Abstract
Background
Routine monitoring of gastric residual in preterm infants on gavage feeds is a common practice used to guide initiation and advancement of feeds. It is believed that an increase in or an altered gastric residual may be predictive of necrotising enterocolitis (NEC). Withholding monitoring of gastric residual may take away the early indicator and thus may increase the risk of NEC. However, routine monitoring of gastric residual as a guide, in the absence of uniform standards, may lead to unnecessary delay in initiation and advancement of feeds and hence might result in a delay in establishing full enteral feeds. This in turn may increase the duration of total parenteral nutrition (TPN) and central venous line usage, increasing the risk of associated complications. Furthermore, delays in establishing full enteral feeds increase the risk of extrauterine growth restriction and neurodevelopmental impairment.
Objectives
• To assess the efficacy and safety of routine monitoring versus no monitoring of gastric residual in preterm infants
• To assess the efficacy and safety of routine monitoring of gastric residual based on two different criteria for interrupting feeds or decreasing feed volume in preterm infants
Search methods
We conducted searches in Cochrane CENTRAL via CRS, Ovid MEDLINE, Embase and CINAHL in February 2022. We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCTs), quasi‐ and cluster‐RCTs.
Selection criteria
We selected RCTs that compared routine monitoring versus no monitoring of gastric residual and trials that used two different criteria for gastric residual to interrupt feeds in preterm infants.
Data collection and analysis
Two authors independently assessed trial eligibility, risk of bias and extracted data. We analysed treatment effects in individual trials and reported risk ratio (RR) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CI). We calculated the number needed to treat for an additional beneficial/harmful outcome (NNTB/NNTH) for dichotomous outcomes with significant results. We used GRADE to assess the certainty of evidence.
Main results
We included five studies (423 infants) in this updated review.
Routine monitoring versus no routine monitoring of gastric residual in preterm infants
Four RCTs with 336 preterm infants met the inclusion criteria for this comparison. Three studies were performed in infants with birth weight of < 1500 g, while one study included infants with birth weight between 750 g and 2000 g. The trials were unmasked but were otherwise of good methodological quality.
Routine monitoring of gastric residual:
‐ probably has little or no effect on the risk of NEC (RR 1.08, 95% CI 0.46 to 2.57; 334 participants, 4 studies; moderate‐certainty evidence);
‐ probably increases the time to establish full enteral feeds (MD 3.14 days, 95% CI 1.93 to 4.36; 334 participants, 4 studies; moderate‐certainty evidence);
‐ may increase the time to regain birth weight (MD 1.70 days, 95% CI 0.01 to 3.39; 80 participants, 1 study; low‐certainty evidence);
‐ may increase the number of infants with feed interruption episodes (RR 2.21, 95% CI 1.53 to 3.20; NNTH 3, 95% CI 2 to 5; 191 participants, 3 studies; low‐certainty evidence);
‐ probably increases the number of TPN days (MD 2.57 days, 95% CI 1.20 to 3.95; 334 participants, 4 studies; moderate‐certainty evidence);
‐ probably increases the risk of invasive infection (RR 1.50, 95% CI 1.02 to 2.19; NNTH 10, 95% CI 5 to 100; 334 participants, 4 studies; moderate‐certainty evidence);
‐ may result in little or no difference in all‐cause mortality before hospital discharge (RR 2.14, 95% CI 0.77 to 5.97; 273 participants, 3 studies; low‐certainty evidence).
Quality and volume of gastric residual compared to quality of gastric residual alone for feed interruption in preterm infants
One trial with 87 preterm infants met the inclusion criteria for this comparison. The trial included infants with 1500 g to 2000 g birth weight.
Using two different criteria of gastric residual for feed interruption:
‐ may result in little or no difference in the incidence of NEC (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence);
‐ may result in little or no difference in time to establish full enteral feeds (MD ‐0.10 days, 95% CI ‐0.91 to 0.71; 87 participants; low‐certainty evidence);
‐ may result in little or no difference in time to regain birth weight (MD 1.00 days, 95% CI ‐0.37 to 2.37; 87 participants; low‐certainty evidence);
‐ may result in little or no difference in number of TPN days (MD 0.80 days, 95% CI ‐0.78 to 2.38; 87 participants; low‐certainty evidence);
‐ may result in little or no difference in the risk of invasive infection (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence);
‐ may result in little or no difference in all‐cause mortality before hospital discharge (RR 3.21, 95% CI 0.13 to 76.67; 87 participants; low‐certainty evidence).
‐ we are uncertain about the effect of using two different criteria of gastric residual on the risk of feed interruption episodes (RR 3.21, 95% CI 0.13 to 76.67; 87 participants; very low‐certainty evidence).
Authors' conclusions
Moderate‐certainty evidence suggests routine monitoring of gastric residual has little or no effect on the incidence of NEC. Moderate‐certainty evidence suggests monitoring gastric residual probably increases the time to establish full enteral feeds, the number of TPN days and the risk of invasive infection. Low‐certainty evidence suggests monitoring gastric residual may increase the time to regain birth weight and the number of feed interruption episodes, and may have little or no effect on all‐cause mortality before hospital discharge. Further RCTs are warranted to assess the effect on long‐term growth and neurodevelopmental outcomes.
Keywords: Humans; Infant; Infant, Newborn; Birth Weight; Enterocolitis, Necrotizing; Enterocolitis, Necrotizing/epidemiology; Enterocolitis, Necrotizing/etiology; Enterocolitis, Necrotizing/prevention & control; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/etiology; Infant, Premature, Diseases/prevention & control; Infections
Plain language summary
Does routine monitoring of stomach aspirates (partially digested milk and gut hormones withdrawn from the feeding tube) avoid necrotising enterocolitis in premature babies?
Key messages
Necrotising enterocolitis is a serious intestinal disease in premature babies that causes damage and death of gut tissue and may result in a hole in the intestine.
• Routine monitoring of stomach aspirates to decide on feeding in premature babies probably has little or no effect on the risk of necrotising enterocolitis.
• Monitoring stomach aspirates probably increases the time taken to reach full feeds, duration of parenteral nutrition (feeding through a vein) and the risk of infections. It may increase the time taken to regain birth weight and feed interruption episodes (time frames when feeds are stopped temporarily) in premature babies. The effect of stomach aspirates monitoring on other important outcomes is uncertain.
• There is uncertainty whether using two different criteria of stomach aspirates to interrupt feeds has an effect on important outcomes in preterm infants.
Background
Monitoring of stomach aspirates is performed by withdrawing the stomach contents via the feeding tube and assessing these contents for quantity and quality at regular intervals. Monitoring of stomach aspirates to diagnose feed intolerance and necrotising enterocolitis is a common practice in premature babies who are on tube feeds. There is inadequate evidence to support routine monitoring of stomach aspirates as a guide for when to start or increase feeds in otherwise healthy premature babies. However, not monitoring stomach aspirates may take away an early warning sign for necrotising enterocolitis and thus may increase its risk in premature infants.
What did we want to find out?
We wanted to look for evidence from studies that assessed whether routine monitoring of stomach aspirates is beneficial or harmful in premature babies.
What did we do?
We searched for studies that looked at monitoring of stomach contents in premature babies. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as the size of the study and the methods used.
What did we find?
We included five studies (423 babies) in this review.
We found four studies on 336 premature babies that compared routine monitoring versus no monitoring of stomach aspirates in premature babies. We found one study comparing the usage of two different sets of criteria based on quantity and quality of stomach aspirates to decide on interrupting feeds while monitoring stomach aspirates.
What are the limitations of the evidence?
We are moderately confident about the evidence on the effect of monitoring stomach aspirates on outcomes such as necrotising enterocolitis, risk of infections, time taken to reach full feeds, and duration of parenteral nutrition.
How up‐to‐date is this evidence?
The search is up‐to‐date as of February 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ routine monitoring vs. no routine monitoring of gastric residual in preterm infants.
| Patient or population: preterm infants Setting: neonatal intensive care unit Intervention: routine monitoring Comparison: no routine monitoring of gastric residual | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no routine monitoring of gastric residual | Risk with routine monitoring | |||||
| Risk of necrotising enterocolitis stage ≥ 2 | 48 per 1000 | 52 per 1000 (22 to 124) | RR 1.08 (0.46 to 2.57) | 334 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Time to establish full enteral feeds (days) | The mean time to establish full enteral feeds (days) was 0 | MD 3.14 higher (1.93 higher to 4.36 higher) | ‐ | 334 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Time to regain birth weight (days) | The mean time to regain birth weight (days) was 0 | MD 1.7 higher (0.01 higher to 3.39 higher) | ‐ | 80 (1 RCT) | ⊕⊕⊝⊝ Lowc | |
| Number of infants with feed interruption episodes (lasting ≥ 12 hours) | 258 per 1000 | 570 per 1000 (394 to 825) | RR 2.21 (1.53 to 3.20) | 191 (3 RCTs) | ⊕⊕⊝⊝ Lowd | |
| Number of total parenteral nutrition days | The mean number of total parenteral nutrition days was 0 | MD 2.57 higher (1.2 higher to 3.95 higher) | ‐ | 334 (4 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Risk of invasive infection | 199 per 1000 | 298 per 1000 (203 to 435) | RR 1.50 (1.02 to 2.19) | 334 (4 RCTs) | ⊕⊕⊕⊝ Moderatee | |
| All‐cause mortality before hospital discharge | 37 per 1000 | 79 per 1000 (29 to 221) | RR 2.14 (0.77 to 5.97) | 273 (3 RCTs) | ⊕⊕⊝⊝ Lowf | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_433148032174868747. | ||||||
a Downgraded by one level for serious imprecision due to wide confidence interval and sample size not meeting the optimal information size criterion b Downgraded by one level for serious imprecision due to lower confidence interval crossing the threshold of clinically meaningful difference c Downgraded by two levels for very serious imprecision due to small sample size not reaching the 'Optimal information size' criteria and confidence interval reaching the line of no difference d Downgraded by two levels for very serious risk of bias due to high risk of bias in all the three studies e Downgraded by one level for serious imprecision due to the lower confidence interval reaching the line of no difference f Downgraded by two levels for very serious imprecision due to small sample size not reaching the optimal information size criteria, and confidence interval crossing the line of no difference
Summary of findings 2. Summary of findings table ‐ Quality and volume of gastric residual compared to quality of gastric residual alone for feed interruption in preterm infants.
| Quality and volume of gastric residual compared to quality of gastric residual alone for feed interruption in preterm infants | ||||||
| Patient or population: feed interruption while monitoring gastric residual Setting: neonatal intensive care unit Intervention: quality + volume of gastric residual Comparison: quality of gastric residual alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with quality of gastric residual alone | Risk with quality + volume of gastric residual | |||||
| Risk of necrotising enterocolitis stage ≥ 2 | 0 per 1000 | 0 per 1000 (0 to 0) | RR 5.35 (0.26 to 108.27) | 87 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Time to establish full enteral feeds | The mean time to establish full enteral feeds was 0 | MD 0.1 lower (0.91 lower to 0.71 higher) | ‐ | 87 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Time to regain birth weight (days) | The mean time to regain birth weight (days) was 0 | MD 1 higher (0.37 lower to 2.37 higher) | ‐ | 87 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Number of infants with feed interruption episodes (lasting ≥ 12 hours) | 0 per 1000 | 0 per 1000 (0 to 0) | RR 3.21 (0.13 to 76.67) | 87 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Number of total parenteral nutrition days | The mean number of total parenteral nutrition days was 0 | MD 0.8 higher (0.78 lower to 2.38 higher) | ‐ | 87 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| Risk of invasive Infection | 0 per 1000 | 0 per 1000 (0 to 0) | RR 5.35 (0.26 to 108.27) | 87 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| All‐cause mortality before hospital discharge | 0 per 1000 | 0 per 1000 (0 to 0) | RR 3.21 (0.13 to 76.67) | 87 (1 RCT) | ⊕⊕⊝⊝ Lowa | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_439094110359236378. | ||||||
a Downgraded by two levels for very serious imprecision due to small sample size not reaching the 'Optimal information size' criteria and confidence interval crossing the line of no difference b Downgraded by one level for serious risk of bias due to 'some concerns' in the only included trial
Background
Description of the condition
Providing adequate nutrition is one of the key components of preterm neonatal care. There is increasing emphasis on early initiation and appropriate advancement of enteral feeds with an aim of achieving full‐volume enteral feeds at the earliest opportunity (Dutta 2015; Stevens 2016). Major hindrances to advancing feed volumes in preterm infants may include feed intolerance and the risk of necrotising enterocolitis (NEC).
Feed intolerance is a common problem in preterm infants and is related to structural and functional immaturity of the gut of these infants. The preterm gut has decreased length, immature motility patterns, and inadequate digestive and absorptive capacity compared to the gut of term infants (Lucchini 2011). Feed intolerance causes frequent interruption and delayed advancement of enteral feeds, resulting in protracted use of total parenteral nutrition (TPN) (providing nutrition through a vein) and central venous lines (CVLs), thus possibly increasing their complication rates (Duro 2011; Hermansen 2005; Kaur 2015). Delay in establishing full enteral feeds is a significant contributor to growth failure in preterm infants, resulting in neurodevelopmental impairment and long‐term metabolic complications (Embleton 2013; Franz 2009; Stevens 2016).
Description of the intervention
Feed intolerance is variously defined by signs such as increased volume of gastric residual, altered gastric residual (bilious‐ or blood‐stained), abdominal distension, or vomiting, or both (Moore 2011). The use of gastric residual as an indicator of feed intolerance is controversial (Li 2014; Parker 2015).
Gastric residual is a measure of gastric contents withdrawn from the feeding tube, which includes milk along with gastrointestinal secretions remaining in the stomach after a certain time interval after feeding (most often assessed before the next feed). Increased gastric residual is common in preterm infants due to intrinsic factors such as the inherent immaturity of the gastrointestinal system in the form of delayed gastric emptying, slower intestinal transit, inadequate secretion of gut hormones and enzymes, and possibly due to increased propensity for duodenogastric reflux (Ittmann 1992; Riezzo 2000). Some extrinsic factors, such as use of formula feeds; drugs, such as theophyllines, mydriatics, and opioids; body positioning and the sickness of the infant, may delay gastric emptying and hence contribute to altered or increased volume of gastric residual (Cohen 2004; Li 2014; Malhotra 1992).
