Abstract
Background and Aims
Widespread dysregulation of long non-coding RNAs [lncRNAs] including a reduction in GATA6-AS1 was noted in inflammatory bowel disease [IBD]. We previously reported a prominent inhibition of epithelial mitochondrial functions in ulcerative colitis [UC]. However, the connection between reduction of GATA6-AS1 expression and attenuated epithelial mitochondrial functions was not defined.
Methods
Mucosal transcriptomics was used to conform GATA6-AS1 reduction in several treatment-naïve independent human cohorts [n=673]. RNA pull-down followed by mass spectrometry was used to determine the GATA6-AS1 interactome. Metabolomics and mitochondrial respiration following GATA6-AS1 silencing in Caco-2 cells were used to elaborate on GATA6-AS1 functions.
Results
GATA6-AS1 showed predominant expression in gut epithelia using single cell datasets. GATA6-AS1 levels were reduced in Crohn’s disease [CD] ileum and UC rectum in independent cohorts. Reduced GATA6-AS1 lncRNA was further linked to a more severe UC form, and to a less favourable UC course. The GATA6-AS1 interactome showed robust enrichment for mitochondrial proteins, and included TGM2, an autoantigen in coeliac disease that is induced in UC, CD and coeliac disease, in contrast to GATA6-AS1 reduction in these cohorts. GATA6-AS1 silencing resulted in induction of TGM2, and this was coupled with a reduction in mitochondrial membrane potential and mitochondrial respiration, as well as in a reduction of metabolites linked to aerobic respiration relevant to mucosal inflammation. TGM2 knockdown in GATA6-AS1-deficient cells rescued mitochondrial respiration.
Conclusions
GATA6-AS1 levels are reduced in UC, CD and coeliac disease, and in more severe UC forms. We highlight GATA6-AS1 as a target regulating epithelial mitochondrial functions, potentially through controlling TGM2 levels.
Keywords: GATA6-AS1 long non-coding RNA, mitochondria, inflammatory bowel disease
1. Introduction
The molecular mechanisms of long non-coding RNAs [lncRNAs] in inflammatory bowel disease [IBD] and coeliac disease are not well understood. lncRNAs play a key role in transcription regulation,1–4 metabolic pathways5,6 and intestinal barrier functions,7,8 and have been implicated in human diseases.9 A large group of lncRNAs are considered antisense transcripts based on their genomic orientation in the vicinity of other genes, but their functions can be uncoupled from those.10 We previously identified widespread dysregulation of lncRNAs in ileal Crohn disease [CD],11 which showed comparable patient classifications to the dysregulated protein-coding genes, linking lncRNAs to CD pathogenesis. One of the CD-associated lncRNAs was GATA6-AS1, which was previously found to epigenetically regulate endothelial gene expression via interaction with LOXL2,12 and its expression was linked to gastric cancer.13 Interestingly, Gata6-as1 in murine models was shown to be highly expressed in intestinal stem cells while its knockout impaired epithelial regeneration.14 A recent study also documented GATA6-AS1 downregulation in a relatively small treatment-naïve ulcerative colitis [UC] cohort.15
Predicting Response to Standardized Pediatric Colitis Therapy [PROTECT] is a large prospective UC inception cohort study that examined factors associated with pathogenesis and responses to standardized therapy. We previously characterized rectal protein-coding gene expression and reported a robust reduction in epithelial mitochondrial genes and energy production pathways in UC,16 and defined genes and pathways linked to UC course.17,18 Here, we confirm the reduction of GATA6-AS1 in CD ileum and UC rectum in independent treatment-naïve cohorts. Using the PROTECT UC cohort patient outcome data, we show that GATA6-AS1 levels were further reduced in cases with more severe forms of UC, and in those with a less favourable UC outcome. Additionally, we show that GATA6-AS1 is predominantly expressed in epithelia in the human gut. We demonstrate a similar reduction in GATA6-AS1 expression in primary patient-derived enteroids and colonoids upon inflammatory stimuli and in gut epithelial cell lines. The GATA6-AS1 interactome included TGM2, an autoantigen in coeliac disease, and showed an overall enrichment for proteins involved in mitochondrial functions. GATA6-AS1 silencing in Caco-2 cells resulted in induction of TGM2, attenuated mitochondrial membrane potential, reduced mitochondrial respiration that was rescued upon TGM2 co-silencing, and affected the cellular metabolome with a reduction in tricarboxylic acid [TCA] cycle metabolites and aerobic respiration that are relevant for mucosal inflammation and tissue damage in the gut.
2. Methods [see also Supplementary Methods]
2.1. Cohorts, study approval and RNAseq analyses
PROTECT UC16,17 [Supplementary Table S1] and SEEM coeliac19 [Supplementary Table S3] were described previously. The Sheba Medical Center Institutional Review Board approved the SOURCE CD cohort [Supplementary Table S2] protocol. Informed consent was obtained. Other cohorts used for validation included the published RISK CD ileal and the UC rectal cohorts16,20 and the coeliac PRJNA52875521 cohort. GATA6-AS1 expression was calculated after uniformly re-analysing the transcriptomics raw FATSQ files. Reads were quantified via kallisto,22 including genes with transcripts per million [TPM] values above 1 in at least 20% of the samples. ToppGene23 and ToppCluster software were used to perform functional annotation enrichments, and Cytoscape v3.0.224 was used for visualization. To test gut cell-type-specific expression, the published human colon UC25 and CD26 single cells datasets were used.
2.2. GATA6-AS1 knockdown in cell culture
GATA6A-AS1 stable knockdown was achieved by infecting Caco-2 cells with GATA6-AS1-specific short hairpin RNA [shRNA] lentiviruses with puromycin selection. TGM2-specific short interfering [siRNAs] were used for transient inhibition of TGM2 expression. Stellaris RNA fluorescence in situ hybridization [FISH] was used to visualize GATA6-AS1 and MALAT1. siRNA/shRNA sequences, and quantitative PCR [qPCR] primers are in given Supplementary Tables S4 and S5, respectively.
2.3. lncRNA pull-down and mass spectrometry
Two pools of six tiling antisense oligos with BiotinTEG at the 3ʹ end were designed and used for identification of the GATA6-AS1 interactome. Bound proteins were analysed by mass spectrometry. GATA6-AS1 RNA pull-down oligos are given in Supplementary Table S6.
2.4. Mitochondrial functions, bioenergy tests and metabolomics
A Mito Stress Test and Glycolysis Stress Test were conducted using the Seahorse XFe96 Analyzer [Agilent Technologies]. Mitochondrial membrane potential was evaluated with JC1 [Rhenium]. Mitochondrial levels of reactive oxygen species [ROS] were assessed using a MitoSOX Red [M36008, ThermoFisher] assay. LC-MS analysis was conducted using a Dionex Ultimate ultra-high-performance liquid chromatography [UPLC] system coupled with an Orbitrap Q-Exactive mass spectrometer [Thermo Fisher Scientific].
2.5. Summary of statistical tests used
Pearson’s correlation was used for continuous variables and two-tailed t-tests were used for categorical variables in GraphPad Prism v9.31. Statistical significance is indicated by: *p < 0.05, **p < 0.01, ***p < 0.001.
