Abstract
Background
Si-Miao-Yong-An decoction (SMYAD) is a conventional therapeutic formula for treat thromboangiitis obliterans (TAO), consisting of four Chinese herbs: Lonicerae japonicae Thunb. (Jinyinhua), Scrophularia ningpoensis Hemsl. (Xuanshen), Angelica sinensis (Oliv.) Diels (Danggui) and Glycyrrhiza uralensis Fisch. (Gancao). However, the mechanism of SMYAD in TAO treatment remains unclear.
Methods
Components, as well as potential targets of SMYAD in TAO therapy, were downloaded from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP). Subsequently, with the Database for Annotation, Visualization, and Integrated Discovery (DAVID) server, the gene ontology (GO) biological processes and the Kyoto encyclopedia of genes and genomes (KEGG) signalling pathways of the targets enrichment were performed. Next, based on STRING online database, the protein interaction network of vital targets was built and analysed. Molecular docking and calculation of the binding affinity were performed using AutoDock. The PyMOL software was employed to observe docking outcomes of active compounds and protein targets. Based on the predicted outcomes of network pharmacology, in vivo and in vitro tests were performed for validation. In vivo experiment, the TAO rats model was established using sodium laurate injection into the femoral artery. The symptoms as well as pathological changes of the femoral artery were observed. Besides, the predicted targets were verified by the RT-qPCR, in vitro experiment. The cell viability in LPS-induced human umbilical vein endothelial cells (HUVECs) was detected using CCK-8 kit, and the predicted targets were also verified by the RT-qPCR.
Results
In the network pharmacology analysis, we obtained 105 chemical components in SMYAD and 24 therapeutic targets. We found that the mechanism SMYAD in TAO therapy was primarily associated with inflammation and angiogenesis by constructing multiple networks. Quercetin, vestitol and beta-sitosterol were important compounds, and interleukin-6 (IL6), MMP9, and VEGFA were key targets. According to molecular docking, active compounds (quercetin, vestitol and beta-sitosterol) and targets (IL6, MMP9 and VEGFA) showed good binding interactions. In in vivo experiment, SMYAD ameliorated the physical signs and pathological changes, inhibited the expression of IL6 and MMP9, and enhanced the expression of VEGFA. In an in vitro experiment, SMYAD increased the cell viability of LPS-induced HUVECs and the expression of VEGFA, and reduced the expression of IL6 and MMP9.
Conclusions
This study showed that SMYAD improves TAO symptoms and inhibits the development of TAO. The mechanism could be associated with anti-inflammatory and therapeutic angiogenesis.
Keywords: Si-Miao-Yong-An decoction, thromboangiitis obliterans, network pharmacology, mechanism, molecular docking
1. Introduction
As a chronic, occlusive, nonatherosclerotic, inflammatory vascular disease, thromboangiitis obliterans (TAO) targets small- and medium-sized arteries as well as veins in the extremities. As a global disease, TAO is distributed all over the globe, but its prevalence is more common in the Middle East and East Asia [1]. In China, the distribution of TAO patients has geographical and ethnic differences; for example, the incidence rate is higher in the north of the Yellow River, especially in the northeast, and the incidence rate of Hui is higher than that of Han in the northwest [2]. The average age of onset of TAO is from 30 to 40 years, and fewer patients are over 40 years old. However, the number of affected elderly patients has increased in recent decades [3].
The etiology and pathogenesis of TAO are unclear. TAO is causally associated with tobacco use, and the disease is also related to cold, malnutrition and trauma [4]. Currently, the main therapeutic drugs for TAO include iloprost, aspirin, cilostazol, etc. [5]. Although the above medicines can partially relieve the clinical features of TAO patients, they are linked to several side effects, including bleeding, weakened fertility and hepatic injury [6]. Traditional Chinese Medicine (TCM) is a crucial complementary and substitute approach with a long history in TAO therapy. It can be tracked back to Yellow Emperor’s Classic of Internal Medicine and is characterized by greater availability, affordability, limited side effects and improved safety [7].
Si-Miao-Yong-An decoction (SMYAD), a classical TCM prescription, was first published in the Qing Dynasty [8], consisting of four Chinese herbs: Lonicerae Japonicae Thunb. (Jinyinhua), Scrophularia ningpoensis Hemsl. (Xuanshen), Angelica sinensis (Oliv.) Diels (Danggui) and Glycyrrhiza uralensis Fisch. (Gancao). SMYAD has been used to successfully treat TAO, which can effectively relieve pain and skin swelling in the lower limbs of TAO patients [9]. It has been included in the Guideline for Diagnosis and Treatment of Common Diseases in the Peripheral Vascular Department (2015) as a recommended prescription for the stasis-toxin-corruption retention syndrome TAO [10]. However, studies on its pharmacological mechanism in the treatment of TAO are limited.
Network pharmacology is a field that projects the active components in prescriptions and elucidates their probable mechanism from a systemic viewpoint. The ‘components-targets-diseases’ network, based on the systematic interaction of herbs, components, targets and diseases, is consistent with the holistic approach to diagnosis and therapy informed by the fundamentals principles of Chinese medicine and proves to be a more appropriate technique to analyse multiple ingredients, targets and pathways of Chinese medicine [11]. However, the outcomes suggested by network pharmacology are simply speculative based on information at hand; they are not confirmed outcomes. As such, experiments were combined to validate the outcomes of network pharmacology.
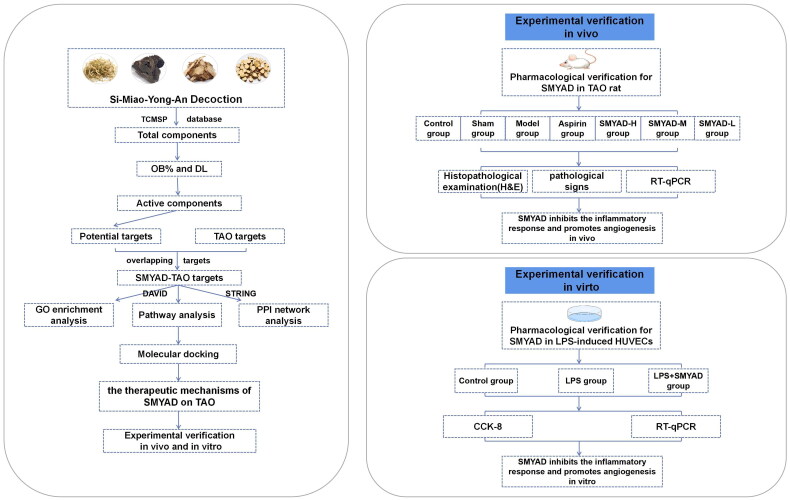
First, the components and targets of SMYAD were obtained in treating TAO. Second, we performed a functional enrichment analysis of potential targets and constructed correlation networks to elucidate the associations between components, targets and pathways. Then, we constructed therapeutic modules based on the ‘pathways-phenotypes’ network, and holistically determined the mode of action of SMYAD in TAO treatment. Immediately, we performed protein–protein interaction (PPI) network analysis to screen out the key targets for SMYAD in the treatment of TAO, and applied molecular docking to calculate the binding energy between the main active ingredients and the key targets. Finally, based on the network pharmacology results, in vivo and in vitro tests were conducted to validate the anti-inflammatory and angiogenic mechanisms of SMYAD. Figure 1 presents the research flowchart for this study.
