. The 2019 Nobel Prize in Physiology and Medicine was awarded to Drs. Kaelin, Ratcliffe, and Semenza for key discoveries in this area. The HIF pathway, and thus VEGF-A secretion, can be deregulated by genomic alterations in the VHL gene, most commonly observed in renal cell carcinoma (RCC) and von-Hippel Lindau disease. Moreover, abnormal activation of receptor tyrosine kinases in tumor cells can also trigger hypoxia-independent increases in HIF-1α and VEGF-A. For example, in non-small cell lung cancer (NSCLC), EGFR activating mutations increase HIF-1α levels and in turn drive VEGF-A production even in normoxic conditions [17–20]. It has been proposed that this enhanced VEGF-A production via hypoxia-independent activation of HIF-1α promotes a more “VEGF-dependent” phenotype, and greater responsiveness to VEGF-A inhibition, in RCC and EGFR-mutant NSCLC [15, 17, 21, 22]. Studies in neuroblastoma have identified that signaling through multiple receptor tyrosine kinases including RET, PDGFR-α and-β, c-Kit, EGFR, and VEGFR-1, −2 and −3 can drive HIF1α and 2α expression in both normoxic and hypoxic conditions [23]. Finally, there are several transcription factors which can directly regulate production of VEGF-A. AP-1, Sp-1, C/EBPB, STAT3, and 17β-estradiol (E2) [24, 25] are known effectors which can modulate transcription by binding to the VEGF-A promoter [26].
Downstream VEGF signaling
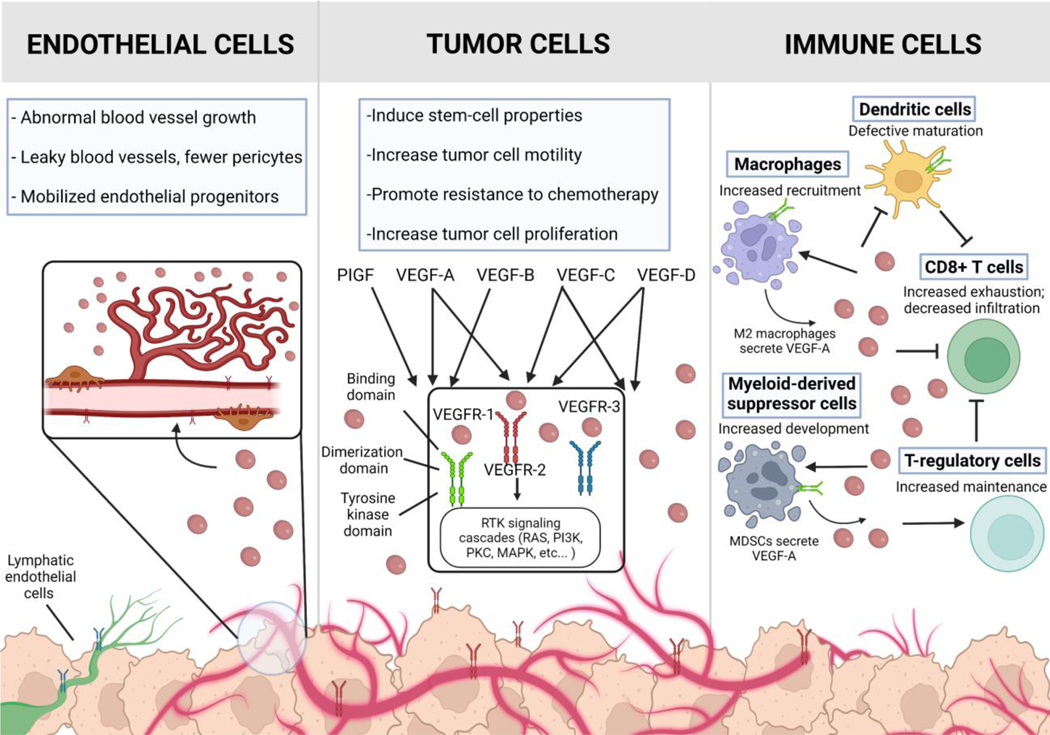
VEGF family members exert their biological effects by binding to VEGFR on target cells and activating downstream signaling pathways (Fig 1). VEGF-A binds VEGFR-1 with a higher affinity than VEGFR-2 though most functional activity results from VEGF-A binding to VEGFR-2 on the tumor endothelium. Ligand binding and receptor dimerization triggers VEGFR-2 auto-phosphorylation [27], and activation of downstream signal transduction including the PI3K, PLC-γ, Akt, Ras, and MAPK pathways promoting proliferation, cell survival, migration, permeability, differentiation, modulation of cell-adhesion molecules [28] and other effects [29]. AXL is a known mediator of VEGF-A dependent activation of PI3K/Akt signaling and the associated changes in vascular permeability [30]. VEGFR signaling is also regulated by the presence of co-receptors such as NRP-1 [31] and co-activating proteins as well as crosstalk with other receptors including fibroblast growth factor receptor (FGFR), AXL, and the Hippo pathway.
Figure 1. The effect of VEGF on the function and growth of endothelial, tumor, and immune cells in the tumor microenvironment.
VEGF drives the formation of new blood vessels within the tumor in part by stimulating endothelial cell sprouting, mitogenesis, and endothelial cell migration. VEGF is not only produced by cancer cells, but also stromal cells, including endothelial cells and fibroblasts. VEGFR-1 is mostly expressed on myeloid cells; VEGFR-2 is primarily expressed on endothelial and sporadically on tumor cells; VEGFR-3 is primarily expressed on lymphatic endothelial cells. Original figure created with Biorender.
MECHANISMS
Impact of VEGF signaling in tumor biology
The initial therapeutic targeting of the VEGF/VEGFR pathway was accomplished by Ferrara and colleagues using a monoclonal antibody against VEGF-A [32]. VEGF blockade resulted in a decrease in tumor vessels, supporting the hypothesis that inhibition of tumor angiogenesis- and the ensuing reduction in tumor perfusion- was the underlying mechanism of tumor growth inhibition. Subsequent studies, however, demonstrated that this hypothesis fails to capture the complex and varied effects of VEGF-A inhibition, especially how anti-VEGF-A therapy can improve the outcome of concurrently administered cytotoxic therapy that requires blood vessels for drug delivery. As noted previously, based on the “vascular normalization” model, VEGF-A inhibitors can transiently normalize the abnormal structure and function of inefficient, tortuous tumor vessels, making them at least temporarily more efficient for oxygen and drug delivery [2, 3]. This model was not accepted initially – as it was against the prevailing hypothesis at that time about using anti-VEGF-A therapy to starve tumors. However, as discussed in more detail below, extensive preclinical and clinical studies provided compelling support for this concept and demonstrated that patients whose tumor perfusion initially increases with anti-VEGF/R agents have better PFS and OS [33, 34]. VEGF-A is also known to directly induce tumor cell invasiveness and motility [23, 35] and to promote immunosuppression in the tumor microenvironment [36]. The major effects VEGF-A signaling on endothelial, tumor, and immune cells are illustrated in Figure 1 and detailed below.
