Abstract
Background and Aims
Thioguanine is a well-tolerated and effective therapy for inflammatory bowel disease [IBD] patients. Prospective effectiveness data are needed to substantiate the role of thioguanine as a maintenance therapy for IBD.
Methods
IBD patients who previously failed azathioprine or mercaptopurine and initiated thioguanine were prospectively followed for 12 months starting when corticosteroid-free clinical remission was achieved (Harvey–Bradshaw Index [HBI] ≤ 4 or Simple Clinical Colitis Activity Index [SCCAI] ≤ 2). The primary endpoint was corticosteroid-free clinical remission throughout 12 months. Loss of clinical remission was defined as SCCAI > 2 or HBI > 4, need of surgery, escalation of therapy, initiation of corticosteroids or study discontinuation. Additional endpoints were adverse events, drug survival, physician global assessment [PGA] and quality of life [QoL].
Results
Sustained corticosteroid-free clinical remission at 3, 6 or 12 months was observed in 75 [69%], 66 [61%] and 49 [45%] of 108 patients, respectively. Thioguanine was continued in 86 patients [80%] for at least 12 months. Loss of response [55%] included escalation to biologicals in 15%, corticosteroids in 10% and surgery in 3%. According to PGA scores, 82% of patients were still in remission after 12 months and QoL scores remained stable. Adverse events leading to discontinuation were reported in 11%, infections in 10%, myelo- and hepatotoxicity each in 6%, and portal hypertension in 1% of patients.
Conclusion
Sustained corticosteroid-free clinical remission over 12 months was achieved in 45% of IBD patients on monotherapy with thioguanine. A drug continuation rate of 80%, together with favourable PGA and QoL scores, underlines the tolerability and effectiveness of thioguanine for IBD.
Keywords: Inflammatory bowel disease, Crohn’s disease, ulcerative colitis, thioguanine, azathioprine, mercaptopurine, nodular regenerative hyperplasia, 6-thioguanine-nucleotides
1. Introduction
Effective, safe and tolerable therapies for ulcerative colitis [UC] and Crohn’s disease [CD] are essential to avoid complications of disease and improve the quality of life of these patients.1 Azathioprine [AZA] and mercaptopurine [MP] are well-established drugs to maintain remission in inflammatory bowel disease [IBD], but their use is increasingly debated in relation to adverse events and effectiveness.2 Anti-tumour necrosis factor [anti-TNF] therapy has strongly been advocated in contemporary IBD regimens, although its use is similarly characterized by specific safety and [long-term] effectiveness issues. Additionally, restricted access still limits prescribing and availability in many countries, notwithstanding the advent of biosimilars.3 One-third of patients do not respond to anti-TNF therapy and loss of response is expected in another third of patients over time.4 Novel biologicals and small molecules have become available for IBD, but still with few data on long-term usage and safety.5
In the past 20 years, there has been an increasing interest in the use of thioguanine [or 6-thioguanine, TG] for treating IBD.6 TG was developed in 1950 [together with AZA and MP] for the treatment of leukaemia, its use decreased substantially over time due to development of newer anti-leukaemic drugs. AZA, MP and TG are all metabolized into the same active metabolites [6-thioguanine nucleotides, 6-TGN], but TG requires less metabolic steps and bypasses several toxicity-associated enzymes and metabolites [including 6-methylmercaptopurines, 6-MMP].7 In several studies, TG appeared effective and well-tolerated in 65–80% of IBD patients with prior intolerance to AZA or MP.8–10 At high dosages, TG has been associated with nodular regenerative hyperplasia [NRH] of the liver, as reported in 2003, accounting for its limited use in general practice since.11,12 In more recent studies, however, it has been suggested that NRH is dose-dependent and relatively uncommon when using the currently recommended therapeutic dosages of maximal 0.2–0.3 mg/kg/day, not exceeding 25 mg/day, for IBD.13–15
To date, TG has mainly been studied in small or retrospective trials. A nationwide prospective TG registry was constructed with the aim to assess sustained effectiveness and safety of TG as a maintenance therapy for IBD patients who failed prior therapy with AZA or MP.
2. Methods
2.1. Study design and patient population
This was a prospective, multicentre study to evaluate the sustained effectiveness and safety of TG in IBD patients. Throughout the Netherlands, patients were recruited between May 2016 and May 2020, in 14 centres. Inclusion criteria were [1] adult patients [18 years or older], [2] diagnosed with UC or CD, [3] prior use of AZA and/or MP, and [4] initiating TG at study entry. Patients with co-therapy with biologicals at baseline were excluded.
Patients had a run-in period up to 6 months to reach corticosteroid-free clinical remission with TG. Patients were monitored for 12 months starting from the time corticosteroid-free clinical remission with TG was achieved. Effectiveness and safety data were collected at study entry and again when corticosteroid-free remission was achieved. Follow-up was documented at all patient visits [e.g. 1, 2, 3, 6, 9 and 12 months] after inclusion during routine clinical practice.
2.2. Effectiveness endpoints
The primary endpoint was the rate of patients with corticosteroid-free clinical remission, defined as a Harvey–Bradshaw Index [HBI] score ≤4 for CD, or Simple Clinical Colitis Activity Index [SCCAI] ≤2 for UC patients, maintained for at least 12 months of TG therapy during all routine patient visits.
Loss of clinical remission was defined as [1] SCCAI score >2 or HBI score >4 measured at any patient visits, or [2] need of intestinal surgery, or [3] escalation of therapy or [4] initiation of long-term corticosteroids for induction of remission. A short course of corticosteroids of less than 3 months was allowed once to extend induction, re-introduce remission, and evade surgery or therapy escalation.