2.6. Data availability
RNA sequencing [RNAseq] data from PROTECT [GSE109142], SEEM [GSE159495], RISK [rectal GSE117993, ileal GSE101794] and coeliac cohort [PRJNA528755[21]] were previously published and are available with the indicated accessions, and SOURCE [GSE199906] data were deposited in GEO.
3. Results
3.1. GATA6-AS1 is linked to epithelial metabolic functions and UC outcome
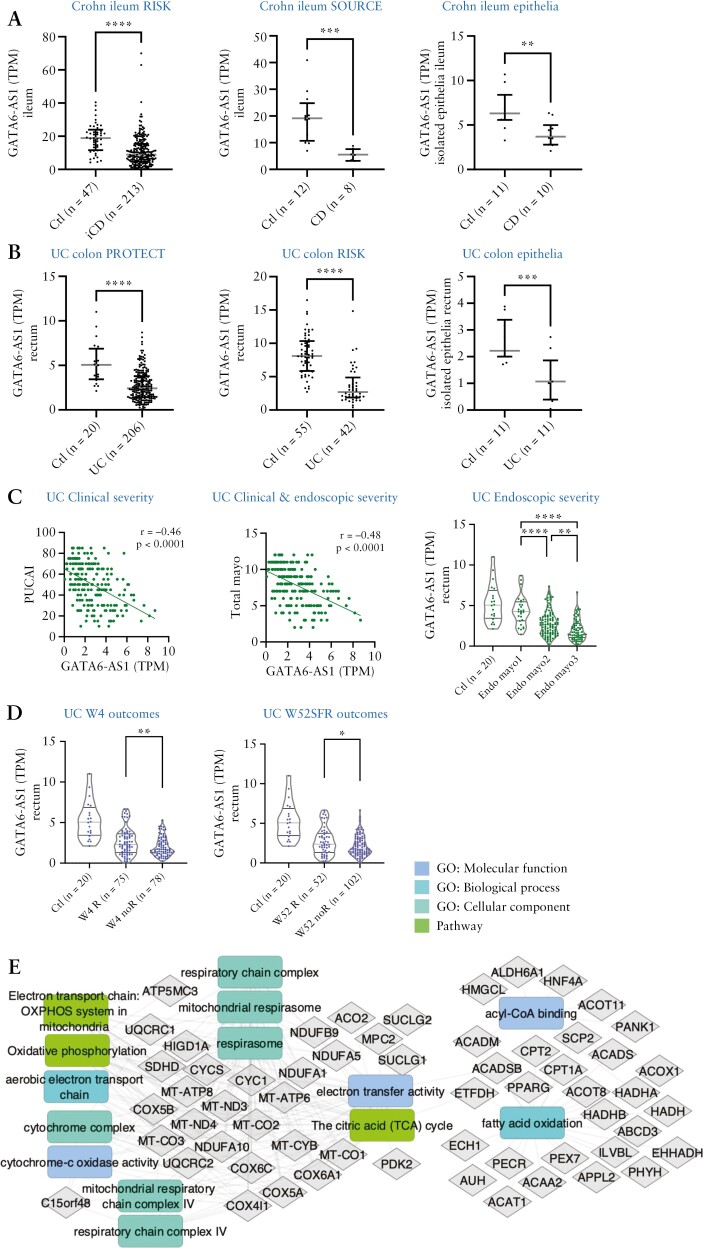
We identified widespread dysregulation of lncRNAs in ileal CD,11 and confirmed GATA6-AS1 reduction in the treatment-naïve paediatric RISK CD ileal cohort and in the adult SOURCE CD cohort [Figure 1A]. Furthermore, we demonstrated a reduction of GATA6-AS1 in the treatment-naïve PROTECT UC [Figure 1B] bulk biopsies and in independent RISK UC rectal samples. Reduction of GATA6-AS1 was noted also in a third cohort that used isolated epithelia from UC colon and CD ileum 27 [Figure 1A and B]. A reduction in GATA6-AS1 was also observed in non-inflamed ileum of CD and in isolated epithelia cells from CD ileum and UC rectum of non-inflamed samples [Supplementary Figure S1]. GATA6-AS1 showed a negative association with both clinical (Pediatric Ulcerative Colitis Activity Index [PUCAI] and total Mayo score; Figure 1C] and endoscopic UC severity [endoscopic Mayo scores; Figure 1C], and with poorer early UC (week 4 remission after 5-aminosalicylic acid [5-ASA]/steroids [W4R]) and late (week 52 steroid-free remission [W52SFR]) disease course [Figure 1D]. These results emphasize that highly inflamed tissue correlated with a higher reduction of GATA6-AS1, and further reduction in GATA6-AS1 within UC cases is linked to a less favourable disease course in UC. Since lncRNA functions remain elusive, protein-coding genes that co-expressed with lncRNA transcripts may offer insight into lncRNA functions that can direct the mechanistic epithelial studies of GATA6-AS1. We performed co-expression analyses within PROTECT, followed by functional annotation enrichment of the 684 co-expressed and better annotated protein coding genes [Figure 1E, Dataset S1]. Enriched terms significantly associated with GATA6-AS1 expression resulting from this analysis included lipid metabolic process (false discovery rate [FDR] < E-18), mitochondrial respirasome [FDR < E-9], cytochrome complex [FDR < E-7], the citric acid [TCA] cycle [FDR < E-10] and respiratory electron transport [FDR < E-9].
Figure 1.
GATA6-AS1 is reduced in UC, CD and coeliac disease, in isolated epithelia in CD and UC, and is further reduced in UC cases with less favourable outcome. [A–C] GATA6-AS1 mRNA expression in CD ileum [A; RISK, SOURCE, isolated epithelia27] and UC colon [B; PROTECT, RISK, isolated epithelia].27 Lines indicate median and upper and lower quartile. [C] GATA6-AS1 is further reduced in more severe UC cases based on clinical [PUCAI], combined clinical–endoscopic [Total Mayo score] and endoscopic disease severity [Endoscopic Mayo score] in the PROTECT cohort. [D] GATA6-AS1 mRNA reduction is linked to less favourable disease outcome [W4R or W52SFR] in the PROTECT cohort. GATA6-AS1 levels in controls are given as a reference. [E] Functional annotation enrichments using ToppGene/ToppCluster58 and Cytoscape24 of the GATA6-AS1 co-expression network in PROTECT using Euclidean distance [full list and FDR values are given in Supplementary Dataset S1].
