Abstract
Aim
There is mounting evidence that eating habits affect sleeping patterns and their quality. The goal of this study was to evaluate the associations between major dietary patterns, identified using principal component analysis (PCA) and insomnia in young women.
Methods
The study subjects comprised 159 healthy young women aged 18–25 years. Neuropsychological assessment was performed using standard instruments, including a cognitive ability questionnaire (CAQ), depression and anxiety stress scales (DASS‐21), insomnia severity index (ISI), Epworth sleepiness scale (ESS), and quality of life questionnaire (QLQ). Dietary patterns were obtained from a 65‐item validated food frequency questionnaire (FFQ) in this study, using PCA.
Results
Two major dietary patterns were identified that were termed: “Traditional” and “Western.” The Western pattern was characterized by a high intake of snacks, nuts, dairy products, tea, fast foods, chicken, and vegetable oils. Subjects with moderate/severe insomnia were found to have lower scores for total cognitive ability task, nocturnal sleep hours, and physical and mental health, but higher scores for depression, anxiety, stress, and daytime sleepiness compared to those without insomnia (p < 0.05). After adjustment for potential confounders, high adherence to the Western dietary pattern was associated with higher odds of insomnia (OR = 5.9; 95% confidence intervals: 1.9–18.7; p = 0.003).
Conclusion
Our findings indicated adherence to Western pattern may increase the odds of insomnia. Prospective research is required to determine the feasibility of targeting dietary patterns to decrease the odds of insomnia.
Keywords: depression, insomnia, quality of life, stress, Western dietary
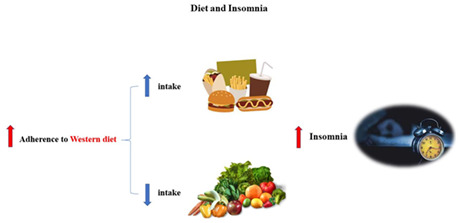
The present study's summary and hypothesis. Adherence to a WD style, characterized by a high intake of fast food, chicken, and snacks and a low intake of vegetables, was associated with a higher risk of insomnia.
1. INTRODUCTION
Sleep, described as a mental state of conceptual disengagement with few responses to the environment, is a crucial part of life. 1 Sleep is necessary for functional, psychological, and emotional health. 2 The quality of sleep is necessary for maximum cognitive performance and sleep disturbances, which include having problems falling or staying asleep, having a regular sleep/wake cycle, and sleepiness may affect an individual's ability to function effectively and cause impairment in cognitive and behavioral abilities. 3 , 4 , 5
It has been reported that sleep disorders may be associated with memory impairment, reduced learning capacity, poor academic function, tiredness, attention deficiency, and depression. 6 Insomnia, described as having trouble falling and maintaining sleep, early awakening, and experiencing no refreshing sleep, 7 is usually linked to adverse consequences in behavioral and occupational areas, including interference in a person's ability to function, exhaustion, overeating, impaired memory, and depression. 8 , 9 , 10 Insomnia, one of the most common sleep‐related complications, also affects the quality of life in a high percentage of people globally. 11 , 12 , 13
It has been suggested that diet is an important determinant of sleep quality and a contributor to insomnia. 14 , 15 , 16 An association between sleep and dietary habits has been found in a cross‐sectional survey of 3129 female workers, suggesting that a diet composed of low vegetable and fish consumption, besides high carbohydrate intake and weak eating habits was linked to poor sleep quality. 9 After a six‐month dietary intervention in obese men with insomnia, Tan et al. showed that the diet group, which was advised to consume optimized nutrients and 300–500 kcal less energy per day, experienced a prolonged total sleep period, and improved objective sleep efficiency, compared with the control group. 17
Previous investigations on sleep disorders and dietary intake have explored the effects of dietary styles, such as a “prudent” or “Mediterranean” pattern, on insomnia, 18 , 19 suggesting that some dietary patterns may affect daily alertness, nocturnal sleep, and have related eating habits with sleep quality. 18 , 20 , 21 , 22 For instance, adherence to the Mediterranean diet (MD) leads to fewer features of insomnia, such as difficulty initiating sleep in females. 23 It has been shown that a high intake of sweets, sugary drinks, and carbohydrates was correlated with poor sleep quality, but vegetables and oily fish consumption, probably due to the great quantity of docosahexaenoic acid (omega‐3) and vitamin D, was correlated with better sleep quality. 15 , 21 , 23 Beigrezaei and co‐workers investigated the relationship between probable insomnia and dietary habits using a food frequency questionnaire (FFQ) with 147 food items among adolescent girls in Iran and showed that a lower intake of spicy foods and eating breakfast and regular meals, was associated with a reduced risk of insomnia. 24 Since the diet is consumed as a complex combination of nutrients, it is challenging to distinguish between the various effects related to confounding and interactions because of the high association between the intake of particular nutrients and foods. 25 To resolve these difficulties, research on nutritional epidemiology has increasing focused on dietary pattern analysis. Dietary patterns are aggregate assessments of many foods or dietary groups that are believed to have a higher impact on disease risk than any single nutrient. 26
An important question, which previous studies have not adequately addressed, is whether dietary patterns can influence sleep complications, especially insomnia. Several studies reported that the “traditional Iranian” dietary pattern was highly loaded with legumes, vegetables, fruits, red meat, potato, egg, refined grain, sugar, French fries, and tea. 27 , 28 , 29 Due to the great economic burden, the potential impact of sleep deprivation on psychological health, and the high prevalence of insomnia, we carried out this investigation to explore the relationship between major dietary patterns and insomnia in young women, a group that is significantly more prone to sleep disorders and sleep insufficiency due to multiple contributing factors such as hormonal fluctuations and menopausal transition. 13 , 30
2. MATERIALS AND METHODS
2.1. Study design
Our study sample comprised 159 female students aged 18–25 y, who were recruited from 5 universities in Birjand province, Iran, between December 2019 and February 2020. 31 Individuals with any severe or persistent systemic disorders or a previous medical record of any psychological conditions that included depression and aggressiveness were excluded. The Ethics Committee of our university approved the study and volunteers gave written informed consent.
2.2. Dietary intake assessment
A validated semi‐quantitative FFQ was used, which contained 65 food items with 5 frequency categories (consumption basis per day, week, month, rarely, and never) for each food item. 32 , 33 The FFQ is a checklist of the dietary history that allows an estimate of long‐term dietary intakes in a simple, cost‐effective, and time‐saving method. The FFQ was completed by an experienced nutritionist via a face‐to‐face interview. The dietary nutrient intakes of participants were calculated, 34 and then dietary patterns were determined based on 24 pre‐defined food groups (Table 1) conforming to the similarity of FFQ food items.
TABLE 1.
