Abstract
Background
Chronic obstructive pulmonary disease (COPD) phenotyping using stable-state blood eosinophil level was shown to have prognostic implication in terms of exacerbation risk. However, using a single cut-off of blood eosinophil level to predict clinical outcome has been challenged. There have been suggestions that variability of blood eosinophil count at stable-state could provide additional information on exacerbation risk.
Methods
A retrospective cohort study was conducted in a major regional hospital and a tertiary respiratory referral centre in Hong Kong, including 275 Chinese patients with COPD, to investigate the possible role of variability of blood eosinophil count at stable-state to predict COPD exacerbation risk in one year.
Results
Higher variability of baseline eosinophil count, which is defined as the difference of the minimal and maximal eosinophil count at stable-state, was associated with increased risk of COPD exacerbation in the follow-up period with adjusted OR (aOR) of 1.001 (95% CI = 1.000–1.003, p-value = 0.050) for 1 unit (cells/µL) increase in variability of baseline eosinophil count, aOR of 1.72 (95% CI = 1.00–3.58, p-value = 0.050) for 1 SD increase in variability of baseline eosinophil count and aOR of 1.06 (95% CI = 1.00–1.13) for 50 cells/µL increase in variability of baseline eosinophil count. The AUC by ROC analysis was 0.862 (95% CI = 0.817–0.907, p-value < 0.001). The cut-off for variability of baseline eosinophil count identified was 50 cells/µL, with sensitivity of 82.9% and specificity of 79.3%. Similar findings were also shown in the subgroup with stable-state baseline eosinophil count below 300 cells/µL.
Conclusion
Variability of baseline eosinophil count at stable-state might predict the exacerbation risk of COPD, exclusively among patients with baseline eosinophil count below 300 cells/µL. The cut-off value for variability was 50 cells/µValidation of the study findings in large scale prospective study would be meaningful.
Keywords: COPD, eosinophil, phenotype, COPD exacerbation
Introduction
Chronic obstructive pulmonary disease (COPD) phenotyping, in particular focusing on the role of blood eosinophil level, has been shown to have prognostic implication in exacerbation risk.1 Cross-sectional data analysis from the National Health and Nutrition Examination Survey (NHANES 2007–2010) cohort demonstrated that 70% of US adults with COPD had blood eosinophil levels >2%.2 Higher blood eosinophil levels during stable disease may also indicate a greater risk of exacerbation.3 Blood eosinophil level at stable-state was also shown to be a predictor of clinical benefits from inhaled corticosteroid (ICS).4–10
However, using a single cut-off of blood eosinophil level to predict clinical outcome has been challenged.11–13 Studies reported that the within-subject biological variation of hourly blood eosinophil counts in healthy individuals was 20.9%. Between-subject diurnal variation was even greater at 46.6%.14 There were studies suggesting that blood eosinophil levels had significant variability throughout the course of COPD, and a single measurement might therefore not be a reliable predictor of ICS response with discordance of eosinophil level up to 77% when using the 2% cutoff.13 A retrospective population-based study suggested the number of exacerbations was slightly higher in patients with larger variability in eosinophil counts, but receiver operator characteristic (ROC) curves did not identify a reliable threshold of absolute blood eosinophil level to discriminate an increased risk of exacerbation.15 The threshold of blood eosinophil variability and COPD exacerbation risk was not assessed in that study. In a population-based study, although various cut-offs of blood eosinophil level did not predict the exacerbation risk of COPD, they found that patients with the largest variability in blood eosinophils were at increased risk of exacerbations.15 While there were suggestions that blood eosinophil count might have prognostic implication, some studies showed an opposite finding. One study suggested that patients with blood eosinophil count above 150 cells/μL, especially based on multiple measurements, had a lower risk of all-cause mortality.16 A Korean study also suggested that patients with persistently high blood eosinophil count had a better survival rate than those with persistently low eosinophil count.17
On the contrary, the role of eosinophil and its variability has been challenged by other studies. A study suggested that there was no significant relationship between the blood eosinophil count, neither in the stable phase nor in the acute phase, and hospital stay, readmissions, deaths during admission, the need for intensive care, or the condition of frequent exacerbator.18
While blood eosinophil count in COPD has been extensively studied and led to changes in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendation for COPD management,19 the role of stable-state blood eosinophil count, especially the variability at stable-state, remains controversial. Using a single cut-off blood eosinophil count may not be able to comprehensively phenotype the patients. While patients who have persistent low blood eosinophil level and persistently elevated blood eosinophil level may represent the conventionally classified non-eosinophilic and eosinophilic subgroup, a further subgroup with variable blood eosinophil count may be present which has distinct clinical characteristics such as exacerbation risks. This study was carried out to explore the value of variability of blood eosinophil count at stable-state on exacerbation risk in COPD.
