Abstract
Background
Amyloidosis is a rare group of diseases in which fibrillar amyloid proteins are deposited in systemic organs to result in functional disorder. However, amyloidosis affecting the cervical spine is very rare. We herein describe a case of systemic amyloidosis including a combination of cervical myelopathy with amyloid deposition and cardiac dysfunction due to cardiac amyloidosis.
Case presentation
An 86-year-old man with cervical myelopathy accompanied with cardiac dysfunction due to cardiac amyloidosis underwent posterior cervical laminectomy from C3 to C4. We were able to identify the patient’s cardiac amyloidosis and significant cardiac dysfunction before surgery and manage his perioperative treatment successfully. Preoperative cervical computed tomography (CT) showed multiple fine calcifications below the lamina, which were later confirmed by pathological analysis as amyloid deposition.
Conclusions
This is a relatively rare report of systemic amyloidosis including a combination of cervical myelopathy with amyloid deposition and cardiac dysfunction from cardiac amyloidosis. CT findings of multiple fine calcifications suggest the possibility of amyloidosis and may warrant further examination of cardiac function.
Keywords: Amyloid, Cervical myelopathy, Calcification, Cardiac dysfunction, Surgical management
Introduction
Amyloidosis is a rare group of diseases in which fibrillar amyloid proteins become deposited in systemic organs to produce functional disorder.1 Amyloidosis can occur in the bones, skin, larynx, lymph nodes, bladder, eyes, tongue, and digestive system. However, amyloidosis affecting the spine is very rare.2,3 We herein present the first reported case of systemic amyloidosis featuring a combination of cervical myelopathy with amyloid deposition and cardiac dysfunction due to cardiac amyloidosis.
Case presentation
An 86-year-old male was diagnosed as having cervical myelopathy. He had been experiencing numbness in his hands for approximately two years. He had a history of hypertension and hyperlipidemia, with no record of renal disorder or dialysis. His clumsiness and gait disorder began progressing three months prior to his first visit. His limb reflexes were increased, and magnetic resonance imaging (MRI) revealed spinal cord compression and signal changes at the C3/4 level (Fig. 1). Computed tomography (CT) showed multiple fine calcifications below the lamina (Fig. 2). Eventually, the patient became unable to stand, with a Japanese Orthopaedic Association (JOA) score of 5 points. After preoperative electrocardiography (ECG) showed conduction disturbances and thorough cardiac examination, technetium-99 m pyrophosphate scintigraphy revealed cardiac amyloidosis (Fig. 3a). Single-photon emission computed tomography showed technetium-99 m pyrophosphate uptake in the myocardium of the left ventricle. His heart to contralateral ratio was 1.56 (Fig. 3(b)). His cardiac function was markedly compromised (ejection fraction: approximately 40%) and required strict management of in–out balance during the perioperative period. We performed posterior cervical laminectomy from C3 to C4 (Fig. 4). Surgical time was 102 min and blood loss volume was 50 g. No perioperative complications, such as heart failure, were noted. The ligamentum flavum at the C3/4 level was found to be markedly thickened. Pathological analysis revealed amyloid deposits with apple-green birefringence under polarized light and Congo red staining (Fig. 5(a,b)). Immunohistochemistry revealed positive transthyretin staining in amyloid deposits (Fig. 5(c)). We explained to the patient that a genetic test would provide useful information for future treatment as well as for his family. However, he resisted such testing due to his advanced age and refusal for any direct amyloidosis treatment, such as disease modifying therapy. Although it was not possible to conclusively diagnose whether he had hereditary variant transthyretin amyloidosis or wild-type transthyretin amyloidosis without a hereditary genotype, we suspected that he harbored the latter form of amyloidosis considering the absence of a family history and his advanced onset age. His postoperative course was good. At 11 days after surgery, he was able to stand with assistance and was transferred to another hospital for continued rehabilitation. Two months postoperatively, he was able to walk with the use of a walker. His JOA score had improved to 10.5 points.
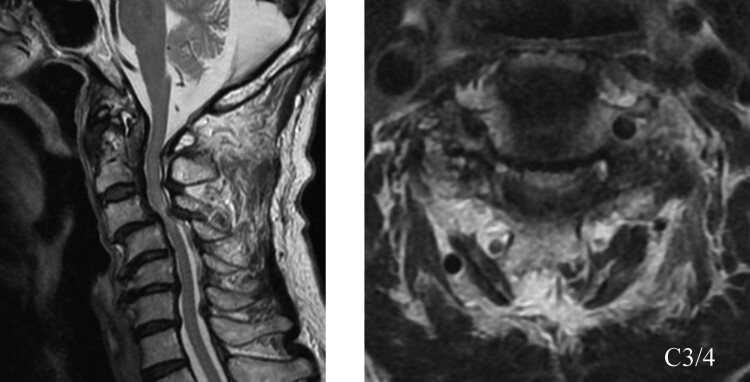
Figure 1.
