Abstract
The endocannabinoid system is widely expressed throughout the body and is comprised of receptors, ligands, and enzymes that maintain metabolic, immune, and reproductive homeostasis. Increasing interest in the endocannabinoid system has arisen due to these physiologic roles, policy changes leading to more widespread recreational use, and the therapeutic potential of Cannabis and phytocannabinoids. Rodents have been the primary preclinical model of focus due to their relative low cost, short gestational period, genetic manipulation strategies, and gold-standard behavioral tests. However, the potential for lack of clinical translation to non-human primates and humans is high as cross-species comparisons of the endocannabinoid system has not been evaluated. To bridge this gap in knowledge, we evaluate the relative gene expression of 14 canonical and extended endocannabinoid receptors in seven peripheral organs of C57/BL6 mice, Sprague-Dawley rats, and non-human primate rhesus macaques. Notably, we identify species- and organ-specific heterogeneity in endocannabinoid receptor distribution where there is surprisingly limited overlap among the preclinical models. Importantly, we determined there were only five receptors (CB2, GPR18, GPR55, TRPV2, and FAAH) that had identical expression patterns in mice, rats, and rhesus macaques. Our findings demonstrate a critical, yet previously unappreciated, contributor to challenges of rigor and reproducibility in the cannabinoid field, which has profound implications in hampering progress in understanding the complexity of the endocannabinoid system and development of cannabinoid-based therapies.
Keywords: Endocannabinoid system, receptors, cannabinoids, mice, rat, non-human primate
Introduction:
The endocannabinoid system (ECS) has been evolutionarily conserved to preserve its importance in maintaining immune, metabolic, and reproductive homeostasis (1–4). This system is present in all vertebrate animals, including rodents, non-human primates (NHP), and humans (4,5). The canonical ECS is comprised of two main cannabinoid receptors (coded by the cnr1 and cnr2 genes), endogenous lipid ligands (endocannabinoids, i.e., anandamide and 2-arachydonoil glycerol), and enzymes involved in endocannabinoid metabolism (coded by the faah and naaa genes, among others not included in this study) (1,6). There are additional extensions to the canonical ECS, termed the “extended” ECS, that are comprised of receptors with primary functions in other pathways that have accessory functions that exist upon interaction with cannabinoids (7,8). Some of these receptors include peroxisome proliferator activated receptors (coded by the ppara and pparg genes, respectively), “endocannabinoid-like” G-protein coupled receptors (i.e., gpr18, gpr55, and gpr119), nociception ion channels (coded by the trpv1 and trpv2 genes, respectively), and transporters (i.e., htr1a, adora2a, and adgrf1) (9,10). Though their primary functions are best characterized in other pathways, the extended ECS receptors functionally interact with endocannabinoid ligands, the phytocannabinoids present in the Cannabis plant, and other endogenous lipid mediators, including oleoyl-ethanolamide (OEA), palmitoyl-ethanolamide (PEA), and linoleoyl-ethanolamide (LEA) (9,10). Together, the canonical and extended ECS, known as the “endocannabinoidome”, consists of many receptors that can interact with multiple ligands, thus creating a complicated network of outcomes during both health and disease and not limited to the brain.
More widespread accessibility of phytocannabinoids for medicinal and recreational use, policy changes that have impacted funding priorities, and the heightened desire for plant-based therapeutics have re-awakened scientific interest in the ECS. As such, preclinical animal models are becoming increasingly important in identifying the health implications of phytocannabinoids and the molecular mechanisms by which the ECS can be therapeutically leveraged. However, challenges exist in the translational capacity of preclinical studies due to conflicting reports that arise because of key differences in study design, including the route of administration, formulation, dose, metabolism, animal species used, the company obtained from, sex, and fasting state (11–16). Further, clinical translation from rodents to primates is often lost due to discrepant findings that exist among preclinical models (17,18). Therefore, a more comprehensive understanding of the distribution of the canonical and extended ECS among preclinical animal models is necessary to increase scientific rigor and provide critical insight into the mechanisms by which phytocannabinoids elicit unexpected or seemingly contradictory findings across research groups.
To address this, we determined the relative expression of the 14 canonical and extended ECS genes (adgrf1, adora2a, cnr1, cnr2, gpr18, gpr55, gpr119, faah, htr1a, naaa, ppara, pparg, trpv1, and trpv2) in seven peripheral organs with metabolic and/or immune functions (colon, heart, kidney, liver, mesenteric lymph node [MLN], spleen, and visceral fat) in three translationally relevant preclinical animal models: C57BL/6 mice (Mus musculus), Sprague Dawley rats (Rattus norvegicus), and rhesus macaques (Macaca mulatta). Of note, while our present focus was on ECS relative gene distribution in the periphery, a subsequent publication will characterize distribution across sub-anatomic brain regions of these same animals.
Materials and Methods:
Ethics statement
Animals and procedures in this study were approved by the Johns Hopkins University Animal Care and Use Committee. Animal handling and euthanasia were conducted as stated under the NIH Guide for the Care and Use of Laboratory Animals and USDA Animal Welfare Regulation. Rats and NHP included in this study were healthy uninfected animals serving as controls in other experiments (13).
2.1: Animal use
2.1.1: Mice
Five C57/BL6 mice (female [n=3] and male [n=2]) were included in this study. Mice were housed in ventilated racks with a 14/10-hours light/dark cycle, with water and standard chow diet (Teklad Diet 2018; IN, USA) ad libitum. Mice were kept in their cages for 13-weeks before they were sedated with isoflurane and euthanized. During necropsy, colon, heart, kidney, liver, spleen, and visceral fat tissue were collected, washed with 1X PBS to remove contaminating blood, and were flash frozen with liquid nitrogen and stored at −80°C until further use. No MLN was included in this study due to the complexity of identifying them due to their small size and dissecting both the brain and the periphery at the time of necropsy.
2.1.2: Rats
Six female Sprague-Dawley rats (Charles River, MA, USA) were single-housed in wire-topped plastic cages in temperature and humidity-controlled facilities with a reverse light cycle (12 hours, lights off from 8:00am-8:00pm). Animals were provided corn-based chow (Teklad Diet 2018; IN, USA), and water ad libitum, except when actively participating as control subjects in behavioral procedures (13). Rats were 52-weeks old at the end of the study. Before necropsy, rats were sedated with isoflurane and euthanized by rapid decapitation. Upon necropsy, pancreas was the first organ to be collected and flash frozen. Afterwards, colon, heart, kidney liver, MLN, and spleen were collected, washed with fresh 1X PBS, flash frozen using dry ice and stored at −80°C until further use. No fat tissue was included in this study.
2.1.3: Non-Human Primates
Four adult, male, pathogen-free Rhesus macaques (RM) (Macaca mulatta) were included in this study (animal identification numbers 560, 561, 562, and 563). Female macaques were not included in this study due to their importance in breeding for maintaining the colony. Macaques were pair-house to minimize any immunologic stress caused by being single-housed and they were fed standard monkey chow (Teklad Diet 2018; IN, USA) (19). Macaques were 7.89, 8.76, 8.59 and 7.95 years old at time of necropsy. During necropsy, animals were sedated using ketamine, and euthanized with an overdose of sodium pentobarbital, according to the American Veterinary Medical Association guidelines (2013). Phosphate buffered saline (1X) was used to perfuse organs and remove blood from organs, tissues, and MLN were taken to analyze the relative expression of the canonical, and the extended endocannabinoid receptors. No colon samples were available at the time of the study.
2.2: RNA extraction & cDNA synthesis
RNA was extracted using RNeasy kit (Qiagen, MD, USA) following manufacturer’s instructions. Briefly, ~200mg of each tissue were added to tubes containing Lysing Matrix D (MP Biomedicals, CA, USA). Tissue was homogenized using MP FastPrep®-24 (MP Biomedicals, CA, USA). Fat tissue was centrifuged after homogenization to remove the top layer of fat as instructed by the manufacturer. Afterwards, the aqueous phase was mixed with 70% ethanol (The Warner Graham Company, MD, USA) at a 1:1 ratio in a clean tube and loaded into the RNeasy columns. RNA-free DNase (Qiagen, CA, USA) was added to the column to digest any DNA present in the sample, as suggested by the manufacturer. RNA concentration and quality parameters were determined using Nanodrop (ThermoFisher Scientific, MA, USA). Extracted RNA was used to synthesize cDNA using iScript cDNA Synthesis kit (Bio-Rad, CA, USA) following manufacturer’s instructions.
2.3: Real-Time Quantitative-Polymerase Chain Reaction
Relative genetic expression was determined using Real Time Quantitative Polymerase Chain Reaction (RT-qPCR) (CFX96™ Real-Time System, Bio-Rad, CA, USA) using commercially available TaqMan primers (Tables 1–3) and TaqMan™ Fast Universal PCR Master Mix (2X) no AMPERASE™ UNG (ThermoFisher Scientific, Catalog#4367846, MA, USA). Amplification was done in 40 cycles with the following conditions (Denaturing at 95°C for 20 seconds and annealing and extending at 60°C for 20 seconds). Cycle threshold values were normalized using Pan Eukaryotic 18S (ThermoFisher Scientific, MA, USA), transformed using the 2−∆CT method, and graphed to represent the relative genetic expression by sample, gene group and species.