Food grouping used in factor analysis of dietary patterns.
| Refined grains | White breads (lavash, baguettes), rice, macaroni, pasta |
|---|---|
| Whole grains | Dark breads (Iranian) |
| Fast foods | Pizza, processed meat |
| Snacks | Biscuits, cakes and pastries, chocolate, ice cream, chips |
| Dairy products | Whole milk, low‐fat milk, yogurt, breakfast cheese, Dough |
| Solid fats | Butter, cream, solid oil, tall, salad dressing |
| Liquid fats | Liquid oil, olive oil |
| Sugars | Honey, sugar loaf, diabetic sugar, sugar |
| Fruits | Tree fruit, seasonal fruit, fruit compote, fruit juice, dried fruits |
| Carbonated beverages | Soft drinks, beer, diet drinks |
| Teas | Tea |
| Coffee drinks | Coffee, coffee and milk, Nescafe |
| Legumes | Beans, soy |
| Pickled foods | Pickles, salty Cucumber |
| Green vegetables | The vegetables, lettuce, spinach |
| Other vegetables | Garlic, onion, tomato, Cucumber, salad (mixed salad of tomato, Cucumber, and onion) |
| Potatos | Boiled potato, other potatoes, French fries, cutlet, and potato cutlets |
| Liquid foods | Soup |
| Eggs | Boiled egg, scrambled eggs |
| Red Meats | Lamb meat, beef, hunting meat |
| Organ meats | Heart, liver and kidney, intestine, and viscera |
| Sea foods | Fish, fish tuna, shrimp |
| Chicken | Chicken feed |
| Nuts | Walnut, all types of nuts |
2.3. Anthropometric and cardiometabolic variables evaluation
Demographic and anthropometric variables were measured using standard methods. 35 Body mass index (BMI) was computed by dividing weight (kg) by height squared (m2). 35 Blood specimens were collected after an overnight fast. A full blood count was determined using a SysmexK‐800 auto‐analyzer.
2.4. Neuropsychological evaluation
2.4.1. Insomnia
The Insomnia Severity Index (ISI) is a short self‐report instrument that quantitatively quantifies the intensity of insomnia based on the patient's perception. The questionnaire contains 7 questions. Each item is rated on a 0 to 4 point to provide a total score ranging from 0 to 28. Higher scores show more severe insomnia. Scores may be classified as no insomnia (0–7), subthreshold or mild 8 , 9 , 10 , 11 , 12 , 13 , 14 insomnia, 15 , 16 , 17 , 18 , 19 , 20 , 21 and severe insomnia. 22 , 23 , 24 , 25 , 26 , 27 , 28 A higher score indicates more degree of insomnia. The Persian version of this test has been validated previously and is used in clinical research. 36 , 37
2.4.2. Sleepiness
The ESS is a validated approach for measuring the level of daytime sleepiness. The ESS is a brief, self‐administered questionnaire, that estimates the probability of falling asleep in numerous situations. It has 8 items with a 4‐point Likert scale which wants the subject to evaluate on a scale of 0–3 the chances that he frequently has dozed in common daily situations. The Sum of scores can range from 0 to 24 and higher scores specify deteriorate sleepiness. 37 , 38
2.4.3. Quality of life (QoL)
Health‐related quality of life was evaluated by the SF‐12. This version used in this study is a shortened version of SF‐36 called SF‐12, which applies 12 questions from SF‐36. 39 The validity of this test has been confirmed by the Iranian people. 40 The questionnaire analyzes 8 aspects of health to determine physical and intellectual health. Higher numbers demonstrate major well‐being. 41
2.4.4. Depression anxiety stress
The DASS is a 42‐item self‐evaluation survey used to scale the intensity of psychological states. DASS‐21 is a shorter form of evaluating 42‐item detail on depression, anxiety, and stress (DASS). 42 This test contains 21 items with 3 subscales with a 4‐Likert measure scored 0–3 and the final score of each subscale should be doubled. A higher score represents poorer emotional reactions. The Persian revision of DASS‐21 has been validated formerly. 43
2.4.5. Cognitive ability assessment
The Cognitive Abilities Questionnaire is a valid instrument that measures cognitive abilities in 7 defined components of memory and cognition and cognitive flexibility. 44 This questionnaire includes 30 items rated on a 5‐point Likert measure. Higher scores reflect better cognition abilities.
2.5. Statistical analysis
All statistical analyses were undertaken using SPSS, version 16 software. Principal component analysis (PCA) was applied to identify major dietary patterns regarding 24 pre‐defined food groups. Varimax rotation was managed to make a simple and final component matrix. We derived 2 factors with Eigenvalues of more than 1 and analysis of a scree plot. So, 2 dietary patterns were distinguished and labeled based on our data explanation and the past reports. For all participants, the factor scores of each identified pattern were accumulated by summing up intakes of foods weighed by their factor loading. Individuals were sub‐grouped based on their insomnia status. First, variables were assessed for normality, by recruiting the Kolmogorov–Smirnov test, and normally distributed variables were compared using parametric tests. Continuous and categorical indices are displayed by mean ± SD and number (percent), respectively. ANOVA test and post hoc Tukey were applied to measure the significant difference in normal variables among groups. Eventually, logistic regression in different models was used for exploring the association between the tertiles of two main dietary patterns with insomnia. All analyses were considered using two‐tailed tests, and a p‐value less than 0.05 was set as significant.
3. RESULTS
3.1. Recognition of main dietary patterns
Principal component analysis (PCA) was used to determine dietary patterns within the study population; two major dietary patterns were identified that we labeled as traditional and Western patterns. A traditional dietary pattern was characterized by a high intake of green leafy vegetables, other vegetables, organic meat, potato, solid oils, fruits, beans, refined grains, red meat, eggs, whole grains, and sugars. A Western dietary (WD) pattern is described by a high intake of snacks, nuts, dairy products, tea, fast foods, chicken, and vegetable oils. The factor‐loading matrixes for 2 dietary patterns are demonstrated in Table 2.
TABLE 2.
Food loading matrix for major dietary patterns.
| Dietary patterns | ||
|---|---|---|
| Food group | Traditional | Western |
| Other vegetables | 0.77 | ‐ |
| Organ's meat | 0.66 | ‐ |
| Potato | 0.66 | ‐ |
| Green vegetables | 0.58 | ‐ |
| Solid oils | 0.46 | ‐ |
| Fruits | 0.44 | ‐ |
| Legumes | 0.37 | ‐ |
| Refined grains | 0.34 | ‐ |
| Red meat | 0.34 | ‐ |
| Eggs | 0.28 | ‐ |
| Whole grains | 0.25 | ‐ |
| Sugars | 0.21 | ‐ |
| Pickled foods | ‐ | ‐ |
| Sea foods | ‐ | ‐ |
| Liquid foods | ‐ | ‐ |
| Snacks | ‐ | 0.82 |
| Nuts | ‐ | 0.78 |
| Dairy products | ‐ | 0.76 |
| Tea | ‐ | 0.51 |
| Fast foods | ‐ | 0.41 |
| Chicken | ‐ | 0.33 |
| Vegetable oils | ‐ | 0.30 |
| Carbonated Beverages | ‐ | ‐ |
| Coffee drinks | ‐ | ‐ |
Values less than 0.20 are not reported.