Secondary endpoints were drug survival, physician global assessment [PGA] scores provided by treating physicians [classified as in remission, mild, moderate or severe active disease], patient-reported outcomes on quality of life [QoL] measured using the validated Short Form Inflammatory Bowel Disease Questionnaire [SIBDQ] and C-reactive protein [CRP] or faecal calprotectin [FCP] concentrations, which were assessed during visits at physicians’ discretion.16
2.3. Safety endpoints
Adverse events that occurred after TG initiation were classified into non-serious or serious events [including death, a life-threatening event, hospitalization, or requiring medical intervention, congenital anomalies or other important medical events] according to pharmacovigilance guidance.17 Physicians further assessed all reported adverse events for any relationship with TG. Attributed severity of the adverse event was categorized as mild, moderate and severe and defined as no, some or serious limitations of physical activity, respectively.
Furthermore, laboratory parameters, including liver and haematological parameters and clinical signs of NRH and/or portal hypertension, and 6-TGN concentrations in red blood cells, were collected according to routine practice.
2.4. Statistics
Statistical analysis was performed using SPSS Version 26 [IBM] and software R [R Core Team 2013]. Data are presented as numbers with percentages or means with standard deviations [SD]. ANOVA on ranks was used to analyse changes in quantitative parameters over time between patient visits. To test differences in means on quantitative parameters between CD and UC patients, the Mann–Whitney U test was used. To test for differences among three or more [sub]groups the Kruskal–Wallis test was performed and differences between two proportions were analysed using the Fisher Exact test.
A sample size calculation of 95 IBD patients [including 55 CD patients] using the Wilson confidence interval was based on an estimated 55% of patients remaining in corticosteroid-free remission for ≥12 months.9 Two-tailed probability [p]-values of <0.05 were considered statistically significant. Determination of remission was based on complete HBI and SCCAI scores without missing values, or with at most one missing value, which would not influence the determination of remission even when the maximum [worst] score was given.
2.5. Ethical approval
The study complied with the definition of the non-interventional [observational] study provided in Article 2[c] of Directive 2001/20/ of the European Commission [EC, 2008] and the 2012 Guideline on Good Pharmacovigilance Practice [GVP] and was approved by the Central Committee on Research Involving Human Subjects [CCMO]. All patients gave informed consent.
2.6. Data availability statements
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
3. Results
3.1. Patient screening
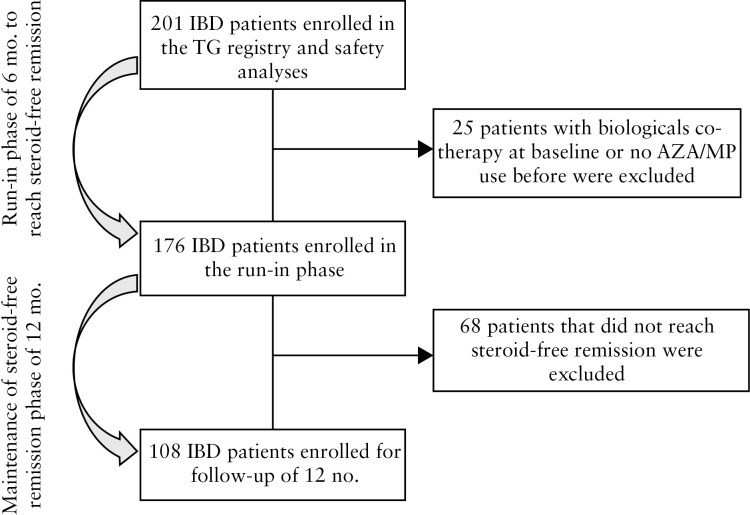
A total of 205 patients were screened and 201 patients gave permission to participate and were enrolled in the study. Of these, 25 [12%] were excluded due to the lack of documented AZA/MP use or to the concomitant use of a biological. The remaining 176 patients were included in the run-in period of 6 months: 108 [61%] reached corticosteroid-free clinical remission within this time period and were followed-up for 12 months, subsequently [Figure 1].
Figure 1.
Flowchart of patient inclusion.
3.2. Baseline characteristics
Out of 108 IBD patients, 67 [62%] were female, the average age was 46 years [SD 14.8] and 66 [61%] had CD [Table 1]. The mean IBD duration was 8.2 years [SD 9.4] in CD and 7.6 years [SD 7.8] in UC patients. Two-thirds of CD patients had a non-stricturing or non-penetrating disease and 62% of UC patients had a proctitis or left-sided colitis. There were no demographic baseline differences between the UC and CD group. Remarkably, almost one-third of CD patients were current smokers as compared to 10% of UC patients. A valid explanation for this unexpected finding was not found.
Table 1.
