Summary
Organisms have homeostatic mechanisms to respond to cold temperature to ensure survival including the activation of the mammalian neuroprotective mild hypothermia response (MHR) at 32°C. We show activation of the MHR at euthermia by an FDA-approved medication Entacapone, proof-of-principle that the MHR can be medically manipulated. Utilizing a forward CRISPR-Cas9 mutagenesis screen, we identify the histone lysine methyltransferase SMYD5 as an epigenetic gatekeeper of the MHR. SMYD5 represses the key MHR gene SP1 at euthermia but not at 32°C. This repression is mirrored by temperature-dependent levels of H3K36me3 at the SP1-locus and globally indicating that the mammalian MHR is regulated at the level of histone modifications. We identified 45 additional SMYD5-temperature dependent genes suggesting a broader MHR-related role for SMYD5. Our study provides an example of how the epigenetic machinery integrates environmental cues into the genetic circuitry of mammalian cells and suggests novel therapeutic avenues for neuroprotection after catastrophic events.
Keywords: Targeted Temperature Management, SP1, Hypothermia, Neonatal Hypoxic-Ischemic Brain Injury, Cardiac Arrest
Introduction
The defining feature of mammals is their ability to maintain a euthermic body temperature (37°C) and most mammals maintain temperature at a relatively tight range to ensure normal cellular homeostasis1. The main exception is the hibernation of some mammals, which is a type of controlled temperature decrease during times of prolonged inactivity2. In clinical practice, physicians imitate hibernation with the use of Targeted Temperature Management (TTM) as a therapeutic strategy to minimize neurological damage following neonatal hypoxic-ischemic brain injury and cardiac arrest3,4. The reasons why lowered internal core temperature (32–36°C) yields benefits are currently not fully understood5,6. Although some argue that the positive neurological benefit from mild hypothermia relates to a lower metabolic rate, others point out that the specific upregulation of several genes during mild hypothermia does not fit that idea7. An alternative hypothesis is that hypothermia is beneficial because of the activation of the mild hypothermia response (MHR) itself, and if so, it would be important for researchers and clinicians to understand both the mechanisms and the extent of the response. In support of this, researchers have found that several genes (e.g., CIRBP/CIRP, RBM3) consistently show increased expression during mild hypothermia.8 Two of these, SP1 and RBM3, appear to have roles in decreasing neuronal death during disease states9–13.
SP1, a transcription factor for many genes14, binds to the promoter region of CIRBP15 upon cold exposure at a sequence called the Mild Cold Response Element (MCRE)15, and its binding leads to increased expression of CIRBP at 32°C, indicating that this regulation occurs at the transcriptional level15. Similarly, RBM3 is known to regulate at least one downstream gene, RTN311, a potential neuroprotective factor in a neurodegeneration model11. Although this suggests that mammalian cells have a pathway culminating in SP1/CIRBP and RBM3/RTN3, little has been done to further elucidate the upstream regulation of this pathway and relatively little knowledge is available regarding mammalian cellular responses to cooling compared to heat shock responses, which have been previously characterized16.
Here, we use complementary unbiased strategies to gain clues into the MHR upstream of SP1. Specifically, we use a novel SP1 mild hypothermia indicator (MHI) containing the SP1 promoter and the MCRE enhancer (SP1+MCRE-MHI) to perform compound screening of 1953 FDA-approved agents. We have successfully identified and validated that the drug Entacapone activates the endogenous MHR at the transcriptional level, which indicates that it is possible to manipulate the response without exposure to hypothermia. To identify additional regulators, we have performed a genome-wide CRISPR-Cas9 forward mutagenesis screen17–19, reading out SP1-promoter activity using the SP1-MHI. Our study has yielded multiple candidate regulators and compounds that may regulate the MHR. We focus on one of these regulators, SMYD5, a histone methyltransferase, and demonstrate that the presence of SMYD5 is temperature dependent and that several other SMYD5-bound genes show significant differential expression upon mild hypothermia exposure. Our insights elucidate how an external cue (mild hypothermia) specifically mediates its effects on the mammalian genome through the actions of the epigenetic machinery and this paves the way towards the development of a novel therapeutic means to treat neonatal hypoxic-ischemic brain injury, a major cause of mortality and morbidity in otherwise healthy newborns20, without exposure to TTM.
Results
Mild hypothermia indicators show increased fluorescence at mild hypothermia (32°C).
Core temperature (euthermia) is kept at a very tight range in humans (36.5–37.8°C)1,21 and by definition, mild hypothermia involves temperature ranges from 32–36°C (Figure S1, bold). To directly visualize the promoter activity of three genes previously shown8,15 to respond to mild hypothermia (CIRBP, RBM3, SP1), we have made eight distinct MHIs (Supplementary Table 1 and Supplementary File 1) all of which have promoter and/or MCRE sequences, driving the expression of either GFP or mCherry (Figure 1A). When transfected into HEK293 cells, the MHIs demonstrate increased fluorescence at 32°C compared to 37°C (Figure 1B–1C). These findings are mirrored by increased endogenous SP1 levels in a Western blot from cells collected after 16 hours (h) at 32°C (Figure 1D). We assessed the temporal dynamics of promoter activity using flow cytometry analysis of the three indicators and observed a maximal increase in fluorescence of SP1-MHI between 6–8 h after a temperature shift to 32°C (P<0.05, Figure 1E). This finding was supported by continuous monitoring of fluorescence from an Incucyte S3 (Sartorius) where again maximal intensity was reached within 6–8 h, with SP1-MHI, rising fastest, followed by RBM3-MHI, and then CIRBP-MHI (Figure S2A–S2C). Indicators were also transiently transfected into HEK293 cells and then exposed to five distinct temperatures (26°C, 29°C, 32°C, 37°C, and 40°C), for 16 h followed by flow cytometry (Figure 1F–1H; Figure S2D–S2E). Consistent with previous literature8,15 and our own validation in HEK293 cells (Figure 1B–D), indicators representing all three genes (SP1, CIRBP, RBM3) had significantly (P<0.0001) increased fluorescence at 32°C compared to 37°C/40°C (Figure 1F–1H) and the response was uniformly strongest at 32°C (Figure 1F–1H). At the same time, the SP1− and CIRBP-MHIs do not show increased activity at lower (26°C, 29°C, Figure S2D–S2E) or higher (40°C) temperatures (Figure 1F–1G), supporting the notion that our MHIs specifically reflect the MHR and are not activated by general cell stress (i.e., moderate hypo- or hyperthermia). These data suggest that all three genes are regulated at the transcriptional level and the time-lag in the response upon hypothermia is measured in hours rather than minutes, indicating that there must be additional regulators operating upstream of these genes. Some would argue that storing cells on ice (i.e., during fluorescent activating cell sorting (FACS)) could possibly lower the temperature of cells to the hypothermic range and activate the MHR, but we found that even prolonged storage on ice (8 h) in a room at room temperature was unable to activate the MHI (Figure S3), ensuring that storage on ice during the study could not act as a potential confounder.
Figure 1. MHIs based on all three genes (SP1, CIRBP, RBM3) show the strongest effect at 32°C.
(A) A schematic of MHI structures. (B-C) Representative images of SP1-Lenti-MHIs and CIRBP-Lenti-MHI, respectively, at 37°C and 32°C as visualized by fluorescent microscope. (D) Western blot quantification and a representative figure demonstrating increased SP1 after 16 h at 32°C compared to 37°C. Shown are data points, each data point is a biological replicate and the mean is depicted. Significance levels were calculated with an unpaired two-tailed t-test in GraphPad Prism. (E) Geometric mean fluorescence (gMFI, flow cytometry) after varying lengths of hypothermia exposure for SP1-MHI. Shown are data points, each data point is a technical replicate, mean and SD where applicable. Significance levels were calculated with an unpaired one-tailed t-test in GraphPad Prism. (F-G) Mean fluorescence (MFI, flow cytometry) of SP1-MHI and CIRBP-MHI, respectively, (16 h at 32°C, 37°C, 40°C). Shown are data points, each point is a technical replicate, mean and SD where applicable. Significance levels were calculated in GraphPad Prism with Šidák’s multiple comparison test. (H) gMFI of RBM3-MHI (16 h at 32°C and 37°C). Significance levels were calculated with an unpaired one-tailed t-test in GraphPad Prism otherwise data shown as in (G-H). * = P<0.05, ** = P<0.01, *** = P<0.001, **** = P<0.0001.
Activation of the MHR at 32°C is uniformly seen in cells of diverse origins.
In addition to HEK293 cells, which have been widely used in prior MHR studies11,15,22, we sought to verify that our novel MHIs also work in diverse cellular systems. We transiently transfected three individual MHIs (SP1−, RBM3−, SP1+MCRE-MHI) into seven distinct cell lines: (HEK293WT, HEK293T, HCT-116, HeLa, Jurkat, K562, and SK-N-SH), representing diverse cellular origins including embryonic kidney, colon, cervix, T lymphocytes, lymphoblasts, and neurons, respectively. After exposing these cells to 26°C, 29°C, 32°C, 37°C, and 40°C for 16 h and quantifying the fluorescence by flow cytometry, we observed highest fluorescence at 32°C independent of cellular origin (Figure S4) and no effect for moderate hypothermia (26–29°C, Figure S5, and Figure S2D–S2E) with the exception of SK-N-SH and K562 cell lines (Figure S5D–S5E). The strongest activation of MHI at 32°C was detected in the cell-types previously described to have neurological characteristics (HEK293 and SK-N-SH)23–25 and these were also the cell lines that showed significant activation of all three MHIs at 32°C (Figure 1F–1H, and Figure S4). This is consistent with prior literature showing that the RBM3-MHR response is strongest in young neurons26. Since many of the key studies in the field have used HEK293 11,15,22 and they have neuronal characteristics23,25 we used this cell line for our screening approaches.
Compound screening reveals MHR activators at euthermia.
In our search for therapeutic targets for TTM, we sought to identify compounds that could activate the MHR at 37°C as this could yield insights into the involved biochemical pathways. To explore this in an unbiased manner, we performed a systematic library drug screen (1953 FDA-approved inhibitors, Figure 2A) using HEK293T cells transfected with the SP1+MCRE-MHI. Cells transfected with SP1+MCRE-MHI were exposed to the drugs (20 μM concentration) and fluorescence was measured after 16, 28, and 40 h of drug exposure (Figure 2A). We focused on drugs that increase the MHI fluorescence at least ≥3.75 times at one of the time points (Supplementary Table 2). Among these drugs we saw an apparent albeit non-significant enrichment of Tyrosine Kinase Inhibitors (TKI, 7/80, p<0.075) including Orantinib, Dasatinib (hydrochloride, monohydrate), Poziotinib, Rociletinib, Afatinib, and Lapatinib27–31. We validated three from this group (Dasatinib, Orantinib, and Poziotinib) as well as selecting another 5 drugs that showed one of the greatest responses in the screen (Cinacalcet, Entacapone, Erythromycin, Naloxone, and Tamoxifen, Figure 2B). For validation of the drug screen HEK293 cells expressing SP1-MHI were individually exposed to each of the 8 compounds (20 μM) for 16 h at either 32°C or 37°C followed by flow cytometry. Two out of the eight (25%), showed significantly (P<0.001, P<0.0001) increased fluorescence at 37°C (Entacapone, Poziotinib, Figure 2C–2D), indicating these two drugs can potentially induce the MHR. Entacapone also had significantly (P<0.01) enhanced fluorescence levels at 32°C (Figure 2C), suggesting that the effect is additive and thus synergistic to the regular response and thus likely independent of the SP1 target itself. Other compounds did not increase the fluorescence of the SP1-MHI for the dose tested (Figure S6A–S6F). To exclude the possibility of cell cycle arrest or increased apoptosis31,32, we evaluated the cell cycle phase in HEK293 cells exposed to Poziotinib and Entacapone, and no apparent signs of cell cycle arrest were observed for either compound, other than a slight increase in the percentage of total cells in the S phase of the cell cycle after Entacapone exposure (Figure S6G). After exposing HEK293 cells to the pro-oxidant agent H2O2 in order to induce apoptosis, we could not see any signs of activation of the SP1-MHI (Figure S6H), but we observed a significant (P<0.01) increase in fluorescence after only 4 h at 32°C (Figure S6H) suggesting that cell stress may potentiate the response. To our surprise, three drugs (Cinacalcet, Dasatinib, and Tamoxifen) showed significantly decreased fluorescence of the SP1-MHI at 32°C during this validation (Figure S6A, S6B, and S6F). To evaluate the endogenous effects on SP1 we carried out Western blotting after 24 h exposure to the compounds at either 32°C or 37°C. For Entacapone (20 μM concentration) we observed a significant increase of SP1 at 37°C (P<0.05, Figure 2E). We were unable to test Poziotinib at 20 μM due to low protein yields, therefore, we performed the Western blot at a lower dose (4 μM) and for this dose we only saw a trend towards increased SP1 at 37°C (Figure 2F). When we looked at mRNA levels for SP1, CIRBP, and RBM3 at 32°C and 37°C after 16 h of Entacapone and Poziotinib exposure (20 μM), we only observed a significant (P<0.01) elevated RBM3 mRNA levels upon Poziotinib exposure at 37°C (Figure S7). This could suggest that the drugs may impact both transcriptional and post-transcriptional levels of key factors of the MHR.
Figure 2. Poziotinib and Entacapone activate the MHR.