Routine monitoring of gastric residual (for assessing the volume or colour, or both) in preterm infants on gavage feeds (tube feeds) is a common practice in many neonatal intensive care units (NICUs) and is used to guide the advancement of gavage feeds (Dorling 2020; Gregory 2012; Perumbil Pathrose 2021; Xu 2019). An increase in or altered gastric residual is putatively considered a sign of feed intolerance or an early sign of NEC (Li 2014). An abnormal gastric residual becomes important when accompanied by other signs such as bilious vomiting, decreased bowel sounds, abdominal distension, abdominal wall erythema (redness of the skin), gross or occult blood in the stool, apnoea, bradycardia, and temperature instability. The significance of increased or altered gastric residual as an isolated finding is uncertain.
The volume or colour, or both, of the gastric residual that definitively indicates feed intolerance, or which is predictive of NEC, is unclear (Bertino 2009; Cobb 2004; Dutta 2015; Gephart 2017; Kenton 2004; Malhotra 1992; Parker 2015). As a consequence, there is wide variation in practice related to this aspect across NICUs (Perumbil Pathrose 2021; Xu 2019). The various cut‐offs used to define significant volume of gastric residual are ≥ 2 mL/kg of the infant’s weight, > 2 mL or 3 mL depending on the infant’s weight, > 30% of the previous feed volume, and > 50% of the cumulative feed volume given during the time interval (Grino 2016; Kaur 2015; Mihatsch 2002; Torrazza 2015). Similarly, there is no standard recommendation for the frequency of assessment of gastric residual.
An increase in abdominal girth is the other commonly used sign of feed intolerance. An increase in abdominal girth of 2 cm or more is considered significant (Kaur 2015; Lucchini 2011; Malhotra 1992). However, measurement of abdominal girth is highly prone to interobserver and intraobserver variability. The evidence to indicate that abdominal girth is a reliable measure of feed tolerance is uncertain (Dutta 2015).
How the intervention might work
Some literature suggests that an increase in, or an altered gastric residual, may be predictive of NEC (Bertino 2009; Cobb 2004; Grino 2016). Withholding monitoring of gastric residual may take away the early indicator and thus may increase the risk of NEC and its associated complications, including mortality. Also, not aspirating at regular intervals may lead to an accumulation of gastric residual in the stomach which will cause gastric distension, and increase the risk of gastro‐oesophageal reflux and aspiration pneumonia.
The practice of routine gastric residual monitoring as a guide in the absence of uniform standards on its usefulness may lead to unnecessary delay in initiation and advancement of feeds or interruption of feeds in preterm infants (Kaur 2015; Shulman 2011). This may result in a delay in reaching full enteral feeds, which in turn may increase the duration of TPN and the risk of parenteral nutrition‐associated liver disease (Duro 2011; Kaur 2015). It may also increase the number of days of CVL usage, thus increasing the risk of late‐onset sepsis and other CVL‐related complications (Hermansen 2005). Delay in achieving full enteral feeds also increases the risk of extrauterine growth restriction and neurodevelopmental impairment (Franz 2009; Leppänen 2014; Morris 1999). The negative pressure created by repeated aspirations, especially when the tip of the nasogastric (NG)/orogastric (OG) tube remains in close contact with the gastric mucosa, has the potential to damage the gastric mucosa (Li 2014). Moreover, the volume of aspirated gastric residual may not be a reliable and accurate measure of residual gastric content, and it varies with the infant's position, size of the nasogastric tube, aspiration technique, and viscosity of feeds (Bartlett 2015; Gozen 2021; Parker 2015).
Uncertainty also exists as to whether to discard or re‐feed (giving again) the aspirated gastric residual (Athalye‐Jape 2020; Dutta 2015; Juvé‐Udina 2009; Williams 2010). This question is addressed in another Cochrane Review (Abiramalatha 2023). The gastric residual contains milk, gastrointestinal enzymes, and hormones that aid in digestion, gastrointestinal motility and maturation (Athalye‐Jape 2020). Hence, discarding this may have a negative influence on the infant's gastrointestinal system.
Why it is important to do this review
Given the potential role of gastric residual monitoring as an early indicator of NEC, as well as the possible risks of its routine monitoring, we undertook a systematic review to identify and appraise data from randomised controlled trials (RCTs) to provide a synthesis of evidence to inform practice and research.
Objectives
To assess the efficacy and safety of routine monitoring versus no monitoring of gastric residual in preterm infants
To assess the efficacy and safety of routine monitoring of gastric residual based on two different criteria for interrupting feeds or decreasing feed volume in preterm infants
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs in the review. We did not include cross‐over trials due to the risk of carry‐over effects.
Types of participants
We included preterm infants (born at < 37 weeks' gestation) who did not have any overt signs of feed intolerance/NEC such as bilious vomiting, decreased bowel sounds, abdominal distension, abdominal wall erythema, gross or occult blood in the stool, apnoea, bradycardia, or temperature instability.
The infant should be on tube feeds. Randomisation should have been done at the time of initiation of enteral feeds. Babies on respiratory support were also eligible.
Types of interventions
Comparison 1
Intervention
Routine monitoring of gastric residual to decide on continuation and advancement of enteral feeds in infants who did not have any sign of feed intolerance/NEC. Gastric residual monitoring could be done at any time interval (e.g. before every feed, before every third feed, etc) at the investigator's discretion.
Note: the investigator could have used predefined criteria for the quantity and quality of gastric residual to decide on feed interruption or to decrease the feed volume.
Control
No monitoring of gastric residual in otherwise healthy infants until any sign of feed intolerance/NEC appeared. The control group could receive no monitoring for any sign of feed intolerance or routine monitoring of other signs of feed intolerance such as an increase in abdominal girth.
Comparison 2
Monitoring of gastric residual was performed in both intervention and control groups, and the decision on feeding (advancement/continuation/decrease/interruption) was based on two different predefined criteria of gastric residual. The criteria for gastric residual could be based on its quality or quantity, or both.
Types of outcome measures
Primary outcomes
Risk of NEC stage ≥ 2 (modified Bell’s staging; Walsh 1986)
Time to establish full enteral feeds ≥ 150 mL/kg/day(d)
Secondary outcomes
Risk of surgical NEC
Time to regain birth weight (days) and subsequent rate of weight gain (g/kg/d), linear growth (cm/week), and increase in head circumference (cm/week) during the initial hospitalisation period
Risk of extrauterine growth restriction at discharge (number of infants who remain below the 10th percentile for the index population for weight, length, and head circumference)
Number of infants with feed interruption episodes (lasting ≥ 12 hours)
Number of TPN days
Risk of parenteral nutrition‐associated liver disease
Number of days of CVL usage
Risk of invasive infection as determined by culture of bacteria or fungus from blood, cerebrospinal fluid, or urine, or from a normally sterile body space
Risk of spontaneous intestinal perforation
Risk of aspiration pneumonia or pneumonitis (clinical or radiological evidence of lower respiratory tract compromise that has been attributed to covert or evident aspiration of gastric contents)
Risk of gastro‐oesophageal reflux diagnosed by clinical features such as post‐feed apnoea (cessation of breathing), desaturation (reduced blood oxygen levels), irritability, vomiting; or oesophageal pH monitoring, or endoscopy
All‐cause mortality before hospital discharge or up to 44 weeks postmenstrual age
Duration of hospital stay (days)
Growth measures following discharge from hospital to latest follow‐up (weight, length, and head circumference)
Neurodevelopmental outcomes assessed after 12 months corrected age: neurological evaluations; developmental scores; and classifications of disability, including auditory and visual disability. We will define neurodevelopmental impairment as the presence of one or more of the following: non‐ambulant cerebral palsy; developmental quotient more than two standard deviations below the population mean; blindness (visual acuity < 6/60) or deafness (any hearing impairment requiring or unimproved by amplification)
Search methods for identification of studies
Electronic searches
The Cochrane Neonatal Information Specialist developed search strategies in consultation with the authors. The MEDLINE strategy was translated, using appropriate syntax, for other databases. Topic terms were combined with terms for the neonatal population and methodological search filters for RCTS and systematic reviews. We searched the following databases without restrictions on date, language or publication type in February 2022:
Cochrane Central Register of Controlled Trials (CENTRAL; 2022 Issue 2) via CRS;
Ovid MEDLINE (R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) 1946 to 23 February 2022;
Ovid Embase 1974 to 23 February 2022;
CINAHL (Cumulative Index to Nursing and Allied Health Literature); 1982 to 24 February 2022.
Search strategies are available: Appendix 1; Appendix 2; Appendix 3; Appendix 4.
We identified trial registration records by using CENTRAL and by independent searches of the following:
ISRCTN registry (https://www.isrctn.com);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (ICTRP) (https://trialsearch.who.int/Default.aspx).
Search strategies are available: Appendix 5.
Searching other resources
We also searched the reference lists of any articles selected for inclusion in this review to identify additional relevant articles. We searched the proceedings of the annual meetings of the
Pediatric Academic Societies (1993 to 2022);
European Society for Paediatric Research (1995 to 2022); and
Perinatal Society of Australia and New Zealand (2000 to 2022).
Trials reported only as abstracts were eligible if sufficient information was available from the report, or by contacting the trial authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of Cochrane Neonatal and Cochrane (Higgins 2022a).
Selection of studies
Two review authors (TA and BR) screened the title and abstract of all studies identified by the above search strategy and independently assessed the full‐text articles for all potentially relevant trials. We excluded those studies that did not meet all of the inclusion criteria, and we stated the reasons for exclusion. We discussed any disagreements until consensus was achieved.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1; Moher 2009), and the Characteristics of excluded studies table.
1.
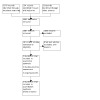
Data extraction and management
Three review authors (TA, ST and SR in pairs of two) extracted data independently using a data collection form to aid extraction of information on the design, methodology, participants, interventions, outcomes, and treatment effects from each included study. We discussed disagreements until we reached a consensus. If data from the trial reports were insufficient, we contacted the trial authors for further information.
Assessment of risk of bias in included studies
Two review authors (TA and VVR) assessed the risk of bias for all included trials using version 2.0 of the Cochrane risk of bias tool (RoB 2) (Higgins 2022b). We assessed risk of bias for the seven priority outcomes listed in Table 1 and Table 2. The effect of interest was intention‐to‐treat or modified intention‐to‐treat analysis. We resolved disagreements by discussion until we reached a consensus.
We assessed the risk of bias for each study outcome using the following RoB 2 criteria:
bias arising from the randomisation process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
For each domain, a series of signalling questions with answers (yes, probably yes, no information, probably no, or no) determine the risk of bias (low risk, some concerns, or high risk). We included relevant text alongside the judgements to provide supporting information for our decisions. We decided the overall risk of bias for an outcome by its performance in all the domains: the overall judgement was 'some concerns' if we assigned a judgement of 'some concerns' for one domain, and 'high risk' if we assigned a judgement of 'some concerns' for multiple domains or 'high risk' for one (or more) domains.
If we include cluster‐RCTs in future, we plan to assess the risk of bias for cluster‐RCTs using the RoB 2 tool with the additional domain 'Bias arising from the timing of identification and recruitment of participants'. We will give additional consideration to the recruitment bias that is unique to cluster‐RCTs.
Measures of treatment effect
We analysed treatment effects in the individual trials using RevMan Web 2022 and reported risk ratio (RR) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CI). We determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for outcomes with statistically significant differences.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials.
We did not identify any cluster RCTs for inclusion in our review. If we identify cluster RCTs in future, we would include and analyse them as long as the trial authors undertook proper adjustment for the intra‐cluster correlation, as described in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022c). In circumstances where trial authors did not adjust appropriately, we would attempt to correct this.
If we identify, in future, any trial that has multiple arms that are compared against the same control condition that will be included in the same meta‐analysis, we will either combine groups to create a single pair‐wise comparison, select one pair of interventions and exclude the others.
Dealing with missing data
We requested additional data from the trial authors if data on important outcomes were missing or were reported unclearly.
Assessment of heterogeneity
We examined treatment effects of individual trials and between‐study heterogeneity by inspecting the forest plots. We calculated the I² statistic for each effect estimate to quantify inconsistency across studies and described the percentage of variability in effect estimates that might be due to heterogeneity rather than sampling error. We classified heterogeneity as follows:
0% to 40% might not be important;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity, and
75% to 100% indicated considerable heterogeneity.
We planned to explore possible causes if we detected substantial or considerable heterogeneity (I² ≥ 60%). However, we did not find substantial or considerable heterogeneity in any analysis.
Assessment of reporting biases
If 10 or more studies were included in a meta‐analysis, we planned to use a funnel plot to detect possible publication bias (Egger 1997). Only four trials, however, were included in the meta‐analysis.
Data synthesis
We analysed all infants randomised on an intention‐to‐treat basis and treatment effects in the individual trials using a fixed‐effect model to combine data. For meta‐analyses of categorical outcomes, we calculated typical estimates of RR, each with 95% CI; for continuous outcomes, we calculated the mean difference (MD). We determined the NNTB or NNTH for analyses with statistically significant differences.
The primary analysis for each outcome included all eligible trials. We planned to conduct sensitivity analyses by excluding studies with high risk of bias.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analysis:
based on gestational age: ≤ 27 weeks, 28 weeks to 31 weeks, ≥ 32 weeks;
based on birth weight: < 1000 g, 1000 g to 1499 g, ≥ 1500 g;
small for gestational age versus appropriate for gestational age infants (classified using birth weight relative to the reference population);
type of feed the infant was receiving (human milk or formula); and
frequency of monitoring of gastric residual (before every feed, before every third feed, etc).
Sensitivity analysis
We planned to undertake sensitivity analyses to determine if the findings were affected by including only studies where the methodology was adequate (i.e. 'low risk' or 'some concerns' in RoB 2 assessments of bias). However, we did not conduct any sensitivity analysis, as it was not required.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of the evidence for the following outcomes.
Risk of NEC
Time to establish full enteral feeds
Time to regain birth weight
Number of infants with feed interruption episodes (lasting ≥ 12 hours)
Number of TPN days
Risk of invasive infection
All‐cause mortality before hospital discharge
Two review authors (TA and VVR) assessed the certainty of the evidence for all the outcomes independently. We considered evidence from RCTs as high‐certainty but downgraded the evidence by one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), inconsistency across studies, indirectness of the evidence, imprecision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development tool to create two summary of findings tables (Table 1; Table 2), to report the certainty of the evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies and Characteristics of studies awaiting classification.