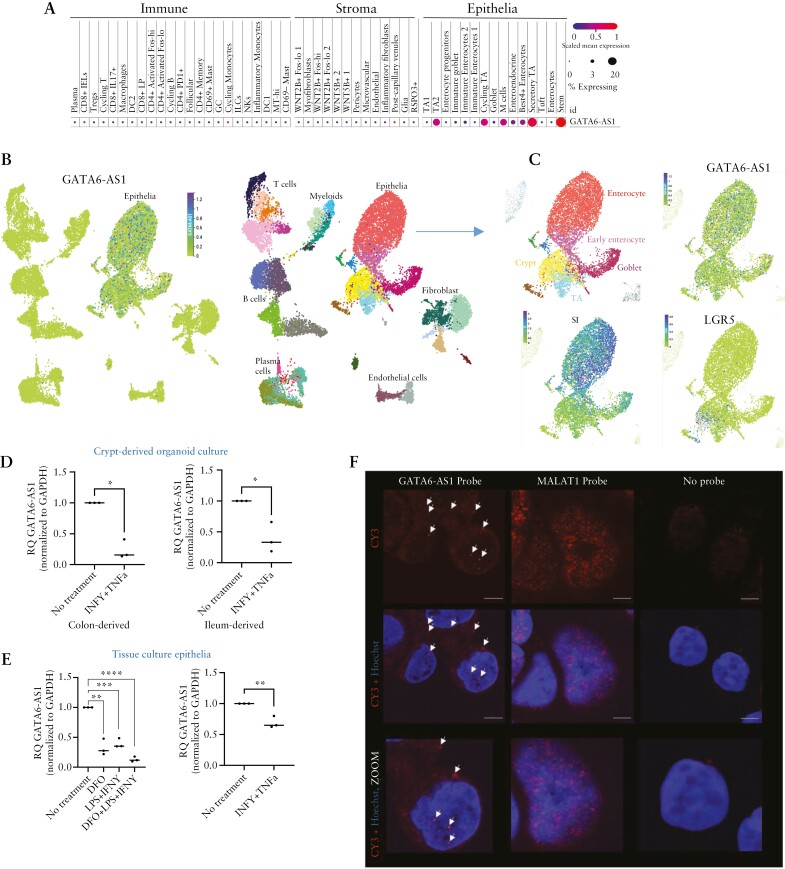
To examine cell-specific trends in gene expression, we analysed a publicly available human colon single-cell data set25 [Figure 2A] and ileum single-cell dataset26 [Figure 2B-C]. This indicated the expression of GATA6-AS1 in gut epithelia, with expression in stem cells/crypts, transient amplifying [TA] cells and early enterocytes. Polarized monolayer human-derived Caco-2 cells express a phenotype resembling that of intestinal enterocytes, including tight junctions and metabolizing enzymes, and can be used as a convenient and robust system to test the effect of genes on epithelial functions and regeneration.28 We have previously shown, using Caco-2 mRNAseq, that many of the lncRNAs11 including GATA6-AS1 are expressed in these cells. Specifically, we demonstrate similar GATA6-AS1 expression patterns between the mRNAseq reads in Caco-2 cells and in enriched epithelia from mucosal biopsies, further supporting the use of this system for functional exploration [Supplementary Figure S1F]. Since interferon-γ [IFNγ] and tumour necrosis factor α [TNFα] are highly upregulated in UC, and lipopolysaccharide [LPS] is a bacterial product increased in the circulation of UC patients,29 we used them in combination to stimulate primary human ileum and rectal differentiated organoids, and human-derived cell lines. Inflammatory triggers resulted in GATA6-AS1 reduction in cultures of primary ileum and rectal differentiated organoids [Figure 2D] and in Caco-2 and HT-29 cells [Figure 2E] similar to the reduction observed in the human cohorts. We also applied deferoxamine [DFO], an iron chelator that can induce mitochondrial toxicity30 and is also used as a hypoxia-mimetic agent that induces hypoxia-inducible factor-1alpha31 [HIF-1A, Supplementary Figure S1] [which is known to be upregulated in IBD16,20], with and without inflammatory triggers, with similar results showing reduction in GATA6-AS1. Unlike MALAT1, a well-described lncRNA with a predominant nuclear expression,32GATA6-AS1 was present in the cytoplasm and the nucleus as indicated by FISH [Figure 2F] and fractionation assays [Supplementary Figure S1E].
Figure 2.
GATA6-AS1 expression in the gut is confined to epithelia. [A–C] Cellular expression of GATA6-AS1 in single-cell dataset of human colon 25 [A] and ileum 26 [B and C]. The size and colour of the dots are proportional to the percentage of cells expressing the gene and the normalized expression level, respectively [A]. [B, C] Uniform manifold approximation and projection [UMAP] plot with the indicated cell types [B, left] and a map coloured by GATA6-AS1 expression [B, right] with further focus on epithelial cell types and expression of the mature epithelial and stem cell markers SI and LGR5 respectively [C], overall showing GATA6-AS1 specificity to epithelia. [D] GATA6-AS1 expression in patient-derived ileum and colon differentiated organoid culture, with and without 40 ng/ml IFNγ plus 20 ng/ml TNFα treatment. [E] GATA6-AS1 expression with and without 100 ng/ml LPS plus 40 ng/ml IFNγ and 100 µM DFO in HT29 [left] and 40 ng/ml IFNγ plus 20 ng/ml TNFα in Caco-2 cells. [F] Fluorescence in situ hybridization [FISH] showing GATA6-AS1 [red, arrows] distribution between the nucleus and cytoplasm in Caco-2 cells, compared to MALAT1 [red], which show predominant nuclear expression. Nuclei are stained with Hoechst [blue]; magnification ×63 oil; scale bar 10 µm. Results of a t-test or Spearman correlation with coefficients [r] are shown. All two-sided: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. GATA6-AS1 regulates epithelial mitochondrial and metabolic functions
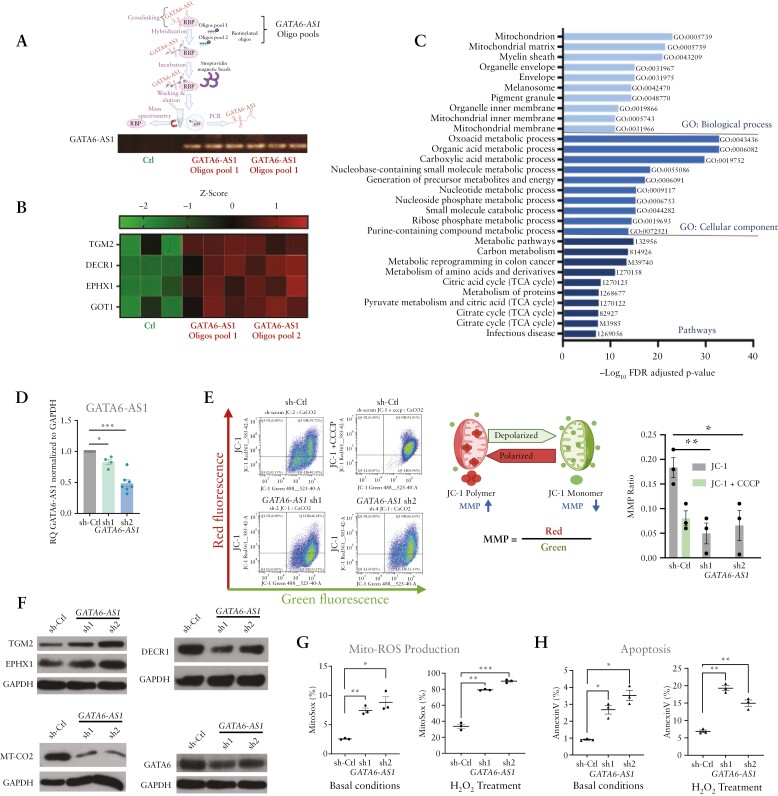
Mass spectrometry analysis of the GATA6-AS1 protein interactome, using affinity capture of the endogenous GATA6-AS1 RNA by two independent tiling GATA6-AS1 antisense oligo pools, identified 285 interacting proteins [FDR < 0.05]. Functional annotation of these proteins indicated strong and significant enrichments for metabolic processes, mitochondria and TCA cycle [Figure 3A–C and Supplementary Dataset S2], which was consistent with the GATA6-AS1 co-expression in patients, which was also significantly enriched for metabolic and mitochondrial functions [Figure 1E; Supplementary Dataset S1]. The GATA6-AS1 interactome included EPHX1, a microsomal epoxide hydrolase, DECR1, a mitochondrial enzyme involved in unsaturated fatty acid oxidation that is reduced in UC,16 and TGM2 tissue transglutaminase, which is an autoantigen in coeliac disease. This prompted us to also characterize GATA6-AS1 expression in coeliac disease and, interestingly, we also observed a reduction of GATA6-AS1 in two independent coeliac treatment-naïve cohorts, coupled with an induction of TGM2 in coeliac as well as in CD and UC epithelia [Supplementary Figure S2], with a significant negative correlation between GATA6-AS1 and TGM2 in vivo in UC, CD and coeliac human cohorts [Supplementary Figure S2].