3.2. Association between the general characteristics of participants across the insomnia category
Participants were categorized into 3 groups based on their ISI scores (Table 3): participants with no insomnia (n = 91), subjects with mild insomnia (n = 46), and cases with moderate/severe insomnia (n = 22). The age was significantly different across insomnia categories, and participants with moderate/severe insomnia were older compared to those without insomnia (p = 0.047). However, we found no significant differences between anthropometric parameters, hematological indices, and cardiometabolic factors across these three groups (p > 0.05).
TABLE 3.
Association between the general characteristics of participants across insomnia category.
| Variables | No insomnia (n = 91) | Mild insomnia (n = 46) | Moderate/severe insomnia (n = 22) | p‐value* |
|---|---|---|---|---|
| Age (y) | 20.9 ± 1.7 | 20.5 ± 1.5 | 21.6 ± 2.1 | 0.047 |
| BMI (Kg/m2) | 21.2 ± 3.1 | 20.8 ± 2.4 | 20.7 ± 2.1 | 0.69 |
| Weight (Kg) | 55.6 ± 8.6 | 54.2 ± 6.8 | 54.8 ± 7.3 | 0.65 |
| WHR | 0.73 ± 0.05 | 0.73 ± 0.03 | 0.73 ± 0.03 | 0.90 |
| SBP (mmHg) | 10.6 ± 0.8 | 10.8 ± 1.0 | 10.7 ± 1.3 | 0.48 |
| DBP (mmHg) | 7.1 ± 0.7 | 7.1 ± 0.8 | 7.1 ± 0.8 | 0.98 |
| Hb (g/dl) | 13.9 ± 1.5 | 14.0 ± 1.3 | 13.6 ± 1.0 | 0.61 |
| Hct (%) | 41.6 ± 4.1 | 41.9 ± 2.8 | 40.9 ± 2.5 | 0.65 |
Abbreviations: BMI, Body mass index; DBP, diastolic blood pressure; Hb, hemoglobin; Hct, hematocrit; SBP, systolic blood pressure; WHR, waist‐to‐hip ratio.
Note: Data presented as mean ± SD.
By using one‐way ANOVA test. Significance of bold values are p < 0.05.
3.3. Association between neuropsychological function and insomnia category
We found an association between the results of the neuropsychological functions and insomnia severity as shown in Table 4. Subjects with moderate/severe insomnia had lower scores for memory, inhibitory control, and selective attention, social cognition, cognitive flexibility, total cognitive ability task, nocturnal sleep hours, physical and mental health, but higher degree of depression, anxiety, stress, and daytime sleepiness compared to those without insomnia (p < 0.05). No significant differences were found in decision‐making, planning, and sustained attention across insomnia between the groups (p > 0.05).
TABLE 4.
Association between neuropsychological function and insomnia category.
| Variables | No insomnia (n = 91) | Mild insomnia (n = 46) | Moderate/severe insomnia (n = 22) | p‐value* |
|---|---|---|---|---|
| Test of cognitive abilities | ||||
| Memory | 25.6 ± 3.5 | 25.9 ± 3.2 | 23.7 ± 4.0 | 0.039 |
| Inhibitory control and selective attention | 21.9 ± 4.1 | 22.7 ± 2.7 | 19.2 ± 4.1 | 0.001 |
| Decision making | 18.6 ± 3.8 | 19.4 ± 3.2 | 17.8 ± 3.4 | 0.182 |
| Planning | 11.3 ± 2.8 | 11.4 ± 2.3 | 9.9 ± 2.8 | 0.064 |
| Sustain attention | 9.6 ± 2.3 | 9.6 ± 2.6 | 8.9 ± 2.3 | 0.42 |
| Social cognition | 10.3 ± 2.0 | 11.3 ± 2.0 | 11.0 ± 2.5 | 0.036 |
| Cognitive flexibility | 14.4 ± 2.7 | 15.3 ± 2.4 | 13.5 ± 3.3 | 0.035 |
| Total cognitive ability task | 111.7 ± 14.3 | 115.6 ± 10.5 | 103.5 ± 14.0 | 0.003 |
| Dass‐21 | ||||
| Depression | 10.2 ± 8.2 | 9.8 ± 8.4 | 17.6 ± 11.0 | 0.001 |
| Anxiety | 7.5 ± 5.4 | 8.7 ± 5.7 | 14.3 ± 7.7 | <0.001 |
| Stress | 16.2 ± 9.8 | 16.4 ± 9.2 | 24.4 ± 10.0 | 0.002 |
| Quality of life (SF‐12) | ||||
| Physical health | 16.5 ± 2.2 | 15.9 ± 1.8 | 13.0 ± 3.1 | <0.001 |
| Mental health | 17.3 ± 3.6 | 15.9 ± 3.8 | 14.2 ± 3.3 | 0.001 |
| SF‐12 score | 33.9 ± 4.7 | 31.9 ± 4.7 | 10.7 ± 5.3 | <0.001 |
| Test of sleep pattern | ||||
| Daytime sleepiness score (ESS) | 4.0 ± 5.5 | 8.9 ± 4.6 | 10.7 ± 53 | <0.001 |
| Nocturnal sleep hours | 7.5 ± 1.2 | 6.9 ± 1.2 | 6.4 ± 1.6 | <0.001 |
Note: Data presented as mean ± SD.
By using one‐way ANOVA test. Significance of bold values are p < 0.05.
3.4. Food group and nutrients intake of study participants across tertiles of major identified dietary patterns
The consumption of food groups and nutrients across tertiles of the 2 dietary patterns are shown in Table 5. Tertile 1 is set as the lowest tertile (lowest adherence), and tertile 3 is the highest tertile (highest adherence). Dietary intake of chicken, total monounsaturated fatty acids (MUFAs), and vitamin A were significantly lower in the third tertile of the traditional pattern versus the first tertile. Moreover, consumption of red meat, organs meat, dairy products, fruits, whole grains, refined grains, vegetable oil, eggs, potato, total fiber, green vegetables, other vegetables, total polyunsaturated fatty acids (PUFAs), and vitamin C was higher in the 3rd tertile of traditional pattern versus the 1st tertile (p < 0.05). Lower intake of vitamin A was seen in the highest tertile of WD compared with the 1st tertile. However, the intake of dairy products, snacks, nuts, carbonated beverages, sugars, MUFAs, PUFA, and vitamin E was increased in the third tertile of WD versus the first tertile.
TABLE 5.