Baseline characteristics of patients who reached steroid-free remission
| CD [N = 66] | UC [N = 42] | Total [N = 108] | |
|---|---|---|---|
| Sex, female | 43 [65%] | 24 [57%] | 67 [62%] |
| Age group | |||
| 18–30 years | 15 [23%] | 10 [24%] | 25 [23%] |
| 30–50 years | 19 [29%] | 17 [40%] | 36 [34%] |
| 50–65 years | 24 [36%] | 10 [24%] | 34 [32%] |
| >65 years | 8 [12%] | 5 [12%] | 13 [12%] |
| Mean age at diagnosis, years | 39 [SD 15.1] | 36 [SD 15.1] | 38 [SD 15.1] |
| Mean age at baseline, years | 47 [SD 14.2] | 44 [SD 15.8] | 46 [SD 14.8] |
| Mean weight, kg | 78.0 [SD 17.2] | 84.2 [SD 16.7] | 80.0 [SD 17.2] |
| Mean BMI, kg/m2 | 26.2 [SD 6.2] | 27.6 [SD 6.2] | 26.8 [SD 6.2] |
| Mean IBD disease duration, years | 8.2 [SD 9.4] | 7.6 [SD 7.8] | 8.0 [SD 8.8] |
| Smoking | |||
| Current | 21 [32%] | 4 [10%] | 25 [23%] |
| Previous | 25 [38%] | 17 [41%] | 42 [39%] |
| Never | 20 [30%] | 21 [50%] | 41 [38%] |
| Alcohol consumption | |||
| None | 31 [47%] | 20 [48%] | 51 [47%] |
| 1–2 drinks per week | 19 [29%] | 9 [21%] | 28 [26%] |
| 3–5 drinks per week | 6 [9%] | 4 [10%] | 10 [9%] |
| 6–9 drinks per week | 4 [6%] | 4 [10%] | 8 [7%] |
| ≥10 drinks per week | 6 [9%] | 3 [7%] | 9 [8%] |
| Unknown | 0 [0%] | 2 [5%] | 2 [2%] |
| Montreal classification CD | |||
| Behaviour | |||
| Non-stricturing/non-penetrating [B1] | 43 [65%] | ||
| Stricturing [B2] | 19 [29%] | ||
| Penetrating [B3] | 2 [3%] | ||
| Peri-anal disease [+P] | 2 [3%] | ||
| Location | |||
| Ileum [L1] | 30 [46%] | ||
| Colon [L2] | 18 [27%] | ||
| Ileocolon [L3] | 16 [24%] | ||
| Upper GI [+L4] | 2 [3%] | ||
| Montreal classification UC | |||
| Extent | |||
| Proctitis [E1] | 5 [12%] | ||
| Left-sided [E2] | 23 [55%] | ||
| Pancolitis [E3] | 12 [29%] | ||
| Other | 2 [5%] | ||
| Severity | |||
| Remission [S0] | 15 [36%] | ||
| Mild [S1] | 11 [26%] | ||
| Moderate [S2] | 14 [33%] | ||
| Severe [S3] | 2 [5%] | ||
| Extra-intestinal manifestations | 12 [18%] | 5 [12%] | 17 [16%] |
| Prior thiopurine use | |||
| AZA | 29 [44%] | 21 [50%] | 50 [46%] |
| MP | 37 [56%] | 18 [43%] | 55 [51%] |
| Both AZA and MP | 0 [0%] | 3 [7%] | 3 [3%] |
| Other therapies in the year before inclusion | |||
| Steroids | 45 [68%] | 26 [62%] | 71 [66%] |
| 5-ASA | 15 [23%] | 31 [74%] | 46 [43%] |
| Methotrexate | 10 [15%] | 3 [7%] | 13 [12%] |
| Anti-TNF therapy | 14 [21%] | 5 [12%] | 19 [18%] |
CD: Crohn’s disease; UC: ulcerative colitis; IBD: inflammatory bowel disease; BMI: body mass index; GI, gastrointestinal; AZA: azathioprine; MP: mercaptopurine; 5-ASA: 5-aminosalicylic acid; TNF, tumour necrosis factor.
All 108 patients had been treated with AZA or MP therapy: 50 [46%] with AZA, 55 [51%] with MP, and three [3%] with both AZA and MP [Table 1]. The reason to switch to TG was due to intolerance to AZA/MP in 72%, insufficient response to AZA/MP in 10%, and both intolerance and non-response in another 18% of patients. During the previous year, 66% of patients were treated with corticosteroids, 43% with 5-amino salicylic acid [5-ASA], 12% with methotrexate, 18% with anti-TNF and 8% with vedolizumab [the last group all failed anti-TNF therapy before vedolizumab].
3.3. TG therapy dosages
At time of achieving corticosteroid-free remission, 86 [80%] patients were using 20 mg TG per day, 12 patients [11%] were using 10 mg/day, one [1%] patient 15 mg/day and nine [8%] patients 25 mg/day [Table 2]. Bodyweight was the main reason for starting a TG dosage other than 20 mg/day. In 76 [70%] patients, TG dosages were not adjusted during therapy, in seven [7%] patients TG dosage was increased and in another seven [7%] patients it was decreased.
Table 2.
Thioguanine dosages and adjustments during therapy
| CD [N = 66] | UC [N = 42] | Total [N = 108] | |
|---|---|---|---|
| TG dosage at baseline | |||
| 10 mg/day | 6 [9%] | 10 [24%] | 16 [15%] |
| 15 mg/day | 1 [2%] | 0 [0%] | 1 [1%] |
| 20 mg/day | 52 [79%] | 30 [71%] | 82 [76%] |
| 25 mg/day | 7 [11%] | 2 [5%] | 9 [8%] |
| TG dosage at remission | |||
| 10 mg/day | 4 [6%] | 8 [19%] | 12 [11%] |
| 15 mg/day | 1 [2%] | 0 [0%] | 1 [1%] |
| 20 mg/day | 53 [80%] | 33 [79%] | 86 [80%] |
| 25 mg/day | 8 [12%] | 1 [2%] | 9 [8%] |
| Adjustments in dosages | |||
| None | 53 [80%] | 23 [55%] | 76 [70%] |
| Increase | 4 [6%] | 3 [7%] | 7 [7%] |
| Decrease | 2 [3%] | 5 [12%] | 7 [7%] |
| Both increase and decrease | 7 [11%] | 11 [26%] | 18 [17%] |
Thioguanine [TG] treatment characteristics at baseline and during follow-up. CD: Crohn’s disease; UC: ulcerative colitis.