VEGF modulates proliferation of endothelial cells, vascular permeability and recruitment of circulating endothelial progenitors
VEGF-A was initially found to regulate endothelial sprouting, migration and differentiation, predominantly through the binding of VEGF-A and VEGFR-2 [27]. Subsequent studies showed that tumors commonly produced high levels of VEGF-A, often driven by tumor-associated hypoxia, and that such vessels take on an aberrant and tortuous pattern [2]. In addition, VEGF-A increases the microvascular permeability and endothelial fenestrations [37], in part through modulation of adhesion factors, P-selectin, E-selectin, ICAM-1, and inducing synthesis of platelet-activating factor (PAF) [28, 38, 39]. Moreover, autocrine VEGF-A signaling by endothelial cells promotes the development of endothelial cell networks, required to support tumor growth [40]. Other sources of VEGF-A include activated fibroblasts [26], endothelial cells [40], and immune cells [41].
VEGF-A can also initiate the recruitment of circulating endothelial progenitor cells (CEPs), which originate in the bone marrow, and can be incorporated into existing vasculature to promote tumor angiogenesis [42–45]. In addition to CEPs, mature circulating endothelial cells (CECs) can be shed from tumor vasculature, a process enhanced by drugs targeting tumor vasculature [46–50]. For this reason, there have been investigations into the utility of CECs and CEPs as biomarkers for the efficacy of agents targeting the VEGF/VEGFR pathway although this approach has not been validated clinically [51, 52].
VEGF acts directly on tumor cells and promotes an aggressive phenotype
It is well known that tumor hypoxia – potentially exacerbated by anti-VEGF/R therapies – can induce EMT, invasiveness and metastatic spread [33]. More recently, there is a growing appreciation that VEGF-A may also exert direct effects on tumor cells via VEGFRs, including the induction of self-renewing cancer stem cells [53]. Preclinical studies in breast cancer have also shown that VEGF/NRP1-mediated activation of the Wnt/β-catenin pathway [54] and VEGF/NRP2-mediated activation of the Hippo pathway [55] induce cancer cell stemness. Other reports have associated epithelial to mesenchymal transition (EMT)-driven secretion of VEGF-A with the propagation of cancer stem cells and increased tumor angiogenesis [56]. Moreover, VEGF-A signaling can directly promote tumor invasiveness [57], motility [58], and migration [59–62]. Studies in colorectal cancer have identified that intracrine VEGF-A signaling mediates cancer cell migration and invasion [58], and in preclinical NSCLC models, tumor cell growth was found to be mediated by autocrine VEGF-A signaling through co-receptor NRP-1 [62]. In addition, VEGF/VEGFR signaling in tumor cells is known to activate survival pathways and promote chemotherapeutic resistance [63, 64]. Taken together these studies suggest that, in addition to its effects on the tumor microenvironment, VEGF may directly impact tumor cell invasiveness, stemness, and survival.
Immunosuppressive effects of VEGF
VEGF-A can act as an immunosuppressive cytokine in the tumor microenvironment, impairing the function of multiple immune cell types. Specifically, VEGF-A impairs maturation and antigen presentation of dendritic cells which in turn can inhibit T cell mediated cytotoxic killing [65, 66]. Similarly, VEGF-A can inhibit activation of NF-κB in hematopoietic progenitor cells (HPCs) which are supporting players in the activation of dendritic cell differentiation [67].
VEGF-A exerts effects on macrophage function as well. Macrophages that typically exhibit immunosuppressive activity are labelled as M2 macrophages while M1 macrophages display a more pro-inflammatory phenotype. VEGF-A is a primary cytokine secreted by tumor-associated M2 macrophages, which in turn promote tumor vascularization and remodeling [68]. Tumor associated macrophages are a major source of degradative matrix metalloproteinase proteins which can assist with metastatic spread and angiogenesis [69].
VEGF-A also serves to mobilize and attract myeloid derived suppressor cells (MDSCs) from bone marrow to the periphery. MDSCs express VEGF-A as well as VEGFR1 and VEGFR2 on their surface which supports a positive autocrine feedback loop [70, 71]. Granulocytic-MDSCs are a subpopulation of immune cells which are significantly reduced by combination of VEGF-A blockade and chemotherapy in peripheral blood of NSCLC patients [72].
VEGF-A also inhibits antitumor immunity via multiple mechanisms, including effects on T cells and immunosuppressive myeloid populations [73]. Specifically, in the lung, VEGF-A has been shown to increase VEGFR2 receptor expression on T cells [74]. CD8+ T cell activation can also be directly inhibited by signal transduction pathways activated by VEGF [75, 76]. Immunosuppressive Foxp3+ T-regulatory cells also express VEGFR-2 and can be recruited to the tumor microenvironment via VEGF secreted by tumor cells [77]. Moreover, high secretion of VEGF by tumor cells results in an abnormal vascular network that can decrease CD8+ T cell infiltration to the tumor [78]. This VEGF-induced abnormal vascular network can compromise the trafficking of immune cells and delivery of therapeutics including immunotherapeutic agents to the tumor [79]. Moreover, T cell exhaustion marker expression is induced by exposure to VEGF [80]. Taken together, these findings indicate that within the tumor microenvironment, VEGF promotes an immunosuppressive microenvironment through multiple mechanisms.
Mechanisms of resistance to VEGF/VEGFR targeting
Therapeutic strategies targeting the VEGF/VEGFR pathway were initially expected to reduce tumor perfusion, hindering the ability for cells to utilize oxygen and nutrients for survival. It was initially hoped that drugs targeting this pathway would be less prone to the development of therapeutic resistance because the primary targets of these drugs, tumor endothelial cells, are genetically stable compared to tumor cells [81, 82]. It became clear from early trials of VEGF-A inhibition that despite the clear improvements in clinical outcomes particularly in renal cell [83] and in colorectal [84] and NSCLC [85] when used in combination with chemotherapy, both de novo (primary) and acquired resistance were common. The mechanisms underlying this resistance are varied, and appear to involve both tumor cell intrinsic and tumor microenvironmental factors [86].
Specific tumor genomic alterations may promote resistance to VEGF/VEGFR inhibition, potentially by rendering tumor cells less sensitive to oxygen or nutrient deprivation, or, alternatively, by enhancing VEGF-independent tumor angiogenesis as well as other modes of recruiting blood vessels [2]. For example, p53 inactivation is known to render tumor cells less sensitive to hypoxia-induced apoptosis [87]; alter HIF-1 stability and VEGF-A expression [88, 89]; and suppress endogenous angiogenesis inhibitors such as TSP-1 [90]. Similarly, loss of LKB1 protein, typically via mutations in the STK11 gene, is associated with worse outcomes in patients treated with bevacizumab combinations in NSCLC [89].