Figure 1.
Experimental technical roadmap.
2. Materials and methods
2.1. Active components and targets collection of SMYAD
Components of the four herbs of SMYAD were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform [12] (TCMSP, https://tcmspw.com/tcmsp.php). As active ingredients, we selected compounds with oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18. Gene names corresponding to targets were identified from the protein database Uniprot (https://www.uniprot.org).
2.2. Collection of therapeutic targets for TAO
The keywords ‘Buerger’s disease’ and ‘thromboangiitis obliterans’ were used to search through the Drugbank database [13] (https://go.drugbank.com/), the genecards database [14] (https://www.genecards.org/) and OMIM database (https://omim.org/).
2.3. Gene ontology (GO) analysis and pathway enrichment analysis
Overlapping drug targets and diseases were imported into Database for Annotation, Visualization, and Integrated Discovery (DAVID) web server (https://david.ncifcrf.gov/) for GO biological processes and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis. The Bioinformatics online visualization program was used to create bubble maps (https://www.bioinformatics.com.cn/).
2.4. Network construction and analysis
To comprehend the complex interactions between herbs, components and targets, CytoScape 3.8.2 was adopted to construct a ‘herbs–components–targets’ network [15]. In the network, nodes denote herbs, components and targets, and the associations between nodes are depicted by edges. The ‘degree’ was used to calculate the edges linked to each node, which indicates the significance of the nodes in the network.
2.5. Protein–protein interaction network
Overlapping targets were mapped into the STRING database (https://cn.string-db.org/), the species was restricted to ‘Homo sapiens’, the least required interaction score was set at ‘high confidence (0.700)’, and save the results. The results were inputted into CytoScape 3.8.2 to create a PPI network, and the node size as well as colour reflects the degree.
2.6. Molecular docking
The molecule structure (Mol2 structure) of the main active compounds of the herbs was downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/). The crystal structure of the core protein targets was extracted from the Protein Data Bank (PDB, https://www1.rcsb.org/) structure. Molecular docking and calculation of the binding affinity were performed using AutoDock (https://ccsb.scripps.edu/projects/). The PyMOL software (version 2.2, https://PyMOL.org/2/) was employed to observe docking outcomes of active compounds and protein targets.
2.7. Experimental validation
2.7.1. Animals and SMYAD preparation
Male Sprague-Dawley rats (6–8 weeks old) with weights ranging from 180 to 220 g were brought from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The animals were kept in an SPF-grade animal room at the Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences, with ad libitum access to food and water, a 12 h light/dark cycle, temperature at 22–26 °C as well as the relative humidity of 40–60%. The Animal Ethics Committee of the Experimental Animal Center, Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences (IBTCMCACMS21) approved all the animal experiments.
SMYAD is composed of four Chinese herbal slices: 15 g of Jinyinhua, 15 g of Xuanshen, 9 g of Danggui and 9 g of Gancao. The four Chinese herbal slices were obtained from CR SANJIU Pharmaceutical Co., Ltd. (Shenzhen, China) to ensure drug consistency. Table 1 shows comprehensive drug information. The preparation method of water decoction is as follows: added 500 ml of distilled water to all the Chinese herbal slices, boiled for 50 min, then filtered the decoction. Boiled four Chinese herbal slices and filtered the decoction again as above. Finally, mix the two decoctions and concentrate them to a concentration of 2 g/ml. The prepared SMYAD was stored at −20 °C. The four Chinese herbal slices of SMYAD were stored in the laboratory of the Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences.
Table 1.
The composition of SMYAD.
| Botanical name | Chinese herbal name | Species | Medicinal parts | Item number (production area) | Weight (g) |
|---|---|---|---|---|---|
| Flos Lonicerae | Jinyinhua | Lonicera japonica Thunb. | Flowers | 20200903 (Anhui, China) | 15 |
| Radix Scrophulariae | Xuanshen | Scrophularia ningpoensis Hemsl. | Roots | 200313 (Hubei, China) | 15 |
| Radix Angelica Sinensis | Danggui | Angelica sinensis (Oliv.) Diels | Roots | 201028 (Gansu, China) | 9 |
| Radix Glycyrrhizae | Gancao | Glycyrrhiza uralensis Fisch. | Roots | 20210201 (Inner Mongolia, China) | 9 |
The preparation method of freeze-dried powders is as follows: the resultant water decoction of SMYAD was concentrated to 4 g/ml and pre-frozen at −20 °C for 8 h before lyophilization. The frozen mixture was sublimated for 10 h (20 Pa, −5 °C), desorbed for 10 h (20 Pa, 45 °C), heat preserved for 4 h (20 Pa, 45 °C) and the complete freeze-drying cycle is 32 h. The freeze-dried powders were sealed and stored in at −20 °C until use. Before cell experiment, a certain amount of freeze-dried powders was weighed and diluted with DMEM.
2.7.2. TAO model and drug treatments
Through a random approach, male SD rats were categorized into seven groups, each group having eight animals: control group, sham-operated group, TAO model group, positive control group (aspirin, 10 mg/kg/d, p.o.), high-dose SMYAD group (SMYAD-H, 9 g/kg/d, p.o.), medium-dose SMYAD group (SMYAD-M, 4.5 g/kg/d, p.o.) and low-dose SMYAD group (SMYAD-L, 2 g/kg/d, p.o.). TAO model was developed as per the experimental protocol of Ashida et al. [16]. The animals were briefly anesthetized, fixed and shaved the medial femoral hair of the left hind leg, and the femoral artery was exposed via surgical incision and muscle retraction. The femoral artery was clamped using an artery clamp at the proximal end, and 0.2 ml of sodium laurate solution (10 mg/ml, adjusted to pH 8.0) (S30244, Yuanye, Shanghai, China) was injected 0.3 cm below the artery clamp; the sham-operated group was injected with 0.2 ml of saline, and the arterial clip was taken out 15 min after the injection. After the surgical wound was closed, penicillin was administered to all groups to prevent infection. The rats were administered SMYAD orally 24 h after surgery, and the same volume of saline was given to the control and sham-operated groups for 28 days. The clinical-equivalent dosage was administered following the U.S. Food and Drug Administration recommendations [17].
2.7.3. Symptoms
After the procedure, regular checks were performed to observe skin temperature, colours, arterial pulse, limb swelling, the level of gangrene, and mummification progression of the hind legs. Photographed every seven days during the study.
2.7.4. Histological examination
The rats were executed, the left hind limb femoral artery was isolated, and the 2–3 cm long femoral artery was intercepted from below the puncture site, soaked in 10% formalin, and stained with haematoxylin and eosin (H&E). Pathological changes were visualized under an optical microscope and captured at ×100 magnification.
2.7.5. Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from the School of Pharmacy, Chengdu University of Traditional Chinese Medicine (Chengdu, China). HUVECs were cultured in 4% DMEM (Gibco, Carlsbad, CA) supplemented with 10% FBS (Gibco, Carlsbad, CA) and 1% penicillin/streptomycin (PYG0016, BOSTER, Wuhan, China) and kept at 37 °C in an incubator containing 5% CO2.