(A) A schematic overview of our drug screen in HEK293T cells transfected with SP1+MCRE-MHI. (B) Representative drugs that were found to cause more than ≥3.75 times increase of SP1+MCRE-MHI fluorescence. The entire list is provided in Supplementary Table 2. (C-D) gMFI of SP1-MHI as measured by flow cytometry after 16 h of either 32°C or 37°C with or without Entacapone (ENT, C, 20 μM) and Poziotinib (POZ, D, 20 μM) exposure. Shown are data points, each point is a technical replicate, mean and SD where applicable. Significance levels were calculated in GraphPad Prism with Šidák’s multiple comparison test. (E-F) Western blot quantification and a representative figure of SP1 after exposure to Entacapone (ENT, E, 20 μM) and Poziotinib (POZ, F, 4 μM) or vehicle (DMSO) after 24 h. Shown are data points, each data point is a biological replicate, mean and SD where applicable. Significance levels were calculated with an unpaired one-tailed t-test in GraphPad Prism. * = P<0.05, ** = P<0.01, *** = P<0.001, **** = P<0.0001.
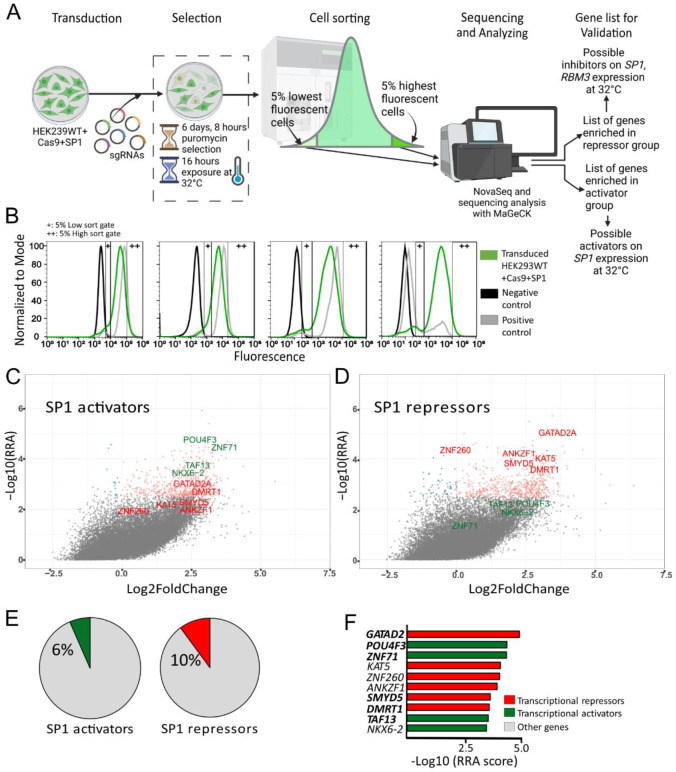
CRISPR-Cas9 Knockout Screen with SP1-MHIs reveals candidate regulators.
To map regulators of the cooling response (SP1) in an unbiased manner, we used an available genome-coverage lentiGuide-Puro pooled sgRNA library17–19 in combination with our SP1-MHI to uncover transcriptional regulators of SP1. We chose to use the SP1-MHI, as our prior data indicate that this gene is regulated at the transcriptional level (Figure 1) and is the quickest key MHR gene to achieve maximal response at 32°C (Figure S2A–S2C). However, given that it does not reach its maximal response until 6–8h of hypothermia exposure, we hypothesized that there may be unknown regulators of the SP1 locus that function earlier in the MHR process. We performed the screen on HEK293WT+Cas9 cells expressing SPI-MHI (HEK293WT+Cas9+SP1), transducing the sgRNA library at the rate of 0.3 sgRNA per cell. After puromycin selection and exposure to hypothermia (32°C) for 16 h (to activate the MHR), we sorted out the 5% most and least fluorescent cells (Figure 3A) to capture repressors and activators of SP1, respectively. We saw a consistent shift towards lower fluorescence for the transduced HEK293WT+Cas9+SP1-MHI (green) compared to the positive control (non-transduced HEK293WT+Cas9+SP1-MHI, grey, Figure 3B). The observation of such a large fluorescent shift of cells (less SP1-MHI fluorescence) at hypothermia suggests the involvement of many sgRNAs, and thus there are likely many genes that are upstream of SP1. Furthermore, this suggests that the majority are activators rather than repressors of the SP1 arm of the MHR. We amplified guides for each sorted group as well as the negative control (transduced HEK293WT+Cas9) and performed deep next-generation sequencing of their distribution from sorted and un-sorted fractions (negative control). To identify guides enriched in the sorted populations, we used the MaGeCK pipeline33 to computationally predict genes with enrichment (Figure 3C–3D). We decided to cast a wide net and look at all genes that had a −Log10(RRA) score above 2.5 to not miss possible regulators (Supplementary Table 3). We excluded 21 genes that were seen in lists for both the high (repressor list) and low (activator list) sorts of transduced HEK293+Cas9+SP1-MHI (Supplementary Table 4), as these may be related to proliferation rather than the MHR itself, and a validation of two of these (RGS21 and MALT1) supported that idea (Figure S8). We were left with a list of 495 genes for the SP1-repressors and 61 for SP1-activators – a clear overrepresentation of repressors to activators (Supplementary Table 3). Of note, there were two microRNAs on the top list of SP1-repressors and activators (hsa-mir-4314 and hsa-mir-6739) and both are predicted to target SP1 (mirdb.org).
Figure 3. Genome wide CRISPR-Cas9 knockout screen on SP1-MHI reveals multiple potential inhibitors and activators of the MHR.
(A) Overview of the genome-wide CRISPR-Cas9 Knockout approach for the HEK293WT+Cas9+SP1 cell line. (B) Fluorescence and sort gates of 4 replicates of transduced HEK293WT+Cas9+SP1 cells (green), negative control (HEK293WT, black) and positive control (HEK293WT+Cas9+SP1, grey). (C-D) Genes marked with their name in red are transcription regulators that have a −Log10(RRA) score >3.5 and have a repressive functions and genes marked with their name in green are transcription regulators that have an activating functions and a −Log10(RRA) score >3.5. Colored dots represent genes that have a −Log10(RRA) score >2.5, where the orange dots indicate genes that have a positive log fold change (LFC) and the blue dots indicate genes that have a negative LFC, from either the SP1 activator (C) or repressor (D) screen. (E) A summary of transcriptional regulators from our screens, activators (green) on left, repressors (red) on right. (F) A summary of top 10 transcriptional regulators ranked by their −Log10(RRA score). Genes in bold are known in the literature to act in the same way as the screen results indicated, that is either as a direct repressor (red) or activator (green).
Since the ERBB2/HER2 signaling is known to regulate SP1 binding through phosphorylation34, and is the target of tyrosine kinases inhibitors such as Poziotinib (Figure S9A), we looked through both lists and noted that ERK was found on the SP1-repressor list (Figure S9A). On the Ingenuity Qiagen ERBB2 Pathway there are 22 genes upstream of SP1, and among those, 3 were found on the SP1-repressor list (RAS, ERK, STAT5, P<0.02). We also used the Molecular Signature Database (MSigDB) to establish likely pathways from genes that had a −Log10(RRA) score above 0.5 from each screen (Figure S9B–S9C) and observed one shared pathway (Pentose Phosphate Pathway (3P)). Of interest there were two categories in the SP1-activator analysis that implicate cytosolic calcium regulation; calcium influx has been previously shown to be an early event in cells exposed to hypothermic stimuli35. As we were particularly interested in a possible transcriptional regulator of SP1, we annotated our lists and identified 50 (10%) on the SP1-repressor list and 4 (6%) on the SP1-activator list (Figure 3E) as transcriptional regulators. Of these, ten (GATAD2, POU4F3, ZNF71, KAT5, ZNF260, ANKZF1, SMYD5, DMRT1, TAF13, NKX6–2) had a −Log10(RRA) score above 3,5 in either the repressor or activator screen (Figure 3F). For a gene from our list to act as a direct repressor or activator for SP1 we reasoned that regulators from the repressor list should have a known function as repressors and genes from the activator list should have known activating functions. Four genes from the list appeared to have opposing functions (KAT5, ZNF260, NKX6–2, ANKZF1, Figure 3F, not bold)36–39 leaving six genes (Figure 3F, bold)40–46 on our list. Histone modification has previously been known to dictate temperature-dependent sex determination in reptiles47 and vernalization in plants48, and one of these six targets (SMYD5) is a histone methyltransferase, known to catalyze H4K20, H3K9, and H3K36 trimethylation44,45. Interestingly, SMYD5 is a likely interactor with KDM6B in fish49 and KDM6B mediates the previously described epigenetic temperature dependent sex determination switch in reptiles47. Also, like CIRBP and RBM3, SMYD5 is a known resident of stress granules50. Since SMYD5 histone tail modifications are known and their levels can be measured at SP1 and other targets we argued that this might be useful for validation purposes. Thus, we decided to further focus our efforts on SMYD5.
SMYD5 is a direct repressor of SP1.
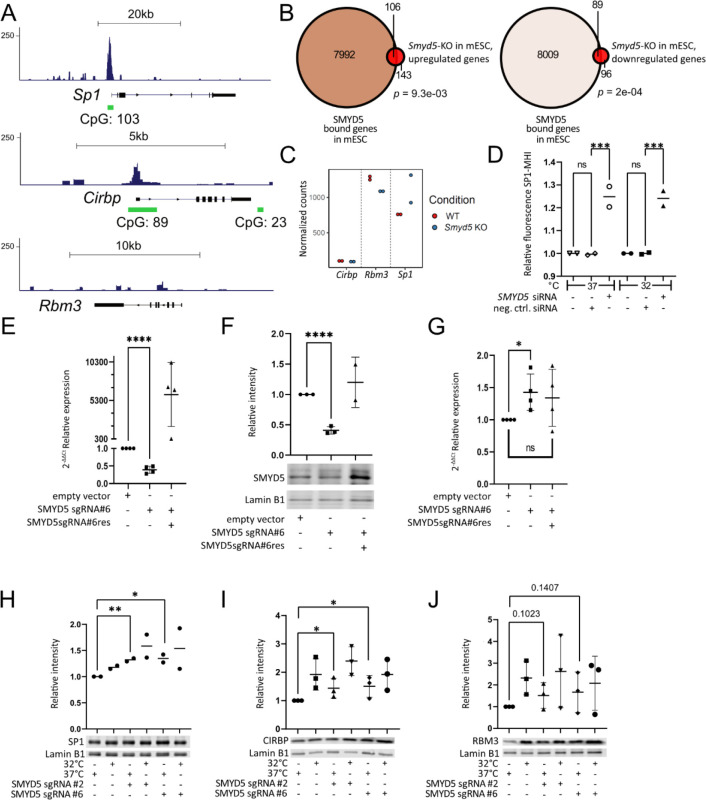
To further explore the role of SMYD5, we took advantage of a newly published dataset44 from a CUT&TAG assay in mouse Embryonic Stem Cells (mES) with an overexpressed SMYD5-Flag plasmid. Upon re-analysis of these data, we noticed that SMYD5 has strong peaks at the promoters of both Sp1 (130K) and Cirbp (70K), but not Rbm3 (Figure 4A). These peaks occur at evolutionarily conserved sites and overlap with CpG islands (Figure 4A). We took advantage of an RNASeq dataset on Smyd5 knockout (KO) mESC from the same study44 and reanalyzed it which yielded approximately equal amounts of upregulated and downregulated genes among SMYD5 bound loci (Figure 4B) supporting prior reports that histone marks deposited by SMYD5 can either repress or activate44,45. When looking at the three loci (Sp1, Cirbp, Rbm3), we found that Smyd5-KO yielded increased expression of Sp1 but not Cirbp or Rbm3 (Figure 4C) suggesting a direct role for SMYD5 as an Sp1 repressor in a murine system. In this dataset, Cirbp showed lower expression compared to Sp1 and Rbm3 (Figure S10) which may impede interpretation. In order to validate these findings in our own system we decided to knockdown SMYD5 with siRNA in HEK293WT+Cas9+SP1 cell lines, we observed an increased fluorescence of SP1-MHI at 32°C and 37°C (Figure 4D). SMYD5-KO with a guide RNA (gRNA) and Cas9 significantly decreased amounts of SMYD5 mRNA expression by qRT-PCR (Figure 4E) and SMYD5 protein levels by Western blot (Figure 4G), which are rescued upon overexpression of a gRNA-resistant Flag-SMYD5 plasmid (Flag-SMYD5 sgRNAres plasmid [labelled SMYD5sgRNA#6res], Figure 4E and 4G). The same experiment revealed de-repression of the SP1 transcript in KO cells by RT-qPCR, with repression restored in rescued cells (Figure 4F). Furthermore, we observed a significant increase of SP1 and CIRBP, but not RBM3 following SMYD5-KO at 37°C using Western blot of the endogenous loci (Figure 4H–4J).
Figure 4. SMYD5 is a repressor of SP1 at 37°C.
