Abstract
A thin layered agarose film on microscope slides provides a versatile support for the preparation of arrayed molecular libraries. An activation step leading to the formation of aldehyde groups in the agarose creates reactive sites that allow covalent immobilization of molecules containing amino groups. Arrays of oligonucleotides and PCR products were prepared by tip printing. After hybridization with complementary fluorescence labeled nucleic acid probes strong fluorescence signals of sequence-specific binding to the immobilized probes were detected. The intensity of the fluorescence signals was proportional to the relative amount of immobilized oligonucleotides and to the concentration of the fluorescence labeled probe. We also used the agarose film-coated slides for the preparation of protein arrays. In combination with specific fluorescence labeled antibodies these protein arrays can be used for fluorescence linked immune assays. With this approach different protein tests can be performed in parallel in a single reaction with minimal amounts of the binding reagents.
INTRODUCTION
In recent years, DNA arrays prepared by the immobilization of nucleic acid fragments on microscope glass slides have become one of the leading methods for the preparation of miniaturized hybridization arrays (1–9). Unfortunately, because of the planar surface, the capacity for immobilization is limited resulting in a relatively low sensitivity of the assay. In comparison, the porous structure of filter membranes allows immobilization of relatively large amounts of nucleic acids providing high sensitivity and a good dynamic range for quantitative comparison. To overcome this problem, several approaches were used to increase the amount of probes that can be bound to the glass surface. Acrylamide gel pads (10,11) or gelatine pads (12), structured by photolithography, as well as dendrimeric linker systems (13) that multiply the coupling sites by introducing additional reactive groups through branched linker molecules, are some examples of methods applied so far to enhance the performance of miniaturized glass slide-based hybridization studies. All approaches try to combine the properties of the glass support, simple handling and detection with the binding capacity of filter membranes. Most procedures require several synthesis steps and in some cases include photolithographic activation (10–13).
To combine the advantage of glass slides and porous structure, we developed a simple procedure for the preparation of glass slides coated with an activated agarose film. These agarose film-coated glass slides can be used for covalent linking of oligonucleotides, PCR products and proteins through reactive (terminal) NH2 groups. Agarose is a widely used support material in molecular biology (14) and it is well known that agarose can provide a support for hybridization reactions (15,16). The activated agarose film presented here can be prepared in any laboratory without special equipment. Moreover, the nature of the agarose film allows the use of any type of spotting technology to deposit the desired molecules. The agarose film has a low fluorescence background and can be used for hybridization with fluorescent dyes. We also demonstrate the use in fluorescence linked miniaturized immune assays with arrays of antibodies or proteins immobilized on the agarose film. This opens a wide range of applications for high-throughput studies of protein expression, antibody specificity and ligand–receptor interactions (17,18).
MATERIALS AND METHODS
Microscope glass slides were obtained from Schütt Labortechnik (Göttingen, Germany; Cat. No. 9161140). These slides were silanized to obtain reactive amino groups on the surface as described previously (19). Agarose was obtained from Gibco BRL (Life Technologies, Karlsruhe, Germany; Cat. No. 14610-08) and NaIO4 from Fluka (Seelze, Germany; Cat. No. 71859). Other chemicals and solvents were purchased from Fluka or Sigma (Deisenhofen, Germany). Fluorescein-5EX-succinimidyl-ester (Molecular Probes Europe BV, Leiden, The Netherlands; Cat. No. F-6130) was used to label antibody 5E6 (20,21).
Oligonucleotides
In experiments with linker variation we used 25mer oligonucleotides of the sequence: 3′-CTG CAT CAG ATC CAA GGG AAC GAG C-5′ that possessed one of the following 5′-end linkers: 2× C18-NH2, 1× C18-NH2, 1× C6-NH2, T15-NH2. Hybridization was performed with a reverse complementary oligonucleotide marked by Cy5 (MWG-Biotech, Ebersberg, Germany).