3.4. Maintenance of corticosteroid-free remission
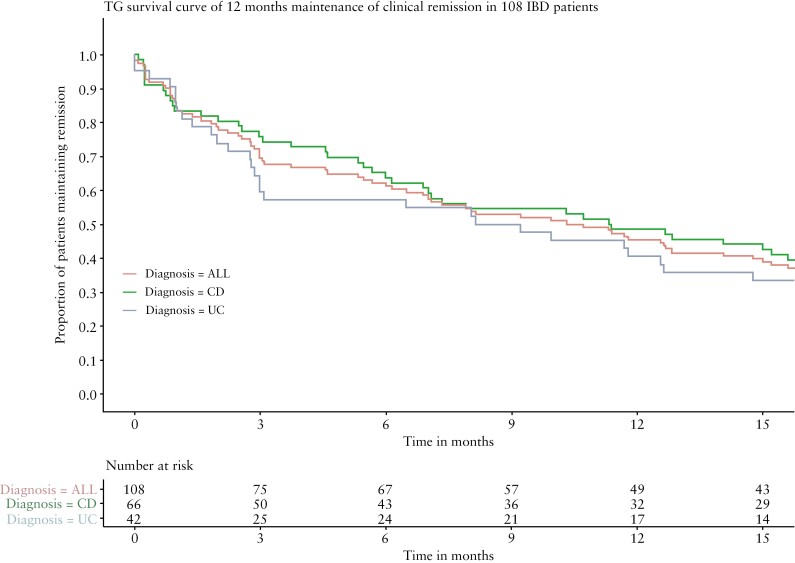
Sustained corticosteroid-free clinical remission at 3, 6 or 12 months was achieved in 75/108 [69%], 66/108 [61%] and 49/108 IBD patients [45%], respectively [Figure 2]. This included two CD patients [2%] who needed a short course of budesonide of a maximum 8 weeks, closely after tapering and being included in the maintenance effectiveness analyses, as was allowed by the protocol. None of the patients [re]initiated corticosteroids. A post-hoc analysis excluding those two patients resulted in sustained clinical remission of 12 months in 47/108 IBD patients [44%].
Figure 2.
TG survival curve of 12 months’ maintenance of clinical remission in 108 IBD patients. Time in steroid-free clinical remission [HBI > 4 or SCCAI > 2] in 108 IBD patients with TG therapy.
The proportion of corticosteroid-free remission at 12 months was 41% for UC and 49% for CD patients, and was not statistically different. There was also no correlation between the reason for TG therapy [intolerance or non-response to AZA/MP] and sustained corticosteroid-free clinical remission.
Concerning the UC patients, there was no difference between the extent of disease [i.e. proctitis or extended colitis] and sustained corticosteroid-free clinical remission [2/5, 40% proctitis vs 15/37, 41% extended colitis]. Sustained clinical remission at 12 months among UC patients was twice as high in patients with no disease activity [S0] at study entry compared to patients with mild to severe disease [60% vs 30%].
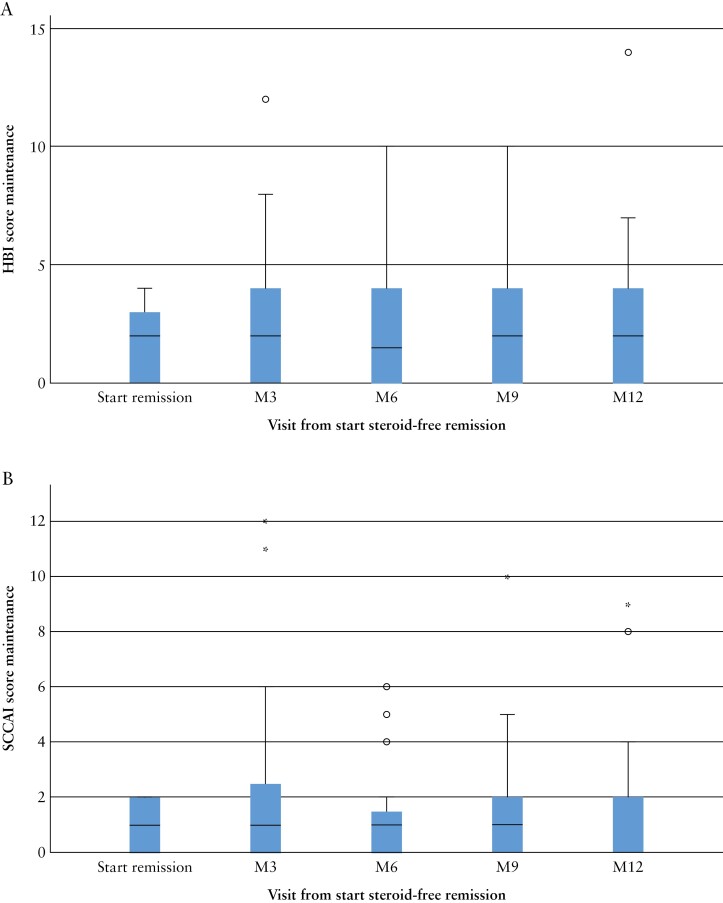
The median HBI score was 2 [0–3] at baseline of remission, 2 [0–4] at 6 months and 2 [0–4] at 12 months [Figure 3A]. The median SCCAI score was 1 [0–2] at baseline of remission, 1 [0–2] at 6 moths and 0 [0–2] at 12 months [Figure 3B].