Dietary intake of study participants by tertiles (T) categories of dietary pattern scores.
| Variables | Traditional pattern | p‐value † | Western pattern | p‐value † | ||
|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 3 | Tertile 1 | Tertile 3 | |||
| Food groups (g) | ||||||
| Red meat | 29.6 ± 26.1 | 62.9 ± 26.6 | 0.005 | 53.3 ± 78.3 | 41.5 ± 38.3 | 0.52 |
| Organ's meat | 0.06 ± 0.24 | 2.6 ± 5.6 | <0.001 | 0.72 ± 2.43 | 1.31 ± 4.8 | 0.66 |
| Dairy product | 156.6 ± 109 | 249.2 ± 137.1 | <0.001 | 146.8 ± 82.8 | 242.6 ± 137.7 | <0.001 |
| Fast foods | 51.7 ± 96.5 | 90.5 ± 119.9 | 0.16 | 51.7 ± 75.3 | 87.6 ± 130.1 | 0.20 |
| Fruit | 138.5 ± 117.2 | 316.0 ± 272.3 | <0.001 | 170.3 ± 141.6 | 236.7 ± 169.2 | 0.22 |
| Chicken | 40.4 ± 34.0 | 21.6 ± 24.0 | 0.001 | 25.1 ± 23.1 | 28.9 ± 31.9 | 0.78 |
| Legumes | 19.7 ± 16.2 | 25.7 ± 30.0 | 0.38 | 27.9 ± 24.2 | 20.7 ± 18.4 | 0.24 |
| Coffee | 301.8 ± 1904 | 34.6 ± 63.5 | 0.44 | 22.5 ± 48.7 | 357 ± 2017 | 0.28 |
| Whole grains | 15.4 ± 17.0 | 39.5 ± 47.5 | 0.014 | 31.5 ± 49.1 | 36.4 ± 47.1 | 0.84 |
| Refined grains | 157.6 ± 74.8 | 266.3 ± 248.9 | 0.003 | 163.9 ± 131 | 199.4 ± 127 | 0.55 |
| See foods | 16.0 ± 18.9 | 16.7 ± 26.4 | 0.98 | 13.1 ± 9.8 | 15.5 ± 21.4 | 0.81 |
| Snacks | 58.6 ± 57.5 | 45.0 ± 34.3 | 0.30 | 25.6 ± 13.7 | 90.7 ± 62.3 | <0.001 |
| Nuts | 17.7 ± 37.6 | 26.4 ± 33.9 | 0.38 | 7.9 ± 12.7 | 44.6 ± 48.7 | <0.001 |
| Vegetable's oil | 26.0 ± 33.7 | 47.0 ± 41.6 | 0.013 | 33.6 ± 38.5 | 45.1 ± 45.5 | 0.27 |
| Eggs | 10.3 ± 8.5 | 23.4 ± 19.7 | <0.001 | 19.4 ± 17.8 | 15.0 ± 14.2 | 0.27 |
| Potato | 28.0 ± 16.7 | 92.4 ± 67.8 | <0.001 | 53.7 ± 55.4 | 63.6 ± 58.2 | 0.57 |
| Pickled food | 55.7 ± 132.8 | 50.7 ± 87.3 | 0.96 | 31.2 ± 51.1 | 58.6 ± 132.2 | 0.31 |
| Carbonated beverage | 50.1 ± 65.6 | 77.4 ± 121.8 | 0.45 | 47.9 ± 75.7 | 118.2 ± 174.7 | 0.005 |
| Green vegetables | 4.4 ± 4.8 | 10.0 ± 8.9 | <0.001 | 13.4 ± 17.1 | 9.6 ± 11.9 | 0.38 |
| Other vegetables | 43.7 ± 36.2 | 221.4 ± 165.5 | <0.001 | 104.3 ± 104.1 | 130.2 ± 144.8 | 0.54 |
| Sugar | 12.4 ± 13.4 | 18.5 ± 29.5 | 0.46 | 7.6 ± 7.2 | 28.6 ± 40.9 | <0.001 |
| Nutrients * | ||||||
| Protein (g) | 61.3 ± 17.3 | 63.4 ± 24.4 | 0.87 | 62.4 ± 24.9 | 57.6 ± 23.9 | 0.34 |
| Total carbohydrates (g) | 113.7 ± 45.5 | 128.3 ± 73.9 | 0.42 | 103.5 ± 51.2 | 125.5 ± 66.4 | 0.14 |
| Total fiber (g) | 11.2 ± 4.0 | 15.9 ± 7.5 | <0.001 | 13.6 ± 5.3 | 12.6 ± 5.4 | 0.68 |
| Total fat (g) | 32.1 ± 21.5 | 23.4 ± 28.5 | 0.19 | 33.8 ± 21.5 | 30.0 ± 27.4 | 0.74 |
| Total SFAs (g) | 17.7 ± 7.0 | 16.9 ± 11.5 | 0.89 | 18.8 ± 8.8 | 16.6 ± 9.6 | 0.47 |
| Total MUFAs (g) | 22.4 ± 24.6 | 13.9 ± 15.9 | 0.049 | 15.1 ± 8.0 | 25.2 ± 27.9 | 0.013 |
| Total PUFAs (g) | 25.4 ± 22.8 | 42.4 ± 26.7 | 0.002 | 29.6 ± 25.1 | 43.4 ± 28.4 | 0.012 |
| Vitamin C (mg) | 84.2 ± 57.0 | 173.7 ± 153 | <0.001 | 114.7 ± 84.8 | 122.9 ± 16.3 | 0.98 |
| Vitamin E (mg) | 24.5 ± 21.1 | 40.0 ± 27.1 | 0.69 | 29.1 ± 25.3 | 40.3 ± 23.9 | 0.035 |
| Vitamin A (μg) | 103.4 ± 67.2 | 38.3 ± 34.1 | <0.001 | 86.9 ± 60.3 | 40.9 ± 40.2 | <0.001 |
Abbreviations: MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acids.
All values are mean ± SD and adjusted for energy intake. †By using one‐way ANOVA. Significance of bold values are p < 0.05.
3.5. Multivariate‐adjusted odds ratios (95% CIs) for insomnia across tertiles of identified dietary patterns
Linear regression analysis showed a direct association between the consumption of red meat, carbonated beverage, and total PUFAs with scores of insomnias (Table 6). Refined grains, total fat, and vitamin E intake were inversely related to insomnia scores (β = −0.006, p = 0.046; β = −0.047; p = 0.029, and β = −0.053; p = 0.047, respectively).
TABLE 6.
Multivariate linear regression between intake of each component of the dietary Pattern and score of insomnia.
| Component of the score | Insomnia | |||
|---|---|---|---|---|
| Crude | Adjusted* | |||
| β | p | β | p | |
| Red meat | 0.022 | 0.007 | 0.019 | 0.010 |
| Dairy product | 0.057 | 0.051 | 0.056 | 0.053 |
| Fast foods | 0.023 | 0.128 | 0.024 | 0.128 |
| Fruit | −0.031 | 0.182 | −0.002 | 0.71 |
| Chicken | −0.042 | 0.111 | −0.040 | 0.130 |
| Legumes | 0.023 | 0.256 | 0.023 | 0.258 |
| Whole grains | −0.001 | 0.938 | 0.0001 | 0.974 |
| Refined grains | −0.007 | 0.009 | −0.006 | 0.046 |
| See foods | −0.036 | 0.085 | −0.036 | 0.086 |
| Snacks | 0.019 | 0.127 | 0.020 | 0.108 |
| Nuts | −0.025 | 0.158 | −0.024 | 0.181 |
| Eggs | −0.040 | 0.130 | −0.041 | 0.295 |
| Carbonated beverage | 0.029 | 0.002 | 0.029 | 0.002 |
| Potato | 0.003 | 0.636 | 0.003 | 0.640 |
| Protein (g) | 0.001 | 0.978 | 0.001 | 0.973 |
| Total carbohydrates (g) | −0.001 | 0.903 | −0.001 | 0.903 |
| Total fiber (g) | 0.066 | 0.716 | 0.063 | 0.726 |
| Total fat (g) | −0.049 | 0.023 | −0.047 | 0.029 |
| Total SFAs (g) | −0.166 | 0.116 | −0.162 | 0.126 |
| Total MUFAs (g) | 0.016 | 0.600 | 0.016 | 0.602 |
| Total PUFAs (g) | 0.052 | 0.048 | 0.049 | 0.049 |
| Vitamin C (mg) | −0.010 | 0.352 | −0.013 | 0.229 |
| Vitamin E (mg) | −0.055 | 0.045 | −0.053 | 0.047 |
| Vitamin A (μg) | 0.005 | 0.139 | 0.005 | 0.139 |
Adjusted for age, BMI and WHR. Significance of bold values are p < 0.05.