Figure 3.
GATA6-AS1 regulates epithelial mitochondrial genes and function. [A] Schematic representation of GATA6-AS1 interactome pull-down experiments by two independent pools of tiling antisense biotinylated oligos. Binding of GATA6-AS1 RNA was confirmed by PCR. [B, C] The GATA6-AS1 interactome in differentiated Caco-2 cells identified 285 proteins using mass spectrometry [t-test: delta LFQ ≥ 0.1 and FDR ≤ 0.05; Supplementary Dataset S2]. [B] Heatmap of GATA6-AS1 top curated interacting targets identified by mass spectrometry; green and red colours indicate low and high binding respectively; Ctl columns are binding to non-specific control oligos. [C] Functional enrichments of the 285 protein targets indicated enrichment of mitochondrial functions [ToppGene/ToppCluster58]. [D] GATA6-AS1 silencing [sh1, sh2] was compared to scrambled non-specific shRNA [Ctl] using qRT-PCR [normalized to GAPDH and to controls]. [E] JC1 staining and FACS analysis were used to define the mitochondrial membrane potential [MMP]; schematic representation of MMP calculated as the ratio of red/green fluorescence. The analysis was performed in the absence or presence of 50 µM CCCP [carbonyl cyanide 3 chlorophenylhydrazone], which reduces MMP. Representative FACS images are presented, and the summarized experiments [n = 3] are shown as a bar-graph. [F] Representative western blots using specific antibodies in GATA6-AS1 knockdown cells indicated reduction of DECR1, MT-CO2 and GATA6, with induction of TGM2 and EPHX proteins. GAPDH was used as a loading control [three to four independent experiments are shown in Supplementary Figure S3]. [G, H] Mitochondrial ROS levels and apoptosis were measured using MitoSOX [G] and annexin V [H] in GATA6-AS1 knockdown cells at baseline or after treatment with 500 µM H2O2 for 3 h. All two-sided paired t-tests: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Schemes were created with biorender.com.
We recently showed a pronounced reduction of the mitochondrial membrane potential [MMP] using JC-1 staining in UC isolated epithelia.16 Therefore, based on the interactome data and the inferred function from the co-expression analyses in patients in the human cohorts, we functionally tested whether GATA6-AS1 reduction regulates associated mitochondrial functions. We observed an almost three-fold reduction in MMP following GATA6-AS1 silencing, from 0.22 in untreated control cells, compared to 0.08 upon GATA6-AS1 suppression with stable silencing using two independent shRNAs [sh1, sh2; Figure 3D], which is similar to the MMP measured with carbonyl cyanide 3-chlorophenylhydrazone [CCCP], which is known to depolarize the mitochondrial membrane and reduce the MMP [Figure 3E], and similar to our previous observation in samples obtained from patients.16 We next tested whether GATA6-AS1 regulates associated epithelial and mitochondrial targets. GATA6-AS1 silencing [sh1, sh2] resulted in induction of EPHX1 and TGM2 at the protein level [Figure 3F and Supplementary Figure S3A showing three to four replications]. By contrast, we noted a reduction of DECR1, which participates in beta-oxidation in the mitochondria, and reduction in the mitochondrially encoded MT-CO2 [part of mitochondria complex IV] and of GATA633 at the mRNA and protein levels [Figure 3F; Supplementary Figure S3B]. Consistent with recent publications, we also noted reduced mRNA levels of LGR5, an intestinal stem cell marker potentially linking mitochondrial genes and functions with epithelial renewal14 and a modest reduction in the ability of cells to form colonies [Supplementary Figure S3B], and a reduction of the nuclear-encoded mitochondrial ornithine transcarbamylase [OTC] that detoxifies ammonia to urea [Supplementary Figure S3C]. Looking at the ratio between isolated ileal enriched villi versus crypts obtained from the same patients indicated the expected reduction in LGR5 upon the villi–crypt axis, as well as a reduction in GATA6-AS1 and a modest induction in TGM2 [Supplementary Figure S3D], correspondingly to the negative correlation between GATA6-AS1 and TGM2 expression seen in patients. Previous studies indicated that TGM2 over-expression induces ROS production and apoptosis.34,35 Similarly, GATA6-AS1 silencing also resulted in higher mitochondrial ROS production [MitoSOX FACS assay] under basal conditions, which was further induced upon H2O2 treatment [Figure 3G], together with increased cell apoptosis [AnnexinV assay; Figure 3H], overall highlighting effects seen upon GATA6-AS1 reduction as seen with inflammatory triggers.