In patients that initially respond to VEGF/VEGFR inhibition, acquired resistance may emerge through multiple mechanisms. Treatment-induced hypoxia can upregulate expression of multiple other pro-angiogenic signaling molecules in tumor cells [91–93] or cancer-associated fibroblasts [94] changes that may be associated with a shift to a mesenchymal phenotype [95]. Secretion of IL-6 and FGF-2, HGF [96] as well as induction of DLL4/Notch signaling, have all been associated with resistance to VEGF-A therapy [97, 98].
Host factors may also impact response. Estrogen has been implicated as a driver of resistance to bevacizumab therapy in NSCLC models [99]. Vasculogenic mimicry, the development of networks of endothelial-like cells which have vascular characteristics, may in part shape a tumor microenvironment that acquires resistance to VEGF-A therapy and furthers immunosuppression. Altered blood vessel development including vessel cooption [100], post-natal vasculogenesis, and intussusception also have been demonstrated or implicated in resistance to anti-angiogenic therapy [8]. Resistance to VEGF therapy can also be mediated by upregulation of alternative pro-angiogenic molecules including Fgf1/2, Efna1, Angpt1, and others [101]. Moreover, a study using multiple tumor model systems identified the correlation between VEGF-A resistance and production of IL-17 which assists the mobilization of immunosuppressive MDSC cells to the tumor microenvironment [102]. The recruitment of bone marrow-derived cells (BMDCs) [103] and pro-angiogenic monocytes [104] have also been implicated in the development of resistance to VEGF therapies. Resistance to VEGF/VEGFR targeting agents can also be mediated by increased pericyte coverage of tumor blood vessels [105], increases in other factors which promote tumor invasiveness and metastasis [106], and increased hypoxia-induced autophagy [107]. Taken as a whole, these studies demonstrate that a wide range of tumor cell, microenvironmental, and host factors can impact response and resistance to VEGF/VEGFR inhibitors, highlighting the challenges in identifying biomarkers for predicting response and developing combination regimens that broadly target potential resistance pathways.
CLINICAL APPLICATIONS
Approved VEGF/VEGFR targeting agents
The first VEGF-targeting agent approved for the treatment of cancer was bevacizumab, largely used in combination with chemotherapy (Table 1). Bevacizumab is a recombinant humanized monoclonal antibody approved for the treatment of colorectal cancer, NSCLC, glioblastoma, renal cell carcinoma, cervical cancer, ovarian cancer, fallopian tube cancer, peritoneal cancer, and hepatocellular carcinoma. Additionally, there are 3 bio-similars of bevacizumab which have been recently FDA approved with many others in trial [108]. Ramucirumab is a monoclonal antibody against VEGFR2 and was first approved for the treatment of gastric cancer or gastro-esophageal junction adenocarcinoma [109] and later in NSCLC in combination with docetaxel [110] or, more recently, with erlotinib for patients with tumors bearing EGFR mutations [21]. Other multi-kinase inhibitors approved include sunitinib, sorafenib, and pazopanib (VEGFR-1, VEGFR-2, and VEGFR-3, PDGFR-α/β, and c-kit). These agents have generally demonstrated higher anticancer activity compared to single-target agents. The “off-target” receptor specificity for each inhibitor, as well as their pharmacokinetics and potency, are likely to be key determinants of their clinical activity. Aflibercept is a recombinant fusion protein of VEGFR1 and VEGFR2 which binds to VEGF-A, VEGF-B, and PIGF1 and 2 which has displayed efficacy in metastatic colorectal cancer [111]. Outside of RCC, VEGF inhibitors typically have limited activity as monotherapy and are approved for use in combination with chemotherapy, immunotherapy, and EGFR inhibitors [15, 17, 21, 22] [112, 113], although combinations with inhibitors of FGFR [113], PDGFR [114], MET [115], and Ang1/2 [116, 117] pathways have been explored.
Table 1.
Current VEGF/VEGFR Therapy Approvals including those approved for use in combination with anti-PD-1/PD-L1 Therapy.
| Approved VEGF/VEGFR Drugs | |||
|---|---|---|---|
| Agent | Type | Target | Approved Indications* |
| Bevacizumab | mAb | VEGF-A | CRC♦, NSCLC♦†, platinum-resistant ovarian cancer†, cervical cancer♦, HCC†, breast cancer (Japan and Europe), and glioblastoma |
| Ramucirumab | mAb | VEGFR-2 | Gastric♦, gastro-esophageal junction adenocarcinoma♦, metastatic NSCLC♦ |
| Aflibercept | decoy receptor | VEGF-A, VEGF-B, PIGF | Metastatic CRC♦ |
| Apatinib | RTKI | VEGFR2 | Adenoid cystic carcinoma, gastric (Approved by CDFA) |
| Axitinib | RTKI | VEGFR-1/2/3, PDGFR | RCC† |
| Cabozantinib | RTKI | VEGFR2, MET, RET, AXL, Flt-3, c-Kit | HCC, RCC† |
| Cediranib | RTKI | VEGFR-1/2/3, PDGFR-a, CSF-1R, Flt3 | Ovarian cancer, BRCA mt HER2- metastatic breast cancer |
| Levantinib | RTKI | VEGFR-2/3 | Thyroid cancer, RCC†, HCC, endometrial carcinoma† |
| Nintedanib | RTKI | VEGFR-1/2/3, PDGFR, FGFR-1/3 | Radiopathic pulmonary fibrosis; NSCLC (EU approval) |
| Pazopanib | RTKI | VEGFR2 | RCC, soft tissue sarcoma |
| Regorafenib | RTKI | VEGFR-2/3, PDGFR-b, FGFR-1/2, c-Kit, RET, B-Raf | CRC, HCC, GIST |
| Sorafenib | RTKI | VEGFR-2/3, PDGFR-b, Flt-3, c-Kit, B-Raf | RCC, hepatocellular cancers†, metastatic differentiated thyroid carcinoma |
| Sunitinib | RTKI | VEGFR-1/2/3, PDGFR, Flt-3, RET | RCC, GIST, NE tumors of the pancreas |
| Vandetanib | RTKI | VEGFR-2, EGFR, RET | MTC |
| Agents approved in Combination with anti-PD1/PD-L1 therapy | ||
|---|---|---|
| Agents | Disease | FDA approval |
| Bevacizumab/ Atezolizumab/ Chemotherapy | NSCLC | 2018 [148] |
| Atixinib/Pembrolizumab | RCC | 2019 [149] |
| Axitinib/Avelumab | RCC | 2019 [150] |
| Bevacizumab/Atezolizumab | HCC | 2020 [151] |
| Cabozantinib/Nivolumab | RCC | 2021 [152] |
| Lenvatinib/Pembrolizumab | Endometrial Cancer (not including MSI-H or dMMR) | 2021 [153] |
| Lenvatinib/Pembrolizumab | RCC | 2021 [154] |
mAb, monoclonal antibody; mt, mutant; RTKI, receptor tyrosine kinase inhibitor; CRC, colorectal cancer; RCC, renal cell carcinoma, GIST, gastrointestinal stromal tumors; NE, neuroendocrine; HCC, hepatocellular carcinoma; MTC, medullary thyroid cancer; EU, European Union. CDFA, China General Administration of Food Drug Administration
FDA-approved unless otherwise indicated.