Odds ratios for insomnia across tertiles of two main dietary patterns are assessed using crude and adjusted models in Table 7. In the first model, regression was adjusted for age, BMI, and WHR. Further adjustments were made for depression, anxiety, and stress. In the other model, additional adjustments were made for daytime sleepiness and cognitive abilities. Using an unadjusted model, adhering to the WD pattern was associated with increased odds of insomnia (OR = 2.25, 95% CI 1.01–4.96; p‐value = 0.003); after controlling for additional potential confounders, the findings remained significant in all 3 adjusted models. No significant relationship was found between adherence to traditional dietary patterns and insomnia in the crude model (OR = 1.4, 95% CI: 0.64–3.05; p‐value = 0.125); and these relationships did not alter post‐adjustment.
TABLE 7.
Multivariate‐adjusted odds ratios (95% CIs) for insomnia across tertiles of dietary pattern scores.
| Crude | Model I | Model II | Model III | |
|---|---|---|---|---|
| Traditional pattern | ||||
| T1 | 1.0 | 1.0 | 1.0 | 1.0 |
| T2 | 0.75 (0.35–1.61) | 0.52 (0.22–1.21) | 0.62 (0.24–1.50) | 0.51 (0.19–1.33) |
| T3 | 1.4 (0.64–3.05) | 1.5 (0.63–3.43) | 1.7 (0.71–4.33) | 1.8 (0.69–4.6) |
| Western pattern | ||||
| T1 | 1.0 | 1.0 | 1.0 | 1.0 |
| T2 | 1.05 (0.48–2.27) | 1.64 (0.68–3.98) | 1.98 (0.78–5.0) | 1.2 (0.44–3.59) |
| T3 | 2.25 (1.01–4.96)* | 4.25 (1.62–11.1)** | 6.0 (2.1‐17.1)*** | 5.9 (1.9‐18.7)** |
Note: Tertile 1 is reference category. Model I: adjusted by age, BMI, and WHR. Model II: additionally, adjusted for depression, anxiety, and stress. Model III: further adjustments for daytime sleepiness and cognitive abilities. *p < 0.05. **p < 0.01. ***p < 0.001.
4. DISCUSSION
The association between dietary habits and sleep is an emerging area of study. In this current study, we have explored the relationship between two dietary patterns, defined by PCA, and insomnia status. The results of the current cross‐sectional investigation suggested that adherence to a WD style, described by high consumption of snacks, fast foods, chicken, and vegetable oil, was correlated with greater odds of insomnia, even after adjustment for several confounding factors. Insomnia is a common sleep disorder that interrupts an individual's life efficiency by decreasing physical and psychological capacity. Previous investigations showed that individuals distressed by sleep complications are prone to a higher risk of being overweight, blood pressure, having diabetes, and having psychological disorders. 16 , 45
Some previous studies have reported remarkable correlations between dietary patterns and insomnia in different populations. 16 , 17 , 46 For instance, adherence to an MD‐style diet was associated with sufficient sleep duration, fewer sleeplessness symptoms, and a lower possibility of changes in sleep duration. 18 , 22 Peltzer et al. found that among Indonesian 15 years and older individuals, the risk of insomnia was higher in those who consumed a low quantity of fruit and vegetables but were high in fast foods and soft drinks. 47 This diet may improve sleep quality because of its high content of fruit, vegetables, dried fruit, grains, and fish and low intake of saturated fat and red meat. 48 Similarly, Lana et al., poor sleep quality in non‐pregnant women was associated with a high intake of meat‐derived protein (red meat or white). Red meat and its by‐products may have a negative impact on how well you sleep due to their high protein content. Tyrosine and tryptophan, two amino acids with the ability to synthesize melatonin, serotonin, and dopamine, may be responsible for the protein's effects on sleep quality (sleep–wake cycle). 49 It has been hypothesized that a high protein intake could lower the tryptophan/tyrosine ratio in the blood, which might lead to a decrease in the production of brain sleep‐inducing substances and, finally, to a worsening of sleep parameters, which is consistent with our results. 49 , 50 , 51
Several studies have demonstrated that a WD has a bidirectional relationship with obesity 47 , 52 and insufficient sleep may lead to an increased risk of being overweight, due to changes in endocrine, behavioral, and neurological systems. 17 , 46 , 53 There is accumulating evidence that suggests that obesity is an important predisposing factor for insomnia and although the nature of this connection is not understood, some longitudinal and cross‐sectional studies indicated that fat individuals had a greater chance of sleep disorders compared to non‐obese ones. 53 , 54 , 55 , 56 Singareddy et al. demonstrated that sleep disorders are two times more frequent in fat individuals, compared to non‐obese. 56 According to epidemiological research, individuals with short sleep duration (SSD) consume more energy‐dense foods with a higher ratio of calories coming from fat or refined carbohydrate than individuals who receive a sufficient amount of sleep. 57 , 58 Also, in comparison to normal sleepers, Indian individuals with insomnia consumed less protein, carbohydrates, thiamine, folate, vitamin B12, and iron. 59 Furthermore, a study has demonstrated that vitamin E can improve sleep quality. 60 Another study investigated the neuroprotective effect of vitamin E on memory damage induced by chronic sleep deprivation in rats, and the results showed that chronic sleep deprivation reduces antioxidant defense mechanisms, and vitamin E treatment through antioxidant activity in the hippocampus prevents this damage. 61 Insufficient sleep, increased intake of high‐energy foods, and beverages such as sugar‐sweetened beverages (SSBs) have all been suggested as predisposing factors for the raised outbreak of obesity. 62
SSBs consumption can cause short sleep duration, due to the effects of sugar and caffeine, and their intake before sleep can influence sleep. 62 , 63 , 64 Caffeine usually prolongs sleep latency, decrees the total sleep period, and worsens sleep quality. Caffeine consumption leads to a reduction in electroencephalographic slow‐wave activity while increasing stage‐1 wakefulness. 65 Additionally, results from a prospective study demonstrated that higher dietary glycemic index (GI) and added sugars may elevate the risk of insomnia occurrence in postmenopausal females. 66 A possible mechanism that explains how a high‐GI diet can elevate insomnia risk is its effects on blood glucose. High blood glucose, occurring after sugar intake, can cause sleepiness. 67 Glycemic load provides insulin demand in healthy persons 68 and hyperinsulinemia can reduce blood sugar to amounts that endanger brain glucose, increasing the production of counter‐regulatory proteins like adrenaline and cortisol. 69 Higher‐GI diets can also trigger inflammatory and immune reactions that inhibit sleep through increasing anti‐inflammatory cytokines secretions. 66 , 70 Lack of sleep can negatively influence physiological and psychological well‐being and affect some behaviors (e.g., food habits). Therefore, health is compromised when an individual has little or insufficient sleep. 71
Although we observed that subjects with elevated adherence to the WD diet, who consume snacks more frequently, are more prone to insomnia, a recent investigation suggested that there is no meaningful statistical relationship between either Western or Iranian traditional dietary styles or insomnia states. However, they indicate that people with healthy dietary habits are less prone to insomnia. 6 Protein shortage has been linked to reduced sleep duration and the amount and source of dietary protein have been reported to characterize the relation between food intake and sleep disturbances and may be the reason for this discrepancy.