Materials and Methods
This was a retrospective cohort study. Patients with COPD followed up in the adult Respiratory Specialty Clinics at Queen Mary Hospital and Grantham Hospital, Hong Kong in the year 2021 were included. Queen Mary Hospital and Grantham Hospital are the major regional hospitals in Hong Kong, and Queen Mary Hospital is a University-affiliated tertiary referral respiratory centre, with a designated clinic for managing patients with COPD of different disease severity. The investigators reviewed clinical records to validate the diagnosis of COPD. Patients’ records (both outpatient and inpatient episodes) were accessed through the Electronic Patient Record (ePR) system of the Hong Kong Hospital Authority. The information available would include demographics, clinical notes, investigation results and treatments.
Chinese patients with COPD at or above 40 years old were included. The diagnosis of COPD was confirmed by spirometry demonstrating post-bronchodilator airflow limitation with forced expiratory volume in one second/forced vital capacity [FEV1/FVC] ratio less than 0.7. Exclusion criteria included co-existing asthma and individuals who were lost to follow up. Demographic data (age, gender, smoking status), clinical data/investigations (comorbidities, treatment records, spirometry results, blood test results), and use of ICS/bronchodilators including long acting beta-agonists (LABA) and long acting muscarinic antagonists (LAMA) were retrieved from clinical records. Regular use of ICS, LABA and LAMA was defined as continuous use for at least 12 months within the study period. Baseline blood eosinophil level was the value taken at clinically stable-state. The interval of 2 complete blood counts at baseline should be more than 3 months but less than 12 months apart. The baseline blood eosinophil level was at least 30 days before an exacerbation episode. The minimal and maximal eosinophil counts at stable-state were collected to calculate the variability of stable-state blood eosinophil levels, which was defined as the difference between the minimal and maximal eosinophil counts. The maximal eosinophil count at stable-state was used to define the stable-state blood eosinophil level as a single cut-off.
The primary outcome was COPD exacerbation within one year of follow-up period. COPD exacerbation was defined as an acute event characterized by worsening of respiratory symptoms beyond the normal day-to-day variations, leading to a change in medications. Compatible symptomatology comprised acute change in one or more of the following: [1] increased frequency and severity of cough; [2] increased sputum volume and/or purulence; [3] increased dyspnea requiring medical attention and treatment.19 Patients who had COPD exacerbations that required in-patient care or out-patient management during the follow-up period were identified from the ePR. The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (Approval reference number: UW 21–172).
Statistical Analysis
The demographic and clinical data were described in actual frequency, mean ± SD or median (interquartile range, IQR) as appropriate. Baseline demographic and clinical data were compared between the two groups (with or without COPD exacerbation) with Chi square tests. Logistic regression was used to estimate the risk of COPD exacerbation among patients with different blood eosinophil counts at stable-state and the variability of blood eosinophil counts in the 1-year follow-up period. Age, gender, COPD severity by GOLD stage and class, baseline CAT score, as well as the use of ICS/LABA/LAMA were adjusted as potential confounders. To define the optimal cut-off value of blood eosinophil count, ROC curve analysis was performed. Statistical significance was determined at the level of p = 0.05. All statistical analyses were performed using the 26th version of SPSS statistical package.
Results
A total of 275 Chinese patients with COPD managed in Queen Mary Hospital and Grantham Hospital were included. Patients with asthma, asthma-COPD overlap and patients who only had one complete blood count measured in the study period were excluded.