We included a total of five trials (423 infants) (Kaur 2015; Parker 2019; Singh 2018; Thomas 2018; Torrazza 2015).
Four trials that evaluated routine monitoring versus no monitoring of gastric residual were included in comparison 1 (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015). Three of these trials were performed in infants with birth weight < 1500 g (Kaur 2015; Parker 2019; Torrazza 2015), while one trial included neonates with birth weight between 750 g and 2000 g (Thomas 2018). In the monitoring group, gastric residual was assessed before each feed in all the studies). In the no monitoring group, routine abdominal girth monitoring was performed in three studies (Kaur 2015; Parker 2019; Thomas 2018), while Torrazza 2015 did not perform proactive monitoring for any sign of feed intolerance in the control group.
In Singh 2018, routine monitoring of gastric residual was performed in both groups and two different criteria of gastric residual for interrupting feeds were used in the two groups. This trial was included in comparison 2. The trial was performed in infants with birth weight ≥ 1500 g.
Results of the search
Database searches identified 3576 references, trial registries 154 records, and 22 records were identified from other sources. After removing 2065 duplicates, 1687 records were screened. We excluded 1668 records during title/abstract screening; assessed 19 full texts or trial registry records. We included 5 studies (2 new); excluded 9 studies; classified 2 as awaiting assessment; and identified 3 ongoing studies. For details see Figure 1.
Included studies
Comparison 1. Routine monitoring of gastric residual versus no monitoring of gastric residual in preterm infants
Kaur 2015 randomised 80 infants with birth weight < 1500 g to the gastric residual monitoring or the abdominal girth monitoring group at the time of initiation of enteral feeds. In the gastric residual group, gastric residuals were measured before each feed. In the abdominal girth group, abdominal girth measurements were performed before each feed. The primary outcome was time taken to achieve full feeds (180 mL/kg/d) that were tolerated for at least 24 hours.
Parker 2019 randomised infants who were born at ≤ 32 weeks' gestation with a birthweight of ≤ 1250 g and were receiving some feeds by 72 hours after birth. In the gastric residual monitoring group, gastric residuals were monitored before each feed. In the no gastric residual monitoring group, gastric residuals were not monitored and infants were assessed for other signs of NEC or feed intolerance, including abdominal girth monitoring. Only human milk (preferably the mother's own milk or donor human milk) was used for feeding. The primary outcome was weekly enteral nutrition measured in mL/kg for six weeks after birth.
Thomas 2018 included infants who were born at 26 to 36 weeks' gestation and 750 g to 2000 g birth weight and were likely to require gavage feeds for at least 48 hours. In the gastric residual monitoring group, gastric residuals were monitored before each feed. While in the abdominal girth monitoring group, abdominal girth was monitored before each feed. Only human milk (the mother's own or donor human milk) was used for feeding. The primary outcome was time to reach full feeds (150 mL/kg/d) that were tolerated for at least 24 hours.
Torrazza 2015 recruited 61 infants born at 23 to 31 weeks' gestational age with ≤ 1250 g birth weight and were receiving some enteral nutrition by 48 hours of age. These infants were randomised to routine monitoring of gastric residuals before every feed or no monitoring of gastric residuals. Both human milk and preterm formula were used for feeding. Primary outcomes were enteral intake at two weeks and days to reach 120 mL/kg/d of enteral feedings.
Comparison 2. Using two different criteria of gastric residual for feed interruption while monitoring gastric residual in preterm infants
Singh 2018 recruited 87 infants with birth weight 1500 g to 2000 g and postnatal age < 48 hours requiring gavage feeds. Routine assessment of gastric residual was done in both groups. In the intervention group, only the quality of gastric residual was assessed; the volume of gastric residual was not assessed. In the control group, both volume and quality of gastric residual were assessed. The primary outcome was time to reach full enteral feeding ≥ 120 mL/kg/d.
Ongoing studies
We found three ongoing studies (ISRCTN98322846; NCT04062851; NCT04064398). Please refer to Characteristics of ongoing studies for details.
Studies awaiting classification
We have placed two studies in the Studies awaiting classification section as we were not able to obtain the full published articles for these studies (Lenfestey 2018; NCT03111329).
Excluded studies
We excluded nine studies in total (see Characteristics of excluded studies). Four were case‐control studies (Bertino 2009; Cobb 2004; Purohit 2022; Riskin 2017). Bertino 2009, Cobb 2004 and Purohit 2022, matched infants with NEC with control infants and studied the role of gastric residuals in early identification of NEC. Riskin 2017 evaluated the time to full enteral feeding and the incidence of NEC in preterm infants after a practice change from routine evaluation of gastric residual volume before each feed to selective evaluation of gastric residual volume. Elia 2022 and Staub 2019 were also before‐and‐after comparison studies evaluating a protocol change from routine monitoring to selective monitoring of gastric residual in preterm neonates. Dubey 2018 was a cohort study, performed in two centres with gastric residual monitoring in one and abdominal girth monitoring in the other centre.
Two were observational studies (Malhotra 1992; Mihatsch 2002). Malhotra 1992 studied the volume of gastric residual in healthy preterm infants prospectively and analysed the various factors influencing gastric residual such as postnatal age, position of the baby, type of milk, and small for gestational age. Mihatsch 2002 evaluated whether the volume of gastric residual and bilious gastric residual was a significant predictor of feeding intolerance in extremely low birth weight infants.
Risk of bias in included studies
A computer‐generated block randomisation sequence with a block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data and this randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. Since the risk is small, we assigned a judgement of 'low risk' to the domain 'bias arising from the randomisation process'.
As there were no data to assess if deviations arose due to the trial context, we assigned 'some concerns' for the domain 'deviations from intended interventions'.
As all 80 randomised infants were included in the analysis, we assigned 'low risk' for the domain 'missing outcome data'.
Masking was not done. Hence, for the domain 'measurement of outcome', we assigned 'low risk' for objective outcomes that are less prone to detection bias and where ascertainment of the outcome could not have differed between the two groups. We assigned the judgement 'some concerns' for the subjective outcome 'feed interruption episodes'. Clinicians' assessment of feed intolerance and the decision to withhold feeds are subjective; hence there is always a risk of surveillance and ascertainment bias in an unmasked trial.
All proposed outcomes were reported, and the reported results for outcome measurement corresponded to intended analyses (personal communication). We, therefore, assigned 'low risk' for the domain 'selection of the reported result'.
The overall risk of bias for the trial was 'some concerns' for objective outcomes and 'high risk' for feed interruption episodes.
Infants were randomised using a computer‐generated sequence with random‐length permuted blocks of sizes (4, 6, or 8) and randomisation was concealed until the intervention was assigned. We, therefore, assigned 'low risk' for the domain 'bias arising from the randomisation process'.
Eighteen (26%) infants in the "no gastric residual" group had one or more gastric residuals evaluated. However, no infant had gastric residuals evaluated for more than one day and hence this deviation was considered unlikely to have affected the outcome. Modified intention‐to‐treat analysis was performed. We, therefore, assigned 'low risk' for the domain 'deviations from intended interventions'.
As all the randomised neonates were accounted for, we assigned 'low risk' for the domain 'missing outcome data'.
Though masking was not done, all reported outcomes were objective and less prone to detection bias. We, therefore, assigned 'low risk' for the domain 'measurement of outcome'
The study protocol had been published. All proposed outcomes were reported and the reported results for outcome measurement corresponded to intended analyses. We, therefore assigned 'low risk' for the domain 'selection of the reported result'.
The overall risk of bias for the trial was 'low risk' for all reported outcomes.
Randomisation was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was performed using sequentially numbered opaque sealed envelopes. We, therefore, assigned 'low risk' for the domain 'bias arising from the randomisation process'.
As there were no deviations from the intended intervention and modified intention‐to‐treat analysis was performed, we assigned 'low risk' for the domain 'deviations from intended interventions'.
As all the randomised neonates were accounted for, we assigned 'low risk' for the domain 'missing outcome data'.
As it was an unmasked trial, for the domain 'measurement of outcome', we assigned 'low risk' for objective outcomes and 'some concerns' for the subjective outcome 'feed interruption episodes' (for reasons discussed above).
As the study protocol had not been published, we assigned 'some concerns' for the domain 'selection of the reported result'.
The overall risk of bias for the trial was 'some concerns' for objective outcomes and 'high risk' for feed interruption episodes.
A computer‐generated block randomisation sequence with variable block sizes was used. The randomisation sequence was kept in sequentially numbered sealed opaque envelopes (personal communication). We, therefore, assigned 'low risk' for the domain 'bias arising from the randomisation process'.
As there were no data to assess if deviations arose due to the trial context, we assigned 'some concerns' for the domain 'deviations from intended interventions'.
As all 61 randomised infants were included in the analysis, we assigned 'low risk' for the domain 'missing outcome data'.
As it was an unmasked trial, for the domain 'measurement of outcome', we assigned 'low risk' for objective outcomes and 'some concerns' for the subjective outcome 'feed interruption episodes' (for reasons discussed above).
All proposed outcomes were reported, and the reported results for outcome measurement corresponded to intended analyses. We, therefore, assigned 'low risk' for the domain 'selection of the reported result'.
The overall risk of bias for the trial was 'some concerns' for objective outcomes and 'high risk' for feed interruption episodes.
The randomisation sequence was computer‐generated and permuted, even‐numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. We, therefore assigned 'low risk' for the domain 'bias arising from the randomisation process'.
As there were no deviations from the intended interventions and modified intention‐to‐treat analysis was performed, we assigned 'low risk' for the domain 'deviations from intended interventions'.
As all 87 randomised infants were included in the analysis, we assigned 'low risk' for the domain 'missing outcome data'.
As masking was not done, for the domain 'measurement of outcome', we assigned 'low risk' for objective outcomes and 'some concerns' for the subjective outcome 'feed interruption episodes' (for reasons discussed above).
The study protocol had been published. All proposed outcomes were reported and the reported results for outcome measurement corresponded to intended analyses. We, therefore, assigned 'low risk' for the domain 'selection of the reported result'.
The overall risk of bias for the trial was 'low risk' for all objective outcomes and 'some concerns' for feed interruption episodes.
Effects of interventions
Comparison 1. Routine monitoring versus no routine monitoring of gastric residual in preterm infants
We included 334 infants from four randomised trials in this comparison (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015).
Risk of NEC stage ≥ 2
Data were available from all four trials for this outcome. Routine monitoring, compared to no monitoring, probably results in little to no difference in the risk of NEC (RR 1.08, 95% CI 0.46 to 2.57; 334 participants; moderate‐certainty evidence; Analysis 1.1; Figure 2). There was no evidence of heterogeneity (I² = 4%).
1.1. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 1: Risk of necrotising enterocolitis stage ≥ 2
2.

Figure 2: Forest plot of comparison: 1 Routine monitoring versus no monitoring of gastric residuals, outcome: 1.1 Necrotising enterocolitis stage 2 or 3
Time to establish full enteral feeds
All four trials reported this outcome. Full enteral feeds were defined as 150 mL/kg/d in Thomas 2018 and Torrazza 2015, 120 mL/kg/d in Parker 2019 and 180 mL/kg/d in Kaur 2015. Routine monitoring when compared to no monitoring probably increases the time to establish full enteral feeds (MD 3.14, 95% CI 1.93 to 4.36 days; 334 participants; moderate‐certainty evidence; Analysis 1.2; Figure 3). There was no significant heterogeneity (I² = 39%).
1.2. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 2: Time to establish full enteral feeds
3.

Forest plot of comparison: 1 Routine monitoring versus no monitoring of gastric residuals, outcome: 1.2 Time to reach full enteral feeds
Risk of surgical NEC
Data were available from three trials for assessment of this outcome (Kaur 2015; Parker 2019; Thomas 2018). Meta‐analysis showed no difference in the risk of surgical NEC between the routine monitoring and no monitoring groups (RR 1.66, 95% CI 0.23 to 12.07; 273 participants; Analysis 1.3). There was no heterogeneity (I2 = 0%).
1.3. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 3: Risk of surgical necrotising enterocolitis
Time to regain birth weight
Data from one trial (Kaur 2015), showed that routine monitoring when compared to no monitoring may increase the time to regain birth weight (MD 1.70, 95% CI 0.01 to 3.39 days; 80 participants; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 4: Time to regain birth weight (days)
Other growth measures during hospital stay
None of the trials reported other growth measures such as subsequent weight gain after regaining birth weight, linear and head growth during hospital stay.
Risk of extrauterine growth restriction at discharge
There was no difference in the risk of extrauterine growth restriction at discharge between the groups based on data from one trial (Kaur 2015), (RR 0.89, 95% CI 0.75 to 1.05; 80 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 5: Risk of extrauterine growth restriction at discharge
Number of infants with feed interruption episodes (lasting ≥ 12 hours)
Data from three trials were available for this outcome (Kaur 2015; Thomas 2018; Torrazza 2015). Routine monitoring when compared to no monitoring may increase the number of infants with feed interruption episodes (RR 2.21, 95% CI 1.53 to 3.20; NNTH 3, 95% CI 2 to 5; 191 participants; low‐certainty evidence; Analysis 1.6; Figure 4). There was no heterogeneity (I² = 0%).
1.6. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 6: Number of infants with feed interruption episodes ≥ 12 hours
4.

Forest plot of comparison: 1 Routine monitoring versus no monitoring of gastric residuals, outcome: 1.6 Number of infants with episodes of feed interruption lasting ≥ 12 hours
Number of TPN days
Meta‐analysis of data from all four trials showed that routine monitoring probably increases the duration of TPN by 2.57 days (MD 2.57, 95% CI 1.20 to 3.95 days; 334 participants; moderate‐certainty evidence; Analysis 1.7; Figure 5). There was no significant heterogeneity (I² = 19%).
1.7. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 7: Number of total parenteral nutrition days
5.

Forest plot of comparison: 1 Routine monitoring versus no monitoring of gastric residuals, outcome: 1.7 Number of total parenteral nutrition days
Risk of parenteral nutrition‐associated liver disease
Data from two trials (Parker 2019; Torrazza 2015), showed no difference in the incidence of parenteral nutrition‐associated liver disease between groups (RR 0.79, 95% CI 0.41 to 1.51; 284 participants; Analysis 1.8). There was no heterogeneity (I² = 0%).