2.7.6. Cell viability assay
Cell viability was detected by Cell Counting Kit-8 (CCK-8) assay (CK-04, Dojindo, Kumamoto, Japan). A total of 5 × 103 cells/well were seeded into 96-well plates and incubated for 24 h. HUVECs were stimulated with 200 μg/ml SMYAD and 10 μg/ml LPS (L6134, Sigma, St. Louis, MO) for 24 h. Then, 10 μl of CCK-8 was added to each well, and the samples were incubated for 2 h. Finally, the samples were evaluated according to the optical density (OD) measured at 450 nm using a microplate reader (DG235, Thermo Fisher Scientific, Waltham, MA).
2.7.7. RT-qPCR mediated analysis of mRNA expression
The RNA Extraction Solution (Servicebio, G3013, Wuhan, China) was utilized to extract total RNA from the femoral artery. The RNA Extraction Kit was (R0024, Beyotime, Shanghai, China) utilized to extract total RNA from HUVECs. RT First Strand cDNA Synthesis Kit (Servicebio, G3330, Wuhan, China) was applied to reverse transcribe the RNA as per the manufacturer’s instructions. Eventually, relative mRNA expression was computed utilizing the 2–ΔΔCT method. The primer sequences are provided in Supplementary Table S1.
2.7.8. Data analysis
The SPSS 23.0 software was used to perform all statistical analyses, and the outcomes of at least three independent tests were taken. The outcomes of each group were displayed as mean ± standard error of the mean (SEM). Data between multiple groups were compared through One-way ANOVA. p < 0.05 represented a statistically significant difference.
3. Results
3.1. Active component and targets collection in SMYAD
After searching and screening (OB ≥ 30%, DL ≥ 0.18), 105 chemical components (Supplementary Table S2) and 253 targets (Supplementary Table S3) for SMYAD were obtained from TCMSP, including 17 chemical components and 210 action targets for Jinyinhua, seven chemical components, and 185 action targets for Xuanshen, two chemical components and 47 action targets for Danggui, 87 chemical components and 227 action targets for Gancao. In addition, beta-sitosterol was shared by Jinyinhua, Xuanshen and Danggui, and quercetin and kaempferol were shared by Jinyinhua and Gancao.
Furthermore, we obtained 601 TAO-related targets from three databases, including four targets from the Drugbank database, 60 targets from the genecards database and 545 targets from the OMIM database (Supplementary Table S4).
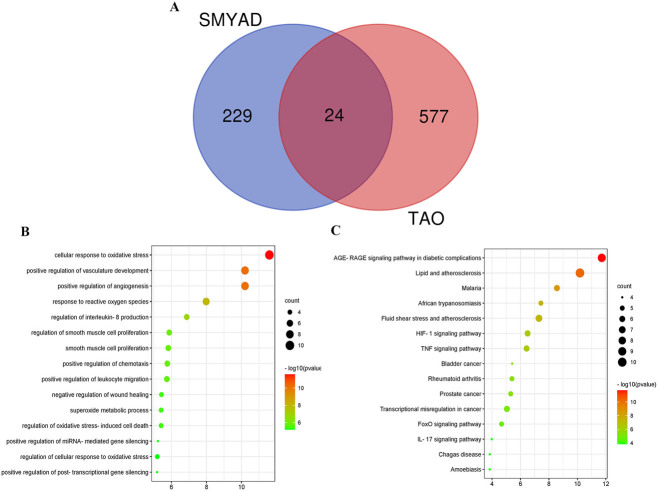
Finally, 253 targets of SMYAD and 601 TAO-related targets are mapped to the Venn. A total of 24 overlapping targets were obtained (Figure 2(A)), details of which are shown in Supplementary Data Table S5.
Figure 2.
Functional enrichment analysis. (A) The Venn diagram of the potential targets in SMYAD and the therapeutic targets for TAO. (B) GP biological process enrichment analysis of 24 TAO treatment targets in SMYAD. (C) KEGG pathway enrichment analysis of 24 TAO treatment targets in SMYAD.
3.2. Gene ontology and KEGG pathway enrichment analysis
We performed GO biological process enrichment analysis to evaluate the SMYAD mechanism for TAO therapy. The top 15 biological process enrichments were plotted in a bubble diagram (Figure 2(B)). The findings revealed that 24 potential therapy targets were implicated in a variety of biological processes, such as cellular response to oxidative stress; positive regulation of angiogenesis, and vasculature development; response to reactive oxygen species; interleukin-8 (IL-8) production regulation; regulation of smooth muscle cell proliferation; smooth muscle cell proliferation; positive regulation of chemotaxis; positive regulation of leukocyte migration; negative regulation of wound healing; superoxide metabolic process; regulation of oxidative stress-induced cell death; positive regulation of miRNA-mediated gene silencing; regulation of cellular response to oxidative stress; positive regulation of post-transcriptional gene silencing.
In addition, pathway enrichment was performed for 24 potential therapeutic targets, and the top 15 significantly enriched pathways are shown in Figure 2(C). Consequently, the major enrichment pathways include AGE-RAGE signalling pathway in diabetic disorders, lipid and atherosclerosis, malaria, African trypanosomiasis, fluid shear stress and atherosclerosis, HIF-1 signalling pathway, tumour necrosis factor (TNF) signalling pathway, bladder cancer, bladder cancer, transcriptional misregulation in cancer, FoxO signalling pathway, IL-17 signalling pathway and Chagas disease. These pathways may regulate TAO-related pathological processes.
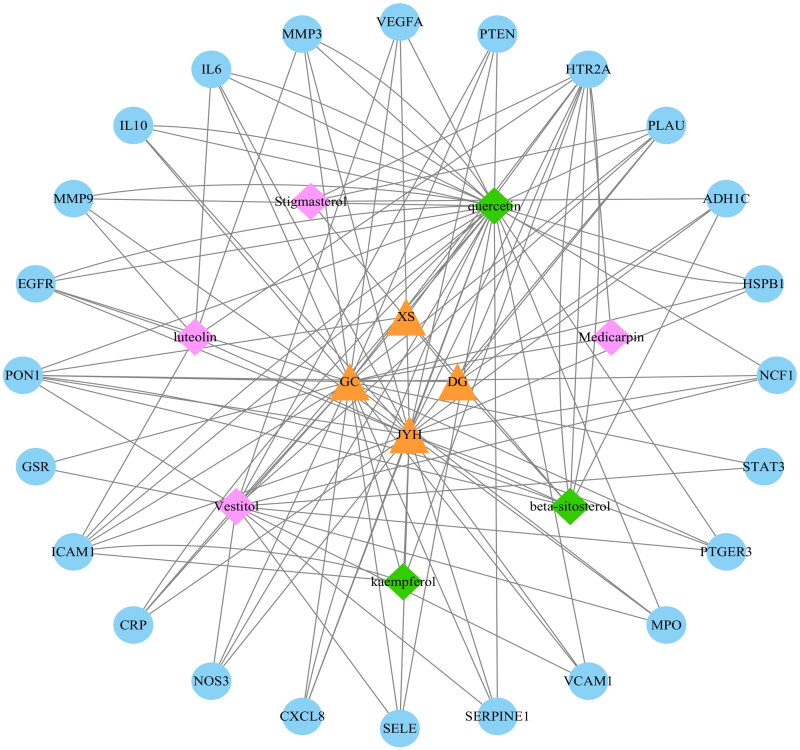
3.3. Herbs–compounds–targets network analysis
As shown in Figure 3, CytoScape 3.8.2 was applied to create an ‘herbs–components–targets’ network to reveal the overall function of SMYAD. The network comprised 35 nodes and 122 edges. Yellow triangle denotes the herbs, pink diamond depicts the components, the green diamond represents the common components, and blue circle denotes the targets. Among the four herbs, the degree value of Jinyinhua (26) was greater than most of the other drugs, and enriched almost all of the TAO therapeutic targets, indicating that Jinyinhua plays a more important role in influencing TAO therapeutic targets than most of the other drugs. Quercetin (31), vestitol (14) and beta-sitosterol (9) were the top three ingredients in terms of degree, which may be the key ingredients for the therapeutic effect of SMYAD. Most of the 24 targets were associated with different drugs and components, suggesting that SMYAD has multi-target effects. Interleukin-6 (IL6) (13), CXCL8 (13) and serotonin receptor 2A gene (HTR2A) (11), as the main enriched targets, may be the main targets of SMYAD for TAO treatment.