Match, mismatch hybridization was performed with immobilized 25mer oligonucleotides: (A) 5′-G AAG GAC TCA TGA CCA CAG TCC ATG-3′, (B) 5′-G AAG GAC TCA CAA CCA CAG TCC ATG-3′ (mismatch). The NH2 group was linked through a C-18 spacer at the 5′ end (MWG-Biotech). As a probe we used an oligonucleotide, reverse complementary to (A), labeled with fluorescein isothiocyanate (FITC) at the 5′ end.
Signal detection
In experiments with fluorescein label and SYBR-Green staining, signals were collected with a 12-bit CCD camera using an Argon-Ne laser (488 nm) for excitation and a 500 nm cut-off polarization filter for reading. In experiments with Cy5-labeled probes, slides were analyzed using a custom built confocal laser scanning device (microscope) using a red laser diode for excitation (635 nm), and an avalance photo diode for detection with polarization filter (700/75 nm) which is comparable with commercial laser scanning devices.
Preparation of the activated agarose film and immobilization of DNA or proteins
Two methods were used for the preparation of the immobilized agarose films. In the first method (I) a 1% agarose solution in purified water was prepared and poured over the surface of the glass slides at 70°C (2.0 ml per slide). After gelling of the agarose, slides were dried in air or in a dryer. The dried slides could be stored or used immediately for immobilization of oligonucleotides after activation of the agarose. For activation we used a 20 mM solution of NaIO4. Slides were submerged in a small bath containing the NaIO4 solution and incubated for 30 min at room temperature then washed in distilled water and dried. In the second method (II), NaIO4 (final concentration 10 mM) was added to the melted agarose and the agarose solution was poured on silanated slides. After gelling, slides were immersed in distilled water for 3 h and dried.
Equal volumes of oligonucleotide or PCR product dissolved in spotting buffer (0.15 M NaCl, 0.1 M NaHCO3, pH 8.5) at concentrations of 0.1 µM–0.1 mM were prepared, and volumes of 0.5–3 nl were spotted onto the activated agarose film using a pin-tool based spotting robot (GeneMachines™). PCR products were denaturated (5 min in boiling water bath and quickly cooled on ice) before spotting. After arraying of the nucleic acids, the slides were incubated overnight in a small humid box and then dried at room temperature. On the area in which the oligonucleotides were arrayed, we dropped several drops of sodium borhydride solution [50 mg NaBH4 in 30 ml phosphate buffered saline (PBS) with 10 ml ethanol]. Slides prepared according to method II were soaked in borhydride solution completely. After 5 min, the slides were washed three times in 0.2% SDS for 2 min, twice in distilled water for 1 min and dried at room temperature. At this stage slides were ready to use for hybridization or for storage in the dark at 25°C.
BAD protein or monoclonal antibodies 6A11 against BAD (20) were spotted at concentrations of 0.3–2.4 µg/µl in bicarbonate buffer (0.01 M, pH 9.6). Slides were incubated overnight at 4°C and washed with PBS with 0.05% Tween20 (PBS+T). After spotting, active aldehyde groups of the agarose were reduced by NaBH4 solution (17 mg NaBH4 in 10 ml PBS with 1 ml ethanol) for 5 min and slides were washed as above. An aliquot of 5 µl of antibody (2 µg/ml) or antigen solution (10 µg/ml) in PBS+T supplemented with 1% bovine serum albumin (BSA) was applied to the area with immobilized antigens or antibodies. Slides were incubated 1 h at room temperature, washed three times with PBS+T and finally incubated with secondary antibodies; goat anti-mouse IgG (H+L)-FITC (Dunn, Asbach, Germany; Cat. No. 1031-02) to detect 6A11 monoclonal antibodies bound to BAD or FITC-marked 5E6 monoclonal antibodies against BAD for detection of antigen (20,21). Subsequent steps were done following standard protocols for fluorescence linked immunoassays.