Table-1:
List of primers used to determine the relative expression of canonical and extended endocannabinoid receptors in mice (Mus musculus).
| Gene Symbol | Assay ID | Company |
|---|---|---|
| adgrf1 | Mm00505409_m1 | ThermoFisher Scientific |
| adora2a | Mm00802075_m1 | ThermoFisher Scientific |
| cnr1 | Mm01212171_s1 | ThermoFisher Scientific |
| cnr2 | Mm00438286_m1 | ThermoFisher Scientific |
| faah | Mm00515684_m1 | ThermoFisher Scientific |
| gpr18 | Mm0122454_m1 | ThermoFisher Scientific |
| gpr55 | Mm03978245_m1 | ThermoFisher Scientific |
| gpr119 | Mm00731497_s1 | ThermoFisher Scientific |
| htr1a | Mm00434106_s1 | ThermoFisher Scientific |
| naaa | Mm01341699_m1 | ThermoFisher Scientific |
| ppara | Mm00440939_m1 | ThermoFisher Scientific |
| pparg | Mm00440940_m1 | ThermoFisher Scientific |
| trpv1 | Mm01246302_m1 | ThermoFisher Scientific |
| trpv2 | Mm00449223_m1 | ThermoFisher Scientific |
Table-3:
List of primers used to determine the relative expression of canonical and extended endocannabinoid receptors in Rhesus macaques (Macaca mulatta).
| Gene Symbol | Assay ID | Company |
|---|---|---|
| adgrf1 | Hs00228100_m1 | ThermoFisher Scientific |
| adora2a | Hs00169123_m1 | ThermoFisher Scientific |
| cnr1 | Hs01038522_s1 | ThermoFisher Scientific |
| cnr2 | Hs00275635_m1 | ThermoFisher Scientific |
| faah | Hs01038678_m1 | ThermoFisher Scientific |
| gpr18 | Hs01649814_m1 | ThermoFisher Scientific |
| gpr55 | Hs00271662_s1 | ThermoFisher Scientific |
| gpr119 | Hs00708890_s1 | ThermoFisher Scientific |
| htr1a | Hs00265014_s1 | ThermoFisher Scientific |
| naaa | Hs01567916_g1 | ThermoFisher Scientific |
| ppara | Hs00231882_m1 | ThermoFisher Scientific |
| pparg | Hs01115513_m1 | ThermoFisher Scientific |
| trpv1 | Hs00218912_m1 | ThermoFisher Scientific |
| trpv2 | Hs00275032_m1 | ThermoFisher Scientific |
2.4: Data Analysis and Statistics
Data were analyzed using PRISM software version 9.0 (GraphPad Software, Inc., San Diego, CA). Determination of relative gene expression was done in duplicates and represented in graphs plotting the mean±SEM. Our limit of detection (LoD) was calculated using an average of each species probing for Pan Eukaryotic 18S (ThermoFisher Scientific, MA USA) with a cycle threshold of 35. Please note that each sample was subtracted each own 18S value and hence can appear below the LoD but its expression was detected in a Ct value below 35. Samples that did not amplify were given an arbitrary value of 39.99. Variance between the relative expression of genes between organs and by species was determined using one-way ANOVA. Post hoc analysis was done to determine the difference between the expression of these genes when there was statistical significance determined by one-way ANOVA. (*p≦0.05, **p≦0.01, ***p≦0.001 & ****p≦0.0001)
Results
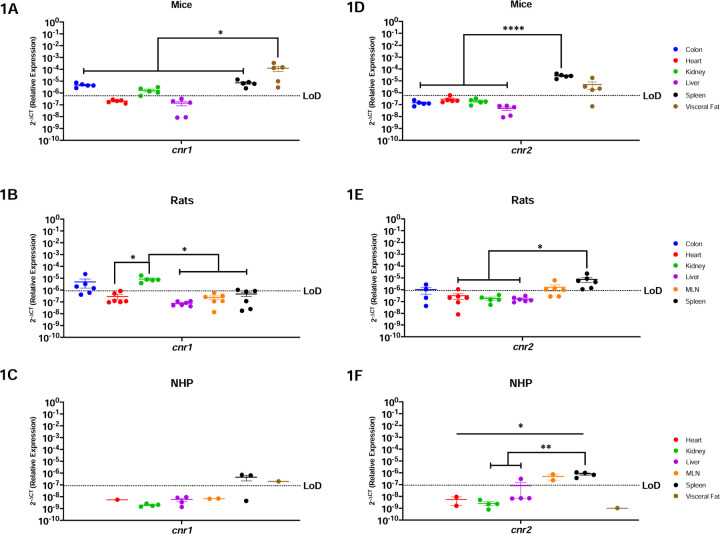
Both Canonical ECS Receptors Are Present In The Spleen Of Mice, Rats and NHP
Cnr1 is primarily expressed and studied in the context of the brain (20,21). Here we show that cnr1 mRNA was detected in both metabolic and secondary immune organs in mice, including the colon, kidney, spleen, and visceral fat (Figure-1A) Interestingly, significantly more cnr1 was present in the visceral fat as compared to all other evaluated organs (p=0.0277 vs. colon, 0.0250 vs. heart, 0.0250 vs. kidney, 0.0250 vs. liver and p=0.302 vs. spleen). In contrast to mice, cnr1 was more restricted in rats where the highest levels occurred in kidney (Figure-1B). Indeed, cnr1 was significantly higher in kidney as compared to heart (p-value=0.0257), liver (p-value=0.0209), MLN (p-value=0.0271), and spleen (p-value=0.0304). While cnr1 was present in colon, MLN, and spleen, it did not occur in all rats with only 4/6, 1/6, and 1/6 rats having detectable expression, respectively. Cnr1 was least abundant in NHP, where it was limited to the spleen and the visceral fat (Figure 1C). Cnr1 was not detectable in the liver or heart in any of the three models evaluated in this study.
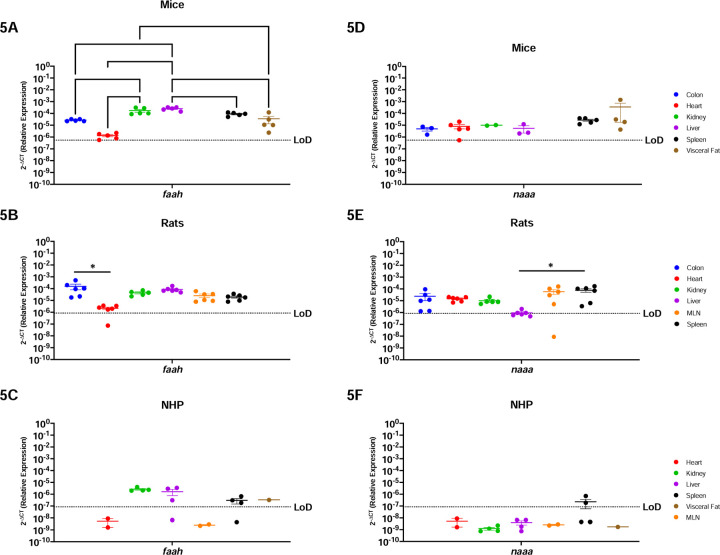
Figure-1: Both Canonical ECS Receptors Are Present In The Spleen Of Mice, Rats and NHP.
Relative expression of cnr1 and cnr2 was determined using qPCR from the colon, heart, kidney, liver, MLN, spleen, and visceral fat from mice, rats and NHP. A) Cnr1 mRNA in mice was detected in the colon, kidney (4/5 mice), spleen, and visceral fat, having the highest levels in the latter. B) Cnr1 was detected in partial samples of the rat model colon (4/6 rats), heart (1/6 rats), kidney, and spleen (1/6 rats), having statistically higher levels of expression in the kidney when compared to the heart, liver, MLN, and spleen. C) In NHP, cnr1 mRNA was detected in the spleen (2/3 rhesus) and visceral fat at comparable levels. D) Cnr2 was detected only in the spleen and visceral fat (4/5 mice), at comparable levels. The spleen had statistically significantly higher levels as compared to the colon, heart, kidney, and liver. E) Cnr2 was detected in the colon (2/4 rats), heart (1/6 rats), MLN (4/6 rats) and spleen of rats. F) In NHP, cnr2 mRNA was detected in the liver (1/4 rhesus), MLN and spleen. Detection levels were significantly higher in the spleen when compared to the heart, kidney, and liver, but with significant differences when compared to the liver (1/4 rhesus), kidney, and heart. Data are represented as the mean ± SEM. (*p-value >0.05, **p-value>0.001, ***p-value>0.0001, ****p-value>0.00001).
Cnr2 is primarily present in the periphery with expression in the brain occurring in the context of disease (20–22). Our findings were consistent with this, where cnr2 mRNA was detected in the spleen and in the visceral fat (4/5 mice) of mice at similar levels. Significant differences were found among the spleen and the colon (p=<0.0001), heart (p=<0.0001), kidney (p=<0.0001), and liver (p=<0.0001). (Figure-1D). In rats, we detected cnr2 mRNA partially in the colon (2/4 rats), the heart (1/6 rats), MLN (4/6 rats), and the spleen (6/6 rats) (Figure-1E). Significant differences were found among the spleen and the heart (p=0.0237), kidney (p=0.0299), and liver (p=0.0201). NHP had restricted cnr2 mRNA, with robust levels in the MLN and spleen (Figure-1F). Cnr2 was partially detected in the liver (1/4 rhesus) in the NHP model. Notably, Cnr2 in the spleen was significantly more highly expressed in NHP when compared to the heart (p=0.0182), kidney (p=0.0043) and liver (p=0.0092).