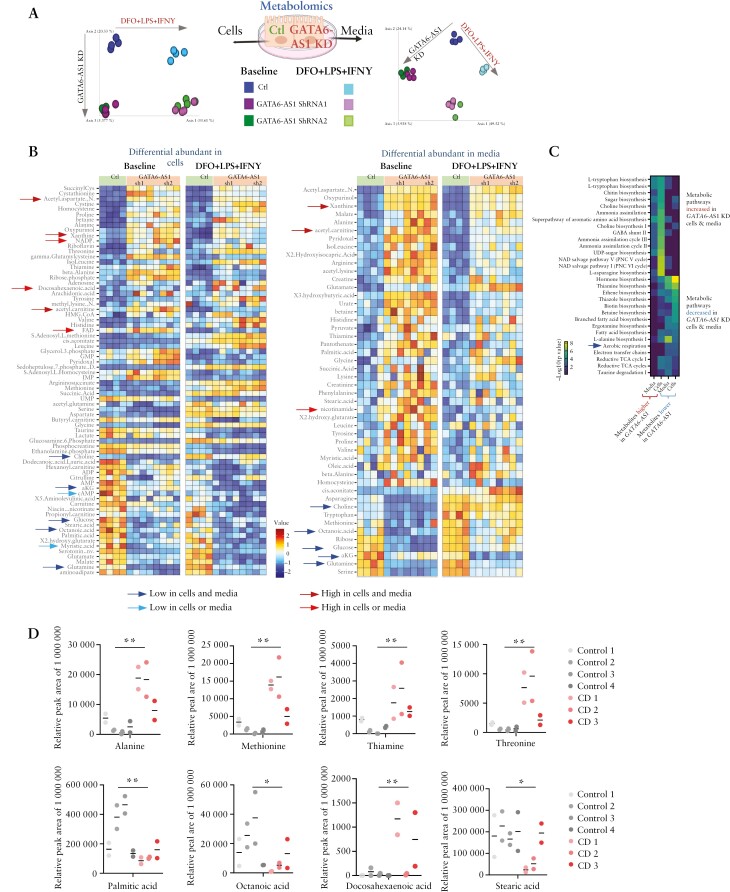
To capture more globally the metabolic state of the cells using a complementary approach, we applied untargeted metabolomics to control and GATA6-AS1-knockdown cells [sh1 and sh2] and their suspending media. Unbiased unsupervised principal coordinates analysis [PCoA] of the metabolomic profiles shows a robust separation by both treatment and GATA6-AS1 knockdown [Figure 4A] in the two independent GATA6-AS1 knockdowns. Seventy-four differentially expressed metabolites were identified in cells and 49 in their media [paired rank mean test, and FDR < 0.1, Supplementary Dataset S3], with a prominent reduction in the TCA cycle metabolites alpha-ketoglutarate [aKG] and malate, and in associated metabolites, including glutamate, aspartate and N-acetyl-aspartate [NAA, Figure 4B] upon GATA6-AS1 knockdown. Applying pathway enrichment using MetaCyc36 to those metabolites that were differentially abundant [increased or decreased] in cells and media between GATA6-AS1 and control indicated that the reduced metabolites in GATA6-AS1 knockdown cells were linked to reduced aerobic respiration, thiamine biosynthesis and branched fatty acid biosynthesis [FDR < 0.005, Figure 4C]. In contrast, increased metabolites in GATA6-AS1 knockdown cells were enriched in pathways that included NAD salvage, ammonia assimilation, and amino acid and sugar metabolism [FDR < 0.005, Figure 4C]. These metabolomic data complement our results, showing strong enrichment of the GATA6-AS1 interactome with mitochondrial metabolic function and TCA cycle, reduction of the mitochondrial membrane potential, and reduced cell respiration. Forty-eight of the 74 metabolites were also detected in the faeces of CD and control patients. Metabolites that were reduced or increased upon GATA6-AS1 silencing [Figure 4B] showed greater persistency in their fold change difference when comparing CD and controls [Mann–Whitney p = 0.014 and binary change χ2p = 0.005 on the fold change between CD and controls], probably suggesting the changes seen in vivo. Examples included higher levels of alanine, methionine, thiamine, threonine and docosahexaenoic acid and a reduction in palmitic, octanoic and stearic acids [Figure 4D].
Figure 4.
GATA6-AS1 regulates cellular metabolism. [A–C] Untargeted metabolomics was used to capture cellular metabolites and metabolites secreted to the media in GATA6-AS1 knockdown and control cells, untreated or treated with 100 µM DFO + 100 ng/ml LPS + 40 ng/ml IFNγ. [A] Schematic representation of the experiment and PCoA plot of the extracted metabolites based on Canberra distance, indicating differences by both GATA6-AS1 expression and by treatment. [B] Heatmap representing significantly different metabolites in cells [left heatmap] and their media [right heatmap, rank-mean test and FDR < 0.1] between GATA6-AS1 knockdown [sh1 and sh2] and control cells, with visualization of the metabolites in both treated and untreated cells [full comparisons in Supplementary Dataset S3]. Blue and red arrows indicate metabolites that were lower or higher in cells and/or media respectively. [C] Heatmap of the –log10[FDR] enriched pathways obtained from metabolites increased [left two columns] or decreased in the media and cells [right two columns]. The pathways in the upper panel are increased and those in the lower panel are decreased in GATA6-AS1 silencing. [D] Peak area of specific faecal metabolites that differed in GATA6-AS1 knockdown vs controls and between CD and controls. Each case submitted two faecal samples at least 2 months apart and those are shown separately for each control and CD subject. Mann–Whitney test with *p < 0.05 and **p < 0.01.
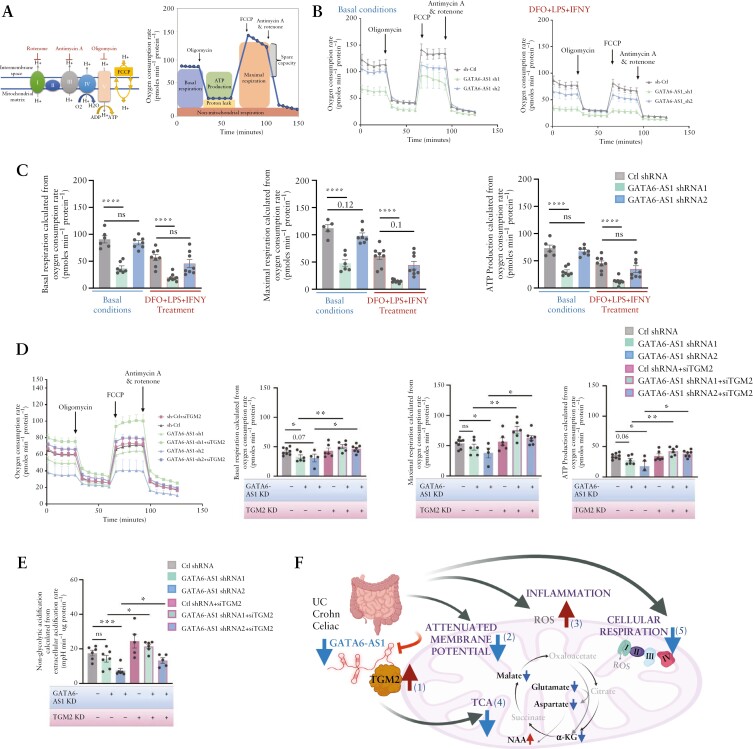
Previous studies indicated that TGM2 over-expression reduced basal mitochondrial respiration.37 A reduction in the oxygen consumption rate [OCR] measured by the Seahorse XFe analyser showed a persistent effect of GATA6-AS1 silencing on mitochondrial respiration [Figure 5A–D], with reduced basal and maximal respiration and ATP production that reached statistical significance in most GATA6-AS1 sh1 as indicated in Figure 5C and D, and showed a similar trend with GATA6-AS1 shRNA2 in Figure 5C and reached significance in maximal respiration and ATP production in Figure 5D. Mitochondrial respiration was also reduced in control cells treated with DFO + LPS + IFNγ, and this was more pronounced upon GATA6-AS1 knockdown. Finally, we examined whether reducing TGM2 in GATA6-AS1 stable knockdown cells can rescue the reduced cellular mitochondrial respiration [OCR] caused by GATA6-AS1 silencing. TGM2 co-silencing recovered the OCR levels of GATA6-AS1 knockdown cells to levels that were at least as high as in controls [Figure 5D] with no effect on GATA6-AS1 levels upon TGM2 reduction [Supplementary Figure S4A and B]. Moreover, measurement of the extracellular acidification rate [ECAR] indicated a reduction in non-glycolytic acidification, linked to CO2 from the TCA cycle or the breakdown of intracellular glycogen [i.e. glycogenolysis], which was again rescued by reducing TGM2 upon GATA6-AS1 knockdown [Figure 5E and Supplementary Figure S4C and D]. In summary, our results indicate that GATA6-AS1 levels were reduced in gut epithelia of UC, CD and coeliac patients. Previous data have already indicated impaired mitochondrial function in UC and CD epithelia.38,39 Here we showed [Figure 5F schematic illustration] that GATA6-AS1 is associated with a complex that includes TGM2 [1], and that GATA6-AS1 silencing resulted in TGM2 induction [1], which resulted in attenuation of mitochondrial membrane potential [2], higher production of ROS [3], reduction of TCA metabolites [4] and reduced cellular respiration [5], which together hamper epithelial barrier function and can exacerbate the mucosal inflammatory cascade.