Figure 3.
The herbs–compounds–targets interaction network.
3.4. KEGG pathway analysis
The phenotypes of 15 pathways were collected to evaluate the mechanism of SMYAD-mediated therapy of TAO after which a ‘pathways–phenotypes’ network was constructed (Figure 4). The 15 pathways corresponded to five different phenotypes, including inflammation, angiogenesis, apoptosis, immunity and oxidative stress. Among them, angiogenesis and inflammation were the two phenotypes with the highest correlation with the above pathways. The pathways involved in angiogenesis are the AGE-RAGE signalling pathway in diabetic complications, Fluid shear stress, and atherosclerosis, the HIF-1 signalling pathway, rheumatoid arthritis and bladder cancer. The pathways involved in inflammation were lipid and atherosclerosis, AGE-RAGE signalling pathway in diabetic complications, fluid shear stress and atherosclerosis, TNF signalling pathway, HIF-1 signalling pathway, rheumatoid arthritis, Chagas disease and IL-17 signalling pathway. This suggested that SMYAD may treat TAO through anti-inflammation and regulation angiogenesis.
Figure 4.
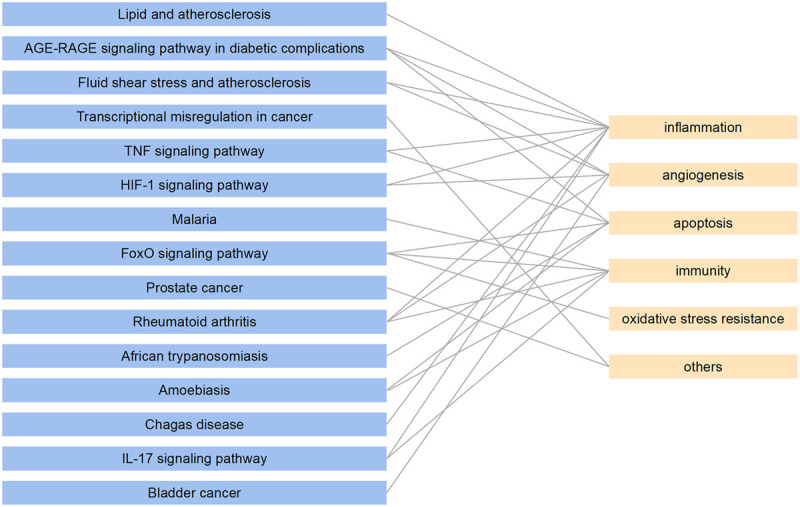
The pathways–phenotypes interaction network.
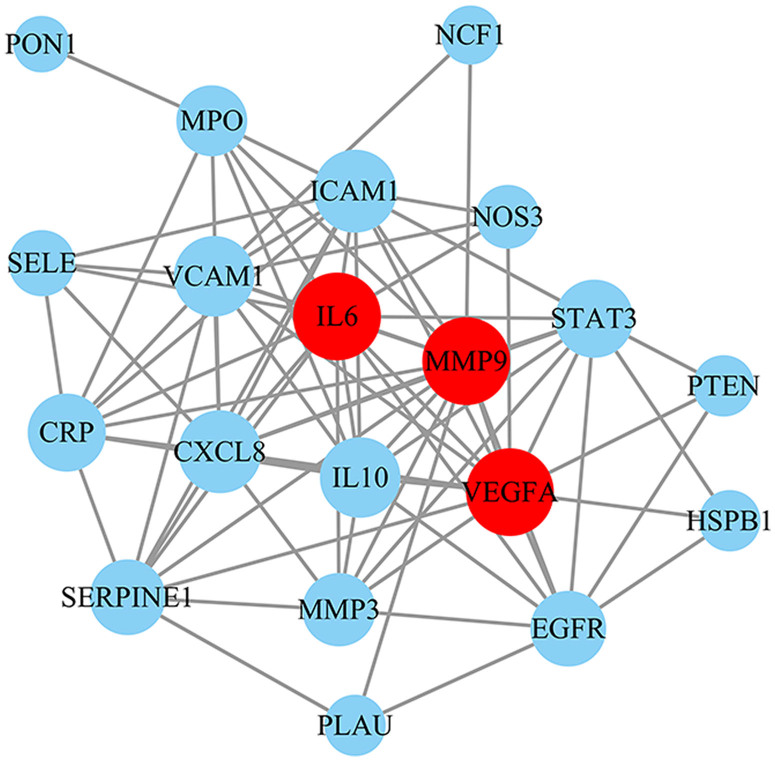
3.5. PPI network analysis
To explore the vital targets for SMYAD treatment of TAO, 24 overlapping targets were inputted into the STRING database to construct the PPI network. The PPI network had 20 nodes (four nodes disconnected from the network graph were removed) and 81 edges with an average node degree of 8.10 (Figure 5). Then, the network was topologically analysed using three algorithms, including degree, betweenness and closeness centralities (Supplementary Table S6). The top three of the degree are also the top five of the betweenness centrality and closeness centrality. Therefore, the top three of the degree are selected as key targets, including matrix metallopeptidase 9 (MMP9), vascular endothelial growth factor A (VEGFA) and IL6.
Figure 5.
Protein–protein interaction network.
3.6. Molecular docking analysis
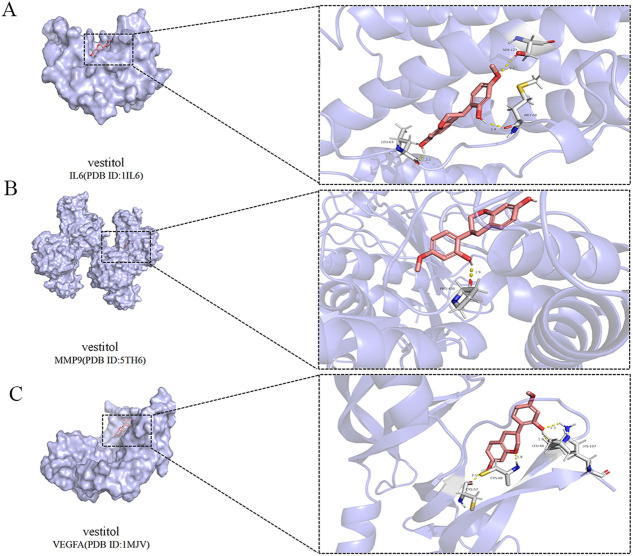
We docked three key targets (IL6, MMP9 and VEGFA) obtained from the PPI with the main compounds in SMYAD (quercetin, vestitol and beta-sitosterol) to verify compound–target interactions. The binding affinity lower than −5.0 kcal/mol revealed good interactions of the confirmations. The results of binding affinity were shown that vital active compounds and major targets showed good binding interactions. The targets IL6, MMP9 and VEGFA showed the strongest affinity to vestitol in the three chemical compositions. Table 2 illustrates the results of binding affinity. Figure 6 shows the conformations of vestitol and major targets.