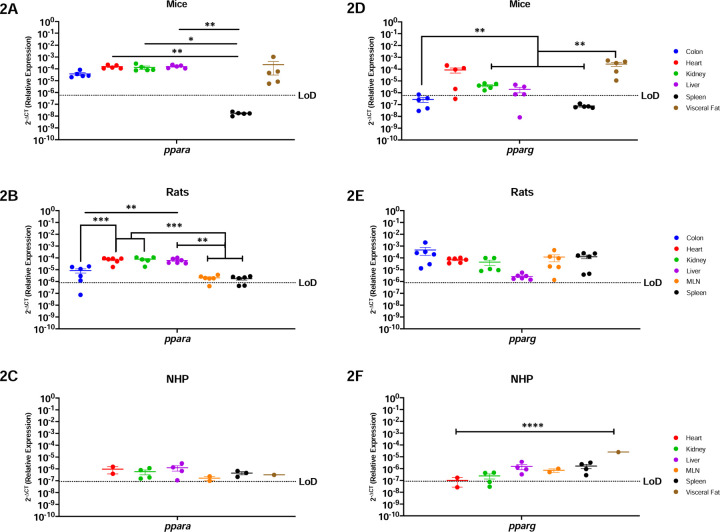
Peroxisome Proliferator Activated Receptors, Ppara and Pparg, Are Generally Well Conserved In All Organs Of Mice, Rats and NHP
Peroxisome proliferator activated receptors mediate several vital functions, and hence are known to be expressed almost ubiquitously (23–25). Our findings corroborated this, where we determined that ppara mRNA was present in every evaluated organ in mice, with the notable exception of the spleen (Figure 2A). These genes were most abundantly expressed in the heart (p=0.0049), kidney (p=0.0484), and liver (p=0.0049). Similar trends occurred in rats, where ppara mRNA was detected in all organs available, having significantly higher expression in the liver vs. secondary immune organs (p=0.0261 for MLN, and p=0.0233 for spleen) and the colon (p=0.0445) (Figure-2B). The NHP model also showed ubiquitous ppara, being detected in all evaluated organs (Figure-2C).
Figure-2: Peroxisome Proliferator Activated Receptors, Ppara and Pparg, Are Generally Well Conserved In All Organs Of Mice, Rats and NHP.
Relative expression of ppara and pparg was determined using qPCR from the colon, heart, kidney, liver, MLN, spleen, and visceral fat from mice, rats, and NHP. A) Ppara mRNA was detected in all the organs tested in mice, except for the spleen. It had significantly higher levels in the kidney when compared with the colon and the spleen. B) In rats, ppara mRNA was detected in the colon (4/6 rats), heart, liver, kidney, MLN (5/6 rats), and spleen (4/6 rats). Ppara in the rat model was significantly higher in the heart, kidney, and liver, when compared to the colon, MLN, and spleen. C) Ppara was detected in all organs tested in the periphery of the NHP model at comparable levels for all the evaluated organs. D) Mouse pparg was similar to ppara, being detected in the colon (3/5 mice), heart (4/5 mice), kidney, liver (4/5 mice) and the visceral fat. Detection of this gene was higher in visceral fat when compared to the colon, kidney, liver, and spleen. E) Pparg was detected in all the organs available for the rat model with no statistical significance among any organ. F) Pparg in the NHP model was also detected in all available organs for the NHP model, having only partial detection in the heart (1/2 rhesus) and the kidney (3/4 rhesus). Interestingly, pparg was significantly higher in the visceral fat when compared with all other tissues. It is worth mentioning that neither of these genes were detected in the spleen of mice, contrary to the other animal models. Data are graphed as the geometric mean ± SEM (*p-value >0.05, **p-value>0.001, ***p-value>0.0001, ****p-value>0.00001).
Pparg mRNA followed a similar trend as ppara in mice, where it was detected in all organs except for the spleen (Figure-2D). Interestingly, pparg was most highly expressed in visceral fat and heart in mice. Significant differences were found among the visceral fat and colon (p=0.0027), kidney (p=0.0031), liver (p=0.0028), and spleen (p=0.0026). Pparg mRNA was also expressed across all organs in rats, where no significant differences occurred across the body (Figure-2E). In contrast, NHP had nuanced pparg expression where it was highly present in visceral fat, when compared to the heart (p=0.0001), kidney (p=0.0001) liver (p=0.0001), MLN (p=0.0001), and spleen (p=0.0001) (Figure-2F). In sum, ppara and pparg were similarly present in all animal models, with high expression detected in all organs, with the notable exception of the spleen of mice.
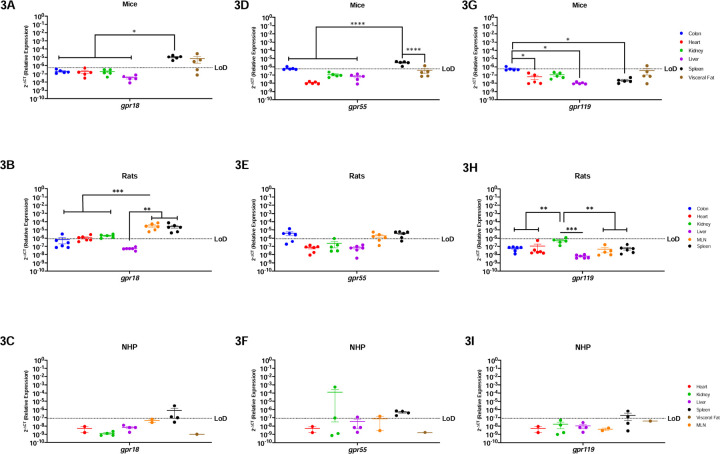
Endocannabinoid-like GPRs Are Preferentially Expressed In Lymphoid Organs and The Visceral Fat
GPRs are mostly considered to be orphan receptors until identification of their specific ligand. Some GPRs (i.e., gpr18, gpr55, and gpr119) are known to interact with cannabinoids and are considered to be endocannabinoid-like GPRs (26–31). Mice had relatively limited gpr18 mRNA, which was present only in the spleen and visceral fat (Figure-3A). Surprisingly, rats had a very different profile where gpr18 was detected among all organs, except for liver (Figure-3B). Notably, gpr18 was most highly expressed in the secondary lymphoid organs in rats, being statistically different from the colon (p=0.0120), heart (p=0.0137), kidney (p=0.0255), and liver (p=0.0092). Gpr18 was only partially detected in the spleen (3/4 rhesus) in the NHP model (Figure-3C).
Figure-3: Endocannabinoid-like GPRs Are Preferentially Expressed In Lymphoid Organs and The Visceral Fat.
Relative expression of gpr18, gpr55, and gpr119 was determined using qPCR from the colon, heart, kidney, liver, MLN, spleen, and visceral fat from mice, rats and NHP. A) Gpr18 was detected in the spleen and visceral fat with significant difference between the spleen and the rest of the organs. B) In rats, gpr18 was highest in the secondary immune organs (MLN and spleen). C) Gpr18 was only detected partially in the spleen (3/4 rhesus) of the NHP model. D) Gpr55 was detected in the colon (1/5 mice), spleen and visceral fat (1/5 mice), having statistical significance among the spleen and all other tissues. E) Gpr55 expression was detected in the colon (4/6 animals), MLN (4/6 rats) and the spleen (5/6 rats). F) In the NHP model, gpr55 was detected in the kidney (1/4 rhesus), liver (1/4 rhesus), MLN (1/2 rhesus), and spleen (2/4 rhesus). G) Gpr119 was detected partially in the colon (1/5 mice) and the visceral fat (1/5 mice), having statistical significance between the colon and the heart, liver, and spleen. H) Gpr119 was only detected in the kidney (1/6 rats) with significant differences with all the other organs included in this study. I) Gpr119 was partially detected in the spleen (2/4 rhesus) of the NHP model. Data represents the geometric mean ± SEM (*p-value >0.05, **p-value>0.001, ***p-value>0.0001, ****p-value>0.00001).
Gpr55 was similar to gpr18 in mice, being highly expressed in the spleen and having significantly higher levels when compared to the colon (p=0.0414), heart (p=0.0278), kidney (p=0.0272), liver (p=0.0275), and visceral fat (p=0.0027) (Figure-3D). Gpr55 also primarily followed a similar expression pattern as gpr18 in rats; however, they did not express gpr55 in the heart or liver (Figure-3E). Interestingly, NHP gpr55 was partially detected in the kidney (2/4 animals), liver (1/4 animals), and MLN (1/2 animals), showing more expression than the other two endocannabinoid-like GPCRs, and a different pattern than the rodent gpr18 and gpr55 (Figure-3F).
Gpr119 generally followed a similar pattern to gpr18 where it was minimally detected in all animal models. Gpr119 was only present in the colon and visceral fat of mice (Figure-3G), was undetectable in the periphery of rats (Figure-3H), and only partially detected in the spleen of NHP (2/4 animals) (Figure-3I).
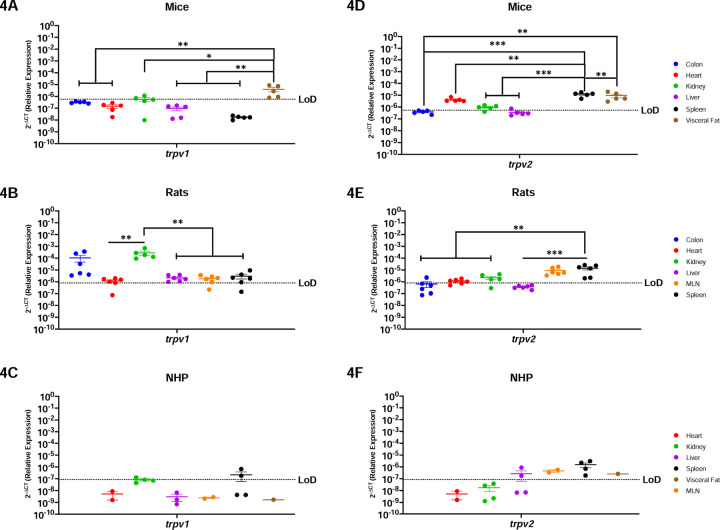
TRPV1 and TRPV2 Nociception Channels Have Limited Translational Applicability to Humans.