(A) Overexpressed FLAG-tagged SMYD5 in mESc binds at promoters of SP1 and CIRBP but not of RBM3. (B) Venn graph showing both up- and down-regulated SMYD5-bound genes in an RNASeq of Smyd5-KO cells. Statistical analysis was done with GeneOverlap package in R using Fisher’s Exact test. (C) Smyd5-KO leads to increased expression of SP1 but not RBM3 or CIRBP in mESCs. (D) SMYD5 knockdown by siRNA yields higher levels of fluorescence of SP1-MHI at 32°C and 37°C compared to empty vector control. Shown are data points, each data point is a biological replicate that has been normalized against the same non-transfected HEK293WT+Cas9+SP1-MHI cell line and a mean of data points. Significance levels were calculated with Šidák’s multiple comparison test in GraphPad Prism. (E) Relative expression of SMYD5 compared to GAPDH from SMYD5-KO cells, measured by RT-qPCR at 37°C, with or without rescue with Flag-SMYD5 sgRNAres plasmid (labelled SMYD5 sgRNA#6res) which demonstrates that SMYD5-KO was successful. Shown are data points, each data point is a biological replicate, mean and SD where applicable. Significance levels were calculated with an unpaired one-tailed t-test in GraphPad Prism. (F) Western blot quantification and representative figure using antibodies against SMYD5 and Lamin B in a SMYD5-KO cell line with and without rescue with Flag-SMYD5 sgRNAres plasmid (labelled SMYD5 sgRNA#6res) shows that SMYD5-KO was successful on protein level at 37°C. Data shown as in (E). (G) Relative expression of SP1 compared to GAPDH from SMYD5-KO cells, measured by RT-qPCR at 37°C, with or without rescue with Flag-SMYD5 sgRNAres plasmid (labelled SMYD5 sgRNA#6res). SMYD5-KO leads to increased expression of SP1 showing that effect is secondary to targeting of SMYD5 but not something else. Data shown as in (E). (H-J) Western blot quantification and a representative figure using antibodies against SP1, CIRBP and RBM3, respectively, in SMYD5-KO cells. Shown are data points, each data point is a biological replicate, mean and SD where applicable. Significance levels were calculated with an unpaired one-tailed t-test in GraphPad Prism. * = P<0.05, ** = P<0.01, *** = P<0.001, **** = P<0.0001.
SMYD5 shows temperature dependent expression in vitro and in vivo.
To test whether SMYD5 shows differential expression levels at 37°C and 32°C, we performed a Western blot on HEK293 for SMYD5 using a custom antibody against SMYD551 that showed significantly decreased SMYD5 at 32°C (P<0.01, Figure 5A). This difference was not observed at the transcriptional level (Figure 5B) indicating that SMYD5, is primarily regulated post-transcriptionally. To validate this further we performed immunofluorescence staining where we observe nuclear and cytoplasmic staining of SMYD5 at 37°C (SMYD5; white or green, DAPI; purple, Figure 5Ca) and at 32°C (Figure 5Cb). The expression of SMYD5 was significantly (p<0.05) decreased at 32°C compared to 37°C (Figure 5C–5D and Figure S11A). We validated this by using an anti-FLAG antibody in cells with overexpression of SMYD5-FLAG44, which yielded a similar result (Figure S11B). We observed a non-significant difference in nuclear to whole cell SMYD5 mean intensity ratio at the two temperatures (Figure 5E), suggesting that the levels are equally affected in both compartments at 32°C. Finally, we show that the brains of mice cooled in a standard regimen used to treat neonatal hypoxic-ischemia52 show a similar temperature-dependent nuclear decrease in SMYD5 after 6 h of cooling, both in neurons of the dentate gyrus (DG) region of the hippocampus (Figure 5Fa–d) and cortical neurons (Figure 5Fe–h), indicating a SMYD5-dependent impact on the MHR during standard TTM regimen in mice.
Figure 5. SMYD5 is depleted at 32°C in vitro and in vivo.
(A) Western blot quantification and a representative figure with a SMYD5 antibody at 37°C and 32°C after 6 h incubation. Significance levels calculated with an unpaired two-tailed t-test in GraphPad Prism. Shown are data points, each data point is a biological replicate, SD and mean are depicted where applicable. (B) qRT-PCR results for SMYD5 expression at 37°C and 32°C after 6 h incubation. Significance levels calculated with an unpaired one-tailed t-test in GraphPad Prism, otherwise data shown as in (A). (C) Intensity of endogenous SMYD5 at 37°C (top, a) and 32°C (bottom, b) after 6 h incubation. Nuclei are marked with a dotted line. (D) Cellular SMYD5 mean intensity levels. Data shown as in (B). (E) SMYD5 nuclear to whole cell mean intensity ratio. Data shown as in (B). (F) SMYD5 staining in brain slices of mice kept at euthermia (37°C) compared to mice that had been cooled down to 32°C for 6 h, mouse model of TTM. Brain slices are from the hippocampal DG (a-d) and cortical (e-h) region. * = P<0.05, ** = P<0.01, *** = P<0.001, **** = P<0.0001.
Epigenetic and transcriptional consequences of temperature-dependent changes in SMYD5.
As SMYD5 is decreased at 32°C and SMYD5-binding peaks overlap a CpG-island (Figure 4A), we performed DNA methylation (DNAm) analysis using the 850 K EPIC Illumina array and observed limited DNAm at 16/18 sites and no differential DNAm at SP1, CIRBP or RBM3 promoters at 32°C for 6 h compared to 37°C at CpGs spanning each of the gene regions (Figure S12 and Supplementary Table 5). However, there was differential DNAm at other CpGs loci after 6 h exposure at 32°C (Supplementary Table 6) supporting the idea that the MHR is regulated by the epigenetic machinery. To further explore the epigenetic consequences of differential SMYD5 availability at 37°C versus 32°C, we performed CUT&RUN using antibodies against H3K36me3, H4K20me3, H3K9me3, H3K4me3, H3K27me3, SMYD5 and H3 (control) in HEK293 cells and human neural progenitor cells (NPCs). We chose H3K36me3, H4K20me3, and H3K9me3 since these modifications have been previously shown to be deposited by SMYD544,45. We also chose to look at H3K4me3 and H3K27me3 as these are general open and closed chromatin modifications that are also known to change during vernalization in plants53. We saw less H3K36me3 over SP1 at 32°C compared to 37°C in NPCs (Figure 6A) and HEK293 cells (Figure S13A) as well as decreased global SMYD5 binding (Figure 6B), all consistent with lower SMYD5 amounts at 32°C (Figure 5). We further observed a global deficiency of H3K36me3 at 32°C compared to 37°C seen in both HEK293 cells (Figure 6C) and NPCs (Figure S13B) albeit more prominent in NPCs indicating that this modification is used to regulate additional genes perhaps through SMYD5 but levels of SMYD5 mirror this effect (Figure 6B and Figure 5). We also observed a global H3K4me3 deficiency at 32°C compared to 37°C (Figure 6D), although there were also 1288 genes that had a unique H3K4me3 modification at 32°C which was not seen at 37°C (Supplementary Table 8). For H3K9me3 we saw a deficiency of histone modification marks upstream of gene bodies in NPCs (Figure 6E). We did not observe a difference for other histone modifications when comparing 32°C and 37°C in NPCs (Figure S13C–S13D). CUT&RUN for H3K36me3 also revealed several significantly differentially modified genes at 32°C when compared to 37°C (Figure 6F, Figure S13E, Figure S14, and Supplementary Table 7) including a number of epigenetic regulators, such as BAZ2A and SETD1B (Figure 6F, Figure S14A, and Figure S14E). To identify additional genes regulated by SMYD5 and temperature, we performed an RNASeq in mouse NPCs (mNPCs) at the two temperatures. This yielded 2890 differentially expressed genes (DEGS) that were upregulated and 3133 DEGS that were downregulated at 32°C compared to 37°C. Interestingly, when we overlap upregulated DEGS in mNPCs at 32°C with SMYD5-bound and repressed genes (Smyd5-KO upregulated) at 37°C in mESCs we observe a statistically significant overlap of 26 genes (P=5.4e-04, Figure 6G). Similarly, when we overlap down-regulated DEGS in mNPCs at 32°C with SMYD5-bound and upregulated genes (Smyd5-KO downregulated) at 37°C in mESC we also observe an overlap of 19 genes (P=0.029, Figure 6H). This suggests that SMYD5 regulates additional genes in a similar manner to SP1 (as a repressor at euthermia) as well as regulating some in an inverse manner (normally as an activator at euthermia) in murine models. This includes several genes upregulated at 32°C with a theoretically appealing role for hypothermia responses such as Klf15 (role in brown adipose tissue metabolism)54 and Thrap3 (role in thyroid metabolism and adipocyte differentiation)55,56. Among the genes normally upregulated at 37°C but downregulated at 32°C, Bnip3L, a pro-apoptotic gene57 is of particular interest given the previously observed cytoprotective role of mild hypotherma9–13. We provide the entire list of 45 SMYD5-temperature dependent genes in Table 1. When we looked more closely at the list of genes that had unique H3K4me3 modification in NPCs at 32°C and overlapped those genes with human orthologs of upregulated DEGS in mNPCs at 32°C we saw a significant overlap of 169 genes (P=2.4e-03, Figure 6J, and Supplementary Table 9). Only 6 genes are bound by SMYD5 and upregulated with Smyd5-KO at 37°C in mESCs as well as upregulated at 32°C in mNPCs (Table 1) and have a unique H3K4me3 peak at 32°C in NPCs (Supplementary Table 8). Interestingly Asxl1, a member of the Polycomb family58 a family which is known to take part in vernalization in plants48, is among these 6 genes. This indicates that there may be additional epigenetic MHR-responses that are independent of SMYD5. Finally, we demonstrate that on average these 45 genes, regulated by SMYD5, have lower H3K36me3 modifications at 32°C versus 37°C at their gene bodies as well as fewer modifications over the 3ˈ-UTR region at 32°C in NPCs and HEK293 cells (Figure S13F–S13G). In addition, these genes also show a different H3K4me3 and H3K9me3 modification pattern at 32°C and 37°C in NPCs when looking at their 3ˈ- and 5ˈ-UTR regions (Figure S13H–S13J).
Figure 6. SMYD5 is a novel epigenetic gatekeeper of the MHR.
(A) Decreased H3K36me3 is observed at the SP1 promoter and gene body at 32°C compared to 37°C. (B) SMYD5 chromatin binding is globally decreased (all genes) at 32°C compared to 37°C. (C) There is a global deficiency of H3K36me3 at 32°C compared to 37°C. (D) There is a global H3K4me3 deficiency at 32°C compared to 37°C. (E) Upstream of gene bodies there is a global H3K9me3 deficiency at 32°C compared to 37°C. (F) At the gene-specific levels there are a number of genes, that have significantly altered H3K36me3 at 32°C compared to 37°C in NPCs. Red circles indicate genes that have Log2 fold change over 1 and p-value less than 10−6, gray circles indicate Log2 fold change over 1 but non-significant p-value (>10−6) and black circles indicate non-significant Log2 fold change and p-values for those genes. (G-H) DEGS from RNASeq in mNPCs at both temperatures show overlap with SMYD5-bound genes that are differentially expressed upon Smyd5-KO in mESCs at 37°C. Statistical analysis was done with GeneOverlap package in R using Fisher’s Exact test. (J) There is a significant overlap of DEGS and genes that have a unique H3K4me3 modification at 32°C. Statistical analysis as in (G-H).
Table 1:
Summary of temperature responsive SMYD5-regulated genes.