Table 2.
The results of molecular docking.
| PBD ID | Target | Mol ID | Chemical name | Binding energy |
|---|---|---|---|---|
| 1IL6 | IL6 | MOL000098 | Quercetin | –3.67 |
| MOL000358 | Beta-sitosterol | –5.43 | ||
| MOL000500 | Vestitol | –5.91 | ||
| 5TH6 | MMP9 | MOL000098 | Quercetin | –4.42 |
| MOL000358 | Beta-sitosterol | –6.03 | ||
| MOL000500 | Vestitol | –7.47 | ||
| 1MJV | VEGFA | MOL000098 | Quercetin | –4.22 |
| MOL000358 | Beta-sitosterol | –6.57 | ||
| MOL000500 | Vestitol | –7.72 |
Figure 6.
Molecular docking of TAO related targets with main compounds of SMYAD. (A) Vestitol binds to protein IL6. (B) Vestitol binds to protein MMP9. (C) Vestitol binds to protein VEGFA.
3.7. Experimental validation of SMYAD in TAO rats
Network pharmacology analysis revealed that SMYAD treatment of TAO involves a variety of potential mechanisms, including inflammation and angiogenesis. IL6, MMP9 and VEGFA are the key targets. Therefore, we verified the three targets through in vivo and in vitro experiments.
3.7.1. SMYAD relieves left hind limb symptoms
Rats in the control and the sham groups had normal skin colour and activity of the left hind limbs, and no ulceration or gangrene appeared during the experiment period. The rats in the other groups revealed different degrees of violet and swelling after modelling, and showed limp and dragline phenomenon, or even ulceration and gangrene, which was in line with symptoms of patients with TAO (Figure 7). On the 7th day, the left hind limb of rats in TAO group fell off, a part of the left hind limb of rats in SMYAD-L/M group fell off, and only the paw of rats in aspirin group and SMYAD-H group fell off. On the 28th day, the rat thighs of the SMYAD-L group fully fell off and ulcers were noted in the rat thighs of the SMYAD-M group; the rats in the aspirin group and SMYAD-H group had no ulcers. Therefore, SMYAD can inhibit the progression of TAO, and achieves similar efficacy to aspirin.
Figure 7.
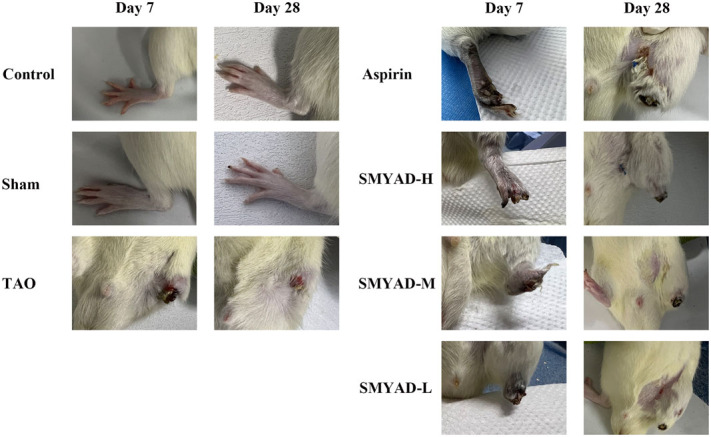
Appearance of the left hind limbs of rats. Control: control group, sham: sham-operated group, aspirin: positive control group, TAO: TAO model group, SMYAD-L: SMYAD low-dose group, SMYAD-M: SMYAD medium-dose group and SMYAD-H: SMYAD high-dose group.
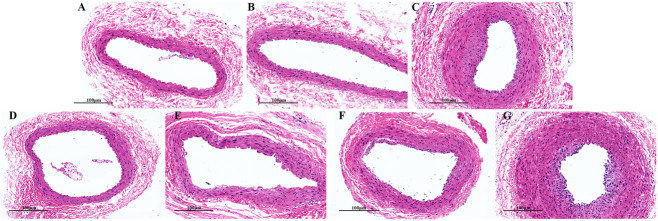
3.7.2. SMYAD ameliorates pathological symptoms of the femoral artery
As illustrated in Figure 8, no significant lesions were noted in the femoral artery of the control and sham groups. In the TAO group, the femoral artery vessel wall was significantly thickened, some of the intima fell off, the arrangement of vascular smooth muscle cells was disturbed, and the arterial wall and perivascular tissue showed inflammatory cell infiltration. The vascular wall of femoral artery was normal, with a small amount of peripheral inflammatory cell infiltration in the aspirin group and SMYAD-H group. The femoral artery vascular wall was mildly thickened in the SMYAD-M group, with a small amount of peripheral inflammatory cell infiltration. In the SMYAD-L group, the femoral artery vascular wall was thickened, with peripheral inflammatory cell infiltration. Therefore, SMYAD relieved pathological symptoms of the femoral artery, and achieved similar efficacy to aspirin.
Figure 8.
Pathological changes in the femoral arterial wall of all group, as observed using optical microscopy (H&E staining, ×200). (A) Control group, (B) sham group, (C) TAO model group, (D) aspirin group, (E) SMYAD-H group, (F) SMYAD-M group and (G) SMYAD-L group. Scale bar = 100 µm.
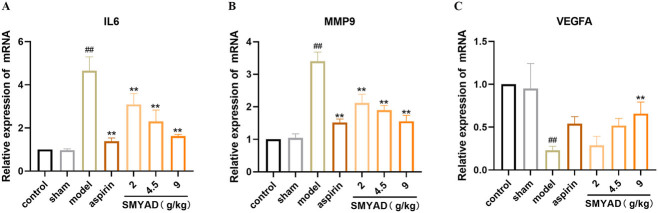
3.7.3. SMYAD effects on expression of IL6, MMP9 and VEGFA in the femoral artery
Target genes related to an inflammatory response by RT-qPCR were detected to confirm the anti-inflammatory effect of SMYAD. In this study, we detected the mRNA expression of IL6 (Figure 9A), MMP9 (Figure 9B) and VEGFA (Figure 9C) in femoral arteries by RT-qPCR, the mRNA levels of IL6 and MMP9 in the model group were markedly higher than that in the control group (p < 0.01), and the mRNA levels of VEGFA was significantly lower (p < 0.01). After administering SMYAD, the mRNA levels of IL6, as well as MMP9 in the each medication group, were reduced, among which the aspirin group and SMYAD-H group had the most obvious effect (p < 0.01). Meanwhile, VEGFA levels was increased in all dosing groups, among which the SMYAD-H group had the most obvious effect (p < 0.05). Consequently, SMYAD exerted anti-inflammatory and pro-angiogenic impacts by suppressing IL6, MMP9 and improving VEGFA expression.
Figure 9.
IL6, MMP9 and VEGFA expressions in the femoral artery wall. (A) IL6 expression. (B) MMP9 expression. (C) VEGFA expression. Data are expressed as mean ± SEM (n = 3 per group). ##p < .01 compared with the control group; **p < .05 compared with the TAO model group.