Baseline Characteristics
The mean age was 74.7 ± 8.6 years, with the majority (77%) being male. There were more males (77%) patients and all the patients were ever-smoker. The mean FEV1 was 1.35 ± 0.52 L (61 ± 22%), and the median CAT score was 8 [IQR = 3–14]. The median blood eosinophil count at stable-state was 70 [IQR 30 −140] cells/µL. There were 105 (38.2%) patients who had COPD exacerbation one year prior to recruitment. There were 111 patients who developed COPD exacerbation during the follow-up period that were treated with systemic steroid. The results are summarized in Table 1. The baseline blood eosinophil count did not significantly correlate with the variability of stable-state blood eosinophil level with Pearson correlation of 0.104 (95% confidence interval [CI] = −0.015–0.219, p-value = 0.087). The variability of stable-state blood eosinophil level was also not shown to be correlated with the lung function, bronchodilator reversibility and symptom burden by CAT or mMRC score, with the results summarized in Supplementary Table 1. The baseline eosinophil count across different subgroups were shown in Supplementary Tables 2 and 3.
Table 1.
Baseline Demographic and Clinical Characteristics
| No COPD Exacerbation of Follow-Up (n = 164) | Has COPD Exacerbation of Follow-Up (n = 111) | P-values | |
|---|---|---|---|
| Age (years), mean ± SD | 74.5 ± 8.3 | 75.5 ± 9.0 | 0.376 |
| Male | 154 (93.9%) | 94 (84.7%) | 0.012* |
| CAT score, median, IQR | 6, (6.-23) | 10 (7–18) | <0.001* |
| mMRC dyspnea scale, median, IQR | 2, (2–3) | 2, (1–3) | <0.001* |
| FEV1 (L), mean ± SD | 1.45 ± 0.51 | 1.15 ± 0.47 | <0.001* |
| FEV1 (% predicted), mean ± SD | 64.2 ± 20.0 | 54.3 ± 19.2 | <0.001* |
| FVC (L), mean ± SD | 2.397± 0.84 | 2.51 ± 0.75 | <0.001* |
| FVC (% predicted), mean ± SD | 96.3 ± 24.9 | 86.6 ± 19.6 | 0.001* |
| FEV1/FVC Ratio (%), mean ± SD | 49.6 ± 11.3 | 46.3 ± 13.2 | 0.033* |
| Bronchodilator responsiveness (mL), mean ± SD | 97 ± 82 | 94 ± 87 | 0.808 |
| Bronchodilator responsiveness (%), mean ± SD | 6.5 ± 7.1 | 11.3 ± 20.8 | 0.009* |
| Exacerbation(s) in the past 1 year | 55 (78.6%) | 50 (74.1%) | 0.329 |
| Minimum baseline blood eosinophil count (x cells/µL) (Median, IQR) | 150 (90–302.5) | 120 (15–130) | 0.120 |
| Maximum baseline blood eosinophil count (x cells/µL) (Median, IQR) | 200 (255–475) | 250 (120–330) | 0.970 |
| Variability of baseline blood eosinophil count (x cells/µL) (Median, IQR) | 20 (2.5–50) | 135 (85–235) | 0.068 |
| Baseline blood eosinophil % (Median, IQR) | 2.06 (1.61–4.14) | 2.08 (1.43–3.80) | 0.707 |
| COPD Group as in GOLD recommendation | <0.001* | ||
| A | 66 (40.2%) | 21 (18.9%) | |
| B | 90 (54.9%) | 70 (63.1%) | |
| C | 2 (1.2%) | 9 (8.1%) | |
| D | 6 (3.7%) | 11 (9.9%) | |
| COPD Stage by spirometry results | <0.001* | ||
| 1 | 24 (24.4%) | 12 (10.8%) | |
| 2 | 82 (50.0%) | 54 (51.4%) | |
| 3 | 41 (25.0%) | 32 (28.8%) | |
| 4 | 1 (0.6%) | 10 (9.0%) | |
| ICS use | 71 (43.3%) | 71 (64.0%) | <0.001* |
| Type of ICS use | |||
| Budesonide | 17 (23.9%) | 7 (9.9%) | |
| Fluticasone furoate | 7 (9.9%) | 57 (80.3%) | |
| Fluticasone propionate | 14 (19.7%) | 4 (5.6%) | |
| Beclomethasone | 33 (46.5%) | 3 (4.2%) | |
| ICS dose | <0.001* | ||
| High dose | 6 (8.5%) | 3 (4.2%) | |
| Medium dose | 46 (64.8%) | 59 (83.1%) | |
| Low dose | 19 (26.8%) | 9 (12.7%) | |
| Theophylline use | 21 (12.8%) | 22 (19.8%) | 0.116 |
Abbreviations: SD, standard deviation; mL, milliliter; *, statistically significant; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; CAT, COPD Assessment Test; mMRC, Modified Medical Research Council; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid.