1.8. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 8: Risk of parenteral nutrition‐associated liver disease
Number of days of central venous line (CVL) usage
Data were available from two trials (Parker 2019; Torrazza 2015). There was no significant difference in the duration of CVL usage between routine monitoring and no monitoring (MD 3.34, 95% CI ‐1.76 to 8.44; 204 participants; Analysis 1.9). There was no heterogeneity (I² = 0%).
1.9. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 9: Duration of central venous lines usage (days)
Risk of invasive infection
Data were available from all four trials for analysis of this outcome. While Kaur 2015 included culture‐positive sepsis alone, the other three trials (Parker 2019; Thomas 2018; Torrazza 2015), included both culture‐positive and probable/clinical sepsis. Routine monitoring probably increases the incidence of invasive infection when compared to no monitoring (RR 1.50, 95% CI 1.02 to 2.19; NNTH 10, 95% CI 5 to 100; 334 participants, 4 studies; moderate‐certainty evidence; Analysis 1.10; Figure 6). There was no heterogeneity (I² = 0%).
1.10. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 10: Risk of invasive infection
6.

Figure 7: Forest plot of comparison: 1 Routine monitoring versus no monitoring of gastric residuals, outcome: 1.10 Incidence of invasive infection
Risk of aspiration pneumonia or pneumonitis
None of the trials reported this outcome.
Risk of gastro‐oesophageal reflux
None of the trials reported this outcome.
All‐cause mortality before discharge
Meta‐analysis of data from three trials (Kaur 2015; Parker 2019; Thomas 2018) showed that routine monitoring when compared to no monitoring may have little or no effect on all‐cause mortality before discharge (RR 2.14, 95% CI 0.77 to 5.97; 273 participants; low‐certainty evidence; Analysis 1.11). There was no significant heterogeneity (I² = 35%).
1.11. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 11: All‐cause mortality before discharge
Duration of hospital stay
Data were available from three trials (Kaur 2015; Parker 2019; Thomas 2018). There was no significant difference in the duration of hospital stay between routine monitoring and no monitoring groups (MD 4.26, 95% CI ‐0.79 to 9.32; 273 participants; Analysis 1.12). There was no significant heterogeneity (I² = 24%).
1.12. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 12: Duration of hospital stay (days)
Risk of spontaneous intestinal perforation
Data from two trials (Parker 2019; Thomas 2018), showed no difference in the risk of spontaneous intestinal perforation between routine monitoring and no monitoring groups (RR 2.80, 95% CI 0.30 to 26.26; 193 participants).
Growth measures following discharge
None of the trials assessed this outcome.
Neurodevelopmental outcomes
None of the trials assessed this outcome.
Comparison 2. Both quality and volume of gastric residual compared to quality of gastric residual alone for feed interruption in preterm infants
We included one trial with 87 infants in this comparison (Singh 2018).
Risk of NEC stage ≥ 2
Data from the trial showed that using both quality and volume compared to quality of gastric residual alone for feed interruption may result in little or no difference in the incidence of NEC (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 1: Risk of necrotising enterocolitis stage ≥ 2
Time to establish full enteral feeds
Using both quality and volume compared to quality of gastric residual alone for feed interruption may result in little or no difference in the time to establish full enteral feeds (MD ‐0.10, 95% CI ‐0.91 to 0.71 days; 87 participants; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 2: Time to establish full enteral feeds
Risk of surgical NEC
The trial showed no difference in surgical NEC (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 3: Risk of surgical necrotising enterocolitis
Time to regain birth weight
Data from the trial showed that using both quality and volume compared to quality of gastric residual alone for feed interruption may result in little or no difference in time to regain birth weight (MD 1.00, 95% CI ‐0.37 to 2.37 days; 87 participants; low‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 4: Time to regain birth weight (days)
Other growth measures during hospital stay
The trial did not report other growth measures such as subsequent weight gain after regaining birth weight, linear and head growth during hospital stay.
Risk of extrauterine growth restriction at discharge
There was no difference in the risk of extrauterine growth restriction at discharge between the two groups (RR 0.54, 95% CI 0.14 to 2.01; 87 participants; low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 5: Risk of extrauterine growth restriction at discharge
Number of infants with feed interruption episodes (lasting ≥ 12 hours)
We are uncertain about the effect of using both quality and volume compared to quality of gastric residual alone on the risk of feed interruption episodes (RR 3.21, 95% CI 0.13 to 76.67; 87 participants; very low‐certainty evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 6: Number of infants with feed interruption episodes ≥ 12 hours
Number of TPN days
Using both quality and volume compared to quality of gastric residual alone feed interruption may result in little or no difference in the number of TPN days (MD 0.80, 95% CI ‐0.78 to 2.38 days; 87 participants; low‐certainty evidence; Analysis 2.7).
2.7. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 7: Number of total parenteral nutrition days
Risk of parenteral nutrition‐associated liver disease
None of the infants in either of the groups developed parenteral nutrition‐associated liver disease.
Number of days of central venous line (CVL) usage
The trial did not report this outcome.
Risk of invasive infection
There was no significant difference in the risk of invasive infection between groups (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence; Analysis 2.8).
2.8. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 8: Risk of Invasive Infection
Risk of aspiration pneumonia or pneumonitis
The trial did not report this outcome.
Risk of gastro‐oesophageal reflux
The trial did not report this outcome.
All‐cause mortality before discharge
Data from the trial showed that using two different criteria for gastric residual for feed interruption may result in little or no difference in all‐cause mortality before discharge (RR 3.21, 95% CI 0.13 to 76.67; 87 participants; low‐certainty evidence; Analysis 2.9).
2.9. Analysis.

Comparison 2: Using two different criteria of gastric residual for feed interruption while monitoring gastric residual, Outcome 9: All‐cause mortality before discharge
Duration of hospital stay
The trial did not report this outcome.
Risk of spontaneous intestinal perforation
The trial did not report this outcome.
Growth measures following discharge
The trial did not assess this outcome.
Neurodevelopmental outcomes
The trial did not assess this outcome.
Subgroup analyses
Based on gestational age (≤ 27 weeks, 28 weeks to 31 weeks, ≥ 32 weeks)
This subgroup analysis was not possible. In comparison 1, two trials (Parker 2019; Torrazza 2015), included infants born at less than 32 weeks' gestational age, Kaur 2015 included infants born at 27 to 34 weeks' gestational age and Thomas 2018 included infants born at 26 to 36 weeks' gestational age. The one trial in comparison 2 used only birth weight and did not use gestational age criteria for recruitment (Singh 2018).
Based on birth weight (< 1000 g, 1000 g to 1499 g, ≥ 1500 g)
This subgroup analysis was not possible. In comparison 1, three trials (Kaur 2015; Parker 2019; Torrazza 2015), included babies with < 1500 g birth weight, and one trial included infants with birth weight between 750 and 2000 g. The one trial included in comparison 2 exclusively included infants with birth weight ≥ 1500 g (Singh 2018).
Small for gestational age versus appropriate for gestational age infants (classified using birth weight relative to the reference population)
This subgroup analysis was not possible. In comparison 1, none of the trials included provided data on small for gestational age infants separately (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015). The trial included in comparison 2 excluded infants with birth weight below the third percentile (Singh 2018).
Type of feed the infant is receiving (human milk vs formula)
This subgroup analysis was not possible. In comparison 1, two studies (Parker 2019; Thomas 2018), used only human milk, while the other two studies used both human milk and preterm formula feeds (Kaur 2015; Torrazza 2015). The only trial in comparison 2 used both human milk and formula to feed the infants (Singh 2018).
Frequency of monitoring of gastric residual (before every feed, before every third feed, etc.)
This subgroup analysis was not possible. All included trials in both comparisons monitored gastric residual before every feed.
Discussion
Summary of main results
We included five trials (423 infants) in this review.
Four RCTs with 336 neonates met the inclusion criteria for the comparison of routine monitoring versus no monitoring of gastric residual in preterm infants (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015). Three studies were performed on infants with birth weight < 1500 g (Kaur 2015; Parker 2019; Torrazza 2015), while one study included neonates with birth weight between 750 g and 2000 g (Thomas 2018). In the monitoring group, gastric residual was assessed before each feed in all the studies (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015). In the no monitoring group, routine abdominal girth monitoring was performed in three studies (Kaur 2015; Parker 2019; Thomas 2018), while no proactive monitoring for feed intolerance was done in Torrazza 2015. All the trials were unmasked but were otherwise of good methodological quality.
Routine monitoring of gastric residual probably has little or no effect on the risk of NEC (RR 1.08, 95% CI 0.46 to 2.57; 334 participants; moderate‐certainty evidence). Routine monitoring probably increases the time to establish full enteral feeds (MD 2.92 days, 95% CI 1.36 to 4.48; 334 participants; moderate‐certainty evidence).
Routine monitoring of gastric residual may increase the time to regain birth weight (MD 1.70 days, 95% CI 0.01 to 3.39; 80 participants; low‐certainty evidence) and the number of infants with feed interruption episodes (RR 2.21, 95% CI 1.53 to 3.20; NNTH 3, 95% CI 2 to 5; 191 participants; low‐certainty evidence). Routine monitoring probably increases the number of TPN days (MD 2.57 days, 95% CI 1.20 to 3.95; 334 participants; moderate‐certainty evidence) and the risk of invasive infection (RR 1.50, 95% CI 1.02 to 2.19; NNTH 10, 95% CI 5 to 100; 334 participants; moderate‐certainty evidence). Routine monitoring may result in little or no difference in all‐cause mortality before hospital discharge (RR 2.14, 95% CI 0.77 to 5.97; 273 participants; low‐certainty evidence).
We found no data for outcomes such as growth measures following discharge and neurodevelopmental outcomes. The three ongoing trials may provide more data on important outcomes of routine monitoring versus no monitoring of gastric residual in preterm infants (ISRCTN98322846; NCT04062851; NCT04064398).
One trial met the inclusion criteria for the comparison of using two different criteria of gastric residual for interrupting feeds, while gastric residual monitoring was performed in both groups (Singh 2018). The trial was performed in infants ≥ 1500 g. The trial was unmasked but was otherwise of good methodological quality. In this trial, both the quality and volume of gastric residual were monitored in the intervention group; interruption of feeds or decreasing the feed volume, or both, was done based on both quality and volume of the residual. In the control group, the quality of gastric residual only was monitored and considered for feed interruption; the volume of gastric residual was not monitored.
Using two different criteria for gastric residual for feed interruption may result in little or no difference in the incidence of:
NEC (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence);
time to establish full enteral feeds (MD ‐0.10, 95% CI ‐0.91 to 0.71; 87 participants; low‐certainty evidence);
time to regain birth weight (MD 1.00, 95% CI ‐0.37 to 2.37; 87 participants; low‐certainty evidence);
number of TPN days (MD 0.80, 95% CI ‐0.78 to 2.38; 87 participants; low‐certainty evidence);
incidence of invasive infection (RR 5.35, 95% CI 0.26 to 108.27; 87 participants; low‐certainty evidence); and
all‐cause mortality before discharge (RR 3.21, 95% CI 0.13 to 76.67; 87 participants; low‐certainty evidence).
We are uncertain about the effect of using two different criteria of gastric residual on the risk of feed interruption episodes (RR 3.21, 95% CI 0.13 to 76.67; 87 participants; very low‐certainty evidence).
Overall completeness and applicability of evidence
For the comparison of routine monitoring versus no monitoring of gastric residual, three trials were performed in infants with birth weight < 1500 g (Kaur 2015; Parker 2019; Torrazza 2015), while one trial included infants with 750 g to 2000 g birth weight. None of the studies excluded small for gestational age infants, and only one trial excluded infants with absent or reversed end‐diastolic flow in the umbilical artery in antenatal Doppler (Kaur 2015). Hence, the results probably apply to small for gestational age infants without absent or reversal of end‐diastolic flow in umbilical artery Doppler.
Two studies (Parker 2019; Thomas 2018), in comparison 1 (routine monitoring versus no monitoring), used only human milk, while the other two studies used both human milk and preterm formula feed (Kaur 2015; Torrazza 2015). Thus, the results are applicable to both exclusive human milk feeding and mixed feeding.
Infants were given intermittent tube feeds, and gastric residuals were monitored before each feed in all four of these trials (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015). In the no gastric residual monitoring group, routine abdominal girth monitoring was performed in three studies (Kaur 2015; Parker 2019; Thomas 2018), while there was no proactive monitoring for feed intolerance in the no gastric residual monitoring group in Torrazza 2015. Hence, the comparison group was not similar across the included trials.
The major concern in not monitoring gastric residual is that it takes away an early indicator of NEC and hence may increase the risk of NEC. However, this meta‐analysis shows that routine monitoring of gastric residual probably has little or no effect on the risk of NEC. Gastric residual monitoring probably increases the duration of TPN and the risk of invasive infection. Furthermore, it probably increases the time to establish full enteral feeds, the time taken to regain birth weight, and may increase feed interruption episodes. Although not shown in this meta‐analysis, a decrease in the number of TPN days would imply a decrease in the number of days of CVL usage, parenteral nutrition‐associated liver disease, and duration of hospital stay.
For the comparison of using two different criteria for gastric residual to interrupt feeds while monitoring gastric residual, the only included trial was conducted in preterm infants with birth weight of 1500 g to 2000 g (Singh 2018). The trial excluded infants with perinatal asphyxia and infants with birth weight less than the third percentile. The trial showed no difference in any of the major outcomes such as NEC, time to establish full enteral feeds, time to regain birth weight, feed interruption episodes, or number of TPN days. This could be because these larger preterm infants usually do not require a long duration of gavage feeds, TPN, or CVL usage, and they are at lesser risk of NEC when compared to very low birth weight infants.
Quality of the evidence
All the included trials were of good methodological quality, except for lack of masking. The certainty of the evidence was moderate for NEC (downgraded by one level for serious imprecision due to wide CIs and small sample not meeting the optimal information size criterion), time to establish full enteral feeds (downgraded by one level for serious imprecision due to CI crossing the threshold of clinically meaningful difference), number of TPN days (downgraded by one level for serious imprecision due to lower CI crossing the threshold of clinically meaningful difference) and incidence of invasive infection (downgraded by one level for serious imprecision due to lower CI reaching the line of no difference). The certainty of the evidence was low for time to regain birth weight (downgraded by two levels for very serious imprecision due to CI reaching the line of no difference and small sample size), number of feed interruption episodes (downgraded by two levels for very serious risk of bias due to high risk of bias in all included studies) and mortality before discharge (downgraded by two levels for very serious imprecision due to the small sample not meeting the optimal information size criterion and CI crossing the line of no difference).