Nociception channels are widely studied in their response to painful stimuli (32,33). Trpv1 mRNA was detected in the colon, kidney, and visceral fat of mice, having significant differences when comparing the visceral fat to the colon (p=0.0076), heart (p=0.0050), kidney (p=0.0139), liver (p=0.0044) and spleen (p=0.0036) (Figure-4A). In contrast, rats had a wider expression of this gene, which was detected in all evaluated organs (Figure-4B). Trpv1 was highest in the rat kidney and statistically increased as compared to the heart (p=0.0038), liver (p=0.0039), MLN (p=0.0039) and spleen (p=0.0041). NHP were comparable to mice and only had detectable trpv1 in the kidney and spleen (Figure-4C).
Figure-4: Peripheral TRPV1 and TRPV2 Nociception Channels Have Limited Translational Applicability to Humans.
Relative expression of trpv1 and trpv2 was determined using qPCR from the colon, heart, kidney, liver, MLN, spleen, and visceral fat from mice, rats and NHP. A) Trpv1 was detected in the colon, partially in the kidney (3/5 mice), and the visceral fat. B) Trpv1 was detected in the colon, heart (5/6 animals), kidney, liver, MLN (5/6 rats) and spleen (5/6 rats). Significant differences were found between the kidney and the other organs, except for the colon which showed comparable expression levels. C) Trpv1 was detected in the kidney (1/4 rhesus) and the spleen (2/4 rhesus). D) Trpv2 mRNA was detected in the heart, kidney (4/5 mice), liver (1/6 mice), spleen, and visceral fat, having higher significant levels in the last two. E) Trpv2 was detected in the colon (1/6 rats), heart (4/6 rats), kidney (4/5 rats), MLN, and spleen. Trpv2 was similar between immune organs, and they are both significantly different when compared with the metabolic organs. F) Trpv2 showed broader detection, being detected in the liver (2/4 rhesus), MLN, spleen, and visceral fat. Data are graphed as the geometric mean ± SEM (*p-value >0.05, **p-value>0.001, ***p-value>0.0001, ****p-value>0.00001).
Trpv2 mRNA was widely present in mouse with expression in the heart, kidney, liver, spleen, and visceral fat (Figure-4D). Even still, trpv2 was not expressed equally but instead was significantly higher in the spleen and the visceral fat as compared to colon (p=0.0002), heart (p=0.0117), kidney (p=0.0003), and liver (p=0.002). Trpv2 was detected throughout all the organs in the rat, except for the liver (Figure-4E). Of the organs in which it was expressed, trpv2 was most highly present in the rat spleen, with comparable levels to MLN, but reaching statistical significance with the colon (p=0.0010), heart (p=0.0016), and kidney (p=0.0071). Interestingly, the NHP model was the only preclinical model where trpv2 was present in liver (Figure-4F). In contrast, trpv2 was detectable in the NHP MLN, spleen, and visceral fat, and was comparably expressed across organs without any significant differences between them.
Endocannabinoid Metabolic Enzymes Are Ubiquitous In Rodents, But More Restricted In NHP
Faah and naaa are ubiquitous enzymes involved in endocannabinoid degradation (34–38). In agreement with this, faah was present in all organs analyzed in mice, having more abundance in the kidney and the liver as compared to the colon (p=0.0067 & p>0.0001, respectively), heart (p=0.0013 & p>0.0001, respectively), visceral fat (p=0.0116 & p>0.0001, respectively), and the spleen, but only when compared to the liver (p=0.0021) (Figure-5A). Similarly, faah was present in all rat organs, having increased expression in the colon and decreased expression in the heart (p=0.0264), following a similar pattern as the mice (Figure-5B). Interestingly, NHP had a different faah expression pattern than the rodents. While widely detected in the kidney, liver (3/4 rhesus), spleen (3/4 rhesus) and visceral fat, faah was not detected in the NHP heart nor MLN (Figure-5C).
Figure-5: Endocannabinoid Metabolic Enzymes Are Ubiquitous In Rodents, But More Restricted In The NHP Model.
Relative expression of faah and naaa was determined using qPCR from the colon, heart, kidney, liver, MLN, spleen, and visceral fat from mice, rats and NHP. A) Faah mRNA was detected in all organs tested, having differential expression between them. The heart of mice had the lowest levels for this gene. B) Faah mRNA was detected in all organs included in this study. The colon of rats showed higher levels of faah, particularly significant when compared to expression in the heart. C) Levels of faah in the NHP model were detected in the kidney, liver (3/4 animals), spleen (3/4 rhesus), and visceral fat. D) Naaa mRNA was detected in all organs with similar levels. E) Expression of naaa mRNA in rats was detected in all organs and was least abundant in liver. F) Naaa mRNA was detected only partially in the spleen (2/4 rhesus). Data are graphed as the geometric mean ± SEM (*p-value >0.05, **p-value>0.001, ***p-value>0.0001, ****p-value>0.00001).
Naaa showed similar trends as faah where rodents had ubiquitous expression while NHP had more restricted expression. Naaa was detected in all organs tested in mice, to comparable levels (Figure-5D). In rats, it was also detected in all organs, but with significantly lower expression in the liver when compared to the spleen (p=0.0289) (Figure-5E). Naaa was only expressed in the NHP spleen (2/4 rhesus).
Htr1a, Adora2a and Adgrf1 are Poorly Conserved Among Mice, Rats, and NHP
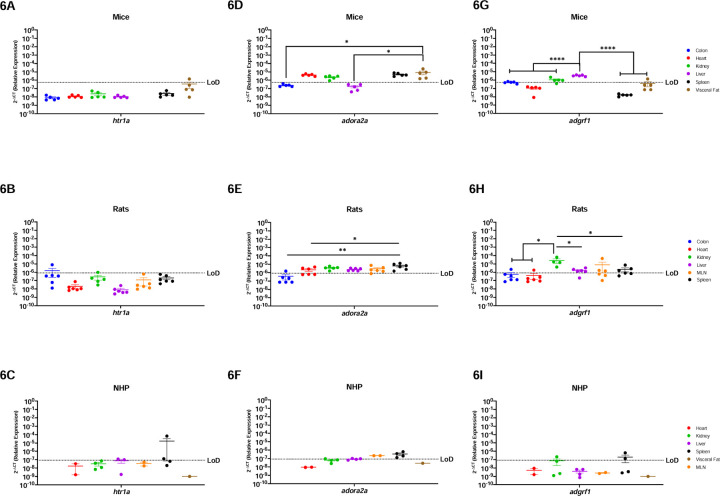
Htr1a is a serotonin receptor primarily studied in the brain, while adora2 and adgrf1 are more widely present and implicated in inflammation, cardiovascular diseases, and cancer (39–45). In general, htr1a mRNA was minimally expressed in the periphery of all three preclinical models. Htr1a was only detected in the visceral fat (1/5 mice), colon (1/5 rats), and liver (2/3 rhesus) and spleen (2/4 rhesus) (Figure-6A-C). Adora2a is present to a greater extent in the periphery and was detected in the heart, kidney, spleen, and visceral fat of mice (Figure-6D). Rats also widely expressed adora2a which was found in all examined organs but was lowly expressed in the colon (p=0.0014) and the heart (p=0.0384) (Figure-6E). NHP also expressed adora2a, however it was present only in the spleen and MLN secondary immune organs (Figure-6F). Adgrf1 was detected in the colon, kidney, liver, and visceral fat from mice, though it was mostly highly expressed in the liver (Figure-6G). Rats had widespread adgrf1 across all organs in rats, with preferential expression in the kidney when compared with the colon (p=0.0286), heart (p=0.0273), liver (p=0.0396), and the spleen (p=0.0472) (Figure-6H). Adgrf1 had the most limited expression in NHP where it was detected only in the kidney (1/4 rhesus) (Figure-6I).
Figure-6a. Adora2a and Adgrf1 are Poorly Conserved Among Mice, Rats, and NHP.
, Relative expression of 5-htr1a, adora2a and adgrf1 was determined using qPCR from the colon, heart, kidney, liver, MLN, spleen, and visceral fat from mice, rats and NHP. A) Htr1a was only detected partially in the in the visceral fat of mice (1/6 mice). B) In rats, htr1a was only detected in one sample of the colon. C) Htr1a in the NHP model was detected in the spleen (2/4 rhesus). D) In mice, Adora2a was detected in the heart, kidney, spleen, and visceral fat. The visceral fat levels were significantly higher as compared to colon and liver. E) Adora2a was detected in all organs screened, with significantly higher levels in the spleen when compared to other organs. F) Adora2a in NHP was detected in the MLN and spleen. G) Adgrf1 was detected in the colon, kidney, liver, and visceral fat (1/6 mice). This gene was higher in the liver when compared to all the other organs. H) Adgrf1 was detected in the kidney with significant higher levels when compared to the colon, liver, and spleen. This gene was also detected in the heart (1/6 rats) and MLN (3/5 rats) I) Adgrf1 was detected partially in the kidney (1/4 rhesus) and the spleen (2/4 rhesus). Data are graphed as the geometric mean ± SEM (*p-value >0.05, **p-value>0.001, ***p-value>0.0001, ****p-value>0.00001).