Risk of COPD Exacerbation with Stable-State Blood Eosinophil Levels
Univariate regression analysis was performed using different cut-offs for stable-state blood eosinophil level. Using single cut-offs at 150 cells/µL, 300 cells/µL, 2% and 3%, they were unable to significantly predict subsequent COPD exacerbation in the follow-up period. The odds ratios (ORs) were 1.386 (95% CI = 0.829–2.316, p-value = 0.213), 1.364 (95% CI = 0.796–2.340, p-value = 0.259), 1.065 (95% CI = 0.644–1.761, p-value = 0.806) and 1.309 (95% CI = 0.805–2.128, p-value = 0.278) for 150 cells/µL, 300 cells/µL, 2% and 3%, respectively.
Univariate logistic regression analysis also did not suggest that the maximal nor minimal baseline eosinophil level at stable-state could predict COPD exacerbation in the follow-up period when analyzed as a continuous variable. The ORs were 1.000 (95% CI = 1.000–1.000, p-value = 0. 974) and 0.999 (95% CI = 0.997–1.000, p-value = 0. 098), respectively.
Risk of COPD Exacerbation with Variability of Baseline Eosinophil Count
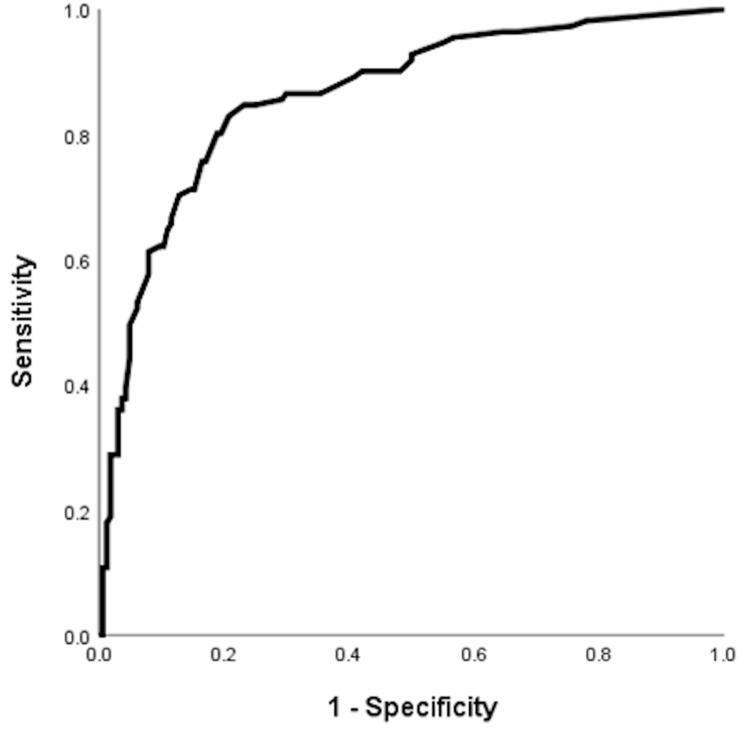
Increased variability of baseline eosinophil count, defined as the difference between the minimal and maximal eosinophil count at stable-state, was associated with increased risk of COPD exacerbation in the follow-up period with OR of 1.002 (95% CI = 1.000–1.003, p-value = 0.018) for 1 unit (cells/µL) increase in variability of baseline eosinophil count, OR of 2.44 (95% CI = 1.17–5.10, p-value = 0.018) for 1 SD increase in variability of baseline eosinophil count and OR of 1.09 (95% CI = 1.02–1.17) for 50 cells/µL increase in variability of baseline eosinophil count in the univariate logistic regression analysis. With multivariate logistic regression adjusted for age, gender, COPD group by GOLD, COPD stage, baseline CAT score, use of LABA, LAMA and ICS, the adjusted OR (aOR) was 1.001 (95% CI = 1.000–1.003, p-value = 0.050) for 1 unit (cells/µL) increase in variability of baseline eosinophil count, aOR of 1.72 (95% CI = 1.00–3.58, p-value = 0.050) for 1 SD increase in variability of baseline eosinophil count and aOR of 1.06 (95% CI = 1.00–1.13) for 50 cells/µL increase in variability of baseline eosinophil count. The area under the curve (AUC) by receiver operating characteristic (ROC) analysis was 0.862 (95% CI = 0.817–0.907, p-value < 0.001), suggesting good predictive power (Figure 1 and Supplementary Table 4).