Figure 3.
Boxplot of [A] HBI scores and [B] SCCAI scores. Median HBI scores in 66 Crohn’s disease patients during 12 months of follow-up. Median SCCAI scores in 42 ulcerative colitis patients during 12 months of follow-up.
The median CRP concentration was 4.0 mg/l [1.8–6.0, 71/108 patients] at baseline of remission, 3.0 mg/l [1.2–5.5, 65/82 patients] at 6 months and 3.1 mg/l [1.4–5.2, 53/72 patients] at 12 months. FCP concentration data were not collected routinely and thus are not reported.
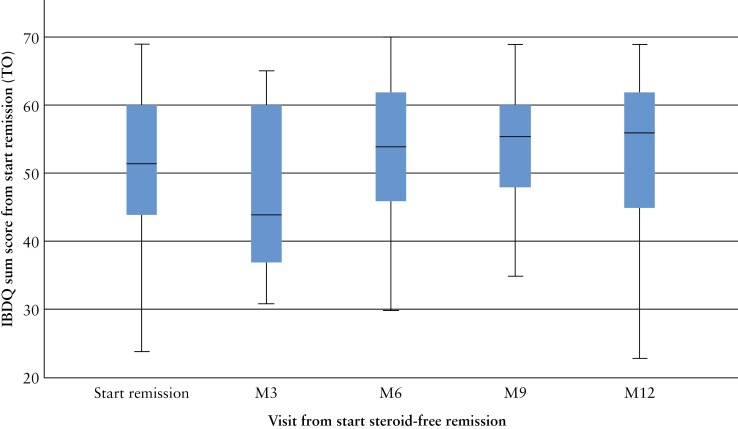
The proportion of patients in remission based on PGA scores was: 61% at baseline of remission [105/108 patients], 71% at 6 months [77/108 patients] and 82% at 12 months [67/108 patients]. SIBDQ scores were completed by all patients at every visit. The mean SIBDQ score was 52 [44–60] at baseline of remission, reaching 54 [46–62] at 6 months and 56 [45–63] at 12 months [Figure 4].
Figure 4.
SIBDQ scores on quality of life. Median Short Form Inflammatory Bowel Disease Questionnaire [SIBDQ] scores in 108 IBD patients during 12 months of follow-up.
3.5. Escalation of therapy
In 16 [15%] out of 108 patients, therapy was escalated to biologicals after initial remission with TG. Seven [6%] of these patients continued with TG as a co-therapy. A long-term course of steroids was required in 11 patients [10%]. Furthermore, three [3%] CD patients needed surgery within 12 months after baseline. One of them continued TG after surgery.
3.6. Drug survival
Out of 108 patients, 22 [20%] patients discontinued TG and the remaining 86 [80%] patients continued TG for at least 12 months [Figure 5]. Withdrawal reasons were adverse events in 14 [13%] patients of which two were classified as unrelated to TG, insufficient therapeutic response in seven [6%] and non-compliance in another patient [1%].
Figure 5.
TG continuation in 108 TG-treated IBD patients.
3.7. Safety profile
From TG initiation until the end of follow-up, a total of 470 adverse events were reported in 88 out of 108 [81%] patients. Of these, 190 adverse events were possibly or probably TG related and reported in 61 [56%] patients. Serious [possibly or probably] related adverse events were reported in 14 [13%] and severe adverse events in five [5%] patients. The most frequently reported adverse events were abdominal pain [12%], arthralgia [12%], infections [10%], diarrhoea [9%], pruritus [8%], flu-like illness [6%], myalgia [6%], alopecia [6%] and nausea [6%] [Table 3]. Four [4%] patients needed hospitalization due to adverse events. This was due to a cystitis in two patients, pancytopaenia in one patient and a collapse with a rib fracture in another patient. Discontinuation of TG due to adverse events occurred in 11% of patients.
Table 3.
Number of adverse events in 108 patients
| Adverse events | Percentage |
|---|---|
| Nausea | 6% |
| Abdominal pain | 12% |
| Arthralgia | 12% |
| Hepatotoxicity | 6% |
| Portal hypertension | 1% |
| Nodular regenerative hyperplasia | 0% |
| Diarrhoea | 9% |
| Pruritus | 8% |
| Flu-like illness | 6% |
| Myalgia | 6% |
| Alopecia | 6% |
| Myelotoxicity | 6% |
| Infections | 10% |
| Squamous cell cancer skin | 1% |
Infections that are expected to occur in the population regardless of IBD status [such as common cold, influenza, pharyngitis] were not counted.
Myelotoxicity was reported in seven [6%] patients, including leukopaenia in two, thrombocytopaenia in four and pancytopaenia in one patient. Two patients discontinued TG; the other five continued TG and the adverse events did resolve over time. Hepatotoxicity was reported in seven [6%] patients, three of whom discontinued TG. Data regarding thiopurine methyltransferase [TPMT] enzyme activity were not available.
There was no histologically confirmed NRH in this cohort. There was a 52-year-old female patient with signs of portal hypertension at ultrasound [but no clinical or biochemical signs] after 1 year of TG use of 25 mg/day. Liver biopsy was not performed. The adverse event was reported as possibly related to TG or previous conventional thiopurine use.