| SMYD5-repressed genes ↑ at 32°C (SMYD5 represses at 37°C) | ||
|---|---|---|
| Gene | Function | Role |
|
| ||
| Mybl2 | Transcription factor | Cell-cycle control |
| Klf15 | Transcription factor | Brown-tissue metabolism |
| Thrap3 | Transcriptional co-activator | Thyroid hormone receptor binding |
| Tex15 | DNA repair protein | Role in testicular meiosis |
| Ewsr1 | RNA binding | Role in Ewing sarcoma |
| Tfcp2l1 | Transcription factor | Pluripotency in stem cells |
| Amotl2 | Angiostatin binding factor | Role in angiogenesis |
| Phf20 | Methyl-lysine-binding protein | Histone modification |
| Cd24a | Sialoglycoprotein | Cell differentiation |
| Nt5e | Nucleotidase | Nucleotides metabolism |
| Neo1 | Cell surface receptor | Immunoglobulin superfamily |
| Gabbr1 | GABA receptor | Inhibitory neurotransmitter |
| Asxl1 | Chromatin binding protein | Polycomb family member |
| Tulp4 | Tubby Superfamily Protein | Ubiquitination function |
| Pkn1 | Serine/threonine-protein kinase | Protein kinase C superfamily |
| Hnrnpul1 | RNA-binding protein | hnRNP family |
| Mier1 | Transcriptional regulator | Mesoderm induction early response |
| Fam21 | WASH complex member | Endosomal protein sorting |
| Trip12 | E3 ubiquitin-protein ligase | Ubiquitin regulation and DNA repair |
| Lamc1 | Extracellular matrix glycoprotein | A laminin |
| Rell1 | RELT-like protein | Regulates the p38MAPK cascade |
| Xrn2 | 5'-3' exonuclease | Promotes transcription termination |
| Hspg2 | Multidomain proteoglycan | Encodes Perlecan protein |
| Ctc1 | Telomere maintenance protein | Limits telomere degradation |
| Stag1 | Component of cohesin complex | Role in cohesion of sister chromatids |
| Ass1 | Enzyme | Role in urea cycle |
| SMYD5-upregulated genes ↓ at 32°C (SMYD5 activates at 37°C) | ||
| Gene | Function | Role |
|
| ||
| Usp28 | Ubiquitin Thioesterase | DNA damage checkpoint |
| Ccnd1 | Cyclin D | Cell cycle regulation |
| Dzip3 | Ubiquitin-protein transferase | Spermatogenesis |
| Rad50 | DNA double-strand break repair | Cell cycle regulation |
| Arglu1 | Enables cadherin binding | Mediator complex |
| Sltm | RNA binding activity | Regulation of transcription |
| Slc16a3 | Solute transporter | Lactic acid and pyruvate transport |
| Ier5l | Unknown function | Parolog of IER5 |
| Acly | Enzyme | Synthesis of cytosolic acetyl-CoA |
| Agps | Enzyme | Lipid biosynthesis |
| Apobec3 | Cytidine deaminase | RNA editing |
| Ivns1abp | Viral binding protein | Response to viral infection |
| Dus3l | Synthesis of dihydrouridine | RNA editing |
| Upp1 | Uridine phosphorylase | Ribonucleosides dynamics |
| Ldha | Enzyme | Lactate to Pyruvate conversion |
| Scd2 | Enzyme | Fatty acid biosynthesis |
| Smarcad1 | Chromatin remodeler | Role in heterochromatin formation |
| Bnip3l | Pro-apoptotic protein | Induces apoptosis |
| Esrra | Nuclear receptor | Mitochondrial biogenesis regulator |
Discussion
Histone modification has previously been found to help integrate the effects of cold temperature exposure in diverse organisms. For example, in plants, vernalization, the basis of how cold exposure influences the rate of flowering, is mediated through a Trithorax-Polycomb switch48. In some reptiles, sex determination is achieved through a temperature dependent mechanism and for one such, the red eared slider turtle (Trachemys Scripta Elegans), this regulation acts through a histone methylation switch47. To uncover regulators of the mammalian MHR we used our novel MHIs to identify an FDA-approved drug, Entacapone, that can induce the SP1-mild hypothermia response as well as conducting a forward CRISPR-Cas9 mutagenesis screen resulting in the identification of SMYD5 as a repressor of the MHR at euthermia. Such insights can be useful to expand the mechanistic understanding of the MHR, but our data also provide both a feasible current strategy (Entacapone) as well as a future therapeutic target (SMYD5) for efforts to activate the MHR without cooling in patients at risk of neurological sequela after catastrophic events. Finally, a detailed understanding of the mammalian MHR is important to understand how temperature can systematically bias results in biology and medicine. One such example involves the process of harvesting tissue from human cadavers but uniformly these are kept at a low temperature which may activate the mild hypothermia response in the cells being harvested. Similarly, countless researchers store samples at room temperature between experimental steps which in some scenarios may activate the MHR. This contrasts with storage on ice but in our own hands that does not appear to activate the MHR. Such data may be of use to a large number of scientists.
Our current MHIs, a novel tool for the MHR community, allow for some temporal dissection, yet they all currently harbor stable fluorescent protein, which may capture average responses over time rather than dynamic difference, but the latter can be detected by real time qPCR. Thus, our current MHIs may be the most useful to map the start of the response (as we have done in Figure 1). However, in future work, we hope to create unstable fluorescent molecules that break down quickly to help us map the kinetics of different parts of the response, for example, how quickly the response is resolved (i.e., termination).
Although our drug screen and validation may have limitations (only three timepoints, single dose), we observe an overrepresentation of TKIs. The reason why some TKIs as well as other drugs did not validate could be that the drug screen was performed using an MHI containing the MCRE (to increase signal) but validation was performed on an MHI lacking the MCRE (the endogenous situation in mammals15). Of the TKIs we have further shown that Poziotinib, a known pan-HER inhibitor29, was able to increase RBM3 mRNA but not SP1 or CIRBP mRNA (Figure S7) or SP1 (Figure 2F) at 37°C with the doses tested. The lack of increased SP1 quantified by Western blot could be caused by the relatively low (4 μM) Poziotinib dose compared to what was used for RT-qPCR and flow cytometry (20 μM). Here, we identify and validate Entacapone as a compound that can activate the SP1-MHR at euthermia (Figure 2E). Entacapone is a COMT-inhibitor, which is frequently used in the clinic due to its ability to increase dopamine availability by blocking its degradation in synapses59. It is interesting that synaptic plasticity is known to be influenced by temperature changes60. This suggests that Entacapone may act at the cell membrane since Entacapone is a neurotransmitter breakdown inhibitor (COMT), which acts at the neural synapse. In fact, several candidates (Figure 3C–3D) from the CRISPR-Cas9 forward mutagenesis screen appear to operate at the neural synapse or bind to Syntaxin (Supplementary table 3) such as STXBP1 (SP1 repressor) and TXLNB (SP1 activator). Both bind to Syntaxin (www.genecards.org) and play a role at the neuronal synapse as well as having a role in dopaminergic synaptic transmission61, and such distal regulation may be worth exploring in future work.
Our data support the notion that SP1 and RBM3 may be parts of distinct arms of the mammalian hypothermia response as SMYD5 only regulates SP1 but not RBM3. We interpret the lag in the response (at least 6 hours to signal) and the obvious shift in fluorescence of mutagenized cells to mean that there are likely many genes upstream of the currently tested point of the response (SP1). Despite a global shift towards activators, the yield from the enrichment screen yielded more putative SP1 repressors compared to SP1 activators. This could be caused by the screen setup as it may be easier in general to find genes that increase fluorescence (repressors) than those that decrease fluorescence (inhibitors), as the latter will overlap with cells that have lost the SP1-MHI for various reasons. Another limitation of our forward screen is that it can only identify cell-autonomous genes and cannot find essential genes. However, it is a starting point, and here we provide 556 candidate regulators that can be subsequently validated using our MHIs or other strategies. Of interest, SMYD5 and ERBB2/HER2 (the receptor Poziotinib blocks) have been previously found to functionally interact in a forward mutagenesis screen of genes that play a role in metastatic reactivation and tumor dormancy62 and additionally, CIRBP transcript levels correlate with prognosis in luminal A/B breast cancer, which is influenced by HER2 status63. In fact, our SP1-repressor screen revealed several downstream factors of ERBB2 signaling (RAS, ERK, STAT5, Figure S9A), indicating that this pathway may be upstream of SMYD5 and worth exploring. Alternatively, SP1 itself may be a target of phosphorylation, and as SP1 is predicted to bind at its own promoter64 and perhaps both signals are required for full effect.
Here, by uncovering SMYD5 as a negative epigenetic regulator of SP1, a key factor of the mammalian MHR, we provide an example of how histone methylation integrates temperature cues into a fundamental mammalian response. Our present model is that SMYD5 exerts its effects at 37°C to ensure that SP1/CIRBP are not upregulated without a cold stimulus, however, upon cold stimulus (32°C), SMYD5 is degraded and SMYD5 repression is released, thus activating the MHR. The nuclear SMYD5 repression release upon mild hypothermia could happen because of one of the following: nuclear exclusion of SMYD5, less SMYD5 mRNA being translated, less protein synthesis, or SMYD5 could undergo proteasomal degradation. Per bioGRID, SMYD5 is known to bind to four ubiquitinators (PARK2, UBA52, TRIM25, USP34) so this may be an avenue for further study as such factors often target proteins to the proteasome. Previously, a generalized decrease of protein production upon cold stimulus has been shown to act through AMPK mediated phosphorylation of eEF2K22, and thus decreased translational efficiency could play a part. However, we are unaware of biological examples of targeted protein degradation in response to mild hypothermia.
SMYD5 itself appears to regulate at least 45 other factors that show temperature dependence, 26 in which SMYD5 acts as a repressor (like SP1) and another 19 where SMYD5 acts as an activator at euthermia. Some of these genes look like very plausible MHR participants, especially BNIP3L,57 a pro-apoptotic factor that is downregulated with hypothermia and may thus contribute to the neuroprotective/cytoprotective effect of mild hypothermia. There are also genes that play a role in thyroid and adipocyte function (THRAP3)55,56, brown adipose tissue (KLF15)54 and testes (TEX15)65,66, all target tissues of physiological mammalian temperature responses. Coinciding with decreased global H3K4me3 at 32°C, we also observed decreased H3K36me3 at 32°C over the gene SETD1B (Figure 6F and S14E) which encodes an H3K4me3-methyltransferase. In oocytes, Setd1b-KO can cause gain or loss of H3K4me367. Accordingly, this could be one potential mechanism underlying H3K4me3 changes at 32°C and warrants further investigation. It is interesting that H3K36me3 has previously been shown to regulate temperature-regulated alternative splicing in plants68 and in the fungus Saccharomyces cerevisiae, H3K4 and H3K36 methyltransferases (Set1 and Set2) regulate alternative polyadenylation69. Furthermore, in mice, H3K36me3 has a direct impact on alternative splicing of neurobiological factors in the brain70. In mammals’ temperature dependent alternative splicing71 and alternative splicing of CIRBP and RBM3 have been observed72–74. Perhaps alternative splicing or polyadenylation play a role in controlling levels of SP1 or one of the other 45 SMYD5-bound factors elucidated here.
In summary, we have developed and validated novel tools to interrogate the MHR and used them to show, for the first time, that a compound exists that can activate the MHR without cooling. We provide a list of 556 candidate of the MHR pathway and validate SMYD5 as a temperature-dependent epigenetic gatekeeper of the MHR and, for the first time, show how histone methylation (H3K36me3, H3K9me3, and H3K4me3) changes in mammals with MHR exposure, providing a specific example of how mammalian cells use epigenetics to respond to temperature cues.
STAR Methods
Creation of novel indicators:
We used the PGL4.10 plasmid (Promega, #9PIE665) as the backbone for our CIRBP- and SP1-MHI, where the luc2 sequence was cut from the plasmid with NcoI (NEB) and XbaI (NEB). Promoter sequence for CIRBP75 was cloned from mouse DNA using CIRBP forward primer 5’-tcgataggtaccTGGCTTCACAAATGCGCCTCAGT-3’ and CIRBP reverse primer 3’-cctaaggcagatctGCGAGGGGGAGCGCAAGAGT-5’75. Restriction enzymes KpnI (NEB) and BglII (NEB) were used to insert the promoter into the plasmid. Promoter sequence for SP176 was cloned from human DNA using the forward primer 5’-tcaagtcaggctagcGCAACTTAGTCTCACACGCCTTGG-3’ and reverse primer 3’-cagtgctgcctcgagGCTCAAGGGGGTCCTGTCCGG-5’76. Restriction enzymes NheI (NEB) and XhoI (NEB) were used to insert the promoter into the plasmid. AcGFP1 was cloned from PT7XbG2-AcGFP1 (Novopro, #V002843) vector using the reverse primer 3’-cggcggagTCTAGAATTACTTGTACAGCTCGTCC-5’ and the forward primer 5’-taagccaccATGGTGAGCAAGGGCGAGGAGC-3’. Restriction enzymes NheI (NEB) and XhoI (NEB) were used to insert the acGFP1 into the plasmid. The neomycin selection cassette was cloned from pROSA26-dest plasmid (Addgene, #21189) using the forward primer 5’-CATTATCGTCGACTCTACCGGGTAGGGGAGGCGCTT-3’ and reverse primer 3’-CGCCGCCGACGATAGTCAAGCTTCTGATGGAATTAGAACTTGGC-5’. Restriction enzymes SalI (NEB) and PshAI (NEB) were used to insert the neomycin cassette into the plasmid. CIRBP- and SP1-MHI were made with and without MCRE15. The MCRE sequence was inserted in 5 repeats in front of the promoter sequences with a linker sequence between. Acc65I (NEB) restriction enzyme was used to insert the MCRE sequence into the CIRBP-MHI and Acc65I (NEB) and SacI (NEB) restriction enzymes were used to insert the MCRE into the SP1-MHI. Sanger Sequencing was used to validate insertion sites, size, and sequence of insertion. The RBM3 promoter with MCRE enhancer (6 repeats) in front of the promoter was cloned into the multiple cloning site pCMV6-AC-GFP (BlueHeron, #PS100010). We also made lentiviral vectors for CIRP, SP1 and RBM3 on a pLV-Ex (VectorBuilder) lentiviral vector. The CIRBP promoter for the CIRBP-lenti-MHI was designed from the human promoter and the SP1 promoter was moved upstream of the TSS for the SP1-lenti-MHI but RBM3 promoter sequence remained unchanged. Included were MCRE (8 repeats for CIRBP-lenti-MHI, five repeats for other), promoter, neomycin selection cassette and a mCherry fluorescent protein behind each promoter sequence. The promoter region, size and backbone plasmid for each indicator is detailed in Supplementary Table 1 and sequence of each MHI in Supplementary File 1. We also observed some variation in absolute levels among individual indicators/experiments, but the pattern was always consistent; given this, we only show data from individual experiments in any figure.