3.7.4. SMYAD effects on the cell viability in LPS-induced HUVECs
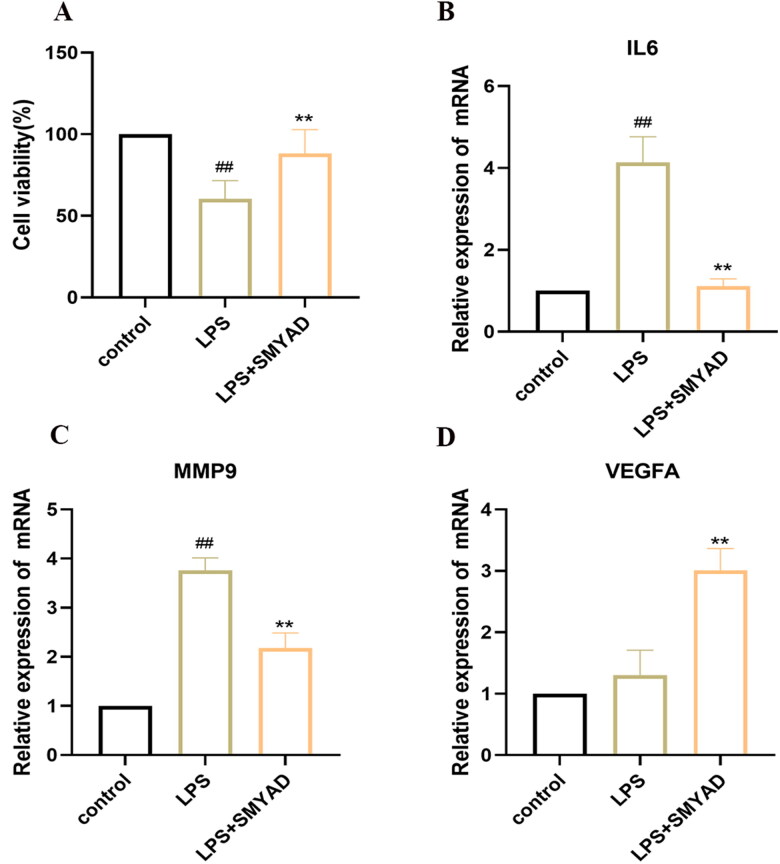
In order to determine the effect on the cell viability of HUVECs, we co-stimulated HUVECs with SMYAD (200 μg/ml) and LPS (10 μg/ml) for 24 h, and the viability was detected by CCK-8 assay. As illustrated in Figure 10(A), compared with the control group, LPS (10 μg/ml) decreased cell viability (p < .01), while SMYAD (200 μg/ml) significantly reversed the LPS-induced decrease in cell viability (p < .01). These data showed that SMYAD protected HUVECs against LPS-induced injury and improved the cell viability.
Figure 10.
SMYAD effects on the cell viability and expression of IL6, MMP9 and VEGFA in LPS-induced HUVECs. (A) Cell viability; (B) IL6 expression; (C) MMP9 expression; (D) VEGFA expression.
3.7.5. SMYAD effects on the expression of IL6, MMP9 and VEGFA in LPS-induced HUVECs
As the in vivo experiment, RT-qPCR was used to detect the mRNA expression of IL6 (Figure 10B), MMP9 (Figure 10C) and VEGFA (Figure 10D) in LPS-induced HUVECs. The results showed that the expression of IL6, MMP9 was significantly higher in HUVECs after 10 μg/ml LPS treatment (p < 0.01). SMYAD reduced the expression of IL6, MMP9 (p < 0.01), and increased expression of VEGFA (p < 0.01) in LPS-induced HUVECs.
4. Discussion
TAO is a peripheral vascular disease with clinical symptoms of numbness, pain, intermittent claudication, ulcers and gangrene gradually appearing as the disease progresses. The etiology and pathogenesis of TAO are unclear, and inflammation, oxidative stress and immunity are considered to be their underlying mechanisms, given the unclear pathogenesis of TAO and the lack of wonder drugs available for comprehensive treatment. Thus, it is crucial to seek effective therapeutic agents that sustainably inhibit the progression of TAO disease.
SMYAD is a popular conventional prescription in ancient China, comprising four herbs, Jinyinhua, Xuanshen, Danggui and Gancao. It removes heat and toxins, cools the blood and activates blood circulation, disperses retention as well as nourishes vessels. SMYAD is commonly used in TAO therapy, and clinical studies have reported its effectiveness in relieving rest pain and ulcers in TAO patients. Nonetheless, its pharmacological effects in TAO treatment remains understudied. This work integrated network pharmacology, molecular docking and experiments geared toward elucidating the mechanism of SMYAD in TAO therapy.
First, a database search generated 24 therapeutic targets. For these 24 treatment targets, we performed GO and pathway enrichment analyses. GO analysis suggests that multiple biological processes played a role in TAO treatment with SMYAD, such as cellular response to oxidative stress, positive regulation of angiogenesis, vasculature development, as well as response to ROS. Oxidative stress is a state of imbalance between oxidation and antioxidant action [18]. The total oxidative status (TOS) levels are markedly higher in TAO patients than that in healthy smoker controls, indicating TAO patients having high oxidative stress. At the same time, TOS/TAC ratio was substantially higher in TAO patients along with increased TOS levels, indicating a substantial imbalance between oxidative and antioxidant systems [19]. In vivo experiments also found that suppresses the PI3K/Akt signalling pathway to increase the oxidative stress level, hence worsening TAO [20]. In addition, therapeutic angiogenesis has become one of the important treatment strategies for TAO. The study found that TAO patients treated with long-term angiogenesis-inducing drugs showed significant improvement in chronic ulcer healing over 1–2 years of treatment [21]. KEGG analysis suggests that SMYAD treats TAO by regulating the AGE-RAGE signalling pathway in diabetic complications, Lipid and atherosclerosis, HIF-1 signalling pathway, TNF signalling pathway, IL-17 signalling pathway, etc. The activation of the TNF pathway can contribute to the release of downstream various pro-inflammatory components (IL6, TNFα), triggering a vascular inflammatory response and leading to the development of TAO [22]. More and more studies suggest that TAO may be an autoimmune disease, and IL17, an important cytokine of Th17, is highly expressed in the plasma of TAO patients and can cause immune disorders in TAO patients [23].