Furthermore, infections were reported in 11 [10%] patients, six of them experiencing infections classified as serious, including a 62-year-old patient with cytomegalovirus [CMV] infection and two patients requiring hospitalization for cystitis. Squamous cell carcinoma of the skin was reported in one patient [1%]. Furthermore, of five pregnancies reported in the study, four resulted in live birth with healthy newborns and one resulted in a terminated pregnancy due to a fetus with Down syndrome.
3.8. 6-TGN concentrations
6-TGN concentrations were not routinely [proactively] determined. Concerning all 201 patients who were screened, the mean 6-TGN concentrations, measured as described by Lennard,18 at baseline, 6 months and 12 months were 344 pmol/8 × 108 red blood cells [RBCs] [data available in 29/201 patients, 14%], 794 pmol/8 × 108 RBCs [data in 23/141 patients, 16%] and 1364 pmol/8 × 108 RBCs [data in 16/119 patients, 13%]. 6-TGN levels did not correlate with effectiveness of therapy or [severity of] adverse events.
4. Discussion
In this prospective, multicentre effectiveness study, the proportion of patients who maintained continuous corticosteroid-free clinical remission with TG therapy at 3, 6 and 12 months was 69, 61 and 45%, respectively. TG was well tolerated and continued in 80% of patients until the end of follow-up. Eighty-two per cent of patients were still in remission after 12 months, as judged by PGA scores, and patient satisfaction was suitable with QoL scores remaining stable over time. Following these results, TG has been licensed as a certified IBD treatment in The Netherlands since 2022.
The effectiveness of conventional thiopurine has been examined previously. In a large retrospective UK cohort of 11 928 IBD patients, AZA or MP monotherapy was effective in 42% of patients [53% in UC and 34% in CD patients].19 In a recent study of 1016 IBD patients, the effectiveness of thiopurines was reported in 40 and 30% of patients after 5 and 10 years, respectively.20 In a systematic review on TG effectiveness in IBD, a therapeutic benefit of TG was described in 228 out of 353 [65%] patients, but varied widely between 22 and 80%.9 In our study, effectiveness of TG was observed in 45% of patients. This rate is comparable to the rate observed with conventional thiopurines. Nonetheless, the pre-specified effectiveness endpoint of 55% was not reached, possibly due to the patients’ characteristics. The population in our study included an AZA/MP-experienced IBD cohort, 18% of whom failed on anti-TNF, 12% failed on methotrexate and 70% received a course oif corticosteroids in the year prior to TG initiation. Thus, this population could be more difficult to control due to refractory disease. A direct comparison with studies using more recent IBD drugs was not possible, due to differences in study populations, study designs and definitions of maintenance treatment success. Given the severity of disease activity of the study participants, the finding that a substantial proportion of patients [45%] experienced continuous remission over a year is of clinical relevance.
Drug survival of TG in this cohort was 80% and similar to previous TG survival rates, such as described in a large Dutch cohort of 274 IBD patients [with 51 months of follow-up] and a UK cohort of 193 IBD patients [with 36 months of follow-up].8,21 By contrast, AZA/MP drug survival was 63% in a large IBD cohort and one-third of patients discontinued therapy within 1 year, especially due to adverse events.2,20 When compared to methotrexate, TG appeared to be better tolerated, as recently reported in a study in which 42% of methotrexate-using patients discontinued therapy due to adverse events as compared to only 19% of TG-treated patients.22
During the study period, serious TG-related adverse events were observed in 13% of patients and severe adverse events in 5% of patients, also in line with previous reports.8 Myelo- and hepatotoxicity were reported in 6 and 6%, respectively, infections in 10%, and skin cancer and portal hypertension [shown by ultrasound but without clinical or biochemical abnormalities] each in one patient. There was no histopathologically confirmed NRH of the liver documented, but a thiopurine-related NRH with non-cirrhotic portal hypertension cannot be excluded. Yet, if comparing the one unconfirmed case to a background incidence of NRH of 2.6% [in an autopsy study], the potential TG-related severe hepatotoxicity risk is low, and probably consistent with previous studies of low-dose use of TG in IBD patients.15,23 In the present study, as in routine clinical practice in the Netherlands, laboratory parameters, including liver enzymes, and adverse events are routinely monitored as with AZA/MP therapy [induction: weeks 0, 1, 2, 4, 8 and 12, maintenance every 3–4 months].24 Imaging or biopsies of the liver are not routinely conducted, but solely in the case of suspicion of liver toxicity [such as persistent elevated liver enzymes and/or signs of portal hypertension].24
In this cohort, all pregnancies except one resulted in a live birth. No other congenital anomalies were reported. This is in line with a recent quite large study describing 117 pregnancies, where reassuring outcomes concerning teratogenicity were provided.25
The main strength of our study was the prospective follow-up of a nationwide IBD cohort treated in routine clinical settings. We used a stringent definition for sustained clinical remission [HBI ≤ 4 or SCCAI ≤ 2 at all patient visits without need for surgery and escalation of therapy or long-term corticosteroids] and observed that sustained corticosteroid-free remission was achieved in 45% of IBD patients. However, extrapolating our findings to other clinical settings may be limited due to the study population being derived from mainly tertiary centres [and thus likely to be sicker than patients treated in primary care] and the strict inclusion criteria [i.e. patients achieving steroid-free remission during a run-in period of 6 months]. A treatment comparator or untreated group was lacking in this study.