Discussion
We report a comparison of the relative expression of 14 genes from the canonical and extended ECS in seven peripheral organs from three animal species and strains widely used in research, including in cannabis and cannabinoid research: C57BL/6 mice, Sprague-Dawley rats, and Rhesus macaque NHP. We identified key differences in the relative expression patterns of these evolutionary conserved, polyfunctional receptors, and found that these preclinical model systems were more dissimilar than has been previously appreciated. Indeed, there were only five receptors (CB2, GPR18, GPR55, TRPV2, and FAAH) that had identical expression patterns in all three preclinical animal models. Of note, these five receptors were consistently expressed in the spleen for all species evaluated, suggesting the importance of endocannabinoid function in this secondary lymphoid organ and potential for therapeutic intervention. This indicates that while the ECS is highly conserved, each of the three animal species included has a differing pattern for receptor composition in their peripheral organs. This is incredibly important and has profound implications for translation to humans and for comparison across research groups. The impact of route of administration, diet, formulation, dose, fasting vs. fed state, biological sex, and metabolite distribution and bioavailability (11) is already implicated in contributing to discrepant findings in the cannabinoid field. We propose that the unique receptor composition patterns of the preclinical model must also be considered to enhance scientific rigor and reproducibility. Indeed, as multiple canonical and extended ECS receptors are simultaneously present within a tissue, the potential for off-target and polypharmacy effects (14,16,46–51,51–67) is staggering as each receptor has its own unique function and signaling processes. Therefore, it is important to understand the nuances of endocannabinoid receptor tissue localization in the most common preclinical animal models.
Our findings also bring attention to the importance of additional receptors that have been understudied thus far. While the canonical ECS receptors, CB1 and CB2, have been most widely studied, our work demonstrates that the extended ECS receptor distribution represents an additional level of complexity that must be considered when performing cannabinoid studies. Indeed, these receptors and/or metabolic enzymes are simultaneously present in peripheral tissues in tandem with CB1 and/or CB2. and are also capable of mediating physiologic effects upon interacting with endo- and phytocannabinoids. These interactions should not be ignored as they result in a complex network of physiological pathways having diverse effects in biologic systems in the chosen preclinical model. Our results are summarized in Tables 4–6.
Table-4:
Summary of the findings of the expression of the canonical and extended ECS in mice (Mus musculus).
| Mice | Colon | Heart | Kidney | Liver | MLN | Spleen | Visceral Fat |
|---|---|---|---|---|---|---|---|
| cnr1 | + | − | + | − | + | + | |
| cnr2 | − | − | − | − | + | + | |
| ppara | + | + | + | + | − | + | |
| pparg | + | + | + | + | − | + | |
| gpr18 | + | − | − | − | + | + | |
| gpr55 | + | − | − | − | + | + | |
| gpr119 | + | − | − | − | − | + | |
| trpv1 | + | − | + | − | − | + | |
| trpv2 | − | + | + | + | + | + | |
| faah | + | + | + | + | + | + | |
| naaa | + | + | + | + | + | + | |
| htr1a | − | − | − | − | − | + | |
| adora2A | − | + | + | - | + | + | |
| adgrf1 | + | − | + | + | − | + |
Table-6:
Summary of the findings of the expression of the canonical and extended ECS in Rhesus macaques (Macaca mulatta).
| NHP | Colon | Heart | Kidney | Liver | MLN | Spleen | Visceral Fat |
|---|---|---|---|---|---|---|---|
| cnr1 | − | − | − | − | + | + | |
| cnr2 | − | − | + | + | + | − | |
| ppara | + | + | + | + | + | + | |
| pparg | + | + | + | + | + | + | |
| gpr18 | − | − | − | − | + | − | |
| gpr55 | − | + | + | + | + | − | |
| gpr119 | − | − | − | − | + | − | |
| trpv1 | − | − | + | + | − | − | |
| trpv2 | − | − | + | + | + | + | |
| faah | − | + | + | − | + | + | |
| naaa | − | − | − | − | + | − | |
| htr1a | − | + | + | − | + | − | |
| adora2A | − | + | + | + | + | − | |
| adgrf1 | − | + | − | − | − | − |
Both Canonical ECS Receptors Are Present In The Spleen Of Mice, Rats and NHP
The CB1R (cnr1 gene) is most widely studied in the brain where it mediates antinociceptive effects, appetite regulation, and interacts with phytocannabinoids (20,21). However, CB1R is also present in multiple peripheral sites, including fat, lungs and reproductive organs where its plays roles in regulating inflammation and obesity (20,70). In contrast, CB2R is present primarily in peripheral organs, such as the spleen and MLN, and is most widely implicated in immune cell functions (71).
Here, we report that cnr1 and cnr2 mRNA were detected in peripheral organs of all three preclinical animal models, in agreement with existing studies. However, we expand on this knowledge to identify similarities and key differences among the model systems. Cnr1 was most highly present in the visceral fat tissue for mice, while in rats its highest levels instead occurred in the kidney and colon. The NHP model had more limited cnr1 where it was detectable only in the spleen and visceral fat. Similar findings occurred with cnr2. While cnr2 was commonly detected in the MLN and spleen of all animal models, as expected, nuanced expression also existed where it was present in the rat colon, but not in the mouse. These findings denote key differences in cnr1 mRNA not only between rodent and NHP models, but also between mice and rats.
Importantly, we determined that both canonical receptors were detected in the spleen of all three preclinical models, suggesting that it is a well conserved candidate to study the implications of CB1 and CB2 in health and disease. However, it must be acknowledged that we also identified important differences between mice, rats, and NHP. Indeed, we identified there was a striking absence of cnr1 mRNA in the liver and of cnr2 in the kidney for all three preclinical animal models, which is inconsistent with human expression patterns (20,72,73). This demonstrates an important limitation in translation across species. Further, this demonstrates the necessity for comparative ECS analyses to identify appropriate preclinical animal models to determine those that are best reflective of what occurs in humans.
Nuclear Transcription Factors, Ppara and Pparg, Are Generally Well Conserved In All Organs Of Mice, Rats and NHP
PPARs are a group of ligand-activated nuclear hormone receptors (ppara, pparb/d and pparg) that interact with Retinoid X Receptor to act as transcription factors that regulate gene expression of genes involved in energy metabolism, glucose and fat metabolism, and inflammation (23,25,74,75). In humans, mice and rats, ppara is ubiquitous, but in rodents it has biased expression in energy requiring organs, including the heart, liver, and kidney (76–81). In contrast, PPARG is primarily present in the fat tissue in humans and mice, while rats have highest expression in the thymus (79–81).
Our PPARA and PPARG findings are in agreement with existing knowledge, except for their notable lack of detection in the spleen of mice. Indeed, except for this occurrence, PPARA and PPARG were the most highly conserved ECS receptor gene evaluated, having comparable detection among all organs across all preclinical models. Interestingly, ppara and pparg mRNA were detected ubiquitously in all evaluated organs in the NHP model. These findings demonstrate high translational potential for ppara and pparg and provide implications for evaluating how cannabinoids may impact energy homeostasis (74), macrophage activation, insulin sensitivity, (24,82,83), and anti-inflammatory pathways through NF-kB inhibition (24,84).
Endocannabinoid-like GPRs Are Preferentially Expressed In Lymphoid Organs and The Visceral Fat
There is limited understanding of the endocannabinoid-like GPRs, which in humans is restricted to detection of gpr18 and gpr55 in lymphoid tissue and reproductive organs (85,86), and gpr119 in the GI tract (87,88). Here we report that, overall, the GPR’s had limited expression across all preclinical models evaluated. Further, when detected, there were marked organ- and species-specific differences. The GPR’s were most consistently detected in the spleen, where gpr18 and gpr55 were expressed in all evaluated animal models. However, there was a noticeable lack of gpr119 in the spleen, which was conserved among the mice, rat, and NHP models. This trend continued, as there was more widespread gpr18 and gpr55 expression among all organs, although there were key species-specific differences. Indeed, gpr18 was primarily restricted to the rat model (heart and spleen), which were not detectable in mice and NHP. In sum, these findings demonstrate that the rat model represents the best preclinical model to evaluate endocannabinoid-mediated GPR activation in vivo. Further, we identify the spleen as the most attractive therapeutic option to target the GPR’s as it has the most consistent expression across all evaluated models.
While their endogenous functions are incompletely understood, the GPR’s have clinically relevant implications, including gpr18’s roles in intracellular calcium, immunomodulation, cancer, metabolism and intraocular pressure (28,89–91); gpr55’s effects on bone physiology and intracellular signal transduction involving the activation of NF-κB, NFAT, CREB and ATF2 (29–31,91–94); and gr119’s involvement in glucose homeostasis and insulin secretion and sensitivity (103–107).
TRPV1 and TRPV2 Nociception Channels Have Limited Translational Applicability to Humans
Trpv1 and trpv2 are ion channels that allow passage of essential ions (i.e., Na2+ and Ca2+) through the cell membrane (32,33). These ionotropic receptors are involved in noxious stimuli such as pain, heat, and inflammation and its expression is ubiquitous in humans (98,99). Here we report marked differences in these receptors. Rats had the most similar trpv1 expression patterns to humans as it was widely expressed, whereas it was more limited in mice and NHP. While trpv2 was more abundant in all three preclinical animal models, the only organ with shared expression among all three preclinical models was the spleen. Expression in the colon, heart and kidney was detected between rodents but not in NHP model. These discrepancies are profound in comparison to humans and demonstrates relatively poor translational potential. While these models are invaluable tools to evaluate the function of these receptors, care must be taken in drawing conclusions to the human condition. This suggests high potential for failure of preclinical endocannabinoid studies that aim to evaluate the roles of trpv1 in hyperalgesia, body temperature control, diabetes, hormone secretion, epilepsy and hearing (98), as well as trpv2 in cancer and cardiovascular dysfunction (99–102).