Culture of cell lines:
HEK293 (gifted and DSMZ, CRL-1573, referred to as 293/293WT) cells were cultured in DMEM/F12 with GlutaMAX (Gibco, 10565018) with 10% filtered FBS (Gibco, 10270106) under 5% CO2. Cells were cultured at 37°C unless otherwise stated, then 37°C served as a control. In addition to HEK293, we used the following cell types HEK293T (gifted, CRL-3216), HCT-116 (gifted, CCL-247), HeLa (gifted, CCL-2), Jurkat (gifted, TIB-152), K562 (gifted, CCL-243), SK-N-SH (gifted, HTB-11). Cells were sub-cultured when they had reached 70–90% confluence and media was changed every other day. Killing curves were used for all cell lines to determine lowest selection concentration for all selection agents. We used culture recommendations from ATCC when possible. We also used human iPS cells (ATCC, ACS-1019) that were used to generate NPCs by neural induction of iPSCs using dual SMAD inhibition and embryoid body generation (StemCell Technologies; 08581, 05832, 05838)77, prior to experiments described here the NPCs were cultured in STEMdiff Neural Progenitor Medium (StemCell Technologies, 05833) on Matrigel (Corning, 354234). As well as murine primary NPCs (see section mNPC isolation for further information).
Transfection of MHI and SMYD5-Flag overexpression plasmid:
HCT116, 293WT, HEK293T, HeLa and SK-N-SH cell lines were transfected with MHI when they had reached 70–90% confluency. We used either Lipofectamine™ 2000 or 3000 (Thermo Fisher Scientific, 11668027, L3000015) according to the manufacturer’s protocol. For transfection of K5622 and Jurkat cell lines we used electroporation for transfecting the MHIs into the cell lines. The fluorescence of the indicators was measured via flow cytometry up to 48 h after transfection. We analyzed flow cytometry data with the FlowJo Software, where we gated living cells, single cells, GFP positive cells and measured the fluorescence for that population. For further analysis, we used Excel from Microsoft, GraphPad Prism (v.9) and FlowJo (v.10.8.1). For transfection of SMYD5-Flag overexpression vector44 we used the same lipofectamine method as described above.
Construction of 293WT+Cas9 and 293WT+Cas9+SP1 cell lines:
We made two cell lines from 293 cells, one that expressed Cas9 (lentiCas9-blast, Addgene #52962), referred to as 293WT+Cas9, and another that expressed both Cas9 and SP1-MHI, referred to as 293WT+Cas9+SP1. These cell lines were made by transfecting the plasmids with lipofectamine as described above and selecting stably transfected cells using Blasticidin selection for Cas9 and Neomycin selection for SP1-MHI.
Drug screen:
Briefly, we used Lipofectamine 3000 (Thermo Fisher Scientific, L3000015) to transfect the SP1+MCRE-MHI into the 293T cell line. 24 h after transfection, 30000 cells were plated into each well of a black 96 well plate. 48 h after transfection the cells were exposed to FDA Approved Drug Screening Library (L1300-Z298012) via the High Throughput Screening Services, ChemCore Facilities (Johns Hopkins University). Each drug was exposed in 20 μM final concentration. Total well GFP was read with the CLARIOStar Plus Plate Reader (BMG LABTECH, settings: gain 1500, 470–15 excitation and emission 515–20 at focal height 2.6) 16, 28, and 40 h after exposure of the library. Ratio of increased fluorescence was calculated by dividing GFP reads from positive control (SP1+MCRE-MHI transfected but untreated) at each time point with GFP reads of each whole well exposed to drug at the same timepoint for wells in the same lane. For further analysis, we used Excel from Microsoft and GraphPad Prism (v.9).
Validation of drug screen:
We individually validated 8 compounds in same concentration (20 μM). Here, we exposed the drug for 16 h either at 32 or 37°C, and evaluated by flow cytometry as previously described. In a 96-well plate 20,000 of either 293 or 293+SP1-MHI cells were seeded per well in a total volume of 200 μL of media. After cells had attached to the wells (the morning after), cells were exposed to 20 μM of each drug, controls were exposed to the same quantity of DMSO as drug, for 16 h and harvested for flow cytometry analysis. Flow cytometry analysis was conducted as previously described. Drugs that were tested for further validation after initial screen were: Orantinib (Selleckchem, S1470), Dasatinib (Selleckchem, S7782), Poziotinib (Selleckchem, S7358), Tamoxifen Mylan (Mylan AB*), Erythromycin (Stragen Pharma GmbH*), Naloxone (B. Braun*), Entacapone (Orion Corporation*) and Cinacalcet (Ratiopharm GmbH*). Drugs labeled with “*” were pharmaceutical grade medications gifted from the Pharmacy at Landspítali University Hospital. Analysis was performed as described above.
Apoptosis assay:
We based our apoptosis assay on a protocol published by Xiang et al.78. We exposed 293WT+Cas9+SP1 cell line either to 1–5 μM H2O2 or vehicle (H2O) for 4 h at 32°C or 37°C. 4 h after exposure gMFI of the cells were analyzed with flow cytometry as described above.
Cell cycle assay:
The cell cycle assay was based on a protocol from Rutgers Flow Cytometry/Cell Sorting & Confocal Microscopy Core Facility (available online)79. HEK293 cells were seeded in four 12-well culture plates. The day after, the media was replaced with fresh media containing Poziotinib, Entacapone, or DMSO (vehicle) either at 32 or 37°C. 16 h later, the cells were harvested. To include dead cells in the analysis, the media and the PBS used to wash the cells were collected with the cells. Cells were fixed with 1% paraformaldehyde for 1 hour at 4°C and then permeabilized with 66% EtOH at 4°C for 2 hours. Next, cells were stained with 1 × propidium iodide + RNase staining solution (Abcam, ab139418) and incubated at 37°C in the dark for 30 minutes. Cells were placed on ice and underwent flow cytometry.
Genome Wide CRISPR-Cas9 screen on fluorescent HEK293WT cell lines that stably express SP1-MHI:
Using the 293WT+Cas9+SP1 and 293WT+Cas9 (negative control) cell lines we performed a genome wide CRISPR-Cas9 knockout screen (GeCKO) screen, where we transduced the sgRNA pool made from library A and B (Addgene, #1000000049) in MOI 0.317–19. We mostly followed the Joung et al. protocol19, the exceptions are as follows. For making the lentivirus, a protocol described by Kutner et al.80 was used. Lentiviral sgRNA library concentration was done with Amicon ultracentrifugal filters (Millipore, UFC9003). 20 h after transduction with the sgRNA library selection was started with Puromycin and continued for 6 days and 8 h before the cells were moved to 32°C, where they were incubated for 16 h before fluorescent activating cell sorting (FACS) with Cell sorter SH800Z (Sony). he 5% highest fluorescent cells for 293WT+Cas9+SP1 and 5% lowest fluorescent cells for 293WT+Cas9+SP1 were sorted. Next, we isolated gDNA and performed a two step PCR for all samples (sorted as well as negative control). According to cell number after puromycin selection, the sgRNA library coverage was >700 times coverage for HEK293WT+Cas9+SP1. Next generation sequencing (NGS) was performed with single read, 80 cycles and 8 indexing cycles with PhiX spike in of 20% on NovaSeq 6000 S4 at deCODE genetics for HEK293WT+Cas9+SP1 and HEK293WT+Cas9, 32–280 million aligned reads per sample. To analyze the screen data, single read fastq files for each replicate and condition were merged using cat command. Then, MAGeCK (v.0.5.9) was used to identify enriched sgRNA’s in sorted samples33,81 and sgRNA counts were normalized to internal control for sgRNA’s (control sgRNAs). We used RStudio (R, v.4.1.1)82 to visualize the results and to filter genes. We exported a list of genes that had both FDR value under 0.25 and LFC value over 2.5 for each condition in both screens. Then, we excluded genes, overrepresented in both the repressor and activators screen when compared to control as they most likely promote cell growth and therefore were present in both lists (Supplementary Table 4). For the pathway enrichment analysis we used EnrichAnalyzer() from MAGeCKFlute (v.1.12.0)81.
Making of SMYD5 knockout (SMYD5-KO) cells:
Two ready-made sgRNA vectors (Genscript, SMYD5 plasmid vector #2 and #6) were packed into lentivirus according to protocol described by Kutner et al.80, for concentration we used the 4x Lentivirus concentrator solution protocol from the MD Anderson Cancer center at University of Texas (available online)83. We selected for stable SMYD5-KO cells with Puromycin selection for at least 7 days.
Making of MALT1 and RGS21 knockout cells:
Ready-made sgRNA vectors (Genscript, plasmid vector MALT1 #1 and #2, RGS21 plasmid vector #2 and #3) were packed into lentivirus and selected as described above in the making of SMYD5-KO cells.
Making of gRNA resistant SMYD5-Flag plasmid (Flag-SMYD5 sgRNAres plasmid):
In an effort to make SMYD5-Flag resistant to CRISPR-Cas9 cutting from the two guides (Genscript, SMYD5 plasmid vector #2 and #6), we used site-directed mutagenesis (Q5® Site-Directed Mutagenesis Kit from NEB, E0554S) to modify predicted PAM sites of the guides. We used the following primers to induce the mutations: SMYD5–2mut-F: 5’-gctctttacgAGGAAGCAGTCAGCCAGT-’3; SMYD5–2-mut-R: 5’-ttccgtaaagAGTCTCCGCAGAAGTTCC-’3; SMYD5–6-mut-F: 5’-accgatatcgAGCCTGTGACCACTGCCT-’3; SMYD5–6-mut-R: 5’-atagagcgtt-CCAGAGAAACTGTGCAGCC-’3. After site-directed mutagenesis, we isolated plasmid with Monarch Midiprep kit (NEB, T1010) and Sanger sequenced to verify changes in PAM-sites. This plasmid was then used for rescue experiments (Figure 4E–4G).
siRNA:
We used the TriFECTa RNAi kit (IDT; TYE 563 Transfection Control DsiRNA, 51–01-20–19 (transfection control); Negative Control DsiRNA, 51–01-14–03 (negative control)) and used predesigned DsiRNA (IDT, hs.Ri.SMYD5.13.8) to knockdown SMYD5 mRNA in two biological cell lines of HEK293+Cas9+SP1-MHI. For the reverse transfection we used Lipofectamine 2000 (Thermo Fisher Scientific, 11668027) where we seeded 1*106 cells per well in a 6 well plate and exposed to siRNA. Around 24 h after the reverse transfection one batch of cells was exposed to 32°C and the other kept at 37°C for 6 hours before the cells underwent flow cytometry. Further statistical analysis of the flow cytometry data was similar to that described above.
Immunofluorescence of cell culture:
HEK293 cells were seeded into two 8-well chamber slides (Falcon, 354118) and cultured for 24–48 h before one of the chamber slides was moved to 32°C. After 6 h cells from both slides at 32°C and 37°C were fixed and permeabilized with 4% PFA and 0.1% Triton-X 100 diluted in PBS for 10 minutes at room temperature. Then cells were washed twice with a blocking solution (PBS with 2.5% BSA and 10% normal goat serum) and followed by blocking for 30 min at room temperature in the blocking solution. Cells were incubated overnight with primary antibodies (rabbit anti-SMYD5, 1:100051; anti-FLAG, 1:500, (Sigma-Aldrich, F1804–200UG)) at 4°C. The following day, cells were incubated at room temperature for 30 mins and then washed three times every 10 min with a washing solution composed of PBS with 0.25% BSA. Cells were incubated with secondary antibodies (goat anti-mouse IgG (H+L) Alexa Fluor™ 488, 1:1000 (Invitrogen™, A-11001); goat anti-rabbit IgG (H+L) Alexa Fluor™ 647, 1:1000 (Invitrogen™, A21244)) for 1 h at room temperature in the dark. Cells were washed three times every 10 mins with the washing solution and mounted with Fluoromount-G Mounting Medium with DAPI (Invitrogen, 00–4959-52). Cells were imaged using confocal microscopy (FV 1200, Olympus Fluoroview) using a 30× silicon objective (NA1.05). Images were processed by ImageJ84,85 and quantified using CellProfiler (v.4.2.5.)86. Statistics were calculated in GraphPad Prism.
Real Time Quantitative Polymerase Chain Reaction (RT-qPCR):
Total RNA from cells incubated at 32 or 37°C was isolated, using Direct-zol™ RNA Microprep (Zymo Research, R2062) according to the manufacturer’s instructions. The concentration of RNA was measured by NanoDrop (Thermo Fisher Scientific) followed by cDNA synthesis with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368814) on MiniAmp™ Thermal Cycler (Applied Biosystems™). RT-qPCR was performed using Luna® Universal qPCR Master Mix (NEB, M3003) on CFX384™ Real-Time PCR Detection System (Bio-Rad). Each biological replicate of the RT-qPCR assay in this study was carried out in technical triplicates. Any technical replicate that deviated from other replicates by ≥ 0.4 cycle threshold (Ct) was removed from calculations of average Ct values. The primers used are as follows: SP1 (fwd): 5’-CACCCAATTCAAGGCCTGCCGT-3’; SP1 (rev): 5’-GGGTTGGGCATCTGGGCTGTTT-3’; RBM3 (fwd): 5’-GAGACTCAGCGGTCCAGGGGTT-3’; RBM3 (rev): 5’-CCTCTGGTTCCCCGAGCAGACT-3’; CIRBP (fwd): 5’-CCGAGTTGACCAGGCTGGCAAG-3’; CIRBP (rev): 5’-TCCATAGCCCCGGTCTCCTCCT-3’; GAPDH (fwd): 5’-TCAAGGCTGAGAACGGGAAG-3’; GAPDH (rev): 5’-CGCCCCACTTGATTTTGGAG-3’. SMYD5 1 (fwd): 5’-GCACTGTGCGCAAAGACCTCCA-3’, SMYD5 2 (fwd): 5’-GGAAACCAGGCCAGGTTCTGCC-3’, SMYD5 3 (fwd) 5’-CGTGGAAGTCCGTTTCGTGA-3’, SMYD5 1 (rev:) 5’-CTGGGCACAGGACCTGGTGGTA-3’, SMYD5 2 (rev): 5’-GGCTGCCAACCGACATTCTGCA-3’, SMYD5 3 (rev) 5’-CCAGAGAAACTGTGCAGCCA-3’.