Furthermore, biomarkers FCP or the routinely assessed 6-TGN concentrations or endoscopic or magnetic resonance enterography [as per protocol] were not assessed. Since all patients were scored during their routine patient visits, we were not able to collect sufficient data on some of these parameters to draw conclusions. Therapeutic drug monitoring as practised with AZA and MP therapy is not routinely performed during TG therapy.24 During TG metabolism, relatively high 6-TGN concentrations in RBCs are measured without inducing myelotoxicity, as could occur with AZA/MP therapy with comparable 6-TGN concentrations. This is caused by differences in drug metabolism, leading to substantially different drug levels in leukocytes versus erythrocytes.26 In one study, clinical response to TG was associated with 6-TGN levels ≥700 pmol/8 × 108 RBCs, but more research is needed to confirm such a cut-off value for TG in IBD.8
In conclusion, in 45% of IBD patients who failed conventional thiopurine derivatives, continuous corticosteroid-free clinical remission was maintained throughout at least 12 months with TG therapy. The continuous use of TG in 80% of the IBD patients, together with favourable PGA and patient-reported scores at 12 months, underlines the tolerability and effectiveness of TG as a potential therapy for IBD patients.
Contributor Information
Melek Simsek, Department of Gastroenterology and Hepatology, AGEM Research Institute, Amsterdam University Medical Centre, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Femke Schepers, Teva Pharmaceutical Industries, Haarlem, The Netherlands.
Sigal Kaplan, Teva Pharmaceutical Industries Ltd, Netanya, Israel.
Dirk van Asseldonk, Department of Gastroenterology and Hepatology, Noordwest ziekenhuisgroep, Alkmaar, The Netherlands.
Petra van Boeckel, Department of Gastroenterology and Hepatology, Sint Antonius, Nieuwegein, The Netherlands.
Paul Boekema, Department of Gastroenterology and Hepatology, Maxima Medical Centre, Veldhoven, The Netherlands.
Gerard Dijkstra, Department of Gastroenterology and Hepatology, University Medical Centre Groningen, Groningen, The Netherlands.
Herma Fidder, Department of Gastroenterology and Hepatology, University Medical Centre Utrecht, Utrecht, The Netherlands.
Ingrid Gisbertz, Department of Gastroenterology and Hepatology, Bernhoven Hospital, Uden, The Netherlands.
Frank Hoentjen, Department of Gastroenterology and Hepatology, Radboud University Medical Centre, Nijmegen, The Netherlands; Division of Gastroenterology, Department of Medicine, University of Alberta, Edmonton, Canada.
Bindia Jharap, Department of Gastroenterology and Hepatology, Meander Medical Centre, Amersfoort, The Netherlands.
Frank Kubben, Department of Gastroenterology and Hepatology, Maasstad Hospital, Rotterdam, The Netherlands.
Marleen de Leest, Department of Gastroenterology and Hepatology, Rijnstate Hospital, Arnhem, The Netherlands.
Maarten Meijssen, Department of Gastroenterology and Hepatology, Isala Clinics, Zwolle, The Netherlands.
Ana Petrak, Teva Pharmaceutical Industries, Haarlem, The Netherlands.
Else van de Poel, Teva Pharmaceutical Industries, Haarlem, The Netherlands.
Maurice Russel, Department of Gastroenterology and Hepatology, Medical Spectrum Twente, Enschede, The Netherlands.
Adriaan A van Bodegraven, Department of Gastroenterology, Geriatrics, Internal and Intensive Care Medicine (CO-MIK), Zuyderland Medical Centre, Heerlen-Sittard-Geleen, The Netherlands.
Chris J J Mulder, Department of Gastroenterology and Hepatology, AGEM Research Institute, Amsterdam University Medical Centre, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Nanne de Boer, Department of Gastroenterology and Hepatology, AGEM Research Institute, Amsterdam University Medical Centre, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Funding
This work was supported by TEVA Nederland B.V.
Conflict of Interest
M. Simsek has received an unrestricted research grant from TEVA. S. Kaplan, A. Petrak, E. van de Poel and F. Schepers are employees of TEVA. D. van Asseldonk served as speaker, adviser and/or principal investigator for DrFalk, Ferring, Galapagos/Gilead and Takeda and received research grants from Janssen, DrFalk and Noordwest Academie. H. Fidder has served a speaker for Ferring and Takeda. She has served a consultant for Takeda, Galapagos, Abbvie and Janssen-Cilag. She has received a grant from Takeda. F. Hoentjen has served on advisory boards or as speaker for Abbvie, Janssen-Cilag, MSD, Takeda, Celltrion, TEVA, Sandoz and Dr Falk. Funding [Grants/Honoraria], Takeda, Janssen-Cilag, Abbvie. Consulting Fees: Celgene. A. van Bodegraven has served as speaker, adviser and/or principal investigator for AbbVie, Arandal, ARENA, BMS, Celgene, Ferring, Galapagos, Janssen, MSD, Pfizer, Roche, Takeda and TEVA and received research grants from TEVA, Eurostars funding, ZonMW, Zuyderland MC and Pfizer. C. Mulder has served a principal investigator for TEVA and as consultant for Douglas Pharma and Arega. N. de Boer has served as a speaker for AbbVie, Takeda and MSD. He has served as consultant and principal investigator for Takeda and TEVA. He has received research grants from Dr. Falk, Takeda, TEVA and MLDS. The remaining authors have nothing to declare.
Authorship Statement
N. de Boer was the guarantor of the article. FS, SK, AP, EvdP, AvB, CM and NdB developed the protocol. All authors collected the data. MS, FS, SK, AP and NdB analysed the data. MS and FS prepared the first draft of the article. All authors reviewed the manuscript for important intellectual content. MS finalized the manuscript. All authors approved the final version of the manuscript.