For the comparison of two different criteria for gastric residual to interrupt feeds, the certainty of evidence from the only included trial was low for outcomes such as NEC, time to establish full enteral feeds, time to regain birth weight, number of TPN days, invasive infection and all‐cause mortality before hospital discharge (downgraded by two levels for very serious imprecision due to the small sample size and wide CIs). The certainty of the evidence was very low for feed interruption episodes (downgraded by two levels for serious imprecision due to the small sample size and wide CIs and by one level for serious risk of bias due to 'some concerns' in the only included trial).
Potential biases in the review process
We found five trials for inclusion in this review. Although we conducted a comprehensive search, we cannot exclude fully the possibility of publication bias because we do not know whether other published (but not indexed) or unpublished trials have been conducted. We did not have a sufficient number of trials to explore symmetry of funnel plots as a means of identifying possible publication bias.
Agreements and disagreements with other studies or reviews
Kumar 2021 is a systematic review that evaluated routine monitoring of gastric residual versus no monitoring in preterm neonates. The review included six trials (Kaur 2015; Lenfestey 2018; Parker 2019; Singh 2018; Thomas 2018; Torrazza 2015). Four of these trials were included in this review (Kaur 2015; Parker 2019; Thomas 2018; Torrazza 2015). Singh 2018 was included in comparison 2 and Lenfestey 2018 was classified under Studies awaiting classification due to lack of adequate data.
Kumar 2021 found no significant difference in the incidence of NEC between routine monitoring and no monitoring of gastric residual. Routine monitoring was associated with delays in achieving full enteral feeds, longer duration of hospitalisation, and greater incidence of late‐onset sepsis. The review did not find any difference in other outcomes, such as time to regain birth weight, TPN days, CVL usage, culture‐positive sepsis and all‐cause mortality. These results were quite similar to those of our meta‐analysis, except that we found a significant increase in TPN duration and time to regain birth weight with routine gastric residual monitoring, and no difference in the duration of hospital stay. These differences are probably due to the two additional studies (Lenfestey 2018; Singh 2018) included in the analysis in the previous review (Kumar 2021).
Riskin 2017 is a case‐control study that included 472 preterm infants of < 34 weeks' gestation and evaluated the effects of routine monitoring versus no monitoring of gastric residual. The study showed no difference in the risk of NEC with routine monitoring of gastric residual. Routine monitoring increased the risk of feed interruption episodes, increased the time to reach full enteral feeds, number of TPN days and duration of hospital stay, and reduced weight gain and weight at discharge.
Dubey 2018 is a cohort study that included 60 very low birth weight infants and assessed gastric residual monitoring versus abdominal girth monitoring. The study found that gastric residual monitoring increased the number of fasting hours and time taken to reach full enteral feeds and resulted in lesser weight gain. Thus, the results of these studies are in agreement with ours.
Authors' conclusions
Implications for practice.
Moderate‐certainty evidence suggests routine monitoring of gastric residual has little or no effect on the incidence of necrotising enterocolitis (NEC). Moderate‐certainty evidence suggests monitoring gastric residual probably increases the time to establish full enteral feeds, the number of total parenteral nutrition (TPN) days and the risk of invasive infection. Low‐certainty evidence suggests monitoring gastric residual may increase the time to regain birth weight and the number of feed interruption episodes, and may have little or no effect on all‐cause mortality before discharge. There are no data on long‐term growth and neurodevelopmental outcomes.
For the comparison of using two different criteria for gastric residual to interrupt feeds, available evidence is insufficient to comment on any of the major outcomes of preterm infants such as NEC, time to establish full enteral feeds, number of TPN days, risk of invasive infection and all‐cause mortality before discharge.
Implications for research.
Further randomised controlled trials (RCTs) on routine monitoring of gastric residuals versus no monitoring that are adequately powered to detect clinical differences in important outcomes are required. These trials should also evaluate long‐term growth and neurodevelopmental outcomes. Trial authors could stratify randomisation based on gestational age, birth weight, small versus appropriate for gestational age, and type of milk used for feeding.
Further RCTs comparing two different criteria for gastric residual to interrupt feeds (based on quality or quantity, or both) are also required to evaluate the impact of these strategies on major outcomes in preterm infants. Trials should indicate clearly the nature of gastric residual (based on quality or quantity, or both) that might predict NEC.
What's new
| Date | Event | Description |
|---|---|---|
| 16 June 2023 | New citation required and conclusions have changed | We included two new trials, which improved the precision of estimates of the outcomes. Three new co‐authors VVR, BR and SR joined this review update. |
| 16 June 2023 | New search has been performed | We conducted a new search in February 2022. |
History
Protocol first published: Issue 1, 2018 Review first published: Issue 7, 2019
Risk of bias
Risk of bias for analysis 1.1 Risk of necrotising enterocolitis stage ≥ 2.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context | Low risk of bias | All 80 randomised infants were included in the analysis | Low risk of bias | Though masking was not done, NEC is an objective outcome and is less prone to detection bias. | Low risk of bias | All proposed outcomes were reported (personal communication) | Some concerns | Some concerns in one domain, low risk in other domains |
| Parker 2019 | Low risk of bias | Infants were randomized to 1 of 2 groups by a computer generated sequence with random‐length permuted blocks of sizes (4, 6, or 8). Randomization was concealed until the intervention was assigned. | Low risk of bias | Part 1: Eighteen (26%) infants in the "no gastric residual" group had one or more gastric residuals evaluated. However, no infant had gastric residuals evaluated for more than one day and hence this deviation was considered unlikely to have affected the outcome. Part 2: Modified intention to treat (mITT) was used (Low risk) |
Low risk of bias | All randomised neonates were accounted for. | Low risk of bias | Though masking was not done, NEC is an objective outcome and is less prone to detection bias. | Low risk of bias | All proposed outcomes were reported | Low risk of bias | Low risk in all domains |
| Thomas 2018 | Low risk of bias | Randomization was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was done using sequentially numbered opaque sealed envelopes | Low risk of bias | Part 1: There were no deviations from intended intervention (Low risk) Part 2: modified intention to treat analysis was used (Low risk) |
Low risk of bias | All randomised neonates were accounted for. | Low risk of bias | Though masking was not done, NEC is an objective outcome and is less prone to detection bias. | Some concerns | Study protocol had not been published | Some concerns | Some concerns in one domain and low risk in other domains |
| Torrazza 2015 | Low risk of bias | A computer‐generated block randomisation sequence with variable block sizes was used. The randomisation sequence was kept in sequentially numbered sealed opaque envelopes (personal communication) | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 61 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, NEC is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains |
Risk of bias for analysis 1.2 Time to establish full enteral feeds.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context | Low risk of bias | All 80 randomised infants were included in the analysis | Low risk of bias | Though masking was not done, 'Time to reach full enteral feeds' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains |
| Parker 2019 | Low risk of bias | Infants were randomized to 1 of 2 groups by a computer generated sequence with random‐length permuted blocks of sizes (4, 6, or 8). Randomization was concealed until the intervention was assigned. | Low risk of bias | Part 1: Eighteen (26%) infants in the "no gastric residual" group had one or more gastric residuals evaluated. However, no infant had gastric residuals evaluated for more than one day and hence this deviation was considered unlikely to have affected the outcome. Part 2: Modified intention to treat (mITT) was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'Time to reach full enteral feeds' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | Low risk in all domains |
| Thomas 2018 | Low risk of bias | Randomization was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was done using sequentially numbered opaque sealed envelopes | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: modified intention to treat analysis was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though the trial was unmasked, time to reach full feeds is an objective outcome and is less prone to detection bias. | Some concerns | Study protocol had not been published. | Some concerns | Some concerns in one domain, low risk in other domains |
| Torrazza 2015 | Low risk of bias | A computer‐generated block randomisation sequence with variable block sizes was used. The randomisation sequence was kept in sequentially numbered sealed opaque envelopes (personal communication) | Some concerns | No data to assess if deviations arouse because of trial context | Low risk of bias | All 61 randomised infants were included in the analysis | Low risk of bias | Though masking was not done, 'Time to reach full enteral feeds' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
Risk of bias for analysis 1.4 Time to regain birth weight (days).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 80 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Time to regain birth weight' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
Risk of bias for analysis 1.6 Number of infants with feed interruption episodes ≥ 12 hours.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 80 randomised infants were included in the analysis. | Some concerns | Masking was not done. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | High risk of bias | 'Some concerns' in two domains |
| Thomas 2018 | Low risk of bias | Randomization was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was done using sequentially numbered opaque sealed envelopes | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: modified intention to treat analysis was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Some concerns | Masking was not done. | Some concerns | Study protocol had not been published | High risk of bias | 'Some concerns' in two domains |
| Torrazza 2015 | Low risk of bias | A computer‐generated block randomisation sequence with variable block sizes was used. The randomisation sequence was kept in sequentially numbered sealed opaque envelopes (personal communication) | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 61 randomised infants were included in the analysis. | Some concerns | Masking was not done | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | High risk of bias | 'Some concerns' in two domains. |
Risk of bias for analysis 1.7 Number of total parenteral nutrition days.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 80 randomised infants were included in the analysis | Low risk of bias | Though masking was not done, 'TPN days' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains |
| Parker 2019 | Low risk of bias | Infants were randomized to 1 of 2 groups by a computer generated sequence with random‐length permuted blocks of sizes (4, 6, or 8). Randomization was concealed until the intervention was assigned. | Low risk of bias | Part 1: Eighteen (26%) infants in the "no gastric residual" group had one or more gastric residuals evaluated. However, no infant had gastric residuals evaluated for more than one day and hence this deviation was considered unlikely to have affected the outcome. Part 2: Modified intention to treat (mITT) was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'TPN days' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | Low risk in all domains |
| Thomas 2018 | Low risk of bias | Randomization was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was done using sequentially numbered opaque sealed envelopes | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: modified intention to treat analysis was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'TPN days' is an objective outcome and is less prone to detection bias. | Some concerns | Study protocol had not been published. | Some concerns | Some concerns in one domain, low risk in other domains |
| Torrazza 2015 | Low risk of bias | A computer‐generated block randomisation sequence with variable block sizes was used. The randomisation sequence was kept in sequentially numbered sealed opaque envelopes (personal communication) | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 61 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'TPN days' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
Risk of bias for analysis 1.10 Risk of invasive infection.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 80 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Invasive infection' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
| Parker 2019 | Low risk of bias | Infants were randomized to 1 of 2 groups by a computer generated sequence with random‐length permuted blocks of sizes (4, 6, or 8). Randomization was concealed until the intervention was assigned. | Low risk of bias | Part 1: Eighteen (26%) infants in the "no gastric residual" group had one or more gastric residuals evaluated. However, no infant had gastric residuals evaluated for more than one day and hence this deviation was considered unlikely to have affected the outcome. Part 2: Modified intention to treat (mITT) was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'Invasive infection' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | Low risk in all domains |
| Thomas 2018 | Low risk of bias | Randomization was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was done using sequentially numbered opaque sealed envelopes | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: modified intention to treat analysis was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'Invasive infection' is an objective outcome and is less prone to detection bias. | Some concerns | Study protocol had not been published. | Some concerns | Some concerns in one domain, low risk in other domains. |
| Torrazza 2015 | Low risk of bias | A computer‐generated block randomisation sequence with variable block sizes was used. The randomisation sequence was kept in sequentially numbered sealed opaque envelopes (personal communication) | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 61 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Invasive infection' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
Risk of bias for analysis 1.11 All‐cause mortality before discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Kaur 2015 | Low risk of bias | A computer‐generated block randomisation sequence with block size of 4 was prepared by a person not involved in clinical care, measurement of outcomes, or analysis of data. This randomisation sequence was kept in sequentially numbered sealed opaque envelopes. However, a fixed block size of 4 gives the chance to guess the allocation of every fourth infant in an unmasked study. | Some concerns | No data to assess if deviations arouse because of trial context. | Low risk of bias | All 80 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Mortality' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
| Parker 2019 | Low risk of bias | Infants were randomized to 1 of 2 groups by a computer generated sequence with random‐length permuted blocks of sizes (4, 6, or 8). Randomization was concealed until the intervention was assigned. | Low risk of bias | Part 1: Eighteen (26%) infants in the "no gastric residual" group had one or more gastric residuals evaluated. However, no infant had gastric residuals evaluated for more than one day and hence this deviation was considered unlikely to have affected the outcome. Part 2: Modified intention to treat (mITT) was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'Mortality' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | Low risk in all domains |
| Thomas 2018 | Low risk of bias | Randomization was completed using a computer‐generated random number table in unequal block sizes ranging from 4 to 12. Allocation concealment was done using sequentially numbered opaque sealed envelopes | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: modified intention to treat analysis was used (Low risk) |
Low risk of bias | All randomsied infants were accounted for. | Low risk of bias | Though masking was not done, 'Mortality' is an objective outcome and is less prone to detection bias. | Some concerns | Study protocol had not been published. | Some concerns | Some concerns in one domain, low risk in other domains. |
Risk of bias for analysis 2.1 Risk of necrotising enterocolitis stage ≥ 2.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviations from the intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'NEC' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | 'Low risk' in all domains. |
Risk of bias for analysis 2.2 Time to establish full enteral feeds.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Time to reah full enteral feeds' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | 'Low risk' in all domains. |
Risk of bias for analysis 2.4 Time to regain birth weight (days).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Time to regain birth weight' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | 'Low risk' in all domains. |
Risk of bias for analysis 2.6 Number of infants with feed interruption episodes ≥ 12 hours.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Some concerns | Masking was not done | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Some concerns | Some concerns in one domain, low risk in other domains. |
Risk of bias for analysis 2.7 Number of total parenteral nutrition days.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'TPN days' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | 'Low risk' in all domains. |
Risk of bias for analysis 2.8 Risk of Invasive Infection.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Invasive infection' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | 'Low risk' in all domains. |
Risk of bias for analysis 2.9 All‐cause mortality before discharge.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Singh 2018 | Low risk of bias | The randomization sequence was computer generated and permuted, even numbered, randomly varying block sizes were generated with a 1:1 allocation ratio. The allocation sequence was concealed using serially numbered opaque sealed envelopes. | Low risk of bias | Part 1: There were no deviation from intended intervention (Low risk) Part 2: Modified intention to treat analysis was used (Low risk) |
Low risk of bias | All 87 randomised infants were included in the analysis. | Low risk of bias | Though masking was not done, 'Mortality' is an objective outcome and is less prone to detection bias. | Low risk of bias | Study protocol had been published. All proposed outcomes were reported. | Low risk of bias | 'Low risk' in all domains. |
Acknowledgements
The methods section of this review is based on a standard template used by Cochrane Neonatal.