Hybridization
Hybridization was made in the SSC buffer system described by Schena et al. (2) with slight modifications. Slides were placed in a hybridization chamber (TeleChem, CA, USA) and 10 µl of hybridization solution (4× SSC, 0.2% SDS) were added. An aliquot of 6 µl of fluorescent probe oligonucleotide (8.5 pmol) in hybridization buffer (4× SSC, 0.2% SDS) was dropped on the area with arrayed oligonucleotides and covered with a piece of polypropylene film (10 × 10 mm). The sealed hybridization chamber was held at 55°C for 4 h. The slides were then washed by vigorous agitation in 1× SSC/0.1% SDS for 5 min at 55°C and in 0.1× SSC/0.1% SDS for 5 min at room temperature. Finally, slides were rinsed with distilled water and then dried. Fluorescence signals were collected in 0.5 M phosphate buffer, pH 7.2. The presence of immobilized DNA was also monitored by SYBR Green I (21). Quantitative estimations of the fluorescence signals were made with ArrayVission™ (Imaging Research Inc., Ontario, Canada) and KaleidaGraph™.
RESULTS
Hybridization of oligonucleotides
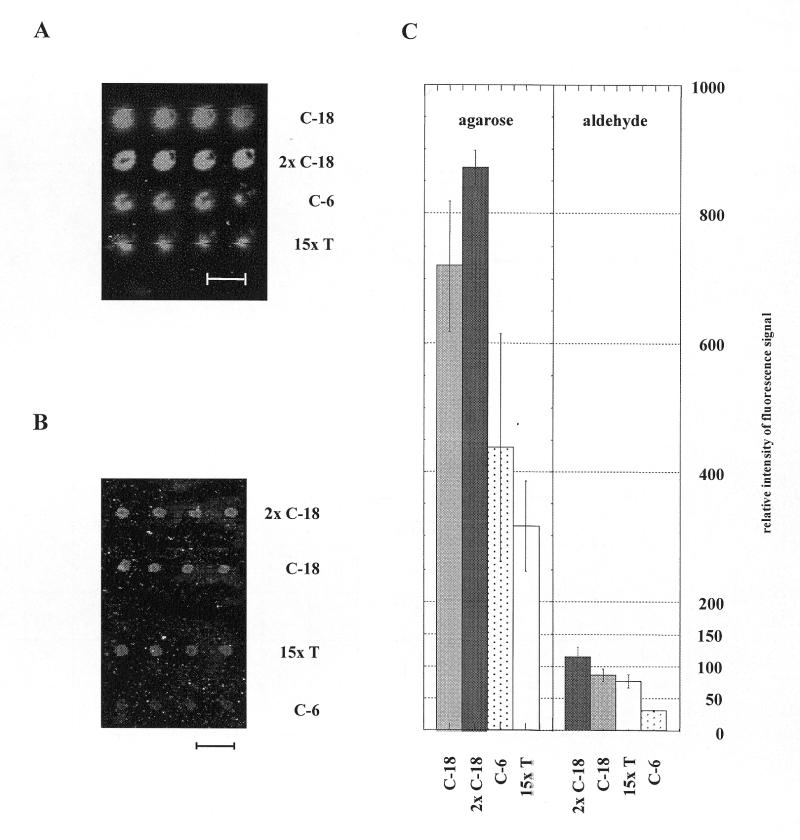
To study the efficiency of hybridization on agarose film-coated slides we immobilized oligonucleotides containing different linkers between the 5′ end of the specific sequence (n = 25) and the terminal primary amino group required for covalent attachment. The respective arrays were hybridized with a Cy5-labeled complementary oligonucleotide. Fluorescence signals measured after hybridization are shown in Figure 1 which shows images of hybridization on an agarose-coated slide (Fig. 1A) and on a silylated slide (Fig. 1B) obtained from TeleChem (www.arrayit.com ). Using identical parameters for signal detection, hybridizations performed on the agarose-coated slides showed more intense fluorescence signals than obtained from parallel experiments with silylated glass slides. This correlates with larger amounts of probe oligonucleotides immobilized in each spot, which we attribute to the three-dimensional porous structure of the agarose. In both experiments, slides were spotted with 0.1 mM oligonucleotide solutions. It can also be seen that in both cases the distance between the terminal amino group and the 5′ end of the immobilized oligonucleotide strongly influences the hybridization signal. Overall, the strength of the hybridization signal improved with longer spacer length. The intensity of fluorescence signals obtained after hybridization not only depended on the length of the spacer but also on the concentration of oligonucleotides in the spotting solution (data not shown). These effects, as well as the specificity of the hybridization signal, are crucial for use in general hybridization applications. Therefore, we assayed the extent of match and mismatch hybridization by the fluorescence signal obtained. Two oligonucleotides varying in only two nucleotides in the middle of a 25-nucleotide sequence were spotted on agarose-coated slides as well as an oligonucleotide of the same length (25 nucleotides) but with unrelated sequence. The slides were hybridized with FITC-labeled oligonucleotides complementary to one of the immobilized oligonucleotides (Fig. 2). The fluorescence signals obtained after hybridization (Fig. 2A) show a clear reduced