Figure 5.
GATA6-AS1 silencing induces TGM2 expression and inhibits mitochondrial respiration while TGM2 co-silencing recovered mitochondrial respiration. [A] Schematic representation of the mitochondrial electron transport chain [I, II, III, IV] and ATP synthase [complex V], with the modulators included in the Seahorse XF Cell Mito Stress Test. [B, C] Mito Stress Test measurement of oxygen consumption rate [OCR] in basal conditions and after sequential injections of oligomycin, FCCP and rotenone/antimycin A [B] in GATA6-AS1 knockdown and control Caco-2 cells without [left] and with [right] 100 µM DFO + 100 ng/ml LPS + 40 ng/ml IFNγ; changes in basal respiration, ATP production and maximal respiration were calculated [C]. [D] OCR [as in Figure 4B and C] in GATA6-AS1 knockdown and control cells transiently transfected with TGM2 or control siRNA to test whether reduction of TGM2 can rescue the GATA6-AS1 knockdown effect of cellular respiration. Changes in basal respiration, ATP production and maximal respiration. [E] Extracellular acidification rate [ECAR] was measured [see also Supplementary Figure S4]; non-glycolytic acidification was calculated and found to differ between GATA6-AS1 knockdown and control cells, and to be rescued by reduction of TGM2. Individual values are shown in the graph with their mean. All two-sided paired t-tests: *p < 0.05, **p < 0.01, ***p < 0.001. [F] Concluding scheme; GATA6-AS1 is reduced in gut epithelia of UC, CD and coeliac cases, and mitochondrial function is impaired in epithelia of UC and CD patients.16,38,39 To model our observations in patients mechanistically, we showed that GATA6-AS1 binds a complex that includes TGM2 [1]. GATA6-AS1 silencing resulted in TGM2 induction, similarly to the induction seen in patients [1]. Reduction of GATA6-AS1 and TGM2 induction caused attenuation of mitochondrial membrane potential [2], higher production of mitochondrial ROS [3], reduction of TCA metabolites [4] and reduced cellular respiration [5]. Schemes were created with biorender.com.
4. Discussion
The human genome encodes thousands of lncRNAs,40 many of which have been shown to be involved in key cellular processes41 and implicated in human disease,9 increasing the motivation to understand their functions. Using several treatment-naïve cohorts, we demonstrated a reduction of GATA6-AS1 in CD, UC and coeliac disease. Isolated epithelial and single-cell datasets implied that GATA6-AS1 shows epithelial-specific expression in the gut. Furthermore, GATA6-AS1 reduction was associated with a more severe UC form, and with less favourable clinical outcomes. Several independent and complementary methodologies [enrichment of co-expressed genes in human patients and the GATA6-AS1 interactome in tissue culture] indicated that GATA6-AS1 plays a key regulatory role in epithelial metabolic and mitochondrial functions. This was further confirmed in vitro upon GATA6-AS1 silencing, resulting in an overall reduction in mitochondrial aerobic respiration. GATA6-AS1 interacting with TGM2, their significant negative correlation in UC, CD and coeliac cohorts, and the induction of TGM2 upon GATA6-AS1 silencing, together with previous studies linking higher TGM2 expression to mitochondrial dysfunction and ROS production,34 suggested that GATA6-AS1 acts as a regulator of TGM2. Reducing TGM2 levels in GATA6-AS1 knockdown cells rescued mitochondrial respiration, indicating that mitochondrial respiration can be regulated by modifying GATA6-AS1 or TGM2 levels, with implications for epithelial integrity and functions relevant to gastrointestinal pathologies. TGM2, also known as tissue transglutaminase and as the autoantigen implicated in coeliac disease, catalyses several key post-translation functions including the crosslinking and polyamination42 of proteins, and the deamidation of gliadin43 [a component of wheat gluten]. It is possible that TGM2 induction causes atypical cross-linking of protein and results in structural changes featuring cell death 44, thereby exposing large aberrant cellular and dietary proteins45 to the immune system, fuelling inflammation.
The consequences of impaired cellular respiration and mitochondrial dysfunction affect inflammation and tissue damage in IBD16,38,39 but the molecular mechanism is not known.38 Abnormal mitochondria function exacerbates barrier dysfunction and inflammation, and various pro-inflammatory46 and anti-inflammatory47 stimuli affect mitochondrial metabolic functions. Mitochondrial dysfunction caused by metabolic damage is prominent mostly in oxidative stress,48 causing excessive production of mitochondrial ROS, which may lead to apoptosis.49 Reduction of GATA6-AS1 in epithelial and mucosal biopsies was noted in UC, CD and coeliac disease, in non-inflamed samples, and with inflammatory triggers in different epithelial model systems, indicating that inflammation and potentially other factors contribute to the regulation of GATA6-AS1 expression. Mimicking GATA6-AS1 reduction in a model system led to mitochondrial ROS accumulation and induction of apoptosis, induction of TGM2, attenuation of mitochondrial membrane potential, reduction of mitochondrial respiration, and a robust effect on cellular metabolomics, with a reduction of several TCA cycle metabolites, including aKG, relevant to disease. GATA6-AS1 silencing showed a significant signal similar to the faecal metabolomics signal seen in CD patients compared to healthy controls. aKG supplementation was reported to protect against epithelial damage and to ameliorate DSS-induced colitis.50 The increased levels of NADP and FAD with reduction of NAD+ and aspartate in GATA6-AS1 knockdown cells and the increased levels of nicotinamide in the media may suggest a effect on complex 1 activity.51 Complex 1 maintains the cellular NAD+ pool and the NAD+/NADH ratio, which sustain activity of mitochondrial malate dehydrogenase [MDH2] and the generation of aspartate,51 and aspartate biosynthesis is an essential function of respiration in proliferating cells.52 These metabolomic data complement our GATA6-AS1 interactome enrichment for proteins linked to the TCA cycle, and the cellular experiments showing attenuation of the mitochondrial membrane potential and cell respiration.
TGM2 has also been shown to regulate Ca2+ flux to mitochondria. TGM2-over-expressing cells were previously shown to exhibit a low basal OCR,37 as seen in our system upon GATA6-AS1 knockdown. Reducing TGM2 levels upon GATA6-AS1 knockdown rescued mitochondrial respiration, indicating that GATA6-AS1 regulation of mitochondrial function may occur through GATA6-AS1’s interaction and regulation of TGM2. We reduced GATA6-AS1 expression using post-transcriptional inhibition with shRNA, which was also shown to affect GATA6 levels as previously noted.14 Interestingly, reducing GATA6 levels in trastuzumab-resistant gastric cell lines53 re-sensitized resistant cells to trastuzumab, and inhibited mitochondrial function and metabolites linked to the TCA cycle. A recent GATA6-AS1 murine model also showed expression of GATA6-AS1 in the small and large intestine, localization to both the nuclear and cytoplasm fraction, expression in dividing epithelial cells, with regulation of epithelial viability, and reduction of LGR5 upon GATA6-AS1 silencing,14 similarly to our findings. Our results align with previous publications showing that higher TGM237 and lower GATA653 are linked to altered mitochondrial metabolic function, and that a reduction of GATA6-AS1 effects GATA6 expression and epithelial viability,14 where we now show that GATA6-AS1 regulates GATA6 and TGM2 levels, thereby resulting in altered mitochondrial function, which was recovered by TGM2 inhibition in our system. Besides using GATA6-AS1 as a biomarkers for diagnosis, severity and outcome, it can be delivered as a therapeutic target using lipid nanoparticles to improve outcome in patients who do not respond to available therapies.