Our results showed that PUFA intake is increased in the WD, and higher PUFA intake may also be associated with elevated risk of insomnia. Magzal et al showed that higher concentrations of acetate, butyrate, and total short‐chain fatty acids (SCFAs), were associated with lower sleep efficiency (SE) and longer sleep onset latency (SOL) after controlling for BMI. 72 Previous studies have evaluated the relationship between energy intake and sleep characteristics. 46 , 73 , 74 Although the mechanisms linking decreased energy intake and better nocturnal sleep aren't totally clear, it has been shown that a reduction in dietary energy intake can accelerate sleep initiation in fat individuals. 15 An animal study showed that chronic fat consumption can reduce pre‐pro‐orexin concentrations in the hypothalamus in mice. 75 Orexin is a neuropeptide involved in alertness and adjusting energy balance. We assume that total energy recessive reduction may enhance orexin signaling in the hypothalamus and this can induce stable alertness and shorter daytime sleepiness. Therefore, calorie reduction may enhance the response of orexin neurons. 76 , 77
We found insomnia has a positive connection with depression, anxiety, and stress. This result is consistent with Goyal et al.'s findings, indicating that delayed sleep onset is firmly related to depressive symptoms. 78 Previously, an association was shown between insomnia and a higher risk of depression, anxiety, and cognitive impairment. 79 , 80 , 81 A study, carried out among perinatal women, has shown that insomnia symptoms are related to symptoms of depression and anxiety. 82 Riemann and colleagues demonstrated that insomnia was one of the major predisposing factors for depression symptoms over time. 79 The significant time spent awake at night can lead to nocturnal rumination and increase depression and anxiety. 83
This study demonstrated an association between WD patterns and insomnia status in young women. Our findings indicate that decreasing adherence to the WD pattern can be an efficient way to alleviate sleep disorders. We estimated dietary intake by FFQ and information was collected with a high level of quality control. To show the correlation of WD style with insomnia, we applied multivariate‐adjusted odds ratios using several scores. However, this investigation was subjected to several limitations. Concerning a cross‐sectional design cannot confirm causality. In addition, students undergoing insomnia can have changed their dietary patterns. Also, we did not assess the physical activity and exercise habits. Applying self‐reported information may lead to recall bias and public desirability bias is a major limitation of the present investigation. However, further investigations on patients who endure insomnia are necessary to confirm our findings.
5. CONCLUSION
We found a significant positive relationship between adherence to a WD and insomnia in young women. We found an association between a WD characterized by high consumption of snacks, tea, fast foods, and chicken as an unhealthy dietary pattern correlated with a significant increase in insomnia, depression, anxiety, and stress, and a decrease in quality of life. Although future longitudinal studies are necessary to validate current findings.
AUTHOR CONTRIBUTIONS
Afsane Bahrami designed the study and developed data collection tools. Samira Karbasi contributed to developing the study proposal and drafting the manuscript. Zahra Asadi, Zabihullah Mohaghegh, and Farhad Saeedi performed material preparation, data collection, and data analysis. Gordon A. Ferns revised the final version of the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by Birjand University of Medical Science (BUMS), Iran (grant No: 5243).
CONFLICT OF INTEREST STATEMENT
No conflict of interest.
ETHICS STATEMENT
Ethical approval was obtained from the Birjand University of Medical Sciences and informed written consent was completed by all participants (code: IR.BUMS.REC.1398.402).
Approval of the Research Protocol by an Institutional Review Board: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal Studies: N/A.
Supporting information
Appendix S1:
ACKNOWLEDGMENTS
We thank all the participating mothers who collaborated in line with the research goals of this project.
Karbasi S, Asadi Z, Mohaghegh Z, Saeedi F, Ferns GA, Bahrami A. The relationship between dietary patterns and insomnia in young women. Neuropsychopharmacol Rep. 2023;43:228–238. 10.1002/npr2.12336
DATA AVAILABILITY STATEMENT
The raw data of the article are available as supplementary Excel data files.
REFERENCES
- 1. Carskadon MA, Dement WC. Normal human sleep: an overview. Sleep Med. 2005;4:13–23. [Google Scholar]
- 2. Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012;32(5):309–19. [DOI] [PubMed] [Google Scholar]
- 3. e Silva JAC . Sleep disorders in psychiatry. Metabolism. 2006;55:S40–S4. [DOI] [PubMed] [Google Scholar]
- 4. Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–38. [DOI] [PubMed] [Google Scholar]
- 5. Ellenbogen JM. Cognitive benefits of sleep and their loss due to sleep deprivation. Neurology. 2005;64(7):E25–E7. [DOI] [PubMed] [Google Scholar]
- 6. Sadat S, Salehi‐Sahlabadi A, Pourmasoumi M, Feizi A, Clark CC, Akkasheh G, et al. A healthy dietary pattern may be associated with primary insomnia among Iranian adults: a case‐control study. Int J Vitam Nutr Res. 2020;91:479–90. [DOI] [PubMed] [Google Scholar]
- 7. Léger D, Poursain B, Neubauer D, Uchiyama M. An international survey of sleeping problems in the general population. Curr Med Res Opin. 2008;24(1):307–17. [DOI] [PubMed] [Google Scholar]
- 8. Association AP . American Psychiatric Association: diagnostic and statistical manual of mental disorders. Arlington: American Psychiatric Association; 2013. p. 81. [Google Scholar]
- 9. Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle‐aged female Japanese workers. J Occup Health. 2014;56:359–68. [DOI] [PubMed] [Google Scholar]
- 10. Cunnington D, Junge MF, Fernando AT. Insomnia: prevalence, consequences and effective treatment. Med J Aust. 2013;199:S36–40. [DOI] [PubMed] [Google Scholar]
- 11. Coleman RM, Roffwarg HP, Kennedy SJ, Guilleminault C, Cinque J, Cohn MA, et al. Sleep‐wake disorders based on a polysomnographic diagnosis: a national cooperative study. JAMA. 1982;247(7):997–1003. [PubMed] [Google Scholar]
- 12. Mai E, Buysse DJ. Insomnia: prevalence, impact, pathogenesis, differential diagnosis, and evaluation. Sleep Med Clin. 2008;3(2):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krystal AD. Insomnia in women. Clin Cornerstone. 2003;5(3):41–50. [DOI] [PubMed] [Google Scholar]