Indicates agent is approved in combination with cytotoxic therapy.
Indicates agent is approved in combination with immunotherapy.
Combinations of VEGF/VEGFR therapy with chemotherapy
Preclinical and clinical studies have demonstrated that anti-angiogenic therapy improves the efficacy of cytotoxic therapies [118, 119]. This might seem counterintuitive given that inhibition of angiogenesis may be expected to reduce drug delivery to tumors. However, the tumor vasculature is known to develop as a disorganized network of abnormal, tortuous vessels during tumorigenesis. These abnormal vascular structures are hyperpermeable and leaky leading to high interstitial fluid pressure [33], which ultimately impairs drug delivery to tumor cells. In preclinical models and in rectal cancer in patients, VEGF/VEGFR inhibition increased mature, pericyte-covered vessels [3, 33, 120], resulting in a vascular normalization or remodeling and decreased tumor interstitial fluid pressure [3, 51, 121–123]. VEGF-A inhibition has also been found to enhance chemotherapy-induced endothelial apoptosis [124]. Combinations of VEGF/VEFR inhibitors with chemotherapy have demonstrated clear benefit in a variety of solid tumor types (Table 1). Moreover, a number of clinical studies have highlighted that patients treated with anti-VEGF/VEGFR therapy alone or in combination with chemotherapy who have increased tumor blood perfusion or oxygenation have better clinical outcomes (Table 2).
Table 2.
Increased blood flow is correlated with better clinical outcome for patients treated with anti-VEGF therapy.
| Agents | Disease | Imaging parameter | Clinical Outcome |
|---|---|---|---|
| Cediranib | Recurrent GBM | Increased blood flow (MRI) | Increased PFS and OS [155] |
| Cediranib + chemoradiotherapy | Newly diagnosed GBM | Increased blood flow (MRI) | Increased PFS and OS [156] |
| Bevacizumab alone and then with chemotherapy | Advanced NSCLC | Increased blood flow (dCT after bevacizumab single agent) |
Increased ORR [157] |
| Neoadjuvant bevacizumab alone and then with chemotherapy | Chemo-naïve breast cancer | Increased oxygenation (FMISO-PET after bevacizumab single agent) |
Increased ORR [158] |
| Neoadjuvant bevacizumab alone and then with chemotherapy | TNBC | Vessel density and pericyte coverage (IHC in serial biopsies after bevacizumab single agent) |
Path response (Miller-Payne score) [159] |
GBM, glioblastoma; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; TNBC, triple negative breast cancer. PFS, progression-free survival; OS, overall survival; ORR, objective response rate.
VEGF/VEGFR therapy in combination with immune checkpoint inhibitors
Immune checkpoint inhibitors prevent the binding of checkpoint proteins (PD-1, CTLA-4, and LAG-3) on T cells, which are negative regulators of immune response, to their binding partners expressed on tumor cells, thus preventing tumor immune evasion. Since its first approval over a decade ago, immune checkpoint blockade (ICB) therapy has provided durable clinical benefit for many cancer patients but the majority do not initially respond and the determinants of response within the tumor microenvironment remain incompletely understood. The VEGF/VEGFR pathway has emerged as an important regulator of the tumor microenvironment because of the interactions between immune cells and vasculature as well as direct effects of the VEGF/VEGFR pathway on immune cells detailed above. VEGF/VEGFR inhibition would therefore be predicted to enhance the effects of various immunotherapies, including ICB, through multiple mechanisms, a hypothesis supported by multiple preclinical studies [79, 125, 126]. These preclinical results have informed the design of clinical trials on combination of VEGF/VEGFR agents with ICB which ultimately led to 7 FDA approvals in the past 3 years (Table 1). The majority of ongoing clinical trials using VEGFR/VEGFR therapeutics are evaluating these agents in combination with ICB.
Emerging therapeutics to target the VEGF/VEGFR pathway
Given the importance of the VEGF/VEGFR pathway for multiple aspects of tumor growth and progression, there continues to be novel VEGF/VEGFR-targeting therapies in development. Sulfatinib, also known as surufatinib, is a novel multi-target inhibitor of VEGFR-1/2/3, FGFR1, and CSF1R. There have been multiple phase II (NCT02267967, NCT02614495) and phase III (NCT02588170) [127] which have demonstrated its clinical efficacy in patients with solid tumors with more trials ongoing (NCT04579757, NCT02549937). Additionally, concurrent inhibition of VEGF-A signaling in combination with other targets such as c-MET is being explored and has shown promising activity in preclinical studies, including models resistant to VEGF/VEGFR inhibition [128]. Other therapies under initial stages of development include bispecific antibodies like CTX-009 which simultaneously targets delta-like ligand 4 and VEGF-A which is currently being evaluated in additional trials (NCT03292783, NCT04492033). Finally fruquintinib, an inhibitor of VEGFR1/2/3 is being evaluated in phase II (NCT04866108) and phase III (NCT04866108, NCT04322539) trials [129, 130]. Other anti-angiogenic treatments have targeted alternate drivers of angiogenesis beyond VEGF including fibroblast growth factors (FGFs) [131], angiopoietins [116], platelet-derived growth factor (PDGF) [93], hepatocyte growth factor (HGF)/c-MET [132], and placental growth factor (PIGF) [10, 133]. Agents targeting these angiogenic factors have displayed anti-tumor activity in preclinical models. More recently, co-targeting agents have been utilized such as the novel humanized VEGF/ANG2 bi-specific monoclonal antibody which demonstrated an acceptable safety and tolerability profile in a recent first-in-human phase I study in patients with advanced solid tumors [134].