Preoperative MRI showed spinal cord compression and signal changes at the C3/4 level.
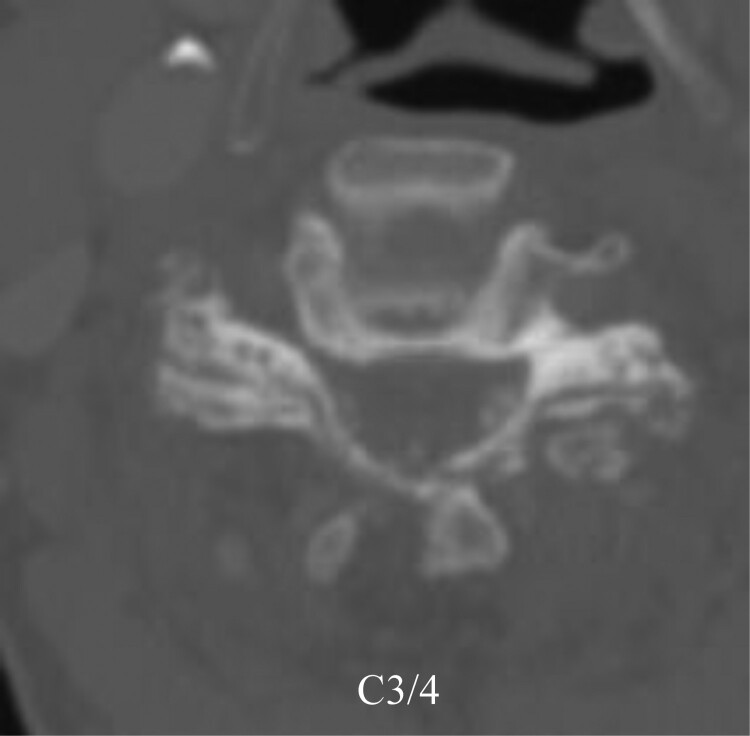
Figure 2.
Preoperative CT showed multiple fine calcifications below the lamina.
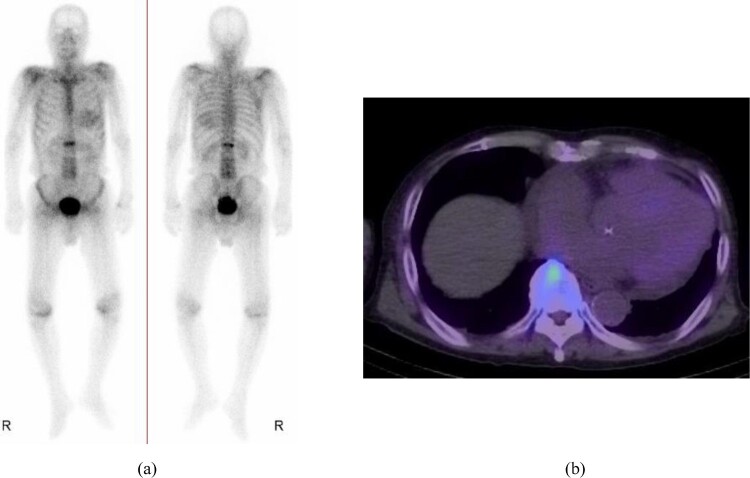
Figure 3.
(a) Technetium-99 m pyrophosphate scintigraphy revealed cardiac amyloidosis. (b) Single-photon emission computed tomography showed technetium-99 m pyrophosphate uptake in the myocardium of the left ventricle.
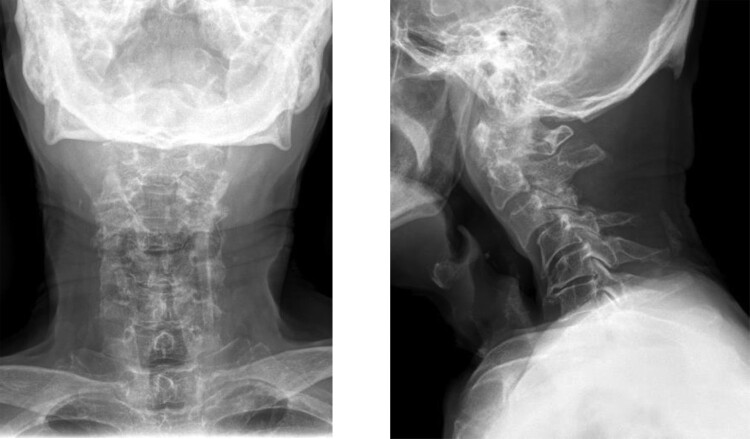
Figure 4.