Western blot assay:
Cells were washed with PBS and then lysed (whole cell lysate) for 30 min on ice in RIPA buffer (50mM Tris HCl ph8, 150mM NaCl, 1% NP-40, 1% Sodium deoxycholate, 0.1% SDS, 2mM EDTA, phosphatase inhibitor (either Cell signaling, 5870S or Thermo Fisher Scientific, 78437)). The lysate was centrifuged at 16,000 ×g for 20 min at 4°C and the supernatant was collected. The supernatant was diluted with 4X loading buffer (LI-COR, 928–40004) and heated at 95°C for 5 min. The protein concentration was measured using Pierce™ BCA Assay Kit (Thermo Fisher Scientific, 23225). 20–30 μg of protein was loaded into each well and separated by SDS-PAGE. They were transferred to polyvinylidene fluoride (PVDF) membranes and blocked in 5% bovine serum albumin in Tris-buffered saline with Tween 20 for 1 h. Primary antibodies: rabbit anti-CIRBP, 1:2000 (Proteintech, 10209-2-AP); rabbit anti-RBM3, 1:1000 (Proteintech, 14363-1-AP); rabbit anti-SP1 1:1000 (Proteintech, 21962-1-AP); mouse anti-Lamin B1, 1:5000 (Proteintech 66095-1-Ig); rabbit anti-SMYD5, 1:100051; mouse anti-GAPDH, 1:5000 (Abcam, ab8245) were applied to respective membranes after washing, and incubated at 4°C overnight. Membranes were incubated with IRDye® secondary antibodies; anti-rabbit (LI-COR,926–32213); anti-mouse (LI-COR, 926–68072), at room temperature for 90 mins. After washing, the protein bands were visualized on the Odyssey® CLx Infrared Imaging System and protein band intensity quantified by Image J84,85 software.
DNAm studies and analysis:
HEK293WT (biological triplicates) were split into two batches and exposed to 37°C and 32°C for 6 h, next cells were harvested, and DNA isolated with the Zymo Quick-DNA™ Midiprep Plus Kit (Zymo Research, D4075). Samples were submitted to the Genetic Resources Core Facility (Johns Hopkins University), bisulfite treated, and run on the Infinium MethylationEPIC BeadChip Kit (Illumina) with two technical controls (50% and 100% DNAm, 8 samples total). Technical controls were created using mixtures of 100% and 0% methylated HCT116 DKO (Zymo Research D50414–1/2). DNA methylation data (IDAT files) containing unmethylated and methylated intensity values were imported to R (v.4.2.1) for analysis82. Raw data was processed using single sample approach with preprocessNoob() function and methylation values (beta values) were obtained using the getBeta() function from minfi package (v.1.42.0)87–89. The model matrix was created for the two temperatures using the model.matrix() function from stats R package and sva() function from sva package (v.3.44.0) was applied with two estimated surrogate variables82,90. The model was fit with contrast matrix using lmFit(), with the estimated coefficients and standard errors generated using contrasts.fit() from limma package (v.3.52.4)91. The p-values were moderated using eBayes() function from limma package (v.3.52.4) and CpG cites ranked according to their p-value91.
CUT&RUN:
293WT and human NPCs (ATCC, ACS-1019) were harvested after 6 h at 32°C or kept at 37°C. CUT&RUN performed according to Epicypher CUTANA protocol (v.1.7)92 on 300.000 cells per sample. We permeabilized the cells with 0.01% digitonin (Sigma, D141). For normalization we spiked in E. Coli DNA (Epicypher, 23618–1401) at the final concentration of 0.2 ng per sample. The library preparation was performed with TrueSeq-Chip Sample preparation Kit (Illumina, IP-202-9001). The following antibodies were used for the CUT&RUN: CTCF antibody (Cell Signaling, 2899S), H3K36me3 (Thermo Fisher Scientific, MA5–24687), H4K20me3 (EpiCypher, 13–0054), H3K9me3 (Abcam, ab176916), H3K4me3 (EpiCypher, 13–0041), H3K27me3 (Thermo Fisher Scientific, MA5–11198), SMYD5 51, and Rabbit IgG Antibody (Epicypher, 23613–0042). The sequencing was performed on a NovaSeq 6000 S4 at deCODE genetics, paired-end 8 cycles of index1, 8 cycles of index 2, 150 cycles read 1 and, 150 cycles read 2, where we aimed for 10 million reads per sample. For the CUT&RUN analysis and the CUT&TAG re-analysis we followed published STAR protocols93. Briefly, first we trimmed the raw Fastq reads in paired mode with TrimGalore (v.0.6.7)94 then we used Bowtie2 (v.2.4.4.)95 to align the reads to Hg38 or mm10 and E. Coli reference genome, all downloaded from UCSC. Overall alignment to Hg38 were from 83.6–98.63% and to E. Coli spike in were from 0.1–4.18% for our data. The aligned Sam files were then converted to Bam files, indexed, and sorted via SAMtools (v.1.15)96 and BedGraphtoBigWig (v.4)97 from BEDTools (v.2.3)98. We used SEACR (v.1.3)99 for peak calling after normalization with the normalization factor calculated as 1/(percent alignment to E. coli). We used DiffBind (v.3.9.6)100 for the differential peak analysis, ChIPseeker (v.1.35.3)101, TxDb.Hsapiens.UCSC.hg38.knownGene (v.3.4.6)102 and org.Hs.eg.db (v.3.8.2)103 for annotation. For further analysis and image creation we used deepTools (v.3.5.1.)104 and R 82.
Mouse NPC isolation:
Mouse NPCs were isolated from the cortex of E17.5 C57BL6/NTac embryos. The protocol was adapted from Bernas et al, 2017105. Embryo cortices were manually dissected from brains and the tissue was dissociated in 1X TrypLE™ Select Enzyme (Thermo Fisher Scientific, A1217701) for 10 min, with manual dissociation using a 1000 μL pipette. The cell suspension was washed in Neurobasal medium (Thermo Fisher Scientific, 21103049) and filtered through a 70 μm cell strainer (Miltenyi Biotech, 130–110-916). Cells were washed twice in 5 mL Neurobasal medium (Gibco, 21103049) by centrifugation at 200 ×g for 10 min. Cells were resuspended in 1 mL Neurobasal growth medium containing 1X B27 supplement (Thermo Fisher Scientific, 17504044), 1X Penicillin/Streptomycin (Thermo Fisher Scientific, 15140122), 1X Glutamax (Thermo Fisher Scientific, 35050038), 20ng/mL FGF-2 (Peprotech, 100–18B), 20 ng/mL EFG (Peprotech, AF-100–15), and 2 μg/mL Heparin (MP Biomedicals, 210193125). Cells were seeded onto 12-well plates, previously coated for 2 h at 37°C in 1:100 dilution of Matrigel (Corning, 354234). After passaging, NPCs were cultured in media described above on plates coated overnight in poly-D-lysine (Sigma, P7280), and then coated for 2 h in laminin (Sigma, L2020). Cells were either sub-cultured or media was changed every 2 days.
RNAseq:
Primary mouse NPC lines from 4 separate embryos were seeded 0.3 × 106 cells per well, in two batches, in a poly-D-lysine/laminin coated 6-well plate after 9 days in vitro. Following 36 h of incubation at 37°C, one plate with 4 lines was incubated at 32°C for 6 h. Cells from both 37°C and 32°C conditions were harvested in tri-reagent and RNA was isolated using the Direct-zol RNA Microprep kit (Zymo Research, R2063). RNAseq library construction and Illumina next generation paired-end sequencing was performed by Novogene, generating 30 million paired-end reads per sample. For the RNAseq analysis, Fastq files were pseudo-aligned to the GRCm39 mouse transcriptome, downloaded from NCBI, using Kallisto (v.0.48.0) with paired-end mode on and 100 bootstraps. Kallisto output files were imported into R using the tximport package106. Transcripts were assigned to genes using the TxDb.Mmusculus.UCSC.mm10.ensGene package (v.3.4.0) 107 in R .
Differential expression analysis was performed using the DESeq2 package (v.3.16)108 in R, after discarding transcripts with less than 10 counts. Differentially expressed genes were defined as those with an adjusted p-value less than 0.1. Overlaps of gene lists were calculated using the GeneOverlap package (v.1.34.0)109 in R, with overlaps tested using Fisher’s exact test. Gene set enrichment analysis was performed using the GSEA-preranked program110,111 with the Mouse Ortholog Hallmark Gene Sets112. For the GSEA-pre-ranked run, we generated a ranked gene list comprising all detected genes assigned to a rank value, which was calculated as −log10(adjusted p-value) × (sign of fold change). The genes were then ranked from the highest rank value to the lowest before inputting into the program.
Mouse cooling followed by immunostaining on brain slices:
C57BL6 mice (Jackson) were bred to create a litter (n=2). At P10, we split littermates into two groups. One group (n=6) was cooled to a core temperature of 32°C and the other (n=6) maintained at 37°C, for 6 hours respectively. These animals were then euthanized, and their brains were isolated immediately after the cooling period and flashed with PBS. One of the hemispheres was immersed in 4% PFA for 72–96 h for fixation and the other hemisphere was dissected for the cerebellum, thalamus/basal ganglia, hippocampus, anterior and posterior cortex. After fixation, samples were cryopreserved in a sucrose gradient and then flash-frozen. Frozen hemispheres were cryosectioned with the help of a cryostat (Keldur, UI). The sections were washed in TBS+ (TBS (LiCor, 927–60001) with 0.05% triton X-100 (Sigma-Aldrich, T8787–100ML)) twice for 10 minutes. Next, the slides were immersed in DAKO (target retrieval solution, sodium citrate buffer, pH 6) at 95°C for 20 minutes. Slides were then washed again in TBS+ twice for 10 minutes. Next, the slides were blocked in TBS++ (TBS (LiCor, 927–60001) with 3% goat serum (Abcam, ab7481)) for 1 h at room temperature. The slides were then stained with primary antibodies and anti-rabbit-SMYD551, 1:200, for 24 h at room temperature and carried out in the dark. On the next day, slides were allowed to reach room temperature before they are washed 3x for 10 minutes in TBS+. The slides were incubated with secondary antibodies, goat anti-mouse IgG (H+L) Alexa Fluor™ 488, 1:1000 (Invitrogen™, A-11001); goat anti-rabbit IgG (H+L) Alexa Fluor™ 647, 1:1000 (Invitrogen™, A21244), for 24 h in the dark and washed 3× for 10 minutes in TBS+. All the liquid on the slides was briefly dried off. Fluoromount-G Mounting Medium with DAPI (Invitrogen, 00–4959-52) was placed on each section and covered with a cover slip. The slides were incubated for 24 h at room temperature before being imaged using confocal microscopy (FV 1200, Olympus Fluoroview) using a 30× silicon objective (NA1.05). Images were processed by ImageJ84,85 and quantified using CellProfiler (v.4.2.5.)86. Statistics were calculated in GraphPad Prism.
Supplementary Material
Supplementary File 1: Sequences of individual MHIs.
Supplementary Table 1: Specifics of our MHI, including sequence material used, vector, selection markers, plasmid size and individual fluorophores.
Supplementary Table 2: Top 91 drug hits from MHI screen.
Supplementary Table 3: Top candidate genes found enriched in mutation screening.
Supplementary Table 4: Genes found in both repressor and activator SP1 lists and thus excluded for further evaluation.
Supplementary Table 5: List of CpGs represented at three genes.
Supplementary Table 6: List of differentially methylated locations from EPIC DNAm array.
Supplementary Table 7: Top differentially modified locations for H3K36me3 in NPCs from CUT&RUN experiment.
Supplementary Table 8: List of genes that have a unique H3K4me3 histone modification peak at 32°C.
Supplementary Table 9: List of genes that show DEGS at 32°C and have a unique H3K4me3 histone modification peak at 32°C.
Acknowledgements:
We thank SCRU lab (UI) for gifting us pMD2.G (Addgene, #12259) and psPAX2 (Addgene, #12260) and H.C. Dietz and D. Valle labs (JHU), for gifting us the HCT-116, SK-N-SH, HeLa, Jurkat and K562 cell lines. We thank Alan Long at the ChemCore facility at JHU and the Pharmacy of Landspitali for support regarding the drug screen and validation. We thank the Immunology department of Landspitali University Hospital for the access and assistance with the FACS sorting. We thank FUNlab at deCODE genetics for the usage of their flow cytometry machines as well as for gifting us the HEK293T cell line. We thank Kári Stefánsson and Ólafur Þ. Magnússon at deCODE Genetics for providing some of the next generation sequencing for this project. We thank Erna Magnúsdóttir for providing pAG-MNase for the CUT&RUN assays as well as guidance with the CUT&RUN experiment and data analysis. This study was supported with funding from the Rannis Technology Development Fund (H.T.B., #2010588), Eimskip University Fund (S.R.), Fulbright Iceland (S.R.), Icelandic Cancer Society Research Fund (H.T.B.), Göngum Saman Research Fund (H.T.B.), Landspitali Research Fund (H.T.B.), Félag Háskólakvenna Research Fund (S.R.). H.T.B. is also supported by funding from Rannís (#217988, #195835, #206806) and the Louma G. Foundation. R.C-V. is supported by Thomas Wilson Foundation and the Johns Hopkins University Pediatric Innovation Grant. R.C-V. and F.J.N. are supported by the NIH R01NS126549, 1R21NS123814, and 1R01 HD110091 and F.J.N. additionally by R01HD074593, U01NS114144. Finally, some images in this manuscript were created with BioRender.com.