signal with the mismatch oligonucleotide (Fig. 2A, row 2) and no signal with the oligonucleotide probe of unrelated sequence (Fig. 2A, row 3). To ensure that DNA probes were present in all three rows spotted and were not removed during hybridization, the agarose slides used were stained with SYBR Green I (Molecular Probes) following hybridization (Fig. 2B). Due to the lower fluorescence of SYBR Green I with single-stranded DNA, a low fluorescence signal is seen for the unrelated probe, which did not hybridize (Fig. 2B, row 3). The high efficiency of double-stranded DNA staining does not allow discrimination between match and mismatch hybridization after SYBR Green I staining (Fig. 2B, rows 1 and 2).
Figure 1.
Hybridization on agarose film-coated slides. (A) Image obtained after hybridization of a micro array spotted on agarose-coated slide. Oligonucleotides with identical 25-nucleotide sequence and various spacers, C-18, 2× C-18, C-6, 15× T were spotted. Array was hybridized with a complementary fluorescence (Cy5) labeled probe. (B) Image of hybridization of a micro array spotted on silylated glass slides using the same oligonucleotides for immobilization and hybridization as for the agarose-coated slides. (C) Densitometric evaluation of the signals obtained as an average from four spots. Data for (A) and (B) were obtained using the same conditions for confocal laser scanning and densitometric evaluation. The distance between the spots was 300 m and is indicated by bars.
Figure 2.

Match and mismatch discrimination. (A) A fluorescence labeled oligonucleotide was hybridized against an array containing oligonucleotides with perfect match (row 1), oligonucleotides with two internal mismatches (row 2) or oligonucleotides with an unrelated sequence (row 3). (B) Signal of SYBR Green I staining of the same array after hybridization demonstrating the presence of oligonucleotide probes in all rows. The higher fluorescence signal in the two hybridized rows is probably due to better staining of double-stranded DNA. Both signals are close to saturation. (C) Densitometric evaluation of signals as an average of five spots. The distance between the spots was 200 m.
Protein micro array
Miniaturized arrays with immobilized different protein antigens or antibodies provide the possibility of monitoring antibody response with small quantities of serum samples as well as the presence of target proteins (antigens) in serum and cell extracts. Thus, antibody and protein expression profiles can be obtained. We performed two types of experiments using activated agarose slides as models for protein (antigen/antibody) micro arrays. In one type, antigens are immobilized and in the other type antibodies are immobilized. As an example for an antigen array we spotted different concentrations of recombinant human BAD (rhBAD) protein on the activated agarose film slides which were then incubated with monoclonal antibodies against BAD (Fig. 3A). At lower concentrations of rhBAD in the spotting solution (0.34, 0.68 and 1.35 µg/µl) an increase of the fluorescence signal was observed (Fig. 3A, rows 1–3) until a saturation of the signal was reached (Fig. 3A, rows 3 and 4). The slight decrease at the highest concentration may be due to steric hindrance for antibody binding.