We are grateful to Drs Leslie A. Parker, Saudamini Nesargi, Satish Saluja, Josef Neu, Roberto Murgas Torrazza, Balpreet Singh, and Sumesh Thomas for providing further details and data.
We thank Cochrane Neonatal: Michelle Fiander and Jane Cracknell, Managing Editors; and Co‐ordinating Editors Roger Soll and Bill McGuire who provided support.
Michelle Fiander, Information Specialist, Cochrane Neonatal, ran searches, wrote search methods and results of search, and developed the PRISMA flow diagram.
We thank the following peer reviewers: Souvik Mitra, Associate Professor, Dalhousie University & IWK Health, Halifax, Canada; and Leslie Choi, Evidence Synthesis Development Editor (Cochrane Central Executive Team).
We thank Anupa Shah, Cochrane Production Services, for copy editing.
We thank Binu Ninan, who was involved in the previous version of this review.
Appendices
Appendix 1. Cochrane CENTRAL strategy
| Cochrane CENTRAL via CRS 24 February 2022 | ||
| 1 | MESH DESCRIPTOR Infant, Newborn EXPLODE ALL AND CENTRAL:TARGET | 17409 |
| 2 | infant or infants or infant’s or "infant s" or infantile or infancy or newborn* or "new born" or "new borns" or "newly born" or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or "pre term" or premies or "low birth weight" or "low birthweight" or VLBW or LBW or ELBW or NICU AND CENTRAL:TARGET | 95692 |
| 3 | preemie OR preemies or pre‐mature or pre‐matures or pre‐maturity AND CENTRAL:TARGET | 54 |
| 4 | #1 OR #2 OR #3 | 95701 |
| 5 | (gastric NEAR/2 residual*):ti,ab,kw OR aspirate*:ti,ab,kw AND CENTRAL:TARGET | 2705 |
| 6 | #4 AND #5 | 346 |
Appendix 2. MEDLINE strategy
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions(R) 1946 to 23 February 2022 | ||
| # | Searches | Results |
| 1 | (gastric adj2 residual*).ti,ab,kw,kf. | 860 |
| 2 | aspirate*.ti,ab,kw,kf. | 32238 |
| 3 | or/1‐2 [Gastric Residual] | 33021 |
| 4 | exp infant, newborn/ or Intensive Care, Neonatal/ or Intensive Care Units, Neonatal/ | 649075 |
| 5 | (baby* or babies or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU).ti,ab,kw,kf. | 961121 |
| 6 | or/4‐5 [Filter: Neonatal Population 01‐2022‐‐MEDLINE] | 1242164 |
| 7 | randomized controlled trial.pt. | 559489 |
| 8 | controlled clinical trial.pt. | 94705 |
| 9 | randomized.ti,ab. | 598220 |
| 10 | placebo.ti,ab. | 231966 |
| 11 | drug therapy.fs. | 2447253 |
| 12 | randomly.ti,ab. | 377572 |
| 13 | trial.ti,ab. | 684606 |
| 14 | groups.ti,ab. | 2342161 |
| 15 | or/7‐14 [Cochrane HSSS‐SM Filter; Box 6.4.a Cochrane Handbook] | 5334468 |
| 16 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1024285 |
| 17 | (control* adj2 (group? or random* or trial? or study)).ti,ab,kw,kf. | 1019197 |
| 18 | or/16‐17 [Additional terms to increase sensitivity] | 1586676 |
| 19 | exp animals/ not humans/ | 4962934 |
| 20 | (or/15,18) not 19 [RCT Filter: Medline] | 4885157 |
| 21 | meta‐analysis/ or "systematic review"/ or network meta‐analysis/ [/ finds same as.pt. syntax] | 260571 |
| 22 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kf,kw. | 259145 |
| 23 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kf,kw. | 33511 |
| 24 | (data synthes* or data extraction* or data abstraction*).ti,ab,kf,kw. | 34084 |
| 25 | (hand search* or handsearch*).ti,ab,kf,kw. | 10330 |
| 26 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kf,kw. | 31338 |
| 27 | meta‐analysis as topic/ or network meta‐analysis/ | 24143 |
| 28 | (met analy* or metanaly* or meta regression* or metaregression*).ti,ab,kf,kw. | 12628 |
| 29 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 279291 |
| 30 | (cochrane or systematic review?).jw. | 19066 |
| 31 | or/21‐30 [SR filter‐Medline; based on CADTHhttps://www.cadth.ca/strings‐attached‐cadths‐database‐search‐filters] | 499471 |
| 32 | 3 and 6 and 20 [Gastric Residual/Apsirate AND Neonate AND RCT] | 841 |
| 33 | 3 and 6 and 31 [Gastric Residual/Aspirate AND Neonate AND Systematic Review Filter] | 37 |
| 34 | or/32‐33 [MEDLINE All results] | 856 |
Appendix 3. Embase strategy
| Ovid Embase 1974 to 23 February 2022 | ||
| # | Searches | Results |
| 1 | (gastric adj2 residual*).ti,ab,kw,kf. | 1345 |
| 2 | aspirate*.ti,ab,kw,kf. | 49004 |
| 3 | or/1‐2 [Gastric Residual] | 50235 |
| 4 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ | 641045 |
| 5 | (baby* or babies or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU).ti,ab,kw,kf. | 1124258 |
| 6 | or/4‐5 [Filter: Neonatal Population 2021‐OVID EMBASE] | 1341740 |
| 7 | Randomized controlled trial/ or Controlled clinical study/ | 886510 |
| 8 | random$.ti,ab,kw. | 1764176 |
| 9 | Randomization/ | 93121 |
| 10 | placebo.ti,ab,kw. | 337398 |
| 11 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 253738 |
| 12 | double blind procedure/ | 192633 |
| 13 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 400691 |
| 14 | parallel group$1.ti,ab. | 28968 |
| 15 | (crossover or cross over).ti,ab. | 114952 |
| 16 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 373656 |
| 17 | (open adj label).ti,ab. | 94726 |
| 18 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1440533 |
| 19 | (control* adj2 (group? or random*)).ti,ab,kw,kf. | 1170580 |
| 20 | or/7‐17 [ Terms based on Cochrane Central strategy‐https://www‐cochranelibrary‐com.ezproxy.uvm.edu/central/central‐creation] | 2524984 |
| 21 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 23325369 |
| 22 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 30171902 |
| 23 | 22 not 21 [Animal Exclusion‐https://community‐cochrane‐org.ezproxy.uvm.edu/sites/default/files/uploads/inline‐files/Embase%20animal%20filter.pdf] | 6846533 |
| 24 | 20 not 23 [Filter: RCT‐EMBASE] | 2255913 |
| 25 | meta‐analysis/ or "systematic review"/ or "meta analysis (topic)"/ [EMTREE] | 486396 |
| 26 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kw. | 316129 |
| 27 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kw. | 47216 |
| 28 | (data synthes* or data extraction* or data abstraction*).ti,ab,kw. | 41746 |
| 29 | (hand search* or handsearch*).ti,ab,kw. | 12572 |
| 30 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kw. | 41438 |
| 31 | (met analy* or metanaly* or meta regression* or metaregression*).ti,ab,kw. | 16192 |
| 32 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 353000 |
| 33 | (cochrane or systematic review?).jn,jx. | 30354 |
| 34 | (overview adj2 reviews).ti. | 101 |
| 35 | or/25‐34 [SR Filter: EMBASE based on CADTH filter: https://www‐cadth‐ca.ezproxy.uvm.edu/strings‐attached‐cadths‐database‐search‐filters] | 723742 |
| 36 | 3 and 6 and 24 [Gastric Residual AND Neonate AND RCT Filter] | 356 |
| 37 | 3 and 6 and 35 [Gastric Residual AND Neonate AND Systematic Review Filter] | 59 |
| 38 | or/36‐37 [EMBASE Results] | 391 |
Appendix 4. CINAHL strategy
| CINAHL Ebsco | ||
| Search date: 24 February 2022 | ||
| 1 | TI (gastric N2 residual*) OR AB (gastric N2 residual*) | 469 |
| 2 | TI aspirate* OR AB aspirate* | 4,306 |
| 3 | S1 OR S2 [GASTRIC RESIDUAL] | 4,730 |
| 4 | (MH "Infant, Newborn+") OR (MH "Infant, Large for Gestational Age") OR (MH "Infant, Low Birth Weight+") OR (MH "Infant, Postmature") OR (MH "Infant, Premature") | 152,930 |
| 5 | (MH "Intensive Care, Neonatal+") OR (MH "Intensive Care Units, Neonatal") | 19,595 |
| 6 | TI (baby* or babies or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) OR AB (baby* or babies or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or LBW or ELBW or NICU) | 243,954 |
| 7 | S4 OR S5 OR S6 [NEONATAL TERMS] | 303,027 |
| 8 | (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Randomized Controlled Trials+") OR (MH "Double‐Blind Studies") | 166,873 |
| 9 | (MH "Clinical Trials+") | 334,894 |
| 10 | TI (randomized or randomised) OR AB (randomized or randomised) OR SU (randomized or randomised) | 318,334 |
| 11 | AB randomly | 102,088 |
| 12 | AB placebo | 63,583 |
| 13 | AB (trial) | 319,953 |
| 14 | AB groups | 848,082 |
| 15 | TI (quasirandom* or quasi‐random*) OR AB (quasirandom* or quasi‐random*) | 2,167 |
| 16 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 | 1,245,004 |
| 17 | (MH "Animal Studies") | 145,125 |
| 18 | (MH "Human") | 2,521,187 |
| 19 | S17 NOT S18 | 120,959 |
| 20 | S16 NOT S19 [RCT FILTER] | 1,206,982 |
| 21 | (MH "Systematic Review") | 107,301 |
| 22 | (MH "Meta Analysis") | 60,739 |
| 23 | ( TI ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) OR ( AB ((systematic* N3 (review* or overview*)) or (methodologic* N3 (review* or overview*))) ) | 130,603 |
| 24 | ( TI ((integrative N3 (review* or overview*)) or (collaborative N3 (review* or overview*)) or (pool* N3 analy*)) ) OR ( AB ((integrative N3 (review* or overview*)) or (collaborative N3 (review* or overview*)) or (pool* N3 analy*)) ) | 17,559 |
| 25 | ( TI (data synthes* or data extraction* or data abstraction*) ) OR ( AB (data synthes* or data extraction* or data abstraction*) ) | 13,086 |
| 26 | AB (hand search* or handsearch*) | 4,762 |
| 27 | AB (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*) | 9,055 |
| 28 | ( TI met analy* or metanaly* or meta regression* or metaregression*) ) OR ( AB met analy* or metanaly* or meta regression* or metaregression*) ) | 4,520 |
| 29 | AB (medline or cochrane or pubmed or medlars or embase OR CINAHL) | 109,882 |
| 30 | S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 [SYSTEMATIC REVIEW FILTER] | 233,105 |
| 31 | S3 AND S7 AND S16 [RCT Results] | 221 |
| 32 | S3 AND S7 AND S30 [SR Results] | 17 |
| 33 | S31 OR S32 [CINAHL All results] | 227 |
Appendix 5. Trial registry strategies
| Date | Source | Terms | Results |
| 02‐18‐2018 | WHO ICTRP | (gastric residual*) AND infant; (gastric residual*) AND neonate | 7 |
| 02‐18‐2018 | Clinicaltrials.gov | (gastric residual* OR aspirate*) AND (infant or neonate) | Child | 11 |
| 02‐18‐2018 | ISRCTN registry | (gastric residual*) AND infant | 4 |
| 02‐28‐2022 | Clinicaltrials.gov | gastric residual AND infant [Other terms] | 21 |
| 02‐28‐2022 | Clinicaltrials.gov | gastric residual AND neonate [Other terms] | 28 |
| 02‐28‐2022 | Clinicaltrials.gov | residual gastric AND infant [Other terms] | 45 |
| 02‐28‐2022 | Clinicaltrials.gov | residual gastric AND neonate [Other terms] | 13 |
| 02‐28‐2022 | ISRCTN | gastric residual AND infant | 1 |
| 02‐28‐2022 | ISRCTN | residual gastric AND infant | 1 |
| 02‐28‐2022 | ISRCTN | gastric residual AND neonate | 2 |
| 02‐28‐2022 | ISRCTN | residual gastric AND infant | 1 |
| 02‐28‐2022 | ICTRP (WHO) | gastric residual AND infant | 8 |
| 02‐28‐2022 | ICTRP (WHO) | gastric residual and neonate | 2 |
| 02‐28‐2022 | ICTRP (WHO) | residual gastric AND infant | 8 |
| 02‐28‐2022 | ICTRP (WHO) | residual gastric AND neonate | 2 |
| Total | 154 | ||
| Dupes | 116 | ||
| Net | 38 | ||
Data and analyses
Comparison 1. Routine monitoring versus no monitoring of gastric residuals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Risk of necrotising enterocolitis stage ≥ 2 | 4 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.46, 2.57] |
| 1.2 Time to establish full enteral feeds | 4 | 334 | Mean Difference (IV, Fixed, 95% CI) | 3.14 [1.93, 4.36] |
| 1.3 Risk of surgical necrotising enterocolitis | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.23, 12.07] |
| 1.4 Time to regain birth weight (days) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.01, 3.39] |
| 1.5 Risk of extrauterine growth restriction at discharge | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.75, 1.05] |
| 1.6 Number of infants with feed interruption episodes ≥ 12 hours | 3 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.53, 3.20] |
| 1.7 Number of total parenteral nutrition days | 4 | 334 | Mean Difference (IV, Fixed, 95% CI) | 2.57 [1.20, 3.95] |
| 1.8 Risk of parenteral nutrition‐associated liver disease | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.51] |
| 1.9 Duration of central venous lines usage (days) | 2 | 204 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [‐1.76, 8.44] |
| 1.10 Risk of invasive infection | 4 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.02, 2.19] |
| 1.11 All‐cause mortality before discharge | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.77, 5.97] |
| 1.12 Duration of hospital stay (days) | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | 4.26 [‐0.79, 9.32] |
| 1.13 Risk of spontaneous intestinal perforation | 2 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.30, 26.26] |
1.13. Analysis.

Comparison 1: Routine monitoring versus no monitoring of gastric residuals, Outcome 13: Risk of spontaneous intestinal perforation
Comparison 2. Using two different criteria of gastric residual for feed interruption while monitoring gastric residual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Risk of necrotising enterocolitis stage ≥ 2 | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.26, 108.27] |
| 2.2 Time to establish full enteral feeds | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.91, 0.71] |
| 2.3 Risk of surgical necrotising enterocolitis | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.26, 108.27] |
| 2.4 Time to regain birth weight (days) | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐0.37, 2.37] |
| 2.5 Risk of extrauterine growth restriction at discharge | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.14, 2.01] |
| 2.6 Number of infants with feed interruption episodes ≥ 12 hours | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 76.67] |
| 2.7 Number of total parenteral nutrition days | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.78, 2.38] |
| 2.8 Risk of Invasive Infection | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.35 [0.26, 108.27] |
| 2.9 All‐cause mortality before discharge | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.13, 76.67] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kaur 2015.