Figure 1.
Receiver operating curve (ROC) for variability of baseline eosinophil count and COPD exacerbation risks in one year.
Subgroup Analysis in Patients with Baseline Eosinophil Count <300 or >300 Cells/uL
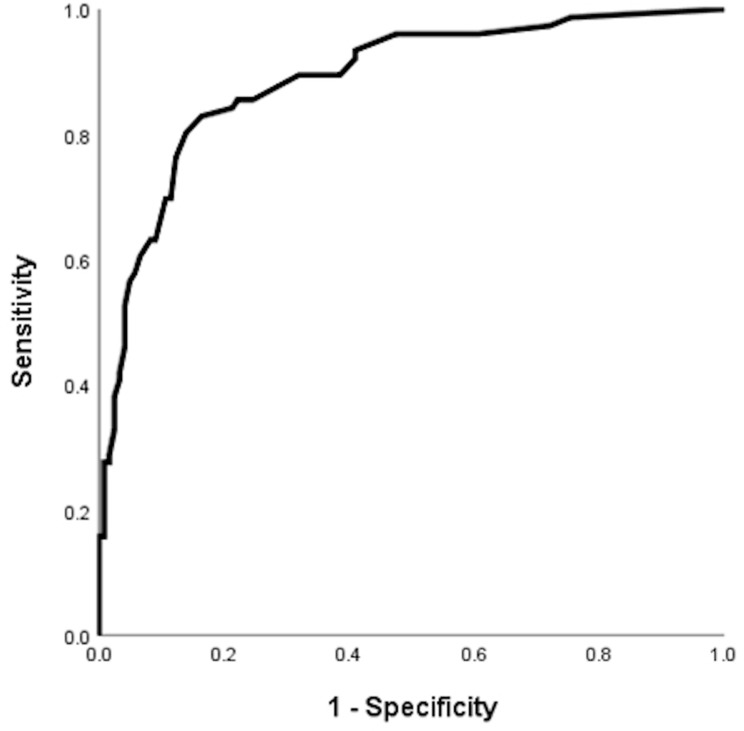
There were 199 patients with baseline eosinophil count <300 cells/µL. In this subgroup, higher variability of baseline eosinophil count was associated with increased risk of COPD exacerbation in the follow-up period with OR of 1.030 (95% CI = 1.021–1.040, p-value <0.001) for 1 unit (cells/µL) increase in variability of baseline eosinophil count, OR of 42.0 (95% CI = 13.9–126.9, p-value = 0.018) for 1 SD increase in variability of baseline eosinophil count and OR of 4.47 (95% CI = 2.87–6.70) for 50 cells/µL increase in variability of baseline eosinophil count in univariate logistic regression. With multivariate logistic regression adjusted for age, gender, COPD group by GOLD, baseline CAT score, COPD stage, use of LABA, LAMA and ICS, the aOR was 1.035 (95% CI = 1.022–1.047, p-value <0.001) and aOR for 1 unit (cells/µL) increase in variability of baseline eosinophil count, OR of 70.8 (95% CI = 15.5–323.0, p-value = 0.018) for 1 SD increase in variability of baseline eosinophil count and aOR of 5.51 (95% CI = 3.00–10.1) for 50 cells/µL increase in variability of baseline eosinophil count. The AUC by ROC analysis was 0.888 (95% CI = 0.840–0.936, p-value <0.001), suggesting good predictive power (Figure 2 and Supplementary Table 5).
Figure 2.
Receiver operating curve (ROC) for variability of baseline eosinophil count and COPD exacerbation risks in one year among the subgroup with baseline eosinophil count below 300 cells/µL.