Figure 3.
Detection of immobilized protein with FITC-labeled antibody. (A) Protein antigens were immobilized and subsequently incubated with the labeled antibody. The fluorescence signal of the bound antibody correlates with the concentration of immobilized rhBAD protein on the agarose film-coated slide. In each spot ~0.5–2 nl of the following protein dilutions were spotted: row 1, 0.34 µg/µl; row 2, 0.68 µg/µl; row 3, 1.35 µg/µl; row 4, 2.7 µg/µl rhBAD in spotting solution. (B) Anti-BAD monoclonal antibody was spotted on agarose film. Signals were obtained by incubation with a FITC-labeled second antibody, specific for a different epitope with or without pre-incubation with rhBAD protein. (C and D) Densitometric evaluation of the fluorescence signal. The distance between the spots was 200 m.
As an example for a miniaturized sandwich immune-fluorescence assay, we immobilized monoclonal antibodies (Mab) against the human BAD protein. Then a small sample of rhBAD protein was applied to the agarose film slide and slide was incubated 1 h at room temperature. After washing by PBS-T buffer, FITC-labeled anti-BAD antibodies, specific for another epitope, were added (Fig. 3B). As a control, an identical slide was stained with the FITC-labeled anti-BAD antibody without rhBAD incubation (Fig. 3B). In the presence of rhBAD, a strong fluorescence signal was observed indicating the formation of antibody/antigen/antibody sandwich (Fig. 3B).
DISCUSSION
One of the major limitations of glass slide-based micro array technology is the immobilization capacity on the planar surface. This significantly limits the amount of probe molecules that can be immobilized. We showed that slides coated with a thin agarose film have a higher binding capacity than conventionally activated glass slides. Other methods, developed with the aim of enhancing binding capacity without changing the fluorescent properties of the glass support, are acrylamide gels (10,11) and dendrimeric branched linker systems (13). Both systems were shown to increase binding capacity but have some disadvantages, like specialized chemistry (13), or require complex preparation technology, like photolithographic masks and devices for the precise immobilization of probe material on prefabricated gel pads (11). We observed that the agarose film does not interfere with fluorescence detection and that standard hybridization protocols (2) are applicable. Our results clearly demonstrated that with the agarose film much higher fluorescence signals are obtained indicating a much more efficient loading capacity compared with silylated glass (Fig. 1). An increase in signal strength was also observed in hybridizations with larger RNA or cDNA probes (data not shown). However, the outcome of hybridizations with longer labeled fragments was less reliable on agarose-coated slides than with conventional aldehyde slides. Therefore, the range of applications in hybridization experiments should be limited to tasks where a high loading capacity is needed and shorter nucleic acids are used as probes. Our experiments also demonstrate that these agarose films are suitable for the preparation of miniaturized systems for antigen/antibody reactions. In combination with an appropriate spotting robot, such agarose film-coated slides are suitable for the fabrication of DNA chips or protein chips that can be used in a wide range of applications.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Ulf R. Rapp and Jakob Troppmair for providing rhBAD, Thomas Munder for helpful discussion and reading the manuscript, Torsten Kroll for technical help with CCD and computer equipment and Hans-Martin Striebel for exchange and discussion. This work was supported by grants from the BMBF and TMWFK and the European Commission Framework IV.