Additional innovative approaches for targeting VEGF/VEGFR signaling are in development. This includes decoy receptor fusion proteins conbercept, which binds VEGFB, PIGF, and VEGFA, and VEGF-grab, which bind to VEGF-A and PIGF. These have both shown promising results in inhibiting tumor growth in models of colon cancer [135, 136]. Monoclonal antibodies such as brolucizumab (DLX1008) have demonstrated pre-clinical activity in Kaposi sarcomas [137], and bispecific antibodies are being evaluated including those which target VEGF-A in combination with Ang-2, PD(L)1, or DLL4 [138–140]. Novel tyrosine kinase inhibitors against VEGFRs like anlotinib, tivozanib, and YLT192 are also being currently investigated [141–143].
FUTURE DIRECTIONS
It is a testament to the progress in the field that there are now more than a dozen VEGF/VEGFR inhibitors approved for routine clinical use (Table 1), covering the majority of common solid tumor types including NSCLC, CRC, RCC, and hepatocellular cancer. At the same time, the magnitude of benefit in most of these tumor types has been relatively modest, and there remain gaping deficiencies in our knowledge about how to optimally use these drugs. For example, to date, there remain no biomarkers in routine clinical use for selecting which patients are likely to benefit from treatment with these drugs, and in many cases it is unclear whether the benefits of treatment occur through effects on tumor angiogenesis, the immune microenvironment, the cancer cells themselves, or some combination of the three (Fig. 1). This remains an active areas of investigation, as the potential use of VEGF-A, VEGFRs, P1GF, IL-6, IL-8, FGF-2, and other angiogenic markers continue to be evaluated as biomarkers to predict clinical outcome and response to VEGF/VEGFR targeted therapies [144–147]. Moving forward, the emergence of next-generation genomic profiling, which is done routinely for many tumor types, as well as growing use of transcriptomic profiling provide the opportunity to examine the association of specific oncogenic drivers (e.g. KRAS, HER2, EGFR), tumor suppressors (e.g. p53, LKB1), or transcriptionally-defined phenotypes (e.g. “inflamed” or “mesenchymal” tumors) and their association with clinical response, and use this information to develop predictive markers and more rational combination regimens. Targeted therapies and immunotherapies are typically being given in a tailored, biomarker-driven manner, and there is an opportunity for VEGF/VEGFR inhibitors to join in this progress.
Acknowledgements:
R.K.J. would like to acknowledge Drs. Zohreh Amoozgar, Dan Duda, Dai Fukumura, and Yuhui Huang for their helpful input on the manuscript.
M.N. would like to acknowledge support from R50CA265307. S.P., M.N., X.L., T.C., and J.H. are supported by SPORE P50 grant, Cancer Center Support Grants (CCSG), and S.P. was supported by the CPRIT Training Award (RP210028) and John Kopchick Fellowship.
R.K.J.’s support - R35CA197743; U01CA224348; U01CA261842; R01CA269672; R01-CA259253; R01-CA208205; R01-NS118929; Ludwig Cancer Center at Harvard; Nile Albright Research Foundation; Jane’s Trust Foundation and National Foundation for Cancer Research.
Disclosures:
XL receives consulting/advisory fees from EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Novartis, Eli Lilly, Boehringer Ingelheim, Hengrui Therapeutics, Janssen, Blueprint Medicines, Sensei Biotherapeutics, and Abbvie, and Research Funding from Eli Lilly, EMD Serono, Regeneron, and Boehringer Ingelheim. TC receives speaker fees/honoraria from The Society for Immunotherapy of Cancer, Bristol Myers Squibb, Roche, Medscape, and PeerView; advisory role/consulting fees from MedImmune/AstraZeneca, Bristol Myers Squibb, EMD Serono, Merck & Co., Genentech, and Arrowhead Pharmaceuticals; and institutional research funding from MedImmune/AstraZeneca, Bristol Myers Squibb, and EMD Serono. RKJ received Consultant fees from Elpis, Innocoll, SPARC, SynDevRx; owns equity in Accurius, Enlight, SynDevRx; Board of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund and received a Research Grant from Boehringer Ingelheim. JVH serves on advisory committees for AstraZeneca, EMD Serono, Boehringer-Ingelheim, Catalyst, Genentech, GlaxoSmithKline, Guardant Health, Foundation medicine, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, Sanofi, Takeda, Mirati Therapeutics, BMS, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, Pneuma Respiratory, Kairos Venture Investments, Roche, Leads Biolabs, RefleXion, Chugai Pharmaceuticals, receives research support from Takeda, AstraZeneca, Boehringer-Ingelheim, and Spectrum, and receives royalties and licensing fees from Spectrum Pharmaceuticals. MBN receives royalties and licensing fees from Spectrum Pharmaceuticals.
References
- 1.Folkman J, Tumor angiogenesis: therapeutic implications. N Engl J Med, 1971. 285(21): p. 1182–6. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med, 2001. 7(9): p. 987–9. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK, Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science, 2005. 307(5706): p. 58–62. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK, Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol, 2013. 31(17): p. 2205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senger DR, et al. , Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science, 1983. 219(4587): p. 983–5. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak HF, et al. , Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci, 1992. 667: p. 101–11. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. and Henzel WJ, Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun, 1989. 161(2): p. 851–8. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. and Jain RK, Molecular mechanisms and clinical applications of angiogenesis. Nature, 2011. 473(7347): p. 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, et al. , VEGF-B promotes cancer metastasis through a VEGF-A-independent mechanism and serves as a marker of poor prognosis for cancer patients. Proc Natl Acad Sci U S A, 2015. 112(22): p. E2900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snuderl M, et al. , Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell, 2013. 152(5): p. 1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biselli-Chicote PM, et al. , VEGF gene alternative splicing: pro- and anti-angiogenic isoforms in cancer. J Cancer Res Clin Oncol, 2012. 138(3): p. 363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Star E, et al. , A drug-repositioning screen using splicing-sensitive fluorescent reporters identifies novel modulators of VEGF-A splicing with anti-angiogenic properties. Oncogenesis, 2021. 10(5): p. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavrou A, et al. , Serine-arginine protein kinase 1 (SRPK1) inhibition as a potential novel targeted therapeutic strategy in prostate cancer. Oncogene, 2015. 34(33): p. 4311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber HP, et al. , Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem, 1997. 272(38): p. 23659–67. [DOI] [PubMed] [Google Scholar]
- 15.George DJ and Kaelin WG Jr., The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N Engl J Med, 2003. 349(5): p. 419–21. [DOI] [PubMed] [Google Scholar]