FAAH and NAAA Endocannabinoid Metabolic Enzymes Are Ubiquitous In Rodents, But More Restricted In The NHP Model
FAAH and NAAA are important components of the ECS through endocannabinoid regulation that are ubiquitously expressed in humans (34,35,38). Our results identify that faah and naaa are ubiquitously expressed in the peripheral organs of rodents. While there were statistically significant differences among the organs, the mRNA for these metabolic enzymes were always detectable in mice and rats. Surprisingly, there was limited expression in the peripheral organs of the NHP model. Notably, faah was not present in the NHP heart and MLN, while naaa was undetectable in all organs except for spleen. This suggests that rodent models may have better preclinical utility to perform cannabinoid studies focused on targets of faah and naaa. This realization is important as these enzymes are essential in regulating endocannabinoid tone, which when dysregulation leads to pathology (103,104). Inhibiting these endocannabinoid catabolic enzymes is of major therapeutic interest as FAAH inhibitors are suggested as therapeutic targets for a group of diseases related to endocannabinoid level deficiencies termed “Clinical Endocannabinoid Deficiency Syndrome”, which have implications for migraine, fibromyalgia, and irritable bowel syndrome (103,104).
Htr1a, Adora2a and Adgrf1 are Poorly Conserved Among Mice, Rats, and NHP
Htr1a and adora2a have been primarily studied in the context of the brain, while adgrf1 is known to be expressed in the kidney (39,40,42,105,106). We identified minimal conservation of htr1a, adora2a, and adgrf1 among the three animal models. Our results corroborated that htr1a was minimally present in the periphery of both mice and rats. However, we were surprised to learn of its more widespread presence in the NHP model where it was detected partially in the kidney, liver, and spleen. In contrast, adora2a was present in the kidney and spleen for all three preclinical animal models. Even with these similarities, we observed marked species differences as adora2a was not present in the liver of mice, while it was expressed in the liver of rats and NHP. Similar trends occurred for adora2a in the heart and visceral fat as they were detected only in the rodent models. While rats had detectable adgrf1 in all organs tested, it was not present in the heart or spleen of mice. Interestingly, NHP had the most limited adgrf1 expression as it was restricted to the kidney, presenting a limiting factor in clinical translation from rodents to NHP and therefore to humans.
Strikingly, there was little overlap among htr1a, adora2a and adgrf1 in the animal models. In fact, each species had only one organ where these genes were co-expressed: visceral fat for mice, colon for rat, and kidney for NHP. This dissimilarity in expression patterns warns that caution must be taken when evaluating cannabinoid-mediated effects on htr1a, adora2a, and adgrf1 in efforts to identify new therapeutic targets, as the potential for limited translation is high. This is the most poignant demonstration of the care that must be taken when selecting preclinical animal models for endocannabinoid studies. The translational limitations of these receptors has clinical implications as htr1a has been extensively studied as a target for mood disorders, adora2a is suggested as a therapeutic target for neurodegenerative disorders, blood brain barrier integrity, immunosuppression, cancer, and angiogenesis (41,42), and adgrf1 is proposed as a novel therapeutic for cancer and inflammation (43,44,107).
Conclusions
The endocannabinoid system is an incredibly attractive therapeutic target for many disorders where phytocannabinoids and receptor agonists/antagonists are being considered as novel treatment strategies. However, our findings demonstrate profound species- and organ-specific effects where there is limited overlap in expression pattern among mice, rat, and rhesus macaque preclinical models. We recommend that cannabinoid studies carefully consider the preclinical model to be included with respect to animal species, strain, genetic background, and even the site of procurement as there are reported variations in the same strains of rats obtained from different vendors (12). Further, cannabinoid formulation, vehicle in which it is reconstituted, metabolism, transport, mechanism of action, and their complex pharmacology should be considered to determine the right dose, formulation, and route of administration (12,108). Additionally, we urge scientists in the cannabinoid field to consider studying the relation between formulation, dosage, route of administration, diet, fed state, water availability, and cannabinoid pharmacokinetics. We also suggest considering age as an important factor as expression of endocannabinoid receptors, ligands and enzymes changes throughout the life course (68,69). It must also be considered that rodents are nocturnal animals, and their circadian rhythm may also impact results that can lead to discrepant findings with NHP and human preclinical studies. Finally, geographical location, time of administration, and time from administration to the actual experiment is performed should be taken into consideration. We anticipate our findings will provide insight for more rigorous experimental design of cannabis and cannabinoid translational research involving these preclinical animal models. Further, we hope that our findings will demonstrate the need to consider the extended ECS receptors that are abundantly expressed, activated by both endo- and phytocannabinoids, and that represent underlying mechanisms of action for these important lipid ligands.
Table-2:
List of primers used to determine the relative expression of canonical and extended endocannabinoid receptors in rats (Ratus norvegicus).
| Gene Symbol | Assay ID | Company |
|---|---|---|
| adgrf1 | Rn01511909_m1 | ThermoFisher Scientific |
| adora2a | Rn00583935_m1 | ThermoFisher Scientific |
| cnr1 | Rn03037213_s1 | ThermoFisher Scientific |
| cnr2 | Rn01637601_m1 | ThermoFisher Scientific |
| faah | Rn00577086_m1 | ThermoFisher Scientific |
| gpr18 | Rn01493247_m1 | ThermoFisher Scientific |
| gpr55 | Rn03037213_s1 | ThermoFisher Scientific |
| gpr119 | Rn01648212_m1 | ThermoFisher Scientific |
| htr1a | Rn01637601_m1 | ThermoFisher Scientific |
| naaa | Rn01768319_m1 | ThermoFisher Scientific |
| ppara | Rn00566193_m1 | ThermoFisher Scientific |
| pparg | Rn00440945_m1 | ThermoFisher Scientific |
| trpv1 | Rn00583117_m1 | ThermoFisher Scientific |
| trpv2 | Rn00567974_m1 | ThermoFisher Scientific |
Table-5:
Summary of the findings of the expression of the canonical and extended ECS in rats (Ratus norvegicus).
| Rat | Colon | Heart | Kidney | Liver | MLN | Spleen | Visceral Fat |
|---|---|---|---|---|---|---|---|
| cnr1 | + | + | + | − | + | + | |
| cnr2 | + | + | − | − | + | + | |
| ppara | + | + | + | + | + | + | |
| pparg | + | + | + | + | + | + | |
| gpr18 | + | + | − | + | + | + | |
| gpr55 | + | − | − | − | + | + | |
| gpr119 | − | − | − | − | − | − | |
| trpv1 | + | + | + | + | + | + | |
| trpv2 | + | + | + | − | + | + | |
| faah | + | + | + | + | + | + | |
| naaa | + | + | + | + | + | + | |
| htr1a | + | − | − | − | − | − | |
| adora2A | + | + | + | + | + | + | |
| adgrf1 | + | + | + | + | + | + |
Acknowledgements
The authors would like to extend their gratitude towards the Retrovirus Laboratory at Johns Hopkins for their support and provision of samples from the historical rhesus macaque NHP samples included in this study, as well as Dr. Janice Clements for providing the funding that supported procurement and goals of the original research study in which these historical animals were involved. We would also like to acknowledge the veterinary staff that handled the rats and rhesus macaque at Johns Hopkins Bayview Campus and East Baltimore Campus, respectively. Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA052859 and U01DA058527 (DWW) and the National Institute of Neurologic Disorders and Stroke award number K00NS118713 (CJW). The authors also acknowledge mentorship to DWW and procurement of pilot funds to ALE from the Johns Hopkins University Center for AIDS Research (P30AI094189), Diversity Supplement funded by R01-DA052859-03S1 to JJRF. REW is a Solomon H. Snyder Fellow at the Neuroscience Training Program at Johns Hopkins University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest:
The authors declare no financial interest, conflict of interest or any competing interest of any kind.