REFERENCES
- 1.. Schena M., Heller,R.A., Theriault,T.P., Konrad,K., Lachenmeier,E. and Davis,R.W. (1998) Trends Biochem. Sci., 16, 301–306. [DOI] [PubMed] [Google Scholar]
- 2.. Schena M., Shalon,D., Heller,R., Chai,A., Brown,P.O. and Davis,R.W. (1996) Proc. Natl Acad. Sci. USA, 93, 10614–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.. Lockhart D.J., Dong,H., Byrne,M.C., Follettie,M.T., Gallo,M.V., Chee,M.S., Mittmann,M., Wang,C., Kobayashi,M., Horton,H. and Brown,E.L. (1996) Nature Biotechnol., 14, 1675–1680. [DOI] [PubMed] [Google Scholar]
- 4.. DeRisi J., Penland,L., Brown,P.O., Bittner,M.L., Meltzer,P.S., Ray,M., Chen,Y., Su,Y.A. and Trent,J.M. (1996) Nature Genet., 14, 457–460. [DOI] [PubMed] [Google Scholar]
- 5.. Heller R.A., Schena,M., Chai,A., Shalon,D., Bedilion,T., Gilmore,J., Woolley,D.E. and Davis,R.W. (1997) Proc. Natl Acad. Sci. USA, 94, 2150–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.. Beier M. and Hoheisel,J.D. (1999) Nucleic Acids Res., 27, 1970–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.. Fodor S.P.A., Rava,R.P., Huang,X.C., Pease,A.C., Holmes,C.P. and Adams,C.L. (1993) Nature, 364, 555–556. [DOI] [PubMed] [Google Scholar]
- 8.. Schena M., Shalon,D., Davis,R.W. and Brown,P.O. (1995) Science, 270, 467–470. [DOI] [PubMed] [Google Scholar]
- 9.. Maskos U. and Southern,E.M. (1992) Nucleic Acids Res., 20, 1679–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.. Mirzabekov A.D. (1994) Trends Biotechnol., 12, 27–32. [DOI] [PubMed] [Google Scholar]
- 11.. Proudnikov D., Timofeev,E. and Mirzabekov,A.D. (1998) Anal. Biochem., 259, 34–41. [DOI] [PubMed] [Google Scholar]
- 12.. Ermantraut E., Wohlfart,K., Wölfl,S., Schulz,T. and Köhler,M. (1997) In Ehrfeld,W. (ed.), Microreaction Technology-Proceedings of the First International Conference on Microreaction Technology. Springer Verlag, Heidelberg, pp. 332–339.
- 13.. Hauser N.C., Vingron,M., Scheideler,M., Krems,B., Hellmuth,K., Entian,K.-D. and Hoheisel,J.D. (1998) Yeast, 14, 1209–1221. [DOI] [PubMed] [Google Scholar]
- 14.. Osterman L.A. (1985) Chromatographija belkov i nukleinovuh kislot. Moskow, Nayka, pp. 339–456.
- 15.. Lindberg U. and Persson,T. (1974) Methods Enzymol., 34, 496–499. [DOI] [PubMed] [Google Scholar]
- 16.. Purrello M. and Balazs,I. (1983) Anal. Biochem., 128, 393–397. [DOI] [PubMed] [Google Scholar]
- 17.. Lueking A., Horn,M., Eickhoff,H., Büssow,K., Lehrach,H. and Walter,G. (1999) Anal. Biochem., 270, 103–111. [DOI] [PubMed] [Google Scholar]
- 18.. Büssow K., Cahill,D., Nietfeld,W., Bancroft,D., Scherzinger,E., Lehrach,H. and Walter,G. (1998) Nucleic Acids Res., 26, 5007–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.. Robinson P.J., Dunnill,P. and Lilly,M.D. (1971) Biochim. Biophys. Acta, 242, 659–661. [DOI] [PubMed] [Google Scholar]
- 20.. Afanassiev V., Troppmair,J., Schuler,M., Weber,Ch. and Rapp,U.R. (1998) Hybridoma, 4, 383–387. [DOI] [PubMed] [Google Scholar]
- 21.. Haugland R.P. (1996) Handbook of Fluorescent Probes and Research Chemicals, Sixth Edition. Molecular Probes Inc., Eugene, OR, USA, p. 164.