Postoperative radiographs following posterior cervical laminectomy from C3 to C4.
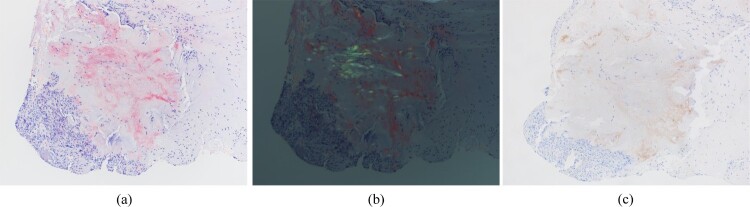
Figure 5.
Pathological analysis showed amyloid deposition with apple-green birefringence under polarized light and Congo red staining. (a) Congo red, 100x; (b) Congo red, polarized, 100x. (c) Immunohistochemistry revealed positive transthyretin staining in amyloid deposits.
Discussion
Amyloidosis is a rare protein misfolding disorder characterized by the extracellular deposition of amyloid fibrils in a variety of tissues. The global incidence of amyloidosis is estimated at five to nine per million patients per year.1 Amyloid deposits are usually found in the bones, skin, larynx, lymph nodes, bladder, eyes, tongue, and digestive system, with spine deposits being extremely rare.2,3 It is well known that cervical myelopathy can result from beta-2-microglobulin deposition in patients undergoing long-term dialysis.4–8 Several cases of amyloidoma confined to the cervical spine of non-dialysis patients have also been reported, but are limited in number.2,3,9–11 A systematic review of 35 patients with localized amyloidoma of the spine showed localization in the craniocervical transition of 6 patients (17.1%), cervical vertebrae of 6 patients (17.1%), cervicothoracic transition of 1 patient (2.9%), thoracic vertebrae of 17 patients (48.6%), lumbar vertebrae of 4 patients (11.4%), and sacral vertebrae of 1 patient (2.9%).12 Elsewhere, reports have described associations of systemic amyloidosis with carpal tunnel syndrome and other orthopedic disease.13–16 Yanagisawa et al. witnessed that lumbar spinal canal stenosis patients often had amyloid deposits in the ligamentum flavum.17 Westermark et al. also reported that amyloid deposits were extremely common in the tissues around the spinal canal in lumbar canal stenosis.18 However, there have been few accounts of amyloid deposition related to cervical myelopathy. Sueyoshi et al. presented a case of amyloidosis of the cervical and lumbar spine, but could not find evidence of amyloid deposits in other tissues.19 The present case was rare in that cardiac amyloidosis was found incidentally during preoperative examination for cervical myelopathy. Pathological analysis of the cervical spine after surgery revealed amyloid deposits corresponding to the areas of calcification detected by CT.
Cardiac amyloidosis is present in many patients with amyloidosis, with approximately 50% of cases presenting with diastolic heart failure at the time of diagnosis.20 Amyloid heart disease is a major prognostic factor as it accounts for approximately 75% of deaths due to heart failure or arrhythmia.21,22 The deposition of amyloid fibrils within the myocardium causes restrictive heart failure with thickening of the ventricular and atrial walls and a gradual increase in asthenia, dyspnea, and lower extremity edema. Amyloid infiltration into the myocardium may also induce conduction defects and ventricular or supraventricular arrhythmias.21–23 In the present case, preoperative ECG indicated conduction disturbances, which led to a preoperative diagnosis of cardiac amyloidosis. Although the patient had significant cardiac dysfunction, we were able to identify it before surgery and manage the patient's perioperative treatment successfully.
Preoperative cervical CT in this patient disclosed multiple fine calcifications below the lamina, after which pathological examination revealed amyloid deposition in tissue taken from the same area. As these characteristic CT findings suggest the possibility of amyloidosis, a thorough preoperative evaluation of cardiac function, including screening for cardiac amyloidosis, may be warranted.
The patient in this case was elderly and had cervical spondylosis. It is a limitation of this report that the amyloidosis reported here was not the causative etiology and may have been coincidental.
Conclusion
This is a relatively rare report of systemic amyloidosis featuring a combination of cervical myelopathy with amyloid deposition and cardiac dysfunction from cardiac amyloidosis. As CT findings of multiple fine calcifications indicate possible amyloidosis, a thorough preoperative evaluation of cardiac function may be necessary for optimal management.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Funding statement
Not applicable.