There were 76 patients with baseline eosinophil count ≥300 cells/µL. In this subgroup, higher variability of baseline eosinophil count was not associated with increased risk of COPD exacerbation in the follow-up period with OR of 1.000 (95% CI = 1.000–1.001, p-value = 0.739) for 1 unit (cells/µL) increase in variability of baseline eosinophil count, OR of 1.08 (95% CI = 0.68–1.74, p-value = 0.739) for 1 SD increase in variability of baseline eosinophil count and OR of 1.00 (95% CI = 0.98–1.03) for 50 cells/µL increase in variability of baseline eosinophil count.
Subgroup Analysis in Patients with or without Bronchodilator Responsiveness
A significant bronchodilator response was defined by a greater than or equal to increase in FEV1 or FVC by 10% of their respective predicted values according to The 2021 European Respiratory Society/American Thoracic Society (ERS/ATS) interpretation standards20 In both the subgroups with or without significant bronchodilator responsiveness, neither single cut-off at 150 cells/µL, 300 cells/µL, 2%, 3% nor variability of baseline eosinophil count was associated with increased risk of COPD exacerbation in the follow-up period.
In the subgroup without significant bronchodilator responsiveness (n = 211), the ORs with univariate logistic regression were 1.386 (95% CI = 0.539–1.884, p-value = 0.982), 1.177 (95% CI = 0.587–2.358, p-value = 0.646), 0.686 (95% CI = 0.383–1.230, p-value = 0.206) and 0.909 (95% CI = 0.503–1.642, p-value = 0.752) and 1.000 (95% CI = 1.000–1.000, p-value = 0.098) for eosinophil count of 150 cells/µL, 300 cells/µL, 2% and 3%, and high variability of baseline eosinophil count, respectively.
In the subgroup with significant bronchodilator responsiveness (n = 64), the ORs with univariate logistic regression were 2.931 (95% CI = 0.926–9.283, p-value = 0.067), 1.447 (95% CI = 0.475–4.410, p-value = 0.515), 2.373 (95% CI = 0.764–7.375, p-value = 0.135) and 2.073 (95% CI = 0.755–5.693, p-value = 0.157) and 1.000 (95% CI = 1.000–1.000, p-value = 0.168) for eosinophil count of 150 cells/µL, 300 cells/µL, 2% and 3%, and high variability of baseline eosinophil count, respectively.
Discussion
In this study, high variability of baseline eosinophil count rather than a single cut-off value was found to be predictive of the risk of COPD exacerbation in the follow-up period of 1 year. Our finding concurs with what was reported in the literature that serial measurements of eosinophil count at stable-state to calculate the variability of baseline eosinophil count, in particular for patients with baseline eosinophil count <300 cells/µL, would be more appropriate in phenotyping COPD with prognostic implication.
COPD exacerbation is one of the most important sequelae of COPD, which is associated with increased morbidity and mortality.21–23 Risk factors for COPD exacerbations include advanced age, longer duration of COPD,24 presence of one or more comorbidities and COPD-related hospitalization within the previous year25 baseline blood eosinophil count above 340 cells/microL.26 While the role of blood eosinophil has been implicated in predicting exacerbation risks of COPD and the potential benefits of ICS treatment, there are factors that would affect the blood eosinophil level. Factors that were reported to be associated with higher blood eosinophil counts included current smoking, positive skin-prick test, elevated total IgE, comorbid allergic rhinitis, age ≤18 years, male sex, spirometric asthma/COPD diagnosis, metabolic syndrome and adiposity.27 Regarding COPD variability, among patients with COPD, the number of asthma-like features (bronchodilator reversibility, blood eosinophilia, and atopy) affects the blood eosinophil count variation patterns.28