A notable strength of our study was the use of large well-designed cohorts of treatment-naïve patients, free from confounding variables of previous therapy. Other strengths include independent validation in other cohorts and in isolated and single-cell intestinal epithelia. We used diverse complementary wet-lab and informatics approaches, including transcriptomics, metabolomics, proteomics and specific mitochondrial functional assays, to cross-validate our results, suggesting that GATA6-AS1 regulates mitochondrial functions. Study limitations include the use of treatment-naïve samples, which are likely to be inflamed, although we supplemented those showing GATA6-AS1 reduction also in non-inflamed samples. We used a tissue culture model rather than an animal model to establish the role of the GATA6-AS1 function as there may be species-related differences in lncRNA functions.54–57 Although our results are consistent with previous murine models showing expression of GATA6-AS1 in epithelia,14 we suggest an alternative regulatory explanation through metabolic regulation for this outcome. Additionally, our study included two different shRNAs for GATA6-AS1 downregulation with different efficacies of knockdown, although both knockdowns resulted in upregulation of TGM2 and similar response in the mitochondrial functions and in the metabolome signals. In conclusion, we show that GATA6-AS1 is reduced in UC rectum, CD ileum and coeliac duodenum, and GATA6-AS1 reduction is linked to a more severe UC form and to less favourable outcomes. Mimicking GATA6-AS1 reduction in a model system led to mitochondrial ROS accumulation and induction of apoptosis, induction of TGM2, attenuation of mitochondrial membrane potential, reduction of mitochondrial respiration and a robust effect on cellular metabolomics. TGM2 reduction in GATA6-AS1 knockdown cells rescued mitochondrial respiration. We therefore suggest that GATA6-AS1 can be used as a biomarker and as a target to boost proper epithelial function and mucosal healing to improve outcomes.
Supplementary Material
Acknowledgements
We gratefully acknowledge PROTECT, RISK and SEEM co-investigators. We also thank all participants in the study.
Contributor Information
Katya E Sosnovski, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Tzipi Braun, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel.
Amnon Amir, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel.
Danielle Moshel, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Marina BenShoshan, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Kelli L VanDussen, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Nina Levhar, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Haya Abbas-Egbariya, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Katia Beider, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel.
Rakefet Ben-Yishay, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel.
Syed Asad Ali, Department of Pediatrics and Child Health, Aga Khan University, Karachi, Pakistan.
Sean R Moore, Department of Pediatrics, University of Virginia, Charlottesville, VA, USA.
Subra Kugathasan, Emory University, Atlanta, GA, USA.
Ifat Abramovich, The Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Bat Galim, Haifa, Israel.
Efrat Glick Saar, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel.
Batya Weiss, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Iris Barshack, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Eyal Gottlieb, The Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Bat Galim, Haifa, Israel.
Tamar Geiger, Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel.
Shomron Ben-Horin, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel.
Igor Ulitsky, Departments of Biological Regulation and Molecular Neuroscience, Weizmann Institute of Science, Rehovot, Israel.
Jeffrey S Hyams, Connecticut Children’s Medical Center, Hartford, CT, USA.
Lee A Denson, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Yael Haberman, Sheba Medical Center, Tel-Hashomer, affiliated with the Tel Aviv University, Tel Aviv, Israel; Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Conference presentation
This wa presented in part at the UEG Week 2021 and received the National Scholar Awards.
Funding
This work was supported by the ERC starting grant [YH, grant No. 758313], the Israel Science Foundation [YH, grant No. 908/15], the I-CORE programme [YH, grant No. 41/11], the Helmsley Charitable Trust, and NIDDK P30 DK078392 [Integrative Morphology and Gene Expression Cores]. PROTECT was supported by the NIDDK 5U01DK095745, RISK was supported by Crohn’s & Colitis Foundation, SEEM by the Bill and Melinda Gates Foundation [OPP1144149 and OPP1138727], and SOURCE is supported by the Helmsley Charitable Trust. The funding sources did not play a role in writing of the manuscript or the decision to submit it for publication, did not play a role in data collection, analysis or interpretation; trial design; patient recruitment; or any aspect pertinent to the study.
Conflict of Interest
The following authors have no conflicts of interest: KES, TB, AA, MB, KLV, NL, HAE, KB, RBY, SAA, SRM, SK, IA, EGS, BW, IB, EG, TG, SBH, IU, LAD, YH. JSH: Advisory Board Janssen, consultant: Takeda, Pfizer, Bristol Myers Squibb, Boehringer Ingleheim, and Eli Lilly.
Author Contributions
KES, TB, AA, JSH, LAD and YH conceived and designed the study, analysed the data, and wrote the first draft of the manuscript. DM, MBS, KVD, NL, HAE, KB, RBY, MS, BW, IB, IA, EGS, EG, TG, SBH and IU generated and analysed the data and participated in drafting the manuscript. SAA, SRM and SK recruited patients, collected and analysed data, and participated in drafting the manuscript. All authors had access to study data and approved the decision to submit the manuscript.
Data Availability
RNA sequencing [RNAseq] data from PROTECT [GSE109142], SEEM [GSE159495], RISK [rectal GSE117993, ileal GSE101794] and coeliac cohort [PRJNA528755[21]] were previously published and are available with the indicated accessions, and SOURCE [GSE199906] data were deposited in GEO.