- 16.Forsythe JA, et al. , Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol, 1996. 16(9): p. 4604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson MB, et al. , Altered Regulation of HIF-1alpha in Naive- and Drug-Resistant EGFR-Mutant NSCLC: Implications for a Vascular Endothelial Growth Factor-Dependent Phenotype. J Thorac Oncol, 2021. 16(3): p. 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng XH, et al. , Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem, 2006. 281(36): p. 25903–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips RJ, et al. , Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem, 2005. 280(23): p. 22473–81. [DOI] [PubMed] [Google Scholar]
- 20.Zhong H, et al. , Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res, 2000. 60(6): p. 1541–5. [PubMed] [Google Scholar]
- 21.Nakagawa K, et al. , Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 2019. 20(12): p. 1655–1669. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, et al. , Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1alpha in non-small cell lung cancer cells. Oncogene, 2010. 29(18): p. 2616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nilsson MB, et al. , Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors. Oncogene, 2010. 29(20): p. 2938–49. [DOI] [PubMed] [Google Scholar]
- 24.Mueller MD, et al. , Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci U S A, 2000. 97(20): p. 10972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min Y, et al. , C/EBP-delta regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1alpha. Oncogene, 2011. 30(49): p. 4901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukumura D, et al. , Tumor induction of VEGF promoter activity in stromal cells. Cell, 1998. 94(6): p. 715–25. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto T. and Claesson-Welsh L, VEGF receptor signal transduction. Sci STKE, 2001. 2001(112): p. RE21. [DOI] [PubMed] [Google Scholar]
- 28.Melder RJ, et al. , During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med, 1996. 2(9): p. 992–7. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki K, et al. , Ras signaling directs endothelial specification of VEGFR2+ vascular progenitor cells. J Cell Biol, 2008. 181(1): p. 131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan GX and Kazlauskas A, Axl is essential for VEGF-A-dependent activation of PI3K/Akt. EMBO J, 2012. 31(7): p. 1692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soker S, et al. , Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell, 1998. 92(6): p. 735–45. [DOI] [PubMed] [Google Scholar]
- 32.Kim KJ, et al. , Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature, 1993. 362(6423): p. 841–4. [DOI] [PubMed] [Google Scholar]
- 33.Jain RK, Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell, 2014. 26(5): p. 605–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukumura D, et al. , Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol, 2018. 15(5): p. 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oommen S, Gupta SK, and Vlahakis NE, Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: identification of a specific {alpha}9{beta}1 binding site. J Biol Chem, 2011. 286(2): p. 1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WS, et al. , Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med, 2020. 52(9): p. 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts WG and Palade GE, Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci, 1995. 108 (Pt 6): p. 2369–79. [DOI] [PubMed] [Google Scholar]
- 38.Sirois MG and Edelman ER, VEGF effect on vascular permeability is mediated by synthesis of platelet-activating factor. Am J Physiol, 1997. 272(6 Pt 2): p. H2746–56. [DOI] [PubMed] [Google Scholar]
- 39.Hippenstiel S, et al. , VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol, 1998. 274(5): p. L678–84. [DOI] [PubMed] [Google Scholar]
- 40.Helmlinger G, et al. , Formation of endothelial cell networks. Nature, 2000. 405(6783): p. 139–41. [DOI] [PubMed] [Google Scholar]
- 41.Khan KA and Kerbel RS, Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol, 2018. 15(5): p. 310–324. [DOI] [PubMed] [Google Scholar]
- 42.Rafii S, et al. , Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer, 2002. 2(11): p. 826–35. [DOI] [PubMed] [Google Scholar]
- 43.Asahara T, et al. , Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res, 1999. 85(3): p. 221–8. [DOI] [PubMed] [Google Scholar]
- 44.Lyden D, et al. , Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med, 2001. 7(11): p. 1194–201. [DOI] [PubMed] [Google Scholar]
- 45.Das S. and Marsden PA, Angiogenesis in glioblastoma. N Engl J Med, 2013. 369(16): p. 1561–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamin LE and Keshet E, Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A, 1997. 94(16): p. 8761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaudry P, et al. , Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res, 2005. 11(9): p. 3514–22. [DOI] [PubMed] [Google Scholar]
- 48.Mehran R, et al. , Tumor endothelial markers define novel subsets of cancer-specific circulating endothelial cells associated with antitumor efficacy. Cancer Res, 2014. 74(10): p. 2731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mancuso P, et al. , Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood, 2001. 97(11): p. 3658–61. [DOI] [PubMed] [Google Scholar]
- 50.Monestiroli S, et al. , Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res, 2001. 61(11): p. 4341–4. [PubMed] [Google Scholar]
- 51.Willett CG, et al. , Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med, 2004. 10(2): p. 145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaked Y, et al. , Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell, 2008. 14(3): p. 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beck B, et al. , A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature, 2011. 478(7369): p. 399–403. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, et al. , VEGF-A/Neuropilin 1 Pathway Confers Cancer Stemness via Activating Wnt/beta-Catenin Axis in Breast Cancer Cells. Cell Physiol Biochem, 2017. 44(3): p. 1251–1262. [DOI] [PubMed] [Google Scholar]
- 55.Elaimy AL, et al. , VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP beta2-chimaerin. Sci Signal, 2018. 11(528). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fantozzi A, et al. , VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res, 2014. 74(5): p. 1566–75. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson MB, et al. , KDR Amplification Is Associated with VEGF-Induced Activation of the mTOR and Invasion Pathways but does not Predict Clinical Benefit to the VEGFR TKI Vandetanib. Clin Cancer Res, 2016. 22(8): p. 1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharya R, et al. , Intracrine VEGF signalling mediates colorectal cancer cell migration and invasion. Br J Cancer, 2017. 117(6): p. 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knizetova P, et al. , Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle, 2008. 7(16): p. 2553–61. [DOI] [PubMed] [Google Scholar]