Footnotes
Declaration of interest: H.T.B. is a consultant for Mahzi therapeutics. S.R. and H.T.B. have a European patent application (number 23167505.9).
References
- 1.Osilla E. V., Marsidi J.L., and Sharma S. (2022). Physiology, Temperature Regulation (StatPearls Publishing; ). [PubMed] [Google Scholar]
- 2.Turbill C., Bieber C., and Ruf T. (2011). Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proceedings of the Royal Society B: Biological Sciences 278, 3355–3363. 10.1098/RSPB.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew J.L., Kaur N., and Dsouza J.M. (2022). Therapeutic hypothermia in neonatal hypoxic encephalopathy: A systematic review and meta-analysis. J Glob Health 12. 10.7189/JOGH.12.04030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luedke M.W., Graffagnino C., McKinney B.G., Piper J., Iversen E., and Kolls B. (2022). Association of time-temperature curves with outcomes in temperature management for cardiac arrest. BMJ Neurol Open 4. 10.1136/BMJNO-2022-000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrichs E.S., and Dietrichs E. (2015). Neuroprotective effects of hypothermia. Tidsskr Nor Laegeforen 135, 1646–1651. 10.4045/tidsskr.14.1250. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y.J., Zhang Z.Y., Fan B., and Li G.Y. (2019). Neuroprotection by Therapeutic Hypothermia. Front Neurosci 13. 10.3389/FNINS.2019.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita J. (1999). Cold shock response in mammalian cells. J Mol Microbiol Biotechnol 1, 243–255. [PubMed] [Google Scholar]
- 8.Zhu X., Bührer C., and Wellmann S. (2016). Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cellular and Molecular Life Sciences, 1–21. 10.1007/s00018-016-2253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu H., Lee J., Olofsson B.A., Mwidau A., Deodoglu A., Escudero M., Flemington E., Azizkhan-Clifford J., Ferrante R.J., and Ratan R.R. (2003). Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci U S A 100, 4281–4286. 10.1073/PNAS.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H.J., Zhuang R.J., Li Y.B., Li T., Yuan X., Lei B.B., Xie Y.F., and Wang M. (2019). Cold-inducible protein RBM3 mediates hypothermic neuroprotection against neurotoxin rotenone via inhibition on MAPK signalling. J Cell Mol Med 23, 7010–7020. 10.1111/JCMM.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastide A., Peretti D., Knight J.R.P., Grosso S., Spriggs R. V., Pichon X., Sbarrato T., Roobol A., Roobol J., Vito D., et al. (2017). RTN3 Is a Novel Cold-Induced Protein and Mediates Neuroprotective Effects of RBM3. Current Biology 27, 638. 10.1016/J.CUB.2017.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chip S., Zelmer A., Ogunshola O.O., Felderhoff-Mueser U., Nitsch C., Bührer C., and Wellmann S. (2011). The RNA-binding protein RBM3 is involved in hypothermia induced neuroprotection. Neurobiol Dis 43, 388–396. 10.1016/j.nbd.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Peretti D., Bastide A., Radford H., Verity N., Molloy C., Martin M.G., Moreno J.A., Steinert J.R., Smith T., Dinsdale D., et al. (2015). RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature 2015 518:7538 518, 236–239. 10.1038/nature14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wierstra I. (2008). Sp1: Emerging roles—Beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 372, 1–13. 10.1016/J.BBRC.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 15.Sumitomo Y., Higashitsuji H., Higashitsuji H., Liu Y., Fujita T., Sakurai T., Candeias M.M., Itoh K., Chiba T., and Fujita J. (2012). Identification of a novel enhancer that binds Sp1 and contributes to induction of cold-inducible RNA-binding protein (cirp) expression in mammalian cells. BMC Biotechnol 12, 72. 10.1186/1472-6750-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Pastor R., Burchfiel E.T., and Thiele D.J. (2018). Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol 19, 4–19. 10.1038/NRM.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanjana N.E., Shalem O., and Zhang F. Improved vectors and genome - wide libraries for CRISPR screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalem O., Sanjana N.E., Hartenian E., Shi X., Scott D.A., Mikkelson T., Heckl D., Ebert B.L., Root D.E., Doench J.G., et al. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joung J., Konermann S., Gootenberg J.S., Abudayyeh O.O., Platt R.J., Brigham M.D., Sanjana N.E., and Zhang F. (2017). Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc 12, 828–863. 10.1038/nprot.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre S., Nelson K.B., Mulkey S.B., Lechpammer M., Molloy E., and Badawi N. (2021). Neonatal encephalopathy: Focus on epidemiology and underexplored aspects of etiology. Semin Fetal Neonatal Med 26. 10.1016/J.SINY.2021.101265. [DOI] [PubMed] [Google Scholar]
- 21.Geneva I.I., Cuzzo B., Fazili T., and Javaid W. (2019). Normal Body Temperature: A Systematic Review. Open Forum Infect Dis 6. 10.1093/OFID/OFZ032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight J.R.P., Bastide A., Roobol A., Roobol J., Jackson T.J., Utami W., Barrett D.A., Smales C.M., and Willis A.E. (2015). Eukaryotic elongation factor 2 kinase regulates the cold stress response by slowing translation elongation. Biochem J 465, 227–238. 10.1042/BJ20141014. [DOI] [PubMed] [Google Scholar]
- 23.Shaw G., Morse S., Ararat M., and Graham F.L. (2002). Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. The FASEB Journal. 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 24.Barnes E.N., Biedler J.L., Spengler B.A., and Lyser K.M. (1981). The fine structure of continuous human neuroblastoma lines SK-N-SH, SK-N-BE(2), and SK-N-MC. In Vitro 17, 619–631. 10.1007/BF02618461. [DOI] [PubMed] [Google Scholar]
- 25.Stepanenko A.A., and Dmitrenko V. V (2015). HEK293 in cell biology and cancer research: phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 569, 182–190. 10.1016/j.gene.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 26.Jackson T.C., Manole M.D., Kotermanski S.E., Jackson E.K., Clark R.S.B., and Kochanek P.M. (2015). Cold stress protein RBM3 responds to temperature change in an ultra-sensitive manner in young neurons. Neuroscience 305, 268–278. 10.1016/j.neuroscience.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann J., Haap M., Kopp H.-G., and Lipp H.-P. (2009). Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr Drug Metab 10, 470–481. 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 28.Nelson V., Ziehr J., Agulnik M., and Johnson M. (2013). Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther 6, 135–143. 10.2147/OTT.S23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim T.M., Lee K.W., Oh D.Y., Lee J.S., Im S.A., Kim D.W., Han S.W., Kim Y.J., Kim T.Y., Kim J.H., et al. (2018). Phase 1 Studies of Poziotinib, an Irreversible Pan-HER Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Cancer Res Treat 50, 835–842. 10.4143/CRT.2017.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erber R., Thurnher A., Katsen A.D., Groth G., Kerger H., Hammes H.P., Menger M.D., Ullrich A., and Vajkoczy P. (2004). Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 18, 338–340. 10.1096/FJ.03-0271FJE. [DOI] [PubMed] [Google Scholar]
- 31.Lee H., Kim J.W., Choi D.K., Yu J.H., Kim J.H., Lee D.S., and Min S.H. (2020). Poziotinib suppresses ovarian cancer stem cell growth via inhibition of HER4-mediated STAT5 pathway. Biochem Biophys Res Commun 526, 158–164. 10.1016/J.BBRC.2020.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Grinstein E., Jundt F., Weinert I., Wernet P., and Royer H.D. (2002). Sp1 as G1 cell cycle phase specific transcription factor in epithelial cells. Oncogene 2002 21:10 21, 1485–1492. 10.1038/sj.onc.1205211. [DOI] [PubMed] [Google Scholar]
- 33.Li W., Xu H., Xiao T., Cong L., Love M.I., Zhang F., Irizarry R.A., Liu J.S., Brown M., and Liu X.S. (2014). MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol 15, 554. 10.1186/s13059-014-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan N.Y., and Khachigian L.M. (2009). Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol 29, 2483–2488. 10.1128/mcb.01828-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai G., and Nakaoka Y. (1998). Cooling sensitive [Ca2+](i) response associated with signaling of G protein-coupled receptors. Biochem Biophys Res Commun 248, 733–737. 10.1006/BBRC.1998.8873. [DOI] [PubMed] [Google Scholar]
- 36.Chelban V., Patel N., Vandrovcova J., Zanetti M.N., Lynch D.S., Ryten M., Botía J.A., Bello O., Tribollet E., Efthymiou S., et al. (2017). Mutations in NKX6–2 Cause Progressive Spastic Ataxia and Hypomyelination. Am J Hum Genet 100, 969–977. 10.1016/J.AJHG.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ZNF260 zinc finger protein 260 [Homo sapiens (human)] - Gene - NCBI https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=339324#gene-expression.
- 38.Burke T.L., and Grant P.A. (2009). Histone Acetylation Complexes. Handbook of Cell Signaling, Second Edition 3, 2369–2378. 10.1016/B978-0-12-374145-5.00285-0. [DOI] [Google Scholar]
- 39.Zhou X., Shang Y.N., Lu R., Fan C.W., and Mo X.M. (2019). High ANKZF1 expression is associated with poor overall survival and recurrence-free survival in colon cancer. 10.2217/fon-2018-0920 15, 2093–2106. . [DOI] [PubMed] [Google Scholar]
- 40.Tawamie H., Martianov I., Wohlfahrt N., Buchert R., Mengus G., Uebe S., Janiri L., Hirsch F.W., Schumacher J., Ferrazzi F., et al. (2017). Hypomorphic Pathogenic Variants in TAF13 Are Associated with Autosomal-Recessive Intellectual Disability and Microcephaly. Am J Hum Genet 100, 555. 10.1016/J.AJHG.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ZNF71 zinc finger protein 71 [Homo sapiens (human)] - Gene - NCBI https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=58491.
- 42.Yu H. V., Tao L., Llamas J., Wang X., Nguyen J.D., Trecek T., and Segil N. (2021). POU4F3 pioneer activity enables ATOH1 to drive diverse mechanoreceptor differentiation through a feed-forward epigenetic mechanism. Proc Natl Acad Sci U S A 118. 10.1073/PNAS.2105137118/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.GATAD2A GATA zinc finger domain containing 2A [Homo sapiens (human)] - Gene - NCBI https://www.ncbi.nlm.nih.gov/gene/54815.
- 44.Zhang Y., Fang Y., Tang Y., Han S., Jia J., Wan X., Chen J., Yuan Y., Zhao B., and Fang D. (2022). SMYD5 catalyzes histone H3 lysine 36 trimethylation at promoters. Nature Communications 2022 13:1 13, 1–19. 10.1038/s41467-022-30940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kidder B.L., He R., Wangsa D., Padilla-Nash H.M., Bernardo M.M., Sheng S., Ried T., and Zhao K. (2017). SMYD5 Controls Heterochromatin and Chromosome Integrity during Embryonic Stem Cell Differentiation. Cancer Res 77, 6729–6745. 10.1158/0008-5472.CAN-17-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murphy M.W., Sarver A.L., Rice D., Hatzi K., Ye K., Melnick A., Heckert L.L., Zarkower D., and Bardwell V.J. (2010). Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc Natl Acad Sci U S A 107, 13360–13365. 10.1073/PNAS.1006243107/SUPPL_FILE/SD01.XLS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber C., Zhou Y., Lee J.G., Looger L.L., Qian G., Ge C., and Capel B. (2020). Temperature-dependent sex determination is mediated by pSTAT3 repression of Kdm6b. Science (1979) 368, 303–306. 10.1126/SCIENCE.AAZ4165/SUPPL_FILE/AAZ4165_S1.MP4. [DOI] [PubMed] [Google Scholar]
- 48.Kim D.H., Doyle M.R., Sung S., and Amasino R.M. (2009). Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25, 277–299. 10.1146/annurev.cellbio.042308.113411. [DOI] [PubMed] [Google Scholar]
- 49.Fellous A., Earley R.L., and Silvestre F. (2019). The Kdm/Kmt gene families in the self-fertilizing mangrove rivulus fish, Kryptolebias marmoratus, suggest involvement of histone methylation machinery in development and reproduction. Gene 687, 173–187. 10.1016/J.GENE.2018.11.046. [DOI] [PubMed] [Google Scholar]
- 50.Youn J.Y., Dyakov B.J.A., Zhang J., Knight J.D.R., Vernon R.M., Forman-Kay J.D., and Gingras A.C. (2019). Properties of Stress Granule and P-Body Proteomes. Mol Cell 76, 286–294. 10.1016/J.MOLCEL.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Aljazi M.B., Gao Y., Wu Y., and He J. (2022). SMYD5 is a histone H3-specific methyltransferase mediating mono-methylation of histone H3 lysine 36 and 37. Biochem Biophys Res Commun 599, 142–147. 10.1016/J.BBRC.2022.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diaz J., Abiola S., Kim N., Avaritt O., Flock D., Yu J., Northington F.J., and Chavez-Valdez R. (2017). Therapeutic hypothermia provides variable protection against behavioral deficits after neonatal HI: A potential role for BDNF. Dev Neurosci 39, 257. 10.1159/000454949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xi Y., Park S.R., Kim D.H., Kim E.D., and Sung S. (2020). Transcriptome and epigenome analyses of vernalization in Arabidopsis thaliana. Plant J 103, 1490. 10.1111/TPJ.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan L., Lesser A.F., Sweet D.R., Keerthy K.S., Lu Y., Chan E.R., Vinayachandran V., Ilkayeva O., Das T., Newgard C.B., et al. (2022). KLF15 controls brown adipose tissue transcriptional flexibility and metabolism in response to various energetic demands. iScience 25, 105292. 10.1016/J.ISCI.2022.105292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katano-Toki A., Satoh T., Tomaru T., Yoshino S., Ishizuka T., Ishii S., Ozawa A., Shibusawa N., Tsuchiya T., Saito T., et al. (2013). THRAP3 Interacts with HELZ2 and Plays a Novel Role in Adipocyte Differentiation. Molecular Endocrinology 27, 769. 10.1210/ME.2012-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.THRAP3 thyroid hormone receptor associated protein 3 [Homo sapiens (human)] - Gene - NCBI https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=9967.