References
- 1. Gomez JA, Wapinski OL, Yang YW, et al. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell 2013;152:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NE II, Heward JA, Roux B, et al. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun 2014;5:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hong SH, Han G, Lee SL, et al., Testicular germ cell-specific lncRNA, Teshl, is required for complete expression of Y chromosome genes and a normal offspring sex ratio. Sci Adv 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allou L, Balzano S, Magg A, et al. Non-coding deletions identify Maenli lncRNA as a limb-specific En1 regulator. Nature 2021;592:93–8. [DOI] [PubMed] [Google Scholar]
- 5. Sirey TM, Roberts K, Haerty W, et al., The long non-coding RNA Cerox1 is a post transcriptional regulator of mitochondrial complex I catalytic activity. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin YH. Crosstalk of lncRNA and cellular metabolism and their regulatory mechanism in cancer. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang ZF, Zhang L.. LncRNA: a potential research direction in intestinal barrier function. Dig Dis Sci 2021;66:1400–8. [DOI] [PubMed] [Google Scholar]
- 8. Chen SW, Wang P-Y, Liu Y-C, et al. Effect of long noncoding RNA H19 overexpression on intestinal barrier function and its potential role in the pathogenesis of ulcerative colitis. Inflamm Bowel Dis 2016;22:2582–92. [DOI] [PubMed] [Google Scholar]
- 9. Wapinski O, Chang HY.. Long noncoding RNAs and human disease. Trends Cell Biol 2011;21:354–61. [DOI] [PubMed] [Google Scholar]
- 10. Guttman M, Rinn JL.. Modular regulatory principles of large non-coding RNAs. Nature 2012;482:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haberman Y, BenShoshan M, Di Segni A, et al. Long ncRNA landscape in the ileum of treatment-naive early-onset Crohn disease. Inflamm Bowel Dis 2018;24:346–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neumann P, Jaé N, Knau A, et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat Commun 2018;9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li ZT, Zhang X, Wang D-W, et al. Overexpressed lncRNA GATA6-AS1 inhibits LNM and EMT via FZD4 through the Wnt/beta-Catenin signaling pathway in GC. Mol Ther Nucleic Acids 2020;19:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Zhu P, Wu J, Wang Y, et al. LncGata6 maintains stemness of intestinal stem cells and promotes intestinal tumorigenesis. Nat Cell Biol 2018;20:1134–44. [DOI] [PubMed] [Google Scholar]
- 15. Ray MK, Fenton CG, Paulssen RH.. Novel long non-coding RNAs of relevance for ulcerative colitis pathogenesis. Noncoding RNA Res 2022;7:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haberman Y, Karns R, Dexheimer PJ, et al. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanisms underlying disease severity and treatment response. Nat Commun 2019;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hyams JS, Davis Thomas S, Gotman N, et al. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study. Lancet 2019;393:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hyams JS, Brimacombe M, Haberman Y, et al., Clinical and host biological factors predict colectomy risk in children newly diagnosed with ulcerative colitis. Inflamm Bowel Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haberman Y, qbal NT, Ghandikota S, et al., Mucosal genomics implicate lymphocyte activation and lipid metabolism in refractory environmental enteric dysfunction. Gastroenterology 2021;160:2055–2071.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haberman Y, Tickle TL, Dexheimer PJ, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest 2014;124:3617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leonard MM, Bai Y, Serena G, et al. RNA sequencing of intestinal mucosa reveals novel pathways functionally linked to celiac disease pathogenesis. PLoS One 2019;14:e0215132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bray NL, Pimentel H, Melsted P, Pachter L.. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 2016;34:525–7. [DOI] [PubMed] [Google Scholar]
- 23. Chen J, Bardes EE, Aronow BJ, Jegga AG.. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 2009;37:W305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biton M, Ordovas-Montanes J,Smillie CS, et al., Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019;178:714–730.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elmentaite R, Ross ADB, Roberts K, et al., Single-cell sequencing of developing human gut reveals transcriptional links to childhood Crohn’s disease. Dev Cell 2020;55:771–783.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howell KJ, Kraiczy J, Nayak KM, et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology 2018;154:585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hidalgo IJ, Raub TJ, Borchardt RT.. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989;96:736–49. [PubMed] [Google Scholar]
- 29. Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D.. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res 2000;6:205–14. [PubMed] [Google Scholar]
- 30. Kim BM, Choi JY, Kim YJ, Woo HD, Chung HW.. Desferrioxamine (DFX) has genotoxic effects on cultured human lymphocytes and induces the p53-mediated damage response. Toxicology 2007;229:226–35. [DOI] [PubMed] [Google Scholar]
- 31. Bianchi L, Tacchini L, Cairo G.. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res 1999;27:4223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tripathi V, Ellis JD, Shen Z, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010;39:925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whissell G, Montagni E, Martinelli P, et al. The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nat Cell Biol 2014;16:695–707. [DOI] [PubMed] [Google Scholar]
- 34. Ruan Q, Quintanilla RA, Johnson GV.. Type 2 transglutaminase differentially modulates striatal cell death in the presence of wild type or mutant huntingtin. J Neurochem 2007;102:25–36. [DOI] [PubMed] [Google Scholar]
- 35. Wang F, Wang L, Qu C, et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021;21:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caspi R, Billington R, Keseler IM, et al. The MetaCyc database of metabolic pathways and enzymes – a 2019 update. Nucleic Acids Res 2020;48:D445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar S, Donti TR, Agnihotri N, Mehta K.. Transglutaminase 2 reprogramming of glucose metabolism in mammary epithelial cells via activation of inflammatory signaling pathways. Int J Cancer 2014;134:2798–807. [DOI] [PubMed] [Google Scholar]
- 38. Mottawea W, Chiang C-K, Mühlbauer M, et al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat Commun 2016;7:13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rath E, Moschetta A, Haller D.. Mitochondrial function - gatekeeper of intestinal epithelial cell homeostasis. Nat Rev Gastroenterol Hepatol 2018;15:497–516. [DOI] [PubMed] [Google Scholar]
- 40. Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 2015;47:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ulitsky I, Bartel DP.. lincRNAs: genomics, evolution, and mechanisms. Cell 2013;154:26–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farrelly LA, Thompson RE, Zhao S, et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 2019;567:535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Molberg O, Mcadam SN, Körner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med 1998;4:713–7. [DOI] [PubMed] [Google Scholar]
- 44. Melino G, Annicchiarico-Petruzzelli M, Piredda L, et al. Tissue transglutaminase and apoptosis: sense and antisense transfection studies with human neuroblastoma cells. Mol Cell Biol 1994;14:6584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willis WL, Wang L, Wada TT, et al. The proinflammatory protein HMGB1 is a substrate of transglutaminase-2 and forms high-molecular weight complexes with autoantigens. J Biol Chem 2018;293:8394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab 2015;21:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R.. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017;356:513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim SH, Kim H.. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction–a mini-review. Nutrients 2018;10:1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khaloian S, Rath E, Hammoudi N, et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut 2020;69:1939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tian Q, Bravo Iniguez A, Sun Q, Wang H, Du M, Zhu M-J.. Dietary alpha-ketoglutarate promotes epithelial metabolic transition and protects against DSS-induced colitis. Mol Nutr Food Res 2021;65:e2000936. [DOI] [PubMed] [Google Scholar]
- 51. Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM.. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015;162:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG.. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015;162:552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chang J, Wang Q, Bhetuwal A, Liu W.. Metabolic pathways underlying GATA6 regulating trastuzumab resistance in gastric cancer cells based on untargeted metabolomics. Int J Med Sci 2020;17:3146–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghanam AR, Bryant WB, Miano JM.. Of mice and human-specific long noncoding RNAs. Mamm Genome 2022;33:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guo CJ, Ma XK, Xing YH, et al., Distinct processing of lncRNAs contributes to non-conserved functions in stem cells. Cell 2020;181:621–636.e22. [DOI] [PubMed] [Google Scholar]
- 56. Paralkar VR, Mishra T, Luan J, et al. Lineage and species-specific long noncoding RNAs during erythro-megakaryocytic development. Blood 2014;123:1927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schorderet P, Duboule D.. Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet 2011;7:e1002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ.. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res 2010;38:W96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing [RNAseq] data from PROTECT [GSE109142], SEEM [GSE159495], RISK [rectal GSE117993, ileal GSE101794] and coeliac cohort [PRJNA528755[21]] were previously published and are available with the indicated accessions, and SOURCE [GSE199906] data were deposited in GEO.