- 60.Spannuth WA, et al. , Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer, 2009. 124(5): p. 1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lesslie DP, et al. , Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br J Cancer, 2006. 94(11): p. 1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barr MP, et al. , Vascular endothelial growth factor is an autocrine growth factor, signaling through neuropilin-1 in non-small cell lung cancer. Mol Cancer, 2015. 14: p. 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riquelme E, et al. , VEGF/VEGFR-2 upregulates EZH2 expression in lung adenocarcinoma cells and EZH2 depletion enhances the response to platinum-based and VEGFR-2-targeted therapy. Clin Cancer Res, 2014. 20(14): p. 3849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang F, et al. , Increased VEGFR-2 gene copy is associated with chemoresistance and shorter survival in patients with non-small-cell lung carcinoma who receive adjuvant chemotherapy. Cancer Res, 2011. 71(16): p. 5512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gabrilovich DI, et al. , Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med, 1996. 2(10): p. 1096–103. [DOI] [PubMed] [Google Scholar]
- 66.Dikov MM, et al. , Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol, 2005. 174(1): p. 215–22. [DOI] [PubMed] [Google Scholar]
- 67.Ohm JE and Carbone DP, VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res, 2001. 23(2–3): p. 263–72. [DOI] [PubMed] [Google Scholar]
- 68.Lai YS, et al. , Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J Cell Mol Med, 2019. 23(2): p. 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiratsuka S, et al. , MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell, 2002. 2(4): p. 289–300. [DOI] [PubMed] [Google Scholar]
- 70.Parker KH, Beury DW, and Ostrand-Rosenberg S, Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res, 2015. 128: p. 95–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norden-Zfoni A, et al. , Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res, 2007. 13(9): p. 2643–50. [DOI] [PubMed] [Google Scholar]
- 72.Koinis F, et al. , Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells’ Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J Thorac Oncol, 2016. 11(8): p. 1263–1272. [DOI] [PubMed] [Google Scholar]
- 73.Ohm JE, et al. , VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood, 2003. 101(12): p. 4878–86. [DOI] [PubMed] [Google Scholar]
- 74.Chapoval SP, et al. , Lung vascular endothelial growth factor expression induces local myeloid dendritic cell activation. Clin Immunol, 2009. 132(3): p. 371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gavalas NG, et al. , VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer, 2012. 107(11): p. 1869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziogas AC, et al. , VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer, 2012. 130(4): p. 857–64. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki H, et al. , VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur J Immunol, 2010. 40(1): p. 197–203. [DOI] [PubMed] [Google Scholar]
- 78.Palazon A, et al. , An HIF-1alpha/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell, 2017. 32(5): p. 669–683 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y, et al. , Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A, 2012. 109(43): p. 17561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voron T, et al. , VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med, 2015. 212(2): p. 139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kerbel RS, Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. Bioessays, 1991. 13(1): p. 31–6. [DOI] [PubMed] [Google Scholar]
- 82.Martin JD, Seano G, and Jain RK, Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu Rev Physiol, 2019. 81: p. 505–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang JC, et al. , A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med, 2003. 349(5): p. 427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hurwitz H, et al. , Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med, 2004. 350(23): p. 2335–42. [DOI] [PubMed] [Google Scholar]
- 85.Sandler A, et al. , Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med, 2006. 355(24): p. 2542–50. [DOI] [PubMed] [Google Scholar]
- 86.Cascone T, et al. , Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest, 2011. 121(4): p. 1313–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu JL, et al. , Effect of p53 status on tumor response to antiangiogenic therapy. Science, 2002. 295(5559): p. 1526–8. [DOI] [PubMed] [Google Scholar]
- 88.Ravi R, et al. , Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev, 2000. 14(1): p. 34–44. [PMC free article] [PubMed] [Google Scholar]
- 89.Bonanno L, et al. , LKB1 Expression Correlates with Increased Survival in Patients with Advanced Non-Small Cell Lung Cancer Treated with Chemotherapy and Bevacizumab. Clin Cancer Res, 2017. 23(13): p. 3316–3324. [DOI] [PubMed] [Google Scholar]
- 90.Dameron KM, et al. , Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science, 1994. 265(5178): p. 1582–4. [DOI] [PubMed] [Google Scholar]
- 91.Ichikawa K, et al. , Activated FGF2 signaling pathway in tumor vasculature is essential for acquired resistance to anti-VEGF therapy. Sci Rep, 2020. 10(1): p. 2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shojaei F, et al. , Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol, 2007. 25(8): p. 911–20. [DOI] [PubMed] [Google Scholar]
- 93.Liu T, et al. , PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat Commun, 2018. 9(1): p. 3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahai E, et al. , A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer, 2020. 20(3): p. 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piao Y, et al. , Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res, 2013. 19(16): p. 4392–403. [DOI] [PubMed] [Google Scholar]
- 96.Cascone T, et al. , The HGF/c-MET Pathway Is a Driver and Biomarker of VEGFR-inhibitor Resistance and Vascular Remodeling in Non-Small Cell Lung Cancer. Clin Cancer Res, 2017. 23(18): p. 5489–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Incio J, et al. , Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci Transl Med, 2018. 10(432). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li JL, et al. , DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res, 2011. 71(18): p. 6073–83. [DOI] [PubMed] [Google Scholar]
- 99.Patel SA, et al. , Estrogen Promotes Resistance to Bevacizumab in Murine Models of NSCLC. J Thorac Oncol, 2021. 16(12): p. 2051–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seano G. and Jain RK, Vessel co-option in glioblastoma: emerging insights and opportunities. Angiogenesis, 2020. 23(1): p. 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergers G. and Hanahan D, Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer, 2008. 8(8): p. 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chung AS, et al. , An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med, 2013. 19(9): p. 1114–23. [DOI] [PubMed] [Google Scholar]
- 103.Asahara T, et al. , Isolation of putative progenitor endothelial cells for angiogenesis. Science, 1997. 275(5302): p. 964–7. [DOI] [PubMed] [Google Scholar]
- 104.Pollard JW, Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer, 2004. 4(1): p. 71–8. [DOI] [PubMed] [Google Scholar]
- 105.Benjamin LE, Hemo I, and Keshet E, A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development, 1998. 125(9): p. 1591–8. [DOI] [PubMed] [Google Scholar]
- 106.Du R, et al. , HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell, 2008. 13(3): p. 206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu YL, et al. , Hypoxia-induced tumor cell autophagy mediates resistance to anti-angiogenic therapy. Autophagy, 2012. 8(6): p. 979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joszt L, FDA Approves Third Bevacizumab Biosimilar. AJMC, 2022.