- 14. Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21(21):591–643. [Google Scholar]
- 15. Tan X, Alén M, Cheng SM, Mikkola TM, Tenhunen J, Lyytikäinen A, et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle‐aged men. J Sleep Res. 2015;24(4):414–24. [DOI] [PubMed] [Google Scholar]
- 16. Rostami H, Khayyatzadeh SS, Tavakoli H, Bagherniya M, Mirmousavi SJ, Farahmand SK, et al. The relationship between adherence to a dietary approach to Stop hypertension (DASH) dietary pattern and insomnia. BMC Psychiatry. 2019;19(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan X, Alén M, Wang K, Tenhunen J, Wiklund P, Partinen M, et al. Effect of six‐month diet intervention on sleep among overweight and obese men with chronic insomnia symptoms: a randomized controlled trial. Nutrients. 2016;8(11):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castro‐Diehl C, Wood AC, Redline S, Reid M, Johnson DA, Maras JE, et al. Mediterranean diet pattern and sleep duration and insomnia symptoms in the multi‐ethnic study of atherosclerosis. Sleep. 2018;41(11):zsy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gianfredi V, Nucci D, Tonzani A, Amodeo R, Benvenuti A, Villarini M, et al. Sleep disorder, Mediterranean diet and learning performance among nursing students: inSOMNIA, a cross‐sectional study. Ann Ig. 2018;30(6):470–81. [DOI] [PubMed] [Google Scholar]
- 20. Bushman BA. Understanding and using the dietary guidelines for Americans. ACSM's Health & Fitness Journal. 2017;21(1):4–8. [Google Scholar]
- 21. Del Brutto OH, Mera RM, Ha J‐e, Gillman J, Zambrano M, Castillo PR. Dietary fish intake and sleep quality: a population‐based study. Sleep Med. 2016;17:126–8. [DOI] [PubMed] [Google Scholar]
- 22. Campanini MZ, Guallar‐Castillón P, Rodríguez‐Artalejo F, Lopez‐Garcia E. Mediterranean diet and changes in sleep duration and indicators of sleep quality in older adults. Sleep. 2017;40(3):1–9. [DOI] [PubMed] [Google Scholar]
- 23. Jaussent I, Dauvilliers Y, Ancelin M‐L, Dartigues J‐F, Tavernier B, Touchon J, et al. Insomnia symptoms in older adults: associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beigrezaei S, Mazidi M, Davies IG, Salehi‐Abargouei A, Ghayour‐Mobarhan M, Khayyatzadeh SS. The association between dietary behaviors and insomnia among adolescent girls in Iran. Sleep Health. 2022;8(2):195–9. [DOI] [PubMed] [Google Scholar]
- 25. Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease?. Oxford, UK: Oxford University Press; 2001;73(1):1–2. [DOI] [PubMed] [Google Scholar]
- 26. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 27. Asghari G, Momenan M, Yuzbashian E, Mirmiran P, Azizi F. Dietary pattern and incidence of chronic kidney disease among adults: a population‐based study. Nutr Metab. 2018;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirmiran P, Bahadoran Z, Vakili AZ, Azizi F. Western dietary pattern increases risk of cardiovascular disease in Iranian adults: a prospective population‐based study. Appl Physiol Nutr Metab. 2017;42(3):326–32. [DOI] [PubMed] [Google Scholar]
- 29. Alizadeh M, Mohtadinia J, Pourghasem‐Gargari B, Esmaillzadeh A. Major dietary patterns among female adolescent girls of talaat intelligent guidance school, Tabriz. Iran Red Crescent Med J. 2012;14(7):436–41. [PMC free article] [PubMed] [Google Scholar]
- 30. Miller EH. Women and insomnia. Clin Cornerstone. 2004;6(1):S6–S18. [PubMed] [Google Scholar]
- 31. Ayadilord M, Mahmoudzadeh S, Hoseini ZS, Askari M, Rezapour H, Saharkhiz M, et al. Neuropsychological function is related to irritable bowel syndrome in women with premenstrual syndrome and dysmenorrhea. Arch Gynecol Obstet. 2020;302(4):915–23. [DOI] [PubMed] [Google Scholar]
- 32. Ahmadnezhad M, Asadi Z, Miri HH, Ferns GA, Ghayour‐Mobarhan M, Ebrahimi‐Mamaghani M. Validation of a short semi‐quantitative food frequency questionnaire for adults: a pilot study. J Nutr Sci. 2017;3(2):49–55. [Google Scholar]
- 33. Asadi Z, Yaghooti‐Khorasani M, Ghazizadeh H, Sadabadi F, Mosa‐Farkhany E, Darroudi S, et al. Association between dietary inflammatory index and risk of cardiovascular disease in the Mashhad stroke and heart atherosclerotic disorder study population. IUBMB Life. 2019;72:706–15. [DOI] [PubMed] [Google Scholar]
- 34. Pehrsson P, Haytowitz D, Holden J, Perry C, Beckler D. USDA's national food and nutrient analysis program: food sampling. J Food Compos Anal. 2000;13(4):379–89. [Google Scholar]
- 35. Sadeghniiat‐Haghighi K, Montazeri A, Khajeh‐Mehrizi A, Ghajarzadeh M, Alemohammad ZB, Aminian O, et al. The STOP‐BANG questionnaire: reliability and validity of the Persian version in sleep clinic population. Qual Life Res. 2015;24(8):2025–30. [DOI] [PubMed] [Google Scholar]
- 36. Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yazdi Z, Sadeghniiat‐Haghighi K, Zohal MA, Elmizadeh K. Validity and reliability of the Iranian version of the insomnia severity index. Malays J Med Sci. 2012;19(4):31–6. [PMC free article] [PubMed] [Google Scholar]
- 38. Haghighi KS, Montazeri A, Mehrizi AK, Aminian O, Golkhandan AR, Saraei M, et al. The Epworth sleepiness scale: translation and validation study of the Iranian version. Sleep Breath. 2013;17(1):419–26. [DOI] [PubMed] [Google Scholar]
- 39. Corle DK, Sharbaugh C, Mateski DJ, Coyne T, Paskett ED, Cahill J, et al. Self‐rated quality of life measures: effect of change to a low‐fat, high‐fiber, fruit and vegetable enriched diet. Ann Behav Med. 2001;23(3):198–207. [DOI] [PubMed] [Google Scholar]
- 40. Juniper E, O′ byrne P, Guyatt G, Ferrie P, King D. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7. [DOI] [PubMed] [Google Scholar]
- 41. Montazeri A, Vahdaninia M, Mousavi SJ, Omidvari S. The Iranian version of 12‐item short form health survey (SF‐12): factor structure, internal consistency and construct validity. BMC Public Health. 2009;9(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parkitny L, McAuley J. The depression anxiety stress scale (DASS). J Physiother. 2010;56(3):204. [DOI] [PubMed] [Google Scholar]
- 43. Sahebi A, Asghari MJ, Salari R. Validation of depression anxiety and stress scale (DASS‐21) for an Iranian population. J Iran Psychol. 2005;1(4):36–54. [Google Scholar]
- 44. Nejati V. Cognitive abilities questionnaire: development and evaluation of psychometric properties. Adv Cogn Psychol. 2013;15(2):11–9. [Google Scholar]
- 45. Siomos KE, Avagianou P‐A, Floros GD, Skenteris N, Mouzas OD, Theodorou K, et al. Psychosocial correlates of insomnia in an adolescent population. Child Psychiatry Hum Dev. 2010;41(3):262–73. [DOI] [PubMed] [Google Scholar]