| Study characteristics | |
| Methods | RCT |
| Participants | 80 infants with birth weight < 1500 g Exclusion criteria: infants with major congenital abnormalities, gestation < 27 or > 34 weeks, absent or reversed end‐diastolic flow in antenatal Doppler, or Apgar score < 3 at 5 minutes |
| Interventions | Infants were randomised to the two groups at the time of initiation of enteral feeds Gastric residual volume monitoring group: gastric residual volume was measured before each feed. Feed intolerance was defined as presence of 1 or more of the following features: bilious/haemorrhagic aspirates or volume of aspirates > 50% of previous feed or > 3 mL, whichever was larger. If gastric residues were between 30% and 50% of previous feeds, the same volume was continued without making a daily increment. Feeds were advanced as per protocol if gastric residues were < 30% of previous feeds. The gastric residues aspirated were discarded. Abdominal circumference monitoring group: abdominal circumference measurement was performed before each feed using a standard, disposable non‐stretchable paper tape with minimum markings of 1 mm. The tape was positioned 1 cm above the umbilicus and was read along its bottom edge. A mark was made along the lower edge as reference for subsequent measurements. An increase in prefeed abdominal circumference by 2 cm from baseline was considered a sign of feed intolerance. Gastric residual volume assessment was not routinely performed unless the abdominal circumference increased by > 2 cm. The decision for feed interruption was merely based on an increase in abdominal girth. The least abdominal circumference during the previous 24 hours was used as the baseline reference Infants in both groups who experienced feed intolerance were kept nil per oral for the next 24 hours. Once abdominal circumference was ≤ baseline (abdominal circumference group) or gastric aspirates were clear and < 10 mL/kg/d (gastric residual volume group), feeds were restarted at 50% of the volume being delivered at the time of feed interruption |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | The enrolled infants were assessed daily from birth for feed initiation. Feeds were initiated when infants were haemodynamically stable with soft abdomen and audible bowel sounds. Intermittent gavage feeds were given at 2‐hourly intervals. Feed was started at 10 mL/kg in infants < 1250 g and 20 mL/kg in infants ≥ 1250 g. Subsequent advancements were made by 20 mL/kg/d as tolerated, to a maximum volume of 180 mL/kg/d. Expressed mother’s milk was preferred; if not available, standard preterm formula with a calorie content of 80 kcal/100 mL was used. Human milk fortifier was added once infants tolerated 100 mL/kg/d feed volume |
Parker 2019.
| Study characteristics | |
| Methods | RCT |
| Participants | 143 infants born at ≤ 32 weeks’ gestation with a birth weight of ≤ 1250 g and were receiving some feeds by 72 hours after birth Exclusion criteria: congenital or chromosomal abnormalities, including complex congenital heart disease or a gastrointestinal condition |
| Interventions |
Gastric residual monitoring group: gastric residuals were aspirated and monitored before each feed. In addition, infants were assessed for other signs such as abdominal distension or tenderness or both, increased abdominal girth, visible bowel loops, emesis and visible blood in the stool No monitoring of gastric residual group: prefeed gastric residuals were not monitored. Infants were assessed for other signs of NEC or feed intolerance, including abdominal girth monitoring, as described above |
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes included:
|
| Notes | Feeds were initiated at up to 20mL/kg/d and advanced daily by ≥ 20mL/kg/d to reach a maximum of 120 to 150 mL/kg/day. Only human milk was used for feeding. Mothers' own breast milk was the first choice followed by donor human milk |
Singh 2018.
| Study characteristics | |
| Methods | RCT |
| Participants | 87 infants with birth weight 1500 g to 2000 g and postnatal age < 48 hours who required gavage feeds Exclusion criteria: perinatal asphyxia (cord blood gas or first blood gas after birth with pH < 7.0 or base excess > –16 mmol/L and Apgar score < 5 at 10 minutes), major congenital malformations/surgical conditions that could interfere with feeding, and severe growth restriction (defined as birth weight below the third percentile) |
| Interventions | Routine assessment of gastric residual was done in both groups Intervention group: only the quality of gastric residual was assessed. A maximum of 0.5 mL of gastric contents was aspirated before each feed. If the residual was haemorrhagic or was repeatedly bilious (more than 1 time) with or without vomiting or abnormal abdominal examination, feed interruption was done. The volume of gastric residual was not assessed Control group: both volume and quality of gastric residual were assessed. The entire volume of gastric residual was aspirated before every feed. If the aspirate was > 50% of feed volume or > 3 mL, whichever was greater, feeds were withheld. Also, if the aspirate was bloody or bile‐stained, feeds were withheld |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Feeds were started on day 1 or later, once the infant was haemodynamically stable. Feeds were started at 3 mL every 3 hours and were increased by 3 mL every 9 hours in infants with birth weight 1500 g to 1750 g. For infants with 1751 g to 2000 g birth weight, feeds were started at 6 mL every 3 hours and were increased by 3 mL every 6 hours. Infants were fed breast milk if available and preterm formula after parental consent was obtained when breast milk was not available. Feeds were fortified when enteral feeds of 150 mL/kg/d were achieved |
Thomas 2018.
| Study characteristics | |
| Methods | RCT |
| Participants | 52 infants born at 26 to 36 weeks' gestational age, with a birth weight between 750 g and 2000 g and likely to require gavage feeds for at least 48 hours Exclusion criteria: life‐threatening congenital anomalies, anomalies of the gastrointestinal tract |
| Interventions |
Gastric residual monitoring group: gastric residuals were aspirated and monitored before each feed Abdominal girth monitoring group: abdominal girth was monitored before each feed |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Trophic feeds of 10 to 20 mL/kg/d were started on day 1 in haemodynamically stable infants. Feeds were advanced by 20 mL/kg/d in infants 750 g to 1249 g and those with abnormal antenatal Dopplers or by 35 mL/kg/d in infants 1250 g to 1499 g and were stable. Infants weighing greater than 1500 g to 2000 g were started on full feeds if they did not have abnormal Dopplers, respiratory distress, asphyxia, or haemodynamic instability. Only human (mothers' or donor human) milk was used for feeding. TPN was continued till 100 mL/kg/d of feeds was reached. Full feeds were defined as 150 mL/kg/d. Feed intolerance was defined as the presence of any one of the following 4 features: increase in abdominal girth by 2 cm or more; vomiting 2 or more episodes in the past 6 hours; blood‐stained or bilious aspirates; more than 2 episodes of voluminous gastric aspirates in a 6‐hour period. Voluminous gastric residuals are defined as more than 50% of previous feed volume if 6 mL per feed or more, or 2 episodes of more than 50% in a 6‐hour period, or a single residue of 100% if feed volume is less than 6 mL per feed |
Torrazza 2015.
| Study characteristics | |
| Methods | RCT |
| Participants | 61 infants born at postmenstrual age > 23 weeks but ≤ 32 weeks with birth weight ≤ 1250 g and without congenital or chromosomal anomalies or gastrointestinal malformations who were receiving some enteral nutrition by 48 hours of age |
| Interventions | Infants were randomised before 48 hours of life Routine monitoring of gastric residuals before every feeding No monitoring of gastric residuals |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | Enteral feeds were started at 20 mL/kg/d and were increased by 20 mL/kg/d. Both human milk and preterm formula were used for feeding. Abdominal distension/discolouration/tenderness, emesis, gastric residual > 50% of the feed volume or bilious aspirates were taken as signs of feed intolerance, and an abdominal radiograph was taken. If the radiograph was normal, feeds were continued; increasing length of feeds to 30 to 50 minutes; decreasing feed volume, or changing to continuous feeds was considered. If the radiograph was abnormal, feeds were withheld for 24 hours followed by reassessment |
BPD: bronchopulmonary dysplasia; CVL: central venous line; NEC: necrotising enterocolitis; PNALD: parenteral nutrition associated liver disease; TPN : total parenteral nutrition; RCT: randomised controlled trial; VAP: ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bertino 2009 | Case‐control study |
| Cobb 2004 | Case‐control study |
| Dubey 2018 | Cohort study |
| Elia 2022 | Before‐and‐after comparison study |
| Malhotra 1992 | Prospective observational study |
| Mihatsch 2002 | Prospective observational study |
| Purohit 2022 | Case‐control study |
| Riskin 2017 | Case‐control study |
| Staub 2019 | Before‐and‐after comparison study |
Characteristics of studies awaiting classification [ordered by study ID]
Lenfestey 2018.
| Methods | RCT |
| Participants | 30 preterm infants born at ≤ 32 weeks' gestation and ≤ 1250 g birth weight |
| Interventions |
Routine monitoring of gastric residual No monitoring of gastric residual |
| Outcomes |
|
| Notes |
NCT03111329.
| Methods | RCT |
| Participants | Infants born at 26 to 30 weeks’ gestational age and birth weight < 1500 g |
| Interventions |
No routine aspiration of prefeed gastric residuals: opening of the nasogastric tube once every 6 hours to relieve possible backflow of gastric content will be allowed Routine monitoring of gastric residuals before each feed |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | www.clinicaltrials.gov/ct2/show/NCT03111329 |
CVL: central venous line; NEC: necrotising enterocolitis; TPN: total parenteral nutrition; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN98322846.
| Study name | Routine gastric residual aspiration in preterm infants and the effect on reaching full feed |
| Methods | RCT |
| Participants | Infants ≤ 32 weeks' gestational age |
| Interventions |
Routine gastric aspiration group: routine prefeed aspiration of gastric residuals every 6 hours No routine gastric residual aspiration group |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | December 2015 |
| Contact information | www.isrctn.com/ISRCTN98322846 |
| Notes | Study completion estimated for December 2018 |
NCT04062851.
| Study name | Routine versus no assessment of gastric residual volumes in preterm infants |
| Methods | RCT |
| Participants | 80 preterm neonates with gestational age < 33 weeks and birth weight < 1250 g |
| Interventions |
Routine monitoring of prefeed gastric residual No monitoring of gastric residual |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 3 May 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT04062851 |
| Notes | Study completed on 26 April 2022 |
NCT04064398.
| Study name | Evaluation of gastric residuals and feedings progression (REGAP) |
| Methods | RCT |
| Participants | 240 preterm neonates born between 26 and 33 weeks' gestational age |
| Interventions | Routine monitoring of gastric residual No monitoring of gastric residual |
| Outcomes | Time to reach full feeds 150 mL/kg/d |
| Starting date | 2019 |
| Contact information | https://clinicaltrials.gov/ct2/show/NCT04064398 |
| Notes |
CVL: central venous line; NEC: necrotising enterocolitis; TPN: total parenteral nutrition; RCT: randomised controlled trial
Differences between protocol and review
For the 2019 published review, we made the following change to the protocol (Abiramalatha 2018).
For the outcome of feed interruption, while we planned to analyse the number of episodes of feed interruption lasting ≥ 12 hours in each group, authors of the included trials reported the number of infants with episodes of feed interruption lasting ≥ 12 hours.
For the 2023 update, we made the following changes.
For the outcome of time to reach full enteral feeds, we defined full enteral feeds as ≥ 150 mL/kg/d. However, the trial authors defined it variably as ≥ 120, ≥ 150 or ≥ 180 mL/kg/d.
For the outcome invasive infection, we defined sepsis as 'culture‐positive sepsis'. However, the included trials defined it variably as either culture‐positive sepsis alone or culture‐positive along with probable/clinical sepsis.
We added a new outcome 'risk of spontaneous intestinal perforation'.
We searched an additional trial registry: the World Health Organization International Clinical Trials Registry Platform (ICTRP) (https://trialsearch.who.int/Default.aspx ). We did not search the conference abstracts of the Royal College of Paediatrics and Child Health.
We used the risk of bias assessment tool RoB 2.
Contributions of authors
TA and BR screened search outputs and assessed study eligibility.
TA, ST and SR extracted and synthesised data.
TA and VVR assessed risk of bias across key domains and undertook GRADE assessment.
All authors approved the final review.
TA and ST developed the protocol (Abiramalatha 2018).
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
Editorial support for Cochrane Neonatal has been funded through a UK National Institute of Health Research (NIHR) Cochrane Programme Grant (16/114/03). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the UK Department of Health.
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
TA is an Associate Editor of Cochrane Neonatal, but was not involved in the editorial processing or acceptance of this review.
ST is an Associate Editor of Cochrane Neonatal, but was not involved in the editorial processing or acceptance of this review.
VVR has no interests to declare.
BR has no interests to declare.