- 109.Fuchs CS, et al. , Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet, 2014. 383(9911): p. 31–39. [DOI] [PubMed] [Google Scholar]
- 110.Garon EB, et al. , Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet, 2014. 384(9944): p. 665–73. [DOI] [PubMed] [Google Scholar]
- 111.Van Cutsem E, et al. , Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol, 2012. 30(28): p. 3499–506. [DOI] [PubMed] [Google Scholar]
- 112.Le X, et al. , Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. J Thorac Oncol, 2021. 16(2): p. 205–215. [DOI] [PubMed] [Google Scholar]
- 113.Lieu C, et al. , Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res, 2011. 17(19): p. 6130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hosaka K, et al. , Therapeutic paradigm of dual targeting VEGF and PDGF for effectively treating FGF-2 off-target tumors. Nat Commun, 2020. 11(1): p. 3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Escudier B, et al. , Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J Clin Oncol, 2018. 36(8): p. 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peterson TE, et al. , Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A, 2016. 113(16): p. 4470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cascone T. and Heymach JV, Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J Clin Oncol, 2012. 30(4): p. 441–4. [DOI] [PubMed] [Google Scholar]
- 118.Gerber HP and Ferrara N, Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res, 2005. 65(3): p. 671–80. [PubMed] [Google Scholar]
- 119.Jain RK, Molecular regulation of vessel maturation. Nat Med, 2003. 9(6): p. 685–93. [DOI] [PubMed] [Google Scholar]
- 120.Willett CG, et al. , Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol, 2005. 23(31): p. 8136–9. [DOI] [PubMed] [Google Scholar]
- 121.Cesca M, et al. , Bevacizumab-Induced Inhibition of Angiogenesis Promotes a More Homogeneous Intratumoral Distribution of Paclitaxel, Improving the Antitumor Response. Mol Cancer Ther, 2016. 15(1): p. 125–35. [DOI] [PubMed] [Google Scholar]
- 122.Turley RS, et al. , Bevacizumab-induced alterations in vascular permeability and drug delivery: a novel approach to augment regional chemotherapy for in-transit melanoma. Clin Cancer Res, 2012. 18(12): p. 3328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dickson PV, et al. , Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res, 2007. 13(13): p. 3942–50. [DOI] [PubMed] [Google Scholar]
- 124.Sweeney CJ, et al. , The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res, 2001. 61(8): p. 3369–72. [PubMed] [Google Scholar]
- 125.Meder L, et al. , Combined VEGF and PD-L1 Blockade Displays Synergistic Treatment Effects in an Autochthonous Mouse Model of Small Cell Lung Cancer. Cancer Res, 2018. 78(15): p. 4270–4281. [DOI] [PubMed] [Google Scholar]
- 126.Kim CG, et al. , VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol, 2019. 4(41). [DOI] [PubMed] [Google Scholar]
- 127.Xu J, et al. , Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol, 2020. 21(11): p. 1500–1512. [DOI] [PubMed] [Google Scholar]
- 128.Sennino B, et al. , Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov, 2012. 2(3): p. 270–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li J, et al. , Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA, 2018. 319(24): p. 2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dasari A, et al. , FRESCO-2: a global Phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Future Oncol, 2021. 17(24): p. 3151–3162. [DOI] [PubMed] [Google Scholar]
- 131.Tsimafeyeu I, et al. , Targeting FGFR2 with alofanib (RPT835) shows potent activity in tumour models. Eur J Cancer, 2016. 61: p. 20–8. [DOI] [PubMed] [Google Scholar]
- 132.Lu KV, et al. , VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell, 2012. 22(1): p. 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bagley RG, et al. , Placental growth factor upregulation is a host response to antiangiogenic therapy. Clin Cancer Res, 2011. 17(5): p. 976–88. [DOI] [PubMed] [Google Scholar]
- 134.Hidalgo M, et al. , First-in-Human Phase I Study of Single-agent Vanucizumab, A First-in-Class Bispecific Anti-Angiopoietin-2/Anti-VEGF-A Antibody, in Adult Patients with Advanced Solid Tumors. Clin Cancer Res, 2018. 24(7): p. 1536–1545. [DOI] [PubMed] [Google Scholar]
- 135.Wang Q, et al. , Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS One, 2013. 8(8): p. e70544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JE, et al. , Novel glycosylated VEGF decoy receptor fusion protein, VEGF-Grab, efficiently suppresses tumor angiogenesis and progression. Mol Cancer Ther, 2015. 14(2): p. 470–9. [DOI] [PubMed] [Google Scholar]
- 137.Eason AB, et al. , DLX1008 (brolucizumab), a single-chain anti-VEGF-A antibody fragment with low picomolar affinity, leads to tumor involution in an in vivo model of Kaposi Sarcoma. PLoS One, 2020. 15(5): p. e0233116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yeom DH, et al. , ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models. Int J Mol Sci, 2020. 22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cui X, et al. , A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front Immunol, 2021. 12: p. 778978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kloepper J, et al. , Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A, 2016. 113(16): p. 4476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xia Y, et al. , YLT192, a novel, orally active bioavailable inhibitor of VEGFR2 signaling with potent antiangiogenic activity and antitumor efficacy in preclinical models. Sci Rep, 2014. 4: p. 6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rini BI, et al. , Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol, 2020. 21(1): p. 95–104. [DOI] [PubMed] [Google Scholar]
- 143.Hu N, et al. , Anlotinib has good efficacy and low toxicity: a phase II study of anlotinib in pre-treated HER-2 negative metastatic breast cancer. Cancer Biol Med, 2021. [DOI] [PMC free article] [PubMed]
- 144.Batchelor TT, et al. , AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell, 2007. 11(1): p. 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azad NS, J.J., Annunziata C, Cao L, Greenberg L, Minasian L, Perroy A, Kotz H, Figg WD, Kohn E, Correlative studies of a phase I trial of combination anti-vascular endothelial growth factor (VEGF) therapy with sorafenib and bevacizumab. Journal of Clinical Oncology, 2008. 26(15). [Google Scholar]
- 146.Burstein HJ, et al. , Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol, 2008. 26(11): p. 1810–6. [DOI] [PubMed] [Google Scholar]
- 147.Rini BI, et al. , Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol, 2008. 26(22): p. 3743–8. [DOI] [PubMed] [Google Scholar]
- 148.Socinski MA, et al. , Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med, 2018. 378(24): p. 2288–2301. [DOI] [PubMed] [Google Scholar]
- 149.Rini BI, et al. , Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med, 2019. 380(12): p. 1116–1127. [DOI] [PubMed] [Google Scholar]
- 150.Motzer RJ, et al. , Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med, 2019. 380(12): p. 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Finn RS, et al. , Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med, 2020. 382(20): p. 1894–1905. [DOI] [PubMed] [Google Scholar]
- 152.Choueiri TK, Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. 2020.
- 153.Makker VCN, Casado H, A multicenter, open-label, randomized phase 3 study to compare the efficacy and safety of lenvatinib in combination with pembrolizumab vs treatment of physician’s choice in patients with advanced endometrial cancer: Study 309/KEYNOTE-775. Soceity of Gynecologic Oncology, 2021.
- 154.Motzer R. and Choueiri TK, Lenvatinib plus Pembrolizumab for Renal Cell Carcinoma. Reply. N Engl J Med, 2021. 385(3): p. 287. [DOI] [PubMed] [Google Scholar]
- 155.Sorensen AG, et al. , Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res, 2012. 72(2): p. 402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Batchelor TT, et al. , Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A, 2013. 110(47): p. 19059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heist RS, et al. , Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc Natl Acad Sci U S A, 2015. 112(5): p. 1547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Garcia-Foncillas J, Martinez P, Lahuerta A, Llombart Cussac A, Gonzalez MG, Sanchez-Gomez RM, Alvarez I, Anton A, Illarramendi JJ, De Juan A, Dynamic contrast-enhanced MRI versus 18F-misonidazole-PET/CT to predict pathologic response in bevacizumab-based neoadjuvant therapy in breast cancer. J Clin Oncol 2012.
- 159.Tolaney SM, et al. , Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc Natl Acad Sci U S A, 2015. 112(46): p. 14325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]