References
- 1. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A.. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part I. Inflamm Bowel Dis 2018;24:742–51. [DOI] [PubMed] [Google Scholar]
- 2. Jharap B, Seinen ML, de Boer NK, et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis 2010;16:1541–9. [DOI] [PubMed] [Google Scholar]
- 3. Pouillon L, Bossuyt P, Peyrin-Biroulet L.. Considerations, challenges and future of anti-TNF therapy in treating inflammatory bowel disease. Expert Opin Biol Ther 2016;16:1277–90. [DOI] [PubMed] [Google Scholar]
- 4. Roda G, Jharap B, Neeraj N, Colombel JF.. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol 2016;7:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Al-Bawardy B, Shivashankar R, Proctor DD.. Novel and emerging therapies for inflammatory bowel disease. Front Pharmacol 2021;12:651415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Boer NK, van Asseldonk DP, van Bodegraven A.. ECCO consensus: evidence-based use of 6-thioguanine therapy in Crohn’s disease? J Crohns Colitis 2010;4:484–5. [DOI] [PubMed] [Google Scholar]
- 7. Bayoumy AB, Simsek M, Seinen ML, et al. The continuous rediscovery and the benefit–risk ratio of thioguanine, a comprehensive review. Expert Opin Drug Metab Toxicol 2020;16:111–23. [DOI] [PubMed] [Google Scholar]
- 8. Simsek M, Deben DS, Horjus CS, et al. Sustained effectiveness, safety and therapeutic drug monitoring of tioguanine in a cohort of 274 IBD patients intolerant for conventional therapies. Aliment Pharmacol Ther 2019;50:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meijer B, Mulder CJ, Peters GJ, van Bodegraven AA, de Boer NK.. Efficacy of thioguanine treatment in inflammatory bowel disease: a systematic review. World J Gastroenterol 2016;22:9012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pavlidis P, Ansari A, Duley J, Oancea I, Florin T.. Splitting a therapeutic dose of thioguanine may avoid liver toxicity and be an efficacious treatment for severe inflammatory bowel disease: a 2-center observational cohort study. Inflamm Bowel Dis 2014;20:2239–46. [DOI] [PubMed] [Google Scholar]
- 11. Dubinsky MC, Vasiliauskas EA, Singh H, et al. 6-Thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology 2003;125:298–303. [DOI] [PubMed] [Google Scholar]
- 12. Ferlitsch A, Teml A, Reinisch W, et al. 6-Thioguanine associated nodular regenerative hyperplasia in patients with inflammatory bowel disease may induce portal hypertension. Am J Gastroenterol 2007;102:2495–503. [DOI] [PubMed] [Google Scholar]
- 13. van Asseldonk DP, Jharap B, Verheij J, et al. The prevalence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with thioguanine is not associated with clinically significant liver disease. Inflamm Bowel Dis 2016;22:2112–20. [DOI] [PubMed] [Google Scholar]
- 14. van Asseldonk DP, Simsek M, de Boer NKH, et al. Limited relevance and progression of histological alterations in the liver during thioguanine therapy in inflammatory bowel disease patients. Scand J Gastroenterol 2019;54:753–60. [DOI] [PubMed] [Google Scholar]
- 15. Toksvang LN, Schmidt MS, Arup S, et al. Hepatotoxicity during 6-thioguanine treatment in inflammatory bowel disease and childhood acute lymphoblastic leukaemia: a systematic review. PLoS One 2019;14:e0212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irvine EJ, Zhou Q, Thompson AK.. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol 1996;91:1571–8. [PubMed] [Google Scholar]
- 17. Guideline on Good Pharmacovigilance Practices (GVP). https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf . Accessed July 28, 2017.
- 18. Lennard L, Maddocks JL.. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol 1983;35:15–8. [DOI] [PubMed] [Google Scholar]
- 19. Stournaras E, Qian W, Pappas A, et al. Thiopurine monotherapy is effective in ulcerative colitis but significantly less so in Crohn’s disease: long-term outcomes for 11 928 patients in the UK inflammatory bowel disease bioresource. Gut 2021;70:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rezazadeh Ardabili A, Jeuring S, Mujagic Z, et al. Classic drugs in the time of new drugs: real-world, long-term outcomes of thiopurine monotherapy in 1016 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2022;56:1030–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bayoumy AB, van Liere E, Simsek M, et al. Efficacy, safety and drug survival of thioguanine as maintenance treatment for inflammatory bowel disease: a retrospective multi-centre study in the United Kingdom. BMC Gastroenterol 2020;20:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savelkoul E, Maas M, Bourgonje AR, et al. Safety and drug survival of methotrexate versus tioguanine after failure of conventional thiopurines in patients with Crohn’s disease. J Crohns Colitis 2022;16:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 1990;11:787–97. [DOI] [PubMed] [Google Scholar]
- 24. Crouwel F, Simsek M, Mulder CJ, Buiter HJ, De Boer NK.. Thioguanine therapy in inflammatory bowel diseases. a practical guide. J Gastrointestin Liver Dis 2020;29:637–45. [DOI] [PubMed] [Google Scholar]
- 25. Crouwel F, Simsek M, de Boer MA, et al. Exposure to thioguanine during 117 pregnancies in women with inflammatory bowel disease. J Crohns Colitis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simsek M, Meijer B, Mulder CJJ, van Bodegraven AA, de Boer NKH.. Analytical pitfalls of therapeutic drug monitoring of thiopurines in patients with inflammatory bowel disease. Ther Drug Monit 2017;39:584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]