SR has no interests to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Kaur 2015 {published and unpublished data}
- Kaur A, Kler N, Saluja S, Modi M, Soni A, Thakur A, et al. Abdominal circumference or gastric residual volume as measure of feed intolerance in VLBW infants. Journal of Pediatric Gastroenterology and Nutrition 2015;60(2):259-63. [DOI: 10.1097/MPG.0000000000000576] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2019 {published and unpublished data}
- Parker LA, Weaver M, Murgas Torrazza RJ, Shuster J, Li N, Krueger C, et al. Effect of aspiration and evaluation of gastric residuals on intestinal inflammation, bleeding, and gastrointestinal peptide level. Journal of Pediatrics 2020;217:165-71.e2. [DOI: 10.1016/j.jpeds.2019.10.036] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Weaver M, Murgas Torrazza RJ, Shuster J, Li N, Krueger C, et al. Effect of gastric residual evaluation on enteral intake in extremely preterm infants: a randomized clinical trial. JAMA Pediatrics 2019;173(6):534-43. [DOI: 10.1001/jamapediatrics.2019.0800] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2018 {published and unpublished data}
- Singh B, Rochow N, Chessell L, Wilson J, Cunningham K, Fusch C, et al. Gastric residual volume in feeding advancement in preterm infants (GRIP Study): a randomized trial. Journal of Pediatrics 2018;200:79-83. [DOI: 10.1016/j.jpeds.2018.04.072] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2018 {published data only}
- Thomas S, Nesargi S, Roshan P, Raju R, Mathew S, P S, Rao S. Gastric residual volumes versus abdominal girth measurement in assessment of feed tolerance in preterm neonates: a randomized controlled trial. Advances in Neonatal Care 2018;18(4):E13-9. [DOI: 10.1097/ANC.0000000000000532] [PMID: ] [DOI] [PubMed] [Google Scholar]
Torrazza 2015 {published and unpublished data}
- Torrazza RM, Parker LA, Li Y, Talaga E, Shuster J, Neu J. The value of routine evaluation of gastric residuals in very low birth weight infants. Journal of Perinatology 2015;35(1):57-60. [DOI: 10.1038/jp.2014.147] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bertino 2009 {published data only}
- Bertino E, Giuliani F, Prandi G, Coscia A, Martano C, Fabris C. Necrotizing enterocolitis: risk factor analysis and role of gastric residuals in very low birth weight infants. Journal of Pediatric Gastroenterology and Nutrition 2009;48(4):437-42. [DOI: 10.1097/mpg.0b013e31817b6dbe] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cobb 2004 {published data only}
- Cobb BA, Carlo WA, Ambalavanan N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics 2004;113(1 (Pt 1)):50-3. [DOI: 10.1542/peds.113.1.50] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dubey 2018 {published data only}
- Dubey SP, Jain A, Kumari N, Bhatnagar A, Devgan V. Pre feed aspirates vs abdominal girth monitoring for detection of feed intolerance in VLBW babies. Indian Journal of Neonatal Medicine & Research 2018;6(2):PO06-12. [DOI: 10.7860/IJNMR/2018/35573.2229] [DOI] [Google Scholar]
Elia 2022 {published data only}
- Elia S, Ciarcià M, Miselli F, Bertini G, Dani C. Effect of selective gastric residual monitoring on enteral intake in preterm infants. Italian Journal of Pediatrics 2022;48(1):30. [DOI: 10.1186/s13052-022-01208-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malhotra 1992 {published data only}
- Malhotra AK, Deorari AK, Paul VK, Bagga A, Singh M. Gastric residuals in preterm babies. Journal of Tropical Pediatrics 1992;38(5):262-4. [DOI: 10.1093/tropej/38.5.262] [PMID: ] [DOI] [PubMed] [Google Scholar]
Mihatsch 2002 {published data only}
- Mihatsch WA, Schoenaich P, Fahnenstich H, Dehne N, Ebbecke H, Plath C, et al. The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics 2002;109(3):457-9. [DOI: 10.1542/peds.109.3.457] [PMID: ] [DOI] [PubMed] [Google Scholar]
Purohit 2022 {published data only}
- Purohit G, Mehkarkar P, Athalye-Jape G, Nathan E, Patole S. Association of gastric residual volumes with necrotising enterocolitis in extremely preterm infants-a case-control study. European Journal of Pediatrics 2022;181(1):253-60. [DOI: 10.1007/s00431-021-04193-x] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riskin 2017 {published data only}
- Riskin A, Cohen K, Kugelman A, Toropine A, Said W, Bader D. The impact of routine evaluation of gastric residual volumes on the time to achieve full enteral feeding in preterm infants. Journal of Pediatrics 2017;189:128-34. [DOI: 10.1016/j.jpeds.2017.05.054] [PMID: ] [DOI] [PubMed] [Google Scholar]
Staub 2019 {published data only}
- Staub E, Van Vuuren A, Priest C, McNaught E, Paradisis M. Introduction of a standardized feeding protocol without gastric asirates improves enteral nutrition in VLBW infants. Journal of Paediatrics and Child Health 2019;55(S1):102. [DOI: 10.1111/jpc.14410_143] [DOI] [Google Scholar]
References to studies awaiting assessment
Lenfestey 2018 {published data only}
- Lenfestey W. Does routine gastric residual aspiration affect the preterm infant fecal microbiome? In: Pediatric Academic Societies 2018 Meeting. Toronto Canada.
NCT03111329 {published data only}
- NCT03111329. Does routine assessment of gastric residuals in pretermneonates influence time taken to reach full enteral feeding? (GRASS). www.clinicaltrials.gov/ct2/show/NCT03111329 (first received 12 April 2017).
References to ongoing studies
ISRCTN98322846 {published data only}
- ISRCTN98322846. Routine gastric residual aspiration in preterm infants and the effect on reaching full feed. https://www.isrctn.com/ISRCTN98322846 (first received 19 December 2017). [CENTRAL: CN-01900301] [DOI: 10.1186/ISRCTN98322846] [DOI]
NCT04062851 {published data only}
- NCT04062851. Routine versus no assessment of gastric residual volumes in preterm infants. https://clinicaltrials.gov/ct2/show/NCT04062851 (first received 20 August 2019). [CENTRAL: CN-01983601]
NCT04064398 {published data only}
- NCT04064398. Evaluation of gastric residuals and feedings progression (REGAP). https://clinicaltrials.gov/ct2/show/NCT04064398 (first received 21 August 2021).
Additional references
Abiramalatha 2023
- Abiramalatha T, Thanigainathan S, Ramaswamy VV, Rajaiah B, Ramakrishnan S. Re-feeding versus discarding gastric residuals to improve growth in preterm infants. Cochrane Database of Systematic Reviews (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Athalye‐Jape 2020
- Athalye-Jape G, Nettleton M, Lai CT, Nathan E, Geddes D, Simmer K, et al. Composition of coloured gastric residuals in extremely preterm infants- a nested prospective observational study. Nutrients 2020;12(9):2585. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2015
- Bartlett Ellis RJ, Fuehne J. Examination of accuracy in the assessment of gastric residual volume: a simulated, controlled study. Journal of Parenteral and Enteral Nutrition 2015;39(4):434-40. [DOI: 10.1177/0148607114524230] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cohen 2004
- Cohen S, Mandel D, Mimouni FB, Solovkin L, Dollberg S. Gastric residual in growing preterm infants: effect of body position. American Journal of Perinatology 2004;21(3):163-6. [DOI: 10.1055/s-2004-823778] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dorling 2020
- Dorling J, Tume L, Arch B, Woolfall K, Latten L, Roper L, et al. Gastric residual volume measurement in British neonatal intensive care units: a survey of practice. BMJ Paediatrics Open 2020;4(1):e000601. [DOI: 10.1136/bmjpo-2019-000601] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duro 2011
- Duro D, Mitchell PD, Kalish LA, Martin C, McCarthy M, Jaksic T, et al. Risk factors for parenteral nutrition–associated liver disease following surgical therapy for necrotizing enterocolitis: a Glaser Pediatric Research Network Study. Journal of Pediatric Gastroenterology and Nutrition 2011;52(5):595-600 [Erratum in:Journal of Pediatric Gastroenterology and Nutrition 2011;53(5):583]. [DOI: 10.1097/MPG.0b013e31820e8396] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutta 2015
- Dutta S, Singh B, Chessell L, Wilson J, Janes M, McDonald K, et al. Guidelines for feeding very low birth weight infants. Nutrients 2015;7(1):423-42. [DOI: 10.3390/nu7010423] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Embleton 2013
- Embleton ND. Early nutrition and later outcomes in preterm infants. World Review of Nutrition and Dietetics 2013;106:26-32. [DOI: 10.1159/000342553] [PMID: ] [DOI] [PubMed] [Google Scholar]
Franz 2009
- Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009;123(1):e101-9. [DOI: 10.1542/peds.2008-1352] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gephart 2017
- Gephart SM, Fleiner M, Kijewski A. The conNECtion between abdominal signs and necrotizing enterocolitis in infants 501 to 1500 g. Advances in Neonatal Care 2017;17(1):53-64. [DOI: 10.1097/ANC.0000000000000345] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gozen 2021
- Gözen D, Erkut Z, Uslubaş R, Bilgin L E. Effect of different positions on gastric residuals in preterm infants initiating full enteral feeding. Nutrition in Clinical Practice 2021;37(4):945-54. [DOI: 10.1002/ncp.10789] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 11 September 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Gregory 2012
- Gregory KE, Connolly TC. Enteral feeding practices in the NICU: results from a 2009 Neonatal Enteral Feeding Survey. Advances in Neonatal Care 2012;12(1):46-55. [DOI: 10.1097/ANC.0b013e3182425aab] [PMID: ] [DOI] [PubMed] [Google Scholar]
Grino 2016
- Grino CU, Miller M, Carrasquilla-Lopez V, Rojas M, Bhutanda A, Rastogi S, et al. Early prediction of severity of necrotizing enterocolitis in preterm infants: a composite scoring tool. Clinical Nursing Studies 2016;4(3):47-53. [DOI: 10.5430/cns.v4n3p47] [DOI] [Google Scholar]
Hermansen 2005
- Hermansen MC, Hermansen MG. Intravascular catheter complications in the neonatal intensive care unit. Clinics in Perinatology 2005;32(1):141-56. [DOI: 10.1016/j.clp.2004.11.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2022a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Higgins 2022b
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Higgins 2022c
- Higgins JPT, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ittmann 1992
- Ittmann PI, Amarnath R, Berseth CL. Maturation of antroduodenal motor activity in preterm and term infants. Digestive Diseases and Sciences 1992;37(1):14-9. [DOI: 10.1007/BF01308336] [PMID: ] [DOI] [PubMed] [Google Scholar]
Juvé‐Udina 2009
- Juvé-Udina ME, Valls-Miró C, Carreño-Granero A, Martinez-Estalella G, Monterde-Prat D, Domingo-Felici CM, et al. To return or to discard? Randomised trial on gastric residual volume management. Intensive and Critical Care Nursing 2009;25(5):258-67. [DOI: 10.1016/j.iccn.2009.06.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kenton 2004
- Kenton AB, Fernandes CJ, Berseth CL. Gastric residuals in prediction of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2004;113(6):1848-9. [DOI: 10.1542/peds.113.6.1848] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kumar 2021
- Kumar J, Meena J, Mittal P, Shankar J, Kumar P, Shenoi A. Routine prefeed gastric aspiration in preterm infants: a systematicreview and meta-analysis. European Journal of Pediatrics 2021;180(8):2367-77. [DOI: 10.1007/s00431-021-04122-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Leppänen 2014
- Leppänen M, Lapinleimu H, Lind A, Matomäki J, Lehtonen L, Haataja L, et al. Antenatal and postnatal growth and 5-year cognitive outcome in very preterm infants. Pediatrics 2014;133(1):63-70. [DOI: 10.1542/peds.2013-1187] [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2014
- Li YF, Lin HC, Torrazza RM, Parker L, Talaga E, Neu J. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatrics and Neonatology 2014;55(5):335-40. [DOI: 10.1016/j.pedneo.2014.02.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lucchini 2011
- Lucchini R, Bizzarri B, Giampietro S, De Curtis M. Feeding intolerance in preterm infants. How to understand the warning signs. Journal of Maternal-Fetal & Neonatal Medicine 2011;24 Suppl 1:72-4. [DOI: 10.3109/14767058.2011.607663] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore TA, Wilson ME. Feeding intolerance: a concept analysis. Advances in Neonatal Care 2011;11(3):149-54. [DOI: 10.1097/ANC.0b013e31821ba28e] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 1999
- Morris BH, Miller-Loncar CL, Landry SH, Smith KE, Swank PR, Denson SE. Feeding, medical factors, and developmental outcome in premature infants. Clinical Pediatrics 1999;38(8):451-7. [DOI: 10.1177/000992289903800802] [PMID: ] [DOI] [PubMed] [Google Scholar]
Parker 2015
- Parker L, Torrazza RM, Li Y, Talaga E, Shuster J, Neu J. Aspiration and evaluation of gastric residuals in the neonatal intensive care unit: state of the science. Journal of Perinatal and Neonatal Nursing 2015;29(1):51-9. [DOI: 10.1097/JPN.0000000000000080] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perumbil Pathrose 2021
- Perumbil Pathrose S, Spence K, Taylor C, Psalia K, Schmied V, Dahlen H, et al. A cross-sectional survey of enteral feeding tube placement and gastric residual aspiration practices: need for an evidence-based clinical practice guideline. Advances in Neonatal Care 2021;21(5):418-24. [DOI: 10.1097/ANC.0000000000000822] [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4. 1 2.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Riezzo 2000
- Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, et al. Gastric electrical activity and gastric emptying in term and preterm newborns. Neurogastroenterology and Motility 2000;12(3):223-9. [DOI: 10.1046/j.1365-2982.2000.00203.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from https://gdt.gradepro.org/app/handbook/handbook.html..
Shulman 2011
- Shulman RJ, Ou CN, Smith EO. Evaluation of potential factors predicting attainment of full gavage feedings in preterm infants. Neonatology 2011;99(1):38-44. [DOI: 10.1159/000302020] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2016
- Stevens TP, Shields E, Campbell D, Combs A, Horgan M, La Gamma EF, et al. Variation in enteral feeding practices and growth outcomes among very premature infants: a report from the New York State Perinatal Quality Collaborative. American Journal of Perinatology 2016;33(1):9-19. [DOI: 10.1055/s-0035-1554794] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2010
- Williams TA, Leslie GD. Should gastric aspirate be discarded or retained when gastric residual volume is removed from gastric tubes? Australian Critical Care 2010;23(4):215-7. [DOI: 10.1016/j.aucc.2010.05.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Xu 2019
- Xu JH, Coo H, Fucile S, Ng E, Ting JY, Shah PS, et al, Canadian Neonatal Network Investigators. A national survey of the enteral feeding practices in Canadian neonatal intensive care units. Paediatrics and Child Health 2019;25(8):529-33. [DOI: 10.1093/pch/pxz112] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Abiramalatha 2018
- Abiramalatha T, Thanigainathan S, Ninan B. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD012937. [DOI: 10.1002/14651858.CD012937] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abiramalatha 2019
- Abiramalatha T, Thanigainathan S, Ninan B. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2019, Issue 7(7):CD012937. Art. No: CD012937. [DOI: 10.1002/14651858.CD012937.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