‘Monarch, minister, assistant, and guide’ is a kind of compatibility method in prescriptions, in which the ‘monarch’ drug reflects the main therapeutic direction of the prescription and is the most potent and indispensable drug in the prescriptions [24]. The ‘herbs–components–targets’ outcomes demonstrated that Jinyinhua has a greater effect on potential targets than most other drugs, which was consistent with the theory of ‘monarch, minister, assistant, and guide’. In addition, Xuanshen, Danggui and Gancao were enriched with all or some of the same targets as Jinyinhua, and exerted a synergistic effect with Jinyinhua in 24 targets, and enhance the efficacy of the whole prescription with Jinyinhua, which also reflected their status as the ‘minister, assistant and guide’ of SMYAD. Among the compounds, quercetin, vestitol and beta-sitosterol were the top three ingredients in terms of degree, suggesting that the three ingredients may be the main compounds of SMYAD to exert therapeutic effects. Natural flavonol quercetin is known for its anti-inflammatory, pro-angiogenic and neuroprotective properties [25]. At the same time, beta-sitosterol enhanced the expressions of proteins related to angiogenesis, namely VEGF, VEGF receptor Flk-1 in ischaemia/reperfusion-damaged gerbils, and has therapeutic angiogenic effects [26]. Vestitol can effectively inhibit LPS-induced neutrophil migration, has good anti-inflammatory activity, and is considered a promising novel anti-inflammatory agent [27]. Beta-sitosterol inhibits significantly vascular cell adhesion molecule 1 (VCAM-1) expression in TNF-α-stimulated HAEC, showing anti-inflammatory activity [28]. This indicates that SMYAD may bring about its therapeutic impact by inhibiting the inflammatory response and promoting angiogenesis. Quercetin and beta-sitosterol are the main components of Jinyinhua, proving again that Jinyinhua is a ‘monarch’ drug in SMYAD. In summary, SMYAD can exert a therapeutic effect on TAO by regulating several components and targets.
To further explore the mechanism of SMYAD therapy of TAO, we constructed a ‘pathways–phenotypes’ network and showed that SMYAD treatment of TAO is mainly through two therapeutic modules: inflammation and angiogenesis. Inflammation is the main pathological feature of TAO. In TAO patients, histopathology includes an inflammatory response of the vessel wall and thrombus. Recent studies have shown that inflammatory cytokines such as IL6, TNF-α, IL-1β and high mobility group box-1 (HMGB1) are highly expressed in the serum of patients with TAO [29]. Therefore, suppression of the inflammatory response is considered an important treatment for TAO. Angiogenesis is a process through which new vessels are created from preexisting ones; it involves endothelial cell proliferation, migration, differentiation, tubular development and regulation of vascular growth factors [30]. After blocking the vascular lumen, the ischemic condition contributes to angiogenesis and arteriogenesis, which result in the formation of collaterals in the affected extremities of TAO patients. Results showed that serum from TAO patients inhibited HUVEC outgrowth, migration and proliferation, exhibiting anti-angiogenic activity [31]. Conversely, in a small clinical trial of TAO patients, intramuscular recombinant VEGF was found to heal ischemic ulcers and relieve rest pain in TAO patients [32]. All of the above suggests that SMYAD may treat TAO through inhibiting inflammation and promoting angiogenesis.
To further focus on the targets of SMYAD for TAO treatment, we performed PPI analysis, and the results showed that MMP9, VEGFA and IL6 were the three key targets. IL-6 is a cytokine with pleiotropic activity that is associated with both inflammation and angiogenesis. It was found that IL6 protein expression was significantly increased in the affected limb tissues of TAO rats [33]. MMP9 is widely expressed in vascular tissues and is involved in several vascular diseases by regulating angiogenesis. It has been reported that showed increased MMP9 activity in TAO patients, especially in active smokers compared with non-TAO patients [34]. VEGFA is the predominant vascular endothelial growth factor (VEGF) and plays a key role in angiogenesis. Target prediction (Figure 3) showed that IL6 and MMP9 are potential targets of luteolin and quercetin, the main components of SMYAD, and VEGFA is a potential target of quercetin and Vestitol, the main components of SMYAD. Luteolin has been shown in vitro and in vivo to effectively inhibit the expression of MMP9 protein [35]. Quercetin reduces the expression of IL6 in LPS-induced RAW264.7 cells and decreases the inflammatory response [36]. According to reports, quercetin [37] and kaempferol [38] can target VEGF, increase its expression levels, and enhance therapeutic angiogenesis. Beta-sitosterol showed strong angiogenic activity [39]. Molecular docking results also showed that quercetin, vestitol and beta-sitosterol, the main components of SMYAD, have good binding power to IL6, MMP9 and VEGFA. The above results again suggest us that inhibition of inflammation and promotion of angiogenesis may be the mechanism of action of anti-TAO in SMYAD. Based on the network pharmacology results, we conducted in vitro and in vivo experiments to validate. The results of in vivo experiment showed that SMYAD ameliorated the physical signs and pathological changes in TAO rats, delayed the progression of TAO disease, inhibited the expression of IL6 and MMP9 in femoral arteries, and increased the expression of VEGFA. The results of in vitro experiment also showed that SMYAD effectively increased cell viability of LPS-induced HUVECs and cell proliferation, while inhibiting LPS-induced HUVECs IL6 and MMP9 expression and increasing VEGFA expression, which was consistent with the results of in vivo experiment. Therefore, the above results suggest that SMYAD exert anti-inflammatory as well as therapeutic angiogenic effects by downregulating IL6 and MMP9 and upregulating VEGFA to treat TAO.
Network pharmacology is currently employed in Chinese medicine research. By screening drug components and constructing multidimensional networks of drugs, targets and diseases, we predict complex drug treatment mechanisms to predict complex drug treatment mechanisms. In this study, based on network pharmacology, in vitro experiments and in vivo experiments were performed to further validate the predicted therapeutic mechanism of SMYAD for TAO. However, there are still some limitations in this study, such as this study only validated the key targets of SMYAD for the treatment of TAO, and did not further validate the role of related pathways. Second, our network pharmacology and experimental validation provide some direction for the treatment of TAO. However, as a complex disease, the specific mechanisms of TAO still need to be further explored.
5. Conclusions
In conclusion, we integrate the methodology of network pharmacology and the authenticity of molecular docking and experiments to explain the mechanisms of SMYAD in treating TAO. First, 105 active ingredients of SMYAD are screened out, and 24 targets of SMYAD for the treatment of TAO are obtained. Then, through multi-data integration analysis, we clarified that IL6, MMP9 and VEGFA are the key targets of SMYAD for TAO treatment, and their mechanism may be related to inflammatory response and angiogenesis. The molecular docking results revealed that key active substances and primary targets demonstrated good binding interactions. Based on the network pharmacology results, we validated them using in vivo and in vitro experiments. The experimental results showed that SMYAD treated TAO by decreasing the expression of IL6 and MMP9, increasing the expression of VEGFA, inhibiting the inflammatory response, and promoting angiogenesis. Collectively, this work offers theoretical justification and evidence in favour of SMYAD use in clinical settings.
Supplementary Material
Funding Statement
This work was funded by grants from the National Key Research and Development Program of China (2019YFC1708501), the National Natural Science Foundation of China (No.82074316), the Young Scientists Fund of the National Natural Science Foundation of China (No.81904065), Foshan Science and technology innovation project (2020001005585), Special project for training outstanding young scientific and technological talents (innovative) in basic scientific research business expenses of the Chinese Academy of Chinese Medicine Sciences (ZZ14-YQ-036), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX21_0718).
Data availability statement
On reasonable requests, the datasets used and/or analysed during the current investigation were available from the corresponding author.
Author contributions
JH and WX conceived the experiments and helped to coordinate support and funding. JZ performed the research and drafted the manuscript. PG, FZ, ZL and YC participated in the experiments. All authors read and approved the final manuscript.
Ethics statement
The Animal Ethics Committee of the Experimental Animal Center, Institute of Basic Theory for Chinese Medicine, China Academy of Chinese Medical Sciences (IBTCMCACMS21) approved all the animal experiments.
Disclosure statement
We confirm that this manuscript has not been published by other journals. All authors have approved the manuscript and agree with its submission to this journal. The authors declare no competing financial interest.