- 57.Guo K., Searfoss G., Krolikowski D., Pagnoni M., Franks C., Clark K., Yu K.T., Jaye M., and Ivashchenko Y. (2001). Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ 8, 367–376. 10.1038/SJ.CDD.4400810. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Wahab O., Adli M., LaFave L.M., Gao J., Hricik T., Shih A.H., Pandey S., Patel J.P., Chung Y.R., Koche R., et al. (2012). ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell 22, 180–193. 10.1016/J.CCR.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaakkola S. (2000). Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs 59, 1233–1250. 10.2165/00003495-200059060-00004. [DOI] [PubMed] [Google Scholar]
- 60.Feng Z., Saha L., Dritsa C., Wan Q., and Glebov O.O. (2022). Temperature-dependent structural plasticity of hippocampal synapses. Front Cell Neurosci 16, 520. 10.3389/FNCEL.2022.1009970/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carvelli L., Blakely R.D., and DeFelice L.J. (2008). Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. Proc Natl Acad Sci U S A 105, 14192–14197. 10.1073/PNAS.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao H., Chakraborty G., Lee-Lim A.P., Mavrakis K.J., Wendel H.G., and Giancotti F.G. (2014). Forward genetic screens in mice uncover mediators and suppressors of metastatic reactivation. Proc Natl Acad Sci U S A 111, 16532–16537. 10.1073/PNAS.1403234111/SUPPL_FILE/PNAS.201403234SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.INDACOCHEA A., GUERRERO S., UREÑA M., ARAUJO F., COLL O., LLEONART M.E., and GEBAUER F. (2021). Cold-inducible RNA binding protein promotes breast cancer cell malignancy by regulating Cystatin C levels. RNA 27, 190–201. 10.1261/RNA.076422.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicolas M., Noe V., Jensen K., and Ciudad CJ(2001). Cloning and characterization of the 5′-flanking region of the human transcription factor Sp1 gene. . J Biol Chem, 22126–22132. [DOI] [PubMed] [Google Scholar]
- 65.Yang F., Eckardt S., Leu N.A., McLaughlin K.J., and Wang P.J. (2008). Mouse TEX15 is essential for DNA double-strand break repair and chromosomal synapsis during male meiosis. J Cell Biol 180, 673. 10.1083/JCB.200709057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.TEX15 testis expressed 15, meiosis and synapsis associated [Homo sapiens (human)] - Gene - NCBI https://www.ncbi.nlm.nih.gov/gene/56154.
- 67.Hanna C.W., Huang J., Belton C., Reinhardt S., Dahl A., Andrews S., Francis Stewart A., Kranz A., and Kelsey G. (2022). Loss of histone methyltransferase SETD1B in oogenesis results in the redistribution of genomic histone 3 lysine 4 trimethylation. Nucleic Acids Res 50, 1993. 10.1093/NAR/GKAC051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pajoro A., Severing E., Angenent G.C., and Immink R.G.H. (2017). Histone H3 lysine 36 methylation affects temperature-induced alternative splicing and flowering in plants. Genome Biol 18, 1–12. 10.1186/S13059-017-1235-X/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michaels K.K., Mostafa S.M., Capella J.R., and Moore C.L. (2020). Regulation of alternative polyadenylation in the yeast Saccharomyces cerevisiae by histone H3K4 and H3K36 methyltransferases. Nucleic Acids Res 48, 5407–5425. 10.1093/NAR/GKAA292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu S.J., Lombroso S.I., Fischer D.K., Carpenter M.D., Marchione D.M., Hamilton P.J., Lim C.J., Neve R.L., Garcia B.A., Wimmer M.E., et al. (2021). Chromatin-mediated alternative splicing regulates cocaine-reward behavior. Neuron 109, 2943–2966.e8. 10.1016/J.NEURON.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Preußner M., Goldammer G., Neumann A., Haltenhof T., Rautenstrauch P., Müller-McNicoll M., and Heyd F. (2017). Body Temperature Cycles Control Rhythmic Alternative Splicing in Mammals. Mol Cell 67, 433–446.e4. 10.1016/J.MOLCEL.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Horii Y., Shiina T., Uehara S., Nomura K., Shimaoka H., Horii K., and Shimizu Y. (2019). Hypothermia induces changes in the alternative splicing pattern of cold-inducible RNA-binding protein transcripts in a non-hibernator, the mouse. Biomed Res 40, 153–161. 10.2220/BIOMEDRES.40.153. [DOI] [PubMed] [Google Scholar]
- 73.Sano Y., Shiina T., Naitou K., Nakamori H., and Shimizu Y. (2015). Hibernation-specific alternative splicing of the mRNA encoding cold-inducible RNA-binding protein in the hearts of hamsters. Biochem Biophys Res Commun 462, 322–325. 10.1016/j.bbrc.2015.04.135. [DOI] [PubMed] [Google Scholar]
- 74.Smart F., Aschrafi A., Atkins A., Owens G.C., Pilotte J., Cunningham B.A., and Vanderklish P.W. (2007). Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem 101, 1367–1379. 10.1111/J.1471-4159.2007.04521.X. [DOI] [PubMed] [Google Scholar]
- 75.Al-Fageeh M.B., and Smales C.M. (2013). Alternative promoters regulate cold inducible RNA-binding (CIRP) gene expression and enhance transgene expression in mammalian cells. Mol Biotechnol 54, 238–249. 10.1007/s12033-013-9649-5. [DOI] [PubMed] [Google Scholar]
- 76.Nicolas M., Noe V., Jensen K.B., and Ciudad C.J. (2001). Cloning and characterization of the 5’-flanking region of the human transcription factor Sp1 gene. J Biol Chem 276, 22126–22132. 10.1074/jbc.M010740200. [DOI] [PubMed] [Google Scholar]
- 77.Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L., and Author N.B. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling HHS Public Access Author manuscript. Nat Biotechnol 27, 275–280. 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiang J., Wan C., Guo R., and Guo D. (2016). Is Hydrogen Peroxide a Suitable Apoptosis Inducer for All Cell Types? Biomed Res Int 2016. 10.1155/2016/7343965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cell cycle staining with Propidium Iodide for the cytoplasmic GFP transfected cells using Paraformaldehyde and Ethanol (2010). 1–2. https://flowcyt.rutgers.edu/wp-content/uploads/2017/10/Cell-cycle-staining-with-PI-for-GFP-transfected-cells-using-PFA_EtOH_2.pdf. [Google Scholar]
- 80.Kutner R.H., Zhang X.Y., and Reiser J. (2009). Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc 4, 495–505. 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 81.Wang B., Wang M., Zhang W., Xiao T., Chen C.H., Wu A., Wu F., Traugh N., Wang X., Li Z., et al. (2019). Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat Protoc 14, 756–780. 10.1038/s41596-018-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Statistical R Core Team (2017). R Foundation for Statistical Computing Vienna Austria. R: A language and environment for computing. [Google Scholar]
- 83.4×Lentivirus Concentrator Solution https://www.mdanderson.org/documents/core-facilities/Functional%20Genomics%20Core/Homemade%204fold%20lentivirus%20concentrator.pdf.
- 84.Rueden C.T., Schindelin J., Hiner M.C., DeZonia B.E., Walter A.E., Arena E.T., and Eliceiri K.W. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 1–26. 10.1186/S12859-017-1934-Z/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods 2012 9:7 9, 676–682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stirling D.R., Swain-Bowden M.J., Lucas A.M., Carpenter A.E., Cimini B.A., and Goodman A. (2021). CellProfiler 4: improvements in speed, utility and usability. BMC Bioinformatics 22. 10.1186/S12859-021-04344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fortin J.P., Triche T.J., and Hansen K.D. (2017). Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 33, 558. 10.1093/BIOINFORMATICS/BTW691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Triche T.J., Weisenberger D.J., Van Den Berg D., Laird P.W., and Siegmund K.D. (2013). Low-level processing of Illumina Infinium DNA Methylation BeadArrays. Nucleic Acids Res 41, e90. 10.1093/NAR/GKT090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., and Irizarry R.A. (2014). Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 30, 1363. 10.1093/BIOINFORMATICS/BTU049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leek J.T., Johnson W.E., Parker H.S., Jaffe A.E., and Storey J.D. (2012). The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28, 882. 10.1093/BIOINFORMATICS/BTS034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., and Smyth G.K. (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. 10.1093/NAR/GKV007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.CUT&RUN Protocol v1.7 (2021). https://www.epicypher.com/content/documents/protocols/cutana-cut&run-protocol_1.7.pdf.
- 93.Kong N.R., Chai L., Tenen D.G., and Bassal M.A. (2021). A modified CUT&RUN protocol and analysis pipeline to identify transcription factor binding sites in human cell lines. STAR Protoc 2. 10.1016/J.XPRO.2021.100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17, 10–12. 10.14806/EJ.17.1.200. [DOI] [Google Scholar]
- 95.Langmead B., and Salzberg S.L. (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., and Durbin R. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. 10.1093/BIOINFORMATICS/BTP352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo Y., Hitz B.C., Gabdank I., Hilton J.A., Kagda M.S., Lam B., Myers Z., Sud P., Jou J., Lin K., et al. (2020). New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res 48, D882–D889. 10.1093/NAR/GKZ1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quinlan A.R., and Hall I.M. (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. 10.1093/BIOINFORMATICS/BTQ033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meers M.P., Tenenbaum D., and Henikoff S. (2019). Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 12, 1–11. 10.1186/S13072-019-0287-4/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stark R., and Brown G. (2012). DiffBind : differential binding analysis of ChIP-Seq peak data. [Google Scholar]
- 101.Yu G., Wang L.G., and He Q.Y. (2015). ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 31, 2382–2383. 10.1093/BIOINFORMATICS/BTV145. [DOI] [PubMed] [Google Scholar]
- 102.Team BC, and Maintainer BP (2019). TxDb.Hsapiens.UCSC.hg38.knownGene: Annotation package for TxDb object(s). [Google Scholar]
- 103.Carlson M (2019). org.Hs.eg.db: Genome wide annotation for Human. . [Google Scholar]
- 104.Ramírez F., Dündar F., Diehl S., Grüning B.A., and Manke T. (2014). deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42, W187. 10.1093/NAR/GKU365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bernas S.N., Leiter O., Walker T.L., and Kempermann G. (2017). Isolation, Culture and Differentiation of Adult Hippocampal Precursor Cells. Bio Protoc 7. 10.21769/BIOPROTOC.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soneson C., Love M.I., and Robinson M.D. (2016). Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Res 4. 10.12688/F1000RESEARCH.7563.2/DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Team BC, and Maintainer BP (2016). TxDb.Mmusculus.UCSC.mm10.ensGene: Annotation package for TxDb object(s).. [Google Scholar]
- 108.Love M.I., Huber W., and Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 1–21. 10.1186/S13059-014-0550-8/FIGURES/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen L, and Sinai ISoMaM (2022). GeneOverlap: Test and visualize gene overlaps. . [Google Scholar]
- 110.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550. 10.1073/PNAS.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34, 267–273. 10.1038/NG1180. [DOI] [PubMed] [Google Scholar]
- 112.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., and Tamayo P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425. 10.1016/J.CELS.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1: Sequences of individual MHIs.
Supplementary Table 1: Specifics of our MHI, including sequence material used, vector, selection markers, plasmid size and individual fluorophores.
Supplementary Table 2: Top 91 drug hits from MHI screen.
Supplementary Table 3: Top candidate genes found enriched in mutation screening.
Supplementary Table 4: Genes found in both repressor and activator SP1 lists and thus excluded for further evaluation.
Supplementary Table 5: List of CpGs represented at three genes.
Supplementary Table 6: List of differentially methylated locations from EPIC DNAm array.
Supplementary Table 7: Top differentially modified locations for H3K36me3 in NPCs from CUT&RUN experiment.
Supplementary Table 8: List of genes that have a unique H3K4me3 histone modification peak at 32°C.
Supplementary Table 9: List of genes that show DEGS at 32°C and have a unique H3K4me3 histone modification peak at 32°C.