The variability of baseline eosinophil count has been reported in the literature, and there were attempts to categorize into various risk groups based on blood eosinophil count. Using a single cut-off of baseline eosinophil count may have the problem of misclassifying a patient into an inappropriate risk group, as well as affecting the choice of ICS use. As such, the variability of baseline eosinophil count has been studied before and yet there was no definite conclusion. There are also controversies in the optimal cut-off, be it the absolute or the percentage eosinophil count. Using variability of baseline eosinophil count compared with a single cut-off of baseline eosinophil count has the advantage of capturing the eosinophil count over a longer period of time. This may allow more precise phenotyping. In our study, the high variability of baseline eosinophil count was shown to predict the risk of COPD exacerbation, exclusively in the subgroup with baseline eosinophil count less than 300 cells/µL. Similar findings were first reported by Miravitlles et al. Despite showing that the number of exacerbations was slightly higher in patients with higher variability in eosinophil counts, the threshold could not be identified using ROC analysis.15 In another study conducted in Korea, it was shown that more than one-fifth of patients had an inconsistent blood eosinophil level after the 1-year follow-up and the COPD exacerbation rate according to ICS differed based on variability in eosinophils. The group with variably increasing blood eosinophil level was shown to have benefits from ICS treatment in preventing COPD exacerbation.29 Another Chinese study also suggested that among patients with at least 2 hospitalizations for COPD exacerbation in 1 year, those with fluctuating blood EOS had a higher risk of readmission. Subjects with eosinophil variability may present a distinct phenotype that confers increased exacerbation risks. Our study demonstrated a variability of baseline eosinophil count of 50 cells/µL could predict the exacerbation risk with good sensitivity (82.9%) and specificity (79.3%). This was mainly shown in the subgroup with baseline eosinophil count below 300 cells/µL (sensitivity of 80.3% and specificity of 86.1%), but not among those with higher eosinophil count (≥300 cells/μL). This could be partly due to the small sample size in our cohort. A high variability of baseline eosinophil count may not have added value to stratify these patients who had elevated risks of exacerbation risks, exclusively in patients with baseline eosinophil count below 300 cells/µL. The finding of this study may suggest future study on the use of variability of baseline eosinophil count to guide the management of COPD, in particular ICS prescription.
Despite knowing the potential role of variability of baseline eosinophil count in risk stratification, there remain unanswered questions on this issue. First of all, what is the optimal timing of repeating baseline eosinophil count? Secondly, is there any role of variability of blood eosinophil count at stable-state and exacerbation in predicting prognosis? Furthermore, does the variability of baseline eosinophil count predict benefits of ICS, especially among those with baseline eosinophil count of below 300 cells/µL but with high variability of baseline eosinophil count? To answer these questions, conducting prospective studies of larger sample size with predefined interval of measuring baseline eosinophil count, measuring eosinophil count at stable-state and exacerbation, as well as focusing on the risks and benefits from ICS would be worthwhile. While our study findings showed a possible relationship of variability of baseline eosinophil count and COPD exacerbation risk, the sample size and follow-up period may not be adequate to confirm this association, hence, resulting in a relatively small increase in OR. To overcome this problem, a larger scale study with an extended follow-up period is warranted. Nonetheless, our study finding provided insight for future researches.
Conclusion
Variability of baseline eosinophil count at stable-state might predict the risk of COPD exacerbation, exclusively among patients with baseline eosinophil count of below 300 cells/µL. The cut-off value for variability was 50 cells/µL. Validation of the study findings in large scale prospective study would be meaningful.
Funding Statement
There is no funding to report.
Abbreviations
aOR, adjusted OR; AUC, area under the curve; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ePR, electronic patient record; ERS/ATS, European Respiratory Society/American Thoracic Society; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; IQR, interquartile range; LABA, long acting beta-agonists; LAMA, long acting muscarinic antagonists; mMRC, Modified Medical Research Council; NHANES National Health and Nutrition Examination Survey; ORs, odds ratios; ROC, receiver operator characteristic curve; SD, standard deviation; US, United States.