References
- 1.Desport E, Bridoux F, Sirac C, et al. . AL amyloidosis. Orphanet J Rare Dis 2012;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickman CA, Sonntag VK.. Posterior C1-C2 transarticular screw fixation for atlantoaxial arthrodesis. Neurosurgery 1998;43:275–280.; discussion 280-1. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SS, Park YH, Kim JY, et al. . Primary amyloidoma of the cervical spine. AJNR Am J Neuroradiol 2000;21:601–603. [PMC free article] [PubMed] [Google Scholar]
- 4.Amoroso E, Vitale C, Silvestro A.. Spinal-cord compression due to extradural amyloidosis of the cervico-occipital hinge, in a hemodialysed patient. A case report. J Neurosurg Sci 2001;45:120–124. [PubMed] [Google Scholar]
- 5.Dalolio M, Lucarella F, Rampini P, et al. . Neurosurgical aspects of dialysis-related spinal amyloidosis: report of three cases and a review of the literature. Neurochirurgie 2017;63:314–319. [DOI] [PubMed] [Google Scholar]
- 6.Inatomi K, Matsumoto T, Tomonaga T, et al. . Histological analysis of the ligamentum flavum of patients with dialysis-related spondyloarthropathy. J Orthop Sci 2004;9:285–290. [DOI] [PubMed] [Google Scholar]
- 7.Moslavac S, Dzidic I, Kejla Z, et al. . Hemodialysis-associated amyloidosis with cervical spinal cord compression and incomplete tetraplegia: a case report. Spinal Cord 2007;45:799–801. [DOI] [PubMed] [Google Scholar]
- 8.Takeshima Y, Kotsugi M, Park YS, et al. . Hemodialysis-related upper cervical extradural amyloidoma presenting with intractable radiculopathy. Eur Spine J 2012;21(21 Suppl 4(Suppl 4)):463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iplikcioglu AC, Bek S, Gokduman CA, et al. . Primary solitary cervical amyloidosis. Spine 2007;32:E45–E47. [DOI] [PubMed] [Google Scholar]
- 10.Rotter J, Dowlati E, Jha RT, et al. . Primary cervical spine AL-κ amyloidoma: A case report and review of the literature. Neuropathology 2019;39:231–239. [DOI] [PubMed] [Google Scholar]
- 11.Werner BC, Shen FH, Shimer AL.. Primary cervical amyloidoma: a case report and review of the literature. Spine J 2013;13:e1–e7. [DOI] [PubMed] [Google Scholar]
- 12.Pinheiro JP, Carneiro D, Tavares S, et al. . Management and outcome of solitary spinal amyloidoma—A systematic literature review. World Neurosurg 2020;140:325–331. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly JP, Hanna M, Sperry BW, et al. . Carpal tunnel syndrome: A potential early, Red-flag sign of amyloidosis. J Hand Surg [Am] 2019;44:868–876. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa M, Sekijima Y, Yazaki M, et al. . Carpal tunnel syndrome: a common initial symptom of systemic wild-type ATTR (ATTRwt) amyloidosis. Amyloid 2016;23:58–63. [DOI] [PubMed] [Google Scholar]
- 15.Sekijima Y, Uchiyama S, Tojo K, et al. . High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol 2011;42:1785–1791. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama S, Sekijima Y, Tojo K, et al. . Effect of synovial transthyretin amyloid deposition on preoperative symptoms and postoperative recovery of median nerve function among patients with idiopathic carpal tunnel syndrome. J Orthop Sci 2014;19:913–919. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa A, Ueda M, Sueyoshi T, et al. . Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol 2015;28:201–207. [DOI] [PubMed] [Google Scholar]
- 18.Westermark P, Westermark GT, Suhr OB, et al. . Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Upsala J Med Sci 2014;119:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sueyoshi T, Ueda M, Sei A, et al. . Spinal multifocal amyloidosis derived from wild-type transthyretin. Amyloid 2011;18:165–168. [DOI] [PubMed] [Google Scholar]
- 20.Ekelund L. Radiologic findings in renal amyloidosis. Am J Roentgenol 1977;129:851–853. [DOI] [PubMed] [Google Scholar]
- 21.Kappor P, Thenappan T, Singh E, et al. . Cardiac amyloidosis: a practical approach to diagnosis and management. Am J Med 2011;124:1006–1015. [DOI] [PubMed] [Google Scholar]
- 22.Selvanayagam JB, Hawkins PN, Paul B, et al. . Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol 2007;50:2101–2110. [DOI] [PubMed] [Google Scholar]
- 23.Dubrey SW, Hawkins PN, Falk RH.. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart 2011;97:75–84. [DOI] [PubMed] [Google Scholar]