Data availability
The data gathered in this study is compiled and stored according to NIH data management, storing and sharing policies. Data will be available one year after the study has been published and can be accessed using the following link doi:10.17632/t6yd6j6bm6.1
References:
- 1.Moreno E, Cavic M, Canela EI. Functional Fine-Tuning of Metabolic Pathways by the Endocannabinoid System-Implications for Health and Disease. Int J Mol Sci. 2021. Apr 1;22(7):3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya N, Penukonda S, Shcheglova T, Hagymasi AT, Basu S, Srivastava PK. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci. 2017. May 9;114(19):5005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPartland JM, Matias I, Di Marzo V, Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006. Mar 29;370:64–74. [DOI] [PubMed] [Google Scholar]
- 4.Elphick MR, Egertová M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond Ser B. 2001. Mar 29;356(1407):381–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodríguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol Oxf Oxfs. 2005;40(1):2–14. [DOI] [PubMed] [Google Scholar]
- 6.Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiatry. 2001. Feb;178(2):101–6. [DOI] [PubMed] [Google Scholar]
- 7.Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. 2020. Jan;16(1):9–29. [DOI] [PubMed] [Google Scholar]
- 8.Veilleux A, Di Marzo V, Silvestri C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr Diab Rep. 2019. Nov 4;19(11):117. [DOI] [PubMed] [Google Scholar]
- 9.Kienzl M, Kargl J, Schicho R. The Immune Endocannabinoid System of the Tumor Microenvironment. Int J Mol Sci. 2020. Nov 25;21(23):8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stasiulewicz A, Znajdek K, Grudzień M, Pawiński T, Sulkowska JI. A Guide to Targeting the Endocannabinoid System in Drug Design. Int J Mol Sci. 2020. Apr 16;21(8):2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharkey KA, Wiley JW. The Role of the Endocannabinoid System in the Brain–Gut Axis. Gastroenterology. 2016. Aug;151(2):252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore CF, Davis CM, Harvey EL, Taffe MA, Weerts EM. Appetitive, antinociceptive, and hypothermic effects of vaped and injected Δ−9-tetrahydrocannabinol (THC) in rats: exposure and dose-effect comparisons by strain and sex. Pharmacol Biochem Behav. 2021. Mar;202:173116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore CF, Weerts EM. Cannabinoid tetrad effects of oral Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in male and female rats: sex, dose-effects and time course evaluations. Psychopharmacology (Berl). 2022. May;239(5):1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE. A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part II: Comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods. 2014. Jul 1;70(1):112–9. [DOI] [PubMed] [Google Scholar]
- 15.Wiley JL, O’connell MM, Tokarz ME, Wright MJ. Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007. Mar;320(3):1097–105. [DOI] [PubMed] [Google Scholar]
- 16.Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE. A vapourized Δ9-tetrahydrocannabinol (Δ9-THC) delivery system part I: Development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods. 2014. Jul 1;70(1):120–7. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki M, Casella GA, Ratner M. Δ9-Tetrahydrocannabinol: EEG changes, bradycardia and hypothermia in the rhesus monkey. Brain Res Bull. 1987. Aug 1;19(2):223–9. [DOI] [PubMed] [Google Scholar]
- 18.McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of Δ9-THC in rhesus monkeys. Behav Pharmacol. 2005. Sep;16(5–6):363. [DOI] [PubMed] [Google Scholar]
- 19.Castell N, Guerrero-Martin SM, Rubin LH, Shirk EN, Brockhurst JK, Lyons CE, et al. Effect of Single Housing on Innate Immune Activation in Immunodeficiency Virus–Infected Pigtail Macaques (Macaca nemestrina) as a Model of Psychosocial Stress in Acute HIV Infection. Psychosom Med. 2022. Oct;84(8):966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackie K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. In: Pertwee RG, editor. Cannabinoids [Internet]. Berlin, Heidelberg: Springer; 2005. [cited 2023 Feb 25]. p. 299–325. (Handbook of Experimental Pharmacology). Available from: 10.1007/3-540-26573-2_10 [DOI] [PubMed] [Google Scholar]
- 21.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997. Jan 1;74(2):129–80. [DOI] [PubMed] [Google Scholar]
- 22.Ellert-Miklaszewska A, Grajkowska W, Gabrusiewicz K, Kaminska B, Konarska L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007. Mar 16;1137(1):161–9. [DOI] [PubMed] [Google Scholar]
- 23.Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021. Dec;18(12):809–23. [DOI] [PubMed] [Google Scholar]
- 24.Remels AHV, Langen RCJ, Gosker HR, Russell AP, Spaapen F, Voncken JW, et al. PPARγ inhibits NF-κB-dependent transcriptional activation in skeletal muscle. Am J Physiol-Endocrinol Metab. 2009. Jul;297(1):E174–83. [DOI] [PubMed] [Google Scholar]
- 25.Rigamonti E, Chinetti-Gbaguidi G, Staels B. Regulation of Macrophage Functions by PPAR-α, PPAR-γ, and LXRs in Mice and Men. Arterioscler Thromb Vasc Biol. 2008. Jun;28(6):1050–9. [DOI] [PubMed] [Google Scholar]
- 26.Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, et al. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am J Physiol-Endocrinol Metab. 2012. Dec 15;303(12):E1469–78. [DOI] [PubMed] [Google Scholar]
- 27.Yang JW, Kim HS, Choi YW, Kim YM, Kang KW. Therapeutic application of GPR119 ligands in metabolic disorders. Diabetes Obes Metab. 2018;20(2):257–69. [DOI] [PubMed] [Google Scholar]
- 28.Therapeutic Exploitation of GPR18: Beyond the Cannabinoids? | Journal of Medicinal Chemistry [Internet]. [cited 2023 Feb 25]. Available from: 10.1021/acs.jmedchem.0c00926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007. Nov 3;362(4):928–34. [DOI] [PubMed] [Google Scholar]
- 30.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A. 2008. Feb 19;105(7):2699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leyva-Illades D, DeMorrow S. Orphan G protein receptor GPR55 as an emerging target in cancer therapy and management. Cancer Manag Res. 2013. Jul 1;5:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bevan S, Quallo T, Andersson DA. TRPV1. In: Nilius B, Flockerzi V, editors. Mammalian Transient Receptor Potential (TRP) Cation Channels: Volume I [Internet]. Berlin, Heidelberg: Springer; 2014. [cited 2023 May 3]. p. 207–45. (Handbook of Experimental Pharmacology). Available from: 10.1007/978-3-642-54215-2_9 [DOI] [Google Scholar]
- 33.Kojima I, Nagasawa M. TRPV2. In: Nilius B, Flockerzi V, editors. Mammalian Transient Receptor Potential (TRP) Cation Channels: Volume I [Internet]. Berlin, Heidelberg: Springer; 2014. [cited 2023 May 3]. p. 247–72. (Handbook of Experimental Pharmacology). Available from: 10.1007/978-3-642-54215-2_10 [DOI] [Google Scholar]
- 34.Piomelli D, Scalvini L, Fotio Y, Lodola A, Spadoni G, Tarzia G, et al. N-Acylethanolamine Acid Amidase (NAAA): Structure, Function, and Inhibition. J Med Chem. 2020. Jul 23;63(14):7475–90. [DOI] [PubMed] [Google Scholar]
- 35.Tsuboi K, Takezaki N, Ueda N. The N-Acylethanolamine-Hydrolyzing Acid Amidase (NAAA). Chem Biodivers. 2007;4(8):1914–25. [DOI] [PubMed] [Google Scholar]
- 36.Tripathi RKP. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur J Med Chem. 2020. Feb 15;188:111953. [DOI] [PubMed] [Google Scholar]
- 37.Van Egmond N, Straub VM, Van Der Stelt M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu Rev Pharmacol Toxicol. 2021. Jan 6;61(1):441–63. [DOI] [PubMed] [Google Scholar]
- 38.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996. Nov;384(6604):83–7. [DOI] [PubMed] [Google Scholar]
- 39.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998. Aug;44(3):151–62. [DOI] [PubMed] [Google Scholar]
- 40.Saini A, Patel R, Gaba S, Singh G, Gupta GD, Monga V. Adenosine receptor antagonists: Recent advances and therapeutic perspective. Eur J Med Chem. 2022. Jan 5;227:113907. [DOI] [PubMed] [Google Scholar]
- 41.Pasquini S, Contri C, Borea PA, Vincenzi F, Varani K. Adenosine and Inflammation: Here, There and Everywhere. Int J Mol Sci. 2021. Jul 19;22(14):7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018. Jul 1;98(3):1591–625. [DOI] [PubMed] [Google Scholar]
- 43.GPR110 (ADGRF1) mediates anti-inflammatory effects of N-docosahexaenoylethanolamine | Journal of Neuroinflammation | Full Text [Internet]. [cited 2023 Feb 17]. Available from: 10.1186/s12974-019-1621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang BX, Hu X, Kwon HS, Fu C, Lee JW, Southall N, et al. Synaptamide activates the adhesion GPCR GPR110 (ADGRF1) through GAIN domain binding. Commun Biol. 2020. Mar 6;3(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzo VD. Correction: Corrigendum: New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018. Sep;17(9):688–688. [DOI] [PubMed] [Google Scholar]
- 46.Fried PA, Nieman GW. Inhalation of cannabis smoke in rats. Pharmacol Biochem Behav. 1973;1(4):371–8. 47. [DOI] [PubMed] [Google Scholar]
- 47.Naef M, Russmann S, Petersen-Felix S, Brenneisen R. Development and pharmacokinetic characterization of pulmonal and intravenous delta-9-tetrahydrocannabinol (THC) in humans. J Pharm Sci. 2004. May;93(5):1176–84. [DOI] [PubMed] [Google Scholar]
- 48.Niyuhire F, Varvel SA, Martin BR, Lichtman AH. Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther. 2007. Sep;322(3):1067–75. [DOI] [PubMed] [Google Scholar]
- 49.Wilson DM, Peart J, Martin BR, Bridgen DT, Byron PR, Lichtman AH. Physiochemical and pharmacological characterization of a Delta(9)-THC aerosol generated by a metered dose inhaler. Drug Alcohol Depend. 2002. Aug 1;67(3):259–67. [DOI] [PubMed] [Google Scholar]
- 50.Austrich-Olivares A, García-Gutiérrez MS, Illescas L, Gasparyan A, Manzanares J. Cannabinoid CB1 Receptor Involvement in the Actions of CBD on Anxiety and Coping Behaviors in Mice. Pharmaceuticals. 2022. Apr;15(4):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.García-Gutiérrez MS, Navarrete F, Gasparyan A, Austrich-Olivares A, Sala F, Manzanares J. Cannabidiol: A Potential New Alternative for the Treatment of Anxiety, Depression, and Psychotic Disorders. Biomolecules. 2020. Nov;10(11):1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiavon AP, Bonato JM, Milani H, Guimarães FS, Weffort de Oliveira RM. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog Neuropsychopharmacol Biol Psychiatry. 2016. Jan 4;64:27–34. [DOI] [PubMed] [Google Scholar]