Ethics Approval and Informed Consent
The study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (Reference number: UW 21-172). All patients had informed consent for this study. The study was conducted in compliance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Brusselle G, Pavord ID, Landis S, et al. Blood eosinophil levels as a biomarker in COPD. Respir Med. 2018;138:21–31. doi: 10.1016/j.rmed.2018.03.016 [DOI] [PubMed] [Google Scholar]
- 2.DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. 2016;112:88–96. doi: 10.1016/j.rmed.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Colak Y, Afzal S, Nordestgaard BG, Lange P. Majority of never-smokers with airflow limitation do not have asthma: the Copenhagen general population study. Thorax. 2016;71(7):614–623. doi: 10.1136/thoraxjnl-2015-208178 [DOI] [PubMed] [Google Scholar]
- 4.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 5.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ. 2017;358:j3772. doi: 10.1136/bmj.j3772 [DOI] [PubMed] [Google Scholar]
- 7.Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018;6(2):117–126. doi: 10.1016/S2213-2600(18)30006-7 [DOI] [PubMed] [Google Scholar]
- 8.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 9.Pavord ID, Lettis S, Anzueto A, Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med. 2016;4(9):731–741. doi: 10.1016/S2213-2600(16)30148-5 [DOI] [PubMed] [Google Scholar]
- 10.Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi: 10.1016/S2213-2600(16)00100-4 [DOI] [PubMed] [Google Scholar]
- 11.Van Rossem I, Vandevoorde J, Hanon S, Deridder S, Vanderhelst E. The stability of blood eosinophils in stable chronic obstructive pulmonary disease: a retrospective study in Belgian primary care. BMC Pulm Med. 2020;20(1):200. doi: 10.1186/s12890-020-01234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogelmeier CF, Kostikas K, Fang J, et al. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir Res. 2019;20(1):178. doi: 10.1186/s12931-019-1130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumann DM, Tamm M, Kostikas K, Stolz D. Stability of the blood eosinophilic phenotype in stable and exacerbated COPD. Chest. 2019;156(3):456–465. doi: 10.1016/j.chest.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 14.Spector SL, Tan RA. Is a single blood eosinophil count a reliable marker for “eosinophilic asthma?”. J Asthma. 2012;49(8):807–810. doi: 10.3109/02770903.2012.713428 [DOI] [PubMed] [Google Scholar]
- 15.Miravitlles M, Monteagudo M, Solntseva I, Alcazar B. Blood eosinophil counts and their variability and risk of exacerbations in COPD: a population-based study. Arch Bronconeumol. 2020. doi: 10.1016/j.arbres.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Liang LR, Zhang S, et al. Blood eosinophilia and its stability in hospitalized COPD exacerbations are associated with lower risk of all-cause mortality. Int J Chron Obstruct Pulmon Dis. 2020;15:1123–1134. doi: 10.2147/COPD.S245056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin SH, Park HY, Kang D, et al. Serial blood eosinophils and clinical outcome in patients with chronic obstructive pulmonary disease. Respir Res. 2018;19(1):134. doi: 10.1186/s12931-018-0840-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Gestoso S, Garcia-Sanz MT, Calvo-Alvarez U, et al. Variability of blood eosinophil count and prognosis of COPD exacerbations. Ann Med. 2021;53(1):1152–1158. doi: 10.1080/07853890.2021.1949489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global Strategy For Prevention. Global strategy for prevention, diagnosis and management of COPD: 2022 report; 2022.
- 20.Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60:1. [DOI] [PubMed] [Google Scholar]
- 21.Colak Y, Afzal S, Marott JL, et al. Prognosis of COPD depends on severity of exacerbation history: a population-based analysis. Respir Med. 2019;155:141–147. doi: 10.1016/j.rmed.2019.07.021 [DOI] [PubMed] [Google Scholar]
- 22.Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007;131(3):696–704. doi: 10.1378/chest.06-1610 [DOI] [PubMed] [Google Scholar]
- 23.Hoogendoorn M, Hoogenveen RT, Rutten-van Molken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi: 10.1183/09031936.00043710 [DOI] [PubMed] [Google Scholar]
- 24.Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000;67(5):495–501. doi: 10.1159/000067462 [DOI] [PubMed] [Google Scholar]
- 25.Rothnie KJ, Mullerova H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464–471. doi: 10.1164/rccm.201710-2029OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med. 2016;193(9):965–974. doi: 10.1164/rccm.201509-1869OC [DOI] [PubMed] [Google Scholar]
- 27.Benson VS, Hartl S, Barnes N, Galwey N, Van Dyke MK, Kwon N. Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis. Eur Respir J. 2022;59(1):2004590. doi: 10.1183/13993003.04590-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abe Y, Suzuki M, Kimura H, et al. Blood eosinophil count variability in chronic obstructive pulmonary disease and severe asthma. Allergol Int. 2022. doi: 10.1016/j.alit.2022.11.012 [DOI] [PubMed] [Google Scholar]
- 29.Yoon JK, Lee JK, Lee CH, et al. The association between eosinophil variability patterns and the efficacy of inhaled corticosteroids in stable COPD patients. Int J Chron Obstruct Pulmon Dis. 2020;15:2061–2070. doi: 10.2147/COPD.S258353 [DOI] [PMC free article] [PubMed] [Google Scholar]