- 46. Cheng FW, Li Y, Winkelman JW, Hu FB, Rimm EB, Gao X. Probable insomnia is associated with future total energy intake and diet quality in men. Am J Clin Nutr. 2016;104(2):462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peltzer K, Pengpid S. Prevalence, social and health correlates of insomnia among persons 15 years and older in Indonesia. Psychol Health Med. 2019;24(6):757–68. [DOI] [PubMed] [Google Scholar]
- 48. Bach‐Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274–84. [DOI] [PubMed] [Google Scholar]
- 49. Lana A, Struijk EA, Arias‐Fernandez L, Graciani A, Mesas AE, Rodriguez‐Artalejo F, et al. Habitual meat consumption and changes in sleep duration and quality in older adults. Aging Dis. 2019;10(2):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr. 2003;77(1):128–32. [DOI] [PubMed] [Google Scholar]
- 51. Lindseth G, Murray A. Dietary macronutrients and sleep. West J Nurs Res. 2016;38(8):938–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naja F, Hwalla N, Itani L, Karam S, Sibai AM, Nasreddine L. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13–19 years): a cross‐sectional study. Br J Nutr. 2015;114(11):1909–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29(5):645–9. [DOI] [PubMed] [Google Scholar]
- 54. Palm A, Janson C, Lindberg E. The impact of obesity and weight gain on development of sleep problems in a population‐based sample. Sleep Med. 2015;16(5):593–7. [DOI] [PubMed] [Google Scholar]
- 55. Vgontzas A, Lin H, Papaliaga M, Calhoun S, Vela‐Bueno A, Chrousos G, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond). 2008;32(5):801–9. [DOI] [PubMed] [Google Scholar]
- 56. Singareddy R, Vgontzas AN, Fernandez‐Mendoza J, Liao D, Calhoun S, Shaffer ML, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13(4):346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond). 2008;32(12):1835–40. [DOI] [PubMed] [Google Scholar]
- 58. Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci. 2002;21(2):115–20. [DOI] [PubMed] [Google Scholar]
- 59. Zadeh SS, Begum K. Comparison of nutrient intake by sleep status in selected adults in Mysore. Nutr Res Pract. 2011;5(3):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gromadzińska J, Peplonska B, Sobala W, Reszka E, Wasowicz W, Bukowska A, et al. Relationship between intensity of night shift work and antioxidant status in blood of nurses. Int Arch Occup Environ Health. 2013;86(8):923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alzoubi KH, Khabour OF , Rashid BA, Damaj IM, Salah HA. The neuroprotective effect of vitamin E on chronic sleep deprivation‐induced memory impairment: the role of oxidative stress. Behav Brain Res. 2012;226(1):205–10. [DOI] [PubMed] [Google Scholar]
- 62. Sampasa‐Kanyinga H, Hamilton HA, Chaput J‐P. Sleep duration and consumption of sugar‐sweetened beverages and energy drinks among adolescents. Nutrition. 2018;48:77–81. [DOI] [PubMed] [Google Scholar]
- 63. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–87. [PubMed] [Google Scholar]
- 64. Prather AA, Leung CW, Adler NE, Ritchie L, Laraia B, Epel ES. Short and sweet: associations between self‐reported sleep duration and sugar‐sweetened beverage consumption among adults in the United States. Sleep Health. 2016;2(4):272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clark I, Landolt HP. Coffee, caffeine, and sleep: a systematic review of epidemiological studies and randomized controlled trials. Sleep Med Rev. 2017;31:70–8. [DOI] [PubMed] [Google Scholar]
- 66. Gangwisch JE, Hale L, St‐Onge M‐P, Choi L, LeBlanc ES, Malaspina D, et al. High glycemic index and glycemic load diets as risk factors for insomnia: analyses from the Women's Health Initiative. Am J Clin Nutr. 2020;111(2):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. St‐Onge M‐P, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Advances in Nutrition. 2016;7(5):938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bao J, Atkinson F, Petocz P, Willett WC, Brand‐Miller JC. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am J Clin Nutr. 2011;93(5):984–96. [DOI] [PubMed] [Google Scholar]
- 69. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. [DOI] [PubMed] [Google Scholar]
- 70. Krueger JM, Majde JA. Humoral links between sleep and the immune system. Ann N Y Acad Sci. 2003;992(1):9–20. [DOI] [PubMed] [Google Scholar]
- 71. Chaput J‐P. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014;134:86–91. [DOI] [PubMed] [Google Scholar]
- 72. Magzal F, Even C, Haimov I, Agmon M, Asraf K, Shochat T, et al. Associations between fecal short‐chain fatty acids and sleep continuity in older adults with insomnia symptoms. Sci Rep. 2021;11(1):4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Perron IJ, Pack AI, Veasey S. Diet/energy balance affect sleep and wakefulness independent of body weight. Sleep. 2015;38(12):1893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, et al. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity. 2014;22(5):E55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tanno S, Terao A, Okamatsu‐Ogura Y, Kimura K. Hypothalamic prepro‐orexin mRNA level is inversely correlated to the non‐rapid eye movement sleep level in high‐fat diet‐induced obese mice. Obes Res Clin Pract. 2013;7(4):e251–e7. [DOI] [PubMed] [Google Scholar]
- 76. Jakubowski KP, Hall MH, Lee L, Matthews KA. Temporal relationships between napping and nocturnal sleep in healthy adolescents. Behav Sleep Med. 2017;15(4):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Owens JF, Buysse DJ, Hall M, Kamarck TW, Lee L, Strollo PJ, et al. Napping, nighttime sleep, and cardiovascular risk factors in mid‐life adults. J Clin Sleep Med. 2010;6(4):330–5. [PMC free article] [PubMed] [Google Scholar]
- 78. Goyal D, Gay CL, Lee KA. Patterns of sleep disruption and depressive symptoms in new mothers. J Perinat Neonatal Nurs. 2007;21(2):123–9. [DOI] [PubMed] [Google Scholar]
- 79. Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76(1–3):255–9. [DOI] [PubMed] [Google Scholar]
- 80. Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40(8):700–8. [DOI] [PubMed] [Google Scholar]
- 81. Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. [DOI] [PubMed] [Google Scholar]
- 82. Swanson LM, Pickett SM, Flynn H, Armitage R. Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. J Womens Health. 2011;20(4):553–8. [DOI] [PubMed] [Google Scholar]
- 83. Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1:
Data Availability Statement
The raw data of the article are available as supplementary Excel data files.