- 53.Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006. Dec 30;30(8):1466–71. [DOI] [PubMed] [Google Scholar]
- 54.Assareh N, Gururajan A, Zhou C, Luo JL, Kevin RC, Arnold JC. Cannabidiol disrupts conditioned fear expression and cannabidiolic acid reduces trauma-induced anxiety-related behaviour in mice. Behav Pharmacol. 2020. Sep;31(6):591–6. [DOI] [PubMed] [Google Scholar]
- 55.Sales AJ, Fogaça MV, Sartim AG, Pereira VS, Wegener G, Guimarães FS, et al. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol Neurobiol. 2019. Feb 1;56(2):1070–81. [DOI] [PubMed] [Google Scholar]
- 56.Zanelati T, Biojone C, Moreira F, Guimarães F, Joca S. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159(1):122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shbiro L, Hen-Shoval D, Hazut N, Rapps K, Dar S, Zalsman G, et al. Effects of cannabidiol in males and females in two different rat models of depression. Physiol Behav. 2019. Mar 15;201:59–63. [DOI] [PubMed] [Google Scholar]
- 58.Shoval G, Shbiro L, Hershkovitz L, Hazut N, Zalsman G, Mechoulam R, et al. Prohedonic Effect of Cannabidiol in a Rat Model of Depression. Neuropsychobiology. 2016;73(2):123–9. [DOI] [PubMed] [Google Scholar]
- 59.Silveira Filho NG, Tufik S. Comparative effects between cannabidiol and diazepam on neophobia, food intake and conflict behavior. Res Commun Psychol Psychiatry Behav. 1981;6:251–66. [Google Scholar]
- 60.Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl). 1990. Apr 1;100(4):558–9. [DOI] [PubMed] [Google Scholar]
- 61.Zuardi AW, Karniol IG. Effects on variable-interval performance in rats of delta 9-tetrahydrocannabinol and cannabidiol, separately and in combination. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. 1983. Jul 1;16(2):141–6. [PubMed] [Google Scholar]
- 62.Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990. Jun 1;253(3):1002–9. [PubMed] [Google Scholar]
- 63.Almeida V, Levin R, Peres FF, Niigaki ST, Calzavara MB, Zuardi AW, et al. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog Neuropsychopharmacol Biol Psychiatry. 2013. Mar 5;41:30–5. [DOI] [PubMed] [Google Scholar]
- 64.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol. 2010. Aug;13(7):861–76. [DOI] [PubMed] [Google Scholar]
- 65.ElBatsh MM, Assareh N, Marsden CA, Kendall DA. Anxiogenic-like effects of chronic cannabidiol administration in rats. Psychopharmacology (Berl). 2012. May 1;221(2):239–47. [DOI] [PubMed] [Google Scholar]
- 66.Resstel LBM, Joca SRL, Moreira FA, Corrêa FMA, Guimarães FS. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav Brain Res. 2006. Sep 25;172(2):294–8. [DOI] [PubMed] [Google Scholar]
- 67.Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017. Jan 3;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piyanova A, Lomazzo E, Bindila L, Lerner R, Albayram O, Ruhl T, et al. Age-related changes in the endocannabinoid system in the mouse hippocampus. Mech Ageing Dev. 2015. Sep;150:55–64. [DOI] [PubMed] [Google Scholar]
- 69.Nidadavolu P, Bilkei-Gorzo A, Effah F, Leidmaa E, Schürmann B, Berger M, et al. Dynamic Changes in the Endocannabinoid System during the Aging Process: Focus on the Middle-Age Crisis. Int J Mol Sci. 2022. Sep 6;23(18):10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.CNR1 cannabinoid receptor 1 [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/1268 [Google Scholar]
- 71.CNR2 cannabinoid receptor 2 [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/1269 [Google Scholar]
- 72.Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Llorente-Berzal A, et al. Species differences in cannabinoid receptor 2 ( CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes Brain Behav. 2009. Jul;8(5):519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackie K. Cannabinoid Receptors: Where They are and What They do. J Neuroendocrinol. 2008;20(s1):10–4. [DOI] [PubMed] [Google Scholar]
- 74.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2(4):236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutr J. 2014. Feb 14;13(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.PPARA peroxisome proliferator activated receptor alpha [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/5465 [Google Scholar]
- 77.Ppara peroxisome proliferator activated receptor alpha [Mus musculus (house mouse)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/19013 [Google Scholar]
- 78.PPARA peroxisome proliferator activated receptor alpha [Macaca mulatta (Rhesus monkey)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/?term=ppara+rhesus [Google Scholar]
- 79.PPARG peroxisome proliferator activated receptor gamma [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/5468 [Google Scholar]
- 80.Pparg peroxisome proliferator activated receptor gamma [Mus musculus (house mouse)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/19016 [Google Scholar]
- 81.Pparg peroxisome proliferator-activated receptor gamma [Rattus norvegicus (Norway rat)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/25664 [Google Scholar]
- 82.Janani C, Ranjitha Kumari BD. PPAR gamma gene – A review. Diabetes Metab Syndr Clin Res Rev. 2015. Jan;9(1):46–50. [DOI] [PubMed] [Google Scholar]
- 83.Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004. Jun;25(6):331–6. [DOI] [PubMed] [Google Scholar]
- 84.Zhang N, Chu ESH, Zhang J, Li X, Liang Q, Chen J, et al. Peroxisome proliferator activated receptor alpha inhibits hepatocarcinogenesis through mediating NF-κB signaling pathway. Oncotarget. 2014. Sep 30;5(18):8330–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.GPR18 G protein-coupled receptor 18 [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/2841 [Google Scholar]
- 86.GPR55 G protein-coupled receptor 55 [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/9290 [Google Scholar]
- 87.GPR119 G protein-coupled receptor 119 [Homo sapiens (human)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene/139760 [Google Scholar]
- 88.Gpr119 G-protein coupled receptor 119 [Mus musculus (house mouse)] - Gene - NCBI [Internet]. [cited 2023 Feb 25]. Available from: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=236781 [Google Scholar]
- 89.Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, et al. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006. Sep 1;347(3):827–32. [DOI] [PubMed] [Google Scholar]
- 90.Bradshaw HB, Rimmerman N, Hu SSJ, Benton VM, Stuart JM, Masuda K, et al. The endocannabinoid anandamide is a precursor for the signaling lipid N-arachidonoyl glycine by two distinct pathways. BMC Biochem. 2009. May 21;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morales P, Reggio PH. An Update on Non-CB1, Non-CB2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017. Oct 1;2(1):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Howlett AC, Blume LC, Dalton GD. CB1 Cannabinoid Receptors and their Associated Proteins. Curr Med Chem. 2010;17(14):1382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G Protein-coupled Receptor Ligand Identification Using β-Arrestin PathHunter™ Assay. J Biol Chem. 2009. May 1;284(18):12328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Henstridge CM, Balenga NAB, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J Off Publ Fed Am Soc Exp Biol. 2009. Jan;23(1):183–93. [DOI] [PubMed] [Google Scholar]
- 95.Ghislain J, Poitout V. Targeting lipid GPCRs to treat type 2 diabetes mellitus — progress and challenges. Nat Rev Endocrinol. 2021. Mar;17(3):162–75. [DOI] [PubMed] [Google Scholar]
- 96.Ye L, Cao Z, Wang W, Zhou N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr Mol Pharmacol. 2019. Aug;12(3):239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manaithiya A, Alam O, Sharma V, Javed Naim Mohd, Mittal S, Khan IA. GPR119 agonists: Novel therapeutic agents for type 2 diabetes mellitus. Bioorganic Chem. 2021. Aug 1;113:104998. [DOI] [PubMed] [Google Scholar]
- 98.Aghazadeh Tabrizi M, Baraldi PG, Baraldi S, Gessi S, Merighi S, Borea PA. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists: TRPV1 RECEPTOR ANTAGONISTS. Med Res Rev. 2017. Jul;37(4):936–83. [DOI] [PubMed] [Google Scholar]
- 99.Gorbunov AS, Maslov LN, Jaggi AS, Singh N, De Petrocellis L, Boshchenko AA, et al. Physiological and Pathological Role of TRPV1, TRPV2 and TRPV4 Channels in Heart. Curr Cardiol Rev. 2019. Sep;15(4):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller C, Morales P, Reggio PH. Cannabinoid Ligands Targeting TRP Channels. Front Mol Neurosci. 2019. Jan 15;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mangal N, Erridge S, Habib N, Sadanandam A, Reebye V, Sodergren MH. Cannabinoids in the landscape of cancer. J Cancer Res Clin Oncol. 2021;147(9):2507–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan A, Khan S, Kim YS. Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr Drug Targets. 2019. Jun 1;20(7):775–88. [DOI] [PubMed] [Google Scholar]
- 103.Russo EB. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016;1(1):154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McPartland JM, Guy GW, Di Marzo V. Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PLoS ONE. 2014. Mar 12;9(3):e89566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prömel S, Waller-Evans H, Dixon J, Zahn D, Colledge WH, Doran J, et al. Characterization and functional study of a cluster of four highly conserved orphan adhesion-GPCR in mouse. Dev Dyn. 2012;241(10):1591–602. [DOI] [PubMed] [Google Scholar]
- 106.Lum AM, Wang BB, Beck-Engeser GB, Li L, Channa N, Wabl M. Orphan receptor GPR110, an oncogene overexpressed in lung and prostate cancer. BMC Cancer. 2010. Feb 11;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdulkareem NM, Bhat R, Qin L, Vasaikar S, Gopinathan A, Mitchell T, et al. A novel role of ADGRF1 (GPR110) in promoting cellular quiescence and chemoresistance in human epidermal growth factor receptor 2-positive breast cancer. FASEB J Off Publ Fed Am Soc Exp Biol. 2021. Jul;35(7):e21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hryhorowicz S, Walczak M, Zakerska-Banaszak O, Słomski R, Skrzypczak-Zielińska M. Pharmacogenetics of Cannabinoids. Eur J Drug Metab Pharmacokinet. 2018. Feb;43(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]