References
- 1.Olin JW, Shih A.. Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol. 2006;18(1):1–15. [DOI] [PubMed] [Google Scholar]
- 2.Li HY, Sun H, Zhang AH, et al. Therapeutic effect and mechanism of Si-Miao-Yong-an-Tang on thromboangiitis obliterans based on the urine metabolomics approach. Front Pharmacol. 2022;13:827733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Małecki R, Zdrojowy K, Adamiec R.. Thromboangiitis obliterans in the 21st century—a new face of disease. Atherosclerosis. 2009;206(2):328–334. [DOI] [PubMed] [Google Scholar]
- 4.Li MD, Wang YF, Yang MW, et al. Risk factors, mechanisms and treatments of thromboangiitis obliterans: an overview of recent research. Curr Med Chem. 2020;27(35):6057–6072. [DOI] [PubMed] [Google Scholar]
- 5.Modaghegh MS, Hafezi S.. Endovascular treatment of thromboangiitis obliterans (Buerger’s disease). Vasc Endovascular Surg. 2018;52(2):124–130. [DOI] [PubMed] [Google Scholar]
- 6.Norona LM, Fullerton A, Lawson C, et al. In vitro assessment of farnesoid X receptor antagonism to predict drug-induced liver injury risk. Arch Toxicol. 2020;94(9):3185–3200. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Zefr C, Yang B, et al. Herbal therapy treatment in thromboangiitis obliterans: a retrospective clinical study. Ann Palliat Med. 2020;9(4):1696–1707. [DOI] [PubMed] [Google Scholar]
- 8.Du A, Xie Y, Ouyang H, et al. Si-Miao-Yong-An decoction for diabetic retinopathy: a combined network pharmacological and in vivo approach. Front Pharmacol. 2021;12:763163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C. The efficacy of Si Miao Yong An Tang in the treatment of vaso-occlusive vasculitis. J Appropriate Clin Med. 2018;11(19):2. [Google Scholar]
- 10.Chen C. Guidelines for diagnosis and treatment of common syndromes in peripheral vascular department (2015). Hebei Med J. 2016;1:151–154. [Google Scholar]
- 11.Wang X, Wang ZY, Zheng JH, et al. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 12.Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1–1.30.33. [DOI] [PubMed] [Google Scholar]
- 15.Otasek D, Morris JH, Bouças J, et al. Cytoscape automation: empowering workflow-based network analysis. Genome Biol. 2019;20(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashida S, Ishihara M, Ogawa H, et al. Protective effect of ticlopidine on experimentally induced peripheral arterial occlusive disease in rats. Thromb Res. 1980;18(1–2):55–67. [DOI] [PubMed] [Google Scholar]
- 17.Nair AB, Jacob S.. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharebiani H, Fazeli B, Maniscalco R, et al. The imbalance among oxidative biomarkers and antioxidant defense systems in thromboangiitis obliterans (Winiwarter-Buerger disease). J Clin Med. 2020;9(4):1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du YM, Du BH, Yang J, et al. Effect of Bradykinin on rats with thromboangiitis obliterans through PI3K/Akt signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(22):10169–10176. [DOI] [PubMed] [Google Scholar]
- 21.Fazeli B, Keramat S, Assadi L, et al. Angiogenesis induction in Buerger’s disease: a disease management double-edged sword? Orphanet J Rare Dis. 2019;14(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Liu H, Ma M, et al. Anti-thrombotic activity of phenolic acids obtained from Salvia miltiorrhiza f. alba in TNF-α-stimulated endothelial cells via the NF-κB/JNK/p38 MAPK signaling pathway. Arch Pharm Res. 2021;44(4):427–438. [DOI] [PubMed] [Google Scholar]
- 23.Keramat S, Sadeghian MH, Keramati MR, et al. Assessment of T helper 17-associated cytokines in thromboangiitis obliterans. J Inflamm Res. 2019;12:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Hu Y, Tan W, et al. Compatibility art of traditional Chinese medicine: from the perspective of herb pairs. J Ethnopharmacol. 2012;143(2):412–423. [DOI] [PubMed] [Google Scholar]
- 25.Shen P, Lin W, Deng X, et al. Potential implications of quercetin in autoimmune diseases. Front Immunol. 2021;12:689044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi S, Kim KW, Choi JS, et al. Angiogenic activity of beta-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med. 2002;68(4):330–335. [DOI] [PubMed] [Google Scholar]
- 27.Franchin M, Cólon DF, Castanheira FV, et al. Vestitol isolated from Brazilian red propolis inhibits neutrophils migration in the inflammatory process: elucidation of the mechanism of action. J Nat Prod. 2016;79(4):954–960. [DOI] [PubMed] [Google Scholar]
- 28.Loizou S, Lekakis I, Chrousos GP, et al. Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res. 2010;54(4):551–558. [DOI] [PubMed] [Google Scholar]
- 29.Dellalibera-Joviliano R, Joviliano EE, Silva JS, et al. Activation of cytokines corroborate with development of inflammation and autoimmunity in thromboangiitis obliterans patients. Clin Exp Immunol. 2012;170(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annex BH, Cooke JP.. New directions in therapeutic angiogenesis and arteriogenesis in peripheral arterial disease. Circ Res. 2021;128(12):1944–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewing B, Stangl V, Stangl K, et al. Circulating angiogenic factors in patients with thromboangiitis obliterans. PLOS One. 2012;7(4):e34717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isner JM, Baumgartner I, Rauh G, et al. Treatment of thromboangiitis obliterans (Buerger’s disease) by intramuscular gene transfer of vascular endothelial growth factor: preliminary clinical results. J Vasc Surg. 1998;28(6):964–973, discussion 73–75. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Li CL, Xia PZ, et al. MiR-223 alleviates thrombus and inflammation in thromboangiitis obliterans rats by regulating NLRP3. Eur Rev Med Pharmacol Sci. 2020;24(20):10605–10611. [DOI] [PubMed] [Google Scholar]
- 34.Dellalibera-Joviliano R, Jacob-Ferreira AL, Joviliano EE, et al. Imbalanced matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 activities in patients with thromboangiitis obliterans. Vasc Med. 2012;17(2):73–78. [DOI] [PubMed] [Google Scholar]
- 35.Fei J, Liang B, Jiang C, et al. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed Pharmacother. 2019;109:1586–1592. [DOI] [PubMed] [Google Scholar]
- 36.Tang J, Diao P, Shu X, et al. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-Induced RAW264.7 cells: in vitro assessment and a theoretical model. Biomed Res Int. 2019;2019:7039802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Hamed AR, Mehanna ET, Hazem RM, et al. Plicosepalus acacia extract and its major constituents, methyl gallate and quercetin, potentiate therapeutic angiogenesis in diabetic hind limb ischemia: HPTLC quantification and LC–MS/MS metabolic profiling. Antioxidants. 2021;10(11):1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu WH, Wang HY, Xia YT, et al. Kaempferol, a major flavonoid in Ginkgo folium, potentiates angiogenic functions in cultured endothelial cells by binding to vascular endothelial growth factor. Front Pharmacol. 2020;11:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon EJ, Lee YM, Lee OH, et al. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis. 1999;3(2):117–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
On reasonable requests, the datasets used and/or analysed during the current investigation were available from the corresponding author.